Abstract
Objective
ICU LOS is an important measure of resource use and economic performance. Our primary aims were to characterize the utilization of PICU beds and to develop a new model for PICU LOS.
Design
Prospective cohort. The main outcomes were factors associated with PICU LOS and the performance of a regression model for LOS.
Setting
Eight pediatric ICUs.
Patients
Randomly selected patients (newborn to 18 years) from 8 PICUs were enrolled from December 4, 2011 to April 7, 2013. Data consisted of descriptive, diagnostic, physiologic, and therapeutic information.
Interventions
none
Measurements and Main Results
The mean LOS for was 5.0 days (SD 11.1), with a median of 2.0 days. The 50.6% of patients with LOS < 2 days consumed only 11.1% of the days of care, while the 19.6% of patients with LOS 4.9 to 19 days and the 4.6% with LOS ≥ 19 days consumed 35.7% and 37.6% of the days of care respectively. Longer LOS was observed in younger children, those with cardiorespiratory disease, post-intervention cardiac patients, and those who were sicker assessed by PRISM scores receiving more intensive therapies. Patients in the cardiac ICU stayed longer than those in the medical ICU. The LOS model using descriptive, diagnostic, severity, and therapeutic factors performed well (R-squared of 0.42). Standardized (observed divided by expected) LOS ratios at the individual sites ranged from 0.87 to 1.09.
Conclusions
PICU bed utilization was dominated by a minority of patients. The 5% of patients staying the longest utilized almost 40% of the bed days. The multivariate LOS model used descriptive, diagnostic, therapeutic and severity factors and has potential applicability for benchmarking.
Keywords: length of stay, outcomes research, critical care, pediatric critical care, quality assessment, health care economics
Introduction
Critical care efficiency and quality are associated with length of stay (LOS).1,2 Intensive care units (ICUs) are a major driver of hospital costs. In 2011, almost 27% of hospital stays involved ICU charges, and the hospital stays that involved the ICU were 2.5 times more costly than other hospital stays.3 The national trend in managing health care costs places a priority on efficient bed utilization. Operational efforts to control LOS include critical pathways, case management, and new management systems.4,5 Managing critical care resources is often difficult because there are complex associations between personnel and facility needs when the provision of timely and sophisticated but unanticipated care by skilled personnel results in important outcome differences. 6,7
ICU LOS is an important measure of resource use and economic performance.8,9 Yet, ICU LOS varies with respect to many factors including severity of illness, diagnostic diversity, and other patient factors.8,10–12 In additional, institutional practices substantially contribute to variability as evidenced by the wide disparity in LOS for both pediatric and adult ICUs despite controlling for patient factors.2,8,11,13,14
Models predicting ICU LOS have been disappointing.8,15–17 Patient descriptive factors along with physiological profiles are statistically important but insufficient to explain the majority of LOS variability. Most models have included therapies and even then, model performances have been modest. For example, the proportion of variance (R2) accounted for by an adult model using physiological profiles, patient admission characteristics, and therapies implemented in the first 24 hours in over 200,000 patients was less than 25%.8
There has not been a recent characterization of pediatric intensive care unit (PICU) utilization described by LOS distributions, and the patient characteristics associated with resource use. Our primary aims for this analysis were to characterize the utilization of PICU beds using the focus of LOS and to develop a multivariatemodel for PICU LOS to better understand the importance of patient and therapeutic factors in a sample of over 10,000 patients from eight institutions. Since model performance has been disappointing using variables only from the early ICU time period, we included therapies used during the entire PICU stay and PICU outcome. Inclusion of these post-admission factors is appropriate for our goal of investigating the relationship of patient and therapeutic factors in a multivariate model with potential applicability to internal and external benchmarking.
Patients and Methods
The data for this analysis originated in the Trichotomous Outcome Prediction in Critical Care (TOPICC) study conducted by the Collaborative Pediatric Critical Care Research Network (CPCCRN) of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Data collection methods and institutional characteristics have been previously described.18 There were seven funded sites, one being composed of two institutions. In brief, patients aged from newborn to less than 18 years were randomly selected and stratified by hospital from December 4, 2011 to April 7, 2013. Patients from both general/medical and cardiac/cardiovascular PICUs were included. Moribund patients (vital signs incompatible with life for the first two hours after PICU admission) were excluded. Only the first PICU admission during a hospitalization was included. The protocol was approved by all participating Institutional Review Boards. Other analyses utilizing this database have been published.18–22
Data included descriptive and demographic information (Supplemental Table 1). LOS was recorded in hours and converted to days of care starting with the first vital sign and ending with the last vital sign, with LOS less than one hour rounded up to one hour. The primary analyses treat days of care as a continuous outcome (for example, a patient in the PICU for exactly 27 hours is analyzed as having 1.125 days of care). For display in histogram form, LOS is presented as the number of 24-hour periods in the PICU (<1 days ≤ 24 hours, 1 day = 24 to < 48 hours, etc.) For model building (below), LOS was truncated at ≤ 30 days to eliminate the effects of outliers and to be consistent with other publications;8,16,17,23 overall, 97.7% of patients had an actual LOS of 30 days or less. In some institutions, infants were admitted prior to cardiovascular interventions to “optimize” their pre-operative status. For these infants, we used their time of admission following the operation as the initiation of intensive care stay. This a priori, objective classification scheme has been published.18
Interventions included both surgery and interventional catheterization. Diagnosis was classified by system of primary dysfunction based on the reason for PICU admission; cardiovascular conditions were classified as congenital or acquired. Cardiac arrest included closed chest massage within 24 hours prior to hospitalization or after hospital admission, but prior to PICU admission. Severity of illness was characterized by physiological profiles using the PRISM score based on the first 4 hours of PICU care.24 Outcomes utilized in this analysis were hospital survival and death. The Functional Status Scale (FSS) was used to describe baseline (pre-illness) and hospital discharge functional status as good/mild dysfunction (FSS 6 – 9) and moderate to very severe dysfunction (FSS >9).25 New functional status morbidity was defined as a change of FSS from baseline to hospital discharge of ≥ 3.22 Therapies included mechanical ventilation, vasoactive agents, neuromuscular blockage, antibiotics, steroids, renal replacement therapies, and extra-corporeal membrane oxygenation (ECMO).
