Abstract
Over the last 50 years, quantitative methodology has made important contributions to our understanding of the cellular composition of the human brain. Not all of the concepts that emerged from quantitative studies have turned out to be true. Here, I examine the history and current status of some of the most influential notions. This includes claims of how many cells compose the human brain, and how different cell types contribute and in what ratios. Additional concepts entail whether we lose significant numbers of neurons with normal aging, whether chronic alcohol abuse contributes to cortical neuron loss, whether there are significant differences in the quantitative composition of cerebral cortex between male and female brains, whether superior intelligence in humans correlates with larger numbers of brain cells, and whether there are secular (generational) changes in neuron number. Do changes in cell number or changes in ratios of cell types accompany certain diseases, and should all counting methods, even the theoretically unbiased ones, be validated and calibrated? I here examine the origin and the current status of major influential concepts, and I review the evidence and arguments that have led to either confirmation or refutation of such concepts. I discuss the circumstances, assumptions and mindsets that perpetuated erroneous views, and the types of technological advances that have, in some cases, challenged longstanding ideas. I will acknowledge the roles of key proponents of influential concepts in the sometimes convoluted path towards recognition of the true cellular composition of the human brain.
Keywords: History, human brain, cell counting, stereology, glia neuron ratio, aging
1. Introduction
Our understanding of the human brain – and of any brain – is based on series of facts and findings that are reported in the scientific literature. Some of the original findings were made decades ago, and typically have been scrutinized in subsequent scientific studies using increasingly sophisticated methodologies. A major mechanism of scientific progress is the replication and acceptance, or refutation and refinement of original findings, with evaluation by peers prior to and after incorporation into the scientific literature. This is how scientific concepts eventually become common knowledge and lay the foundation and framework for further research. The review and acceptance of new facts by peer-review is an indispensable tool of scientific advance that validates scientific knowledge and is supposed to be self-correcting (Committee on the Conduct of Science, 1989; Ioannidis, 2012). It is common that technological advances challenge old concepts and occasionally lead to refutation or amendments. Accordingly, some concepts remain in flux, to be molded by future investigators.
The human brain poses special challenges for quantification of its cellular composition. This is due not only to its large size, the large number of cells involved, and the surprisingly large variation in cell numbers between individuals, but also to the fact that, for obvious ethical reasons, researchers have much less control and access to well-preserved human brains as compared to animal brains (Peters et al., 1998; Pannese, 2011). The latter constraint has consequences for the quality and morphology of the tissues and their optimal processing for quantitative analyses.
Although studies attempted to quantify aspects of the human brain as early as the 1800s (reviewed in: Haug, 1986; von Bartheld et al., 2016), the first serious and systematic studies, with increasingly powerful methodologies, appeared about 50 years ago. Parts of the human brain were quantified using one of four basic types of methodologies, as recently reviewed (von Bartheld et al., 2016): 1. Histology of thin sections for counting of stained cell profiles, with estimates adjusted by correction factors (Abercrombie, 1946; Clarke and Oppenheim, 1995); 2. Stereology (design-based methods) using thick sections and systematic random sampling (Pakkenberg and Gundersen, 1997; Schmitz and Hof, 2005; Geuna and Herrera-Rincon, 2015); 3. DNA extraction to calculate total DNA and extrapolation to estimate cell numbers (Heller and Elliot, 1954; Dobbing and Sands, 1973); and 4. Collection of nuclei in suspension, and calculation of cell numbers based on volume samples (Nurnberger and Gordon, 1957; Johnson and Erner, 1972), an approach that was recently optimized with the design of the isotropic fractionator (Herculano-Houzel and Lent, 2005; Azevedo et al., 2009; Herculano-Houzel et al., 2015).
In this review, I examine the origin, original evidence, replication or challenges, arguments, and current status of some of the most influential concepts and questions about the quantitation and cellular composition of the human brain. I provide the historical context, explain the arguments, evaluate or assess the quality of evidence, and categorize the concepts as either refuted, confirmed, questionable or unsure when the evidence is conflicting and further research is needed. I refer to the original publications that form the basis of my evaluation so that readers can verify statements and can confirm conclusions by consulting relevant sources.
2.1. The concept of a 10:1 glia-neuron ratio (GNR)
The concept of a 10:1 GNR for the entire human brain has dominated the field over the last 50 years (Fig. 1). It originated with Holger Hyden's assertions in the 1960s (Hyden, 1960, 1967) (Fig. 2A), probably based on his model system of the vestibular nuclei in the brainstem which indeed have a large GNR. The claims of an overabundance of glia over neurons were not backed up by experimental evidence, and this may have contributed to the fact that the claims evaded customary peer review. Despite having an unvalidated status, the concept was incorporated into all major textbooks, including the most prominent books by leading neuroscientists such as Stephen Kuffler and Eric Kandel (Fig. 2B, C) (Kuffler and Nicholls, 1976; Kandel and Schwartz, 1981, 1985; Kandel et al., 1991, 2000; reviewed in: von Bartheld et al., 2016), thereby allowing the erroneous concept of a 10:1 GNR to become “common knowledge” for five decades. Instances of conflicting evidence reported by others were rare (e.g., Haug, 1986; Andersen et al., 1992), and they were basically overlooked. Different components of the brain have different GNRs (for reasons yet to be determined), and accordingly the GNR for the entire human brain is a composite of the different GNRs for its individual parts. Since GNRs are strongly conserved in evolution, they are thought to be physiologically important (Herculano-Houzel, 2014). A common mistake was to examine just one part of the brain, and to assume that the rest of the brain is composed in the same way, which prompted false conclusions (von Bartheld et al., 2016). For these reasons, the myth of a 10:1 GNR persisted until 2009, when a novel technical advance, the isotropic fractionator, revealed and effectively communicated that the GNR was less than 1:1 in human brains (Azevedo et al., 2009) – a finding that turned out to be also supported by data obtained with histological methods, including stereology (von Bartheld et al., 2016). The report by Azevedo et al. (2009) challenged and refuted the original concept of an overabundance of glial cells, and eventually led to a reversal of the GNR from 10:1 to 0.7:1, as first advocated by Herbert Haug (1986) (Fig. 3A). The concept of a lower number of glial cells than neurons in human brains is now becoming increasingly accepted in the field (Hilgetag and Barbas, 2009; Lent et al., 2012; Yuhas and Jabr, 2012; Zorzetto, 2012; Jarrett, 2015; von Bartheld et al., 2016) (Fig. 1).
Fig. 1.
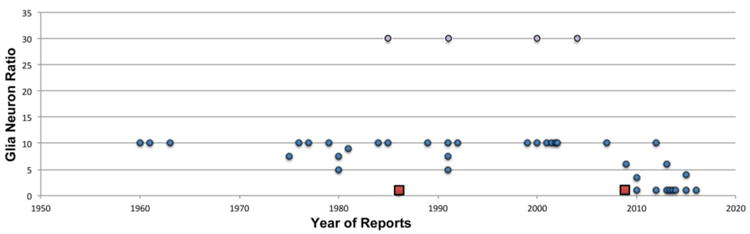
Reports of the glia neuron ratio (GNR) in human brains from the 1960s to the current time. Note the obvious outliers by Kandel's and other's text books with a GNR of 30 (10–50), indicated by purple dots, and the pioneering reports of a GNR of 0.7–1 by Haug (1986), and by Azevedo et al. (2009) that used the isotropic fractionator, indicated by red squares. Data are compiled from the Table 5 published by von Bartheld et al. (2016).
Fig. 2.

A-C. Photographs of individuals who had a major influence on concepts regarding the cellular composition of the human brain. This composite shows the three main proponents of the 10:1 glia-neuron ratio and the notion of one trillion glia cells in the human brain. A. Holger Hydén was a professor at the University of Goteborg, Sweden, and the first to claim a 10:1 glia-neuron ratio in the 1960s (Hyden, 1960, 1967). Photo: Courtesy of Anders Hamberger, photography: Lennart Nilsson. Reproduced with permission. B. Stephen Kuffler, professor at Harvard and the “father of modern neuroscience,” was the first to promote the “at least 10:1 ratio” in his influential textbook in 1976 (Kuffler and Nicholls, 1976). Photo by Bachrach, Boston. Reproduced with permission. C. Eric Kandel, professor at Columbia University and Nobel laureate (2000), contributed to the perpetuation of the notion of one trillion glial cells by stating that glia outnumber neurons 10–50fold in his many editions of “Principles of Neural Science” – the “bible of neuroscience” visible in the bookshelf (Kandel and Schwarz, 1981 Kandel and Schwarz, 1985). Photo credit: Columbia University. Reproduced with permission.
Fig. 3.
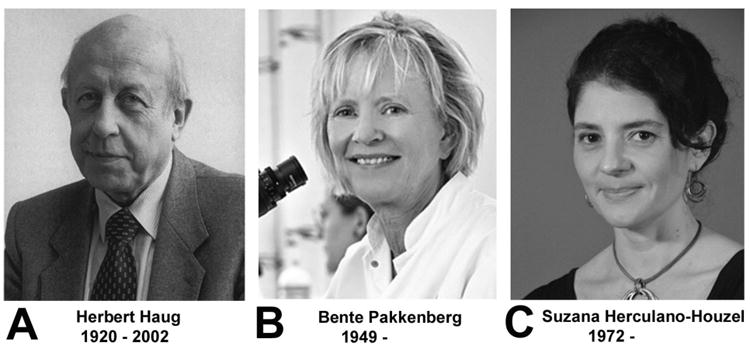
A-C. Photographs of individuals who had a major influence on concepts regarding the cellular composition of the human brain. This composite shows the three main investigators who's work debunked several myths, including the claim of a 10:1 glia-neuron ratio and the trillion glia myth. A. Herbert Haug, professor at the University of Lübeck, Germany, correctly estimated in 1986 the glia-neuron ratio to be less than 1, based on his own cell counts, and he refuted the prevailing myth of neuronal fallout with normal aging. Photograph originally published by Wolfgang Kühnel (Annals of Anatomy, 185:293, 2003), and reproduced with permission by Elsevier. B. Bente Pakkenberg, professor at Bispebjerg University Hospital, Denmark, a pioneer in the use of stereological methods, determined cell numbers in numerous human brain structures, including cerebral cortex, cerebellum, and the effect of aging, diseases, and alcohol use on neuron numbers in human brains. Photograph by Claus Peuckert, claus peuckert photography, reproduced with permission. C. Suzana Herculano-Houzel, professor at Vanderbilt University, developed a highly efficient alternative counting method, the isotropic fractionator. Historically, this method was the first that revealed and effectively communicated the true number of non-neuronal cells and the true ratio of non-neuronal to neuronal cells in human brains. Photo credit: Luiza Herculano-Houzel. Reproduced with permission.
2.2. The concept of 1–50 trillion glial cells in the human brain
Glial cells are smaller than most neurons and therefore are more difficult to identify and count in histological sections than neurons. Information about glial cell numbers has always been less certain than information about neuron numbers. However, the ratio between glia and neurons could be determined with a reasonable degree of certainty in distinct regions of the brain. Neuron numbers were estimated within relatively narrow ranges for certain brain regions; however, no stereological study has yet attempted to estimate glia numbers for the entire human brain. Therefore, prior to the development of the isotropic fractionator counting method, glia numbers for the whole brain were largely based on the presumed fact of a 10:1 glia-neuron ratio (GNR), which was used for calculations. Accordingly, glia numbers were postulated for the whole brain to be at least 10 times the number of neurons (which was known to be about 85–100 billion), yielding numbers of 1 trillion or more for glial cells. Several prominent textbooks increased that number by an additional factor of 10–50, notably the Kandel text book editions from 1981 and 1985 (Kandel and Schwartz, 1981, 1985), where the neuron number was erroneously stated at 1 trillion, so that a total of up to 50 trillion glial cells was postulated to exist in the human brain. These numbers conflicted with histology-based estimates of maximally 130 billion glial cells (Blinkov and Glezer, 1968) or 50 billion glial cells (Haug, 1986), but these discrepancies were not discussed in the textbooks or reviews. It was not until 2009 that the dogma of abundant glia numbers was challenged, in the paper by Azevedo et al. (2009), when the isotropic fractionator technique revealed no more than 85 billion non-neuronal cells in the human brain, with only a fraction of these – albeit a major fraction – being glial cells. Thus, the new methodology supported the estimates of 40–50 billion glial cells, as published by Herbert Haug (1986) (Fig. 3A). His estimate has been shown to be plausible based on both histological evidence as well as evidence from the isotropic fractionator, which was recently compiled (von Bartheld et al., 2016). Accordingly, the total glia number in human brains is now thought to be about 40–50 billion glial cells, which is a small fraction of the 1–50 trillion postulated in major text books and reviews just a few years ago.
2.3. The concept of a 5:3:1 ratio for neurons, glia, endothelial cells in the human brain
In the history of cell counting, a consensus about the total number of neurons in the human brain developed earlier than for glial cells. The review of Williams and Herrup (1988) provided a plausible number of about 85 billion neurons. This number of just under 100 billion neurons was widely reported in peer-reviewed articles and text books (Soper and Rosenthal, 1988; von Bartheld et al., 2016). It was supported by studies using histological/stereological techniques (Haug, 1986) as well as isotropic fractionator methodology (Azevedo et al., 2009; Andrade-Moraes et al., 2013), a technique that was developed in 2005 (Herculano-Houzel and Lent, 2005) (Fig. 3C). Once a plausible number of glial cells in the human brain had been established at about 40–50 billion, the remaining major cell type left to be quantified were the endothelial cells. Since the number of endothelial cells is essentially the difference between the total non-neuronal cells and the total glial cells, this number can be calculated by simple subtraction. Alternatively, it can be determined by histological analyses, when percentages of glial cells and endothelial cells are compiled separately based on histological appearance (Garcia-Cabezas et al., 2016). Such percentages are tissue-specific and range from 30% of all non-neuronal cells in neocortex (= 23.6% of all cells in neocortex; Nurnberger, 1958; Blinkov and Glezer, 1968; Brasileiro-Filho et al., 1989; Bjugn and Gundersen, 1993; Lyck et al., 2009; Garcia-Amado and Prensa, 2012; Bahney and von Bartheld, 2014) to 85% of all non-neuronal cells in the cerebellum (a relatively high percentage, because of the low glia number) (= 16–19% of all cells in the cerebellum, Andersen et al., 1992; Andersen et al., 2012; Azevedo et al., 2009; Andrade-Moraes et al., 2014). Contrary to the notion that endothelial cell numbers are negligible (Bass et al., 1971; Herculano-Houzel, 2011), it is now thought that endothelial cells in the whole human brain make up about 25% of all non-neuronal cells, with the rest (75%) of non-neuronal cells being glial cells, thus generating a ratio of about 5:3:1 for neurons, glia and endothelial cells in the human brain (Bahney and von Bartheld, 2017) (Fig. 4).
Fig. 4.
