Abstract
In a recent publication by Henry and Royston [J. Acoust. Soc. Am. 142, 1774–1783 (2017)], an algorithm was introduced to calculate the acoustic response to externally introduced and endogenous respiratory sounds within a realistic, patient-specific subglottal airway tree. This work is extended using an efficient numerical boundary element (BE) approach to calculate the resulting radiated sound field from the airway tree into the lung parenchyma taking into account the surrounding chest wall. Within the BE model of the left lung parenchyma, comprised of more than 6000 triangular surface elements, more than 30 000 monopoles are used to approximate complex airway-originated acoustic sources. The chest wall is modeled as a boundary condition on the parenchymal surface. Several cases were simulated, including a bronchoconstricted lung that had an internal acoustic source introduced in a bronchiole, approximating a wheeze. An acoustic source localization algorithm coupled to the BE model estimated the wheeze source location to within a few millimeters based solely on the acoustic field at the surface. Improved noninvasive means of locating adventitious respiratory sounds may enhance an understanding of acoustic changes correlated to pathology, and potentially provide improved noninvasive tools for the diagnosis of pulmonary diseases that uniquely alter acoustics.
I. INTRODUCTION
A. Background and motivation
Respiratory sounds are categorized as normal and abnormal (adventitious). Normal respiratory sounds are generated in healthy airways by physiological unforced breathing. These sounds are generally subdivided into tracheobronchial and vesicular; the former originate in the trachea and larger bronchial airways, and the latter may originate in small branches of the airway tree further from the trachea or from other mechanisms at distal regions of the lung parenchyma.1 The absence or deficiency of normal breath sounds or the manifestation of adventitious sounds may be an indicator of a pulmonary disease. Adventitious respiratory sounds have been classified into several different types, depending on their spectral-temporal characteristics and their location. Different types of adventitious sounds correlate with different pathologies. Conventional classifications of some of the more common adventitious sounds, their multiple pathological connections, and prior strategies to identify their spatial location are reviewed in Sec. I, followed by a summary of the objectives of the present study and the structure of the remainder of this article.
1. Common adventitious respiratory sounds
Crackles, also known as crepitations and rales, correspond to short, discontinuous, and non-stationary sounds.2 They are correlated to numerous pathologies, including bronchiectasis, chronic obstructive pulmonary disease (COPD), edema, and fibrosis.1–4 Crackles are generated when airways that were abnormally closed (due to accumulation of secretions or to airway collapse) open in the inspiration phase or, to a lesser extent, close during the expiration phase.5 The sudden opening of an obstructed airway causes an immediate re-equilibration of the pressures on both sides creating vibrations in the airway walls. Fredberg and Holford6 suggested that the cross-section of the airways that are subjected to the sudden closing and opening is one of the possible factors affecting the spectral-temporal character of crackling sounds. Crackles are generally differentiated into two categories, one being “fine,” having a short duration (∼5 ms) and a higher pitch, with frequency content dominant on the order of 600–700 Hz, but ranging up to 2 kHz.5,6 Often, fine crackles are repetitive, originate in the basal part of the lung, and are altered by changes in body habitus, but not by coughing; they are not transmitted to the mouth. “Coarse” crackles have a longer duration (∼15 ms) and a lower pitch, with spectral content dominant in the range of 300–400 Hz. There is no specific location from where coarse crackles primarily originate. They are often altered by coughing, but not changes in body habitus, and they can be transmitted to the mouth. “Biphasic” crackles are a combination of coarse and fine crackles.
Wheezes are adventitious “continuous” sounds that are typically heard at end of the inspiratory phase or in the early expiratory phase. They are often detected in subjects affected by obstructive diseases, in particular, asthma and COPD,3,4,7,8 as well as in sickle cell patients experiencing an acute pain crisis. Wheezes can be considered the result of the gradual reopening (during inspiration) or closure (during expiration) of a collapsed airway lumen. The reduction of the cross-section of the airway lumen causes the air flow velocity to increase, generating a periodic vibration (flutter) of the airway wall. It has been suggested that their origination is more likely to happen between two to seven generations branching down the tracheobronchial tree.3 Their duration is typically between 100 and 250 ms, and they are generally represented as sinusoidal oscillations with some harmonic distortion. The dominant spectral content is usually between 100 and 1000 Hz with higher harmonics above 1 kHz. The application of theoretical models of flutter in a flow-limited collapsible conduit has shown that the frequency of wheezing could be affected by parameters like the airway wall bending elasticity, thickness, and the tension in the longitudinal direction.7
Stridor, often considered a type of wheezing, refers to intense continuous monophonic sounds best heard over extra-thoracic airways. Stridor tends to be more recognizable during inspiration due to the collapse of extra-thoracic airways because of the lower internal lumen pressure. Stridor is usually considered as an indicator of upper airway obstruction. Signal analysis reveals that stridor tends to have a sinusoidal waveform with fundamental frequency generally above 500 Hz.3,9
Bronchial breath sounds are blasting sounds and are audible throughout inspiration and expiration. They occur due to the lack of air in the lung tissue between the chest wall and the large airways. This is likely to happen in the consolidated lung of pneumonia or pulmonary fibrosis. They are commonly heard in the anterior part over the manubrium of the sternum and in the posterior part between the vertebrae C7 and T3. Generally, they have a higher frequency content with respect to normal sounds: this is because consolidation causes a reduction of the low-pass filtering role of the alveolar region. When auscultated, they are similar to normal tracheal breathing and therefore particularly difficult to recognize.3
Ronchi are defined as musical low-pitched sounds. They are characterized by rapidly damping periodic waveforms. The duration is generally higher than 100 ms and frequency content lower than 300 Hz. They are associated with abnormal airway collapsibility and the creation of breach of fluid films. As they generally clear with coughing, clinical practice suggests that secretions in larger airways play an important role in generating these sounds. Ronchi can be considered as indicators of airway lumen constriction associated with the thickening of the mucosa, edema, or bronchospasm (e.g., bronchitis and COPD).3
Squawks are defined as mixed sound since they are characterized by a crackle followed by a short musical component resembling a wheeze. They are likely to occur in fibrotic pulmonary disorders. Acoustically, their waveform is similar to that of short wheezes, but they are often preceded by a crackle. Squawk mean duration is approximately 90–320 ms. It has been suggested that squawks are produced when an obstructed airway abruptly opens in inspiration, while, for a short amount of time, the airway walls remain coupled in a way similar to the production of wheezes.3
2. Adventitious respiratory sound localization
As reviewed in the last subsection, a variety of lung pathologies and injuries result in adventitious respiratory sounds and/or alter sound transmission pathways, both with spectrally and regionally differing effects that, if properly quantified, might provide additional information about the severity and location of the trauma or disease. The sound characteristics vary from sharp and transient, like crackles, which will have broad spectral content, to sounds that are more “musical” in nature, lasting longer with a dominant frequency or frequencies. In addition to their spectral-temporal character, their location of origin may indicate or at least narrow the possible types of pathological causes and identify the specific lung region that is afflicted. This information can be useful in diagnosis and treatment planning.
