Abstract
Biofilms are microbial communities embedded within an extracellular matrix, forming a highly organized structure that causes many human infections. Dental caries (tooth-decay) is a polymicrobial biofilm disease driven by the diet and microbiota-matrix interactions that occur on a solid surface. Sugars fuel the emergence of pathogens, the assembly of the matrix, and the acidification of the biofilm microenvironment, promoting ecological changes and concerted multispecies efforts that are conducive for acid damage of the mineralized tooth tissue. Here, we discuss recent advances on the role of the biofilm matrix and interactions between opportunistic pathogens and commensals in the pathogenesis of dental caries. In addition, we highlight the importance of matrix-producing organisms in fostering a pathogenic habitat where inter-species competition and synergies occur to drive the disease process, which could have implications to other infections associated with polymicrobial biofilms
Oral Biofilms: More than Just the Sum of its Bacterial Parts
Many infectious diseases are caused or exacerbated by biofilms [1, 2]. Oral infectious diseases are prime examples of the consequences of dynamic interactions between microorganisms, their host and the host’s diet, leading to microbial colonization of oral surfaces and the establishment of pathogenic biofilms (or dental plaque) [3]. Biofilms are defined as structured communities of microorganisms that are attached to a surface and enmeshed in an extracellular polymeric matrix [1, 4]. Advances in DNA- and RNA-sequencing technologies are revealing important information about the diversity in composition, genome content and behaviors of the biofilm microbiota at different oral sites (Box 1). In parallel, the knowledge about the biological impacts of the extracellular matrix in governing cell-cell interactions, creating microenvironments, and modifying the virulence of the biofilms continues to augment our understanding of the pathogenesis of infectious diseases [1, 5].
Box 1. The microbiomes and oral diseases: the need for mechanistic studies.
Defining the ‘microbiome’ in the oral cavity may be far more complex and challenging than initially envisioned, which in addition to many bacterial species (bacteriome) also harbors fungi (mycobiome), virus (virome) and even ultrasmall organisms (candidate phyla radiation group) [69]. The total surface of the adult mouth is ~215cm2 of which 20% is comprised of tooth surfaces, 50% keratinized and 30% non-keratinized epithelium. The surface topography, including different areas of teeth, fissures in the tongue and gingival crevices, provide unique retention sites for microbes, which are continuously bathed by saliva. Microbiome-profiling have revealed a remarkable variety of species in different oral biofilm communities, allowing researchers to make associations between microbial composition and health status. Yet, it remains uncertain whether these ‘microbiome snap-shots’ demonstrate ‘causation or correlation’ with disease or health. Sometimes, a particular bacterial group or distinctive species may merely be bystanders or opportunistic colonizers with little or no contribution to the onset or severity of disease. In-depth mechanistic studies are required to precisely define the role of microbial components in the pathological process by omitting specific taxa from reconstituted communities, or by assessing the pathogenic potential of specific interacting species using in vivo models. For example, robust molecular and in vivo studies have helped to establish periodontitis as an exemplar of polymicrobial synergy and dysbiosis (PSD) on the basis of animal model mechanistic studies and human periodontal metagenomics/metatranscriptomic data [16]. A similar PSD concept, sometimes overlapping with ecological models, has been proposed to explain the etiopathogenesis of dental caries, where microbial community members might play roles comparable to those determined in periodontitis (keystones, accessory pathogens/pathobionts). This hypothesis certainly merits rigorous investigation as research in this direction has thus far been at best descriptive lacking mechanistic understanding. However, there are indeed fundamental differences. The diet (sugars) is a major driving force for the development of cariogenic biofilm communities. The biofilm also harbors abundant extracellular polysaccharides that enmesh the microorganisms, forming a diffusion-modifying matrix that profoundly changes the chemical and physical microenvironment on a non-shedding surface (instead of soft-tissues with localized immune responses). These distinct factors raise the question whether caries can be characterized using the PSD/keystone model. Nevertheless, the available data point towards the assembly of a localized ‘pathogenic habitat’ via matrix production, where polymicrobial interactions between pathogens and commensals occur within highly structured biofilms to modulate the disease process. As much as departing from reductionism to holism created great strides to understand the complexity of oral microbiomes, a return to a reductionist approach will be essential to understand the pathogenic mechanisms, as well as to validate the emerging concepts in longitudinal clinical studies.
There are many factors that affect the composition of the microbiota found on various surfaces of the mouth, especially when teeth begin to erupt, providing novel, non-shedding surfaces for colonization by commensals and opportunistic pathogens. These include, but are not limited to, age, diet, oral hygiene, systemic and immune conditions, and the use of certain medications that induce for example hyposalivation. The critical role that diet plays in microbial colonization is well-illustrated in patients or experimental animals [6, 7]. When hosts are over-exposed to dietary sugars, the structure and composition of biofilms formed on teeth changes significantly and the residing microbial communities become highly fit to metabolize carbohydrates and produce acids leading to dental caries (see Glossary) [8, 9]. Sequencing of ancient dental plaque provides additional evidence that shifts in oral microbiota are associated with dietary changes [10]. For example, Streptococcus mutans (a cariogenic bacterium) was not detected in plaque samples from Neolithic or Mesolithic fossils [10]. However, only when humans adopted agriculture with a sugar-rich diet, did S. mutans begin to appear in the fossil record and became more prevalent as refined sugars in the diet continuously increased, as did the incidence of dental caries [10].
Although early studies focused on microbial composition of biofilms, it is now clear that microorganisms residing within biofilms are embedded in a matrix containing extracellular polymeric substances (EPS). The importance of the matrix in the collective microbial behavior and virulence, as well as for tolerance of antimicrobials, is being increasingly recognized and considered integral to the biofilm lifestyle [1, 2, 4]. EPS production directly mediates microbial adherence to a surface and cell-to-cell adhesion, while forming a polymeric matrix that enhances mechanical stability of biofilms. Furthermore, the diffusion-modifying properties of EPS matrix cause chemical/nutrient gradients to form, thereby creating microenvironments within biofilms that can vary widely from other sites in key environmental inputs known to affect microbial behaviors, including pH, redox, and nutrient availability. Thus, the matrix allows the cells to organize into cohesive multicellular ecosystems where cooperative and antagonistic interactions occur within a heterogeneous chemical and physical milieu [1], helping to create localized niches with differing pathogenic potentials.
As the science of microbiomics and matrix biology evolved, it became clear that polymicrobial interactions and the local biofilm microenvironment play instrumental roles in modulating health and disease conditions [11–13]. Conversely, decades of research have clearly demonstrated that pathogens have developed an arsenal of mechanisms to enhance the virulence potential of the biofilm [14–16]. Changes experienced by the host (e.g. increased consumption of sugars or alterations in immune responses) can trigger pathogens to reshape the local microenvironment and the microbial community. Still, little is known about how pathogens modify the spatial-temporal organization and communal behavior to create localized pathological niches. Furthermore, how commensals and pathogens co-exist and battle with one another within a biofilm matrix to mediate the disease process remains poorly defined. Here, we review and provide new perspectives about the role of pathogens based on current knowledge of matrix biology and the data accumulated from microbiome studies using dental caries as an exemplar. Recognizing that opportunistic pathogens evolved in intimate association with not only the human host and resident microbiota, but also with a constantly changing diet, we focus on their collective impact on the plaque microbiota and its virulence potential. We hope this article will stimulate new dialogue, hypotheses and approaches that establish a more robust framework to understand and resolve a persistent and costly oral disease, while concurrently providing new insights to other polymicrobial biofilm-associated infections.