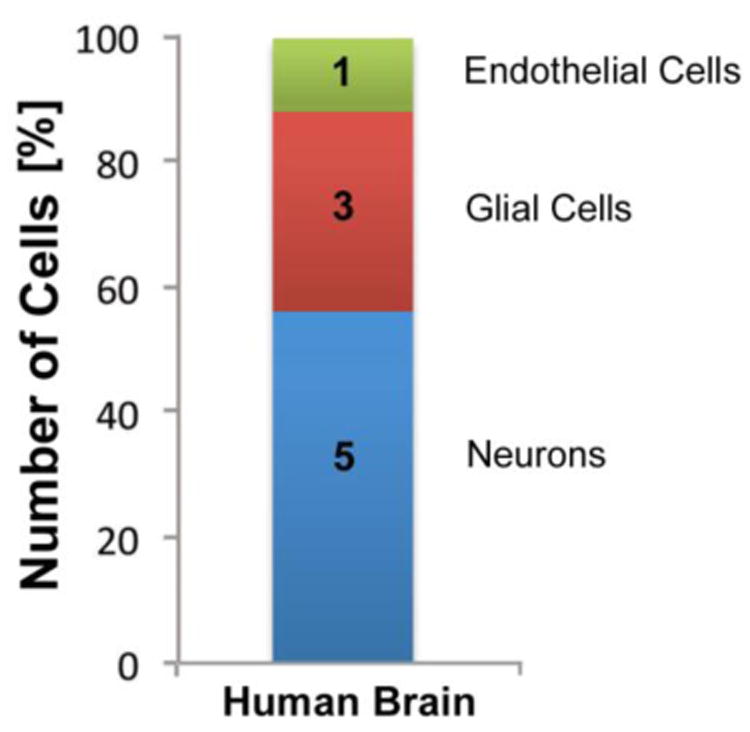
Cellular composition of the human brain: the concept of a 5:3:1 numerical ratio of neurons (blue), glial cells (red), and endothelial cells (green). Data and concept as originally designed in Bahney and von Bartheld (2017), and based on current estimates of the numbers of neurons, glia and endothelial cells (von Bartheld et al., 2016).
2.4. The concept of loss (“fall-out”) of cortical neurons with normal aging
The concept that humans lose a substantial number of cortical neurons during normal aging (“neuronal fall-out”) was based on cell counting studies in the 1950s to 1980s (Brody, 1955; Brody, 1970; Colon, 1972; Hanley, 1974; Devaney and Johnson, 1980; Henderson et al., 1980; Curcio et al., 1982; Anderson et al., 1983). Animal studies had suggested that at least some aged animals have significantly reduced numbers of neurons in their brains when compared to younger animals (Johnson and Erner, 1972; reviewed by Hanley, 1974), and Brody's and other's cell counting studies appeared to confirm this for human neocortex. These studies indicated that between 35% and 55% of cortical neurons were lost during adulthood, corresponding to a nearly 1% loss per year, and additional studies by Devaney and Johnson (1980), Henderson et al. (1980), and Anderson et al. (1983) reported similar losses (Fig. 5). Accordingly, this fall-out of about half of all cortical neurons was deemed in the 1970s and 1980s to be a normal consequence of aging, and senility was seen as an inevitable consequence of cortical neuron loss, which was thought responsible for the expected decline in intellectual abilities (Anderton, 1997; Kausler et al., 2007; Pannese, 2011). This made for a depressing outlook on life for senior citizens. Although correlation alone cannot be regarded as evidence for causation, it may not be a coincidence that suicidal ideation in the elderly spiked in the 1970s and 1980s, since mental decline is among the disabilities most feared in old age (Meehan et al., 1991; McKeown et al., 2006; Schmutte et al., 2009; Deary, 2012).
Fig. 5.
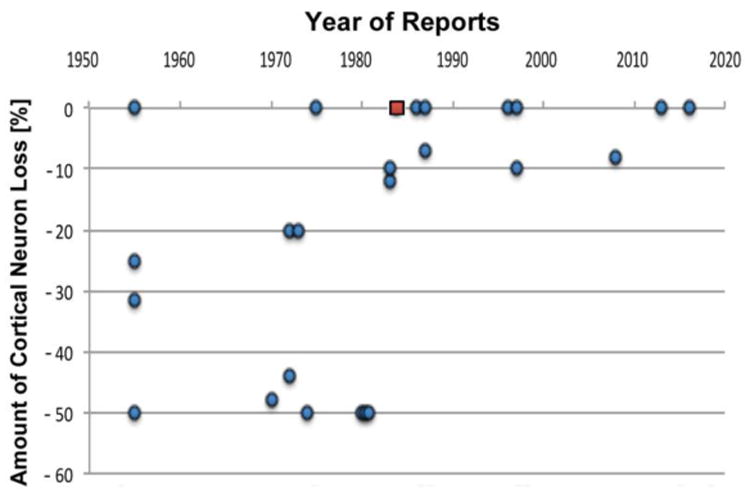
Reports of neuron death in the human cerebral cortex during normal aging. Note that in the 1950s through 1980s reports prevailed that claimed substantial neuron death (“neuronal fall-out”) during normal aging, until the report of Haug et al. (1984) (indicated with a red square) convincingly exposed this concept to be a technical artifact. It is now well established that there is no significant global cortical neuron loss with normal aging (see also Fig. 6).
At the time when fall-out of cortical neurons was the prevailing concept, Herbert Haug (Fig. 3A) examined cell numbers in human brains and discovered that brains from older individuals had different patterns of shrinkage during fixation than younger brains (Haug, 1980; Haug et al., 1984). His re-examination of the studies claiming cell loss with normal aging showed that the apparent neuron loss was almost entirely due to a technical artifact: age-dependent differences in shrinkage, with a change in neuronal density, but not neuronal numbers. Knowledge about shrinkage is essential when neuronal densities rather than absolute numbers of neurons are examined. When corrected for actual shrinkage, the older brains showed essentially the same numbers of neurons as the younger brains (Haug et al., 1984). Haug's work corrected a major concept that had misled researchers as well as lay people (Fig. 5).
Subsequent studies have confirmed that there is either no loss, or a much smaller loss, of cortical neurons during normal aging (Terry et al., 1987; Pakkenberg and Gundersen, 1997; Peters et al., 1998; Pannese, 2011). The work of Haug (1987), Leuba and Kraftsik (1994a) and Gomez-Isla et al. (1996, 1997) found no loss, while Terry et al. (1987), Pakkenberg and Gundersen (1997), and Pelvig et al. (2008) reported a minor decrease of less than 10% during normal aging. When one combines the data from all available stereology studies into one large meta-analysis dataset (Fig. 6B, for details of the regression analysis, see legend to panel B), it becomes obvious that there is only a small decrease (2–4%) of the number of cortical neurons with age, in both males and females. The numbers for males are 24.6 ± 4.2 billion (standard deviation) for ages 20–50 vs. 23.6 ± 3.4 billion for ages 60–86; and the numbers for females are 20.6 ± 3.6 billion for ages 20–50 vs. 19.5 ± 3.6 billion for ages 70–105 (Fig. 6B). Several additional factors may be responsible for the differences between results when compared with the earlier studies: (1) large variability between individuals, making large cohorts necessary; (2) some cohorts may have included individuals with preclinical stages of Alzheimer's disease where neuron loss precedes clinical signs, although the recent work of Andrade-Moraes et al. (2013) argues against this possibility; (3) secular (generational) changes in neuron numbers may explain some of the differences in neuron number when younger and older brains are compared; (4) abnormal diploid neuron fall-out may contribute to a small decline in neuron numbers (Fischer et al., 2012). In the most recent studies, very old females (94–105 years old) were found to have surprisingly large (and presumably stable) numbers of cortical neurons (Fabricius et al., 2013; Walloe et al., 2014). These old individuals may be a subpopulation with increased cell numbers at birth that correlate with, or may cause, longevity. As detailed by Peters et al. (1998) and Pannese (2011), changes in neuron numbers would ideally be determined in a longitudinal study, in vivo, but that is not technically possible. Based on the more recent reports and the meta-analysis presented in Figure 6B, it now appears well-established that cortical neuron numbers in humans decline very little during normal aging, and intellectual decline with age may rather be due to numerical reductions of relatively small, specific neuronal populations, or changes in aging neurons' chemistry or morphology (Morrison and Hof, 1997; Peters et al., 1998; Uylings and de Brabander, 2002; Hof and Mobbs, 2009; Pannese, 2011).
Fig. 6.
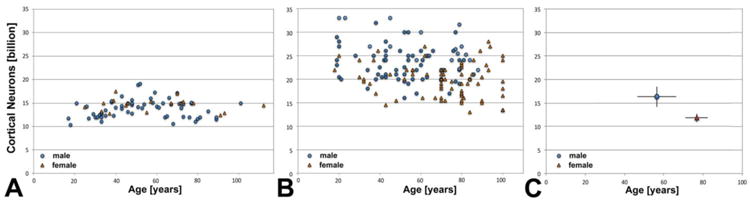
A-C. Graphs of the number of cortical neurons in male and female brains with normal aging, based on one study using an early form of stereology (A), four studies using conventional stereology (B), and two studies using the isotropic fractionator (C). Comparison of the results of a total of seven studies: by Haug (1987) (A), (Braendgaard et al., 1990; Pakkenberg and Gundersen, 1997; Pelvig et al., 2008; Fabricius et al., 2013) (B), and the isotropic fractionator (IF) (Azevedo et al., 2009; Andrade-Moraes et al., 2013) (C). The bars in panel C denote ranges with n=4 for males, and n=5 for females; exact individual data points could not be shown for these two studies, because the data were reported only in aggregate in the original publications. Note that Haug's estimates correspond well in numbers with those obtained by the IF methodology, while the subsequent stereology estimates are somewhat higher. Note also that numbers do not decline significantly with age (panel A: y = 13.25 billion + 0.0099x for males, and y = 15.0 billion - 0.0071x for females), while in panel B the regression analyses show a minor decline of 2–4% (y = 24.6 billion - 0.0211x for males, and y = 21.7 billion - 0.0245x for females). The regression analysis is similar when up to seven possible duplicate data points are removed in panel B (because some of the same brains were used in multiple studies): y = 25.7 billion - 0.0336x for males, and y = 21.8 billion - 0.0266x for females, or when the very old females (ages 94–105) from the Fabricius et al. (2013) study are omitted (y = 21.5 billion - 0.0209x). Female numbers are significantly lower (by about 15–16%) than male numbers (p<0.0005), based on Gundersen and Pakkenberg's (1997) stereology work (panel B).
2.5. Do female brains have significantly fewer cortical neurons than male brains?
Reports are conflicting whether female human brains have fewer cortical neurons than male brains. Haug (1987) presented data indicating that female brains were smaller but had a higher density of cortical neurons, resulting in similar numbers of neurons between the genders, while Pakkenberg and Gundersen (1997) concluded that male brains have about 15% more neurons in the cerebral cortex than female brains. It is possible that the discrepancy between conclusions is due to differences between techniques: Haug used an early version of stereology (volume estimation and sampling of densities), while Pakkenberg and Gundersen applied one of the newly developed, now conventional stereological methods: the optical disector. Haug presented data for a total of 78 brains (60 male and 18 female), while Pakkenberg and Gundersen examined a total of 94 brains (62 male and 32 female). When one combines the 1997 data with those from three additional stereology studies – Braendgaard et al., 1990 (five male brains), Pelvig et al., 2008 (13 male brains and 18 female brains), and Fabricius et al., 2013 (23 female brains) – it becomes apparent in this meta-analysis of 80 male brains and 73 female brains that the stereological studies support a gender difference of about 16% (23.7 billion cortical neurons in males vs. 19.8 billion cortical neurons in females) (Fig. 6B). This difference is maintained when it is taken into account that up to seven of these brains were used in more than one study (see legend to Fig. 6B). The work of Leuba and Kraftsik (1994a) examined the visual cortex of 17 adult brains (ages 18–96 years, 10 male, 7 female), and Gomez-Isla et al. (1996) examined the entorhinal cortex, with an age range from 60–89 years (4 male, 6 female). In these studies, no differences between genders were apparent, but the numbers of subjects were too low to be conclusive.
2.5.1. Absolute numbers of cortical neurons
Figure 6 shows that the total number of cortical neurons differs significantly between Haug's 1987 study and the subsequent stereology studies; Haug's estimates were between 10 and 20 billion, while the stereology work estimated between 15 and 30 billion. Interestingly, the third method of cell counting, the isotropic fractionator (IF), reports estimates that are comparable to Haug's numbers rather than the later stereology data. However, relatively few normal brains have been examined with the IF to date (Fig. 6C). One of the two IF studies, in 2009, examined four normal brains, from 50–71 year-old males (Azevedo et al., 2009), while the other study examined five normal brains, from 71–84 year-old females (Andrade-Moraes et al., 2013). These studies reported 16.3 billion cortical neurons for the group of younger males, and 12.7 billion neurons for the older female group: a difference of 22%. Based on Pakkenberg and Gundersen's (1997) study and the meta-analysis shown in Figure 6B, these differences in numbers seem to reflect mostly gender, rather than age differences. The stereology study by Pakkenberg and Gundersen (1997) estimated 22 billion cortical neurons for male brains, and 19 billion for females. This is at the high end of numerical estimates for cortical neurons (von Bartheld et al., 2016). The studies of Haug (1987), Herculano-Houzel (Azevedo et al., 2009) and Lent (Andrade-Moraes et al., 2013) reported lower numbers: Haug estimated about 15 billion for males, Herculano-Houzel about 16 billion for males, and Lent about 13 billion for older females. Although these numbers are consistent with a gender difference, the number of subjects examined with the isotropic fractionator (IF) is too small to conclusively confirm the results from the histological studies, especially in light of the considerable variability between individuals. Wide ranges between individuals were shown in the data of Haug (1987), West (1993a) and Pakkenberg and Gundersen (1997), with a variability that greatly exceeded the small difference between ages. The gender difference appears to be robust, since it is apparent in the later stereology studies and presumably also with the IF approach (Fig. 6B, C). Accordingly, it is likely, but has yet to be conclusively shown, that female brains have about 15% fewer cortical neurons than male brains.
2.5.2. Brain size, body size, intelligence and gender
After 180 years of research, it is now well-established that female human brains are significantly smaller, on average about 60–150 g, than male human brains (Tiedemann, 1836; Haug, 1984, 1987; Ankney, 1992; Rushton and Ankney, 2009; Deary, 2012; Pietschnig et al., 2015), but the degree to which this difference persists when adjusted for body height or size is less clear (Ankney, 1992; Rushton and Ankney, 2009; Pakkenberg and Gundersen, 1997). The question whether brain size correlates positively with intelligence (and the direction of causality) has been a matter of considerable debate (Tiedemann, 1836; Ankney, 1992; McDaniel, 2005; Rushton and Ankney, 2009; Pietschnig et al., 2015; Saini, 2017). Based on the more recent meta-analyses and reviews that take into account publication and recruitment bias (Deary, 2012; Pietschnig et al., 2015), it has been concluded that the major difference in intelligence between genders is with variance (more males than females distribute at the extremes), while the mean is virtually identical between genders (Jensen, 1980; Ankney, 1992; Deary, 2012; Pietschnig et al., 2015). In this context, recent work on the Neanderthal brain is of interest. Neanderthal brains are thought to have been about 10% larger than the brains of anatomically modern humans (Jerison, 1973; Roth and Dicke, 2005), assuming that brain mass estimates based on endocranial volumes are accurate (see Ridgway et al., 2016, for a recent challenge), yet the intelligence of the large-bodied and big-brained Neanderthals is thought to have been similar, if not inferior to Homo sapiens (Wynn and Coolidge, 2011; Villa and Roebroeks, 2014). One interesting solution to this apparent paradox is that the Neanderthal brain may have been larger because of certain specializations, such as an enlarged visual cortex, that evolved to adapt to low light intensity at high latitudes (Pearce et al., 2013), while the smaller modern humans may have had superiority in parts of the brain devoted to social cognition (Pearce et al., 2013; Villa and Roebroeks, 2014) and social skills (prosociality, Hare, 2017). However, it is controversial whether sociality or diet drives cognitive complexity and larger brain sizes (DeCasien et al., 2017), and how this makes species more adaptive to a changing environment and ultimately more successful (Wynn and Coolidge, 2011; Villa and Roebroeks, 2014). Likewise, it is thought that some forms of cognition (e.g., visuospatial recognition), where males have superiority, require larger brain capacity (Ankney, 1992; Rusthon and Ankney, 2009). This may explain why males have increased brain sizes over females. Brain size correlates positively with cortical neuron numbers according to Haug (1987, r = 0.48) and Pakkenberg and Gundersen (1997, r = 0.56). Since brain size correlates with cortical neuron number, and brain size correlates with body size (Haug, 1984; Rushton and Ankney, 2009), one would expect that cortical neuron number correlates with body size. However, the data on this correlation is conflicting (compare Pakkenberg and Gundersen, 1997, with Rushton and Ankney, 2009). Haug (1987) found that the smaller female brains had a higher density of cortical neurons, resulting in similar numbers of cortical neurons as their male counterparts, while Pakkenberg and Gundersen (1997) found that female cortical neuron numbers were about 15% lower than those in males, so this issue is not yet resolved. It is also possible that instead of neuron number, the number of synapses and/or circuits is the crucial parameter for intelligence. As recently reviewed by Dicke and Roth (2016), studies in this respect are controversial, and reliable studies on numbers of synapses are essentially lacking. Accordingly, proper assessment of this interesting aspect has to await further work.