To better assess location, simultaneous multi-sensor auscultation methods have been advanced to “map” sounds on the thoracic surface by several groups.7,10–13 Beyond this mapping process, Kompis et al.14 attempted to form a three-dimensional (3D) acoustic image of the likely sound source location(s) by using multiple sensors and assuming “ray acoustic,” i.e., “incident field,” models for how sound propagated away from these sources. In their study, they noted that a useful imaging system for the human lung should (1) be robust with respect to acoustic properties, especially speed of sound which varies and is not precisely known; (2) provide 3D data sets and resulting images that are intuitively interpretable; and (3) be robust with respect to missing sensors or noisy data in individual sensors. The algorithm employed assumed spherically symmetric sound radiation away from a source and propagation throughout the thorax without reflections or standing waves, only dependent on sound speed and a per unit length damping factor; it then triangulated on the likely sources of the sound. In five human subject studies, the algorithm indicated differences in acoustic source location between inspiration and expiration and in one case, the presence of severe consolidation in the lower left lung. Parametric computer simulation studies showed that their imaging algorithm lacked some robustness with respect to acoustic properties, specifically speed of sound. In their idealized mechanical phantom study the algorithm demonstrated a location resolution of nominally 2 cm.14
In modeling the transmission of sound throughout the pulmonary system and chest region, the modeling challenge may be viewed as having two main components: (1) transmission in air through the tracheobronchial tree and (2) coupling to and transmission through the surrounding biological tissues to reach the thoracic surface—namely, the parenchyma, free air, or water/blood region in the case of a pneumothorax or hydro/hemothorax, surrounding muscle and rib cage regions, and outer soft tissues. Many studies have focused on the transmission of sound in the respiratory tract, the tracheobronchial airway tree, with some also considering coupling to modes of wave propagation in the parenchyma.
In previous work of the last author,15 the focus of the study was related to the second component: transmission through the surrounding biological tissues to reach the chest surface. Previous studies of this part of the problem had assumed simplified geometries and homogenized material properties, such as simulating it as a two-dimensional planar geometry,16 an axisymmetric cylindrical geometry, with the outer tissue regions of the chest treated simply as a uniform mass load on the parenchyma,17 or an axisymmetric layered model for the thorax that includes annular regions for the parenchyma, rib cage, soft outer tissue, and skin.18
In the work of Ozer et al.,15 an acoustic boundary element (BE) model was used to simulate sound propagation in the lung parenchyma, neglecting the bronchial airway tree. The BE approach was computationally validated and then compared with experimental studies on lung phantom models devoid of airway tree structure. Parametric studies quantified the effect of different model parameters on the resulting acoustic field within the lung phantoms. The BE model was then coupled with a source localization algorithm to predict the position of an acoustic source within the phantom based on an array of measurements of sound transmitted to the surface of the phantom. Experimental studies validated the BE-based source localization algorithm and showed that the same algorithm does not perform as well if the BE simulation is replaced with an incident field assumption that neglects acoustic reflections and standing wave patterns created within the finite-size lung phantom. The BE model and source localization procedure were then applied to actual lung geometry (lacking airway structure) taken from the National Library of Medicine's Visible Human Project. These numerical studies were in agreement with the studies on simpler geometry in that use of a BE model in place of the incident field assumption improved the predicted acoustic field and source localization results.
B. Objectives
In the present study, the objective is to merge the source localization work of Ozer et al.15 with more recent developments in simulating normal and adventitious respiratory sound generation and transmission within a complex, realistic airway tree.19 The goal is to have a realistic ability to simulate adventitious sound generation, propagation and the resulting acoustic field within the thorax, in order to design and optimize acoustic sensor array measurements of this field, for example using an array of measurements on the thoracic surface (e.g., via stethoscopes), that can localize adventitious sounds spectrally and spatially to provide enhanced diagnostic information noninvasively.
To achieve this goal, we extend the work recently reported,19 which showcased normal ventilatory and adventitious sound propagation throughout a realistic model of the conducting airways, comprised of about 60 000 airway segments. The sound field created on the surface as a result of normal ventilatory and wheeze sounds is calculated using the BE approach. Acoustic source localization is also calculated based on an array of measurements of the sound field on the surface. Like in Ref. 15, simulation results using the BE model are compared to results using an incident field assumption.