The Pathogens and Dietary Sugars: Modulating Biofilm Virulence
Tooth surfaces are coated with a proteinaceous film known as acquired enamel pellicle, which is derived from host and microbial sources, such as salivary proteins and bacterial exoenzymes. Within the oral microbiome, a small group of bacteria (such as streptococci and Actinomyces spp.) are known to adhere to pellicle-coated surfaces via combinations of highly specific adhesin-receptor interactions augmented by hydrophobic or electrostatic forces, followed by co-adhesion events that drive microbial colonization and biofilm initiation on teeth [17, 18]. During this process the various bacterial species interact physically and metabolically to shape the initial biofilm community structure. Certain microbial interactions are beneficial as they interfere with caries pathogens, whereas others can function in synergy with cariogenic bacteria to accelerate the disease progression. The dynamic balance between commensals and opportunistic pathogens can be disrupted by frequent sugar consumption and poor oral care, which promotes the development of virulent biofilms in close proximity to the tooth surface.
Dental caries is a classic biofilm-induced disease that causes the destruction of the mineralized tooth tissue [8, 9, 13]. The microorganisms in the oral cavity are required, but not sufficient, to cause dental caries because the formation of cariogenic biofilms is dependent on the host diet [8, 9]. A diet rich in sugar fuels the assembly of EPS matrix and enhance accumulation of acidogenic and acid-tolerant microbiota, which can explain microscopic images of plaque-biofilms collected from caries active sites, revealing bacteria enmeshed in EPS (Fig 1). S. mutans, a member of the mutans streptococci (MS) group, has long been implicated with dental caries in humans. Extensive clinical, epidemiological and experimental animal studies have shown, conclusively, though not exclusively, that MS are strongly associated with the disease, especially in early childhood caries [19]. One of the primary adaptations that allow S. mutans to become such an efficient opportunistic pathogen within the oral microbiota resides with its exceptional capacity to utilize a wide variety of carbohydrates to produce EPS and acids, and to live a biofilm lifestyle, including stress resistance and bacterial competence mechanisms (Box 2). One sugar in particular, sucrose, the ordinary table sugar and the historical sweetener in cooking, is most cariogenic as the component hexoses (glucose and fructose) provide the building blocks of EPS and are efficiently fermented to produce acids. Sucrose is essential to the organism’s success as a pathogen because of the unique ability of S. mutans to convert sucrose to extracellular insoluble glucans that enhance bacterial adhesion-cohesion, and form the core of the EPS matrix [20]. S. mutans clearly does not act alone to cause dental caries [21]. Rather, it interacts with other organisms in a dynamic and concerted polymicrobial effort to assemble a cariogenic biofilm.
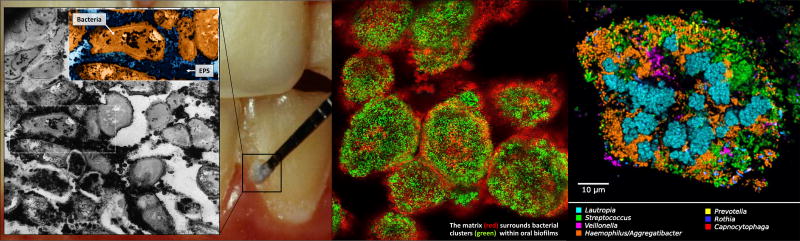
Figure 1. Dental plaque architecture: the EPS matrix, spatial organization and polymicrobial composition.
Plaque biofilm from caries-active subject (photo courtesy of Dr. Jaime A. Cury): microscopic image (inset) of plaque-biofilm showing a selected area containing bacterial cells (highlighted in orange) enmeshed in EPS (in dark blue); the image was pseudo-colored using ADOBE Photoshop software for visualization purposes (adapted from Hajishengallis et al., 2016 [19]). The middle panel shows bacterial clusters (green) surrounded by EPS matrix (red) detected in mature mixed-species oral biofilms formed in sucrose (adapted from Koo & Yamada, 2016 [5]). The right panel displays the spatial organization of human dental plaque showing multiple clusters of varying sizes containing different microbial species (adapted from Mark Welch et al., 2016 [31]).
Box 2. Streptococcus mutans biofilm lifestyle: an avid sugar consumer and EPS/acid producer.
Streptococcus mutans and dental caries became more prevalent after dietary shifts of the Neolithic and Industrial revolutions, which were characterized by the introduction of sugar-rich diets [10]. Co-evolution of S. mutans with the increased sugar consumption largely explains how well-adapted S. mutans is to colonize and thrive on teeth when human host ingests sugars. S. mutans has at least 7 enzymes that hydrolyze sucrose, some yielding polymers and free glucose or fructose, and one that cleaves the sucrose-6-phosphate generated by sucrose transporters. S. mutans secretes multiple exoenzymes, particularly glucosyltransferases (Gtfs), which are able to cleave sucrose to produce extracellular glucans (homopolymers of glucose) and free fructose. The glucans are key constitutes of the matrix in cariogenic biofilms, and associated with enhanced virulence in experimental animals and in humans. The Gtfs make a polymer that is adhesive, relatively water-insoluble and rich in (α1,3)linkages, with (α1,6)branching, which in turn are important for biofilm scaffolding and stability. While there are numerous oral microbial species, most of them are incapable of producing insoluble glucans until they are coated by secreted Gtfs. Interestingly, the ratio of 1,3-to-1,6 linkages increases by the action of secreted dextranases attacking the (α1,6)chains as glucans are produced [20]. However, no oral bacteria have yet been found that can hydrolyze the (α1,3)bonds, including S. mutans. Expression of Gtfs and production of glucan-matrix are dependent on environmental cues, including pH and carbohydrate source, involving signaling, such as the second messenger c-di-AMP and possibly unique small non-coding RNAs called microRNAs [70–72]. The discovery of the latter may provide new understanding of the matrix biology and regulation of biofilm formation. How S. mutans modulates this integrative regulatory network to respond to environmental challenges and coordinate expression of genes required for matrix synthesis remains poorly defined. Furthermore, all isolates of S. mutans harbor high-affinity, high-capacity transport systems for a variety of carbohydrates, which are primarily mediated by the sugar:phosphotransferase system (PTS). The PTS can rapidly internalize mono- and di-saccharides, including glucose, mannose, fructose among others, which can be further metabolized into acids. When sugar is present in excess, the cells produce mostly lactic acid via a lactate dehydrogenase. In low-carbohydrate conditions, a pyruvate-formate lyase funnels the pyruvate to acetate and formate, gaining an additional ATP, compared to lactate-pathway [73]. By efficiently utilizing sucrose, S. mutans can orchestrate EPS production to help bacteria accumulate and form a matrix. In turn, the adherent bacteria rapidly transport and efficiently metabolize different sugars to produce acids via concerted actions of transporters and catabolic enzymes. These properties (with acid-tolerance) allow S. mutans to build-up, interact and live in an acidified habitat that becomes a hallmark of cariogenic biofilms.
Over the years, the ecological plaque hypothesis, whereby microbial community shifts occur due to environmental acidification caused by sugar metabolism, has provided a logical and a tractable model for studying polymicrobial communities inhabiting the oral cavity [3]. However, an often forgotten question arises as to how localized acidification within biofilms and resultant caries on tooth surface occurs in the presence of powerful buffering saliva and the slightly alkaline environment of the human mouth. One explanation is that saliva cannot gain access to the acid produced in the depths of plaque formed on teeth, while ‘the fuel’ (dietary sugars) can readily diffuse throughout the biofilm [13, 22, 23]. The presence of an EPS matrix with diffusion-modifying properties plays an important role in creating the pathological (acidic) microenvironment adjacent to the tooth surface. Thus, dental caries, though undoubtedly a result of polymicrobial acidogenesis, can be better understood conceptually as a pathological process that relies not only on microbial composition and metabolic activity, but also on the milieu within which the organisms interact and acids accumulate. In this regard, S. mutans can play a key role in biofilm matrix assembly as the main producer of insoluble glucans among oral bacteria (Box 2), ‘resetting’ the microenvironment for other aciduric-cariogenic bacteria to thrive and become established, possibly at the expense of S. mutans itself, consistent with the variable levels of the bacterium in human plaque (<1% to 30%) as the disease progresses [21, 24, 25].
