Abstract
Age‐specific incidence estimates are important and useful facts in psychiatric epidemiology, but incidence estimation can be challenging. Methods artifacts are possible. In the United States, where the minimum legal drinking age is 21 years, recent cross‐sectional field research on 12‐ to 25‐year‐olds applied conventional “age‐at‐assessment” approaches (AAA) for incidence estimation based on 12‐month recall. Estimates disclosed unexpected nonlinear patterns in age‐specific incidence estimates for both drinking onset and for transitioning from first drink to heavy drinking. Here, our aim is to draw attention to an “age of onset” (AOO) alternative to AAA approaches and to verify whether the AOO approach also discloses nonlinearity. Yearly data are from U.S. nationally representative samples drawn and assessed for National Surveys on Drug Use and Health, 2002–2014, with standardized audio computer‐assisted self‐interview assessments for drinking outcomes. Both AAA and AOO approaches show nonlinearities, with an unexpected dip in drinking incidence rates after age 18 and before the age 21 minimum legal drinking age. The AOO and the AAA approaches disclosed similar age‐specific patterns. We discuss advantages of the AOO approach when nonlinear incidence patterns can be anticipated, but we conclude that the AAA approach has not created an artifactual nonlinear pattern.
Keywords: alcohol drinking, heavy drinking, incidence, psychiatric epidemiology
1. INTRODUCTION
Estimation of age‐specific incidence is a fundamental task in psychiatric epidemiology, because it speaks directly to the risk of becoming a new case (Kramer, 1957; Lapouse, 1967). In addition, for anyone planning a new prospective or longitudinal study, accurate age‐specific incidence estimates are needed—for example, to ensure that the planned sample size will yield statistically precise and powerful numbers of newly incident cases during follow‐up intervals.
In psychiatric epidemiology field studies, calculation for age‐specific disease incidence rates generally draws upon William Farr's vital statistics tradition (Farr, 1885), with an adaptation to the onset age of a psychiatric disorder of interest. To illustrate, in 1989, Eaton and colleagues published age‐specific incidence estimates for specific subtypes of mental disorders based on the two‐wave Epidemiologic Catchment Area (ECA) studies (Eaton et al., 1989). The denominators for these incidence estimates were created by stratifying the ECA samples by age at baseline assessment (“Wave 1”), followed by removal of all persons with a baseline Diagnostic Interview Schedule‐identified lifetime history of the disorder. The numerator for each age stratum consisted of the number of individuals who became newly incident cases between the baseline assessment and the follow‐up assessment, roughly 1 year apart. It is important to note that the assigned age for incidence estimation was the baseline “age at assessment” (AAA). It was not the age of onset (AOO) of the disorder, as might be measured by taking into account the month of the next birthday occurring after baseline but before follow‐up. Akin to an age‐specific “attack rate” in communicable disease epidemiology, the resulting incidence estimate is an age‐specific proportion with counts in the numerator and in the denominator. That is, the denominator is not a person‐years denominator as one might construct when estimating incidence rates for becoming a case via the “incidence density” formulation.
A somewhat different tradition for psychiatric epidemiology field studies is more closely aligned with Farr's vital statistics AOO approach, as exemplified in the discrete‐time survival analysis described by Willett and Singer (Willett & Singer, 1993), and often applied when incidence estimates are derived from cross‐sectional field survey data based on information about the AOO. Using this approach, the “origin” of the time axis is set either as the date of birth or as some other meaningful event (Degenhardt et al., 2008; Hasin, Stinson, Ogburn, & Grant, 2007). Individuals remain candidates for becoming a newly incident case, and retain a zero (0) value of a binary case variable across each unit of post‐birth study time, until the AOO of the disorder, at which time the zero value shifts to a value of one (1) corresponding with a specific AOO value. This approach readily lends itself to estimation of the incidence density formulation (with units of person‐time in the denominator).
Challenges faced when using the prospective AAA approach include disorder‐related attrition, as well as response reactivity occurring when inconsistent and possibly untruthful answers are elicited when the same assessment is administered more than one time (Morrison et al., 1997; Thygesen, Johansen, Keiding, Giovannucci, & Grønbæk, 2008). For example, in the ECA study, some respondents provided positive answers at Wave 1 but negative answers at Wave 2 for their lifetime histories (Eaton et al., 1989). These types of memory errors might become more substantial in prospective studies when the follow‐up interval extends beyond 2 years (Engels, Knibbe, & Drop, 1997).
Memory and reactivity problems also can work together in prospective studies. To illustrate, when asked about lifetime history in the ECA study, some respondents provided negative answers at Wave 1 but positive answers at Wave 2 with an onset age dated prior to Wave 1 (Eaton et al., 1989).
Challenges to the cross‐sectional AOO approach based on recalled information include prominent memory errors (e.g., “forward telescoping”), without sample attrition and reactivity issues faced in the prospective research approach. With respect to telescoping, individuals with a longer elapsed time since the onset may recall a more recent onset age than the actual onset age, as compared to individuals with a shorter elapsed time since onset (Engels et al., 1997; Kuntsche, Rossow, Engels, & Kuntsche, 2016; Shillington, Woodruff, Clapp, Reed, & Lemus, 2012). This sort of “telescoping” issue becomes especially salient when the task is to estimate age‐specific incidence rates for event onsets. For example, in drinking incidence estimation, age of drinking onset has only 13 possible values (0,1,…,12) when a 12‐year‐old drinker is answering an age of onset question. The corresponding 22‐year‐old drinker has 23 possible options (0,1,…,22).
There is a variant of the AAA approach for cross‐sectional data to estimate incidence rates based upon information about recent and newly incident experiences. For this variant, the estimated incidence for alcohol drinking is conceptualized as the number of new drinkers who had their first full drink during the prior 12 months, arising from an “at‐risk” population composed of never drinkers as of the assessment date plus newly incident drinkers. Applying this AAA approach to national survey data for the United States (U.S.), we have found an interesting nonlinear pattern in age‐specific incidence for alcohol drinking, characterized by an increment until age 16 years, something of a plateau between 16 and 18, after which the estimated incidence pattern shows a dip (i.e., smaller incidence estimate) for age 19–20 years, which then is followed by a sharp increase at age 21 years, and a decline afterwards (Cheng, Cantave, & Anthony, 2016a, 2016b). This finding is consistent with our a priori hypothesis that the age‐specific incidence of drinking would show a monotonic increase age by age until 21 years (the legal minimum drinking age in the U.S.), at which time there could be the equivalent of a step function jump in the incidence estimate, reflective of the large number of adolescents in the U.S. who delay their first drink until the legal drinking age.