Model
Statistical analyses utilized SAS 9.4® for descriptive statistics, model development, and fit assessment. The statistical analysis was conducted under the direction of R.H. Patient characteristics were evaluated for univariate association with LOS using nonparametric approaches (Kruskal-Wallis test for binary or unordered categorical variables, and Jonckeheere-Terpstra test for ordered categorical variables; continuous factors were categorized into a modest number of clinically relevant levels for these assessments.)
The final model was constructed using a nonautomated (examined by biostatistician and clinician at each step) forward stepwise selection approach from the factors significantly associated with truncated, untransformed LOS in the univariate analyses. Survival or death at PICU discharge was included as a predictor. Several categorizations of age and baseline FSS were considered in the model, as was an alternative diagnostic categorization predictive of mortality in a previous report.24 The reported model includes variables, as described above, entered sequentially with an F-statistic of significance <0.05 at each step; this model also achieved optimal cross-validated performance in the sequence of candidate models as assessed by the predicted residual sum of squares criterion.26
Modifications of the above specific selection criteria, which were examined to assess robustness of the analyses, sometimes generated slightly different “final” models with very similar performances. We considered modeling log-transformed LOS as outcome, as well as using generalized linear models, and found that the predictive ability of these models for the original LOS outcome ranged from somewhat worse to only slightly better than standard regression on truncated LOS. Others have reported similar results.16 We therefore report results of the standard regression model, in part because unlike for other approaches, untransformed linear regression coefficients are directly interpretable as magnitude of change in LOS attributable to differences in a factor. Use of this untransformed model, whose residuals are not normally distributed, is acceptable for our aim of gauging whether mean PICU LOS appears to be higher or lower than expected at an aggregate, institution-wide level in this large dataset.27 Standard errors and significance tests reported for our model use heteroscedasticity-consistent covariance estimates robust to residual nonnormality.28
Results
Overall, the 10,078 patients stayed in the PICU for a total of 50,621 days. Supplemental Table 1 shows the distribution of the patient characteristics. In the total sample, 27.7% of patients were less than 1 year of age. Most patients had good pre-illness functional status (78.6%), were emergency admissions (63.6%), were medical patients not receiving a surgical or catheterization intervention (62.3%), and the most common primary systems of dysfunction were cardiorespiratory (57.6%), and neurological (20.1%). ICU therapies ranged from common to very uncommon including mechanical ventilation (38.1%), vasoactive agent infusions (23.7%), neuromuscular blockade (13.6%) and ECMO (1.1%). Most patients were cared for in medical-surgical ICUs (80.8%) while 19.2% were cared for in cardiac ICUs. A total of 53.8% of patients had government insurance. Outcomes included death (2.7%), new significant functional status morbidity (4.6%), and intact survival (92.7%).