The EPS Matrix: Assembling a Complex Biochemical Microenvironment
Recent advances in the understanding of the EPS matrix biology have revealed a ‘multi-functional scaffold’ essential for the biofilm lifestyle [1, 13]. The structural and biochemical properties of the matrix provide the emergent properties of biofilms, including surface adhesion, spatial and chemical heterogeneities, synergistic/competitive interactions and increased tolerance to antimicrobials [1] (Fig 2). The formation of EPS matrix depends on substrate availability, the synthesis and secretion of extracellular materials, shear and other stresses. The major matrix components in oral biofilms associated with dental caries are polysaccharides, particularly S. mutans-derived glucans [20]. Furthermore, soluble glucans and fructans produced by other species (e.g. Actinomyces, S. salivarius and S. gordonii) and hybrid starch-glucans are also present. Like other matrices, cariogenic biofilms also appear to contain eDNA [26], bacterially-derived proteins that possess amyloid-like properties [27], as well as host proteins and glycoproteins, that can contribute to the matrix scaffold often in association with glucans (e.g. eDNA-glucan complexes) [28]. However, the function and structural organization of these other exopolymers in the matrix remain poorly understood and further studies are required to elucidate their role on the microbial composition and cariogenic potential of the biofilm. Glucans are comprised of glucose moieties linked primarily by α1,3 and α1,6 glycosidic bonds produced by a concerted action of streptococcal exoenzymes termed glucosyltransferases (Gtfs) [20, 29]. Intriguingly, Gtf enzymes released extracellularly can bind to tooth surface in active form producing glucans in situ that provide novel bacterial binding sites. Furthermore, secreted Gtfs also bind to other oral microbes (commensal streptococci, Actinomyces, lactobacilli, and even C. albicans), thereby converting them into glucan producers. The EPS glucans formed on surrogate surfaces enhances microbial accumulation on teeth, while forming new interspecies interactions and increasing cell-cell cohesion. Using direct incorporation of fluorescent probes during the synthesis of glucans by Gtfs, a detailed portrait of the spatio-temporal order of EPS-matrix assembly and its spatial arrangement with bacterial cells in mixed-species biofilms have emerged [30]. These extracellular polymers accumulate at a different location on the apatitic surface and on the cell membrane, each with complementary roles to form a nascent EPS matrix and coordinate biofilm development including: surface adherence, cell-cell adhesion and formation of cell clusters similar to microcolonies found in other biofilm systems [4]. As the biofilm matures, the continued EPS production in situ expands the matrix tri-dimensionally, encasing cells clusters, and creating a bridge from one to another forming a highly compartmentalized yet cohesive structure within a 3D matrix scaffold. Such spatial organization and heterogeneities shaped by EPS synthesis could explain the detection of different microbial clusters varying in size and composition found in human oral biofilms [31, 32] (Fig. 1).
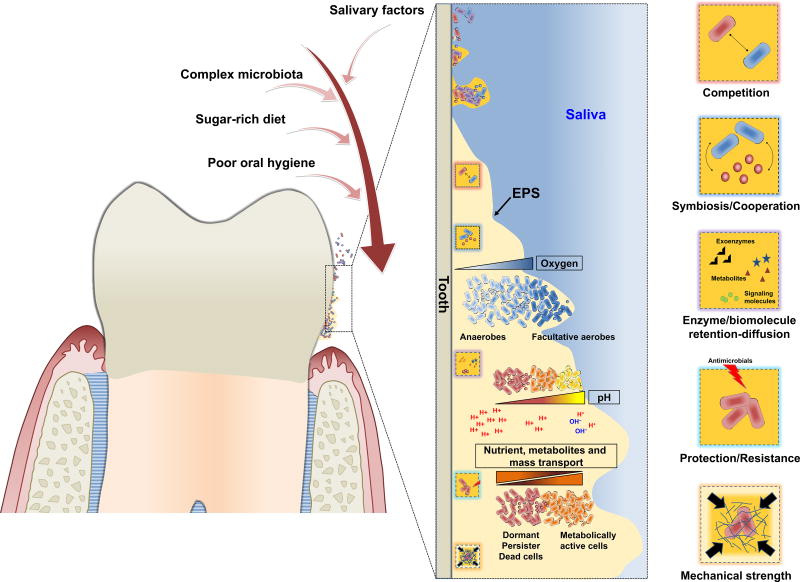
Figure 2. The biofilm properties: assembling a complex microenvironment.
In the oral cavity, a diet rich in sugar, particularly sucrose, provide substrate for the production of extracellular polysaccharides, which forms the core of the extracellular matrix in cariogenic biofilms. The matrix drastically changes the physical and biological properties of the biofilm. The exopolysaccharides enhance microbial adhesion-cohesion and accumulation on the tooth surface, while forming a polymeric matrix that embeds the cells. The matrix provides a multi-functional scaffold for structured organization and stability of the biofilm microbial community, where microorganisms co-exist and compete with each other. The diffusion-modifying properties of the matrix combined with the metabolic activities of embedded organisms help create a variety of chemical microenvironments, including localized gradients of oxygen and pH. Furthermore, the matrix can trap or sequester a diverse range of substances, including nutrients, metabolites and quorum sensing molecules. Similarly, enzymes can be retained and stabilized, transforming the matrix into a de facto external digestive system [1]. These properties provide the distinctive characteristics of the biofilm lifestyle, including mechanical stability, spatial and chemical heterogeneity and drug tolerance [1].
EPS deposition on surfaces and development into polymeric matrix also affect the mechanical properties of biofilms, such as increasing adhesive strength to surfaces and cohesiveness [33]. Well-established biofilms are mechanically difficult to remove from tooth surfaces and often display enhanced viscoelasticity, which can help them persist by partially yielding rather than detaching when subjected to fluid shear stresses. However, the EPS matrix can be constantly remodeled locally though the actions of dextranases, DNAses and proteolytic enzymes dynamically altering the mechanical properties and/or exposing new binding sites to additional microorganisms that were unavailable during biofilm initiation [20]. Indeed, matrix stiffness appears to increase as the biofilm matures. At later stages, mature biofilms can release small aggregates or even individual cells (a process termed dispersal), often through matrix degradation, to seed uncolonized sites and reinitiate the biofilm life cycle [34]. The physicochemical properties of the biofilm matrix can also provide protection to embedded bacteria by reducing drug access and triggering antimicrobial tolerance. For example, the EPS can bind cationic antimicrobials such as chlorhexidine and antimicrobial peptides preventing penetration into the deeper layers of the biofilm, and thereby reducing killing efficacy [30, 35].
The formation of chemical and nutritional ‘gradients’ within most biofilms involves a balance between the matrix acting as a physical barrier (sequestering molecules or affecting diffusion of substances in and out of the biofilms) and local microbial consumption/metabolism (Fig. 2). These processes can create numerous tailored biological niches with varying concentrations of pH, O2, inorganic ions, signaling molecules, metabolites and other solutes [1, 2, 4]. Thus, the positioning of microorganisms and their stress response mechanisms may correlate with their susceptibility to, or affinity for, low pH or hypoxic environments and availability of specific ligand, nutrients or biomolecules. Such heterogeneous milieu can modulate gene expression locally and influence the metabolic exchange and intercellular signaling among different species or between different clusters of cells distributed within the biofilm structure, orchestrating their communal ‘social behavior’, spatial organization and/or physiological heterogeneity [1, 2, 4, 31, 32] to develop pathogenic niches. Recent studies have shown that bacterial aggregates can create pH or O2 micro-gradients and induce transcriptomic changes in a subpopulation of cells within the biofilm [22, 36, 37].