Intrigued by this nonlinear pattern, and trying to “explain” the observed phenomenon, we decided to re‐approach the estimation task using the AOO approach. We do so in this study seeking to discover whether the AAA approach induced an artifactual dip in what otherwise might be expected as a monotonically increasing pattern of age‐specific incidence estimates. To make the circumstances more concrete, when the AAA approach is used, the precise AOO value is ignored, and the age at assessment is substituted for that value. Here, the AAA approach counts its incidence numerator based on newly incident drinkers found among individuals assessed at age 21 years, irrespective of whether drinking onset age is 20 or 21 years. In contrast, the AOO approach counts its incidence numerator based on newly incident drinkers who started drinking at age 21 years, irrespective of whether assessment age is 21 or 22 years. The AOO approach takes into account that some individuals assessed at age 21 years actually had started drinking at age 20 years; some of those assessed at age 22 years actually had started drinking at age 21 years. The AAA approach, published in Cheng and Anthony (2016), does not take into account this AOO variation.
For a domain of research that is focused on a specific age value or “threshold age” (e.g., setting or evaluating a minimum legal age for a societal privilege such as drinking), the estimates based on an AOO approach should speak more directly to the research questions under study, as compared with estimates based on the AAA approach. To provide a second illustration, we also compare the incidence estimates with a focus on rapid transitioning from first full drink to the first heavy drinking episode (HDE), in a deliberate comparison of the AAA and AOO approaches.
Methodological choices between AAA and AOO approaches may have important implications for research on behavioral and mental conditions when an abrupt shift in a suspected causal influence occurs at a specific age. Examples include a minimum age set for lawful use of a psychoactive drug (e.g., alcohol, tobacco, or cannabis), as well as exposure to developmentally sensitive traumatic events such as a terrorism event (Eaton et al., 1989; Fontalba‐Navas et al., 2017). The previously described peak incidence rate for drinking onset at age 21 years serves as an example of these types of nonlinear patterns in incidence estimates (Cheng, Cantave, & Anthony, 2016a, 2016b).
2. MATERIALS AND METHODS
2.1. Study population and sample
The study population was specified to include noninstitutionalized civilian residents of the U.S., 12 years of age and older, as sampled and assessed for the National Surveys on Drug Use and Health (NSDUH). The NSDUH cross‐sectional surveys were conducted from 2002 to 2014 with multistage area probability sampling to draw nationally representative samples and with an oversampling of 12‐ to 17‐year‐olds. Assessments were conducted after child assent and parental consent obtained via an IRB‐approved protocol (overall n = ~55,000 each year with response levels varying from 72% to 76%; United States Substance Abuse and Mental Health Services Administration, 2012).
The final analytic sample for drinking incidence across the years 2002–2014 includes 208,766 12–25 year olds (because few start drinking after age 25 years), who were “at risk” for drinking onset during the 12 months prior to the assessment. The date of first episode of heavy drinking was first assessed in 2006. Therefore, the final sample for studying the transition to HDE includes the 24,340 12‐to‐25‐year‐olds newly incident drinkers who had their first full drink during the 12 months prior to the interview, sampled from 2006 to 2014. (Table S1 provides a more detailed overview of sample size for this study.)
2.2. Assessment
Confidential audio computer‐assisted self‐interviews were used to collect information about the month and year of the first full drink, the age of first full drink, and the month and year of first episode of heavy drinking defined as the consumption of at least five drinks in one occasion via a standard NSDUH multi‐item alcohol module (United States Substance Abuse and Mental Health Services Administration, 2012). Age was based on self‐reported date of birth. In the NSDUH publicly downloadable dataset, age was binned into age pairs for 22–25 year olds. As in previous published estimates, newly incident drinkers are individuals who consumed their first full drink within the 12 months prior to the assessment (Cheng & Anthony, 2016; Cheng, Chandra, Alcover, & Anthony, 2016).
2.3. Analysis
In this study,
That is, in both the AAA approach and the AOO approach, newly incident drinkers are individuals who consumed their first full drink during the 12 months prior to the assessment. When estimating age‐specific drinking incidence, the difference between the AAA and AOO approaches is that the AAA new drinkers include all qualified new drinkers assessed at a certain age with no attention paid to age at first drink, whereas the AOO new drinkers are individuals who consumed the first drink at a specific age. For example, to estimate drinking incidence for those who are 19 years old when assessed, the AAA configuration of new drinkers includes those who had their first full drink during the past 12 months and were 19 at assessment even though they might have been either 18 or 19 years old when they had their first full drink. In contrast, new drinkers under the AOO configuration are those who had their first full drink during the past 12 months and had reached age 19 years at the time of that drink, and were either 19 years old or 20 years old when assessed. The denominators of both approaches include never drinkers who were assessed at 19 plus the new drinkers in the corresponding numerator.
For estimation of the rapid transition from drinking to the first HED, the AAA approach is conceptualized as the proportion of HDE cases among new drinkers who had the first full drink during the 12 months prior to the assessment and were assessed at a stated age. The AOO approach involved conceptualization of incidence in relation to the proportion of HDE cases among new drinkers who had their first full drink during the 12 months prior to the assessment with drinking onset stated at a certain age. Therefore, the numerator for both approaches includes individuals who had the first HDE during the 12 months prior to the assessment. For example, to estimate the transition from drinking to HDE for age 21, the denominator of the AAA approach includes all newly incident drinkers who were assessed at 21 years, who might have been either age 20 or 21 when the first drink was consumed. The AOO approach includes newly incident drinkers who consumed their first full drink at 21 years of age, who might have been either age 21 years or age 22 years on the assessment date.
In this study, standard errors and 95% confidence intervals (CI) are from Taylor Series linearization. The age‐specific AAA and AOO estimates have been derived for each year based on NSDUH year‐specific analysis weights that account for sample selection probabilities and poststratification adjustment factors (PSAF) based upon U.S. Census subpopulation counts. Data from years 2002–2014 were not pooled for a single analysis due to year‐by‐year variability in methodological approaches that sometimes involves changes in the assessment protocol and that almost always involved variations in the PSAF used to construct the analysis weights. For example, the analysis weight for 2002 is based on the U.S. census completed in 2000, whereas the analysis weight for 2012 is based on the U.S. census completed in 2010, with between‐census adjustments in the PSAF every 1–3 years.
