Abstract
The list of Mendelian disorders of the epigenetic machinery has expanded rapidly during the last five years. A few missense variants in the chromatin remodeler CHD1 have been found in several large scale sequencing efforts focused on uncovering the genetic etiology of autism. Here we describe CHD1 heterozygous missense variants in a cohort of patients with autism, speech apraxia, developmental delay and facial dysmorphic features. Importantly three of these variants occurred de novo. We also report on a patient with a de novo deletion covering a large fraction of the CHD1 gene without any obvious neurological phenotype. Our results suggest that variants in CHD1 can lead to diverse phenotypic outcomes; however, the neurodevelopmental phenotype appears to be limited to patients with missense variants, which is compatible with a dominant negative mechanism of disease.
Keywords: human disease, neurological dysfunction, epigenetic machinery, chromatin, speech apraxia
There has been rapid discovery of the genetic etiologies of intellectual disability with the advent of single nucleotide polymorphism microarray and clinical trio-based exome sequencing in recent years,[1]. Many of the newly discovered genetic variants are found in components of the epigenetic machinery,[2,3]. Patients with these disorders often display variable intellectual disability, growth dysregulation, and facial/limb dysmorphic features,[3]; the variability may be caused by individual variants but also by interaction with genetic or epigenetic variation at target genes,[3,4]. There are approximately 300 known epigenetic factors to date and currently about fifty (17%) have been associated with discernible phenotypes,[3,5–9]. The epigenetic machinery consists of readers, writers, and erasers of epigenetic modifications, as well as remodelers of chromatin, of which the latter three classes are enzymes. Enzymes are a class of proteins which is usually tolerant to the loss of a single allele, however, a deleterious pathogenic variant on a single allele of components of the epigenetic machinery appears to be sufficient to cause a clinical phenotype in a large majority of these diseases. This suggests that dosage may be critically important for enzymatic components of the epigenetic machinery,[3].
A number of well-known disease entities have been found to be associated with dysfunctional chromatin remodelers, including Coffin-Siris syndrome (MIM:135900),[10], CHARGE syndrome (MIM: 214800),[11] and ATR-X syndrome (MIM: 301040),[12]. Among the CHD (chromodomain, helicase, DNA binding) family of ATP-dependent chromatin remodelers, Mendelian disease phenotypes have so far been linked to four genes, CHD2, CHD4, CHD7, and CHD8, and common features are intellectual disability, autism, and abnormal head size (Supplemental Table 1). For three of these (CHD2, CHD7 and CHD8) the predominant variant type appears to be loss of function. In contrast, only missense variants have been found in CHD4. Sequencing of tumors has also revealed variants in the CHD gene family in cancer; variants in CHD5 have been found in neuroblastoma,[13] and variants in the other CHD genes (1–4 and 7–9) have been found in tumors of the gut (gastric and colorectal cancers),[14,15].
CHD1 is an ATP-dependent chromatin remodeler,[16] encoded on the long arm of chromosome 5,[17]. CHD1 has two chromodomains,[18,19] that bind to H3K4me3/H3K4me2,[19]. CHD1 regulates the opening of chromatin and contributes to the pluripotency of embryonic stem cells,[20]. It may play a role in transcript elongation,[21] and help to deposit the H3.3 histone variant,[22]. CHD1 is expressed in many tissues including in brain, where the highest level of expression is found in the cerebellum and basal ganglia (Supplemental Figure 1).
In our Epigenetics and Chromatin Clinic (https://igm.jhmi.edu/ecc-clinic), we saw two unrelated individuals with missense variants in CHD1 (Subjects 1 and 2). We submitted an entry into GeneMatcher,[23] and made contact with several clinical groups and the genetic testing company GeneDx. This resulted in six individuals described here with single nucleotide variants, all of which underwent whole exome sequencing at GeneDx which has performed more than 50,000 clinical whole exome sequencing tests to date (January 2017).
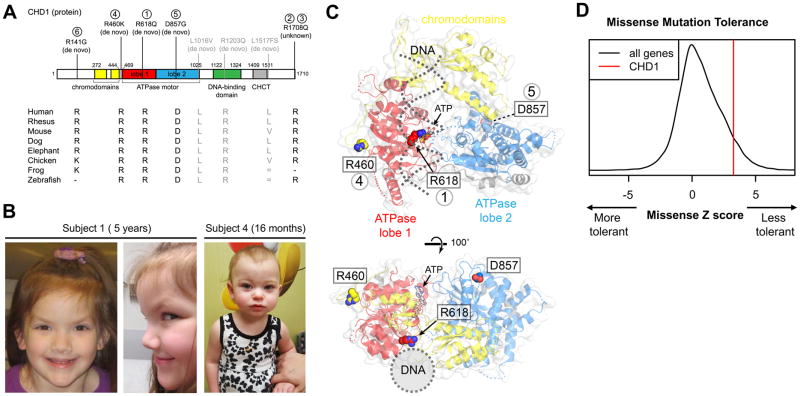
In addition to the three new individuals we identified with de novo heterozygous missense variants in CHD1 (Figure 1A Subjects 1, 4, 5), we also found that de novo missense (L1016V, R1203Q),[24–26] and nonsense (L1517fs*),[26–27] variants in CHD1 were previously described in three autistic patients (Figure 1A, gray) identified in large surveys of autistic patients,[24–27]. However, the available phenotypic information is limited, so it is unknown whether these patients show phenotypic overlap beyond autism.