In the context of dental caries, how and where acidic microenvironments are formed, maintained and protected within the 3D biofilm architecture may be the key determinant because the buffering saliva surrounding the tooth surfaces is capable of neutralizing acids produced in the mouth. The heterogeneous spatial distribution of pH across oral biofilm structure has been long appreciated [13]. Fluorescent pH probe directly immobilized into the biofilm matrix revealed a fascinating 3D pH distribution within intact biofilms despite exposure to neutral pH buffer [30]. Localized regions of low pH values (4.5–5.5) throughout the biofilm structure and at the biofilm-apatite interface suggest that the acids accumulated and confined in these specific areas are not readily neutralized. The EPS matrix has been shown to limit diffusion of charged ions in buffers, whereas uncharged solutes such as sucrose can diffuse into biofilms, which can be readily metabolized into acids by the embedded bacteria [22]. Furthermore, extracellular glucans appear to directly trap proton to help retain and accumulate acids within biofilms [23]. The biofilm matrix can also act as an external digestion system by immobilizing exoenzymes, allowing them to metabolize substrates in close proximity to cells while also participating in matrix remodeling. For instance, soluble fructans and glucans present in the matrix can be degraded by fructanase and dextranase, providing readily fermentable carbohydrates on-site and thereby extending the duration of the acid challenge [20]. Thus, EPS can modulate persistent acidification at the tooth interface by helping biofilms to adhere, spatially localize metabolites and possibly restrict access to buffering saliva, which help create a cariogenic microenvironment.
Biofilm Microbiota: Pathogens, Commensals and Interspecies Interactions
Commensals have a significant advantage over cariogenic pathogens when the host’s diet is not rich in fermentable carbohydrates, particularly sucrose. Many commensal bacteria associated with oral health can adhere more avidly to saliva-coated tooth surfaces, can grow substantially better than S. mutans and many other aciduric species, and have multiple mechanisms to interfere with their establishment and growth. However, the ‘microbial battlefield’ can change dramatically if sugar is supplied frequently promoting EPS matrix synthesis, acid production and the creation of localized acidic microenvironments, where S. mutans can work synergistically with other aciduric species and cause ecological changes to shape the biofilm community, structure and metabolism conducive to caries development (Fig 3).
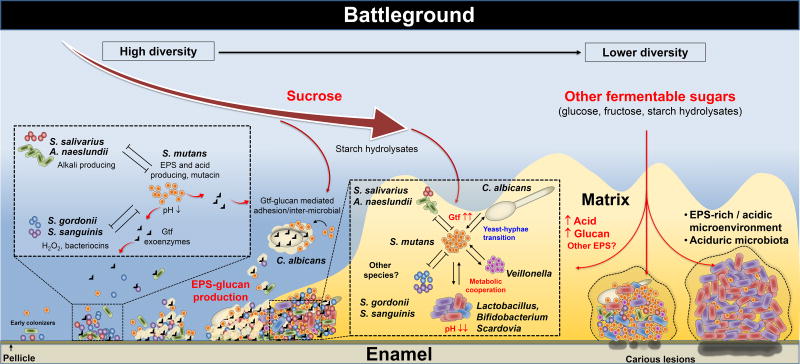
Figure 3. The biofilm battleground: antagonistic, synergistic and mutualistic interactions to build a pathogenic habitat.
The social interactions of the oral microbial community start with early colonizers that can rapidly adhere to the tooth surface, and then co-adhere with other microorganisms. During this process the various species interact physically and metabolically to shape the initial biofilm community. The interactions can be both antagonistic and cooperative, which can dynamically change depending on the host. Certain interactions are beneficial as S. gordonii/S. salivarius and A. naeslundii interfere with caries pathogens such as S. mutans by secreting bacteriocins and hydrogen peroxide as chemical weapons or counter the deleterious effects of acidification by producing alkali. However, the balance between commensals and pathogens can be disrupted by frequent sugar consumption and poor oral care. When sucrose is available, EPS-producing exoenzymes such as Gtfs present in the pellicle and also bound to different microbes (including C. albicans) produce copious amounts of glucans. Furthermore, the Gtfs can also use starch hydrolysates (from α-amylase activity on pellicle and bacterial surfaces) to produce hybrid glucan polymers. The surface-formed glucans provide novel binding sites for adhesion and co-adhesion, which mediates new interspecies interactions and microbial clustering on the tooth surface, while assembling a polymeric matrix that provides protection and mechanical stability, making biofilm recalcitrant to antimicrobials and difficult to remove. Competitive and synergistic interactions continue to develop between the microbes embedded in the biofilm structure. If the biofilm remains on teeth and the consumption of carbohydrate-rich diets persist, the amount of EPS and extent of acidification of the matrix increases. The diffusion-modifying properties of the matrix combined with microbial metabolic activities help create a highly acidic and increasingly anaerobic (hypoxic) niche. Such conditions elicit biochemical, ecological, and structural changes that favor the survival and dominance of highly acid-stress tolerant organisms that can synergize with each other. The microbial diversity can further reduce in favor of an aciduric microbiota, help maintaining an acidified microenvironment. The low-pH condition at tooth-biofilm interface promotes demineralization of the enamel leading to the onset and progression of dental caries. This model may explain the rapid accumulation of cariogenic plaque in the presence of sucrose (and other fermentable sugars) in the diet, even if the initial population of pathogens such as S. mutans is numerically low.
Competition/Antagonism
The inverse association between the abundance of commensals with mutans streptococci has been well-documented, indicating that these organisms can control growth of cariogenic bacteria. The simplest mechanism involves the production of basic (alkaline) compounds that maintain pH values near neutrality, allowing commensals to outcompete S. mutans and acid tolerant organisms that are able to grow and dominate at lower pH conditions. The two prevailing pathways for alkali production are urea metabolism by bacterial ureases, mainly Streptococcus salivarius, certain Actinomyces species, and a few oral haemophili [38]. Urea hydrolysis yields ammonia and CO2. While the CO2 can provide some modest buffer capacity, ammonia can rapidly equilibrate with a proton to yield NH4+, raising the pH and providing the bacteria with a source of nitrogen. Arginine can be metabolized by numerous oral bacteria via various pathways. A dominant route for arginine catabolism by oral commensals is the arginine deiminase system (ADS), which metabolizes internalized arginine yielding ornithine, ammonia, CO2 and ATP. Urea and arginine metabolism not only result in alkalinization of oral biofilms, preventing demineralization and promoting remineralization, they also provide bioenergetic advantages to the organisms that harbor ureases and the ADS. This can provide ecological advantages to commensals and deter the growth of caries pathogens, promoting the development of healthy oral biofilms.
In addition to adhesion and alkali generation, many commensals have more ‘active measures’ to interfere with the growth and biological processes of caries pathogens, and S. mutans in particular. The production of H2O2 via pyruvate oxidase and other enzymes, and secretion of bacteriocins and other antimicrobial compounds by commensals are important ‘chemical weapons’ that are inhibitory to the growth of S. mutans [39]. Interestingly, S. mutans can ‘counter-attack’ by releasing mutacins (lantibiotic and non-lantibiotic peptide antibiotics), which are effective against a variety of commensals. However, certain commensal streptococci (e.g. S. gordonii) have the ability to interfere with intercellular signaling systems required for the production of mutacin [40]. In particular, a recent clinical isolate designated as Streptococcus A12, produces a protease (similar to S. gordonii challisin) that breaks down competence stimulating peptide (CSP), which activates mutacin production by S. mutans via a two-component signal transduction systems [41]. Alkali-generating or bacteriocin/toxin-producing bacteria within biofilms can influence interspecies interactions, local pH, and microenvironment dynamics [42].
