Abstract
Background
Studies on multiple dimensions of the symptom experience of patients with gastrointestinal cancers are extremely limited. Purpose was to evaluate for changes over time in the occurrence, severity, and distress of seven common symptoms in these patients.
Methods
Patients completed Memorial Symptom Assessment Scale, six times over two cycles of CTX. Changes over time in occurrence, severity, and distress of pain, lack of energy, nausea, feeling drowsy, difficulty sleeping, and change in the way food tastes were evaluated using multilevel regression analyses. In the conditional models, effects of treatment group (i.e., with or without targeted therapy (TT)), age, number of metastatic sites, time from cancer diagnosis, number of prior cancer treatments, cancer diagnosis, and CTX regimen on enrollment levels, as well as the trajectories of symptom occurrence, severity, and distress were evaluated.
Results
While the occurrence rates for pain, lack of energy, feeling drowsy, difficulty sleeping, and change in the way food tastes declined over the two cycles of CTX, nausea and numbness/tingling in hands/feet had more complex patterns of occurrence. Severity and distress ratings for the seven symptoms varied across the two cycles of CTX.
Conclusions
Demographic and clinical characteristics associated with differences in enrollment levels as well as changes with changes over time in occurrence, severity, and distress of these seven common symptoms were highly variable. These findings can be used to identify patients who are at higher risk for more severe and distressing symptoms during CTX and to enable the initiation of pre-emptive symptom management interventions.
Keywords: gastrointestinal cancer, chemotherapy, targeted therapy, severity, distress, pain, fatigue, sleep disturbance, taste changes, feeling drowsy
INTRODUCTION
Patients with gastrointestinal (GI) cancers who receive chemotherapy (CTX), with or without targeted therapy (TT), experience multiple co-occurring symptoms.1 In fact, in a recent cross-sectional study,2 our research team evaluated the occurrence, severity, and distress of acute symptoms in the week following CTX administration using the Memorial Symptom Assessment Scale (MSAS). While significant differences were found in the symptom experience of GI cancer patients who received CTX alone compared to CTX with TT, both groups reported an average of 12.5 symptoms. After controlling for a number of clinically meaningful covariates, patients who received CTX with TT reported lower occurrence rates for lack of energy, cough, feeling drowsy, and difficulty sleeping. In addition, these patients reported lower severity scores for dry mouth and change in the way food tastes. However, they reported significantly higher severity scores for worrying and “I don’t look like myself”. No differences in symptom distress scores were found between the two treatment groups.
As noted by an expert panel convened by the National Institute of Nursing Research,33 additional information is needed on changes over time in multiple dimensions (i.e., occurrence, severity, distress) of patients’ symptom experiences. In addition, consideration should be given to an evaluation of how pertinent demographic (e.g., age) and clinical (e.g., diagnosis, type of treatment) influence initial levels as well as the trajectories of various symptoms.
However, longitudinal studies of the symptom experience of patients with GI cancers are extremely limited. To our knowledge, only one study evaluated for changes over time in the symptom experience of patients with GI cancers. In this study,4 changes in the occurrence of depressive symptoms and associated predictors were assessed in patients with metastatic GI (n=376) and lung (n=166) cancer who were receiving CTX. While the occurrence and severity of depressive symptoms increased in the final months of life, results were not reported separately for the two cancer diagnoses.
Given the paucity of longitudinal studies of the symptom experience of patients with GI cancers, the purpose of this study was to evaluate for changes over time in the occurrence, severity, and distress of seven common symptoms (i.e., pain, lack of energy, nausea, feeling drowsy, numbness/tingling in hands/feet, difficulty sleeping, change in the way food tastes) in a sample of patients with GI cancers who were assessed over two cycles of CTX. In addition, the effects of a number of demographic and clinical characteristics that are known to influence cancer patients’ symptom experience (i.e., treatment group (i.e., CTX with and without TT,2,5 age,6,7 number of metastatic sites,8,9 time from cancer diagnosis,8,9 total number of prior cancer treatments,8,9 cancer diagnosis,10 CTX regimen11) on enrollment scores, as well as on changes over time in the various dimensions of the symptom experience were evaluated.
METHODS
Patients and Settings
This study is part of a larger descriptive, longitudinal study of the symptom experience of oncology outpatients who received CTX.12,13 Patients were included if they were ≥18 years of age; had a diagnosis of breast, GI, lung, or gynecological cancer; had received CTX within the preceding four weeks; were scheduled to receive at least two additional cycles of CTX; were able to read, write, and understand English; and provided written informed consent. Patients were recruited from two Comprehensive Cancer Centers, one Veterans Affairs hospital, and four community based oncology programs. The study was approved by the Committee on Human Research at the University of California at San Francisco and by the Institutional Review Board at each of the study sites.
Instruments
A demographic questionnaire obtained information on age, gender, ethnicity, marital status, living alone (yes/no), education, employment status, and income. Functional status was assessed using the Karnofsky Performance Status (KPS) scale, which is widely used in patients with cancer and has well established validity and reliability.14 Patients rated their functional status using the KPS scale that ranged from 30 (I feel severely disabled and need to be hospitalized) to 100 (I feel normal; I have no complaints or symptoms).15,16
Self-Administered Comorbidity Questionnaire (SCQ) consists of 13 common medical conditions simplified into language that can be understood without prior medical knowledge.17 Patients indicated if they had the condition; if they received treatment for it (proxy for disease severity); and if it limited their activity (indication of functional limitations). For each condition, patients can receive a maximum of 3 points. The total SCQ score ranges from 0 to 39. The SCQ has well established validity and reliability.17
A modified version of the MSAS was used to evaluate the occurrence, severity, and distress of 38 symptoms commonly associated with cancer and its treatment. In addition to the original 32 MSAS symptoms, the following six symptoms, found on other symptom assessment instruments, were assessed: hot flashes, chest tightness, difficulty breathing, abdominal cramps, increased appetite, and weight gain. These symptoms were added to the MSAS because they are common symptoms in oncology patients.