Figure 1. Missense variants in the chromatin remodeler CHD1 result in a distinct neurological syndrome with dysmorphic features.
(A) We have identified five unrelated subjects with missense variants at highly conserved locations within the coding region of the CHD1 gene. Three of these variants are de novo. Three other de novo variants previously described in large cohorts of subjects with autism,[24–27] are noted in gray. Two of these variants occur at heavily conserved sites (1016, 1203) and the other (1517) leads to a frameshift at the C-terminal end of the protein. CHCT, which stands for CHD1 helical C-terminal, is an alpha-helical domain of unknown function,[43]. Domain boundaries are based on Chd1 structures,[43–45]. R = Arginine, K = Lysine, D = Aspartic Acid, G = Glycine, Q = Glutamine. (B) Representative images of some of the phenotypic facial features in two of the subjects. (C) Locations of the de novo missense sites on the chromodomain-ATPase motor, based on the yeast Chd1 structure,[44]. Two orthogonal views are shown, with the location of the DNA duplex binding highlighted with dotted gray lines. Note that the Arg618Gln variant occurs at the interface between DNA and ATP, and also is expected to pack against ATPase lobe 2 in the active state. (D) This gene does not tolerate missense variation particularly well as it has a relatively high missense Z score compared to all genes. The data here are based on data from the Exome Aggregation Consortium,[35].
Likely disease causing variants were also identified in three other individuals, though it could not be determined whether the change was de novo or not. These included two affected sisters (Subjects 2 and 3) conceived by separate in vitro fertilization using eggs from a single, presumably healthy, egg donor for which CHD1 sequencing is unavailable and a female (Subject 6) for whom parents had not been tested yet (Figure 1A). Phenotypic data of these individuals is described in Table 1, but biological samples are unavailable.
Table 1.
Clinical Findings in five subjects with missense variants in CHD1
| Subject 1 | Subject 2 | Subject 3 | Subject 4 | Subject 6 | |
|---|---|---|---|---|---|
| CHD1 variant | c.1853G>A; p.Arg618Gln | c.5123G>A; p.Arg1708Gln | c.5123G>A; p.Arg1708Gln | c.1379G>A; p.Arg460Lys | c.421A>G, p.Arg141Gly |
| PolyPhen-2 | PrD (1.000) | PrD (0.994) | PrD (0.994) | PoD (0.558) | Ben (0.08) |
| de novo | + | Unknown | Unknown | + | Unknown |
| Gender, age at last exam | female, 5 years | female, 6 years | female, 10 years | female, 7 months | female, 4 years |
| Height | 103.1 cm (10–25%ile) | 99.1 (<3rd%ile) | 128 cm (<3%ile) | 66.4 cm (63.1%ile) | 104 cm (75%ile) |
| Weight | 18.4 kg (50%ile) | 18.2 kg (25%ile) | 23.4 kg (<3%ile) | 7.5 kg (59.7%ile) | 17.7 kg (75%ile) |
| Developmental delay | + | + | regression | + | + |
| Speech apraxia | + | + | + | − | + |
| Diagnosis of Autism | + | + | + | − | − |
| Stereotypies | + | + | + | − | − |
| Hypotonia | + | +/dyspraxia | +/dyspraxia | + | + |
| Intellectual disability | − | + | + | n/a | n/a |
| Macrocephaly | + | + | − | − | − |
| Immune abnormalities | + | − | + | − | − |
| Depressed midface | + | + | + | − | − |
| Postnatal growth retardation | − | + (on GH) | + (on GH) | − | − |
| Translucent skin | + | + | + | − | − |
| Allergic shiners | + | + | + | − | − |
| Seizures | absence seizures | on seizure medication | abnormal EEG | + | − |
| Almond shaped eyes | + | +, long eyelashes | − | − | − |
| Flaring of eyebrows | + | + | − | − | − |
| Down-slanting palpebral fissures | + | + | + | − | − |
| Periorbital fullness | + | + | − | + | − |
| Fetal finger or toepads | + | − | + | − | − |
PrD = probably damaging, PoD = possibly damaging, Ben = benign; Reference transcript for CHD1 is NM_001270.2. Subject 1 had frequent episodes of fevers and inflammation, with fever ranging up to 104 with swelling of face, and bags under eyes. Immune testing showed incomplete vaccination to pneumococcus but responded to extra booster. Also, had elevated IgE and reduced NK cell function. Subject 2 and 3 both have immune problems which include IgA deficiency and hypogammaglobulinemia. Other features observed in Subject 1 include constipation, flexibility (family history of EDS) and high pain threshold. Although Subject 1 never had growth retardation she is small compared to other family members and had poor growth after being 8 pounds at birth. Subject 1 inherited another variant of unknown significance from her unaffected mother (CACNA1H, c.5608 G>A, p.A1870T; reference transcript: NM_021098.2). Subject 2 has been treated with a ketogenic diet and carries another variant of unknown significance from her unaffected father (DEPDC5, c.1355 C>T, p.A452V; reference transcript: NM_001242896.1). Subject 2 and 3 are siblings. Subject 4 was seen again at 16 months and had less delay at that time (5–10 words, waves bye) although was still receiving supportive services (occupational therapy). Her measurements at that point included a height of 77.5 cm (33rd %ile), a weight of 10 kg (56th %ile) and a head circumference of 44.5 cm (16th %ile). One of the parents of Subject 6 also has intellectual disability but has not been available for genetic testing.