For comparability with prior publications on this topic, estimates are populated into epidemiologic mutoscopic tables with rows representing age strata and columns representing survey years. What we call a “mutoscopic” cohort view can be obtained by tracing down the diagonals (Seedall & Anthony, 2015). Via the mutoscopic view, we are able to assess whether the age pattern gained by tracing the experience of each cohort is congruent with the age pattern observed for each year's cross‐sectional view without prospective follow‐up. A previous publication provides more historical and contextual details about the mutoscope approach (Cheng, Cantave, & Anthony, 2016a). We return to this topic in Section 4.
Thereafter, meta‐analysis was used to summarize year‐specific estimates (log transformed and back transformed). Random‐effects estimators for the meta‐analysis were used when heterogeneity was detected (DerSimonian & Laird, 1986; Higgins, Thompson, Deeks, & Altman, 2003). An alternative to our meta‐analysis approach might be to pool the data across years. As noted above, the data‐pooling approach assumes that there is a way to correct for year‐to‐year variations such as differences in PSAF used to produce each year's analysis weights in the NSDUH public use files. The meta‐analysis approach takes into account this variation, year‐to‐year, whereas the pooling‐of‐data approach fails to take it into account (e.g., see Cheng, Cantave, & Anthony, 2016a).
3. RESULTS
Table 1 presents the age‐ and year‐specific estimates as well as age‐specific meta‐analytic summary estimates for 12‐month drinking incidence using the AAA and AOO approaches, and Figure 1 provides a visual presentation of these estimates. The age and cohort views reveal highly congruent patterns; for example, there generally is a dip in the incidence estimate right after the value estimated for age 18 years, especially when using the AOO configuration.
Table 1.
Age‐, cohort‐, and year‐specific risk estimates for newly incident drinking (%) and their 95% confidence intervals. Data from U.S. National Surveys on Drug Use and Health (2002–2014; unweighted n = 208,766 12‐ to 25‐year‐olds)
| 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22–23 | 24–25 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | Panel 1. Estimates using the age‐at‐assessment approach | |||||||||||
| 2002 | 3 (2,4) | 8 (7,10) | 15 (13,17) | 22 (20,25) | 27 (25,30) | 28 (25,32) | 31 (27,35) | 23 (19,28) | 23 (17,29) | 43 (37,49) | 11 (8,15) | 5 (3,9) |
| 2003 | 3 (3,5) | 9 (7,10) | 15 (13,17) | 25 (23,28) | 25 (22,29) | 26 (23,30) | 31 (27,36) | 27 (21,33) | 27 (21,33) | 41 (35,48) | 17 (13,22) | 6 (4,11) |
| 2004 | 3 (2,4) | 8 (6,10) | 16 (14,18) | 23 (21,25) | 27 (24,31) | 28 (25,32) | 34 (29,39) | 26 (22,31) | 20 (15,26) | 43 (36,49) | 14 (10,19) | 2 (1,5) |
| 2005 | 2 (2,3) | 8 (6,9) | 15 (13,17) | 23 (21,25) | 27 (24,30) | 27 (24,30) | 30 (27,34) | 29 (25,34) | 22 (18,28) | 40 (34,47) | 11 (8,16) | 3 (2,8) |
| 2006 | 3 (2,3) | 7 (6,9) | 14 (12,16) | 20 (18,22) | 26 (23,28) | 29 (26,32) | 35 (31,38) | 31 (26,37) | 26 (20,33) | 39 (34,45) | 16 (11,21) | 4 (2,7) |
| 2007 | 3 (2,4) | 7 (6,8) | 14 (12,16) | 21 (19,24) | 25 (22,28) | 28 (25,31) | 32 (27,36) | 27 (22,33) | 23 (18,28) | 50 (43,57) | 14 (11,19) | 4 (2,7) |
| 2008 | 2 (2,3) | 6 (5,7) | 12 (11,14) | 20 (17,23) | 25 (23,28) | 24 (21,27) | 32 (29,35) | 29 (25,34) | 22 (18,27) | 45 (40,50) | 16 (12,21) | 6 (4,11) |
| 2009 | 3 (2,4) | 7 (6,8) | 12 (11,14) | 19 (17,21) | 27 (24,29) | 27 (23,30) | 33 (30,37) | 30 (26,34) | 26 (21,32) | 48 (42,54) | 17 (13,23) | 5 (3,8) |
| 2010 | 2 (1,3) | 6 (5,8) | 12 (10,13) | 20 (18,22) | 24 (21,26) | 23 (20,26) | 34 (30,39) | 27 (24,31) | 24 (19,30) | 56 (50,62) | 17 (13,22) | 5 (3,10) |
| 2011 | 2 (1,3) | 6 (5,8) | 11 (10,13) | 20 (18,22) | 23 (21,25) | 25 (22,28) | 27 (24,30) | 25 (21,30) | 21 (16,26) | 53 (47,58) | 20 (16,26) | 5 (3,9) |
| 2012 | 3 (2,4) | 5 (4,6) | 12 (10,14) | 17 (15,19) | 21 (19,23) | 23 (20,26) | 29 (25,33) | 27 (22,32) | 20 (16,25) | 50 (44,56) | 23 (18,28) | 4 (2,7) |