The MSAS is a self-report questionnaire designed to measure the multidimensional experience of symptoms. Patients were asked to indicate whether they experienced each symptom within the past week (i.e., symptom occurrence). If they experienced the symptom, they were asked to rate its severity and distress. Severity was rated using a 4-point Likert scale (i.e., 1 = slight, 2 = moderate, 3 = severe, 4 = very severe). Distress was rated using a 5-point Likert scale (i.e., 0 = not at all, 1 = a little bit, 2 = somewhat, 3 = quite a bit, 4 = very much). The validity and reliability of the MSAS are well established.18–20
Study Procedures
A total of 2,234 patients were approached and 1,343 consented to participate (60.1% response rate) in the larger study. The major reason for refusal was being overwhelmed with their cancer treatment. For this study, only patients with a diagnosis of a GI cancer were include (n=404). A research staff member in the infusion unit approached eligible patients and discussed participation in the study. Written informed consent was obtained from all patients. Patients were recruited during the second or third cycle of CTX. Based on the length of the CTX cycle (i.e., 14 days, 21 days, 28 days) GI cancer patients completed self-report questionnaires in their homes, a total of six times over two cycles of CTX, namely: before CTX administration (i.e., recovery from previous CTX cycle, Times 1 [T1] and 4 [T4]), approximately one week after CTX administration (i.e., acute symptoms, Times 2 [T2] and 5 [T5]), and approximately two weeks after CTX administration [i.e., potential nadir, Times 3 [T3] and 6 [T6]).
Statistical Analysis
All analyses were done using SPSS Version 23 (IBM, Armonk, NY) and Stata/SE 14 (StataCorp LP, College Station, TX). Descriptive statistics as means and standard deviations for quantitative variables and frequencies and percentages for categorical variables were calculated for all of the study variables.
While the modified MSAS evaluated 38 symptoms, changes over time in occurrence, severity, and distress of the seven most common symptoms (i.e., symptoms that occurred in ≥50% of the patients at the initiation of their next CTX cycle), namely, pain, lack of energy, nausea, feeling drowsy, numbness/tingling in hands/feet, difficulty sleeping, change in the way food tastes, were evaluated in these longitudinal analyses. Based on a review of the literature of characteristics that are known to influence the symptom experience of patients with cancer, treatment group (i.e., CTX with and without TT2,5) age,6,7 number of metastatic sites,8,9 time from cancer diagnosis,8,9 total number of prior cancer treatments,8,9 cancer diagnosis,10 and CTX regimen11 were included as covariates in our longitudinal analyses.
For each of the seven symptoms, multilevel regression analysis was used to estimate changes over time in symptom occurrence, severity, and distress (i.e., a total of six assessments over two cycles of CTX). Symptom occurrence was coded as a binary variable (yes = 1 and no = 0) and changes were evaluated using multilevel logistic regression. Symptom severity was coded as an ordinal variable, with increasing severity ratings from 1 to 4. Symptom distress was coded as an ordinal variable (i.e., with 0 = not at all, 1 = a little bit, and increasing distress ratings from 2 to 4). Changes over time in symptom severity and distress were examined with multilevel ordinal logistic regression employing the logistic cumulative distribution function.21–23
First, unconditional models were examined to estimate the linear change in each of the symptoms. Given the possibility that the growth trajectory might not be only linear, quadratic effects were examined. In addition, because the length of treatment and two treatment cycles invited the examination of shifts in the growth trajectories (also called “discontinuities),22 piecewise models were examined. The piecewise model had four segments: enrollment (T1) to the T2, T2 to T3, T4 to T5, and T5 to T6. After identifying the best fitting growth trajectory for each symptom (i.e., linear, linear plus quadratic, or piecewise), conditional models were fit to examine the association between each of the covariates (i.e., treatment group, age, number of metastatic sites, time from cancer diagnosis, number of prior cancer treatments, cancer diagnosis, CTX regimen) and each of the symptoms at enrollment and on changes in each of the symptoms over time (i.e., cross-level interaction).22 A p-value of <0.05 was considered statistically significant.
RESULTS
Demographic and Clinical Characteristics
A total of 404 patients with GI cancers consented to participate and 401 (99%) completed the MSAS at T1. As shown in Table 1, the majority of the patients were male (55.2%), married/partnered (67.5%), and had a diagnosis of colon, rectal, or anal cancer (63.7%). The patients had a mean age of 58.0 (±11.8) years, reported an average of 5.4 (±2.9) comorbidities, and had a KPS score of 80.8 (±12.5).
Table 1.
Demographic and Clinical Characteristics of the Sample (n=404)
| Characteristics | Mean (SD) |
|---|---|
|
| |
| Age (years) | 58.0 (11.8) |
|
| |
| Education (years) | 16.0 (3.1) |
|
| |
| Karnofsky Performance Status score | 80.8 (12.5) |
|
| |
| Self-Administered Comorbidity Questionnaire score | 5.4 (2.9) |
|
| |
| Time since cancer diagnosis (years) | 1.4 (2.9) |
|
| |
| Time since diagnosis (median) | 0.42 |
|
| |
| Number of prior cancer treatments | 1.4 (1.3) |
|
| |
| Number of metastatic sites including lymph node involvement | 1.5 (1.1) |
|
| |
| % (n) | |
|
| |
| Female | 44.8 (181) |
|
| |
| Married/Partnered (% yes) | 67.5 (270) |
|
| |
| Lives alone (% yes) | 19.0 (76) |
|
| |
| Currently employed (% yes) | 34.3 (136) |
|
| |
| Type of prior cancer treatment | |
| No prior treatment | 29.0 (113) |
| Only surgery, CTX, or RT | 38.3 (149) |
| Surgery & CTX, or surgery & RT, or CTX & RT | 21.9 (85) |
| Surgery & CTX & RT | 10.8 (42) |
|
| |
| Cancer diagnosis | |
| Colon/rectum/anal | 63.7 (252) |
| Pancreatic/liver/gall bladder/esophageal/gastric/small intestine/and other | 36.3 (144) |
|
| |
| Genetic testing (% yes) | |
| BRAF detected | 2.3 (9) |
| KRAS detected | 12.7 (50) |
|
| |
| Metastatic sites | |
| No metastasis | 19.3 (77) |
| Only lymph node metastasis | 19.6 (78) |
| Only metastatic disease in other sites | 28.6 (114) |
| Metastatic disease in lymph nodes/and other sites | 32.4 (129) |
|
| |
| Chemotherapy regimen | |
| FOLFIRI | 13.9 (56) |
| FOLFOX | 43.6 (176) |
| FOLFIRINOX | 10.9 (44) |
| Other | 31.7 (128) |
|
| |
| Targeted therapy | |
| Yes | 23.4 (93) |
| No | 76.6 (304) |
Abbreviations: BRAF = B-Raf proto-oncogene, serine/threonine kinase, CTX = chemotherapy, FOLFIRI = leucovorin/5-fluorouracil/irinotecan, FOLFIRINOX = leucovorin/5-fluorouracil/irinotecan/oxaliplatin, FOLFOX = leucovorin/5-fluorouracil/oxaliplatin, KRAS = Kristen rat sarcoma viral oncogene homolog, RT = radiation therapy, SD = standard deviation
Changes in Symptom Occurrence, Severity, and Distress Ratings
Tables 2, 3, and 4 present the results of the multilevel regression analyses for occurrence, severity, and distress ratings, respectively for the seven symptoms that were evaluated in this study. Most of the results are presented in tabular format with accompanying figures. The figures for the unconditional model of the occurrence of the various symptoms illustrate the change in the observed and predicted probability of the occurrence of that symptom over time (Figures 1 and 2). For the conditional models, each of the figures displays the probability that a patient would report the occurrence of each symptom conditioned on the values of the predictor. This predicted probability can be compared with the observed responses at each time point to determine how well the estimated trajectories match the observed responses.