Four of our subjects with CHD1 mutations carried a diagnosis of speech apraxia. Three of these patients also received a diagnosis of autism, although one (Subject 1) no longer carries this diagnosis. Speech apraxia is a relatively rare (1–2/1000 children) diagnosis in the general population,[28]. However, a recent study suggests that speech apraxia is seen in a large portion of children with autism,[29]. Furthermore, both apraxia and autistic phenotypes have in recent years been linked more heavily to the cerebellum,[30–32] and the CHD1 chromatin factor is highly expressed in the cerebellum (Supplemental Figure 1). All five patients had developmental delay and hypotonia, all are female and some had epileptiform abnormalities on an EEG (Table 1). Although autism is in general more common in males, the 6 subjects we observe with CHD1 mutations are all female. Attention should be given in future studies to the sex of patients harboring CHD1 mutations and if a female skew is observed in larger cohorts, consideration should be given to the mechanism of male intolerance of CHD1 mutations. A subset of patients had dysmorphic features including a pointed chin, frontal bossing and arched eyebrows (Figure 1B). Although we include information about the de novo variant found in subject 5 (D857G), this individual also carried compound heterozygous variants in the WDR62 gene which were thought to be disease causing. Variants in WDR62 are the cause of microcephaly 2, primary, autosomal recessive, with or without cortical malformation (MIM: 604317). Therefore no phenotypic information about this patient (Subject 5) is included, as her notable cortical malformation likely accounted for her global developmental delay. Additional phenotypic contributions of the de novo D857G variant in the CHD1 gene in this patient could not be discerned at 2 years of age.
It was curious that all patients we identified in this study have missense mutations in CHD1 and we hypothesize that there is a dominant negative mechanism of disease in the case of CHD1 mutations and their association with neurodevelopmental disability. Five of the six new variants described here involved a loss of an arginine and several are located in structurally important regions. This recurrent loss of arginine may offer a potential clue towards the mechanism of the pathogenicity as even when we take into account the arginine richness of this protein (7% of all amino acids), there is still a statistically significant enrichment of these missense changes involving arginine over what could be expected by chance (p = 9.3x10−5). One of these changes, Arg618Gln, substitutes a glutamine for a conserved arginine residue that is adjacent to the Walker B helicase motif which is central for the hydrolysis of ATP. Although the precise function of this arginine is not presently known, its positioning suggests it may help couple DNA binding to ATP hydrolysis (Figure 1C). All of these variants occurred at highly conserved amino acids (Figure 1A) and most were deemed pathogenic by both PolyPhen,[33] (Table 1) and SIFT (data not shown),[34]. No variants have been described at amino acid position 460 and 618 in the ExAC database,[35] of healthy individuals. However, there was a single description of a variant changing the arginine at amino acid position 141 to a serine (frequency of <1/10−5) but no variants have been described that change arginine at 141 to a glycine in CHD1. The change to glycine at 141 disrupts the conserved basic character at this residue, which may disrupt the protein structure or function. Similarly, there are 23 occurrences of a substitution from arginine to tryptophan at position 1708 (less than 0.03% of the ExAC database) but no observed changes of arginine to glutamine at this position in CHD1. Constraint data from the ExAC database also revealed that missense variants are generally poorly tolerated in this gene (z = 3.26, Figure 1D), as are loss of function variants (pLI =1). In three of the unrelated subjects, the variants were found to be de novo in the probands, further supporting the potential significance of these variants for the phenotype of our subjects. Additionally, CHD1 has been shown to bind to numerous key factors involved in transcriptional regulation, such as FACT, SPT4–5, RTF1, and components of large complexes such as Mediator and the spliceosome,[36–38]. It is possible that an inactivating mutation, such as many missense mutations, in the CHD1 protein could titrate out important factors or reduce the occupancy of active CHD1 at targeted sites, which could offer an explanation for a dominant negative mechanism of disease.