2.6. The concept that excessive alcohol consumption kills cortical neurons
It was widely believed in the 1970s and 1980s that alcoholism caused cortical neuron loss and behavioral dysfunction due to that neuron loss (Courville, 1955; Harper, 1983; Harper and Kril, 1985; Harper et al., 1985, 1987; Kril and Harper, 1989; Harper and Kril, 1990). Such beliefs were reinforced by studies showing significant loss of neurons in brains of animals exposed to alcohol (e.g., Walker et al., 1980). However, in humans, neuropathological studies reported that white matter of chronic alcoholics seemed more reduced than gray matter (Harper et al., 1985) and suggested regional differences in the loss of neurons (Kril and Harper, 1989; Kril and Halliday, 1999). Using the newly-developed stereological counting methods, the group of Bente Pakkenberg (Fig. 3B) reported that the total number of cortical neurons was not significantly reduced with excessive alcohol consumption (Jensen and Pakkenberg, 1993). This finding received attention, because it challenged the previously established concept of significant cortical neuron loss due to chronic alcohol abuse. However, the 1993 study only examined global (cortex-wide) loss, and was not designed to detect relatively small losses that may be restricted to distinct cortical regions, layers or cell types. Subsequent stereological studies that focused on such regional changes revealed that some cortical and subcortical regions were vulnerable to alcohol-induced neuronal losses, while other regions, such as motor cortex and hippocampus – in contrast to results from previous animal studies (Walker et al., 1980) – were not affected (Harding et al., 1997). Accordingly, a more refined concept of alcohol-induced neuronal loss now prevails (Kril and Halliday, 1999): While white matter atrophy dominates, gray matter is differentially affected, with neuron losses primarily in frontal cortex. Additional losses of gray matter are seen in certain conditions as a result of alcohol abuse: Wernicke's encephalopathy and Korsakoff syndrome are associated with neuron losses in the hypothalamus, thalamus, and cerebellum (Harper and Matsumoto, 2005). This refined concept of regional, restricted losses provides more hope to former and recovering alcoholics, because axonal (white matter) and myelination deficits may be potentially reversible, while dead neurons in the central nervous system generally cannot be replaced.
2.7. Are there secular (generational) changes in cell numbers in human brains?
Herbert Haug pointed out that some of the reported changes in cell numbers in normal aging brains may be due to generational changes in bodies and brains of the population, as body height increased from the 20th to the 21st century (Haug, 1984; Haug, 1987; reviewed by Peters et al., 1998), possibly due to improved nutrition. Several studies claim that body height is positively related to brain weight (reviewed in Rushton and Ankney, 2009), and Haug assumed that therefore, body height was also positively related to cortical neuron number (Haug, 1987). However, surprisingly, Pakkenberg and Gundersen (1997) concluded that cortical neuron number was not significantly related to body height, at least not when this was calculated separately for each gender. This has interesting implications, as Pakkenberg and Gundersen (1997) observe: “body size … has no relationship to neocortical neuron number for individuals of a particular sex: small men may have large neocortical neuron numbers, and large men may have small neocortical neuron numbers,” presumably because neuron number is determined very early in life, while body height manifests 15 to 20 years later, and is in part determined by environmental influences. It should be noted that these findings disagree with the concept of the encephalization quotient (Jerison, 1973), as recently discussed (Herculano-Houzel, 2017). Nevertheless, the apparent lack of correlation between body height and cortical neuron number in humans undermines Haug's idea about secular changes as a relevant factor to explain some studies' neuron loss with age. Even though Haug did not find any significant loss of neurons in older individuals in his own data, he cited secular acceleration as a factor that may make it look as if cortical neurons are lost, as had been reported by others (Brody, 1955; Brody, 1970; Colon, 1972; Hanley, 1974; Devaney and Johnson, 1980; Henderson et al., 1980; Anderson et al., 1983), because younger individuals with a higher base level (larger number of neurons) are compared to individuals with a lower base level (lower number of neurons) that is not due to loss of neurons (Peters et al., 1998). The meta-analysis presented in Figure 6B indicates rather small numerical differences (decreases of about 3%) in cortical neurons between cohorts with increasing age. Although body height and brain weight are undergoing secular changes in most populations, with mean body height increasing by 1 mm per year and brain weight by 50–60 g per century, changes which appear to have been occurring only in the last 200 years (Haug, 1984; Haug, 1987; Miller and Corsellis, 1977), one must take into account that it is not clear whether cortical neuron number correlates with body-size and height, given the negative results by Pakkenberg and Gundersen (1997). Accordingly, Haug's concern about the effect of secular acceleration for “pseudo” neuron loss in normal aging is now mitigated for two reasons: (1) there is minimal loss of cortical neurons with normal aging, and (2) there may not be a relation between body height and cortical neuron number. Relative stability of neuron numbers with age has also been documented for subcortical human neuronal populations, such as brainstem catecholaminergic neurons (Mouton et al., 1994; Kubis et al., 2000) and the cochlear nucleus (Sharma et al., 2014).
2.8. Do glia make you smart?
There has been a longstanding notion, based on comparisons between vertebrate species, that increased numbers of glial cells in the brain correlate with, and possibly contribute to, superior intelligence. As discussed in the section 2.5.2, the relationships between brain size, neuron number, and intelligence are still controversial. The GNR in cerebral cortex was observed to increase with increasing brain sizes between species, suggesting a correlation and possible causation between high GNRs and superior intelligence (Friede, 1954; Jerison, 1973; Araque et al., 2001; Fields, 2009; Koob, 2009; Verkhratsky and Butt, 2013). The idea gained further traction when Albert Einstein's brain was found to contain normal neuron numbers, but appeared to have slightly increased glia numbers in some cortical regions (Diamond et al., 1985; Witelson et al., 1995), although the methodology of estimating cell densities based on profile counts is problematic according to current knowledge. Furthermore, animal studies showed that injecting human glia into mouse brains made those mice smarter in learning tests (Han et al., 2013; Fields, 2013). However, the notion of an increasing GNR with increasing brain mass (supported by Stolzenburg et al., 1989; Nedergaard et al., 2003; Marino, 2006; Sherwood et al., 2006; Bahney and von Bartheld, 2017) has recently been questioned (Herculano-Houzel et al., 2007; Herculano-Houzel, 2011). An alternative interpretation of the GNR's correlation with intelligence is that the correlation is with increasing brain size and not necessarily intelligence. This was supported by studies on whale brains, which showed even higher GNRs than human brains (Hawkins and Olszewski, 1957; Tower and Young, 1973; Haug, 1987; Eriksen and Pakkenberg, 2007). The concept was further clarified by comprehensive scaling studies using the isotropic fractionator: such studies indicated that the GNR depends primarily on neuron size (which is related to brain size and also neuron density), and is not determined by intelligence per se (Herculano-Houzel, 2011, 2015). Furthermore, the spinal cord's GNR increases similarly or even more with increasing brain size than cortical GNRs (Bahney and von Bartheld, 2017), showing that not only cortical GNRs increase with brain size, but also subcortical and even spinal cord GNRs. Thus, the reason for GNR increases with increasing brain size does not appear to be directly linked to intelligence.
2.9. Should all cell counting methods be calibrated, even when they are theoretically unbiased? How can biases be identified and avoided?
It became apparent in the 1980s that cell counting methods can generate widely diverging results (Haug, 1986; Schmalbruch, 1987, Table 1). This gave rise to the idea that these methods should be validated by calibration (Schmalbruch, 1987). To address this problem, comparison with a serial section reconstruction – the so-called “gold standard” – was considered to be the most useful approach. In this context, calibration means that estimates obtained by a histological counting method are directly compared with the true number of particles in that same tissue obtained by exhaustive counting in a 3D serial section reconstruction (Coggeshall et al., 1990; Pover and Coggeshall, 1991). Such a comparison would show whether a method is biased, and if so, by how much. Accordingly, any bias of a counting method can be assessed and the method thereby validated – although it has to be kept in mind that numerous parameters can contribute to bias (as discussed below). For the isotropic fractionator (IF), validation is not as straightforward, because the same tissue cannot be used for direct comparison as for the histological counting methods. Therefore, the IF can only be compared with other, preferably already validated, counting methods. One needs to avoid validation that is “circular”, such that method A is validated with method B, and method B is validated by comparison with method A. This is not the case in the examples discussed below, because stereology was validated against an exhaustive count (3D serial reconstruction), the “gold standard”, and then the IF was compared with the (already validated) stereology method, thus preventing circular reasoning.
Table 1. Variability in numerical estimates between quantitative studies.
| Nervous System Structure | Species | Method | Numerical Ranges | References |
|---|---|---|---|---|
| Cerebral cortex | Human | HIS | 0.6-16.5 ×109 | Haug, 1986 |
| Dorsal root ganglion, Lumbar 4 | Rat | HIS | 5.3-12.5 ×103 | Schmalbruch, 1987 |
| Dorsal root ganglion, Lumbar 5 | Rat | HIS | 2.0-14.8 ×103 | Schmalbruch, 1987 |
| Dorsal root ganglion, Lumbar 6 | Rat | HIS | 4.1-14.1 ×103 | Schmalbruch, 1987 |
| Purkinje cells | Rat | STE | 2.1-6.1 ×105 | Guillery & Herrup, 1997 |
| Cerebral cortex | Human | STE | 13.7-22.8 ×109 | Peters et al., 1998 |
| Dentate gyrus | Mouse strains | STE | 0.38-0.94 ×106 | Abusaad et al., 1999 |
| Pyramidal cell layer | Mouse strains | STE | 0.43-0.93 ×106 | Abusaad et al., 1999 |
| Dorsal root ganglion, Lumbar 5 | Rat | STE | 14.1-19.2 ×103 | Tandrup et al., 2004 |
| Hippocampus | Human | STE | 22.6-40.4 ×106 | Korbo et al., 2004 |
| Granule cells (Dentate gyrus) | Rat | STE | 1.2-1.55 ×106 | Schmitz & Hof, 2005 |
| Pyramidal cells (CA1-3) | Rat | STE | 615-930 ×103 | Schmitz & Hof, 2005 |
| Cerebral cortex | Rhesus monkey | STE | 1.35-2.95 ×109 | Charvet et al., 2015 |
| Cerebral cortex | Rhesus monkey | IF | 0.825 ×109 | Charvet et al., 2015 |
| Purkinje cells | Rat | STE | 2.1-6.1 ×105 | Herculano-Houzel et al., 2015 |
| Lateral geniculate nucleus | Human | STE | 1.99-3.48 ×106 | Herculano-Houzel et al., 2015 |
| Neocortex | Human | IF | 12.7-16.3 ×109 | Herculano-Houzel et al., 2015 |
| Cerebellum | Human | IF | 54-65 ×109 | Herculano-Houzel et al., 2015 |
| Whole Brain | Human | IF | 67.3-86 ×109 | Herculano-Houzel et al., 2015 |
| Purkinje cells | Human | HIS | 0.88-26 ×106 | von Bartheld et al., 2016 |
| Purkinje cells | Human | STE | 15.4-30.5 ×106 | von Bartheld et al., 2016 |
| Dorsomedial thalamic nucleus (Schizophrenia) | Human | STE | 0-40% loss | Dorph-Petersen & Lewis, 2017 |
| Spiral ganglion (Young Adult) | Human | HIS | 23.1-33.7 ×103 | Kaur et al., 2018 |
| Spiral ganglion (Young Adult) | Human | STE | 26.7-41.7 ×103 | Kaur et al., 2018 |
HIS, histology (profile counting = 2D Method); IF, isotropic fractionator; STE, stereology (= 3D Method)
Coggeshall and his colleagues pioneered the validation approach by first calibrating various 2D methods (Coggeshall et al., 1990), followed by calibration of the physical disector (Pover and Coggeshall, 1991). Coggeshall realized that such a calibration need not to be performed on an entire peripheral ganglion or nucleus in the brain, which would be prohibitively time-consuming to process and analyze in serial sections, but that a small sample of tissue sections could be serially reconstructed as the gold standard to validate counting methods (Coggeshall et al., 1990; Pover and Coggeshall, 1991; Coggeshall, 1992; Farel, 2002; Delaloye et al., 2009). Such analyses proved effective in revealing practical features important in minimizing biases in the counting methods (Pover and Coggeshall, 1991; Popken and Farel, 1996; Hatton and von Bartheld, 1999; von Bartheld, 2001, 2002). When the physical disector was calibrated, it was discovered that estimates generated from disectors with adjacent reference and look-up sections require an optimal distance between the consecutive disector pairs (Pover and Coggeshall, 1991; Delaloye et al., 2009). Pover and Coggeshall emphasized that “we would not have known this was a problem if we did not verify our counts.” Likewise, when Farel and colleagues calibrated the physical disector, they found that the recommended sampling of 100–200 particles generated an unacceptable variability, and that sampling of over 300 particles was necessary (Farel, 2002). When Hatton and von Bartheld (1999) calibrated the optical disector, they found that the use of recommended guard zones introduced a deficit of over 20% in numerical estimates, apparently due to compression of the margins of soft tissue sections – the unsampled guard zones (Baryshnikova et al., 2006).
Historically, stereologists have had a mixed reaction to efforts of calibrating the new counting methods. Some, such as Mark West (West, 1999), Rob Williams (Williams et al., 2003) and Jens Nyengaard embraced this approach (Basgen et al., 2006: “We consider the Exhaustive Count method to be the gold standard for cell counting, since there is no sampling … and every nucleus is counted directly”), while others, such as Cruz-Orive, Gundersen, and Howard claimed that the new stereological methods did not need calibration, because they were inherently (theoretically) unbiased (Cruz-Orive, 1994; Mayhew and Gundersen, 1996; Howard and Reed, 1998). This notion prompted extensive discussions and concerns about a “double standard” – why would only 2D methods need validation, but not 3D methods (Guillery and Herrup, 1997; Saper, 1999; Benes and Lange, 2001a; von Bartheld, 2001; von Bartheld, 2012)? Table 2 compiles studies that calibrated 2D and 3D methods as well as the IF. As detailed below, these efforts have led to the identification of several types and sources of potential bias, some of them generally applicable (relevant for all counting methods), while others are specific to certain methodology.