Table 2.
Results of the Multilevel Regression Analyses of Occurrence Ratings for the Seven Symptoms that Occurred in ≥50% of the Patients
| Pain – Occurrence (n=401) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Unconditional Model | Conditional Model | |||||||
| OR | SE | CI | p-value | OR | SE | CI | p-value | |
| Linear | 0.91 | 0.04 | 0.84–0.99 | 0.022 | ||||
| Treatment group | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Age | ||||||||
| Linear | 0.91 | 0.04 | 0.84–0.99 | 0.021 | ||||
| Enrollment | 0.94 | 0.01 | 0.92–0.97 | <0.001 | ||||
| Cross-level interaction | NS | |||||||
| Number of metastatic sites | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Time from cancer diagnosis | ||||||||
| Linear | 0.91 | 0.04 | 0.83–0.98 | 0.020 | ||||
| Enrollment | 1.13 | 0.07 | 1.005–1.27 | 0.041 | ||||
| Cross-level interaction | NS | |||||||
| Number of prior cancer treatments | ||||||||
| Linear | 0.91 | 0.04 | 0.83–0.98 | 0.019 | ||||
| Enrollment | 1.80 | 0.24 | 1.38–2.34 | <0.001 | ||||
| Cross-level interaction | NS | |||||||
| Cancer diagnosis | ||||||||
| Linear | 1.01 | 0.07 | 0.89–1.15 | 0.884 | ||||
| Enrollment (Reference is colon = no) | 1.94 | 0.81 | 0.86–4.38 | 0.108 | ||||
| Cross-level interaction | 0.84 | 0.07 | 0.71–0.998 | 0.047 | ||||
| Chemotherapy regimen | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Lack of Energy – Occurrence (n=401) | ||||||||
| Linear | 0.91 | 0.04 | 0.84–0.99 | 0.022 | ||||
| Treatment group | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Age | ||||||||
| Linear | 0.91 | 0.04 | 0.84–0.99 | 0.022 | ||||
| Enrollment | 0.97 | 0.01 | 0.95–0.999 | 0.038 | ||||
| Cross-level interaction | NS | |||||||
| Number of metastatic sites | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Time from cancer diagnosis | ||||||||
| Linear | 0.91 | 0.04 | 0.84–0.98 | 0.016 | ||||
| Enrollment | 1.17 | 0.08 | 1.03–1.33 | 0.016 | ||||
| Cross-level interaction | NS | |||||||
| Number of prior cancer treatments | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Cancer diagnosis | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Chemotherapy regimen | ||||||||
| Linear (FOLFIRI as reference) | 0.83 | 0.09 | 0.66–1.04 | 0.099 | ||||
| Enrollment | NS | |||||||
| Cross-level interaction: omnibus test | X2 = 9.02; p = 0.029 | |||||||
| Cross-level interaction: pairwise comparisons* | ||||||||
| FOLFIRI vs FOLFOX (1 v 2) | 1.00 | 0.13 | 0.71–1.41 | >0.999 | ||||
| FOLFIRI vs FOLFIRINOX (1 v 3) | 1.48 | 0.25 | 0.95–2.30 | 0.125 | ||||
| FOLFIRI vs Other (1 v 4) | 1.16 | 0.16 | 0.81–1.66 | >0.999 | ||||
| FOLFOX vs FOLFIRINOX (2 v 3) | 1.47 | 0.20 | 1.02–2.12 | 0.039 | ||||
| FOLFOX vs Other (2 v 4) | 1.16 | 0.11 | 0.90–1.49 | 0.760 | ||||
| FOLFIRINOX vs Other (3 v 4) | 0.79 | 0.11 | 0.54–1.15 | 0.581 | ||||
| Nausea – Occurrence (n=401) | ||||||||
| P1 assessments | 1.68 | 0.34 | 1.13–2.50 | 0.011 | ||||
| P2 assessments | 0.32 | 0.09 | 0.18–0.55 | <0.001 | ||||
| P3 assessments | 4.47 | 1.34 | 2.48–8.06 | <0.001 | ||||
| P4 assessments | 0.15 | 0.06 | 0.07–0.32 | <0.001 | ||||
| Treatment group | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Age | ||||||||
| P1 assessments | 1.67 | 0.34 | 1.12–2.49 | 0.012 | ||||
| P2 assessments | 0.32 | 0.09 | 0.18–0.56 | <0.001 | ||||
| P3 assessments | 4.45 | 1.34 | 2.47–8.01 | <0.001 | ||||
| P4 assessments | 0.15 | 0.06 | 0.07–0.32 | <0.001 | ||||
| Enrollment | 0.94 | 0.01 | 0.91–0.96 | <0.001 | ||||
| Cross-level interaction | NS | |||||||
| Number of metastatic sites | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Time from cancer diagnosis | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Number of prior cancer treatments | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Cancer diagnosis | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Chemotherapy regimen | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Feeling Drowsy – Occurrence (n=401) | ||||||||
| Linear | 0.85 | 0.03 | 0.79–0.91 | <0.001 | ||||
| Treatment group | ||||||||
| Linear | 0.84 | 0.03 | 0.79–0.91 | <0.001 | ||||
| Enrollment (Reference is targeted therapy = no) | 0.47 | 0.15 | 0.25–0.88 | 0.018 | ||||
| Cross-level interaction | NS | |||||||
| Age | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Number of metastatic sites | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Time from cancer diagnosis | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Number of prior cancer treatments | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Cancer diagnosis | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Chemotherapy regimen | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Numbness/Tingling in Hands/Feet – Occurrence (n=401) | ||||||||
| Piecewise model | ||||||||
| P1 assessments | 1.32 | 0.28 | 0.87–2.01 | 0.192 | ||||
| P2 assessments | 0.52 | 0.15 | 0.29–0.92 | 0.025 | ||||