II. METHODS
A. Geometry acquisition and preparation
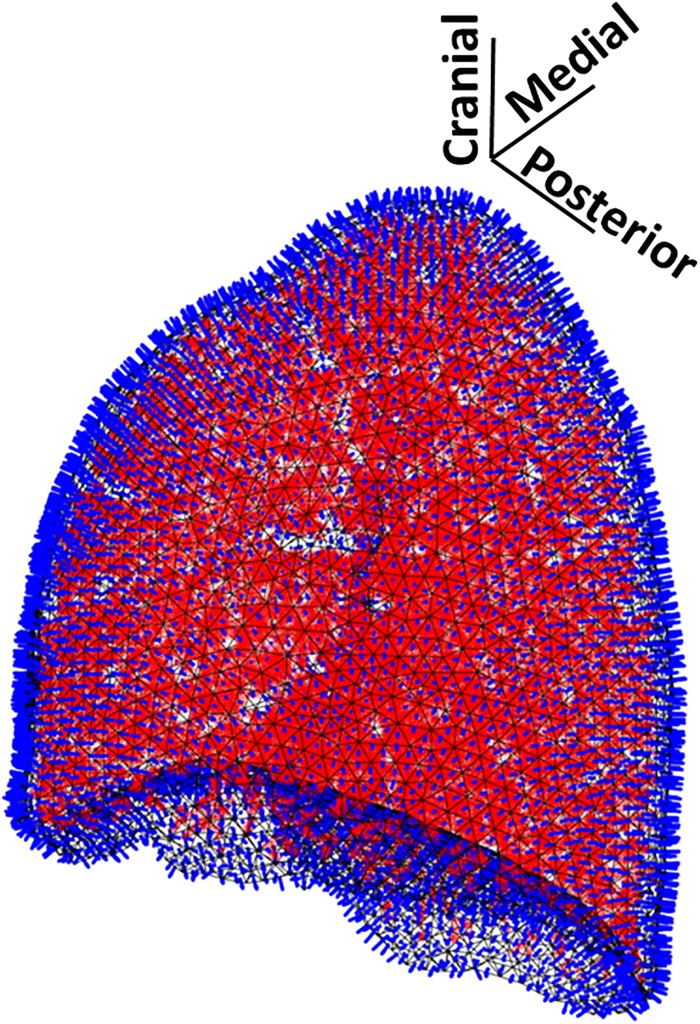
The healthy human conducting airway model and lung segmentation used in this study was obtained from Miyawaki et al.20 The airway tree was generated via a hybrid method including image segmentation plus algorithmic generation that extends beyond the limit of x-ray computed tomography (CT) resolution.21 The full model consisted of 15 generations with 59 834 conducting airway segments. In the current study, the left lung was used as the host volume for the BE model and the left lung airway segments (31 429) were each treated as a single monopole source as described in Sec. II B. Airway segments were identified in matlab by checking if their midpoint resided within the left lung volume (Fig. 1).
FIG. 1.
(Color online) Left lung with 31 429 monopole sources shown as red dots within black parenchymal surface mesh of 6188 BEs. Blue “rods” depict outward normal vectors at BE centroids.
The left lung had 6188 surface (boundary) elements. The volume of the lung is 4.17 liters. The orientation of BEs on the left lung was also checked to ensure that all centroid normals pointed outwards. The left lung was then surrounded by a finite element (FE) model of the thorax that delineated soft tissue, ribcage, and right lung regions (Fig. 2). This was done to calculate external mass loads on each BE of the left lung based on the thickness (as measured along the BE normal) and type of material (bone, lung, and/or soft tissue) that separated its centroid from the thoracic surface. In this study, two modes of acoustic excitation were investigated: (1) sound introduced at the top of the trachea (insonification); and (2) peripheral airway (bronchiole) adventitious sound generation. In the insonification mode, two pathological states were investigated: healthy and fibrotic. In the adventitious sound mode, one pathologic state was investigated: a wheeze generated in a peripheral bronchiole with systemic bronchial constriction present. All cases had geometric and material property changes recently documented in this journal.19 For insonification, a 1 Pa planar sinusoidal wave was applied as the inlet boundary condition at the top of the trachea at 200, 400, or 800 Hz, and the airway acoustic response in the airways was calculated as described previously.19 Once the acoustic field in the airway tree was determined, it was approximated in the BE model of the lung parenchyma using monopole sources as described in Sec. II B 2.
FIG. 2.

(Color online) Thorax geometry: anterior open to depict internal geometry.
B. BE method
1. Primary equations
In previous work15,22 a BE model was introduced for simulation of sound (fast compression wave) propagation within the lung parenchyma governed by the following equation in the frequency () domain:
| (1) |
Here, and are vectors of length of the velocity potentials and normal velocities, respectively, defined at the centroids of the BEs comprising the discretized closed surface of the parenchyma. The acoustic pressure at the ith BE is given by , where denotes the density of the bounded medium (parenchyma) and . Note, implicit in this formulation is that we are multiplying by and taking the real part. Coefficients of the matrices, and , are calculated based on integral expressions (integrating over the surface of a BE) involving and and the Green's function, which relates the acoustic response on the surface of one of the BEs to a source located on another BE. Calculation of and can be found in the Refs. 15, 22, and 23; is the identity matrix.