| 2013 | 1 (1,2) | 4 (3,5) | 9 (7,10) | 16 (14,18) | 24 (22,26) | 25 (22,28) | 29 (26,33) | 25 (22,29) | 19 (15,24) | 55 (48,62) | 19 (14,23) | 3 (2,6) |
| 2014 | 1 (1,2) | 4 (3,6) | 8 (7,10) | 17 (15,18) | 21 (19,24) | 24 (21,27) | 30 (25,35) | 23 (18,28) | 27 (22,33) | 53 (47,59) | 20 (16,26) | 5 (3,10) |
| MAS | 2 (2,3) | 6 (6,7) | 12 (11,14) | 20 (19,22) | 25 (24,26) | 26 (25,27) | 31 (30,33) | 27 (26,29) | 23 (22,25) | 47 (44,51) | 17 (15,19) | 5 (4,5) |
| Panel 2. Estimates using the age‐of‐onset approach | ||||||||||||
| 2002 | 5 (4,6) | 10 (8,11) | 17 (15,19) | 23 (20,25) | 25 (23,28) | 28 (24,32) | 28 (25,31) | 17 (13,22) | 21 (16,26) | 43 (38,49) | 7 (4,10) | 4 (3,8) |
| 2003 | 5 (4,6) | 11 (10,12) | 17 (16,19) | 25 (22,27) | 23 (21,27) | 26 (22,30) | 29 (25,34) | 21 (17,26) | 27 (21,33) | 40 (33,48) | 11 (8,16) | 5 (3,9) |
| 2004 | 5 (4,6) | 9 (8,11) | 18 (16,20) | 23 (21,25) | 27 (24,29) | 28 (25,32) | 29 (25,33) | 21 (18,26) | 19 (14,25) | 43 (37,49) | 9 (6,13) | 2 (1,5) |
| 2005 | 4 (4,5) | 9 (8,10) | 18 (16,20) | 23 (21,26) | 26 (24,29) | 25 (22,28) | 30 (26,35) | 22 (19,27) | 18 (14,24) | 41 (35,47) | 5 (4,8) | 3 (1,7) |
| 2006 | 4 (3,5) | 10 (8,11) | 15 (14,17) | 22 (20,24) | 24 (22,27) | 28 (26,31) | 33 (29,38) | 21 (17,25) | 24 (19,31) | 40 (35,46) | 9 (6,15) | 3 (1,6) |
| 2007 | 4 (3,5) | 9 (7,10) | 17 (16,19) | 21 (18,24) | 25 (22,28) | 26 (22,29) | 30 (27,34) | 19 (15,25) | 20 (16,26) | 51 (44,58) | 8 (6,12) | 3 (2,6) |
| 2008 | 4 (3,5) | 7 (6,9) | 15 (13,17) | 23 (20,26) | 22 (19,25) | 25 (22,28) | 30 (27,34) | 21 (18,26) | 17 (14,21) | 47 (41,52) | 11 (8,17) | 6 (3,10) |
| 2009 | 4 (3,6) | 9 (8,10) | 14 (12,16) | 21 (19,23) | 26 (23,29) | 26 (23,29) | 33 (29,36) | 22 (18,26) | 22 (17,28) | 51 (45,56) | 9 (6,14) | 4 (2,7) |
| 2010 | 3 (2,4) | 8 (6,9) | 14 (12,16) | 22 (19,24) | 23 (20,26) | 23 (20,26) | 32 (28,36) | 21 (18,24) | 24 (20,29) | 57 (50,63) | 7 (5,10) | 4 (2,8) |
| 2011 | 3 (2,4) | 8 (7,10) | 13 (12,15) | 21 (19,23) | 23 (20,26) | 24 (21,27) | 26 (23,30) | 18 (14,22) | 18 (14,23) | 55 (50,60) | 11 (7,16) | 5 (3,8) |
| 2012 | 3 (2,4) | 7 (5,8) | 14 (12,15) | 18 (16,20) | 21 (19,24) | 22 (19,26) | 29 (25,33) | 20 (16,24) | 20 (16,24) | 51 (45,56) | 12 (8,17) | 3 (1,5) |
| 2013 | 2 (2,3) | 6 (5,7) | 11 (9,12) | 19 (17,21) | 22 (20,25) | 26 (23,29) | 27 (24,31) | 18 (14,22) | 21 (16,26) | 56 (49,62) | 9 (7,13) | 2 (1,5) |
| 2014 | 2 (1,3) | 6 (5,7) | 11 (9,12) | 18 (16,20) | 21 (19,24) | 25 (21,29) | 28 (24,33) | 17 (14,21) | 23 (18,28) | 56 (50,62) | 8 (5,13) | 4 (2,9) |
| MAS | 4 (3,4) | 8 (7,9) | 15 (13,16) | 21 (20,22) | 24 (23,25) | 26 (24,27) | 30 (29,31) | 20 (19,21) | 21 (19,22) | 49 (45,52) | 9 (8,10) | 4 (3,5) |
Note. MAS = meta‐analysis summary.
Figure 1.
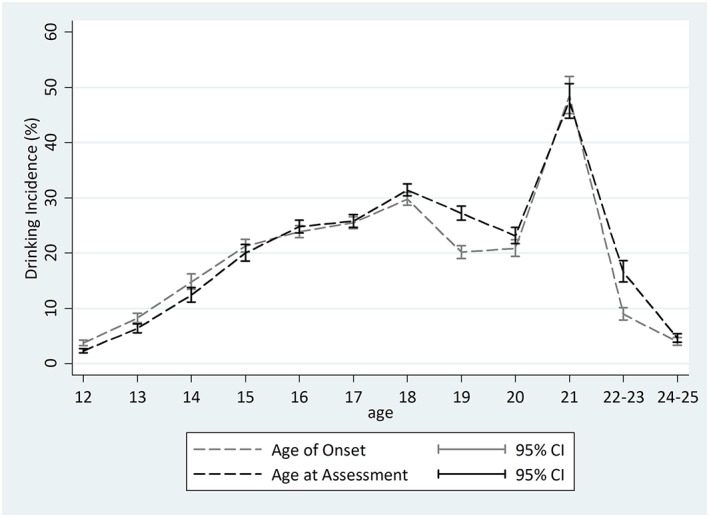
Comparison of meta‐analytic summary of age‐specific estimates of incidence of drinking among 12‐ to 25‐year‐olds using age‐of‐onset and age‐at‐assessment approaches. Data from United States National Survey on Drug Use and Health (2002–2014; unweighted n = 208,766). The lack of heterogeneity across replications motivated use of the fixed‐effects variance estimation approach for the age‐at‐assessment estimates for 18‐ to 20‐year‐olds and 24‐ to 25‐year‐olds, and age‐of‐onset estimates for 22‐ to 25‐year‐olds
The meta‐analysis summaries of age‐specific incidence using the AAA and AOO approaches reveal generally congruent patterns. Two exceptions are noteworthy. First, smaller AOO estimates are seen for 19‐ and 22‐to‐23‐year‐olds, as compared with the corresponding AAA estimates (AOO incidence estimate for 19‐year‐olds = 20.2%, 95% CI [19.1%, 21.4%]; AAA incidence estimate for 19‐year‐olds = 27.2%, 95% CI [26.2%, 28.5%]; AOO incidence estimate for 22‐to‐23‐year‐olds = 8.9%, 95% CI [7.9%, 10.2%]; AAA incidence estimate for 22‐to‐23‐year‐olds = 16.5%, 95% CI [14.8%, 18.6%]). Second, one can see a tendency of larger AOO incidence estimates when incidence is on a rise and lower estimates when age‐specific incidence is on a decline.