Moreover, data from DECIPHER, as well as the high predicted intolerance of CHD1 to loss of function variants (pLI = 1 in ExAC), lend further evidence to the contribution of CHD1 to disease. For instance, seven deletions overlapping the CHD1 gene are available from DECIPHER,[39]; six of these ranged from 5.64 to 12.10 Mb and involved a large number of genes. The smallest of these copy number variants was a 2.95 Mb deletion with a loss of six genes in a patient with hypotonia, constipation, and language delay (Supplemental Table 2). However, in addition to CHD1, there were other candidate genes within this deletion that could potentially explain the observed phenotype (Supplemental Table 2). Interestingly, there is also a recent description of an individual with isolated talipes equinovarus and a de novo deletion of the entire sequence of RGMB and the final nine exons of CHD1 (Hg18, Chr5:97916544–98250268),[40]. However, we now confirm that this previously described individual, a 9 year old male, has no obvious neurodevelopmental phenotype at this time and that he is generally healthy other than the club foot and asthma. This evidence suggests that deletions of CHD1 may not cause a consistent neurological phenotype, but missense changes in CHD1 may, through a dominant negative mechanism. Alternatively, changes in CHD1 (both deletions and missense changes) could lead to a predisposition towards disease similar to what has been described for CHD8, but may not be fully penetrant.
In addition, Chd1 has been found to be essential for the high transcriptional output needed for rapid growth of the mouse epiblast,[41], and it has also been found to play a role in later murine development,[42]. Despite this, mice with loss of a single CHD1 allele (Chd1+/−) are healthy, fertile and phenotypically normal,[41]. Collectively, these observations highlight that further research is needed to elucidate the consequences of loss of function mutations in CHD1. However, we think that together these data are compatible with the hypothesis that the neurodevelopmental phenotype is associated with a dominant negative disease mechanism of missense mutations inCHD1.
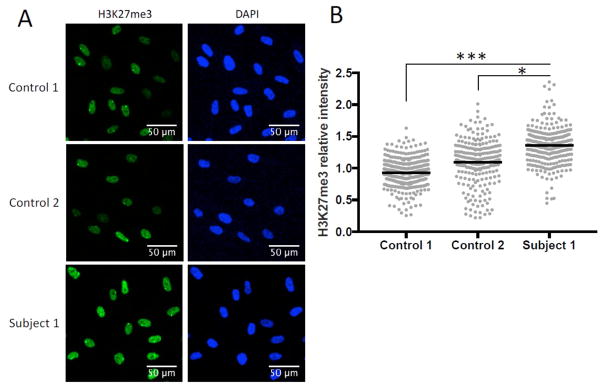
Since prior studies demonstrate that dysregulation of CHD1 leads to global changes in chromatin,[20], we explored the functional consequences of one of these variants in fibroblasts from Subject 1. In these fibroblasts, which carry a heterozygous de novo variant (Arg618Gln), we observed a global increase of a closed chromatin modification (H3K27me3) compared to fibroblasts from control individuals (Figure 2). These data support the notion that missense changes such as Arg618Gln have functional effects on CHD1 function.
Figure 2. Patient fibroblasts demonstrate increased amounts of a closed chromatin modification (H3K27me3) compared to fibroblasts from control subjects.
Since CHD1 is thought to play an active role in the process of opening chromatin we examined the amount of H3K27me3 in fibroblasts from one of our subjects (Subject 1) and fibroblasts from two control individuals. Briefly, samples were cultured in triplicate and stained with antibodies against H3K27me3 (green) and DAPI (blue). Intensity level of H3K27me3 in each nucleus was quantified using ImageJ. (A) Representative images of immunofluorescence staining from Subject 1 and two controls. (B) Quantification of H3K27me3 intensity, normalized to the average control intensity. Gray points represent H3K27me3 intensity in each nucleus and black bars represent the mean intensity of all nuclei measured. Controls were age and sex matched (Karyotypes: 46,XX and 46,XX,del(22)(q11.2q11.2)). * P<0.05, ***P<0.0005, one-way ANOVA with post hoc Tukey’s HSD analysis.
In summary, our data show that missense variants in the chromatin remodeler CHD1 are associated with a novel neurodevelopmental disorder with intellectual disability, autism, seizures, speech apraxia, and dysmorphic features.
Supplementary Material
Acknowledgments
We are grateful to the patients and their families for traveling to the Epigenetics and Chromatin Clinic and to agree to participate in this publication. There was no specified funding for this work but H.T.B. is funded through an Early Independence Award from the National institute of health (DP5OD017877). Confocal images (Figure 2) were taken at the Johns Hopkins Microscope Facility on LSM780 FCS which was supported by a NIH grant (S10OD016374). We would like to thank Elizabeth Wohler for her help with growing up control cell lines. We would like to acknowledge the IDDRC Tissue Culture Repository for providing age and sex matched control fibroblasts; this center is supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health (U54HD079123). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We would like to thank Kasper Hansen for statistical advice for Figure 2. The authors disclose the following: M.T.C., L.B.H., I.M.W., B.B., and M.J.G.S. are employees of GeneDx, which derives revenue from molecular testing of clinical samples.
Footnotes
Author Contributions:
HTB and HJV conceived study; GOP, HTB and GDB wrote the paper; GOP performed cell culture experiments; LB performed computational analysis; GDB performed structural analysis; HJV, CDA, MTC, CAG, PJB, EB, JMH, LM, IDK, MA, DN, LBH, IMW, BB, MJGS, and HTB provided clinical information regarding patients.