| P3 assessments | 2.45 | 0.75 | 1.34–4.48 | 0.004 | ||||
| P4 assessments | 0.41 | 0.17 | 0.18–0.93 | 0.034 | ||||
| Treatment group | ||||||||
| Enrollment | NS | |||||||
| Cross level interaction | NS | |||||||
| Age | ||||||||
| P1 assessments | 1.31 | 0.28 | 0.86–2.01 | 0.201 | ||||
| P2 assessments | 0.52 | 0.15 | 0.29–0.93 | 0.027 | ||||
| P3 assessments | 2.47 | 0.77 | 1.34–4.54 | 0.004 | ||||
| P4 assessments | 0.41 | 0.18 | 0.18–0.938 | 0.035 | ||||
| Enrollment | NS | |||||||
| Cross-level interaction: omnibus test | X2 = 16.04; p = 0.003 | |||||||
| P1 by Age | 1.00 | 0.02 | 0.97–1.04 | 0.989 | ||||
| P2 by Age | 1.03 | 0.03 | 0.98–1.08 | 0.210 | ||||
| P3 by Age | 0.94 | 0.03 | 0.89–0.99 | 0.027 | ||||
| P4 by Age | 1.05 | 0.04 | 0.98–1.13 | 0.203 | ||||
| Number of metastatic sites | ||||||||
| P1 assessments | 1.32 | 0.28 | 0.87–2.01 | 0.189 | ||||
| P2 assessments | 0.52 | 0.15 | 0.29–0.92 | 0.025 | ||||
| P3 assessments | 2.45 | 0.76 | 1.34–4.49 | 0.004 | ||||
| P4 assessments | 0.41 | 0.17 | 0.18–0.93 | 0.033 | ||||
| Enrollment | 0.72 | 0.10 | 0.54–0.96 | 0.026 | ||||
| Cross level interaction | NS | |||||||
| Time from cancer diagnosis | ||||||||
| Enrollment | NS | |||||||
| Cross level interaction | NS | |||||||
| Number of prior cancer treatments | ||||||||
| Enrollment | NS | |||||||
| Cross level interaction | NS | |||||||
| Cancer diagnosis | ||||||||
| P1 assessments | 1.34 | 0.29 | 0.87–2.04 | 0.180 | ||||
| P2 assessments | 0.51 | 0.15 | 0.29–0.91 | 0.023 | ||||
| P3 assessments | 2.44 | 0.76 | 1.33–4.48 | 0.004 | ||||
| P4 assessments | 0.41 | 0.17 | 0.18–0.94 | 0.036 | ||||
| Enrollment (Reference is colon = no) | 2.16 | 0.73 | 1.11–4.20 | 0.023 | ||||
| Cross level interaction | NS | |||||||
| Chemotherapy regimen | ||||||||
| P1 assessments | 1.34 | 0.29 | 0.88–2.03 | 0.177 | ||||
| P2 assessments | 0.51 | 0.15 | 0.29–0.91 | 0.022 | ||||
| P3 assessments | 2.47 | 0.76 | 1.35–4.51 | 0.003 | ||||
| P4 assessments | 0.41 | 0.17 | 0.18–0.93 | 0.033 | ||||
| Enrollment: omnibus test | X2 = 39.86; p <0.001 | |||||||
| Enrollment: pairwise comparisons* | ||||||||
| FOLFIRI vs FOLFOX (1 v 2) | 9.70 | 4.76 | 2.66–35.38 | <0.001 | ||||
| FOLFIRI vs FOLFIRINOX (1 v 3) | 3.16 | 2.00 | 0.60–16.71 | 0.409 | ||||
| FOLFIRI vs Other (1 v 4) | 1.11 | 0.55 | 0.30–4.07 | >0.999 | ||||
| FOLFOX vs FOLFIRINOX (2 v 3) | 0.33 | 0.17 | 0.08–1.33 | 0.216 | ||||
| FOLFOX vs Other (2 v 4) | 0.11 | 0.04 | 0.04–0.31 | <0.001 | ||||
| FOLFIRINOX vs Other (3 v 4) | 0.35 | 0.19 | 0.08–1.49 | 0.336 | ||||
| Cross-level interaction | NS | |||||||
| Difficulty Sleeping – Occurrence (n=401) | ||||||||
| Linear | 0.89 | 0.04 | 0.83–0.96 | 0.004 | ||||
| Treatment group | ||||||||
| Linear | 0.89 | 0.04 | 0.82–0.96 | 0.003 | ||||
| Enrollment (Reference is targeted therapy = no) | 0.40 | 0.16 | 0.19–0.86 | 0.018 | ||||
| Cross-level interaction | NS | |||||||
| Age | ||||||||
| Linear | 0.89 | 0.04 | 0.83–0.96 | 0.004 | ||||
| Enrollment | 0.94 | 0.01 | 0.92–0.97 | <0.001 | ||||
| Cross-level interaction | NS | |||||||
| Number of metastatic sites | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Time from cancer diagnosis | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Number of prior cancer treatments | ||||||||
| Linear | 0.89 | 0.04 | 0.82–0.96 | 0.002 | ||||
| Enrollment | 1.56 | 0.20 | 1.22–2.00 | <0.001 | ||||
| Cross-level interaction | NS | |||||||
| Cancer diagnosis | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Chemotherapy regimen | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Change In the Way Food Tastes – Occurrence (n=401) | ||||||||
| Linear | 0.91 | 0.04 | 0.84–0.99 | 0.024 | ||||
| Treatment group | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Age | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Number of metastatic sites | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Time from cancer diagnosis | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Number of prior cancer treatments | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Cancer diagnosis | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Chemotherapy regimen | ||||||||
| Linear (FOLFIRI is reference) | 0.91 | 0.04 | 0.84–0.99 | 0.023 | ||||
| Enrollment: omnibus test | X2 = 9.41; p=0.024 | |||||||
| Enrollment: pairwise comparisons* | ||||||||
| FOLFIRI vs FOLFOX (1 v 2) | 3.72 | 1.87 | 0.99–14.01 | 0.053 | ||||
| FOLFIRI vs FOLFIRINOX (1 v 3) | 1.65 | 1.10 | 0.29–9.49 | >0.999 | ||||
| FOLFIRI vs Other (1 v 4) | 1.54 | 0.80 | 0.39–6.10 | >0.999 | ||||
| FOLFOX vs FOLFIRINOX (2 v 3) | 0.44 | 0.25 | 0.10–1.95 | 0.888 | ||||
| FOLFOX vs Other (2 v 4) | 0.41 | 0.16 | 0.15–1.14 | 0.130 | ||||
| FOLFIRINOX vs Other (3 v 4) | 0.93 | 0.54 | 0.20–4.31 | >0.999 | ||||
| Cross-level interaction | NS | |||||||