Table 2. Calibration of counting methods against a serial section reconstruction (“gold standard”) or against other counting methods.
| Structure | Embedding | Species | Method | Numerical Differences* | References |
|---|---|---|---|---|---|
| Against “Gold standard” (Exhaustive Counting) | |||||
| DRG | Paraffin | Rat | 2D: Floderus | +39% | Coggeshall et al., 1990 |
| DRG | Paraffin | Rat | 2D: Abercrombie | +25.5 | Coggeshall et al., 1990 |
| DRG | Paraffin | Rat | 2D: Konigsmark | +27.8% | Coggeshall et al., 1990 |
| DRG | Paraffin | Rat | 2D: Devor | +29.8% | Coggeshall et al., 1990 |
| DRG | Paraffin | Rat | 2D: Empirical | -0.5% | Coggeshall et al., 1990 |
| DRG | Paraffin | Rat | 2D: Rose & Rohrlich | -30.3% | Coggeshall et al., 1990 |
| DRG | Paraffin | Rat | All 2D Methods | -30 to +39% | Coggeshall et al., 1990 |
| DRG | Paraffin | Rat | Physical Disector | ±8% to 12% | Pover & Coggeshall, 1991 |
| DRG | Paraffin | Rat | 2D: Empirical | -3.2% to +1% | Pover & Coggeshall, 1991 |
| DRG | Paraffin | Frog | 2D Method | -8% to -30% | St Wecker & Farel, 1994 |
| DRG | Paraffin | Frog | Physical Disector | ±4% to -26%** | Popken & Farel, 1996 |
| Trochlear nucleus | Paraffin | Chicken | Optical Disector | -22.8% | Hatton & von Bartheld, 1999 |
| Trochlear nucleus | Paraffin | Chicken | 2D Method | -9.1% | Hatton & von Bartheld, 1999 |
| Trochlear nucleus | Paraffin | Chicken | Modified Optical Disector | -1.0% | Hatton & von Bartheld, 1999 |
| DRG | Paraffin | Frog | Physical Disector | ±4% to ±26% | Farel, 2002 |
| Glomerulus | Resin | Mouse | Physical Disector | -1.7% [-17 to +17] | Basgen et al., 2006 |
| Glomerulus | Resin | Mouse | 2D Method | +10.2% [+1.2 to +20] | Basgen et al., 2006 |
| Trochlear nucleus | Various | Chicken | Optical Disector | -26% to +0.7% | Ward et al., 2008 |
| Substantia nigra | Paraffin | Mouse | Optical Disector | +5% | Basquet et al., 2009 |
| Substantia nigra | Paraffin | Mouse | 2D Method | -4% | Basquet et al., 2009 |
| DRG | Epon | Rat | Physical Disector | ±6 to -27% *** | Delaloye et al., 2009 |
| IF Against Other Counting Methods | |||||
| White matter | Paraffin | Various | IF vs. Optical Disector | ±0 | Bahney & von Bartheld, 2014 |
| Cerebral cortex | Frozen | Chimpanzee | IF vs. Optical Disector | +5% | Miller et al., 2014 |
| Various | Frozen | Chicken | IF vs. Optical Disector | ±0 | Ngwenya et al., 2017 |
Abbreviations: DRG, dorsal root ganglion; IF, isotropic fractionator; 2D, 2D Method; [ ], ranges.
Note that numerical differences are composed of both, potential biases as well as statistical variability.
In this study, the orientation of sectioning appeared to be correlated with the extent of numerical differences, a finding that has no stereological explanation and does not seem to have been replicated.
the larger deviation was seen only when the separating distance between consecutive pairs of sections was larger than 60 μm.
2.9.1 Issues with counting methods – some revealed by calibration analyses
General issues
Particles to be counted require unambiguous identification and clear inclusion criteria (Ward et al., 2008; Kaplan et al., 2010), specifically for the distinction between glia and small neurons (Flood, 1994; von Bartheld et al., 2016). The region of interest needs a clear delineation and/or definition (Thune and Pakkenberg, 2000; Dorph-Petersen and Lewis, 2011, 2017). This is straightforward for nuclei with well-defined borders, but can be challenging for nuclei with diffuse or geometrically bizzare borders, e.g., the nucleus subceruleus (von Bartheld and Bothwell, 1992), or the mesencephalic nucleus of the trigeminal nerve (von Bartheld and Bothwell, 1993). Operator and inter-observer variability and bias, including errors in the implementation of counting procedures and generation of estimates, need to be minimized (Guillery, 2002; Mouton, 2002; Kaplan et al., 2010; Dorph-Petersen and Lewis, 2011).
Stereology-specific (mostly thick-section) issues
Regarding the originally recommended sampling scheme in which only 150–200 particles are counted (Braendgaard et al., 1990; Coggeshall and Lekan, 1996; Howard and Reed, 1998; West, 1999), it is important to note that several investigators believe that larger samples are needed, especially when the tissues are heterogeneous (Kordower, 2000; Farel, 2002; Schmitz and Hof, 2000, 2005). Measurement of section thickness is not a trivial undertaking (Guillery, 2002; Guillery and August, 2002). Dealing with differential tissue section shrinkage, and the appropriate placement of guard zones (to prevent sampling in compromised zones of tissue sections) is a highly complex issue, because tissue sections can be initially differentially deformed with subsequent loss of particles when treated in harsh conditions (Hatton and von Bartheld, 1999; Gardella et al., 2003; Baryshnikova et al., 2006; Carlo and Stevens, 2011; von Bartheld, 2012). The problem that particle distribution may be distorted in the z-axis is a potential source of bias that unfortunately is ignored in many reviews (e.g., Mouton, 2002; Dorph-Petersen and Lewis, 2011), possibly because not all types of tissues are affected. While some investigators outside the von Bartheld lab have reported such distortions (e.g., Andersen and Gundersen, 1999; Baryshnikova et al., 2006; Carlo and Stevens, 2011), others did not (Pelvig et al., 2003; Hosseini-Sharifabad and Nyengaard, 2007). The behavior of tissues in the hands of different investigators is remarkably variable, and it is not yet known why some tissue sections do not lose caps, while others show a marked loss – which was the reason to implement the now controversial guard zones in the first place (Andersen and Gundersen, 1999). For a detailed examination why distortion of tissue sections can be difficult to prove, see Baryshnikova et al. (2006).
Some proponents of nonstereological methods have stated that design-based stereology requires tissues to be homogenous, with particles distributed in a completely random fashion (Benes and Lange, 2001a; Herculano-Houzel and Lent, 2005; Herculano-Houzel et al., 2015). This is a misconception (Baddeley, 2001). It is true that heterogeneity in tissues will increase variance and will enlarge the coefficient of error (Dorph-Petersen and Lewis, 2011), making a larger number of samples necessary (Benes and Lange, 2001b). The use of relatively few samples and actual neurons counted in 3D methods has prompted questions whether stereological analysis of tissues with heterogeneous distribution of particles can be accomplished with the generally recommended sampling schemes (Benes and Lange, 2001a,b; Guillery, 2002; Guillery and August, 2002; Herculano-Houzel and Lent, 2005). For example, there have been concerns that counting only 300–400 particles in a structure as heterogeneous as cerebral cortex may not be sufficient to generate estimates of 26 billion neurons (Braendgaard et al., 1990; Benes and Lange, 2001b). The recently developed Proportionator tool (Gardi et al., 2008a,b) was designed to enhance sampling efficiency and to thereby facilitate stereological analysis of tissues with cells that are heterogeneously distributed.
IF-specific issues
The concerns with the IF counting method are that nuclear membranes may be ruptured, and destroyed nuclei may be discarded as debris, or that the neuron-specific antibody may not recognize all neuronal nuclei, both of which could lead to an undercount of nuclei (Yuhas and Yabr, 2012; Carlo and Stevens, 2013; Charvet et al., 2015). Autolytic or necrotic cells, especially in human tissues, may not incorporate fluorescent dyes or may not be recognized by antibodies, thus requiring stringent quality control, although the fixed cell nuclei appear to be remarkably robust in this respect (von Bartheld et al., 2016). Empirical studies show that superior tissue preservation is more important for morphological than for biochemical analysis, making the IF the more versatile method when tissue quality varies (Bahney and von Bartheld, 2014).
Some profile counting work has resulted in large variations between studies (Table 1), although the most egregious examples (outliers) were presumably due to no or inadequate use of correction factors (Clarke, 1992; von Bartheld, 2001). It was hoped that stereology would reduce that variability, but the results are mixed: A survey of reviews shows that numerical estimates can be within reasonably tight ranges (for example in rat dorsal root ganglia compiled by Tandrup, 2004, or in rat hippocampus compiled by Schmitz and Hof, 2005), while others are more divergent (Herculano-Houzel et al., 2015, see examples in Table 1). Therefore, it has been argued that stereology is not necessarily preferable to properly conducted profile counting (von Bartheld, 2001). How does the width of ranges yielded by stereological estimates compare with the new method, the isotropic fractionator (IF)? The IF was recently validated (Bahney and von Bartheld, 2014; Miller et al., 2014; Herculano-Houzel et al., 2015; Ngwenya et al., 2017), and so far the results are encouraging. However, it has to be kept in mind that relatively few groups have applied this new method, and variability in results can be expected as more investigators use it. To my knowledge, only two substantive challenges have emerged: Charvet et al. (2015) pointed out that IF-derived neuronal numbers for cerebral cortex, in particular the visual cortex of rhesus monkeys, was lower (by 50%) than stereology-derived numbers. However, some of the stereology-derived numbers appear to be unusually high, and the authors fail to mention corroborating lower numbers from the older literature. The second challenge (Bahney and von Bartheld, 2017) is that a series of estimates for neuron numbers in the primate spinal cord appear to have been unusually low (Burish et al., 2010), for reasons that are not entirely clear.
It may be considered fortunate that the histological and the IF methods have different types of limitations. A major problem for stereology is that small neurons are difficult to distinguish from glial cells (recently reviewed in: von Bartheld et al., 2016). Several groups have attempted to solve this problem by using neuron-specific antibodies such as NeuN that recognize a neuronal antigen present within the nucleus of most neuronal populations (Lyck et al., 2009; Giannaris and Rosene, 2012; Zhu et al., 2015; Ngwenya et al., 2017). However, there are conflicting reports about the success of identifying neurons in tissue sections by using NeuN immunocytochemistry. When Lyck et al. (2009) compared the number of immunolabeled cells in human tissue sections with the identification of cells by morphological criteria, only 18–57% of otherwise neuronal-appearing cells were labeled by the NeuN antibody. On the other hand, Giannaris and Rosene (2012) and Zhu et al. (2015) reported that all neurons, and only neurons, were NeuN-labeled in their mammalian forebrain studies, and likewise Iwaniuk's group found the same in their chicken brain sections (Ngwenya et al., 2017). Apparently, tissue processing, cell types, and neuronal physiology may all play a role in how much NeuN is actually present and can be identified by immunocytochemistry in tissue sections (Mullen et al., 1992; Tsai et al., 2009; Carlo and Stevens, 2013), while the antibody labeling appears to work in a more robust fashion for cell nuclei in suspension (when using the IF approach).
Taken together, this survey shows that indeed, all counting methods benefit from calibration and validation. More work is needed to identify and to minimize sources of bias. It is essential that authors consider all types of potential biases, acknowledge differences in results between studies, try to resolve discrepancies by calibration analyses, discuss their new data in the context of relevant previous studies, and report all important parameters that inform how the sampling and counting was conducted and estimates were obtained (Schmitz and Hof, 2005; Dorph-Petersen and Lewis, 2011).
2.10. How does the cellular composition of the brain change in neurological and psychiatric disease conditions?
Besides the classical neurodegenerative diseases with clearly defined neuron losses, such as in Huntington's and Parkinson's disease (Vonsattel and DiFliglia, 1998; Kordower et al., 2013), several additional neurological and psychiatric diseases have been implicated with abnormal glia numbers, neuron numbers, or GNRs (Rowland and Mettler, 1949; Benes, 1993, 1997; Ongür et al., 1998; Harrison, 1999; Coyle and Schwarcz, 2000; Vawter et al., 2000; Todtenkopf et al., 2005; Bernstein et al., 2015; Elsayed and Magistretti, 2015). While the degree and localization of abnormalities differ between studies, some changes in glial cell number, densities or GNRs have been confirmed in discrete brain regions by recent studies employing stereological or IF methods for conditions including autism spectrum disorders, mood disorder, depression, schizophrenia, and Alzheimer's disease (Rajkowska, 2000; Cotter et al., 2001; Hof et al., 2003; Vostrikov et al., 2007; Morgan et al., 2010; Karlsen and Pakkenberg, 2011; Andrade-Moraes et al., 2013; Verkhratsky et al., 2014; Dorph-Petersen and Lewis, 2017).
The lack of reliability, validity and therefore trust in quantitative data has been a major impediment to progress in defining the potential roles of numerical glia or neuron abnormalities in neurological and psychiatric diseases (Williams and Rakic, 1988; Stark et al., 2005). There have been multiple examples where initial reports of numbers or ratios of glial cells and neurons could not be replicated or had to be substantially revised, even within the same group of investigators or when using the same type of counting technique (Guillery and Herrup, 1997; von Bartheld, 2001; Herculano-Houzel et al., 2015; Dorph-Petersen and Lewis, 2017; Kaur et al., 2018). Meta-analyses have not been able to resolve all controversies about glia numbers and ratios in human neurological and psychiatric diseases (Harrison, 1999; Rajkowska, 2000, 2002; Hof et al., 2003; Lyness et al., 2003; Palmen et al., 2004; Todtenkopf et al., 2005; Courchesne et al., 2007; Amaral et al., 2008). It is beyond the scope of this contribution to review all reports of numerical glia or neuron abnormalities in neurological or psychiatric diseases. I will focus instead on the following two examples that are particularly instructive: the question of neuron loss in the thalamus of people with schizophrenia, and the question of cortical neuron loss in Alzheimer's disease.
Schizophrenia researchers have examined multiple brain regions for abnormalities of neuron and glia numbers as well as ratios between cell types such as the GNR, including various cortical regions as well as subcortical structures (Benes, 1993; Harrison, 1999; Todtenkopf et al., 2005; Dorph-Petersen et al., 2007; Bernstein et al., 2015). The dorsomedial thalamus was implicated in schizophrenia when stereological studies reported a 30–40% decrease in neuron numbers (Pakkenberg et al., 1990, 1992, 1993; Popken et al., 2000). However, subsequent studies were unable to replicate these findings, including a study by the same lead author, Bente Pakkenberg (Nielsen et al., 2008; reviewed in Dorph-Petersen and Lewis, 2017). On the other hand, the pulvinar seems to have abnormal neuron numbers in schizophrenia that are supported by the majority of studies (Dorph-Petersen and Lewis, 2017). The review by Dorph-Petersen and Lewis comprehensively examined the potential reasons for the discrepant results for the dorsomedial thalamus. Since the reviewed studies employed stereology, there should not have been – theoretically – any issues due to different technical approaches (although this still leaves observer errors and biological variation). The authors discuss the heterogeneity of the schizophrenia cases, with the initial cohorts consisting of hospitalized patients with somewhat older ages, while the subsequent studies obtained brains from younger subjects in (non-hospital) community settings. While there was no single conclusive reason for the discrepancies, this example does show how difficult it is, with the small cohorts available and possibly a large biological variability, to find convincing evidence for relatively subtle abnormalities in neuron or glial cell numbers.