Supplemental data include two tables and two figures which can be found with this article online.
Web Resources
DECIPHER, http://decipher.sanger.ac.uk/
1000 Genomes, http://www.1000genomes.org/
ExAC Browser, http://exac.broadinstitute.org
GenBank, http://www.ncbi.nlm.nih.gov/genbank/
GeneMatcher, https://genematcher.org/
OMIM, http://www.omim.org/
UCSC Genome Browser, http://genome.ucsc.edu/
GTEx Portal, http://www.gtexportal.org/home/
PolyPhen-2, http://genetics.bwh.harvard.edu/pph2/
References
- 1.Vissers LE, Gilissen C, Veltman JA. Genetic studies in intellectual disability and related disorders. Nat Rev Genet. 2016;17(1):9–18. doi: 10.1038/nrg3999. [DOI] [PubMed] [Google Scholar]
- 2.Fahrner JA, Bjornsson HT. Mendelian disorders of the epigenetic machinery: tipping the balance of chromatin states. Annu Rev Genomics Hum Genet. 2014;15:269–93. doi: 10.1146/annurev-genom-090613-094245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjornsson HT. The Mendelian disorders of the epigenetic machinery. Genome Res. 2015;25(10):1473–81. doi: 10.1101/gr.190629.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Law MJ, Lower KM, Voon HP, Hughes JR, Garrick D, Viprakasit V, Mitson M, De Gobbi M, Marra M, Morris A, Abbott A, Wilder SP, Taylor S, Santos GM, Cross J, Ayyub H, Jones S, Ragoussis J, Rhodes D, Dunham I, Higgs DR, Gibbons RJ. ATR-X syndrome protein targets tandem repeats and influences allele-specific expression in a size-dependent manner. Cell. 2010;143(3):367–78. doi: 10.1016/j.cell.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 5.Weiss K, Terhal PA, Cohen L, Bruccoleri M, Irving M, Martinez AF, Rosenfeld JA, Machol K, Yang Y, Liu P, Walkiewicz M, Beuten J, Gomez-Ospina N, Haude K, Fong CT, Enns GM, Bernstein JA, Fan J, Gotway G, Ghorbani M, Study DDD, van Gassen K, Monroe GR, van Haaften G, Basel-Vanagaite L, Yang XJ, Campeau PM, Muenke M. De Novo Mutations in CHD4, an ATP-Dependent Chromatin Remodeler Gene, Cause an Intellectual Disability Syndrome with Distinctive Dysmorphisms. Am J Hum Genet. 2016;99(4):934–41. doi: 10.1016/j.ajhg.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan K, Rousseau J, Littlejohn RO, Kiss C, Lehman A, Rosenfeld JA, Stumpel CT, Stegmann AP, Robak L, Scaglia F, Nguyen TT, Fu H, Ajeawung NF, Camurri MV, Li L, Gardham A, Panis B, Almannai M, Sacoto MJ, Baskin B, Ruivenkamp C, Xia F, Bi W, Study DDD, Study C, Cho MT, Potjer TP, Santen GW, Parker MJ, Canham N, McKinnon M, Potocki L, MacKenzie JJ, Roeder ER, Campeau PM, Yang XJ. Mutations in the Chromatin Regulator Gene BRPF1 Cause Syndromic Intellectual Disability and Deficient Histone Acetylation. Am J Hum Genet. 2017;100(1):91–104. doi: 10.1016/j.ajhg.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arboleda VA, Lee H, Dorrani N, Zadeh N, Willis M, Macmurdo CF, Manning MA, Kwan A, Hudgins L, Barthelemy F, Miceli MC, Quintero-Rivera F, Kantarci S, Strom SP, Deignan JL, Center UCG, Grody WW, Vilain E, Nelson SF. De novo nonsense mutations in KAT6A, a lysine acetyl-transferase gene, cause a syndrome including microcephaly and global developmental delay. Am J Hum Genet. 2015;96(3):498–506. doi: 10.1016/j.ajhg.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tham E, Lindstrand A, Santani A, Malmgren H, Nesbitt A, Dubbs HA, Zackai EH, Parker MJ, Millan F, Rosenbaum K, Wilson GN, Nordgren A. Dominant mutations in KAT6A cause intellectual disability with recognizable syndromic features. Am J Hum Genet. 2015;96(3):507–13. doi: 10.1016/j.ajhg.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shang L, Cho MT, Retterer K, Folk L, Humberson J, Rohena L, Sidhu A, Saliganan S, Iglesias A, Vitazka P, Juusola J, O’Donnell-Luria AH, Shen Y, Chung WK. Mutations in ARID2 are associated with intellectual disabilities. Neurogenetics. 2015;16(4):307–14. doi: 10.1007/s10048-015-0454-0. [DOI] [PubMed] [Google Scholar]