Sound input is approximated in this expression via the incident velocity potential field created by a finite number of fundamental acoustic sources, such as monopoles, that represent the distribution of sound generated within the bronchial airways located within the parenchyma. An “incident field” assumption is that the incident wave field is in fact the steady state response created throughout the lung to this sound input. This neglects any wave reflections and scattering caused by the lung's boundary conditions. The BE method takes into consideration these boundary effects.
For Eq. (1) to be solved, one also needs boundary conditions relating and . These boundary conditions may range from simple, such as a free or fixed surface for , to more complex with and related via coupled linear algebraic expressions, which themselves may be determined from analysis of another domain exterior to and bordering the BE domain via another BE implementation or FE analysis. In this study, FE analysis of the surrounding chest components was used to determine the boundary conditions placed on the BE model of the lung. An FE approach was favored as it can more easily account for all types of structural wave motion expected (particularly with ribs present), not only compression (dilatational) waves, but also shear (distortional) and surface or interface-related (e.g., Rayleigh, Rayleigh–Lamb, Lamb, Stoneley, etc.) waves.
If using an FE formulation surrounding the BE domain, master degrees of freedom (DOFs) can be chosen to be coincident with the BE DOFs, in other words at the centroid of each BE where and are defined and in an outward direction normal to the BE surface. With this approach, by application of Newton's law we can write that
| (2) |
Here, , , and are structural stiffness, mass, and damping matrices, respectively, for what surrounds the lung parenchyma (chest wall and mediastinum). The matrix is diagonal, with being the area of the ith BE. The length vector represents forces applied externally (if any), such as percussive excitation on the surface.
Generally, , , and can be non-diagonal matrices that couple the DOFs of the BE model. Simplifying assumptions though can lead to decoupled boundary conditions represented by diagonal matrices. For example, consider the case of an inertia load only on the elements. In other words, we will neglect the stiffness and damping on the parenchyma caused by the chest wall and mediastinum; however, we will take into consideration the mass loading. Suppose a layer of material of density and thickness resides on the ith element. Then, is diagonal with and and are neglected. If , Eq. (2) reduces to decoupled scalar equations
| (3) |
This is the approach taken in the present study. Elasticity and damping of the mediastinum and chest wall were neglected. However, the density of material and thickness residing on the ith BE were taken into consideration, including whether this was purely soft tissue, with a density of 1000 kg/m3 or was partially bone, with a density of 1800 kg/m3.
To solve the above set of coupled equations [Eqs. (1) and (3)] to calculate the parenchymal wall normal acoustic velocity requires inverse matrix multiplication in matlab. Even using the backslash operator, the large complex-valued non-sparse matrix size made this step computationally expensive. To reduce computation time c++ MEX files were compiled and ran alongside the rest of the matlab application. In doing so, the complex matrix division compute time was reduced from ∼10 to ∼3 s for the present application. The computation was then sent into a parfor loop to calculate the 6188 complex divisions in parallel, reducing the final runtime for source localization (see Sec. II C) using 2370 grid points spaced 1 cm apart throughout the entire left lung to about 1 h and 30 min (64-bit Windows computer with two 4-core processors at 2.7 GHz and 16 GB RAM).
2. Monopole approximation of the incident field
The acoustic strength, in m3/s for a particular airway segment can be calculated based on the length and radius of that segment, and the complex amplitude of its acoustic radial velocity [obtained from the one-dimensional (1D) branching waveguide calculation]19
| (4) |
If we define a finite (spherical) monopole located at the centroid of an airway segment to have the same acoustic strength and radius r, then its strength is
| (5) |
| (6) |
where is the monopole radial velocity amplitude. In this manner, the midpoint of the cylindrical segments located in the left lung were approximated as monopole sources.
Additionally, the Green's function that relates how the acoustic response at a point is affected by an acoustic monopole point source located at can be evaluated via the following expression:15
| (7) |
| (8) |
where is at the BE centroid and is the lung complex wavenumber for compression waves. The incident field created by a single monopole within the parenchyma is found by multiplying its strength by the Green's function. Thus, the incident field at the centroid of the ith BE created by an array of S monopoles within the parenchyma is given by the following expression:
| (9) |
The value of for each airway segment is calculated via the modified 1D waveguide, and is unique per excitation mode and pathological type.19 Figure 3 shows average monopole strengths for 200, 400, and 800 Hz for all cases considered where each airway segment is classified as of a certain Horsfield order based on the connectivity from terminal segments to the trachea.15 There were S = 31 429 distinct monopole sources located in the left lung, one for each airway segment.
FIG. 3.
(Color online) Average monopole strengths for airway segments categorized by Horsfield order from the trachea (order >30) to the smallest modeled bronchioles (order = 8).
The complex valued wavenumber was calculated based on the fast compression wave speed and attenuation via application of Biot theory24,25 as described in Dai et al.26 The wavenumber was calculated for 200, 400, and 800 Hz. Then, an accompanying wavenumber was calculated for a fibrotic subject by increasing the shear viscoelasticity of the lung tissue by a factor of 5. See Table I for values.
TABLE I.
Parenchymal compression wavenumber values (m−1).
| Frequency (Hz) | Healthy | Fibrotic |
|---|---|---|
| 200 | 24.4 + 8.94j | 24.8 + 8.14j |
| 400 | 28.0 + 29.0j | 28.0 + 27.5j |
| 800 | 37.6 + 67.6j | 37.6 + 67.6j |
C. Source localization
A form of the beamformer equation that works well in this application is15
| (10) |
Here, is a vector of length of the predicted responses at sensor locations due to a monopole source located at of frequency ; is a vector of the measured responses at those sensor locations. Superscript H denotes the Hermitian of the vector. First, a grid is created within the volume where the source will be searched. A hypothetical monopole source is placed at grid locations and the Bartlett processor, Eq. (10), is evaluated.