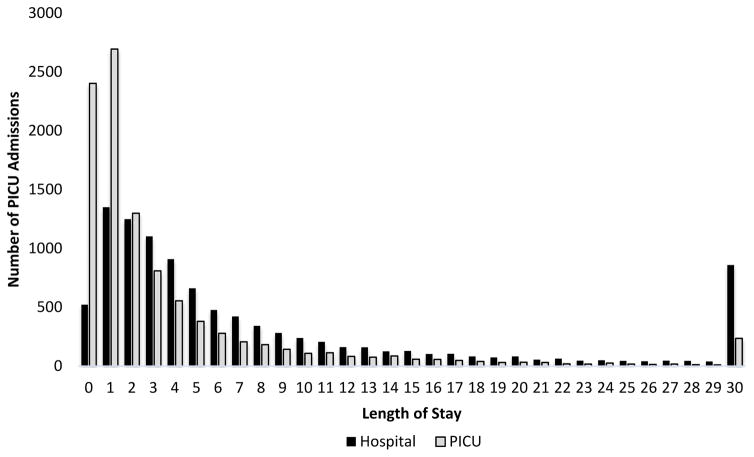
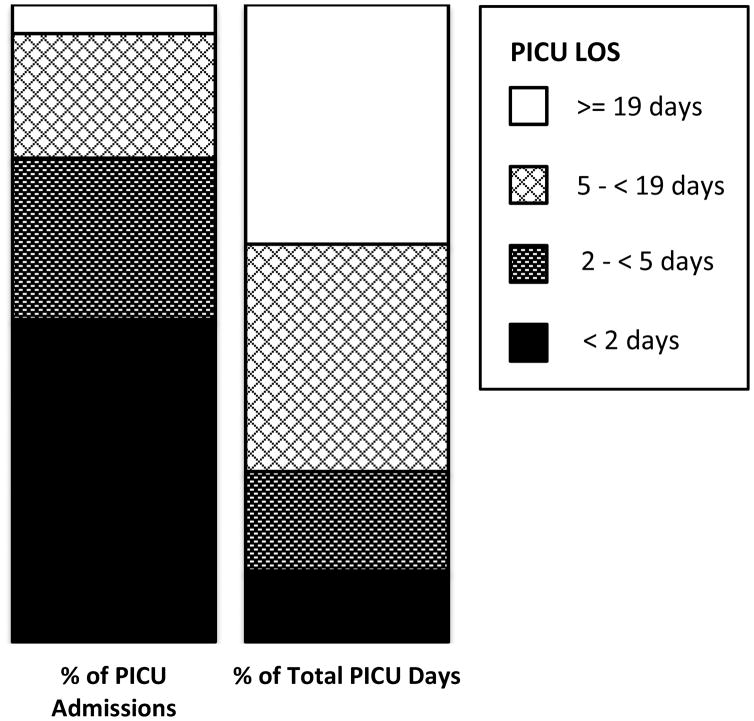
The mean LOS for all patients was 5.0 days (standard deviation 11.1), with a median length of 2.0 days. A total of 9842 patients (97.7%) had PICU stays of 30 days or less. Truncating LOS to a maximum of 30 days accounted for 43,918 PICU days (86.8% of the days of care); the truncated LOS variable had a mean of 4.4 days (standard deviation 6.1), and median of 2.0 days. Figure 1 illustrates the distributions of PICU LOS as well as LOS in the hospital. Of note, 50.6% of patients stayed in the PICU for less than 48 hours. Figure 2 illustrates that a large number of PICU days of care are used by a relatively small proportion of the population. For example, the 50.6% of patient with LOS fewer than 2 days consumed only 11.1% of the days of care, while the 19.6% with LOS 4.9 to 19 days and the 4.6% with LOS of 19 days or longer consumed 35.7% and 37.6% of the days of care respectively.
Figure 1.
PICU and Hospital Length of Stay Distributions. PICU lengths of stay are skewed to short stays compared to hospital stays.
Figure 2.
Utilization of PICUs by Percent of Admissions and Percent of Total PICU Days. Four ranges are displayed for approximately the shortest 50%, next 25%, next 20% and longest 5% LOS admissions. The corresponding percentage of days of PICU care used by these groups are indicated by identical fill patterns. Half of the study population (50.6%) with the shortest PICU LOS (less than 2 days) consumed only 11.1% of the days of care. In contrast, the 19.6% of patients with LOS 4.9 to 19 days and the 4.6% with LOS of 19 days or longer consumed 35.7% and 37.6% of the days of care respectively.
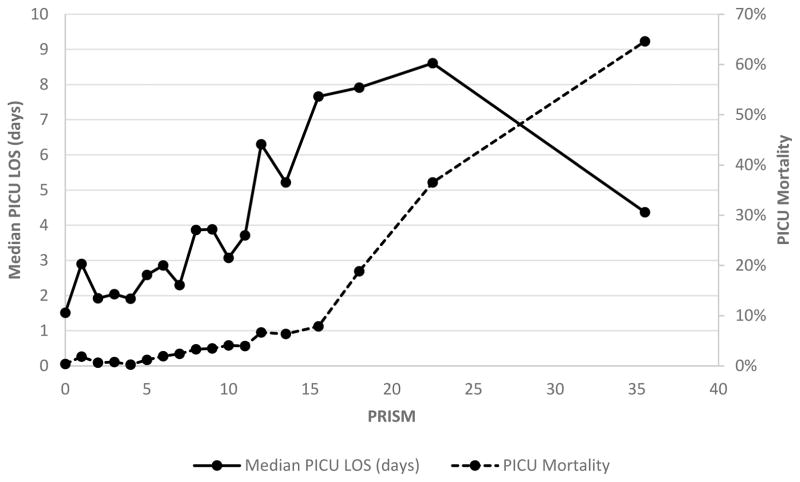
Supplemental Table 1 reports the mean and median LOS data by descriptive category, primary system of dysfunction, functional status on admission, severity of illness categories, for selected critical care therapies and for hospital outcome. Overall, there were significant LOS differences in all patient categories except elective/emergency admission status. In general, longer LOS was observed in younger children, those with cardiorespiratory disease, post-intervention cardiac patients, patients who had a cardiac arrest prior to admission, those with the highest severity of illness, and those receiving the most intensive therapies. Patients in the cardiac ICU stayed longer than those in the medical ICU (median 3.2 vs. 1.8, p<0.001), and patients discharged from the hospital with a new significant functional morbidity stayed longer than deaths or those discharged without a new significant functional status morbidity. As the PRISM score increased, median LOS increased in parallel with mortality risk until a PRISM score of 20–25 when median LOS decreased due to earlier mortality (Figure 3).
Figure 3.
Length of Stay and Mortality Risk Versus Severity of Illness (PRISM). The median pediatric intensive care unit (PICU) LOS (solid line) and PICU mortality (hashed line) are plotted relative to the Pediatric Risk of Mortality (PRISM) score. As the PRISM score increases, the median LOS increases in parallel with mortality risk until a PRISM score of 20–25 when median LOS decreases due to increasing deaths.