- 10.Hoyer J, Ekici AB, Endele S, Popp B, Zweier C, Wiesener A, Wohlleber E, Dufke A, Rossier E, Petsch C, Zweier M, Gohring I, Zink AM, Rappold G, Schrock E, Wieczorek D, Riess O, Engels H, Rauch A, Reis A. Haploinsufficiency of ARID1B, a member of the SWI/SNF-a chromatin-remodeling complex, is a frequent cause of intellectual disability. Am J Hum Genet. 2012;90(3):565–72. doi: 10.1016/j.ajhg.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vissers LE, van Ravenswaaij CM, Admiraal R, Hurst JA, de Vries BB, Janssen IM, van der Vliet WA, Huys EH, de Jong PJ, Hamel BC, Schoenmakers EF, Brunner HG, Veltman JA, van Kessel AG. Mutations in a new member of the chromodomain gene family cause CHARGE syndrome. Nat Genet. 2004;36(9):955–7. doi: 10.1038/ng1407. [DOI] [PubMed] [Google Scholar]
- 12.Gibbons RJ, Picketts DJ, Villard L, Higgs DR. Mutations in a putative global transcriptional regulator cause X-linked mental retardation with alpha-thalassemia (ATR-X syndrome) Cell. 1995;80(6):837–45. doi: 10.1016/0092-8674(95)90287-2. [DOI] [PubMed] [Google Scholar]
- 13.White PS, Thompson PM, Gotoh T, Okawa ER, Igarashi J, Kok M, Winter C, Gregory SG, Hogarty MD, Maris JM, Brodeur GM. Definition and characterization of a region of 1p36.3 consistently deleted in neuroblastoma. Oncogene. 2005;24(16):2684–94. doi: 10.1038/sj.onc.1208306. [DOI] [PubMed] [Google Scholar]
- 14.Kim MS, Chung NG, Kang MR, Yoo NJ, Lee SH. Genetic and expressional alterations of CHD genes in gastric and colorectal cancers. Histopathology. 2011;58(5):660–8. doi: 10.1111/j.1365-2559.2011.03819.x. [DOI] [PubMed] [Google Scholar]
- 15.Liu W, Lindberg J, Sui G, Luo J, Egevad L, Li T, Xie C, Wan M, Kim ST, Wang Z, Turner AR, Zhang Z, Feng J, Yan Y, Sun J, Bova GS, Ewing CM, Yan G, Gielzak M, Cramer SD, Vessella RL, Zheng SL, Gronberg H, Isaacs WB, Xu J. Identification of novel CHD1-associated collaborative alterations of genomic structure and functional assessment of CHD1 in prostate cancer. Oncogene. 2012;31(35):3939–48. doi: 10.1038/onc.2011.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lusser A, Urwin DL, Kadonaga JT. Distinct activities of CHD1 and ACF in ATP-dependent chromatin assembly. Nat Struct Mol Biol. 2005;12(2):160–6. doi: 10.1038/nsmb884. [DOI] [PubMed] [Google Scholar]
- 17.Woodage T, Basrai MA, Baxevanis AD, Hieter P, Collins FS. Characterization of the CHD family of proteins. Proc Natl Acad Sci U S A. 1997;94(21):11472–7. doi: 10.1073/pnas.94.21.11472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delmas V, Stokes DG, Perry RP. A mammalian DNA-binding protein that contains a chromodomain and an SNF2/SWI2-like helicase domain. Proc Natl Acad Sci U S A. 1993;90(6):2414–8. doi: 10.1073/pnas.90.6.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flanagan JF, Mi LZ, Chruszcz M, Cymborowski M, Clines KL, Kim Y, Minor W, Rastinejad F, Khorasanizadeh S. Double chromodomains cooperate to recognize the methylated histone H3 tail. Nature. 2005;438(7071):1181–5. doi: 10.1038/nature04290. [DOI] [PubMed] [Google Scholar]
- 20.Gaspar-Maia A, Alajem A, Polesso F, Sridharan R, Mason MJ, Heidersbach A, Ramalho-Santos J, McManus MT, Plath K, Meshorer E, Ramalho-Santos M. Chd1 regulates open chromatin and pluripotency of embryonic stem cells. Nature. 2009;460(7257):863–8. doi: 10.1038/nature08212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sims RJ, 3rd, Belotserkovskaya R, Reinberg D. Elongation by RNA polymerase II: the short and long of it. Genes Dev. 2004;18(20):2437–68. doi: 10.1101/gad.1235904. [DOI] [PubMed] [Google Scholar]
- 22.Konev AY, Tribus M, Park SY, Podhraski V, Lim CY, Emelyanov AV, Vershilova E, Pirrotta V, Kadonaga JT, Lusser A, Fyodorov DV. CHD1 motor protein is required for deposition of histone variant H3.3 into chromatin in vivo. Science. 2007;317(5841):1087–90. doi: 10.1126/science.1145339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sobreira N, Schiettecatte F, Valle D, Hamosh A. GeneMatcher: a matching tool for connecting investigators with an interest in the same gene. Hum Mutat. 