In the 1980s, it seemed fairly well-established that cortical neurons, especially the larger neuron types, were significantly reduced in Alzheimer's disease, based on the work of Terry and others (Terry et al., 1981; Terry and Davis, 1983; Mountjoy et al., 1983; Price, 1986; Braak and Braak, 1986; Terry and Hansen, 1988; West et al., 1993b; Mann, 1996). It was assumed that this neuron death contributed to many clinical manifestations of the disease (Price, 1986). Therefore, it came as a surprise, if not a shock, when the first major stereological study, by Pakkenberg's group (Regeur et al., 1994), reported that there was no significant global loss (the observed loss of 6% was not statistically significant) of neurons in the cerebral cortex of Alzheimer's patients when compared with a similarly-aged control group. This study examined the cerebral cortex of 11 women with severe Alzheimer's, compared with 10 cognitively normal women of a similar age (mean of 83–84 years). This paper was published in Neurobiology of Aging, and because it prompted reversal of the thinking about the pathology of Alzheimer's disease, the editors invited eighteen leading Alzheimer's researchers to comment and express their thoughts about the validity and the implications of the paper in the same issue of the journal (e.g., Braak and Braak, 1994; Flood, 1994; Hyman and Gomez-Isla, 1994; Mufson and Benzig, 1994), and other experts expressed their opinions in additional publications (e.g., Everall et al., 1997; Peters et al., 1998). While some voiced concerns about the technique, by and large the consensus was to accept the findings, because the data were generated by a so-called unbiased method (although it had not yet been calibrated at the time – see section 2.9). The main new conclusions were that (1) neuronal cortical death was restricted to certain cortical regions, so neuron losses were not apparent in a study that examined neuronal losses globally (cortex-wide); (2) neurons may shrink rather than die, so they would appear in a different size category (or even be counted as glial cells in the older studies), and (3) reductions in neurotransmitters and synapse numbers, rather than degeneration and death of cell bodies, may underlie the clinical deterioration (Peters et al., 1998; Uylings and de Brabander, 2002; Pannese, 2011). Remarkably, during the next 20 years, the findings of Regeur et al. (1994) were neither challenged nor confirmed. Some studies examined specific regions of cortex or specific neuronal populations, and found some losses (Leuba and Kraftsik, 1994b; Gomez-Isla et al., 1996, 1997). Such results were reconciled, because some investigators still believed in the only recently debunked “fallout of cortical neurons with normal aging” (see Section 2.4), and assumed that losses in older patients with Alzheimer's disease would be relatively small (because many neurons would have already been lost due to normal aging, Mann, 1996). Others believed that the specific losses were too small to be apparent as a significant global neuron loss (Duyckaerts et al., 2009).
However, in 2013, Roberto Lent's group published a study (Andrade-Moraes et al., 2013) where they used the new counting method, the IF, to examine the same question that Regeur et al. had addressed 20 years earlier: are there significant changes “globally” in neuron and non-neuronal numbers in the entire cerebral cortex of Alzheimer's patients? In this study, the brains of four subjects with asymptomatic disease and five subjects with dementia were compared with five normal controls. With the IF method, Andrade-Moraes et al. showed that there was a statistically significant loss (of 29%) of neurons globally in the entire cortex of demented patients, and also in specific lobes of cortex, amounting to a loss of 41% for frontal lobe, and 24% loss for the other lobes, consistent with the studies and conclusions prior to 1994 (Braak and Braak, 1986; Mann, 1996). Surprisingly, Andrade-Moraes et al., did not comment on the earlier study by Regeur et al. and did not mention the extensive debate and series of commentaries it had prompted in 1994. In fact, the Regeur study was not even cited. Possibly, Andrade-Moraes et al. (2013) were reluctant to point out the apparent discrepancy between methods. In 2013, the IF had not yet been validated and had never been side-by-side directly compared with stereology. Based on the validation studies that appeared just a short while later (Bahney and von Bartheld, 2014; Miller et al., 2014), one would have expected that IF and stereology would produce similar results. Since two convincing and supposedly methodologically equivalent studies yielded discrepant results on this important question, it now appears unclear which group and conclusion is correct. Without additional studies, it is impossible to decide whether the IF study, that corroborates the older studies and results prior to 1994, is more believable, or the 1994 stereology study. The 1994 study was conducted and published prior to the work that revealed a number of hidden biases (see above), and maybe would have faced more scrutiny if it had been known that the theoretically unbiased methods can have significant biases when applied to real world tissue sections and constraints (von Bartheld, 2012). Obviously, we do not yet know the answer to this question, but what appeared to be generally accepted for 20 years about the persistence of cortical neurons in Alzheimer's disease now seems less certain and has to await further scrutiny and resolution.
3. Conclusions
In summary, this survey of ten influential concepts in quantitative methodology, designed to elucidate the cellular composition of the human brain, teaches several lessons. We need to be vigilant about assumptions that are taken for granted, as shown by the evidence that “text-book knowledge” may not be correct, or the hubris that calibration and validation of counting methods may not be needed. Methodologies will continue to be developed and refined, and it is of utmost importance to relate new findings to previous relevant reports, to discuss differences in results, and attempt to identify underlying mechanisms of discrepancies. It is important to keep an open mind and to be inquisitive and creative, in order to separate truths from myths and to approximate the cellular composition of brains, including that of our own species.
Highlights.
The myth of a 10:1 glia-neuron ratio in human brains has been debunked
The myth of one trillion glial cells in human brains has been debunked
The number of cortical neurons does not decline significantly in normal aging
All counting methods benefit from calibration and validation
Proof is needed for altered cell numbers in neurological and psychiatric disorders
Acknowledgments
This work was supported by NIH grants NS079884, GM104944 and GM103554 (Center of Biomedical Research Excellence, funded by the National Institute for General Medical Science, to CSvB). I thank Jami Bahney for the bench work that prompted this review, and Andrea Agarwal and Wei Yang for comments. I also thank several of my colleagues for collaborating on quantitation of cells: Stefano Geuna, Suzana Herculano-Houzel, Jon Kaas, Suleyman Kaplan, Ron Oppenheim, Glenn Rosen, Oliver von Bohlen und Halbach, and Rob Williams. Special thanks go to Suleyman Kaplan for his inspiration, encouragement and support.
Grant sponsors: National Institutes of Health (NIH); Grant numbers: NS079884, GM104944, and COBRE grant GM103554.
Abbreviations
- CNS
central nervous system
- GNR
glia-neuron ratio
- IF
isotropic fractionator
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Abusaad I, MacKay D, Zhao J, Stanford P, Collier DA, Everall IP. Stereological estimation of the total number of neurons in the murine hippocampus using the optical disector. J Comp Neurol. 1999;408:560–566. doi: 10.1002/(sici)1096-9861(19990614)408:4<560::aid-cne9>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008;31:137–145. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Andersen BB, Gundersen HJ. Pronounced loss of cell nuclei and anisotropic deformation of thick sections. J Microsc. 1999;196:69–73. [PubMed] [Google Scholar]
- Andersen BB, Korbo L, Pakkenberg B. A quantitative study of the human cerebellum with unbiased stereological techniques. J Comp Neurol. 1992;326:549–560. doi: 10.1002/cne.903260405. [DOI] [PubMed] [Google Scholar]
- Andersen K, Andersen BB, Pakkenberg B. Stereological quantification of the cerebellum in patients with Alzheimer's disease. Neurobiol Aging. 2012;33:197.e11–20. doi: 10.1016/j.neurobiolaging.2010.06.013. [DOI] [PubMed] [Google Scholar]
- Anderson JM, Hubbard BM, Coghill GR, Slidders W. The effect of advanced old age on the neurone content of the cerebral cortex. Observations with an automatic image analyser point counting method. J Neurol Sci. 1983;58:235–246. doi: 10.1016/0022-510x(83)90220-4. [DOI] [PubMed] [Google Scholar]
- Anderton BH. Changes in the ageing brain in health and disease. Philos Trans R Soc Lond B Biol Sci. 1997;352:1781–1792. doi: 10.1098/rstb.1997.0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade-Moraes CH, Oliveira-Pinto AV, Castro-Fonseca E, da Silva CG, Guimarães DM, Szczupak D, Parente-Bruno DR, Carvalho LR, Polichiso L, Gomes BV, Oliveira LM, Rodriguez RD, Leite RE, Ferretti-Rebustini RE, Jacob-Filho W, Pasqualucci CA, Grinberg LT, Lent R. Cell number changes in Alzheimer's disease relate to dementia, not to plaques and tangles. Brain. 2013;136:3738–3752. doi: 10.1093/brain/awt273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankney CD. Sex differences in relative brain size: the mismeasure of woman, too? Intelligence. 1992;16:329–336. [Google Scholar]
- Araque A, Carmignoto G, Haydon PG. Dynamic signaling between astrocytes and neurons. Annu Rev Physiol. 2001;63:795–813. doi: 10.1146/annurev.physiol.63.1.795. [DOI] [PubMed] [Google Scholar]
- Azevedo FAC, Carvalho LRB, Grinberg LT, Farfel JM, Ferretti REL, Leite REP, Jacob Filho W, Lent R, Herculano-Houzel S. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol. 2009;513:532–541. doi: 10.1002/cne.21974. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Is stereology ‘unbiased’? Trends Neurosci. 2001;24:375–376. doi: 10.1016/s0166-2236(00)01833-6. author reply 378-80. [DOI] [PubMed] [Google Scholar]
- Bahney J, von Bartheld CS. Validation of the isotropic fractionator: Comparison with unbiased stereology and DNA extraction for quantification of glial cells. J Neurosci Methods. 2014;222:165–174. doi: 10.1016/j.jneumeth.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahney J, von Bartheld CS. The cellular composition and glia-neuron ratio in the spinal cord of a human and nonhuman primate: comparison with other species and brain regions. Anat Rec. 2017 doi: 10.1002/ar.23728. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baquet ZC, Williams D, Brody J, Smeyne RJ. A comparison of model-based (2D) and design-based (3D) stereological methods for estimating cell number in the substantia nigra pars compacta (SNpc) of the C57BL/6J mouse. Neuroscience. 2009;161:1082–1090. doi: 10.1016/j.neuroscience.2009.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baryshnikova LM, von Bohlen und Halbach O, Kaplan S, von Bartheld CS. Two distinct events, section compression and loss of particles (“lost caps”), contribute to z-axis distortion and bias in optical disector counting. Microsc Res Tech. 2006;69:738–756. doi: 10.1002/jemt.20345. [DOI] [PubMed] [Google Scholar]
- Basgen JM, Nicholas SB, Mauer M, Rozen S, Nyengaard JR. Comparison of methods for counting cells in the mouse glomerulus. Nephron Exp Nephrol. 2006;103:e139–e148. doi: 10.1159/000092905. [DOI] [PubMed] [Google Scholar]
- Bass NH, Hess HH, Pope A, Thalheimer C. Quantitative cytoarchitectonic distribution of neurons, glia, and DNA in rat cerebral cortex. J Comp Neurol. 1971;143:481–490. doi: 10.1002/cne.901430405. [DOI] [PubMed] [Google Scholar]
- Benes FM. Neurobiological investigations in cingulate cortex of schizophrenic brain. Schizophr Bull. 1993;19:537–549. doi: 10.1093/schbul/19.3.537. [DOI] [PubMed] [Google Scholar]
- Benes FM. Is there evidence for neuronal loss in schizophrenia? Int Rev Psychiatry. 1997;9:429–436. [Google Scholar]
- Benes FM, Lange N. Two-dimensional versus three-dimensional cell counting: a practical perspective. Trends Neurosci. 2001a;24:11–17. doi: 10.1016/s0166-2236(00)01660-x. [DOI] [PubMed] [Google Scholar]
- Benes FM, Lange N. Benes and Lange respond: reconciling theory and practice in cell counting. Trends Neurosci. 2001b;24:378–380. doi: 10.1016/s0166-2236(00)01660-x. [DOI] [PubMed] [Google Scholar]
- Bernstein HG, Steiner J, Guest PC, Dobrowolny H, Bogerts B. Glial cells as key players in schizophrenia pathology: recent insights and concepts of therapy. Schizophr Res. 2015;161:4–18. doi: 10.1016/j.schres.2014.03.035. [DOI] [PubMed] [Google Scholar]
- Bjugn R, Gundersen HJ. Estimate of the total number of neurons and glial and endothelial cells in the rat spinal cord by means of the optical disector. J Comp Neurol. 1993;328:406–414. doi: 10.1002/cne.903280307. [DOI] [PubMed] [Google Scholar]
- Blinkov SM, Glezer II. A Quantitative Handbook. New York: Plenum Press; 1968. The Human Brain in Figures and Tables; p. 482. [Google Scholar]
- Braak H, Braak E. Ratio of pyramidal cells versus non-pyramidal cells in the human frontal isocortex and changes in ratio with ageing and Alzheimer's disease. Progress Brain Res. 1986;70:185–211. doi: 10.1016/s0079-6123(08)64305-8. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Morphological criteria for the recognition of Alzheimer's disease and the distribution pattern of cortical changes related to this disorder. Neurobiol Aging. 1994;15:355–356. doi: 10.1016/0197-4580(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Braendgaard H, Evans SM, Howard CV, Gundersen HJ. The total number of neurons in the human neocortex unbiasedly estimated using optical disectors. J Microsc. 1990;157:285–304. doi: 10.1111/j.1365-2818.1990.tb02967.x. [DOI] [PubMed] [Google Scholar]
- Brody H. Organization of the cerebral cortex. III. A study of aging in the human cerebral cortex. J Comp Neurol. 1955;102:511–556. doi: 10.1002/cne.901020206. [DOI] [PubMed] [Google Scholar]
- Brody H. Structural changes in the ageing nervous system. Interdiscipl Top Gerontol. 1970;7:9–21. [Google Scholar]
- Burish MJ, Peebles JK, Baldwin MK, Tavares L, Kaas JH, Herculano-Houzel S. Cellular scaling rules for primate spinal cords. Brain Behav Evol. 2010;76:45–59. doi: 10.1159/000319019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlo CN, Stevens CF. Analysis of differential shrinkage in frozen brain sections and its implications for the use of guard zones in stereology. J Comp Neurol. 2011;519:2803–2810. doi: 10.1002/cne.22652. [DOI] [PubMed] [Google Scholar]