The development of dental caries is also intertwined intimately with stress tolerance by caries pathogens. In addition to the aforementioned competitive interactions with commensals, coping with a large influx of carbohydrates in the diet by rapidly shifting metabolism can induce a variety of stress pathways. Perhaps, though, the most significant stress under cariogenic conditions is acid. The in situ measurements of plaque pH following a carbohydrate challenge reveal that the pH can drop within 2 to 3 minutes to values as extreme as 4.0 and below [43]. This is a true “acid shock” that would be intolerable to most health-associated commensals as they cease growth below 6 or 5.5, and are rapidly killed at pH 4.0 [44, 45]. In contrast, S. mutans, lactobacilli and other aciduric bacteria can cope such suddenly dropped pH using various adaptive strategies. A primary determinant of acid tolerance in S. mutans is the proton-extruding F1FO-ATPase pump, which is highly active, has a much lower optimal pH than the same enzyme in commensal streptococci, and is produced in greater quantity when the organisms are challenged with low pH [45]. However, acid tolerance is a complex trait with many factors contributing to constitutional and adaptive acid resistance, including restructuring the bacterial membrane structure-composition. These mechanisms have been reviewed elsewhere [43, 46].
Cooperation/Synergism
If the environmental acidic stress persists, other acidogenic and aciduric bacteria such as non-mutans streptococci, actinomyces, lactobacilli, bifidobacteria, and Scardovia species are detected, which can synergize to enhance acidification of the biofilm milieu as detailed in excellent in-depth review articles [8, 11, 47]. Cariogenic biofilms initiated by S. mutans are acidic, hypoxic, but rich in carbohydrates, which creates an ideal microenvironment for opportunistic organisms like lactobacilli to grow and accelerate caries progression. Concomitantly, EPS production by S. mutans Gtfs is induced under acidic pH. Lactobacillus casei, frequently isolated from the cariogenic plaque with S. mutans, are known for their high capacity to produce and tolerate acid, although they have poor ability to colonize teeth. The presence of S. mutans and sugar exposure promotes colonization by certain lactobacilli [48] through Gtf binding and glucan-mediated adhesion mechanism, increasing accumulation of both organisms within biofilms. Further some L. reuteri strains possess two different enzymes, 4,6-α-glucanotransferase that uses starches to synthesize α-glucan-type polysaccharides while the other is a typical glucansucrase that synthesizes α-glucans from sucrose [49]. In addition, the presence of amylase bound on bacterial (e.g. S. gordonii, S. parasanguinis) and tooth surfaces produce a variety of starch hydrolysates in situ that can be metabolized into acids and incorporated into glucans synthesized by S. mutans Gtfs forming hybrid polymers [50, 51, 52]. Additional EPS produced by other oral bacteria may also contribute to biofilm matrix assembly even if the proportion of S. mutans is declining as the biofilm matures and the caries lesion worsens. Thus, we speculate that co-colonization with a diverse group of EPS-producing or EPS-modifying organisms can expand the substrate repertoire that allows oral bacteria to build complex EPS matrix with properties that can enhance caries development that glucans alone cannot achieve.
The creation of acidic microenvironments benefits not only acid producers and strongly acid-tolerant species [53], but also those that use lactate as a carbon source, whereas other species that are acid sensitive or that cannot metabolize the acids present in their surrounding may perish. Veillonella, an obligate anaerobic Gram-negative bacterium, is considered a bridge organism in the oral biofilm [54]. Veillonella does not utilize carbohydrates as a source of energy, instead it utilizes lactate. Such nutritional preference renders this organism dependent on streptococci that produce copious amounts of lactic acid. In fact, Veillonella often co-aggregates with oral streptococci and, not surprisingly, it is often identified as a signature organism in caries-active subjects [55]. It has been long perceived that the presence of this bacterium in plaque neutralizes the acidic pH of the oral biofilm, thereby preventing enamel demineralization. However, evidence from microbiome studies demonstrates that both Veillonella and S. mutans are highly associated with caries lesions [56]. Acetate produced from Veillonella lactate catabolism can be damaging to enamel, while Veillonella promotes S. mutans growth despite the presence of antagonistic S. gordonii in vitro [57]. Conversely, some bacteria found in cariogenic biofilms do not fit the classical profile of being acidogenic-aciduric, such as Prevotella and Atopobium [58]. Whether they are just bystanders or play an active role in caries pathogenesis remains to be elucidated.
Intriguingly, results from several clinical studies reveal that the fungus Candida albicans is frequently detected in higher numbers in plaque-biofilms from toddlers with early childhood caries (ECC) as reviewed in [19]. In the mouth, C. albicans is known to form mixed microbial communities on soft-tissue and prosthetic surfaces, causing mucosal infections. However, C. albicans can co-adhere with S. mutans and colonize tooth surfaces in the presence of sucrose [59, 60]. Specifically, this cross-kingdom interaction appears to be largely mediated by S. mutans-derived Gtfs that bind avidly onto Candida surface, and produce large amounts of glucans on the fungal surface, boosting the ability of both microbes to form biofilm together while increasing the amount of EPS-matrix. Once together within biofilms, these organisms can cooperate by providing substrates/metabolites and growth stimulating factors [61, 62], while enhancing Gtfs production [60, 62, 63]. Using a rodent model, a synergistic enhancement of biofilm virulence was observed when S. mutans was co-infected with C. albicans and exposed to sucrose-rich diet, leading to rampant caries on teeth similar to those found clinically in ECC [60]. Further investigation into how other metabolic pathways contribute to the symbiotic interactions and matrix production may offer additional insights into the disease process.
Dental caries is a highly dynamic pathological process where the host’s diet fuels the assembly of virulent biofilms by promoting EPS matrix assembly and polymicrobial interactions that cause profound changes in the local environment. EPS-producing pathogens such as S. mutans appear to battle with commensal organisms and, when conditions are conducive, build-up a ‘habitat’ to work synergistically with other aciduric species to acidify the biofilm milieu and cause demineralization of the tooth surface. Yet, it is indeed difficult to fully assess the absolute contribution of many species to the caries process because of the spatial/chemical heterogeneity, the continually changing microenvironments and the fact that organisms could be beneficial or detrimental depending on the conditions and degree to which a lesion had progressed (e.g. proteolytic bacteria could accelerate the caries process in advanced lesions where dentin is exposed). Nevertheless, this evolving view of ecological battles and polymicrobial synergies within a heterogeneous yet structured environment has direct implications in understanding the pathogenic mechanisms of dental caries and in developing effective therapeutics (Box 3).
Box 3. Challenges and therapeutic opportunities for polymicrobial biofilm oral infections.
Oral biofilms are one of the most complex polymicrobial communities found in nature, where ecological and dysbiosis principles have been applied to explain their ability to cause diseases. Multiple biofilm types are formed on mucosal, abiotic (e.g. implants and restorative materials) or tooth surfaces in the oral cavity, where interspecies and even cross-kingdom associations occur while interacting with host saliva, diet and immunity. This unique milieu provides challenges for design and delivery of therapeutics, but also offers fertile ground for development of innovative anti-biofilm strategies targeting host-microbe-diet interactions that could have broader implications. Saliva and the diet of the host play key roles in modulating biofilm structure, spatial organization, microenvironments, and development of microbial communities. Sugar-rich diets fuel opportunistic pathogens such as S. mutans to create an acidic microenvironment protected by an EPS-rich matrix. Conversely, keystone pathogens such as Porphyromonas gingivalis inhabiting the subgingival environment modulate the host immune response and polymicrobial balance resulting in dysbiotic biofilm community that causes periodontitis. Conventional antimicrobial elimination of the pathogen or for biofilm control has been proven difficult. The complexity of biofilm biology highlights the need for new therapeutic strategies to effectively control oral biofilms. Emerging technologies can modulate polymicrobial or host-microbe interactions. Community manipulation through depleting pathogens and favoring the growth of antagonizing commensal organisms could reduce the overall biofilm virulence. For example, L-arginine can be used for alkali production by arginolytic bacteria (e.g. Streptococcus gordonii), which can neutralize acids and modulate pH homeostasis within oral biofilms in vivo [74]. Similarly, probiotic approaches that increase the proportions of beneficial bacteria at the expense of pathogens may be another viable strategy to modulate the pathogenic potential of oral biofilms. Furthermore, species-specific targeting antimicrobial peptides or small molecules can selectively inhibit S. mutans from multispecies biofilms to promote a healthy microbiome in vitro [75, 76]. Likewise, modulation of the immune response can limit destructive inflammation and deplete the source for dysbiosis in periodontitis [77]. However, multi-targeted approaches may be needed to disrupt both the microbes and the surrounding matrix. New pH-sensitive multifunctional nanoparticles are capable of simultaneous EPS degradation and bacterial killing once activated by acidic pH values found within cariogenic biofilm microenvironment [78, 79]. Antimicrobial peptides combined with anti-EPS strategies may further increase the access and permeabilizing properties of the peptides once in the biofilm [35]. These targeted therapies may lead to more effective and precise oral biofilm control, while providing important therapeutic insights against other polymicrobial infections.