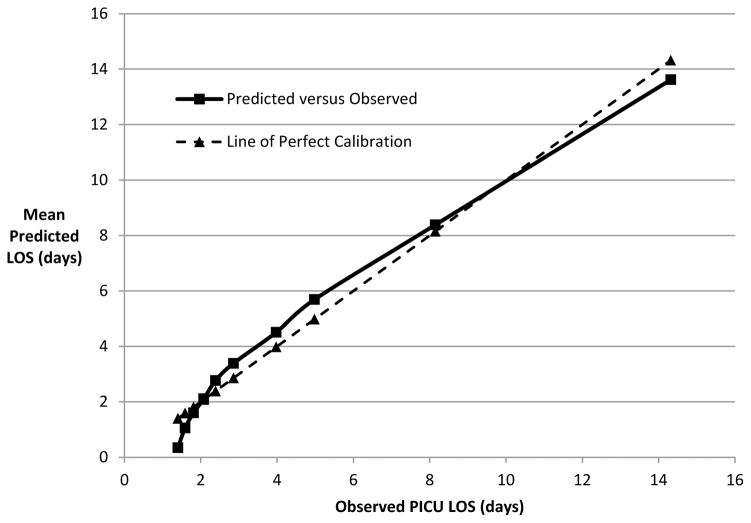
Table 1 reports the final model for LOS truncated at 30 days from data obtained during the entire PICU stay (Methods). All significant variables from Supplemental Table 1 were included except race which was missing in >20% of patients, and cardiac versus non-cardiac ICU type whose association was largely subsumed by diagnosis. Age, admission source, system of primary dysfunction, baseline functional status (dichotomized as normal/mild dysfunction versus worse), PRISM score, survival/death at PICU discharge, and the critical care therapies were included as predictors of LOS in the final regression model. The R-squared for LOS truncated at 30 days is 0.42, indicating that this model predicts truncated LOS moderately well overall.16 Notably, the model relies on therapies received during the PICU stay for a large amount of its performance. During model construction, the first four variables entered were therapies (neuromuscular blockade, vasoactive infusions, mechanical ventilation, and ECMO), and a model with these four therapies alone achieves an R-squared of 0.365. Modeling using only patient factors at admission and the admission PRISM score lead to substantially lower R-squared values around 0.16. Overall, the model generally over-predicted for short stays and under-predicted for longer stays (Figure 4).
Table 1.
Final Linear Regression Model for PICU Length of Stay.1 The model R-squared was 0.42.
| Predictor | Coefficient (SE3) | p-value for predictor (F test3) |
|---|---|---|
| Intercept | 1.28 (0.25) | <0.001 |
| Age at PICU Admission | <0.001 | |
| 0 day to < 14 days | 3.03 (0.35) | |
| 14 days to < 1 month | 2.39 (0.57) | |
| 1 month to < 12 months | 0.91 (0.13) | |
| >12 months | Reference | |
| Admission Source | <0.001 | |
| OR/PACU for post-intervention care after cardiac surgery | −3.08 (0.24) | |
| OR/PACU for post-intervention care after non-cardiac surgery | −0.57 (0.10) | |
| Non-post-intervention admission | Reference | |
| Primary System of Dysfunction | 0.001 | |
| Cancer | Reference | |
| Cardiovascular/respiratory | −0.59 (0.24) | |
| Low risk (endocrine, hematologic, musculoskeletal, renal) | −0.95 (0.26) | |
| Neurologic | −0.74 (0.25) | |
| Other | −0.48 (0.28) | |
| Baseline FSS Score Categorized as Moderate or Severe (2) | 1.28 (0.15) | <0.001 |
| PRISM III Total Score | 0.06 (0.02) | <0.001 |
| Died in PICU (vs. Discharged Alive) | −3.66 (0.71) | <0.001 |
| ECMO during PICU Stay (vs. No) | 7.15 (0.93) | <0.001 |
| Renal Replacement Therapy during PICU Stay (vs. No) | 2.53 (0.67) | <0.001 |
| Mechanical Ventilation during PICU Stay (vs. no) | 2.09 (0.13) | <0.001 |
| Vasoactive Infusions during PICU Stay (vs. No) | 3.27 (0.21) | <0.001 |
| Antibiotics during PICU Stay (vs. No) | 1.27 (0.09) | <0.001 |
| Neuromuscular Blockade during PICU Stay (vs. No) | 4.79 (0.27) | <0.001 |
| Steroids during PICU Stay (vs. No) | 0.97 (0.12) | <0.001 |
Length of stay in the model was truncated to a minimum of one hour and a maximum of 30 days.
Baseline (pre-illness) FSS score > 9.
Coefficient standard error estimates and F-tests were calculated using the heteroscedasticity-consistent approach of White as noted in text.
Figure 4.
Mean Observed Versus Predicted Length of Stay. Data are displayed by deciles of predicted LOS.
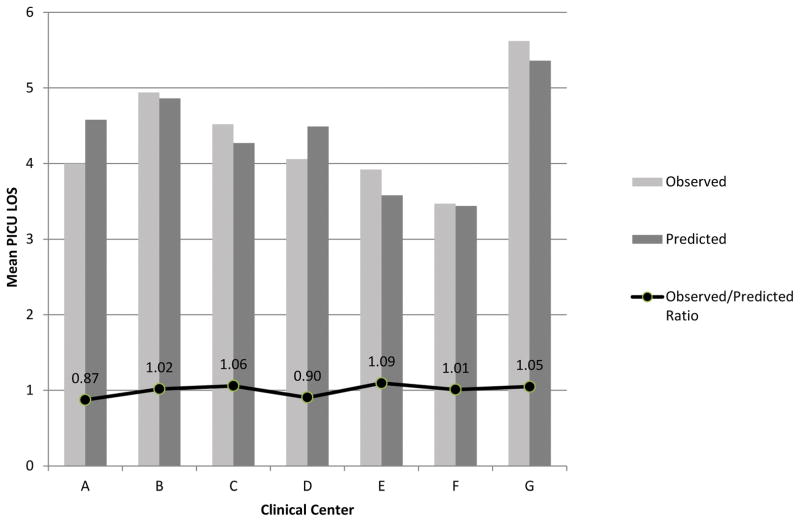
Figure 5 illustrates the LOS observed and predicted by the model when it is applied to all patients at each of the sites. The overall results are summarized as the standardized ratio of observed divided by predicted LOS (SLOSR). Among the sites, the overall the SLOSR ranged from 0.87 to 1.09. There are some centers (A, D) where mean truncated LOS is over predicted by 0.4–0.6 days, and others (C, E, G) where mean LOS is under predicted. These observed differences in model fit by center are statistically significant (p<0.0001 by F test) when center is added as a predictor to the model in Table 1.