For the rapid transition from drinking to HDE among newly incident drinkers with no more than 12 months of drinking experience, the AAA and AOO approaches yield age‐specific meta‐analysis summary estimates that do not differ appreciably, as shown in Table 2 and Figure 2. One exception is a somewhat larger AOO estimate compared to the AAA estimate for 14‐year‐olds (AOO transition estimate for 14‐year‐olds = 26.6%, 95% CI [24.7%, 28.6%]; AAA transition estimate for 14‐year‐olds =22.1%, 95% CI [20.2%, 24.3%]). The AOO estimate for 22‐ to 23‐year‐olds is smaller than the AAA estimate (AOO transition estimate = 20.4%, 95% CI [15.9%, 26.1%]; AAA transition estimate = 29.6%, 95% CI [25.8%, 34.0%]).
Table 2.
Age‐, cohort‐, and year‐specific risk estimates for transitioning from drinking to heavy drinking episode (%) and their 95% confidence intervals among newly incident drinkers. Data from U.S. National Surveys on Drug Use and Health (2006–2014; unweighted n = 24,340 12‐ to 25‐year‐olds)
| 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22–23 | 24–25 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | Panel 1. Estimates using the age‐at‐assessment approach | |||||||||||
| 2006 | 13 (5,27) | 23 (15,33) | 26 (20,32) | 32 (27,38) | 35 (30,40) | 31 (26,37) | 35 (29,41) | 37 (27,47) | 35 (22,52) | 34 (25,46) | 23 (13,39) | 14 (3,45) |
| 2007 | 7 (3,15) | 18 (12,25) | 23 (18,29) | 35 (29,41) | 36 (31,42) | 36 (30,42) | 38 (31,45) | 32 (23,43) | 35 (25,48) | 30 (23,38) | 18 (10,29) | 8 (1,37) |
| 2008 | 22 (12,38) | 25 (17,36) | 21 (16,28) | 29 (25,34) | 35 (29,41) | 35 (30,41) | 41 (34,49) | 27 (20,36) | 33 (23,45) | 33 (26,41) | 25 (13,43) | 10 (2,34) |
| 2009 | 31 (15,53) | 26 (19,36) | 23 (17,30) | 27 (22,33) | 34 (28,39) | 34 (29,41) | 42 (36,50) | 34 (26,43) | 40 (28,53) | 31 (24,38) | 28 (19,39) | 18 (5,49) |
| 2010 | 6 (2,15) | 21 (13,32) | 19 (15,25) | 30 (26,35) | 30 (24,36) | 31 (25,38) | 39 (32,47) | 45 (36,53) | 29 (20,39) | 32 (25,40) | 28 (18,41) | 12 (3,33) |
| 2011 | 24 (9,53) | 18 (11,27) | 23 (17,30) | 29 (24,34) | 28 (23,33) | 37 (31,43) | 36 (29,44) | 30 (22,38) | 24 (15,36) | 39 (31,48) | 39 (27,53) | 14 (3,44) |
| 2012 | 11 (4,27) | 18 (10,30) | 24 (17,33) | 31 (26,37) | 35 (31,39) | 34 (28,41) | 32 (24,40) | 43 (34,52) | 33 (22,47) | 38 (30,47) | 31 (21,43) | 12 (3,40) |
| 2013 | 19 (8,41) | 19 (12,30) | 18 (13,26) | 26 (21,32) | 28 (23,34) | 33 (28,39) | 31 (25,38) | 33 (26,40) | 26 (16,40) | 32 (24,41) | 17 (9,29) | 4 (<1,25) |
| 2014 | 11 (3,32) | 21 (11,37) | 19 (13,27) | 25 (19,32) | 35 (29,41) | 36 (30,42) | 32 (27,39) | 50 (37,63) | 28 (20,39) | 44 (36,51) | 39 (28,51) | 11 (2,48) |
| MAS | 15 (10,22) | 21 (19,25) | 22 (20,24) | 30 (28,32) | 33 (31,35) | 34 (32,36) | 37 (34,39) | 36 (32,42) | 32 (28,36) | 36 (33,38) | 30 (26,34) | 12 (7,19) |
| Panel 2. Estimates using the age‐of‐onset approach | ||||||||||||
| 2006 | 18 (10,30) | 26 (19,34) | 31 (24,39) | 36 (32,41) | 30 (25,35) | 32 (26,39) | 35 (29,42) | 42 (31,55) | 35 (24,47) | 27 (19,38) | 22 (8,47) | 7 (1,40) |
| 2007 | 12 (6,21) | 28 (21,36) | 29 (23,35) | 34 (28,40) | 37 (31,43) | 40 (33,47) | 32 (26,40) | 32 (21,45) | 28 (18,42) | 29 (22,38) | 14 (6,29) | 7 (1,39) |
| 2008 | 17 (10,28) | 30 (22,39) | 25 (20,31) | 31 (27,36) | 41 (34,48) | 34 (27,41) | 35 (28,42) | 31 (21,42) | 29 (16,47) | 33 (26,42) | 13 (6,26) | 14 (2,59) |
| 2009 | 31 (18,47) | 28 (21,36) | 26 (22,31) | 32 (26,38) | 32 (27,37) | 38 (32,45) | 40 (33,48) | 36 (25,49) | 26 (19,36) | 31 (24,38) | 26 (13,44) | 12 (2,54) |
| 2010 | 17 (11,26) | 22 (15,30) | 24 (19,30) | 30 (24,36) | 31 (25,37) | 38 (30,47) | 37 (30,43) | 41 (31,53) | 27 (19,36) | 31 (23,40) | 31 (17,50) | 11 (3,36) |
| 2011 | 23 (12,39) | 18 (12,25) | 28 (22,35) | 30 (24,36) | 30 (25,35) | 38 (32,44) | 34 (28,42) | 25 (16,36) | 34 (22,48) | 39 (31,47) | 24 (10,46) | 11 (2,49) |
| 2012 | 21 (11,36) | 19 (11,32) | 28 (22,34) | 34 (29,40) | 33 (27,39) | 35 (27,44) | 37 (30,44) | 36 (26,47) | 42 (28,57) | 35 (28,43) | 16 (8,31) | 24 (5,65) |
| 2013 | 23 (12,39) | 19 (12,27) | 24 (19,31) | 29 (23,35) | 30 (25,36) | 30 (25,37) | 33 (26,40) | 24 (16,34) | 31 (21,43) | 29 (22,38) | 9 (2,37) | 0 (.,.) |
| 2014 | 12 (5,26) | 22 (13,34) | 23 (17,31) | 29 (22,36) | 40 (32,47) | 32 (25,40) | 37 (30,46) | 47 (32,62) | 26 (17,38) | 42 (34,50) | 18 (6,45) | 17 (3,59) |
| MAS | 19 (16,23) | 25 (22,27) | 27 (25,29) | 32 (30,34) | 33 (31,36) | 36 (33,38) | 36 (33,38) | 36 (32,40) | 31 (27,35) | 34 (32,37) | 20 (16,26) | 13 (7,23) |
Note. MAS = meta‐analysis summary.