Concluding Remarks and Future Perspectives
The diet-microbe interactions in the oral cavity play a key role in determining the fate of the colonizing microbiota from birth. The available evidence clearly indicate that the pathogenesis of dental caries involve the assembly of an EPS matrix and synergistic multispecies efforts triggered by the host’s dietary sugars that promote cariogenic biofilm development. Although the complex microbiota and its function should be viewed as a whole, ‘the battleground’ where the ecological battles and polymicrobial synergy ensues to drive the disease process is equally important (Fig 2/3). The EPS enhance bacterial adhesion-cohesion and interspecies binding interactions, while forming a matrix that embeds the cells into spatially organized microbial clusters. The EPS matrix act as a diffusion-controlling barrier by modulating the access of solutes to the interior and by trapping produced acids inside the biofilm, while also serving as an endogenous source for acid production in addition to creating oxygen gradients. The embedded microbes must then cope with a wide range of stresses (acidic, hypoxia) and large fluctuations in nutrients availability to persist in oral biofilms, a pre-requisite for contributing to the onset of caries. Thus, the matrix helps cariogenic biofilms to ‘cling’ on teeth despite exposure to shear forces and create an acidic pH microenvironment in spite of being ‘bathed’ by buffering saliva.
In this context, the primary role of S. mutans as a pathogen may reside with its exceptional ability to alter the local physico-chemical environment by utilizing dietary sugar to assemble an insoluble polymeric matrix, thereby providing mechanical stability and protecting the acid milieus within which commensals may perish and more potent acidogenic/aciduric organisms flourish to become dominant, making them difficult to treat. Although the immediate cause of dental caries is certainly acid production, the absence of the ‘sheltering effect’ of the EPS matrix would argument the ability of acids to demineralize in the presence of saliva. We propose that EPS-producing pathogens can be considered ‘biofilm environment conditioners’ that help build-up a pathological niche (habitat) within a complex microbial community (Fig. 3). This concept can be integrated with current understanding about the biofilm matrix biology and microbiome-based data, which may have import to other polymicrobial infections.
Future studies need to elucidate the molecular and functional diversity of extracellular matrix and their relationship with spatial-temporal changes of the polymicrobial interactions during the transition between health and disease. Furthermore, it remains unclear how the EPS matrix provides structural scaffolds, and governs microbial clustering, positioning and activity either directly or through generation of local microenvironments (niches). Matrix-mediated changes can modify cell-cell interactions, specifically between different microbial species. In turn, dynamic reciprocity between cells and matrix, or cell-cell interactions and matrix, may provide a complex, interconnected molecular network governing cellular functionality at both single-cell and multicellular levels. Technological advances in microscopy and spectroscopy-based methods have provided impressive details about EPS secretion and interactions to form higher-order matrix structures at single-polymer/protein precision or biofilm formation in vivo [5, 64–66]. In vivo-like environments afforded by microfluidic devices/3D printing (e.g. organ-on-chip) may help assess mechanisms of biofilm formation in conditions mimicking clinical situations. Finally, how host factors/genetics associated with saliva composition, tooth defects (e.g. hypoplasia) [67], immunity among others influence pathogenic biofilm development remain to be fully elucidated [68]. Further in-depth analysis of the structural and functional interaction between the many components of the biofilm matrix, the local microbiome and the host factors shall advance our understanding of the pathogenic mechanisms of oral and other polymicrobial diseases, and lead to precise targeted therapies to prevent or treat them.
Acknowledgments
The authors are also grateful to Dr. Dongyeop Kim for conceiving and designing the diagrams in Figure 2 and 3. The research is supported in part by the National Institute for Dental and Craniofacial Research grants DE012236, DE025832 (RAB), DE022350 (HW), DE018023 and DE025220 (HK). We regret that several important studies could only indirectly be acknowlegded through recent review articles due to space and reference number limitations.
Glossary
- Dental caries
a polymicrobial- and diet-dependent disease that is characterized be the development of acidogenic biofilms (or dental plaque) that causes demineralization of the enamel surface over time eventually leading to the clinical onset of cavitation or tooth-decay.
- Early childhood caries
a virulent type of dental caries that disproportionately afflict underprivileged preschool children. The onset and progression of ECC is aggressive, leading to rampant destruction of primary teeth that are painful and recurring, which may require total oral rehabilitation under general anesthesia if left untreated.
- Extracellular polymeric substances
extracellular biomolecules often termed EPS that forms the biofilm matrix. EPS can be exopolysaccharides, fibrous and globular proteins (including extracellular enzymes), lipids and nucleic acids/eDNA, which can be microbial surface-associated, secreted extracellularly or deposited on abiotic and biotic surfaces
- Pathogens
any organism that supports or enhances the virulence potential of the biofilm to cause infectious diseases.
- Ecological plaque hypothesis
a model that encompasses microbiological, biochemical and ecological properties of biofilms associated with oral diseases. Host-derived changes (e.g. frequent dietary sugar exposure) trigger biofilm environmental acidification and selection of an acidogenic-aciduric microbiota, resulting in net mineral loss and the onset of dental caries.
- Emergent properties
novel structures, activities, patterns and properties that arise during the process, and as a consequence, of self-organization in complex systems, which in the biofilm context include surface adhesion-cohesion, spatial organization, physical and social interactions, chemical heterogeneity and increased tolerance to antimicrobials.
- eDNA
extracellular DNA material that can be either actively secreted or resultant of cell lysis. eDNA can bind to other EPS components contributing to the biofilm matrix structural organization while also serving as a nutrient source for resident microbes.
- F1FO-ATPase
a membrane associated proton-translocating enzyme that is key for bacterial acid tolerance, particularly in S. mutans. During glycolysis, protons are pumped out by F-ATPase to help maintain ΔpH across the cell membrane, preventing acidification of the cytoplasm, which would typically inhibit intracellular enzymes.
- Bacterial competence
the ability of bacterial cells to take up foreign genetic material (DNA) from the environment.