Figure 5.
Observed and Predicted Length of Stay at the Participating Centers. The standardized length of stay ratio is the observed divided by the expected length of stay.
Discussion
The efficient utilization of critical care services has important practical implications. Inefficient use of critical care beds, if it limits bed or staff availability, may result in delays in care, patient care in suboptimal care areas, or patients diverted to other facilities with the associated risks of transport and delayed therapies. Reliable methods that assess institutional bed utilization could prevent or ameliorate these suboptimal care issues if they improve efficiency. In addition, better utilization of critical care beds could improve the economics of critical care units if it reduces labor needs, prevents the allocation of capital to new bed construction, and/or improves the utilization of beds by those waiting for critical care services.29
We assessed PICU bed utilization in over 10,000 patients utilizing over 50,000 days of PICU care. The mean patient LOS was 5.0 days (standard deviation 11.1), with a median LOS of 2.0 days. In this large dataset, LOS was significantly associated with essentially all patient characteristics. It displayed an association with physiological status previously found in both pediatric and adult patients where LOS increases with physiological instability until patient deaths decreases the LOS.9,23 Notably, while most patients stayed a relatively short time in the PICU, PICU bed utilization was dominated by a minority of patients. The 5% of patients staying the longest utilized almost 40% of the bed days. The patients with the highest 25% LOS utilized approximately 75% of the PICU bed days.
Our regression model for LOS utilized age, admission source, primary system of dysfunction, baseline (pre-illness) FSS, the PRISM score based on the first 4 hours of PICU care, outcome (survival or death) and seven therapies as predictors. For the outcome LOS truncated at 30 days, the model R-squared of 0.42, indicates reasonable model performance, and compares favorably to other LOS models. Our model’s performance is comparable to that reported in a Finnish study in adult ICUs that also used ICU survival and treatment intensity throughout the ICU stay as independent variables.14 The three factors with the largest influence in long ICU stay were all therapies associated with prolonged stay including ECMO, neuromuscular blockade, and vasoactive infusions. These therapies are associated with severe but often treatable cardiopulmonary disease and the need for sophisticated life support methods. Notably, when therapies were included in the modeling, they became the dominant factors with only 4 therapies (neuromuscular blockade, vasoactive infusions, mechanical ventilation, and ECMO) achieving an R-squared of 0.365. The factor with the greatest influence on reducing LOS was death. Physiological status (PRISM) was a significant but relatively weak factor in this model, probably because other measures of severity of illness such as therapies and outcome were included in the model. Overall, the model worked well with SLOSR ranging from 0.89 to 1.09. The model performance indicates its potential widespread applicability for internal and external benchmarking.
Comparison of critical care outcomes has been unsatisfactory when the outcomes have not been adjusted for patient characteristics unless these characteristics remain constant, an uncommon event even in single units. Therefore, statistical models generating benchmarks that are case-mix or risk-adjusted are important in assessing comparative performance. In pediatrics, this has been successful for one critical care outcome – mortality,24 and has been suggested for another outcome – morbidity.18 However, these approaches have had limited success in assessing LOS.9 To improve model performance to a level that has applicability to individual units, we made several important decisions. First, we truncated the LOS data to 30 days or less to eliminate outliers. This included 97.7% of the patients but only 86.8% of the days of care. Exclusion of outliers has been a common decision in benchmarking and is consistent with federal programs that have special reimbursement structures for outliers.30 Second, we elected to include information from the entire ICU stay including outcomes, selected therapies, physiological profiles, and patient characteristics to improve model performance. While this decision did result in improved model performance, it also limits the model to internal and external benchmarking as it eliminates real-time management of ICU resources. Use in real-time ICU management may require a new analytic method or a different conceptual approach.31
Our reported model was primarily designed for assessing LOS for at least modestly large institution-wide cohorts. While the model’s overall performance is equivalent or better than previous efforts, its performance will not be as good in subgroups, especially those with longer LOS and we do not recommend it for evaluation of individual patients. Since the model is dependent on data accumulated during the entire PICU stay, interpreting clinical factors must be done in the context of the therapeutic variables. Importantly, deviations from predicted may be controlled by factors beyond the ICU control such as bed availability or institutional practice patterns. Despite these issues, we believe our model has potential utility in assessing LOS utilization for pediatric ICUs and their institutions.
Institutional factors not assessed in our study are a likely cause of much of the inter-institutional variability in LOS and the limitations of statistical models predicting LOS. Institutional practice patterns and factors such as the ability of institutions to care for patients in less intense environments, nurse: patient ratios, practice patterns of physician groups, the availability of intermediate care, open vs. closed units, clinical protocols and pathways, end-of-life practices, as well as other factors will significantly influence when and for how long patients are cared for in ICUs.32 These are some of the important factors potentially impacting bed utilization that simultaneously support the need for LOS benchmarking while making generalizeable models difficult. Models may need to be limited to internal benchmarking due to inter-institutional variability.