- Carlo CN, Stevens CF. Structural uniformity of neocortex, revisited. Proc Natl Acad Sci USA. 2013;110:1488–93. doi: 10.1073/pnas.1221398110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charvet CJ, Cahalane DJ, Finlay BL. Systematic, cross-cortex variation in neuron numbers in rodents and primates. Cereb Cortex. 2015;25:147–160. doi: 10.1093/cercor/bht214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PG. How inaccurate is the Abercrombie correction factor for cell counts? Trends Neurosci. 1992;15:211–212. doi: 10.1016/0166-2236(92)90036-8. [DOI] [PubMed] [Google Scholar]
- Clarke PG, Oppenheim RW. Neuron death in vertebrate development: in vitro methods. Methods Cell Biol. 1995;46:277–321. [PubMed] [Google Scholar]
- Coggeshall RE. A consideration of neural counting methods. Trends Neurosci. 1992;15:9–13. doi: 10.1016/0166-2236(92)90339-a. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE, Lekan HA. Methods for determining numbers of cells and synapses: a case for more uniform standards of review. J Comp Neurol. 1996;364:6–15. doi: 10.1002/(SICI)1096-9861(19960101)364:1<6::AID-CNE2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE, La Forte R, Klein CM. Calibration of methods for determining numbers of dorsal root ganglion cells. J Neurosci Methods. 1990;35:187–194. doi: 10.1016/0165-0270(90)90123-w. [DOI] [PubMed] [Google Scholar]
- Colon EJ. The elderly brain. A quantitative analysis in the cerebral cortex of two cases. Psychiatr Neurol Neurochir. 1972;75:261–270. [PubMed] [Google Scholar]
- Committee on the Conduct of Science, National Academy of Sciences of the United States of America. On being a scientist. Proc Natl Acad Sci USA. 1989;86:9053–9074. doi: 10.1073/pnas.86.23.9053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter DR, Pariante CM, Everall IP. Glial cell abnormalities in major psychiatric disorders: the evidence and implications. Brain Res Bull. 2001;55:585–595. doi: 10.1016/s0361-9230(01)00527-5. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Pierce K, Schumann CM, Redcay E, Buckwalter JA, Kennedy DP, Morgan J. Mapping early brain development in autism. Neuron. 2007;56:399–413. doi: 10.1016/j.neuron.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Courville CB. Effects of alcohol in the nervous system of man. Los Angeles: San Lucas Press; 1955. [Google Scholar]
- Coyle JT, Schwarcz R. Mind glue: implications of glial cell biology for psychiatry. Arch Gen Psychiatry. 2000;57:90–93. doi: 10.1001/archpsyc.57.1.90. [DOI] [PubMed] [Google Scholar]
- Cragg BG. The density of synapses and neurons in normal, mentally defective ageing human brains. Brain. 1975;98:81–90. doi: 10.1093/brain/98.1.81. [DOI] [PubMed] [Google Scholar]
- Cruz-Orive LM. Toward a more objective biology. Neurobiol Aging. 1994;15:377–378. doi: 10.1016/0197-4580(94)90039-6. discussion 379-80. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Buell SJ, Coleman PD. The Aging Motor System Advances in Neurogerontology. Vol. 3. Mortimer JA, Pirozzolo FJ, Maletta GJ: New York: Praeger; 1982. Morphology of the aging central nervous system: Not all downhill; pp. 7–35. [Google Scholar]
- Deary IJ. Intelligence. Annu Rev Psychol. 2012;63:453–482. doi: 10.1146/annurev-psych-120710-100353. [DOI] [PubMed] [Google Scholar]
- DeCasien AR, Williams SA, Higham JP. Primate brain size is predicted by diet but not sociality. Nat Ecol Evol. 2017;1:0112. doi: 10.1038/s41559-017-0112. [DOI] [PubMed] [Google Scholar]
- Delaloye S, Kraftsik R, Kuntzer T, Barakat-Walter I. Does the physical disector method provide an accurate estimation of sensory neuron number in rat dorsal root ganglia? J Neurosci Methods. 2009;176:290–297. doi: 10.1016/j.jneumeth.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Devaney KO, Johnson HA. Neuron loss in the aging visual cortex of man. J Gerontol. 1980;35:836–841. doi: 10.1093/geronj/35.6.836. [DOI] [PubMed] [Google Scholar]
- Diamond MC, Scheibel AB, Murphy GM, Jr, Harvey T. On the brain of a scientist: Albert Einstein. Exp Neurol. 1985;88:198–204. doi: 10.1016/0014-4886(85)90123-2. [DOI] [PubMed] [Google Scholar]
- Dicke U, Roth G. Neuronal factors determining high intelligence. Philos Trans R Soc Lond B Biol Sci. 2016;371:20150180. doi: 10.1098/rstb.2015.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Quantitative growth and development of human brain. Arch Dis Child. 1973;48:757–767. doi: 10.1136/adc.48.10.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorph-Petersen KA, Pierri JN, Wu Q, Sampson AR, Lewis DA. Primary visual cortex volume and total neuron number are reduced in schizophrenia. J Comp Neurol. 2007;501:290–301. doi: 10.1002/cne.21243. [DOI] [PubMed] [Google Scholar]
- Dorph-Petersen KA, Lewis DA. Stereological approaches to identifying neuropathology in psychosis. Biol Psychiatry. 2011;69:113–126. doi: 10.1016/j.biopsych.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorph-Petersen KA, Lewis DA. Postmortem structural studies of the thalamus in schizophrenia. Schizophr Res. 2017;180:28–35. doi: 10.1016/j.schres.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duyckaerts C, Delatour B, Potier MC. Classification and basic pathology of Alzheimer disease. Acta Neuropathol. 2009;118:5–36. doi: 10.1007/s00401-009-0532-1. [DOI] [PubMed] [Google Scholar]
- Elsayed M, Magistretti PJ. A new outlook on mental illnesses: Glial involvement beyond the glue. Front Cell Neurosci. 2015;9:468. doi: 10.3389/fncel.2015.00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen N, Pakkenberg B. Total neocortical cell number in the mysticete brain. Anat Rec (Hoboken) 2007;290:83–95. doi: 10.1002/ar.20404. [DOI] [PubMed] [Google Scholar]
- Everall IP, DeTeresa R, Terry R, Masliah E. Comparison of two quantitative methods for the evaluation of neuronal number in the frontal cortex in Alzheimer disease. J Neuropathol Exp Neurol. 1997;56:1202–1206. doi: 10.1097/00005072-199711000-00004. [DOI] [PubMed] [Google Scholar]
- Fabricius K, Pakkenberg H, Pakkenberg B. No changes in neocortical cell volumes or glial cell numbers in chronic alcoholic subjects compared to control subjects. Alcohol Alcohol. 2007;42:400–406. doi: 10.1093/alcalc/agm007. [DOI] [PubMed] [Google Scholar]
- Fabricius K, Jacobsen JS, Pakkenberg B. Effect of age on neocortical brain cells in 90+ year old human females - a cell counting study. Neurobiol Aging. 2013;34:91–99. doi: 10.1016/j.neurobiolaging.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Farel PB. Trust, but verify: the necessity of empirical verification in quantitative neurobiology. Anat Rec. 2002;269:157–161. doi: 10.1002/ar.10111. [DOI] [PubMed] [Google Scholar]
- Fields RD. The Other Brain. New York: Simon & Schuster; 2009. [Google Scholar]
- Fields ED. Human brain cells make mice smart. [Accessed on February 2, 2017];Scientific American, Guest Blog. 2013 https://blogs.scientificamerican.com/guest-blog/human-brain-cells-make-mice-smart/
- Fischer HG, Morawski M, Brückner MK, Mittag A, Tarnok A, Arendt T. Changes in neuronal DNA content variation in the human brain during aging. Aging Cell. 2012;11:628–633. doi: 10.1111/j.1474-9726.2012.00826.x. [DOI] [PubMed] [Google Scholar]
- Flood DG. Thoughts on no neocortical neuronal loss but loss of volume in AD. Neurobiol Aging. 1994;15:363–365. doi: 10.1016/0197-4580(94)90035-3. [DOI] [PubMed] [Google Scholar]
- Friede R. Der quantitative Anteil der Glia and der Cortexentwicklung. Acta Anat. 1954;20:290–296. [PubMed] [Google Scholar]
- García-Amado M, Prensa L. Stereological analysis of neuron, glial and endothelial cell numbers in the human amygdaloid complex. PLoS One. 2012;7:e38692. doi: 10.1371/journal.pone.0038692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Cabezas MÁ, John YJ, Barbas H, Zikopoulos B. Distinction of neurons, glia and endothelial cells in the cerebral cortex: An algorithm based on cytological features. Front Neuroanat. 2016;10:107. doi: 10.3389/fnana.2016.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Marín V, Blazquez-Llorca L, Rodriguez JR, Gonzalez-Soriano J, DeFelipe J. Differential distribution of neurons in the gyral white matter of the human cerebral cortex. J Comp Neurol. 2010;518:4740–4759. doi: 10.1002/cne.22485. [DOI] [PubMed] [Google Scholar]
- Gardella D, Hatton WJ, Rind HB, Rosen GD, von Bartheld CS. Differential tissue shrinkage and compression in the z-axis: implications for optical dissector counting in vibratome-, plastic- and cryosections. J Neurosci Methods. 2003;124:45–59. doi: 10.1016/s0165-0270(02)00363-1. [DOI] [PubMed] [Google Scholar]
- Gardi JE, Nyengaard JR, Gundersen HJ. The proportionator: unbiased stereological estimation using biased automatic image analysis and non-uniform probability proportional to size sampling. Comput Biol Med. 2008a;38:313–328. doi: 10.1016/j.compbiomed.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Gardi JE, Nyengaard JR, Gundersen HJ. Automatic sampling for unbiased and efficient stereological estimation using the proportionator in biological studies. J Microsc. 2008b;230:108–120. doi: 10.1111/j.1365-2818.2008.01963.x. [DOI] [PubMed] [Google Scholar]
- Geuna S, Herrera-Rincon C. Update on stereology for light microscopy. Cell Tissue Res. 2015;360:5–12. doi: 10.1007/s00441-015-2143-6. [DOI] [PubMed] [Google Scholar]
- Giannaris EL, Rosene DL. A stereological study of the numbers of neurons and glia in the primary visual cortex across the lifespan of male and female rhesus monkeys. J Comp Neurol. 2012;520:3492–3508. doi: 10.1002/cne.23101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Isla T, Price JL, McKeel DW, Jr, Morris JC, Growdon JH, Hyman BT. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer's disease. J Neurosci. 1996;16:4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Isla T, Hollister R, West H, Mui S, Growdon JH, Petersen RC, Parisi JE, Hyman BT. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer's disease. Ann Neurol. 1997;41:17–24. doi: 10.1002/ana.410410106. [DOI] [PubMed] [Google Scholar]
- Guillery RW, Herrup K. Quantification without pontification: choosing a method for counting objects in sectioned tissues. J Comp Neurol. 1997;386:2–7. doi: 10.1002/(sici)1096-9861(19970915)386:1<2::aid-cne2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Guillery RW. On counting and counting errors. J Comp Neurol. 2002;447:1–7. doi: 10.1002/cne.10221. [DOI] [PubMed] [Google Scholar]
- Gundersen HJG, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, Møller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sørensen FB, Vesterby A, West MJ. The new stereological tools: Disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. Acta Pathol Microbiol Immunol Scand. 1988;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Han X, Chen M, Wang F, Windrem M, Wang S, Shanz S, Xu Q, Oberheim NA, Bekar L, Betstadt S, Silva AJ, Takano T, Goldman SA, Nedergaard M. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell. 2013;12:342–353. doi: 10.1016/j.stem.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley T. ‘Neuronal fall-out’ in the ageing brain: a critical review of the quantitative data. Age Ageing. 1974;3:133–151. doi: 10.1093/ageing/3.3.133. [DOI] [PubMed] [Google Scholar]
- Hansen LA, Natelson BH, Lemere C, Niemann W, De Teresa R, Regan TJ, Masliah E, Terry RD. Alcohol-induced brain changes in dogs. Arch Neurol. 1991;48:939–942. doi: 10.1001/archneur.1991.00530210065025. [DOI] [PubMed] [Google Scholar]
- Harding AJ, Wong A, Svoboda M, Kril JJ, Halliday GM. Chronic alcohol consumption does not cause hippocampal neuron loss in humans. Hippocampus. 1997;7:78–87. doi: 10.1002/(SICI)1098-1063(1997)7:1<78::AID-HIPO8>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Hare B. Survival of the friendliest: Homo sapiens evolved via selection for prosociality. Annu Rev Psychol. 2017;68:155–186. doi: 10.1146/annurev-psych-010416-044201. [DOI] [PubMed] [Google Scholar]
- Harper C. The incidence of Wernicke's encephalopathy in Australia—a neuropathological study of 131 cases. J Neurol Neurosurg Psychiatry. 1983;46:593–598. doi: 10.1136/jnnp.46.7.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper C. The neuropathology of alcohol-specific brain damage, or does alcohol damage the brain? J Neuropathol Exp Neurol. 1998;57:101–110. doi: 10.1097/00005072-199802000-00001. [DOI] [PubMed] [Google Scholar]
- Harper CG, Kril JJ, Holloway RL. Brain shrinkage in chronic alcoholics: a pathological study. Br Med J (Clin Res Ed) 1985;290:501–504. doi: 10.1136/bmj.290.6467.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper C, Kril J, Daly J. Are we drinking our neurones away? Br Med J (Clin Res Ed) 1987;294:534–536. doi: 10.1136/bmj.294.6571.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper CG, Kril JJ. Neuropathology of alcoholism. Alcohol Alcohol. 1990;25:207–216. doi: 10.1093/oxfordjournals.alcalc.a044994. [DOI] [PubMed] [Google Scholar]
- Harper C, Matsumoto I. Ethanol and brain damage. Curr Opin Pharmacol. 2005;5:73–78. doi: 10.1016/j.coph.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999;122:593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- Hatton WJ, von Bartheld CS. Analysis of cell death in the trochlear nucleus of the chick embryo: calibration of the optical disector counting method reveals systematic bias. J Comp Neurol. 1999;409:169–186. [PubMed] [Google Scholar]
- Haug H. Die Abhängigkeit der Einbettungsschrumpfung des Gehirngewebes vom Lebensalter. Verh Anat Ges. 1980;74:699–700. [Google Scholar]
- Haug H. Der Einfluss der säkularen Akzeleration auf das Hirngewicht des Menschen und dessen Änderung wahrend der Alterung. Gegenbaurs Morphol Jahrb. 1984;130:481–500. [PubMed] [Google Scholar]
- Haug H. Gibt es Nervenzellverluste wahrend der biologischen Alterung in der menschlichen Hirnrinde? Ein morphometrischer Beitrag zu dieser Frage. Nervenheilkunde. 1985;4:103–109. [Google Scholar]
- Haug H. History of neuromorphometry. J Neurosci Methods. 1986;18:1–17. doi: 10.1016/0165-0270(86)90110-x. [DOI] [PubMed] [Google Scholar]
- Haug H. Brain sizes, surfaces, and neuronal sizes of the cortex cerebri: a stereological investigation of man and his variability and a comparison with some mammals (primates, whales, marsupials, insectivores, and one elephant) Am J Anat. 1987;180:126–142. doi: 10.1002/aja.1001800203. [DOI] [PubMed] [Google Scholar]
- Haug H, Kühl S, Mecke E, Sass NL, Wasner K. The significance of morphometric procedures in the investigation of age changes in cytoarchitectonic structures of human brain. J Hirnforsch. 1984;25:353–374. [PubMed] [Google Scholar]
- Hawkins A, Olszewski J. Glia/nerve cell index for cortex of the whale. Science. 1957;126:76–77. doi: 10.1126/science.126.3263.76. [DOI] [PubMed] [Google Scholar]
- Heller IH, Elliott KA. Desoxyribonucleic acid content and cell density in brain and human brain tumors. Can J Biochem Physiol. 1954;32:584–592. [PubMed] [Google Scholar]
- Henderson G, Tomlinson BE, Gibson PH. Cell counts in human cerebral cortex in normal adults throughout life using an image analysing computer. J Neurol Sci. 1980;46:113–136. doi: 10.1016/0022-510x(80)90048-9. [DOI] [PubMed] [Google Scholar]