Figure 2.
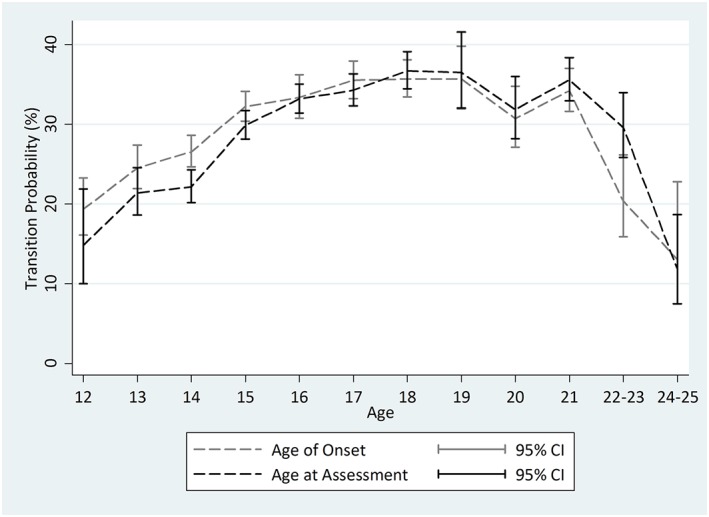
Comparison of age‐specific rapid transition from drinking to heavy episodic drinking among 12‐ to 25‐year‐old newly incident drinkers using age of onset and age at assessment. Data from United States National Survey on Drug Use and Health (2006–2014; n = 24,340). Heterogeneity across replications motivated use of the random‐effects variance estimation approach for the age‐at‐assessment estimates for 12‐ and 19‐year‐olds, and age‐of‐onset estimates for 16‐year‐olds
In postestimation exploratory data analysis steps, we conducted an age–period–cohort analyses using a constrained regression model. This work disclosed no evidence of potential cohort‐ or period‐related variations (data available upon request).
4. DISCUSSION
The main findings of this study may be summarized succinctly. First, we found that the AOO approach disclosed more prominent drops of estimated annual drinking incidence at 19 and 22–23 years of age compared to the AAA approach. This finding rules out the possibility that the previously documented drop in drinking incidence at 19 is a methodological artifact due to ambiguity about the age of first full drink. Instead, if anything, the AAA approach might be understating the drop in age‐specific incidence at age 19 years. As for the peak at 21 years of age, the two approaches yielded results that do not differ appreciably.
Second, for the rapid transition from drinking to HDE among newly incident drinkers who had their first full drink during within the prior 12 months, in the AOO approach, there was a more prominent drop among those who had their first full drink immediately after the legal minimum drinking age (i.e., among 22–23 years of age). Third, the AOO approach seems to produce slightly larger estimates during early adolescence for both newly incident drinking and for the rapid transition to HDE. We note that there is rising incidence for both drinking onsets and HDE onsets in early adolescents.
Before the detailed discussion of these results, several of the more important study limitations merit attention. Of central concern is the self‐report nature of the assessment. A potential problem of self‐report in surveys is socially desirable responding (Paulhus, 1984). If taking the first full drink at 21 years of age is considered a socially desirable behavior by the participant, those who truly started drinking at age 20 might report the age at first drink as 21. This process might exaggerate a drinking incidence that dips at 20 and promote a peak at 21. If this is the case, it is not difficult to imagine that more 21‐year‐olds would report drinking onset at 21 instead of the true value of 20 years. By comparison, those with onsets at age 22–23 years would not be subject to the same process but might not wish to disclose a delayed onset beyond the legal age of 21 years. In consequence, the peak at age 21 years might be observed to be larger than it actually is. Our observation is one of no appreciable variation in the age 21 incidence estimates between the AAA and AOO approaches.
For surveys such as NSDUH, it is possible that some drinkers might experience rapid‐onset alcohol dependence and thereby become less likely to appear on sampling rosters or to participate in survey assessments, as has been observed for individuals with severe mental disorders (Wing, Mann, Leff, & Nixon, 1978). One suspects that this kind of missingness might be more likely to be faced among 19‐to‐20‐year‐old drinkers who live alone compared to 12‐to‐18‐year‐old drinkers (to the extent that younger adolescent drinkers are less likely to live alone and therefore should be included on sampling rosters listed by parents and adult caregivers). Nonetheless, only a small proportion of 19‐to‐20‐year‐olds live alone, and only a small proportion of new drinkers experience alcohol dependence within the first 12 months after drinking onset (Cheng et al., 2016). Therefore, we suspect that differential rostering and participation do not have a large enough effect to completely account for the observed pattern of a dip in incidence at age 19–20 years, followed by an increase in incidence at age 21 years.
When we turned to the AOO approach, we made an assumption that newly incident drinkers who start drinking at age n and assessed at age n are exchangeable with those who started drinking at age n and assessed at age n + 1. We counted on no substantial cohort effects or period effects on drinking incidence when age shifts from n to n + 1 and year shifts from m to m + 1. Another assumption is no substantial drinking‐related differential survival (i.e., newly incident drinkers are as likely to survive from age n to age n + 1 as never drinkers). Against a background of a relatively low mortality rate in the U.S. adolescent population, there is little evidence about substantially differential survival for drinkers versus nondrinkers in this age group (Neumark, Van Etten, & Anthony, 2000).
Notwithstanding limitations such as these, the comparison of two estimation methods for drinking incidence and the rapid transition from drinking to first HDE has some strengths. The use of nationally representative survey data and audio computer‐assisted self‐interviews enhances internal and external validity. Estimating incidence based on newly incident cases observed in cross‐sectionally sampled populations offers a useful alternative to the creation of synthetic time‐to‐event estimates.
In this work, we borrowed information across survey years in order to improve the precision of incidence estimates and to shed light on the nonlinear age‐specific patterns for drinking onsets. Elsewhere, we have described how early epidemiologist Wade Hampton Frost elaborated the 19th century Lexis diagram approach in order to focus attention on experiences of birth cohorts grouped in 10‐year increments, and we have shown how this “epidemiologic mutoscope” approach can be used to check whether the experience of individual year‐by‐year birth cohorts is or is not congruent with the overall age‐specific pattern of incidence rates (Seedall & Anthony, 2015; Cheng, Cantave, & Anthony, 2016a). A similar approach to rearrangement of repeated cross‐sectional survey estimates has been taken in the U.S. National Crime Victimization Survey with respect to recurrent events such as being the victim of a crime once or on multiple occasions (United States Department of Justice, 2017).