The length of hospital stay for pediatric patients has been relatively constant over the last three decades even though the average national hospital LOS has been reduced by almost 50%.33 Reductions in LOS for adults have been most marked in patients over 65 years.33 Our challenge in pediatrics will be to participate in these needed national efforts to improve efficiency and utilization while maintaining and improving quality of care.
Conclusion
PICU bed utilization was dominated by a minority of patients. The 5% of patients staying the longest utilized almost 40% of the bed days. Longer LOS was observed in younger children, those with cardiorespiratory disease, post-intervention cardiac patients, and those who were sicker receiving more intensive therapies. Patients in the cardiac ICU stayed longer than those in the medical ICU. The multivariate LOS model used descriptive, diagnostic, therapeutic and severity factors and has potential applicability for benchmarking.
Supplementary Material
Acknowledgments
Funding Source: Supported, in part, by the following cooperative agreements from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services: U10HD050096, U10HD049981, U10HD049983, U10HD050012, U10HD063108, U10HD063106, U10HD063114 and U01HD049934. This content is solely the responsibility of the authors and does not necessarily represent the views of the National Institutes of Health.
Abbreviations
- ICU
Intensive Care Unit
- PICU
Pediatric Intensive Care Unit
- LOS
Length of Stay
- SLOS
Standardized Length of Stay Ratio
- TOPICC
Trichotomous Outcome Prediction in Critical Care
- FSS
Functional Status Scale
- PRISM
Pediatric Risk of Mortality
- SD
Standard Deviation
- CPCCRN
Collaborative Pediatric Critical Care Research Network
- ECMO
Extra-Corporeal Membrane Oxygenation
Individuals Acknowledged and Roles
Teresa Liu, MPH, CCRP; University of Utah (project management, Data Coordinating Center)
Jean Reardon, MA, BSN, RN; Children’s National Medical Center (institutional project management, data collection)
Elyse Tomanio, BSN, RN; Children’s National Medical Center (institutional project management, data collection)
Morella Menicucci, MD, CCRP; Children’s National Medical Center (data collection)
Fidel Ramos, BA; Children’s National Medical Center (institutional project management, data collection)
Aimee Labell, MS, RN; Phoenix Children’s Hospital (institutional project management, data collection)
Courtney Bliss, BS, DTR; Phoenix Children’s Hospital (data collection)
Jeffrey Terry, MBA; Children’s Hospital Los Angeles (data collection)
Margaret Villa, RN; Children’s Hospital Los Angeles and Mattel Children’s Hospital UCLA (institutional project management, data collection)
Jeni Kwok, JD; Children’s Hospital Los Angeles and Mattel Children’s Hospital (institutional project management, data collection)
Amy Yamakawa, BS; Children’s Hospital Los Angeles and Mattel Children’s Hospital UCLA (data collection)
Ann Pawluszka, BSN, RN; Children’s Hospital of Michigan (institutional project management)
Symone Coleman, BS, MPH; Children’s Hospital of Michigan (data collection)
Melanie Lulic, BS; Children’s Hospital of Michigan (data collection)
Mary Ann DiLiberto, BS, RN, CCRC; Children’s Hospital of Philadelphia (institutional project management, data collection)
Carolann Twelves, BSN, RN; Children’s Hospital of Philadelphia (data collection)
Monica S. Weber, RN, BSN, CCRP; University of Michigan (institutional project management, data collection)
Thomas Shanley, MD; Department of Pediatrics, University of Michigan, Ann Arbor, MI
Lauren Conlin, BSN, RN, CCRP; University of Michigan (data collection)
Alan C. Abraham, BA, CCRC; Children’s Hospital of Pittsburgh of University of Pittsburgh Medical Center (institutional project management, data collection)
Jennifer Jones, RN; Children’s Hospital of Pittsburgh of University of Pittsburgh Medical Center (data collection)
Jeri Burr, MS, RN-BC, CCRC; University of Utah (project management, Data Coordinating Center)
Nichol Nunn, BS, MBA; University of Utah (project management, Data Coordinating Center)
Alecia Peterson, BS, CMC; University of Utah (project management, Data Coordinating Center)
Carol Nicholson, MD (former Project Officer, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, for part of the study period)
Copyright form disclosure
All authors received support for article research from the National Institutes of Health (NIH). Drs. Pollack, Dean, Meert, Newth, Berger, Harrison, and Wessel’s institutions received funding from the NIH. Drs. Holubkov, Reeder, Berg, Carcillo, and Dalton’s institutions received funding from the National Institute of Child Health and Human Development. Dr. Holubkov received funding from DSMB memberships for Pfizer, Medimmune, and Armaron, and he disclosed he was a biostatistical consultant for St Jude Medical (past), is on the Physicians Committee for Responsible Medicine (current), and was a DSMB Member for American Burn Association (past). Dr. Newth received funding from Philips Research North America. Dr. Dalton received funding from Innovate ECMO Concepts Inc (consultant) and Maquet Speakers Bureau. Drs. Jenkins and Tamburro disclosed government work. Dr. Jenkins’ disclosed that she completed this manuscript as a federal employee at the NIH. Dr. Tamburro’s institution received funding from Ony, Inc. and the US Food and Drug Administration Office of Orphan Product Development, and he received funding from Springer Publishing.
Footnotes
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Conflicts of Interest Statement: The authors have no conflicts of interest relevant to this article to disclose.
Clinical Trials Registration: Not Applicable
Contributors’ Statements
Dr. Pollack participated in conceptualization, design, data acquisition, and data analysis.
Drs. Holubkov, Reeder and Dean participated in conceptualization, design and data analysis.