CI = confidence interval; FOLFIRI = leucovorin/5-fluorouracil/irinotecan; FOLFIRINOX = leucovorin/5-fluorouracil/irinotecan/oxaliplatin; NS = not significant; OR = odds ratio; P1 = enrollment to time 2; P2 = time 2 to time 3; P3 = time 4 to time 5; P4 = time 5 to time 6; SE = standard error
Estimates of odds ratios from three models, each with a different reference category in order to obtain Bonferroni post hoc pairwise comparisons: alpha’ for six comparisons controlled at .0083 (.05/6).
For chemotherapy regiment: 1 = FOLFIRI; 2 = FOLFOX; 3 = FOLFIRINOX; 4 = Other
Table 3.
Results of the Multilevel Regression Analyses of Severity Ratings for the Seven Symptoms that Occurred in ≥50% of the Patients
| Pain – Severity (n=293) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Unconditional Model | Conditional Model | |||||||
| OR | SE | CI | p-value | OR | SE | CI | p-value | |
| Linear | 0.95 | 0.04 | 0.88–1.03 | NS | ||||
| Treatment group | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Age | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Number of metastatic sites | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Time from cancer diagnosis | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Number of prior cancer treatments | ||||||||
| Linear | 0.95 | 0.04 | 0.87–1.03 | 0.232 | ||||
| Enrollment | 1.32 | 0.15 | 1.06–1.65 | 0.015 | ||||
| Cross-level interaction | NS | |||||||
| Cancer diagnosis | ||||||||
| Linear | 0.81 | 0.06 | 0.70–0.94 | 0.006 | ||||
| Enrollment (Reference is Colon=no) | 0.91 | 0.34 | 0.43–1.91 | 0.793 | ||||
| Cross-level interaction | 1.27 | 0.12 | 1.06–1.52 | 0.009 | ||||
| Chemotherapy regimen | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Lack of Energy – Severity (n=370) | ||||||||
| Piecewise model | ||||||||
| P1 assessments | 1.56 | 0.28 | 1.10–2.23 | 0.013 | ||||
| P2 assessments | 0.41 | 0.10 | 0.25–0.67 | <0.001 | ||||
| P3 assessments | 3.56 | 0.96 | 2.09–6.04 | <0.001 | ||||
| P4 assessments | 0.23 | 0.08 | 0.11–0.47 | <0.001 | ||||
| Treatment group | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Age | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Number of metastatic sites | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Time from cancer diagnosis | ||||||||
| P1 assessments | 1.58 | 0.32 | 1.06–2.34 | 0.024 | ||||
| P2 assessments | 0.48 | 0.13 | 0.28–0.83 | 0.008 | ||||
| P3 assessments | 2.37 | 0.73 | 1.30–4.33 | 0.005 | ||||
| P4 assessments | 0.29 | 0.12 | 0.13–0.64 | 0.002 | ||||
| Enrollment | 0.998 | 0.06 | 0.88–1.13 | 0.972 | ||||
| Cross-level interaction: omnibus test | X2 = 10.54; p = 0.032 | |||||||
| P1 by Time from cancer diagnosis | 0.99 | 0.06 | 0.88–1.11 | 0.854 | ||||
| P2 by Time from cancer diagnosis | 0.92 | 0.07 | 0.79–1.08 | 0.299 | ||||
| P3 by Time from cancer diagnosis | 1.28 | 0.12 | 1.06–1.54 | 0.010 | ||||
| P4 by Time from cancer diagnosis | 0.87 | 0.10 | 0.69–1.09 | 0.212 | ||||
| Number of prior cancer treatments | ||||||||
| P1 assessments | 1.55 | 0.28 | 1.09–2.21 | 0.015 | ||||
| P2 assessments | 0.42 | 0.10 | 0.26–0.68 | <0.001 | ||||
| P3 assessments | 3.47 | 0.94 | 2.04–5.90 | <0.001 | ||||
| P4 assessments | 0.23 | 0.08 | 0.11–0.47 | <0.001 | ||||
| Enrollment | 1.33 | 0.15 | 1.07–1.65 | 0.011 | ||||
| Cross-level interaction | NS | |||||||
| Cancer diagnosis | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Chemotherapy regimen | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Nausea – Severity (n=282) | ||||||||
| Piecewise model | ||||||||
| P1 assessments | 1.09 | 0.26 | 0.69–1.72 | 0.706 | ||||
| P2 assessments | 0.80 | 0.25 | 0.43–1.49 | 0.485 | ||||
| P3 assessments | 2.68 | 0.93 | 1.36–5.29 | 0.004 | ||||
| P4 assessments | 0.14 | 0.06 | 0.06–0.34 | <0.001 | ||||
| Treatment group | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Age | ||||||||
| P1 assessments | 1.10 | 0.26 | 0.69–1.73 | 0.693 | ||||
| P2 assessments | 0.80 | 0.25 | 0.43–1.48 | 0.476 | ||||
| P3 assessments | 2.67 | 0.93 | 1.36–5.27 | 0.005 | ||||
| P4 assessments | 0.14 | 0.07 | 0.06–0.35 | <0.001 | ||||
| Enrollment | 0.96 | 0.01 | 0.93–0.99 | 0.008 | ||||
| Cross-level interaction | NS | |||||||
| Number of metastatic sites | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Time from cancer diagnosis | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Number of prior cancer treatments | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Cancer diagnosis | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Chemotherapy regimen | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Feeling Drowsy – Severity (n=316) | ||||||||
| Linear | 0.97 | 0.04 | 0.89–1.05 | NS | ||||
| Treatment group | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Age | ||||||||
| Linear | 0.97 | 0.04 | 0.89–1.06 | 0.481 | ||||
| Enrollment | 0.98 | 0.01 | 0.96–0.999 | 0.038 | ||||
| Cross-level interaction | NS | |||||||