2015;36(10):928–30. doi: 10.1002/humu.22844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Rubeis S, He X, Goldberg AP, Poultney CS, Samocha K, Cicek AE, Kou Y, Liu L, Fromer M, Walker S, Singh T, Klei L, Kosmicki J, Shih-Chen F, Aleksic B, Biscaldi M, Bolton PF, Brownfeld JM, Cai J, Campbell NG, Carracedo A, Chahrour MH, Chiocchetti AG, Coon H, Crawford EL, Curran SR, Dawson G, Duketis E, Fernandez BA, Gallagher L, Geller E, Guter SJ, Hill RS, Ionita-Laza J, Jimenz Gonzalez P, Kilpinen H, Klauck SM, Kolevzon A, Lee I, Lei I, Lei J, Lehtimaki T, Lin CF, Ma’ayan A, Marshall CR, McInnes AL, Neale B, Owen MJ, Ozaki N, Parellada M, Parr JR, Purcell S, Puura K, Rajagopalan D, Rehnstrom K, Reichenberg A, Sabo A, Sachse M, Sanders SJ, Schafer C, Schulte-Ruther M, Skuse D, Stevens C, Szatmari P, Tammimies K, Valladares O, Voran A, Li-San W, Weiss LA, Willsey AJ, Yu TW, Yuen RK, Study DDD, Cook EH, Freitag CM, Gill M, Hultman CM, Lehner T, Palotie A, Schellenberg GD, Sklar P, State MW, Sutcliffe JS, Walsh CA, Scherer SW, Zwick ME, Barett JC, Cutler DJ, Roeder K, Devlin B, Daly MJ, Buxbaum JD Homozygosity Mapping Collaborative for A, Consortium UK. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature. 2014;515(7526):209–15. doi: 10.1038/nature13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neale BM, Kou Y, Liu L, Ma’ayan A, Samocha KE, Sabo A, Lin CF, Stevens C, Wang LS, Makarov V, Polak P, Yoon S, Maguire J, Crawford EL, Campbell NG, Geller ET, Valladares O, Schafer C, Liu H, Zhao T, Cai G, Lihm J, Dannenfelser R, Jabado O, Peralta Z, Nagaswamy U, Muzny D, Reid JG, Newsham I, Wu Y, Lewis L, Han Y, Voight BF, Lim E, Rossin E, Kirby A, Flannick J, Fromer M, Shakir K, Fennell T, Garimella K, Banks E, Poplin R, Gabriel S, DePristo M, Wimbish JR, Boone BE, Levy SE, Betancur C, Sunyaev S, Boerwinkle E, Buxbaum JD, Cook EH, Jr, Devlin B, Gibbs RA, Roeder K, Schellenberg GD, Sutcliffe JS, Daly MJ. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature. 2012;485(7397):242–5. doi: 10.1038/nature11011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Roak BJ, Stessman HA, Boyle EA, Witherspoon KT, Martin B, Lee C, Vives L, Baker C, Hiatt JB, Nickerson DA, Bernier R, Shendure J, Eichler EE. Recurrent de novo mutations implicate novel genes underlying simplex autism risk. Nat Commun. 2014;5:5595. doi: 10.1038/ncomms6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iossifov I, O’Roak BJ, Sanders SJ, Ronemus M, Krumm N, Levy D, Stessman HA, Witherspoon KT, Vives L, Patterson KE, Smith JD, Paeper B, Nickerson DA, Dea J, Dong S, Gonzalez LE, Mandell JD, Mane SM, Murtha MT, Sullivan CA, Walker MF, Waqar Z, Wei L, Willsey AJ, Yamrom B, Lee YH, Grabowska E, Dalkic E, Wang Z, Marks S, Andrews P, Leotta A, Kendall J, Hakker I, Rosenbaum J, Ma B, Rodgers L, Troge J, Narzisi G, Yoon S, Schatz MC, Ye K, McCombie WR, Shendure J, Eichler EE, State MW, Wigler M. The contribution of de novo coding mutations to autism spectrum disorder. Nature. 2014;515(7526):216–21. doi: 10.1038/nature13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shriberg LD, Aram DM, Kwiatkowski J. Developmental apraxia of speech: I. Descriptive and theoretical perspectives. J Speech Lang Hear Res. 1997;40(2):273–85. doi: 10.1044/jslhr.4002.273. [DOI] [PubMed] [Google Scholar]
- 29.Tierney C, Mayes S, Lohs SR, Black A, Gisin E, Veglia M. How Valid Is the Checklist for Autism Spectrum Disorder When a Child Has Apraxia of Speech? J Dev Behav Pediatr. 2015;36(8):569–74. doi: 10.1097/DBP.0000000000000189. [DOI] [PubMed] [Google Scholar]
- 30.Hampson DR, Blatt GJ. Autism spectrum disorders and neuropathology of the cerebellum. Front Neurosci. 2015;9:420. doi: 10.3389/fnins.2015.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marien P, van Dun K, Verhoeven J. Cerebellum and apraxia. Cerebellum. 2015;14(1):39–42. doi: 10.1007/s12311-014-0620-1. [DOI] [PubMed] [Google Scholar]
- 32.Fatemi SH, Aldinger KA, Ashwood P, Bauman ML, Blaha CD, Blatt GJ, Chauhan A, Chauhan V, Dager SR, Dickson PE, Estes AM, Goldowitz D, Heck DH, Kemper TL, King BH, Martin LA, Millen KJ, Mittleman G, Mosconi MW, Persico AM, Sweeney JA, Webb SJ, Welsh JP. Consensus paper: pathological role of the cerebellum in autism. Cerebellum. 2012;11(3):777–807. doi: 10.1007/s12311-012-0355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7(4):248–9. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc. 2009;4(7):1073–81. doi: 10.1038/nprot.2009.86. [DOI] [PubMed] [Google Scholar]