With respect to implications for future research, the observed findings on drinking incidence support the use of the AOO approach when incidence might change dramatically from one age to the next. When using the AAA approach, the fact that many 22‐year‐old newly incident drinkers actually had their first drink at 21 obscures the magnitude of the drop immediately after the high peak. Similarly, many newly incident drinkers who were assessed at 19 years of age had their first full drink at 18 when drinking incidence has its second peak. To a lesser extent, the AAA approach also yielded slight underestimates for early adolescents among whom the drinking incidence is on a sharp rise (i.e., more 14‐year‐olds had their first drink at 13 than 13‐year‐olds who had their first drink at 12). Nonetheless, the overall age patterns for incidence estimates are generally congruent.
As for the transition from drinking to HDE, the AOO approach can be used to avoid what otherwise might be an unnecessary mixture of drinkers who start drinking at two separate age values. This consideration will be especially important for alcohol research in locations where the legal minimum drinking age creates a divide between underage drinkers and “postponers,” who might have different personality and behavioral profiles. For this reason, in the context of research on minimum legal drinking ages or age‐related privileges in general, incidence estimation based on the AOO approach might be more valuable than incidence estimation based on the AAA approach.
In a previous study, we estimated drinking incidence using the AAA approach with the restricted version of NSDUH data (i.e., Restricted‐Use Data Analysis System, R‐DAS; Cheng, Cantave, & Anthony, 2016a). In the present study, we used the publicly downloadable version of the NSDUH data to estimate drinking incidence. The exact date of the assessment is available in R‐DAS, whereas only the quarter of the year when the assessment was conducted is available in the publicly downloadable NSDUH data (https://www.icpsr.umich.edu/icpsrweb/NAHDAP/series/64/studies?archive=NAHDAP&sortBy=7, accessed June 30, 2017). In order to identify newly incident drinkers who had their first full drink during the past 12 months as accurately as possible, our team has developed a standard method using the assessment year and quarter variables supplemented with a recency filter as described in detail in previous publications (Cheng & Anthony, 2016; Cheng, Cantave, & Anthony, 2016a). A comparison between the previously published R‐DAS estimates and this study's AAA drinking incidence estimates reveals almost identical age‐specific patterns (Cheng, Cantave, & Anthony, 2016a). This finding supports the validity of our standard approach using the publicly downloadable NSDUH with the interview quarter variable.
For this methods inquiry, we turned to alcohol‐related outcomes as an example. A consideration of these issues is pertinent in studies on age‐specific incidence of other forms of drug use and for AOO research on other behaviors and neuropsychiatric conditions in general. The use of both approaches (AAA and AOO) might become possible whenever cross‐sectionally derived and assessed samples are tapped for approximations for estimates of incidence rates in the absence of or prior to prospective studies. In this study, we chose to specify the recall interval as the prior 12 months because (a) there has been evidence that a recall interval as short as 2–3 years can produce substantial inconsistencies for drinking onset among adolescents (Engels et al., 1997; Kuntsche et al., 2016) and (b) our interest is the age‐specific incidence pattern. When applying this method to other neuropsychiatric conditions, the choice of the length of the recall interval should be guided by the study aim as well as knowledge about the reliability of the assessment of the disorder or behavior of interest.
There is an increasing focus on community field surveys of alcohol and other drug use, as well as neuropsychiatric conditions, with surveys being completed in many countries of the world that previously have been neglected (Morley, Lynskey, Moran, Borschmann, & Winstock, 2015). In addition, many countries have initiated periodic, sometimes annual, surveys to monitor drug use behaviors and other health‐related outcomes with consistent methods. Our estimation approaches may facilitate these cross‐country and cross‐region comparisons.
DECLARATION OF INTEREST STATEMENT
The authors have no conflict of interest to declare.
Supporting information
Table S1. Sample size description. Data from United States National Survey on Drug Use and Health.
ACKNOWLEDGEMENTS
The study is supported by funds from the National Institute on Drug Abuse Grants K05DA015799 (to JCA) and T32DA021129 (to HGC and CLQ), as well as Michigan State University. The content is the sole responsibility of the authors and does not necessarily represent the official views of MSU, the National Institute on Drug Abuse, or the National Institutes of Health. The authors wish to thank the United States Substance Abuse and Mental Health Services Administration Office of Applied Studies (now the Center for Behavioral Health Statistics and Quality) for completion of its annual nationally representative surveys on drug use and health, as well as its direction and supervision of the annual data gathering and preparation of public use datasets.