| Number of metastatic sites | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Time from cancer diagnosis | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Number of prior cancer treatments | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Cancer diagnosis | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Chemotherapy regimen | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Numbness/Tingling in Hands/Feet – Severity (n=314) | ||||||||
| Piecewise model | ||||||||
| P1 assessments | 1.35 | 0.28 | 0.90–2.04 | 0.152 | ||||
| P2 assessments | 0.55 | 0.16 | 0.31–0.96 | 0.036 | ||||
| P3 assessments | 2.04 | 0.63 | 1.11–3.72 | 0.021 | ||||
| P4 assessments | 0.50 | 0.20 | 0.23–1.09 | 0.084 | ||||
| Treatment group | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Age | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Number of metastatic sites | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Time from cancer diagnosis | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Number of prior cancer treatments | ||||||||
| P1 assessments | 1.33 | 0.28 | 0.88–2.01 | 0.170 | ||||
| P2 assessments | 0.56 | 0.16 | 0.32–0.97 | 0.040 | ||||
| P3 assessments | 2.06 | 0.63 | 1.13–3.76 | 0.019 | ||||
| P4 assessments | 0.49 | 0.20 | 0.22–1.09 | 0.080 | ||||
| Enrollment | 1.57 | 0.22 | 1.20–2.07 | 0.001 | ||||
| Cross-level interaction | NS | |||||||
| Cancer diagnosis | ||||||||
| P1 assessments | 1.33 | 0.28 | 0.88–2.01 | 0.174 | ||||
| P2 assessments | 0.55 | 0.16 | 0.31–0.97 | 0.037 | ||||
| P3 assessments | 2.12 | 0.65 | 1.16–3.88 | 0.015 | ||||
| P4 assessments | 0.48 | 0.20 | 0.22–1.07 | 0.074 | ||||
| Enrollment (Reference is Colon = no) | 2.66 | 1.03 | 1.25–5.68 | 0.011 | ||||
| Cross-level interaction | NS | |||||||
| Chemotherapy regimen | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Difficulty Sleeping – Severity (n=311) | ||||||||
| Linear | 0.99 | 0.04 | 0.91–1.07 | NS | ||||
| Treatment group | ||||||||
| Linear | 1.04 | 0.05 | 0.95–1.14 | 0.425 | ||||
| Enrollment (Reference is targeted therapy = no) | 2.79 | 1.28 | 1.13–6.88 | 0.026 | ||||
| Cross-level interaction | 0.75 | 0.08 | 0.61–0.92 | 0.007 | ||||
| Age | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Number of metastatic sites | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Time from cancer diagnosis | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Number of prior cancer treatments | ||||||||
| Linear | 0.98 | 0.04 | 0.91–1.07 | 0.683 | ||||
| Enrollment | 1.44 | 0.17 | 1.14–1.82 | 0.002 | ||||
| Cross-level interaction | NS | |||||||
| Cancer diagnosis | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Chemotherapy regimen | ||||||||
| Linear | 0.99 | 0.04 | 0.91–1.07 | 0.750 | ||||
| Enrollment: omnibus test | X2 = 8.59; p = 0.035 | |||||||
| Enrollment: pairwise comparisons* | ||||||||
| FOLFIRI vs FOLFOX (1 v 2) | 0.33 | 0.17 | 0.08–1.25 | 0.168 | ||||
| FOLFIRI vs FOLFIRINOX (1 v 3) | 0.16 | 0.11 | 0.03–0.90 | 0.031 | ||||
| FOLFIRI vs Other (1 v 4) | 0.29 | 0.15 | 0.07–1.16 | 0.113 | ||||
| FOLFOX vs FOLFIRINOX (2 v 3) | 0.49 | 0.26 | 0.12–2.01 | >0.999 | ||||
| FOLFOX vs Other (2 v 4) | 0.88 | 0.33 | 0.33–2.35 | >0.999 | ||||
| FOLFIRINOX vs Other (3 v 4) | 1.79 | 0.98 | 0.42–7.61 | >0.999 | ||||
| Cross-level interaction | NS | |||||||
| Change In the Way Food Tastes – Severity (n=271) | ||||||||
| Piecewise model | ||||||||
| P1 assessments | 1.06 | 0.23 | 0.69–1.63 | 0.782 | ||||
| P2 assessments | 0.60 | 0.18 | 0.33–1.09 | 0.093 | ||||
| P3 assessments | 3.78 | 1.25 | 1.98–7.24 | <0.001 | ||||
| P4 assessments | 0.28 | 0.12 | 0.12–0.67 | 0.004 | ||||
| Treatment group | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Age | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Number of metastatic sites | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Time from cancer diagnosis | ||||||||
| P1 assessments | 1.09 | 0.24 | 0.71–1.68 | 0.700 | ||||
| P2 assessments | 0.58 | 0.18 | 0.32–1.05 | 0.070 | ||||
| P3 assessments | 4.10 | 1.37 | 2.13–7.88 | <0.001 | ||||
| P4 assessments | 0.26 | 0.12 | 0.11–0.63 | 0.003 | ||||
| Enrollment | 0.90 | 0.05 | 0.81–0.999 | 0.048 | ||||
| Cross-level interaction | NS | |||||||
| Number of prior cancer treatments | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Cancer diagnosis | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Chemotherapy regimen | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
CI = confidence interval; FOLFIRI = leucovorin/5-fluorouracil/irinotecan; FOLFIRINOX = leucovorin/5-fluorouracil/irinotecan/oxaliplatin; NS = not significant; OR = odds ratio; P1 = enrollment to time 2; P2 = time 2 to time 3; P3 = time 4 to time 5; P4 = time 5 to time 6; SE = standard error
Estimates of odds ratios from three models, each with a different reference category in order to obtain Bonferroni post hoc pairwise comparisons: alpha’ for six comparisons controlled at .0083 (.05/6).