- Herculano-Houzel S. The human brain in numbers: a linearly scaled-up primate brain. Front Hum Neurosci. 2009;3:31. doi: 10.3389/neuro.09.031.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herculano-Houzel S. Not all brains are made the same: new views on brain scaling in evolution. Brain Behav Evol. 2011;78:22–36. doi: 10.1159/000327318. [DOI] [PubMed] [Google Scholar]
- Herculano-Houzel S. The remarkable, yet not extraordinary, human brain as a scaled-up primate brain and its associated cost. Proc Natl Acad Sci USA. 2012;109(1):10661–10668. doi: 10.1073/pnas.1201895109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herculano-Houzel S. The glia/neuron ratio: How it varies uniformly across brain structures and species and what that means for brain physiology and evolution. Glia. 2014;62:1377–1391. doi: 10.1002/glia.22683. [DOI] [PubMed] [Google Scholar]
- Herculano-Houzel S. Numbers of neurons as biological correlates of cognitive capability. Curr Opin Behav Sci. 2017;16:1–7. [Google Scholar]
- Herculano-Houzel S, Lent R. Isotropic fractionator: a simple, rapid method for the quantification of total cell and neuron numbers in the brain. J Neurosci. 2005;25:2518–2521. doi: 10.1523/JNEUROSCI.4526-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herculano-Houzel S, Collins CE, Wong P, Kaas JH. Cellular scaling rules for primate brains. Proc Natl Acad Sci USA. 2007;104:3562–3567. doi: 10.1073/pnas.0611396104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herculano-Houzel S, von Bartheld CS, Miller DJ, Kaas JH. How to count cells: the advantages and disadvantages of the isotropic fractionator compared with stereology. Cell Tissue Res. 2015;360:29–42. doi: 10.1007/s00441-015-2127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgetag CC, Barbas H. Are there ten times more glia than neurons in the brain? Brain Struct Funct. 2009;213:365–366. doi: 10.1007/s00429-009-0202-z. [DOI] [PubMed] [Google Scholar]
- Hof PR, Haroutunian V, Friedrich VL, Jr, Byne W, Buitron C, Perl DP, Davis KL. Loss and altered spatial distribution of oligodendrocytes in the superior frontal gyrus in schizophrenia. Biol Psychiatry. 2003;53:1075–1085. doi: 10.1016/s0006-3223(03)00237-3. [DOI] [PubMed] [Google Scholar]
- Hof PR, Mobbs CV. Handbook of the Neuroscience of Aging (Preface: xvii- xix) London: Elsevier; 2009. [Google Scholar]
- Hosseini-Sharifabad M, Nyengaard JR. Design-based estimation of neuronal number and individual neuronal volume in the rat hippocampus. J Neurosci Methods. 2007;162:206–214. doi: 10.1016/j.jneumeth.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Howard CV, Reed MG. Unbiased Stereology Three–Dimensional Measurement in Microscopy. New York: Springer; 1998. [Google Scholar]
- Hyden H. The Neuron. In: Brachet J, Mirsky AE, editors. The Cell: Biochemistry, Physiology, Morphology. Vol. 4. New York: Acad Press; 1960. pp. 215–323. [Google Scholar]
- Hyden H. Dynamic aspects of the neuron-glia relationship - a study with microchemical methods. In: Hyden H, editor. The Neuron. Amsterdam: Elsevier; 1967. pp. 179–217. [Google Scholar]
- Hyman BT, Gomez-Isla T. Alzheimer's disease is a laminar, regional, and neural system specific disease, not a global brain disease. Neurobiol Aging. 1994;15:353–354. doi: 10.1016/0197-4580(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Ioannidis JP. Why science is not necessarily self-correcting. Perspect Psychol Sci. 2012;7:645–654. doi: 10.1177/1745691612464056. [DOI] [PubMed] [Google Scholar]
- Jarrett C. Great Myths of the Brain. Chichester: Wiley Blackwell; 2015. [Google Scholar]
- Jensen AR. Bias in Mental Testing. New York: Free Press; 1980. [Google Scholar]
- Jensen GB, Pakkenberg B. Do alcoholics drink their neurons away? Lancet. 1993;342:1201–1204. doi: 10.1016/0140-6736(93)92185-v. [DOI] [PubMed] [Google Scholar]
- Jerison HJ. Evolution of the Brain and Intelligence. New York: Academic Press; 1973. [Google Scholar]
- Johnson HA, Erner S. Neuron survival in the aging mouse. Exp Gerontol. 1972;7:111–117. doi: 10.1016/0531-5565(72)90005-8. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH. Principles of Neural Science. 1st. New York: Elsevier; 1981. [Google Scholar]
- Kandel ER, Schwartz JH. Principles of Neural Science. 2nd. New York: Elsevier; 1985. [Google Scholar]
- Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science. 3rd. Norwalk, CT: Appleton & Lange; 1991. [Google Scholar]
- Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science. 4th. New York: McGraw-Hill; 2000. [Google Scholar]
- Kaplan S, Geuna S, Ronchi G, Ulkay MB, von Bartheld CS. Calibration of the stereological estimation of the number of myelinated axons in the rat sciatic nerve: a multicenter study. J Neurosci Methods. 2010;187:90–99. doi: 10.1016/j.jneumeth.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsen AS, Pakkenberg B. Total numbers of neurons and glial cells in cortex and basal ganglia of aged brains with Down syndrome - a stereological study. Cereb Cortex. 2011;21:2519–2524. doi: 10.1093/cercor/bhr033. [DOI] [PubMed] [Google Scholar]
- Kaur C, Pal I, Saini S, Jacob TG, Nag TC, Thakar A, Bhardwaj DN, Roy TS. Comparison of unbiased strereological estimation of total number of cresyl violet stained neurons and parvalbumin positive neurons in the adult human spiral ganglion. J Chem Neuroanat. 2018 doi: 10.1016/j.jchemneu.2017.06.004. in press. [DOI] [PubMed] [Google Scholar]
- Kausler DH, Kausler BC, Krupsaw JA. The Essential Guide to Aging in the Twenty-First Century: Mind, Body, and Behavior. St Louis: University of Missouri Press; 2007. [Google Scholar]
- Kettenmann H, Verkhratsky A. Glial Cells. In: Pfaff DW, editor. Neuroscience in the 21st Century. New York: Springer; 2013. pp. 475–506. [Google Scholar]
- Koob A. The Root of Thought: Unlocking Glia – the Brain Cell That Will Help Us Sharpen Our Wits, Heal Injury, and Treat Brain Disease. Upper Saddle River: Pearson Education; 2009. [Google Scholar]
- Korbo L, Amrein I, Lipp HP, Wolfer D, Regeur L, Oster S, Pakkenberg B. No evidence for loss of hippocampal neurons in non-Alzheimer dementia patients. Acta Neurol Scand. 2004;109:132–139. doi: 10.1034/j.1600-0404.2003.00182.x. [DOI] [PubMed] [Google Scholar]
- Kordower JH. Making the counts count: the stereology revolution. J Chem Neuroanat. 2000;20:1–2. doi: 10.1016/s0891-0618(00)00079-x. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Olanow CW, Dodiya HB, Chu Y, Beach TG, Adler CH, Halliday GM, Bartus RT. Disease duration and the integrity of the nigrostriatal system in Parkinson's disease. Brain. 2013;136:2419–2431. doi: 10.1093/brain/awt192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kril JJ, Harper CG. Neuronal counts from four cortical regions of alcoholic brains. Acta Neuropathol. 1989;7:200–204. doi: 10.1007/BF00294379. [DOI] [PubMed] [Google Scholar]
- Kril JJ, Halliday GM. Brain shrinkage in alcoholics: a decade on and what have we learned? Prog Neurobiol. 1999;58:381–387. doi: 10.1016/s0301-0082(98)00091-4. [DOI] [PubMed] [Google Scholar]
- Kubis N, Faucheux BA, Ransmayr G, Damier P, Duyckaerts C, Henin D, Forette B, Le Charpentier Y, Hauw JJ, Agid Y, Hirsch EC. Preservation of midbrain catecholaminergic neurons in very old human subjects. Brain. 2000;123:366–373. doi: 10.1093/brain/123.2.366. [DOI] [PubMed] [Google Scholar]
- Kühnel W. In memoriam des Anatomen Herbert W. Haug (1920-2002) Ann Anat. 2003;185:293–295. [Google Scholar]
- Kuffler SW, Nicholls JG. From Neuron to Brain. Sunderland: Sinauer Associates; 1976. [Google Scholar]
- Lent R, Azevedo FA, Andrade-Moraes CH, Pinto AV. How many neurons do you have? Some dogmas of quantitative neuroscience under revision. Eur J Neurosci. 2012;35:1–9. doi: 10.1111/j.1460-9568.2011.07923.x. [DOI] [PubMed] [Google Scholar]
- Leuba G, Garey LJ. Evolution of neuronal numerical density in the developing and aging human visual cortex. Hum Neurobiol. 1987;6:11–8. [PubMed] [Google Scholar]
- Leuba G, Garey LJ. Comparison of neuronal and glial numerical density in primary and secondary visual cortex of man. Exp Brain Res. 1989;77:31–38. doi: 10.1007/BF00250564. [DOI] [PubMed] [Google Scholar]
- Leuba G, Kraftsik R. Changes in volume, surface estimate, three-dimensional shape and total number of neurons of the human primary visual cortex from midgestation until old age. Anat Embryol (Berl) 1994a;190:351–366. doi: 10.1007/BF00187293. [DOI] [PubMed] [Google Scholar]
- Leuba G, Kraftsik R. Visual cortex in Alzheimer's disease: occurrence of neuronal death and glial proliferation, and correlation with pathological hallmarks. Neurobiol Aging. 1994b;15:29–43. doi: 10.1016/0197-4580(94)90142-2. [DOI] [PubMed] [Google Scholar]
- Lyck L, Santamaria ID, Pakkenberg B, Chemnitz J, Schrøder HD, Finsen B, Gundersen HJ. An empirical analysis of the precision of estimating the numbers of neurons and glia in human neocortex using a fractionator-design with sub-sampling. J Neurosci Methods. 2009;182:143–156. doi: 10.1016/j.jneumeth.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Lyness SA, Zarow C, Chui HC. Neuron loss in key cholinergic and aminergic nuclei in Alzheimer disease: a meta-analysis. Neurobiol Aging. 2003;24:1–23. doi: 10.1016/s0197-4580(02)00057-x. [DOI] [PubMed] [Google Scholar]
- Mann DM. Pyramidal nerve cell loss in Alzheimer's disease. Neurodegeneration. 1996;5:423–427. doi: 10.1006/neur.1996.0057. [DOI] [PubMed] [Google Scholar]
- Marino L. Absolute brain size: did we throw the baby out with the bathwater? Proc Natl Acad Sci USA. 2006;103:13563–13564. doi: 10.1073/pnas.0606337103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhew TM, Gundersen HJ. ‘If you assume, you can make an ass out of u and me’: A decade of the disector for stereological counting of particles in 3D space. J Anat. 1996;188:1–15. [PMC free article] [PubMed] [Google Scholar]
- McDaniel MA. Big-brained people are smarter: a meta-analysis of the relationship between in vivo brain volume and intelligence. Intelligence. 2005;33:337–346. [Google Scholar]
- McKeown RE, Cuffe SP, Schulz RM. US suicide rates by age group, 1970-2002: an examination of recent trends. Am J Public Health. 2006;96:1744–1751. doi: 10.2105/AJPH.2005.066951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan PJ, Saltzman LE, Sattin RW. Suicides among older United States residents: epidemiologic characteristics and trends. Am J Public Health. 1991;81:1198–1200. doi: 10.2105/ajph.81.9.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AK, Corsellis JA. Evidence for a secular increase in human brain weight during the past century. Ann Hum Biol. 1977;4:253–257. doi: 10.1080/03014467700007142. [DOI] [PubMed] [Google Scholar]
- Miller DJ, Balaram P, Young NA, Kaas JH. Three counting methods agree on cell and neuron number in chimpanzee primary visual cortex. Front Neuroanat. 2014;8:36. doi: 10.3389/fnana.2014.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JT, Chana G, Pardo CA, Achim C, Semendeferi K, Buckwalter J, Courchesne E, Everall IP. Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biol Psychiatry. 2010;68:368–376. doi: 10.1016/j.biopsych.2010.05.024. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science. 1997;278:412–419. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- Mountjoy CQ, Roth M, Evans NJ, Evans HM. Cortical neuronal counts in normal elderly controls and demented patients. Neurobiol Aging. 1983;4:1–11. doi: 10.1016/0197-4580(83)90048-9. [DOI] [PubMed] [Google Scholar]
- Mouton PR. Principles and practices of unbiased stereology An introduction for bioscientists. Baltimore: Johns Hopkins University Press; 2002. [Google Scholar]
- Mouton PR, Pakkenberg B, Gundersen HJ, Price DL. Absolute number and size of pigmented locus coeruleus neurons in young and aged individuals. J Chem Neuroanat. 1994;7:185–190. doi: 10.1016/0891-0618(94)90028-0. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Benzing WC. Lack of neocortical nerve cell loss in Alzheimer's disease: reality or methodological artifact. Neurobiol Aging. 1994;15:361–362. doi: 10.1016/0197-4580(94)90034-5. discussion: 379-380. [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 2003;26:523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Ngwenya A, Nahirney J, Brinkman B, Williams L, Iwaniuk AN. Comparison of estimates of neuronal number obtained using the isotropic fractionator method and unbiased stereology in day old chicks (Gallus domesticus) J Neurosci Methods. 2017;287:39–46. doi: 10.1016/j.jneumeth.2017.05.025. [DOI] [PubMed] [Google Scholar]
- Nielsen RD, Abitz M, Pakkenberg B. Neuron and glial cell numbers in the mediodorsal thalamic nucleus in brains of schizophrenic subjects. Image Anal Stereol. 2008;27:133–141. [Google Scholar]
- Nurnberger JI. Direct enumeration of cells of the brain. In: Windle WF, editor. Biology of neuroglia. Springfield, IL: Charles C Thomas; 1958. pp. 193–202. [Google Scholar]
- Nurnberger JI, Gordon MW. The cell density of neural tissues: direct counting method and possible applications as a biologic referent. Prog Neurobiol. 1957;2:100–128. discussion 128-138. [PubMed] [Google Scholar]
- Oliveira-Pinto AV, Andrade-Moraes CH, Oliveira LM, Parente-Bruno DR, Santos RM, Coutinho RA, Alho AT, Leite RE, Suemoto CK, Grinberg LT, Pasqualucci CA, Jacob-Filho W, Lent R. Do age and sex impact on the absolute cell numbers of human brain regions? Brain Struct Funct. 2016;221:3547–3559. doi: 10.1007/s00429-015-1118-4. [DOI] [PubMed] [Google Scholar]
- Ongür D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci USA. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmen SJ, van Engeland H, Hof PR, Schmitz C. Neuropathological findings in autism. Brain. 2004;127:2572–2583. doi: 10.1093/brain/awh287. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B. Pronounced reduction of total neuron number in mediodorsal thalamic nucleus and nucleus accumbens in schizophrenics. Arch Gen Psychiatry. 1990;47:1023–1028. doi: 10.1001/archpsyc.1990.01810230039007. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B. Stereological quantitation of human brains from normal and schizophrenic individuals. Acta Neurol Scand Suppl. 1992;137:20–33. doi: 10.1111/j.1600-0404.1992.tb05034.x. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B. Leucotomized schizophrenics lose neurons in the mediodorsal thalamic nucleus. Neuropathol Appl Neurobiol. 1993;19:373–380. doi: 10.1111/j.1365-2990.1993.tb00457.x. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B. Total nerve cell number in neocortex in chronic schizophrenics and controls estimated using optical disectors. Biol Psychiatry. 1993;34:768–772. doi: 10.1016/0006-3223(93)90065-l. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B, Gundersen HJ. Total number of neurons and glial cells in human brain nuclei estimated by the disector and the fractionator. J Microsc. 1988;150:1–20. doi: 10.1111/j.1365-2818.1988.tb04582.x. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B, Gundersen HJ. New stereological method for obtaining unbiased and efficient estimates of total nerve cell number in human brain areas. Exemplified by the mediodorsal thalamic nucleus in schizophrenics. Acta Pathol Microbiol Immunol Scand. 1989;97:677–681. doi: 10.1111/j.1699-0463.1989.tb00462.x. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B, Gundersen HJ. Neocortical neuron number in humans: effect of sex and age. J Comp Neurol. 1997;384:312–320. [PubMed] [Google Scholar]