- 35.Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, O’Donnell-Luria AH, Ware JS, Hill AJ, Cummings BB, Tukiainen T, Birnbaum DP, Kosmicki JA, Duncan LE, Estrada K, Zhao F, Zou J, Pierce-Hoffman E, Berghout J, Cooper DN, Deflaux N, DePristo M, Do R, Flannick J, Fromer M, Gauthier L, Goldstein J, Gupta N, Howrigan D, Kiezun A, Kurki MI, Moonshine AL, Natarajan P, Orozco L, Peloso GM, Poplin R, Rivas MA, Ruano-Rubio V, Rose SA, Ruderfer DM, Shakir K, Stenson PD, Stevens C, Thomas BP, Tiao G, Tusie-Luna MT, Weisburd B, Won HH, Yu D, Altshuler DM, Ardissino D, Boehnke M, Danesh J, Donnelly S, Elosua R, Florez JC, Gabriel SB, Getz G, Glatt SJ, Hultman CM, Kathiresan S, Laakso M, McCarroll S, McCarthy MI, McGovern D, McPherson R, Neale BM, Palotie A, Purcell SM, Saleheen D, Scharf JM, Sklar P, Sullivan PF, Tuomilehto J, Tsuang MT, Watkins HC, Wilson JG, Daly MJ, MacArthur DG Exome Aggregation C. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536(7616):285–91. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simic R, Lindstrom DL, Tran HG, Roinick KL, Costa PJ, Johnson AD, Hartzog GA, Arndt KM. Chromatin remodeling protein Chd1 interacts with transcription elongation factors and localizes to transcribed genes. EMBO J. 2003;22(8):1846–1856. doi: 10.1093/emboj/cdg179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin JJ, Lehmann LW, Bonora G, Sridharan R, Vashisht AA, Tran N, Plath K, Wohlschlegel JA, Carey M. Mediator coordinates PIC assembly with recruitment of CHD1. Genes Dev. 2011;25(20):2198–209. doi: 10.1101/gad.17554711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sims RJ, Millhouse S, Chen CF, Lewis BA, Erdjument-Bromage H, Tempst P, Manley JL, Reinberg D. Recognition of trimethylated histone H3 lysine 4 facilitates the recruitment of transcription postinitiation factors and pre-mRNA splicing. Mol Cell. 2007;28(4):665–76. doi: 10.1016/j.molcel.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bragin E, Chatzimichali EA, Wright CF, Hurles ME, Firth HV, Bevan AP, Swaminathan GJ. DECIPHER: database for the interpretation of phenotype-linked plausibly pathogenic sequence and copy-number variation. Nucleic Acids Res. 2014;42(Database issue):D993–D1000. doi: 10.1093/nar/gkt937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alvarado DM, Buchan JG, Frick SL, Herzenberg JE, Dobbs MB, Gurnett CA. Copy number analysis of 413 isolated talipes equinovarus patients suggests role for transcriptional regulators of early limb development. Eur J Hum Genet. 2013;21(4):373–80. doi: 10.1038/ejhg.2012.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guzman-Ayala M, Sachs M, Koh FM, Onodera C, Bulut-Karslioglu A, Lin CJ, Wong P, Nitta R, Song JS, Ramalho-Santos M. Chd1 is essential for the high transcriptional output and rapid growth of the mouse epiblast. Development. 2015;142(1):118–27. doi: 10.1242/dev.114843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suzuki S, Nozawa Y, Tsukamoto S, Kaneko T, Manabe I, Imai H, Minami N. CHD1 acts via the Hmgpi pathway to regulate mouse early embryogenesis. Development. 2015;142(13):2375–84. doi: 10.1242/dev.120493. [DOI] [PubMed] [Google Scholar]
- 43.Mohanty B, Helder S, Silva AP, Mackay JP, Ryan DP. The Chromatin Remodelling Protein CHD1 Contains a Previously Unrecognised C-Terminal Helical Domain. J Mol Biol. 2016;428(21):4298–314. doi: 10.1016/j.jmb.2016.08.028. [DOI] [PubMed] [Google Scholar]
- 44.Hauk G, McKnight JN, Nodelman IM, Bowman GD. The chromodomains of the Chd1 chromatin remodeler regulate DNA access to the ATPase motor. Mol Cell. 2010;39(5):711–23. doi: 10.1016/j.molcel.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ryan DP, Sundaramoorthy R, Martin D, Singh V, Owen-Hughes T. The DNA-binding domain of the Chd1 chromatin-remodelling enzyme contains SANT and SLIDE domains. EMBO J. 2011;30(13):2596–609. doi: 10.1038/emboj.2011.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.