For chemotherapy regiment: 1 = FOLFIRI; 2 = FOLFOX; 3 = FOLFIRINOX; 4 = Other
Table 4.
Results of the Multilevel Regression Analyses of Distress Ratings for the Seven Symptoms that Occurred in ≥50% of the Patients
| Pain – Distress (n=292) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Unconditional Model | Conditional Model | |||||||
| OR | SE | CI | p-value | OR | SE | CI | p-value | |
| Linear | 0.90 | 0.03 | 0.84–0.97 | 0.007 | ||||
| Treatment group | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Age | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Number of metastatic sites | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Time from cancer diagnosis | ||||||||
| Linear | 0.86 | 0.04 | 0.79–0.94 | 0.001 | ||||
| Enrollment | 0.93 | 0.05 | 0.83–1.04 | 0.217 | ||||
| Cross-level interaction | 1.03 | 0.01 | 1.005–1.05 | 0.017 | ||||
| Number of prior cancer treatments | ||||||||
| Linear | 0.90 | 0.03 | 0.84–0.97 | 0.007 | ||||
| Enrollment | 1.29 | 0.15 | 1.02–1.62 | 0.030 | ||||
| Cross-level interaction | NS | |||||||
| Cancer diagnosis | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Chemotherapy regimen | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Lack of Energy – Distress (n=366) | ||||||||
| Piecewise model | ||||||||
| P1 assessments | 1.47 | 0.24 | 1.07–2.03 | 0.017 | ||||
| P2 assessments | 0.41 | 0.09 | 0.27–0.64 | <0.001 | ||||
| P3 assessments | 2.85 | 0.68 | 1.79–4.53 | <0.001 | ||||
| P4 assessments | 0.43 | 0.13 | 0.23–0.79 | 0.007 | ||||
| Treatment group | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Age | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Number of metastatic sites | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Time from cancer diagnosis | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Number of prior cancer treatments | ||||||||
| P1 assessments | 1.47 | 0.24 | 1.06–2.01 | 0.019 | ||||
| P2 assessments | 0.42 | 0.09 | 0.27–0.64 | <0.001 | ||||
| P3 assessments | 2.84 | 0.68 | 1.78–4.54 | <0.001 | ||||
| P4 assessments | 0.42 | 0.13 | 0.23–0.79 | 0.007 | ||||
| Enrollment | 1.27 | 0.13 | 1.03–1.55 | 0.025 | ||||
| Cross-level interaction | NS | |||||||
| Cancer diagnosis | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Chemotherapy regimen | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Nausea – Distress (n=281) | ||||||||
| Linear | 0.90 | 0.04 | 0.83–0.98 | 0.015 | ||||
| Treatment group | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Age | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Number of metastatic sites | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Time from cancer diagnosis | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Number of prior cancer treatments | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Cancer diagnosis | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Chemotherapy regimen | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Feeling Drowsy – Distress (n=313) | ||||||||
| Piecewise model | ||||||||
| P1 assessments | 1.39 | 0.28 | 0.94–2.06 | 0.095 | ||||
| P2 assessments | 0.53 | 0.14 | 0.31–0.91 | 0.020 | ||||
| P3 assessments | 2.61 | 0.81 | 1.42–4.78 | 0.002 | ||||
| P4 assessments | 0.30 | 0.13 | 0.13–0.69 | 0.004 | ||||
| Treatment group | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Age | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Number of metastatic sites | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Time from cancer diagnosis | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Number of prior cancer treatments | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Cancer diagnosis | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Chemotherapy regimen | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Numbness/Tingling in Hands/Feet – Distress (n=315) | ||||||||
| Piecewise model | ||||||||
| P1 assessments | 1.01 | 0.19 | 0.70–1.46 | 0.941 | ||||
| P2 assessments | 0.79 | 0.20 | 0.48–1.29 | 0.342 | ||||
| P3 assessments | 2.05 | 0.56 | 1.20–3.52 | 0.009 | ||||
| P4 assessments | 0.45 | 0.16 | 0.22–0.91 | 0.027 | ||||
| Treatment group | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Age | ||||||||
| P1 assessments | 1.01 | 0.19 | 0.70–1.46 | 0.950 | ||||