Cheng HG, Lopez‐Quintero C, Anthony JC. Age of onset or age at assessment—that is the question: Estimating newly incident alcohol drinking and rapid transition to heavy drinking in the United States, 2002–2014. Int J Methods Psychiatr Res. 2018;27:e1587 10.1002/mpr.1587
REFERENCES
- Cheng, H. G. , & Anthony, J. C. (2016). Does our legal minimum drinking age modulate risk of first heavy drinking episode soon after drinking onset? Epidemiological evidence for the United States, 2006‐2014. PeerJ, 4, e2153. 10.7717/peerj.2153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, H. G. , Cantave, M. D. , & Anthony, J. C. (2016a). Alcohol experiences viewed mutoscopically: Newly incident drinking of twelve‐ to twenty‐five‐year‐olds in the United States, 2002‐2013. Journal of Studies on Alcohol and Drugs, 77(3), 405–412. 10.15288/jsad.2016.77.405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, H. G. , Cantave, M. D. , & Anthony, J. C. (2016b). Taking the first full drink: Epidemiological evidence on male‐female differences in the United States. Alcoholism, Clinical and Experimental Research, 40(4), 816–825. 10.1111/acer.13028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, H. G. , Chandra, M. , Alcover, K. C. , & Anthony, J. C. (2016). Rapid transition from drinking to alcohol dependence among adolescent and young‐adult newly incident drinkers in the United States, 2002‐2013. Drug and Alcohol Dependence, 168, 61–68. 10.1016/j.drugalcdep.2016.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt, L. , Chiu, W. T. , Sampson, N. , Kessler, R. C. , Anthony, J. C. , Angermeyer, M. , … Wells, J. E. (2008). Toward a global view of alcohol, tobacco, cannabis, and cocaine use: Findings from the WHO World Mental Health Surveys. PLoS Medicine, 5(7), e141. 10.1371/journal.pmed.0050141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerSimonian, R. , & Laird, N. (1986). Meta‐analysis in clinical trials. Controlled Clinical Trials, 7(3), 177–188. 10.1016/0197-2456(86)90046‐2 [DOI] [PubMed] [Google Scholar]
- Eaton, W. W. , Kramer, M. , Anthony, J. C. , Dryman, A. , Shapiro, S. , & Locke, B. Z. (1989). The incidence of specific DIS/DSM‐III mental disorders: Data from the NIMH Epidemiologic Catchment Area Program. Acta Psychiatrica Scandinavica, 79(2), 163–178. 10.1111/j.1600-0447.1989.tb08584.x [DOI] [PubMed] [Google Scholar]
- Engels, R. C. M. E. , Knibbe, R. A. , & Drop, M. J. (1997). Inconsistencies in adolescents' self‐reports of initiation of alcohol and tobacco use. Addictive Behaviors, 22(5), 613–623. 10.1016/S0306-4603(96)00067‐6 [DOI] [PubMed] [Google Scholar]
- Farr, W. (1885). Vital statistics: A memorial volume of selections from the reports and writtings. London, UK: Office of the Sanitary Institute. [PMC free article] [PubMed] [Google Scholar]
- Fontalba‐Navas, A. , Lucas‐Borja, M. E. , Gil‐Aguilar, V. , Arrebola, J. P. , Pena‐Andreu, J. M. , & Perez, J. (2017). Incidence and risk factors for post‐traumatic stress disorder in a population affected by a severe flood. Public Health, 144, 96–102. 10.1016/j.puhe.2016.12.015 [DOI] [PubMed] [Google Scholar]
- Hasin, D. S. , Stinson, F. S. , Ogburn, E. , & Grant, B. F. (2007). Prevalence, correlates, disability, and comorbidity of DSM‐IV alcohol abuse and dependence in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of General Psychiatry, 64(7), 830–842. 10.1001/archpsyc.64.7.830 [DOI] [PubMed] [Google Scholar]
- Higgins, J. P. T. , Thompson, S. G. , Deeks, J. J. , & Altman, D. G. (2003). Measuring inconsistency in meta‐analyses. BMJ, 327(7414), 557–560. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer, M. (1957). A discussion of the concepts of incidence and prevalence as related to epidemiologic studies of mental disorders. Am J Public Health Nations Health, 47(7), 826–840. Retrieved from. http://www.ncbi.nlm.nih.gov/pubmed/13435387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntsche, E. , Rossow, I. , Engels, R. , & Kuntsche, S. (2016). Is “age at first drink” a useful concept in alcohol research and prevention? We doubt that. Addiction, 111(6), 957–965. 10.1111/add.12980 [DOI] [PubMed] [Google Scholar]
- Lapouse, R. (1967). Problems in studying the prevalence of psychiatric disorder. Am J Public Health Nations Health, 57(6), 947–954. Retrieved from. http://www.ncbi.nlm.nih.gov/pubmed/6067351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley, K. I. , Lynskey, M. T. , Moran, P. , Borschmann, R. , & Winstock, A. R. (2015). Polysubstance use, mental health and high‐risk behaviours: Results from the 2012 Global Drug Survey. Drug and Alcohol Review, 34(4), 427–437. 10.1111/dar.12263 [DOI] [PubMed] [Google Scholar]
- Morrison, T. C. , Wahlgren, D. R. , Hovell, M. F. , Zakarian, J. , Burkham‐Kreitner, S. , Hofstetter, C. R. , … Jones, J. A. (1997). Tracking and follow‐up of 16,915 adolescents: Minimizing attrition bias. Controlled Clinical Trials, 18(5), 383–396. 10.1016/S0197-2456(97)00025‐1 [DOI] [PubMed] [Google Scholar]
- Neumark, Y. D. , Van Etten, M. L. , & Anthony, J. C. (2000). “Alcohol dependence” and death: survival analysis of the Baltimore ECA sample from 1981 to 1995. Substance Use & Misuse, 35(4), 533–549. Retrieved from. http://www.ncbi.nlm.nih.gov/pubmed/10741540 [DOI] [PubMed] [Google Scholar]
- Paulhus, D. L. (1984). Two‐component models of socially desirable responding. J Pers Soc Psychol, 14, 598–609. 10.1037/0022-3514.46.3.598 [DOI] [Google Scholar]
- Seedall, R. B. , & Anthony, J. C. (2015). Monitoring by parents and hypothesized male‐female differences in evidence from a nationally representative cohort re‐sampled from age 12 to 17 years: An exploratory study using a “mutoscope” approach. Prevention Science, 16(5), 696–706. 10.1007/s11121-014-0517-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shillington, A. M. , Woodruff, S. I. , Clapp, J. D. , Reed, M. B. , & Lemus, H. (2012). Self‐reported age of onset and telescoping for cigarettes, alcohol, and marijuana across eight years of the National Longitudinal Survey of Youth. Journal of Child & Adolescent Substance Abuse, 21(4), 333–348. 10.1080/1067828X.2012.710026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Substance Abuse and Mental Health Services Administration . (2012). Comparing and evaluating youth substance use estimates from the National Survey on Drug Use and Health and other surveys ()HHS Publication No. SMA 12‐4727, Methodology Series M‐9 Rockville, MD: Substance Abuse and Mental Health Services Administration. [PubMed] [Google Scholar]
- Thygesen, L. C. , Johansen, C. , Keiding, N. , Giovannucci, E. , & Grønbæk, M. (2008). Effects of sample attrition in a longitudinal study of the association between alcohol intake and all‐cause mortality. Addiction, 103(7), 1149–1159. 10.1111/j.1360-0443.2008.02241.x [DOI] [PubMed] [Google Scholar]
- U.S. Department of Justice . (2017). Survey Methodology for Criminal Victimization in the United States. See https://www.bjs.gov/content/pub/pdf/ncvs_methodology.pdf (accessed on 20 June 2017).
- Willett, J. B. , & Singer, J. D. (1993). Investigating onset, cessation, relapse, and recovery: Why you should, and how you can, use discrete‐time survival analysis to examine event occurrence. Journal of Consulting and Clinical Psychology, 61(6), 952–965. 10.1037/0022-006X.61.6.952 [DOI] [PubMed] [Google Scholar]
- Wing, J. K. , Mann, S. A. , Leff, J. P. , & Nixon, J. M. (1978). The concept of a “case” in psychiatric population surveys. Psychological Medicine, 8(2), 203–217. 10.1017/S0033291700014264 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Sample size description. Data from United States National Survey on Drug Use and Health.