The grid of hypothetical monopole sources (localization grid) was created using a meshgrid of equidistant spaced points in a cube defined by the range of the lung volume. In the lung localization grid, the interior of the lung was evenly spaced in Cartesian dimensions such that each point was spaced 1 cm from nearest neighbors. Points were then checked in matlab to see if they existed in the lung space or not, and points outside of the lung volume were discarded. The localization grid used in this study can be seen in Fig. 4 and included points.
FIG. 4.
(Color online) The localization grid including hypothetical monopole sources, shown in (top) an axial plane, and (bottom) a sagittal plane. Hypothetical monopole sources shown as red dots.
The Bartlett processor was then evaluated via calculation of the acoustic field per equations [Eqs. (1) and (3)] for each of the hypothetical monopole sources in the localization grid , and via the actual acoustic field . Because each of the acoustic fields estimated from the localization grid required the complex matrix division step, this algorithm was computationally expensive, involving 2370 iterations.
Once the Bartlett processor was evaluated for each of the points in the localization grid, results were indexed into bins via the matlab command histcounts. This is referred to as the Bartlett confidence regions, and signify the predicted areas where the (dominant) acoustic source is most likely situated. Insonification cases were more diffuse because source attenuation occurs over a longer distance as compared to the wheeze case. So, the Bartlett confidence regions for insonification cases were divided into the top 5%, the top 15%–6%, and the top 25%–16%. The Bartlett confidence regions for the wheeze case were divided into the top 10%, the top 20%–11%, and the top 30%–21%.
III. RESULTS
A. BE acoustic fields
Shown in Fig. 5 are the BE calculations of the parenchymal surface acoustic velocity at 200, 400, and 800 Hz for normal and fibrotic cases with sound introduced at the top of the trachea (insonification). Shown in Fig. 6 are the BE and incident field calculations of the parenchymal surface acoustic velocity at 200, 400, and 800 Hz for the bronchoconstricted wheeze case, with sound introduced in a bronchiole in the lower lobe. (Note the lack of any effect from the ribs in the incident field assumption, which neglects boundary conditions.) Table II compares maximum values for the healthy, fibrotic, and wheeze cases calculated using the BE method.
FIG. 5.
(Color online) Normal velocity amplitude at parenchyma surface caused by insonification at 200, 400, and 800 Hz for (a)–(c) healthy and (d)–(f) fibrotic lung cases. Anterior direction to the left, lateral coming out of the page, and cranial at the top of the figure.
FIG. 6.
(Color online) Normal acoustic velocity amplitude at parenchyma surface caused by a wheeze in a lower left lobe bronchiole with system bronchoconstriction at 200 Hz, 400 Hz, and 800 Hz calculated using the BE model (a)–(c) and using incident field assumption (d)–(f). Anterior direction to the left, lateral coming out of the page, and cranial at the top of the figure.
TABLE II.
Maximum velocity amplitude on left parenchyma surface in mm/s using BE method.
| Frequency (Hz) | Normal | Fibrosis | Wheeze |
|---|---|---|---|
| 200 | 2.30 | 2.50 | 1.43 × 10−3 |
| 400 | 0.394 | 2.30 | 2.36 × 10−3 |
| 800 | 0.297 | 0.0965 | 3.82 × 10−3 |
B. Sound localization studies
Shown in Fig. 7 are the results of the source localization algorithm at 400 Hz for the insonified healthy and fibrotic left lungs using the centroid of every BE as a sensor location. Table III provides additional quantitative comparisons between these two cases. Figure 8 shows an overlay of the Bartlett confidence regions of Fig. 7 with the insonified main bronchial airways. Shown in Fig. 9 are the results of the source localization algorithm at 400 Hz for the wheeze case using the BE formulation versus the incident field assumption, this time using the centroid of only nine BEs as sensor locations, specifically a three-by-three grid of sensor locations on the lateral surface with a mean distance of 12.4 mm between them and covering the surface region near the wheeze source with the highest amplitude of response shown in Fig. 6. Figure 10 is an overlay of the Bartlett confidence regions using the BE model using the centroid of every BE as a sensor location, with the wheeze source and acoustic field strength in the upstream airways near the source shown. Table IV provides additional comparisons of source localization computation time and accuracy for the 200 and 400 Hz wheeze cases. Use of the BE formulation is compared to the incident field assumption for both the case of using every BE centroid as a sensor location and using only the three-by-three grid of sensors located near or far from the source and with small (12.4 mm) or large (40 mm) mean spacing between each sensor.
FIG. 7.
(Color online) Source localization for insonified healthy (a)–(c) and fibrotic (d)–(f) lung at 400 Hz. Left lung shown as a light blue (gray) transparent isosurface, with source localizations shown from the thorax front, side, and isometrically. Green (darker gray), yellow (light gray), and red (medium gray) dots represent Bartlett confidence regions in the top 5% (>95%), 85%–95%, and 75%–85%, respectively.
TABLE III.
Bartlett confidence regions as percent of lung volume for insonification at 400 Hz.
| Bartlett confidence regions | Healthy Lung | Fibrotic Lung | Percent Increase |
|---|---|---|---|
| >95% | 1.22% | 3.67% | 301% |
| >85% | 21.7% | 30.1% | 39.0% |
| >75% | 48.1% | 56.5% | 17.5% |
FIG. 8.
(Color online) Bartlett confidence regions of Fig. 7 overlaid with major airway branches with the top 30% of airway wall radial velocity amplitude, colored by magnitude in dB ref 1 m/s. Insonified (a) healthy and (b) fibrotic lung.