References
- 1.Flemming HC, et al. Biofilms: an emergent form of bacterial life. Nat Rev Microbiol. 2016;14(9):563–75. doi: 10.1038/nrmicro.2016.94. [DOI] [PubMed] [Google Scholar]
- 2.Lebeaux D, et al. Biofilm-related infections: bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol Mol Biol Rev. 2014;78(3):510–43. doi: 10.1128/MMBR.00013-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marsh PD, Zaura E. Dental biofilm: ecological interactions in health and disease. J Clin Periodontol. 2017;44(Suppl 18):S12–s22. doi: 10.1111/jcpe.12679. [DOI] [PubMed] [Google Scholar]
- 4.Hobley L, et al. Giving structure to the biofilm matrix: an overview of individual strategies and emerging common themes. FEMS Microbiol Rev. 2015;39(5):649–69. doi: 10.1093/femsre/fuv015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koo H, Yamada KM. Dynamic cell-matrix interactions modulate microbial biofilm and tissue 3D microenvironments. Curr Opin Cell Biol. 2016;42:102–112. doi: 10.1016/j.ceb.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.David LA, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–63. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maslowski KM, Mackay CR. Diet, gut microbiota and immune responses. Nat Immunol. 2011;12(1):5–9. doi: 10.1038/ni0111-5. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi N, Nyvad B. The role of bacteria in the caries process: ecological perspectives. J Dent Res. 2011;90(3):294–303. doi: 10.1177/0022034510379602. [DOI] [PubMed] [Google Scholar]
- 9.Pitts NB, et al. Dental caries. Nat Rev Dis Primers. 2017;3:17030. doi: 10.1038/nrdp.2017.30. [DOI] [PubMed] [Google Scholar]
- 10.Adler CJ, et al. Sequencing ancient calcified dental plaque shows changes in oral microbiota with dietary shifts of the Neolithic and Industrial revolutions. Nat Genet. 2013;45(4):450–5. 455e1. doi: 10.1038/ng.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mira A, et al. Role of microbial communities in the pathogenesis of periodontal diseases and caries. J Clin Periodontol. 2017;44(Suppl 18):S23–s38. doi: 10.1111/jcpe.12671. [DOI] [PubMed] [Google Scholar]
- 12.Dewhirst FE. The Oral Microbiome: Critical for Understanding Oral Health and Disease. J Calif Dent Assoc. 2016;44(7):409–10. [PMC free article] [PubMed] [Google Scholar]
- 13.Koo H, et al. The exopolysaccharide matrix: a virulence determinant of cariogenic biofilm. J Dent Res. 2013;92(12):1065–73. doi: 10.1177/0022034513504218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall-Stoodley L, Stoodley P. Evolving concepts in biofilm infections. Cell Microbiol. 2009;11(7):1034–43. doi: 10.1111/j.1462-5822.2009.01323.x. [DOI] [PubMed] [Google Scholar]
- 15.Van Acker H, et al. Molecular mechanisms of antimicrobial tolerance and resistance in bacterial and fungal biofilms. Trends Microbiol. 2014;22(6):326–33. doi: 10.1016/j.tim.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Lamont RJ, Hajishengallis G. Polymicrobial synergy and dysbiosis in inflammatory disease. Trends Mol Med. 2015;21(3):172–83. doi: 10.1016/j.molmed.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nobbs AH, et al. Streptococcus adherence and colonization. Microbiol Mol Biol Rev. 2009;73(3):407–50. doi: 10.1128/MMBR.00014-09. Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmer RJ., Jr Composition and development of oral bacterial communities. Periodontol. 2014;64(1):20–39. doi: 10.1111/j.1600-0757.2012.00453.x. 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hajishengallis E, et al. Advances in the microbial etiology and pathogenesis of early childhood caries. Mol Oral Microbiol. 2017;32(1):24–34. doi: 10.1111/omi.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bowen WH, Koo H. Biology of Streptococcus mutans-derived glucosyltransferases: role in extracellular matrix formation of cariogenic biofilms. Caries Res. 2011;45(1):69–86. doi: 10.1159/000324598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simon-Soro A, Mira A. Solving the etiology of dental caries. Trends Microbiol. 2015;23(2):76–82. doi: 10.1016/j.tim.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 22.Hwang G, et al. Simultaneous spatiotemporal mapping of in situ pH and bacterial activity within an intact 3D microcolony structure. Sci Rep. 2016;6:32841. doi: 10.1038/srep32841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo L, et al. The well-coordinated linkage between acidogenicity and aciduricity via insoluble glucans on the surface of Streptococcus mutans. Sci Rep. 2015;5:18015. doi: 10.1038/srep18015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gross EL, et al. Beyond Streptococcus mutans: dental caries onset linked to multiple species by 16S rRNA community analysis. PLoS One. 2012;7(10):e47722. doi: 10.1371/journal.pone.0047722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johansson I, et al. The microbiome in populations with a low and high prevalence of caries. J Dent Res. 2016;95(1):80–6. doi: 10.1177/0022034515609554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rostami N, et al. A Critical Role for Extracellular DNA in Dental Plaque Formation. J Dent Res. 2017;96(2):208–216. doi: 10.1177/0022034516675849. [DOI] [PubMed] [Google Scholar]
- 27.Besingi RN, et al. Functional amyloids in Streptococcus mutans, their use as targets of biofilm inhibition and initial characterization of SMU_63c. Microbiology. 2017;163(4):488–501. doi: 10.1099/mic.0.000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein MI, et al. Streptococcus mutans-derived extracellular matrix in cariogenic oral biofilms. Front Cell Infect Microbiol. 2015;5:10. doi: 10.3389/fcimb.2015.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu F, et al. Glycosyltransferase-mediated Sweet Modification in Oral Streptococci. J Dent Res. 2015;94(5):659–65. doi: 10.1177/0022034515574865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao J, et al. The exopolysaccharide matrix modulates the interaction between 3D architecture and virulence of a mixed-species oral biofilm. PLoS Pathog. 2012;8(4):e1002623. doi: 10.1371/journal.ppat.1002623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mark Welch JL, et al. Biogeography of a human oral microbiome at the micron scale. Proc Natl Acad Sci U S A. 2016;113(6):E791–800. doi: 10.1073/pnas.1522149113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stacy A, et al. The biogeography of polymicrobial infection. Nat Rev Microbiol. 2016;14(2):93–105. doi: 10.1038/nrmicro.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peterson BW, et al. Viscoelasticity of biofilms and their recalcitrance to mechanical and chemical challenges. FEMS Microbiol Rev. 2015;39(2):234–45. doi: 10.1093/femsre/fuu008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guilhen C, et al. Biofilm dispersal: multiple elaborate strategies for dissemination of bacteria with unique properties. Mol Microbiol. 2017 doi: 10.1111/mmi.13698. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, et al. Topical delivery of low-cost protein drug candidates made in chloroplasts for biofilm disruption and uptake by oral epithelial cells. Biomaterials. 2016;105:156–66. doi: 10.1016/j.biomaterials.2016.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wessel AK, et al. Oxygen limitation within a bacterial aggregate. MBio. 2014;5(2):e00992. doi: 10.1128/mBio.00992-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.von Ohle C, et al. Real-time microsensor measurement of local metabolic activities in ex vivo dental biofilms exposed to sucrose and treated with chlorhexidine. Appl Environ Microbiol. 2010;76(7):2326–34. doi: 10.1128/AEM.02090-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu YL, et al. Progress toward understanding the contribution of alkali generation in dental biofilms to inhibition of dental caries. Int J Oral Sci. 2012;4(3):135–40. doi: 10.1038/ijos.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qi F, Kreth J. Methods to Study Antagonistic Activities Among Oral Bacteria. Methods Mol Biol. 2017;1537:203–218. doi: 10.1007/978-1-4939-6685-1_12. [DOI] [PubMed] [Google Scholar]