- Pakkenberg B, Evans SM, Møller A, Brændgaard H, Gundersen HJG. Total number of neurons in human neocortex related to age and sex estimated by way of optical disectors. Acta Stereologica. 1989;8:251–256. [Google Scholar]
- Pakkenberg B, Pelvig D, Marner L, Bundgaard MJ, Gundersen HJ, Nyengaard JR, Regeur L. Aging and the human neocortex. Exp Gerontol. 2003;38:95–99. doi: 10.1016/s0531-5565(02)00151-1. [DOI] [PubMed] [Google Scholar]
- Palmen SJ, van Engeland H, Hof PR, Schmitz C. Neuropathological findings in autism. Brain. 2004;127:2572–2583. doi: 10.1093/brain/awh287. [DOI] [PubMed] [Google Scholar]
- Pannese E. Morphological changes in nerve cells during normal aging. Brain Struct Funct. 2011;216:85–89. doi: 10.1007/s00429-011-0308-y. [DOI] [PubMed] [Google Scholar]
- Pearce E, Stringer C, Dunbar RI. New insights into differences in brain organization between Neanderthals and anatomically modern humans. Proc Biol Sc. 2013;280:20130168. doi: 10.1098/rspb.2013.0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelvig DP, Pakkenberg H, Regeur L, Oster S, Pakkenberg B. Neocortical glial cell numbers in Alzheimer's disease. A stereological study. Dement Geriatr Cogn Disord. 2003;16:212–219. doi: 10.1159/000072805. [DOI] [PubMed] [Google Scholar]
- Pelvig DP, Pakkenberg H, Stark AK, Pakkenberg B. Neocortical glial cell numbers in human brains. Neurobiol Aging. 2008;29:1754–1762. doi: 10.1016/j.neurobiolaging.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Peters A, Morrison JH, Rosene DL, Hyman BT. Feature article: are neurons lost from the primate cerebral cortex during normal aging? Cereb Cortex. 1998;8:295–300. doi: 10.1093/cercor/8.4.295. [DOI] [PubMed] [Google Scholar]
- Pietschnig J, Penke L, Wicherts JM, Zeiler M, Voracek M. Meta-analysis of associations between human brain volume and intelligence differences: How strong are they and what do they mean? Neurosci Biobehav Rev. 2015;57:411–432. doi: 10.1016/j.neubiorev.2015.09.017. [DOI] [PubMed] [Google Scholar]
- Popken GJ, Farel PB. Reliability and validity of the physical disector method for estimating neuron number. J Neurobiol. 1996;31:166–174. doi: 10.1002/(SICI)1097-4695(199610)31:2<166::AID-NEU3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Popken GJ, Bunney WE, Jr, Potkin SG, Jones EG. Subnucleus-specific loss of neurons in medial thalamus of schizophrenics. Proc Natl Acad Sci USA. 2000;97:9276–9280. doi: 10.1073/pnas.150243397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pover CM, Coggeshall RE. Verification of the disector method for counting neurons, with comments on the empirical method. Anat Rec. 1991;231:573–578. doi: 10.1002/ar.1092310419. [DOI] [PubMed] [Google Scholar]
- Price DL. New perspectives on Alzheimer's disease. Annu Rev Neurosci. 1986;9:489–512. doi: 10.1146/annurev.ne.09.030186.002421. [DOI] [PubMed] [Google Scholar]
- Rajkowska G. Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biol Psychiatry. 2000;48:766–777. doi: 10.1016/s0006-3223(00)00950-1. [DOI] [PubMed] [Google Scholar]
- Rajkowska G. Cell pathology in mood disorders. Semin Clin Neuropsychiatry. 2002;7:281–292. doi: 10.1053/scnp.2002.35228. [DOI] [PubMed] [Google Scholar]
- Regeur L, Jensen GB, Pakkenberg H, Evans SM, Pakkenberg B. No global neocortical nerve cell loss in brains from patients with senile dementia of Alzheimer's type. Neurobiol Aging. 1994;15:347–352. doi: 10.1016/0197-4580(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Ridgway SH, Carlin KP, Van Alstyne KR, Hanson AC, Tarpley RJ. Comparison of dolphins' body and brain measurements with four other groups of cetaceans reveals great diversity. Brain Behav Evol. 2016;88:235–257. doi: 10.1159/000454797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth G, Dicke U. Evolution of the brain and intelligence. Trends Cogn Sci. 2005;9:250–257. doi: 10.1016/j.tics.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Rowland LP, Mettler FA. Cell concentration and laminar thickness in the frontal cortex of psychotic patients; studies on cortes removed at operation. J Comp Neurol. 1949;90:255–280. doi: 10.1002/cne.900900302. [DOI] [PubMed] [Google Scholar]
- Rushton JP, Ankney CD. Whole brain size and general mental ability: a review. Int J Neurosci. 2009;119:691–731. doi: 10.1080/00207450802325843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini A. How Science got Women Wrong – and the New Research That's Rewriting the Story. Boston: Beacon Press; 2017. INFERIOR. [Google Scholar]
- Saper CB. Book Reviews. Unbiased Stereology: Three-Dimensional Measurement in Microscopy. Trends Neurosci. 1999;22:94–95. [Google Scholar]
- Schmalbruch H. The number of neurons in dorsal root ganglia L4-L6 of the rat. Anat Rec. 1987;219:315–322. doi: 10.1002/ar.1092190313. [DOI] [PubMed] [Google Scholar]
- Schmitz C, Hof PR. Recommendations for straightforward and rigorous methods of counting neurons based on a computer simulation approach. J Chem Neuroanat. 2000;20:93–114. doi: 10.1016/s0891-0618(00)00066-1. [DOI] [PubMed] [Google Scholar]
- Schmitz C, Hof PR. Design-based stereology in neuroscience. Neuroscience. 2005;130:813–831. doi: 10.1016/j.neuroscience.2004.08.050. [DOI] [PubMed] [Google Scholar]
- Schmutte T, O'Connell M, Weiland M, Lawless S, Davidson L. Stemming the tide of suicide in older white men: a call to action. Am J Mens Health. 2009;3:189–200. doi: 10.1177/1557988308316555. [DOI] [PubMed] [Google Scholar]
- Sharma S, Nag TC, Bhardwaj DN, Vanamail P, Roy TS. Changing population of neurons and glia in the human cochlear nucleus with progressive age – a stereological study. J Anat Soc India. 2014;63:142–150. [Google Scholar]
- Sherwood CC, Stimpson CD, Raghanti MA, Wildman DE, Uddin M, Grossman LI, Goodman M, Redmond JC, Bonar CJ, Erwin JM, Hof PR. Evolution of increased glia-neuron ratios in the human frontal cortex. Proc Natl Acad Sci USA. 2006;103:13606–13611. doi: 10.1073/pnas.0605843103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soper B, Rosenthal G. The number of neurons in the brain: how we report what we do not know. Tech Psychol. 1988;15:153–156. [Google Scholar]
- Stark AK, Pelvig DP, Jørgensen AM, Andersen BB, Pakkenberg B. Measuring morphological and cellular changes in Alzheimer's dementia: a review emphasizing stereology. Curr Alzheimer Res. 2005;2:449–481. doi: 10.2174/156720505774330528. [DOI] [PubMed] [Google Scholar]
- Stolzenburg JU, Reichenbach A, Neumann M. Size and density of glial and neuronal cells within the cerebral neocortex of various insectivorian species. Glia. 1989;2:78–84. doi: 10.1002/glia.440020203. [DOI] [PubMed] [Google Scholar]
- St Wecker PG, Farel PB. Hindlimb sensory neuron number increases with body size. J Comp Neurol. 1994;342:430–438. doi: 10.1002/cne.903420309. [DOI] [PubMed] [Google Scholar]
- Tandrup T. Unbiased estimates of number and size of rat dorsal root ganglion cells in studies of structure and cell survival. J Neurocytol. 2004;33:173–192. doi: 10.1023/b:neur.0000030693.91881.53. [DOI] [PubMed] [Google Scholar]
- Terry RD, Peck A, DeTeresa R, Schechter R, Horoupian DS. Some morphometric aspects of the brain in senile dementia of the Alzheimer type. Ann Neurol. 1981;10:184–192. doi: 10.1002/ana.410100209. [DOI] [PubMed] [Google Scholar]
- Terry RD, DeTeresa R, Hansen LA. Neocortical cell counts in normal human adult aging. Ann Neurol. 1987;21:530–539. doi: 10.1002/ana.410210603. [DOI] [PubMed] [Google Scholar]
- Terry RD. The pathology of Alzheimer's disease: numbers count. Ann Neurol. 1997;41:7. doi: 10.1002/ana.410410104. [DOI] [PubMed] [Google Scholar]
- Thune JJ, Pakkenberg B. Stereological studies of the schizophrenic brain. Brain Res Rev. 2000;31:200–204. doi: 10.1016/s0165-0173(99)00038-7. [DOI] [PubMed] [Google Scholar]
- Tiedemann F. On the brain of the Negro, compared with that of the European and the Orang-outang. Phil Trans R Soc Lond. 1836;126:497–527. [Google Scholar]
- Todtenkopf MS, Vincent SL, Benes FM. A cross-study meta-analysis and three-dimensional comparison of cell counting in the anterior cingulate cortex of schizophrenic and bipolar brain. Schizophr Res. 2005;73:79–89. doi: 10.1016/j.schres.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Tower DB, Young OM. The activities of butyrylcholinesterase and carbonic anhydrase, the rate of anaerobic glycolysis, and the question of a constant density of glial cells in cerebral cortices of various mammalian species from mouse to whale. J Neurochem. 1973;20:269–278. doi: 10.1111/j.1471-4159.1973.tb12126.x. [DOI] [PubMed] [Google Scholar]
- Tsai PS, Kaufhold JP, Blinder P, Friedman B, Drew PJ, Karten HJ, Lyden PD, Kleinfeld D. Correlations of neuronal and microvascular densities in murine cortex revealed by direct counting and colocalization of nuclei and vessels. J Neurosci. 2009;29:14553–14570. doi: 10.1523/JNEUROSCI.3287-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uylings HB, de Brabander JM. Neuronal changes in normal human aging and Alzheimer's disease. Brain Cogn. 2002;49:268–276. doi: 10.1006/brcg.2001.1500. [DOI] [PubMed] [Google Scholar]
- Vawter MP, Freed WJ, Kleinman JE. Neuropathology of bipolar disorder. Biol Psychiatry. 2000;48:486–504. doi: 10.1016/s0006-3223(00)00978-1. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Butt A. Glial Neurobiology: A Text Book. Chichester, UK: John Wiley & Sons; 2007. [Google Scholar]
- Verkhratsky A, Rodríguez JJ, Parpura V. Neuroglia in ageing and disease. Cell Tissue Res. 2014;357:493–503. doi: 10.1007/s00441-014-1814-z. [DOI] [PubMed] [Google Scholar]
- Villa P, Roebroeks W. Neandertal demise: an archaeological analysis of the modern human superiority complex. PLoS One. 2014;9:e96424. doi: 10.1371/journal.pone.0096424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bartheld CS, Bothwell M. Development and distribution of noradrenergic and cholinergic neurons and their trophic phenotypes in the avian ceruleus complex and midbrain tegmentum. J Comp Neurol. 1992;320:479–500. doi: 10.1002/cne.903200406. [DOI] [PubMed] [Google Scholar]
- von Bartheld CS, Bothwell M. Development of the mesencephalic nucleus of the trigeminal nerve in chick embryos: target innervation, neurotrophin receptors, and cell death. J Comp Neurol. 1993;328:185–202. doi: 10.1002/cne.903280203. [DOI] [PubMed] [Google Scholar]
- von Bartheld CS. Systematic bias in an “unbiased” neuronal counting technique. Anat Rec. 1999;257:119–120. doi: 10.1002/(SICI)1097-0185(19990815)257:4<119::AID-AR2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- von Bartheld CS. Comparison of 2-D and 3-D counting: the need for calibration and common sense. Trends Neurosci. 2001;24:504–506. doi: 10.1016/s0166-2236(00)01960-3. [DOI] [PubMed] [Google Scholar]
- von Bartheld CS. Counting particles in tissue sections: choices of methods and importance of calibration to minimize biases. Histol Histopathol. 2002;17:639–648. doi: 10.14670/HH-17.639. [DOI] [PubMed] [Google Scholar]
- von Bartheld CS. Distribution of particles in the z-axis of tissue sections: Relevance for counting methods. Neuroquantology. 2012;10:66–75. [PMC free article] [PubMed] [Google Scholar]
- von Bartheld CS, Bahney J, Herculano-Houzel S. The search for true numbers of neurons and glial cells in the human brain: A review of 150 years of cell counting. J Comp Neurol. 2016;524:3865–3895. doi: 10.1002/cne.24040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonsattel JP, DiFiglia M. Huntington disease. J Neuropathol Exp Neurol. 1998;57:369–384. doi: 10.1097/00005072-199805000-00001. [DOI] [PubMed] [Google Scholar]
- Vostrikov VM, Uranova NA, Orlovskaya DD. Deficit of perineuronal oligodendrocytes in the prefrontal cortex in schizophrenia and mood disorders. Schizophr Res. 2007;94:273–280. doi: 10.1016/j.schres.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Walker DW, Barnes DE, Zornetzer SF, Hunter BE, Kubanis P. Neuronal loss in hippocampus induced by prolonged ethanol consumption in rats. Science. 1980;209:711–713. doi: 10.1126/science.7394532. [DOI] [PubMed] [Google Scholar]
- Walloe S, Pakkenberg B, Fabricius K. Stereological estimation of total cell numbers in the human cerebral and cerebellar cortex. Front Hum Neurosci. 2014;8:508. doi: 10.3389/fnhum.2014.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward TS, Rosen GD, von Bartheld CS. Optical disector counting in cryosections and vibratome sections underestimates particle numbers: effects of tissue quality. Microsc Res Tech. 2008;71:60–68. doi: 10.1002/jemt.20525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ. New stereological methods for counting neurons. Neurobiol Aging. 1993a;14:275–285. doi: 10.1016/0197-4580(93)90112-o. [DOI] [PubMed] [Google Scholar]
- West MJ. Regionally specific loss of neurons in the aging human hippocampus. Neurobiol Aging. 1993b;14:287–293. doi: 10.1016/0197-4580(93)90113-p. [DOI] [PubMed] [Google Scholar]
- West MJ. Stereological methods for estimating the total number of neurons and synapses: issues of precision and bias. Trends Neurosci. 1999;22:51–61. doi: 10.1016/s0166-2236(98)01362-9. [DOI] [PubMed] [Google Scholar]
- Williams RW, Herrup K. The control of neuron number. Annu Rev Neurosci. 1988;11:423–453. doi: 10.1146/annurev.ne.11.030188.002231. [DOI] [PubMed] [Google Scholar]
- Williams RW, Rakic P. Three-dimensional counting: accurate and direct method to estimate numbers of cells in sectioned material. J Comp Neurol. 1988;278:344–352. doi: 10.1002/cne.902780305. [DOI] [PubMed] [Google Scholar]
- Williams RW, von Bartheld CS, Rosen GD. Counting cells in sectioned material: a suite of techniques, tools, and tips. Curr Protoc Neurosci. 2003;Chapter 1(Unit 1):11. doi: 10.1002/0471142301.ns0111s24. [DOI] [PubMed] [Google Scholar]
- Witelson SF, Kigar DL, Harvey T. The exceptional brain of Albert Einstein. Lancet. 1999;35:2149–2153. doi: 10.1016/S0140-6736(98)10327-6. [DOI] [PubMed] [Google Scholar]
- Wynn T, Coolidge FL. How To Think Like A Neandertal. Oxford; Oxford University Press; 2011. p. 224. [Google Scholar]
- Yuhas D, Jabr F. Know your neurons Chapter 4: What is the ratio of glia to neurons in the brain? [Accessed February 28, 2017];2012 http://blogs.scientificamerican.com/brainwaves/2012/06/13/know-your-neurons-what-is-the-ratio-of-glia-to-neurons-in-the-brain/
- Zhu Y, Liu F, Zou X, Torbey M. Comparison of unbiased estimation of neuronal number in the rat hippocampus with different staining methods. J Neurosci Methods. 2015;254:73–79. doi: 10.1016/j.jneumeth.2015.07.022. [DOI] [PubMed] [Google Scholar]
- Zorzetto R. How many neurons do we really have? (And why we may have 10× fewer glia than we previously thought. [Accessed February 28 2017];2012 https://neuroamer.com/2012/05/19/453/