FIG. 9.
(Color online) Source localization for a wheeze with bronchoconstriction at 400 Hz. Left lung shown as a light blue (gray) transparent isosurface, with source localizations shown from the thorax front, side, and isometrically. Green (darker gray), yellow (light gray), and red (medium gray) dots represent Bartlett confidence regions in the top 10% (>90%), 80%–90%, and 70%–80%, respectively. Calculated using (a) BE model and (b) incident field assumption, with a 3 × 3 grid of sensors located on the lateral parenchymal surface near the wheeze origination sight.
FIG. 10.
(Color online) Bartlett confidence regions for wheeze case using all BE centroids as sensor locations overlaid with peripheral airway branches within the top 30% of airway wall radial velocity amplitude, colored by magnitude in dB ref 1 m/s. Boxed region of Fig. 9 for BE model case is shown (magnified). The circle denotes the actual wheeze source location, and the × denotes the predicted wheeze source location. Green (darker gray), yellow (light gray), and red (medium gray) dots represent Bartlett confidence regions in the top 10% (>90%), 80%–90%, and 70%–80%, respectively.
TABLE IV.
Comparison of incident field assumption and BE model in localization algorithm for wheeze at 200 and 400 Hz, with less sensors in the localization algorithm: 3 × 3 grid place directly over (near) or 13 cm away cranially (far), with mean grid spacing of 12.4 mm (small) or 40 mm (large).
| Computation Time (Seconds) | Accuracy (mm) | ||||
|---|---|---|---|---|---|
| Incident Field | BE | Incident Field | BE | ||
| With All 6188 BE centroids | 200 Hz | 2.68 | 5,330 | 45.0 | 2.6 |
| 400 Hz | 2.68 | 5,370 | 45.0 | 2.6 | |
| Small 3 × 3 grid near source | 200 Hz | 0.25 | 0.23 | 44.3 | 9.8 |
| 400 Hz | 0.26 | 0.64 | 39.1 | 9.8 | |
| Small 3 × 3 grid far from source | 200 Hz | 0.23 | 0.19 | 107.6 | 66.6 |
| 400 Hz | 0.27 | 0.25 | 104.9 | 126.8 | |
| Large 3 × 3 grid near source | 200 Hz | 0.24 | 0.22 | 143.1 | 6.0 |
| 400 Hz | 0.23 | 0.22 | 147.4 | 6.0 | |
| Large 3 × 3 grid far from source | 200 Hz | 0.23 | 0.24 | 124.5 | 68.0 |
| 400 Hz | 0.23 | 0.23 | 124.6 | 91.5 | |
IV. DISCUSSION
An acoustic BE model of the lung parenchyma was used to calculate the acoustic field on the parenchymal surface caused by the distributed sound field within the complex bronchial airway tree, which, for implementation in the BE model, was approximated using an array of more than 30 000 monopoles of appropriate strength and location. The BE approach was chosen over a FE approach, as the construction of a FE model with the number of acoustic sources needed in this study, in conjunction with the complex parenchymal geometry, would be impractical. The BE model of the parenchyma accounted for mass loading on its surface from the chest wall and mediastinum, including differentiating regions under the ribs versus intercostal spaces.
It is posited that the acoustic response calculated on the parenchymal surface with this mass loading profile may approximate the expected response on the thoracic surface (skin) right above it, if attenuation is added. In other words, while the response amplitude levels may be an overestimate, the relative amplitudes for different regions of the parenchymal surface may correlate to the thoracic surface, including whether or not the acoustic path intersects a rib or is in an intercostal space There is precedence for treating the chest wall as a simple mass load on the parenchyma.17 However, future studies should also take into account the effect of rib cage stiffness and damping, not just its mass, using the approach articulated in Sec. II B and as our group has done previously for simplified phantom model studies.15,22
In the fibrotic case, as compared to the baseline healthy case (Fig. 5), larger amplitudes of acoustic velocity on the lung surface were observed. In addition, regions with high acoustic velocity amplitudes were more dispersed, covering more of the lung surface. This can be attributed to the larger incident field strength on the parenchymal surface as compared to the baseline case, reflective of the spatially broader acoustic field for fibrosis. With stiffer airway walls, more sound energy reaches further into the bronchial tree closer to the parenchymal surface before being transferred to the airway walls and radiating throughout the parenchyma. Aspects of this trend are especially evident in Fig. 3, where the fibrotic pathology significantly increased monopole strength over a broader range of Horsfield orders for 200 and 400 Hz.
In the mass-loaded BE simulations of insonification for baseline healthy and fibrotic cases, there is very little difference in the parenchymal surface normal acoustic velocity based on the minor change in the Kp values alone (Table I). The main source of difference is the input 1D branching waveguide calculations for the airway tree producing different monopole strengths. These differences in BE parenchymal surface velocities also produced significant differences in sound source localization. As observed in Fig. 7 and quantified in Table III, the Bartlett confidence region, an estimate of the location of the most dominant sound sources, increased in the fibrotic case, relative to the baseline healthy case. While it generally may not be desirable in a sound localization strategy to have an increased region of probable sound source locations, determining that sound sources are more widely distributed may be diagnostically useful.
In the wheeze case, even though there were over 30 000 monopole sources, the Bartlett beamformer accurately predicted the location of the dominant (originating) source when coupled with the mass-loaded BE calculation of the parenchymal sound field (Fig. 9 and Table IV). The distance between the actual originating location of the wheeze source and predicted location using the BE model was consistently 2.6 mm at 200, 400, and 800 Hz, an inevitable error due to the grid spacing of 1 cm used in the beamformer algorithm. When coupled with the incident field assumption, the Bartlett beamformer algorithm showed significantly less precision in estimating the sound source location at all frequencies considered.