- 40.Merritt J, Qi F. The mutacins of Streptococcus mutans: regulation and ecology. Mol Oral Microbiol. 2012;27(2):57–69. doi: 10.1111/j.2041-1014.2011.00634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang X, et al. A Highly Arginolytic Streptococcus Species That Potently Antagonizes Streptococcus mutans. Appl Environ Microbiol. 2016;82(7):2187–201. doi: 10.1128/AEM.03887-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kreth J, et al. Bacterial and host interactions of oral streptococci. DNA Cell Biol. 2009;28(8):397–403. doi: 10.1089/dna.2009.0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lemos JA, Burne RA. A model of efficiency: stress tolerance by Streptococcus mutans. Microbiology. 2008;154(Pt 11):3247–55. doi: 10.1099/mic.0.2008/023770-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bender GR, et al. Acid tolerance, proton permeabilities, and membrane ATPases of oral streptococci. Infect Immun. 1986;53(2):331–8. doi: 10.1128/iai.53.2.331-338.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sturr MG, Marquis RE. Comparative acid tolerances and inhibitor sensitivities of isolated F-ATPases of oral lactic acid bacteria. Appl Environ Microbiol. 1992;58(7):2287–91. doi: 10.1128/aem.58.7.2287-2291.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baker JL, et al. Acid-adaptive mechanisms of Streptococcus mutans-the more we know, the more we don't. Mol Oral Microbiol. 2017;32(2):107–117. doi: 10.1111/omi.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanner AC, et al. Understanding caries from the oral microbiome perspective. J Calif Dent Assoc. 2016;44(7):437–446. [PubMed] [Google Scholar]
- 48.Wen ZT, et al. Biofilm formation and virulence expression by Streptococcus mutans are altered when grown in dual-species model. BMC Microbiol. 2010;10:111. doi: 10.1186/1471-2180-10-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bai Y, et al. Crystal Structure of 4,6-alpha-Glucanotransferase Supports Diet-Driven Evolution of GH70 Enzymes from alpha-Amylases in Oral Bacteria. Structure. 2017;25(2):231–242. doi: 10.1016/j.str.2016.11.023. [DOI] [PubMed] [Google Scholar]
- 50.Liang X, et al. A distinct sortase SrtB anchors and processes a streptococcal adhesin AbpA with a novel structural property. Sci Rep. 2016;6:30966. doi: 10.1038/srep30966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vacca-Smith AM, et al. Interactions of streptococcal glucosyltransferases with alpha-amylase and starch on the surface of saliva-coated hydroxyapatite. Arch Oral Biol. 1996;41(3):291–8. doi: 10.1016/0003-9969(95)00129-8. [DOI] [PubMed] [Google Scholar]
- 52.Nikitkova AE, et al. Taking the starch out of oral biofilm formation: molecular basis and functional significance of salivary alpha-amylase binding to oral streptococci. Appl Environ Microbiol. 2013;79(2):416–23. doi: 10.1128/AEM.02581-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Richards VP, et al. The microbiome of site-specific dental plaque of children with different caries status. Infect Immun. 2017 doi: 10.1128/IAI.00106-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Knapp S, et al. Natural Competence Is Common among Clinical Isolates of Veillonella parvula and Is Useful for Genetic Manipulation of This Key Member of the Oral Microbiome. Front Cell Infect Microbiol. 2017;7:139. doi: 10.3389/fcimb.2017.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou J, et al. Exploration of Human Salivary Microbiomes--Insights into the Novel Characteristics of Microbial Community Structure in Caries and Caries-Free Subjects. PLoS One. 2016;11(1):e0147039. doi: 10.1371/journal.pone.0147039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiang S, et al. Salivary Microbiome Diversity in Caries-Free and Caries-Affected Children. Int J Mol Sci. 2016;17(12) doi: 10.3390/ijms17121978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mashima I, Nakazawa F. The influence of oral Veillonella species on biofilms formed by Streptococcus species. Anaerobe. 2014;28:54–61. doi: 10.1016/j.anaerobe.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 58.Teng F, et al. Prediction of Early Childhood Caries via Spatial-Temporal Variations of Oral Microbiota. Cell Host Microbe. 2015;18(3):296–306. doi: 10.1016/j.chom.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 59.Metwalli KH, et al. Streptococcus mutans, Candida albicans, and the human mouth: a sticky situation. PLoS Pathog. 2013;9(10):e1003616. doi: 10.1371/journal.ppat.1003616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Falsetta ML, et al. Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Infect Immun. 2014;82(5):1968–81. doi: 10.1128/IAI.00087-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sztajer H, et al. Cross-feeding and interkingdom communication in dual-species biofilms of Streptococcus mutans and Candida albicans. ISME J. 2014;8(11):2256–2271. doi: 10.1038/ismej.2014.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim D, et al. Candida albicans stimulates Streptococcus mutans microcolony development via cross-kingdom biofilm-derived metabolites. Sci Rep. 2017;7:41332. doi: 10.1038/srep41332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hwang G, et al. Candida albicans mannans mediate Streptococcus mutans exoenzyme GtfB binding to modulate cross-kingdom biofilm development in vivo. PLoS Pathog. 2017;13(6):e1006407. doi: 10.1371/journal.ppat.1006407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Turnbull L, et al. Explosive cell lysis as a mechanism for the biogenesis of bacterial membrane vesicles and biofilms. Nat Commun. 2016;7:11220. doi: 10.1038/ncomms11220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Van de Vyver H, et al. A Novel Mouse Model of Staphylococcus aureus Vascular Graft Infection: Noninvasive Imaging of Biofilm Development in Vivo. Am J Pathol. 2017;187(2):268–279. doi: 10.1016/j.ajpath.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 66.Merritt J, et al. Let there be bioluminescence: development of a biophotonic imaging platform for in situ analyses of oral biofilms in animal models. Environ Microbiol. 2016;18(1):174–90. doi: 10.1111/1462-2920.12953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Caufield PW, et al. Hypoplasia-associated severe early childhood caries--a proposed definition. J Dent Res. 2012;91(6):544–50. doi: 10.1177/0022034512444929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Divaris K. Predicting Dental Caries Outcomes in Children: A "Risky" Concept. J Dent Res. 2016;95(3):248–54. doi: 10.1177/0022034515620779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baker JL, et al. Ecology of the Oral Microbiome: Beyond Bacteria. Trends Microbiol. 2017;25(5):362–374. doi: 10.1016/j.tim.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Smith EG, Spatafora GA. Gene regulation in S. mutans: complex control in a complex environment. J Dent Res. 2012;91(2):133–41. doi: 10.1177/0022034511415415. [DOI] [PubMed] [Google Scholar]
- 71.Peng X, et al. Cyclic di-AMP mediates biofilm formation. Mol Microbiol. 2016;99(5):945–59. doi: 10.1111/mmi.13277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peng X, et al. Effects of diadenylate cyclase deficiency on synthesis of extracellular polysaccharide matrix of Streptococcus mutans revisit. Environ Microbiol. 2016;18(11):3612–3619. doi: 10.1111/1462-2920.13440. [DOI] [PubMed] [Google Scholar]
- 73.Moye ZD, et al. Fueling the caries process: carbohydrate metabolism and gene regulation by Streptococcus mutans. J Oral Microbiol. 2014;6 doi: 10.3402/jom.v6.24878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nascimento MM, et al. The effect of arginine on oral biofilm communities. Mol Oral Microbiol. 2014;29(1):45–54. doi: 10.1111/omi.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guo L, et al. Precision-guided antimicrobial peptide as a targeted modulator of human microbial ecology. Proc Natl Acad Sci U S A. 2015;112(24):7569–74. doi: 10.1073/pnas.1506207112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Garcia SS, et al. Targeting of Streptococcus mutans Biofilms by a Novel Small Molecule Prevents Dental Caries and Preserves the Oral Microbiome. J Dent Res. 2017;96(7):807–814. doi: 10.1177/0022034517698096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hajishengallis G, et al. Complement inhibition in pre-clinical models of periodontitis and prospects for clinical application. Semin Immunol. 2016;28(3):285–91. doi: 10.1016/j.smim.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Horev B, et al. pH-activated nanoparticles for controlled topical delivery of farnesol to disrupt oral biofilm virulence. ACS Nano. 2015;9(3):2390–404. doi: 10.1021/nn507170s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gao L, et al. Nanocatalysts promote Streptococcus mutans biofilm matrix degradation and enhance bacterial killing to suppress dental caries in vivo. Biomaterials. 2016;101:272–84. doi: 10.1016/j.biomaterials.2016.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]