Drs. Meert, Berg, Newth, Berger, Harrison, Carcillo, Dalton and Wessel participated in conceptualization, design, and data acquisition.
Ms. Jenkins participated in conceptualization and design.
Dr. Tamburro participated in data analysis.
All authors participated in data interpretation and drafting/revision.
All authors have given final approval and have agreed to be accountable for all aspects of the work.
Contributor Information
Murray M. Pollack, Department of Pediatrics, Children’s National Health System and the George Washington University School of Medicine and Health Sciences, Washington DC.
Richard Holubkov, Department of Pediatrics, University of Utah School of Medicine, Salt Lake City, UT.
Ron Reeder, Department of Pediatrics, University of Utah School of Medicine, Salt Lake City, UT.
J. Michael Dean, Department of Pediatrics, University of Utah School of Medicine, Salt Lake City, UT.
Kathleen L. Meert, Department of Pediatrics, Children’s Hospital of Michigan, Detroit, MI.
Robert A. Berg, Department of Pediatrics, Children’s Hospital of Philadelphia, Philadelphia, PA.
Christopher J. L. Newth, Department of Anesthesiology and Critical Care Medicine, Children’s Hospital Los Angeles, University of Southern California Keck School of Medicine, Los Angeles, CA.
John T. Berger, Department of Pediatrics, Children’s National Medical Center, Washington DC.
Rick E. Harrison, Department of Pediatrics, University of California at Los Angeles, Los Angeles, CA.
Joseph Carcillo, Department of Critical Care Medicine, Children’s Hospital of Pittsburgh, Pittsburgh, PA.
Heidi Dalton, Department of Child Health, Phoenix Children’s Hospital and University of Arizona College of Medicine-Phoenix, Phoenix, AZ (currently INOVA Fairfax Medical Center and George Washington University).
David L. Wessel, Department of Pediatrics, Children’s National Medical Center, Washington DC.
Tammara L. Jenkins, Pediatric Trauma and Critical Illness Branch, Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Institutes of Health (NIH), Bethesda, MD.
Robert Tamburro, Pediatric Trauma and Critical Illness Branch, Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Institutes of Health (NIH), Bethesda, MD.
References
- 1.Breslow MJ, Badawi O. Severity scoring in the critically ill: part 2: maximizing value from outcome prediction scoring systems. Chest. 2012 Feb;141(2):518–527. doi: 10.1378/chest.11-0331. [DOI] [PubMed] [Google Scholar]
- 2.Gemke RJ, Bonsel GJ. Comparative assessment of pediatric intensive care: a national multicenter study. Pediatric Intensive Care Assessment of Outcome (PICASSO) Study Group. Crit Care Med. 1995 Feb;23(2):238–245. doi: 10.1097/00003246-199502000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Barrett MLSM, Elixhauser A, Honigman LS, Pines JM. Utilization of Intensive Care Services, 2011. Statistical Brief #185. Agency for Healthcare Research and Quality; 2014. [PubMed] [Google Scholar]
- 4.Ahmed S, Manaf NH, Islam R. Effects of Lean Six Sigma application in healthcare services: a literature review. Reviews on environmental health. 2013;28(4):189–194. doi: 10.1515/reveh-2013-0015. [DOI] [PubMed] [Google Scholar]
- 5.Zander K. A 30-Year Retrospective: Degrees of Difficulty in Decreasing LOS. Professional case management. 2016 Sep-Oct;21(5):233–242. doi: 10.1097/NCM.0000000000000164. [DOI] [PubMed] [Google Scholar]
- 6.Pollack MM, Alexander SR, Clarke N, Ruttimann UE, Tesselaar HM, Bachulis AC. Improved outcomes from tertiary center pediatric intensive care: a statewide comparison of tertiary and nontertiary care facilities. Critical Care Medicine. 1991 Feb;19(2):150–159. doi: 10.1097/00003246-199102000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Pollack MM, Cuerdon TT, Patel KM, Ruttimann UE, Getson PR, Levetown M. Impact of quality-of-care factors on pediatric intensive care unit mortality. Jama. 1994 Sep 28;272(12):941–946. [PubMed] [Google Scholar]
- 8.Kramer AA, Zimmerman JE. The relationship between hospital and intensive care unit length of stay. Crit Care Med. 2011 May;39(5):1015–1022. doi: 10.1097/CCM.0b013e31820eabab. [DOI] [PubMed] [Google Scholar]
- 9.Ruttimann UE, Patel KM, Pollack MM. Length of stay and efficiency in pediatric intensive care units. Journal of Pediatrics. 1998 Jul;133(1):79–85. doi: 10.1016/s0022-3476(98)70182-9. [DOI] [PubMed] [Google Scholar]
- 10.Ruttimann UE, Pollack MM. Variability in duration of stay in pediatric intensive care units: a multiinstitutional study. Journal of Pediatrics. 1996 Jan;128(1):35–44. doi: 10.1016/s0022-3476(96)70425-0. [DOI] [PubMed] [Google Scholar]
- 11.Ruttimann UE, Patel KM, Pollack MM. Length of stay and efficiency in pediatric intensive care units. The Journal of pediatrics. 1998 Jul;133(1):79–85. doi: 10.1016/s0022-3476(98)70182-9. [DOI] [PubMed] [Google Scholar]
- 12.Ruttimann UE, Patel KM, Pollack MM. Relevance of diagnostic diversity and patient volumes for quality and length of stay in pediatric intensive care units. Pediatr Crit Care Med. 2000 Oct;1(2):133–139. doi: 10.1097/00130478-200010000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Straney L, Clements A, Alexander J, Slater A, Group APS. Quantifying variation of paediatric length of stay among intensive care units in Australia and New Zealand. Qual Saf Health Care. 2010 Dec;19(6):e5. doi: 10.1136/qshc.2008.028811. [DOI] [PubMed] [Google Scholar]