Reducing the number of sensor locations used in the beamforming calculations from 6188 to only nine near the source significantly decreased the computation time from more than one hour to less than a second (comparable to incident field assumption calculation times), while still maintaining better than 1 cm accuracy for the localization calculation based on the BE model. The number of sensors had little effect on source localization accuracy based on the incident field assumption. (And source localization accuracy may worsen when rib cage viscoelasiticity is accounted for in future studies.) A reduced sensor set is more realistic for experimental studies and clinical adaptation in the future. Additional studies summarized in Table IV show that, while distance of the sensor array from the source does decrease accuracy, in all cases, localization using the BE model outperforms use of an incident field model.
A wheeze with a dominant frequency at 200, 400, or 800 Hz was studied here as a representative example of adventitious sounds. Other adventitious sounds described in Sec. I, such as crackles with broadband spectral content, could also be analyzed in the same way by identifying prominent frequencies within their spectrum and conducting the analysis single frequency by single frequency. Or, the strategies reported herein could be adapted to the time domain using the inverse Fourier transform followed by convolution and correlation, and other strategies applicable to systems governed by linear system theory. This is left for future work.
V. CONCLUSION
In a recent publication19 an efficient analytical algorithm was introduced for calculating the acoustic response to externally introduced and endogenous respiratory sounds generated within a realistic, patient-specific subglottal airway tree comprised of nearly 60 000 distinct airway segments. This work was extended using an efficient numerical BE approach to calculate the resulting radiated sound field within the lung parenchyma and surrounding chest wall. Within the BE model of the left lung parenchyma, comprised of more than 6000 triangular surface elements, more than 30 000 elementary acoustic sources, monopoles, were used to approximate the complex airway-originated acoustic source field. The surrounding chest wall and mediastinum are modeled as a complex boundary condition on the parenchymal surface. Three cases were considered: (1) a healthy lung, with sound introduced externally into the airway lumen at the top of the trachea (insonification); (2) a fibrotic lung with the same sound introduction as the healthy case; and (3) a bronchoconstricted lung that had an internal acoustic source introduced in one of its bronchioles, approximating a wheeze. An acoustic source localization algorithm, based on a Bartlett beamforming approach, was then used to estimate the sound source location based solely on the acoustic field at the parenchymal surface or a small portion of it, which correlates with the field present on the thoracic surface. For the wheeze case, when the beamforming algorithm was coupled with the BE model, the source location was accurately predicted regardless of its frequency content and for as little as nine sensors. The beam-formed predictions were less accurate when, in place of the BE model, a simplified “ray acoustics” approximation (incident field) was used that neglects boundary effects and assumes sound propagates and attenuates uniformly with distance from the source (spherical symmetry).
Future studies could incorporate a more accurate model of chest wall mechanical structure to include coupled viscoelastic properties. Then, the output of the BE simulation could be used as the input to a FE model of the chest wall to predict the acoustic field on the thoracic surface. Source localization could then be based on a finite set of sensor locations on the thoracic surface.
Also, while individual components of the realistic complex bronchial tree acoustic modeling, the BE approach for parenchymal acoustics and source localization have been experimentally and numerically validated in previous studies, validation of their combination is needed, using phantom models that incorporate some degree of complex airway structure within a lung-like material surrounded by material mimicking soft and hard (rib-like) tissue. This would provide confidence in the approach, before it is adapted and translated to the clinic for patient-specific analysis of respiratory sounds to improve noninvasive diagnosis and monitoring of pulmonary pathology.
ACKNOWLEDGMENTS
Financial support from NIH Grant No. R01-EB012142 is acknowledged. Conducting airway geometry provided by C.-L. Lin supported by NIH Grants Nos. R01-HL094315 and U01-HL114494. L. Aliboni is acknowledged for his assistance with the review of the literature.
References
- 1. Bahoura M., “Pattern recognition methods applied to respiratory sounds classification into normal and wheeze classes,” Comput. Biol. Med. 39, 824–843 (2009). 10.1016/j.compbiomed.2009.06.011 [DOI] [PubMed] [Google Scholar]
- 2. Siddiqui A. and Ahmed S., “Pulmonary manifestations of sickle cell disease,” Postgrad. Med. J. 79, 384–390 (2003). 10.1136/pmj.79.933.384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bohadana A., Izbicki G., and Kraman S. S., “Fundamentals of lung auscultation,” N. Engl. J. Med. 370, 744–751 (2014). 10.1056/NEJMra1302901 [DOI] [PubMed] [Google Scholar]
- 4. Wang Z., Jean S., and Bartter T., “Lung sound analysis in the diagnosis of obstructive airway disease,” Respiration 77, 134–138 (2009). 10.1159/000178023 [DOI] [PubMed] [Google Scholar]
- 5. Piirila P. and Sovijarvi A. R. A., “Crackles: Recording, analysis and clinical significance,” Eur. Respir. J. 8, 2139–2148 (1995). 10.1183/09031936.95.08122139 [DOI] [PubMed] [Google Scholar]
- 6. Fredberg J. J. and Holford S., “Discrete lung sounds: Crackles (rales) as stress-relaxation quadrupoles,” J. Acoust. Soc. Am. 73, 1036–1046 (1983). 10.1121/1.389151 [DOI] [PubMed] [Google Scholar]
- 7. Pasterkamp H., Kraman S. S., and Wodicka G. R., “State of the art advances beyond the stethoscope,” Am. J. Respir. Crit. Care Med. 156, 974–987 (1997). 10.1164/ajrccm.156.3.9701115 [DOI] [PubMed] [Google Scholar]
- 8. Taplidou S. A. and Hadjileontiadis L. J., “Wheeze detection based on time-frequency analysis of breath sounds,” Comput. Biol. Med. 37, 1073–1083 (2007). 10.1016/j.compbiomed.2006.09.007 [DOI] [PubMed] [Google Scholar]
- 9. Reichert S., Gass R., Brandt C., and Andrès E., “Analysis of respiratory sounds: State of the art,” Clin. Med. Circ. Respirat. Pulm. Med. 2, 45–58 (2008). 10.4137/CCRPM.S530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Benedetto G., Dalmasso F., and Spagnolo R., “Surface distribution of crackling sounds,” IEEE Trans. Biomed. Eng. 35, 406–412 (1988). 10.1109/10.1403 [DOI] [PubMed] [Google Scholar]
- 11. Bergstresser T., Ofengeim D., Vyshedskiy A., Shane J., and Murphy R., “Sound transmission in the lung as a function of lung volume,” J. Appl. Physiol. 93, 667–674 (2002). 10.1152/japplphysiol.00050.2002 [DOI] [PubMed] [Google Scholar]
- 12. Charleston-Villalobos S., Cortes-Rubiano S., Gonzalez-Camarena R., Chi-Lem G., and Aljama-Corrales T., “Respiratory acoustic thoracic imaging RATHI: Assessing deterministic interpolation techniques,” Med. Biol. Eng. Comput. 42, 618–626 (2004). 10.1007/BF02347543 [DOI] [PubMed] [Google Scholar]
- 13. Paciej R., Vyshedskiy A., Shane J., and Murphy R., “Transpulmonary speed of sound input into the supraclavicular space,” J. Appl. Physiol. 94, 604–611 (2003). 10.1152/japplphysiol.00568.2002 [DOI] [PubMed] [Google Scholar]
- 14. Kompis M., Pasterkamp H., and Wodicka G. R., “Acoustic imaging of the human chest,” Chest 120, 1309–1321 (2001). 10.1378/chest.120.4.1309 [DOI] [PubMed] [Google Scholar]
- 15. Ozer M. B., Acikgoz S., Royston T. J., Mansy H. A., and Sandler R. H., “Boundary element model for simulating sound propagation and source localization within the lungs,” J. Acoust. Soc. Am. 122, 657–671 (2007). 10.1121/1.2715453 [DOI] [PubMed] [Google Scholar]
- 16. Royston T. J., Zhang X., Mansy H. A., and Sandler R. H., “Modeling sound transmission through the pulmonary system and chest with application to diagnosis of a collapsed lung,” J. Acoust. Soc. Am. 111, 1931–1946 (2002). 10.1121/1.1452742 [DOI] [PubMed] [Google Scholar]
- 17. Wodicka G. R., Stevens K. N., Golub H. L., Cravalho E. G., and Shannon D. C., “A model of acoustic transmission in the respiratory system,” IEEE Trans. Biomed. Eng. 36, 925–934 (1989). 10.1109/10.35301 [DOI] [PubMed] [Google Scholar]
- 18. Vovk V., Grinchenko V. T., and Oleinik V. N., “Modeling the acoustic properties of the chest and measuring breath sounds,” Acoust. Phys. 41, 667–676 (1995). [Google Scholar]
- 19. Henry B. and Royston T. J., “A multiscale analytical model of bronchial airway acoustics,” J. Acoust. Soc. Am. 142, 1774–1783 (2017). 10.1121/1.5005497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miyawaki S., Choi S., Hoffman E., and Lin C.-L., “A 4DCT imaging-based breathing lung model with relative hysteresis,” J. Comput. Phys. 326, 76–90 (2016). 10.1016/j.jcp.2016.08.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Miyawaki S., Tawhai M., Hoffman E., Wenzel S., and Lin C.-L., “Automatic construction of subject-specific human airway geometry including trifurcations based on a CT-segmented airway skeleton and surface,” Biomech. Mod. Mechanobiol. 16, 583–596 (2016). 10.1007/s10237-016-0838-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Acikgoz S., Ozer M. B., Royston T. J., Mansy H. A., and Sandler R. H., “Experimental and computational models for simulating sound propagation within the lungs,” ASME J. Vib. Acoust. 130, 021010 (2008). 10.1115/1.2827358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Royston T. J., Ozer M. B., Acikgoz S., Mansy H. A., and Sandler R. H., “Advances in computational modeling of sound propagation in the lungs and torso with diagnostic applications,” in Vibration and Acoustics in Biomedical Applications: Imaging, Characterization and Diagnostics, edited by Fatim M. and Al-Jumaily A. ( ASME, New York, 2008), Chap. 9, pp. 217–248. [Google Scholar]
- 24. Biot M. A., “Theory of propagation of elastic waves in a fluid-saturated porous solid. I. Low-frequency range,” J. Acoust. Soc. Am. 28, 168–178 (1956). 10.1121/1.1908239 [DOI] [Google Scholar]
- 25. Biot M. A., “Theory of propagation of elastic waves in a fluid-saturated porous solid. II. Higher frequency range,” J. Acoust. Soc. Am. 28, 179–191 (1956). 10.1121/1.1908241 [DOI] [Google Scholar]
- 26. Dai Z., Peng Y., Mansy H. A., Sandler R. H., and Royston T. J., “Comparison of poroviscoelastic models for sound and vibration in the lungs,” ASME J. Vib. Acoust. 136, 051012 (2014). 10.1115/1.4026436 [DOI] [PMC free article] [PubMed] [Google Scholar]