- 14.Niskanen M, Reinikainen M, Pettila V. Case-mix-adjusted length of stay and mortality in 23 Finnish ICUs. Intensive Care Med. 2009 Jun;35(6):1060–1067. doi: 10.1007/s00134-008-1377-0. [DOI] [PubMed] [Google Scholar]
- 15.Suistomaa M, Niskanen M, Kari A, Hynynen M, Takala J. Customized prediction models based on APACHE II and SAPS II scores in patients with prolonged length of stay in the ICU. Intensive Care Med. 2002 Apr;28(4):479–485. doi: 10.1007/s00134-002-1214-9. [DOI] [PubMed] [Google Scholar]
- 16.Verburg IW, de Keizer NF, de Jonge E, Peek N. Comparison of regression methods for modeling intensive care length of stay. PloS one. 2014;9(10):e109684. doi: 10.1371/journal.pone.0109684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vasilevskis EE, Kuzniewicz MW, Cason BA, et al. Mortality probability model III and simplified acute physiology score II: assessing their value in predicting length of stay and comparison to APACHE IV. Chest. 2009 Jul;136(1):89–101. doi: 10.1378/chest.08-2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pollack MM, Holubkov R, Funai T, et al. Simultaneous Prediction of New Morbidity, Mortality, and Survival Without New Morbidity From Pediatric Intensive Care: A New Paradigm for Outcomes Assessment. Crit Care Med. 2015 Aug;43(8):1699–1709. doi: 10.1097/CCM.0000000000001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pollack MM, Dean JM, Butler J, et al. The ideal time interval for critical care severity-of-illness assessment. Pediatr Crit Care Med. 2013 Jun;14(5):448–453. doi: 10.1097/PCC.0b013e31828a7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pollack MM, Holubkov R, Funai T, et al. Relationship between the functional status scale and the pediatric overall performance category and pediatric cerebral performance category scales. JAMA Pediatrics. 2014 Jul;168(7):671–676. doi: 10.1001/jamapediatrics.2013.5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pollack MM, Holubkov R, Funai T, et al. Simultaneous Prediction of New Morbidity, Mortality, and Survival Without New Morbidity From Pediatric Intensive Care: A New Paradigm for Outcomes Assessment. Crit Care Med. 2015 Aug;43(8):1699–1709. doi: 10.1097/CCM.0000000000001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pollack MM, Holubkov R, Funai T, et al. Pediatric Intensive Care Outcomes: Development of New Morbidities During Pediatric Critical Care. Pediatr Crit Care Med. 2014;15(9):821–827. doi: 10.1097/PCC.0000000000000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zimmerman JE, Kramer AA, McNair DS, Malila FM, Shaffer VL. Intensive care unit length of stay: Benchmarking based on Acute Physiology and Chronic Health Evaluation (APACHE) IV. Crit Care Med. 2006 Oct;34(10):2517–2529. doi: 10.1097/01.CCM.0000240233.01711.D9. [DOI] [PubMed] [Google Scholar]
- 24.Pollack MM, Holubkov R, Funai T, et al. The Pediatric Risk of Mortality Score: Update 2015. Pediatr Crit Care Med. 2016 Jan;17(1):2–9. doi: 10.1097/PCC.0000000000000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pollack MM, Holubkov R, Glass P, et al. Functional Status Scale: new pediatric outcome measure. Pediatrics. 2009 Jul;124(1):e18–28. doi: 10.1542/peds.2008-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allen DM. The Relationship Between Variable Selection and Data Augmentation and a Method for Prediction. Technometics. 1974;16(125–127) [Google Scholar]
- 27.Lumley T, Diehr P, Emerson S, Chen L. The importance of the normality assumption in large public health data sets. Annu Rev Public Health. 2002;23:151–169. doi: 10.1146/annurev.publhealth.23.100901.140546. [DOI] [PubMed] [Google Scholar]
- 28.White H. Heteroskedasticity-Consistent Covariance Matrix Estimator and a Direct Test for Heteroskedasticity. Econometrics. 1980;48:817–838. [Google Scholar]
- 29.Weeks WB, Resar R. Does reducing length of stay make a business case? Jt Comm J Qual Patient Saf. 2008 Nov;34(11):627–628. doi: 10.1016/s1553-7250(08)34079-3. [DOI] [PubMed] [Google Scholar]
- 30.Silberstein GS, Paulson AS. Summary of prospective quantification of reimbursement recovery from inpatient acute care outliers. Journal of health care finance. 2011 Fall;38(1):83–98. [PubMed] [Google Scholar]
- 31.Levin SR, Harley ET, Fackler JC, et al. Real-time forecasting of pediatric intensive care unit length of stay using computerized provider orders. Crit Care Med. 2012 Nov;40(11):3058–3064. doi: 10.1097/CCM.0b013e31825bc399. [DOI] [PubMed] [Google Scholar]
- 32.Gruenberg DA, Shelton W, Rose SL, Rutter AE, Socaris S, McGee G. Factors influencing length of stay in the intensive care unit. American journal of critical care : an official publication, American Association of Critical-Care Nurses. 2006 Sep;15(5):502–509. [PubMed] [Google Scholar]
- 33.DeFrances CJ, Hall MJ. 2005 National Hospital Discharge Survey. Advance data. 2007 Jul 12;(385):1–19. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.