| P2 assessments | 0.79 | 0.20 | 0.48–1.30 | 0.358 | ||||
| P3 assessments | 2.01 | 0.55 | 1.17–3.45 | 0.011 | ||||
| P4 assessments | 0.45 | 0.16 | 0.22–0.92 | 0.029 | ||||
| Enrollment | NS | |||||||
| Cross-level interaction: omnibus test | X2 = 16.62; p = 0.002 | |||||||
| Cross-level P1 assessments | 1.03 | 0.02 | 1.0002–1.07 | 0.048 | ||||
| Cross-level P2 assessments | 0.95 | 0.02 | 0.91–0.998 | 0.039 | ||||
| Cross-level P3 assessments | 1.08 | 0.03 | 1.03–1.13 | 0.003 | ||||
| Cross-level P4 assessments | 0.92 | 0.03 | 0.86–0.98 | 0.016 | ||||
| Number of metastatic sites | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Time from cancer diagnosis | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Number of prior cancer treatments | ||||||||
| P1 assessments | 1.01 | 0.19 | 0.70–1.46 | 0.937 | ||||
| P2 assessments | 0.79 | 0.20 | 0.48–1.29 | 0.340 | ||||
| P3 assessments | 2.05 | 0.56 | 1.20–3.52 | 0.009 | ||||
| P4 assessments | 0.45 | 0.16 | 0.22–0.91 | 0.027 | ||||
| Enrollment | 1.36 | 0.18 | 1.05–1.77 | 0.022 | ||||
| Cross-level interaction | NS | |||||||
| Cancer diagnosis | ||||||||
| P1 assessments | 1.02 | 0.19 | 0.70–1.47 | 0.934 | ||||
| P2 assessments | 0.78 | 0.20 | 0.47–1.28 | 0.323 | ||||
| P3 assessments | 2.09 | 0.58 | 1.22–3.59 | 0.007 | ||||
| P4 assessments | 0.45 | 0.16 | 0.22–0.92 | 0.029 | ||||
| Enrollment (Reference is Colon=no) | 2.46 | 0.92 | 1.18–5.12 | 0.017 | ||||
| Cross-level interaction | NS | |||||||
| Chemotherapy regimen | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Difficulty Sleeping – Distress (n=312) | ||||||||
| Linear | 0.73 | 0.09 | 0.57–0.93 | 0.010 | ||||
| Quadratic | 1.05 | 0.03 | 1.001–1.10 | 0.046 | ||||
| Treatment group | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Age | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Number of metastatic sites | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Time from cancer diagnosis | ||||||||
| Linear | 0.64 | 0.09 | 0.48–0.83 | 0.001 | ||||
| Quadratic | 1.08 | 0.03 | 1.02–1.14 | 0.004 | ||||
| Enrollment | 0.91 | 0.05 | 0.81–1.03 | 0.123 | ||||
| Linear – cross level interaction | 1.09 | 0.04 | 1.02–1.18 | 0.021 | ||||
| Quadratic – cross-level interaction | 0.98 | 0.01 | 0.97–0.996 | 0.014 | ||||
| Number of prior cancer treatments | ||||||||
| Linear | 0.53 | 0.10 | 0.36–0.77 | 0.001 | ||||
| Quadratic | 1.12 | 0.04 | 1.04–1.20 | 0.003 | ||||
| Enrollment | 1.10 | 0.15 | 0.84–1.44 | 0.504 | ||||
| Linear – cross level interaction | 1.24 | 0.12 | 1.03–1.49 | 0.022 | ||||
| Quadratic – cross-level interaction | 0.96 | 0.02 | 0.93–0.99 | 0.021 | ||||
| Cancer diagnosis | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Chemotherapy regimen | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Change In the Way Food Tastes – Distress (n=271) | ||||||||
| Piecewise model | ||||||||
| P1 assessments | 1.36 | 0.29 | 0.90–2.07 | 0.148 | ||||
| P2 assessments | 0.49 | 0.14 | 0.28–0.87 | 0.014 | ||||
| P3 assessments | 2.94 | 0.92 | 1.59–5.43 | 0.001 | ||||
| P4 assessments | 0.31 | 0.13 | 0.14–0.71 | 0.005 | ||||
| Treatment group | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Age | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Number of metastatic sites | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Time from cancer diagnosis | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Number of prior cancer treatments | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Cancer diagnosis | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
| Chemotherapy regimen | ||||||||
| Enrollment | NS | |||||||
| Cross-level interaction | NS | |||||||
CI = confidence interval; NS = not significant; OR = odds ratio; P1 = enrollment to time 2; P2 = time 2 to time 3; P3 = time 4 to time 5; P4 = time 5 to time 6; SE = standard error
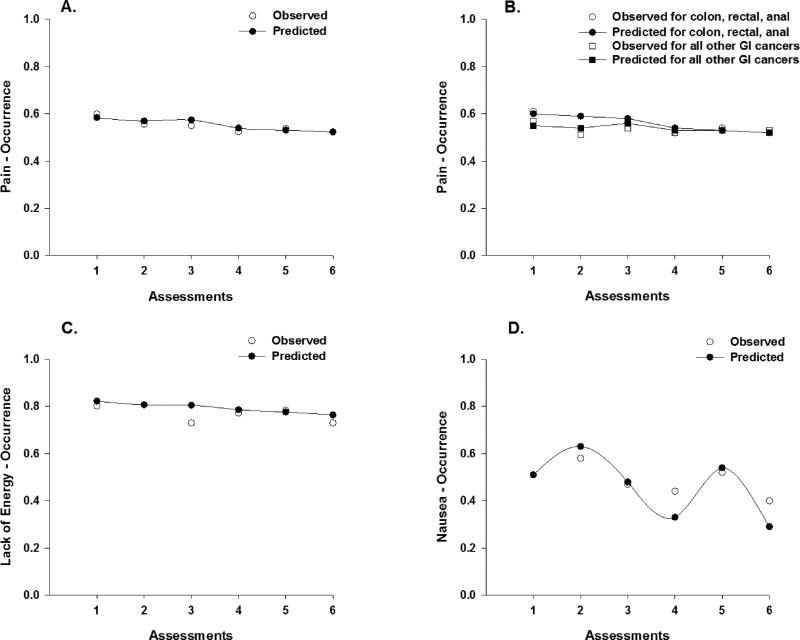
Figure 1.
Observed (open circles) and predicted (filled circles) trajectories for the probability of occurrence of pain (a), lack of energy (c), and nausea (d) across the six assessments. Observed and predicted trajectories for the occurrence of pain across the six assessments for patients with colon, rectal, and anal cancers versus patients with all other gastrointestinal (GI) cancers (b).
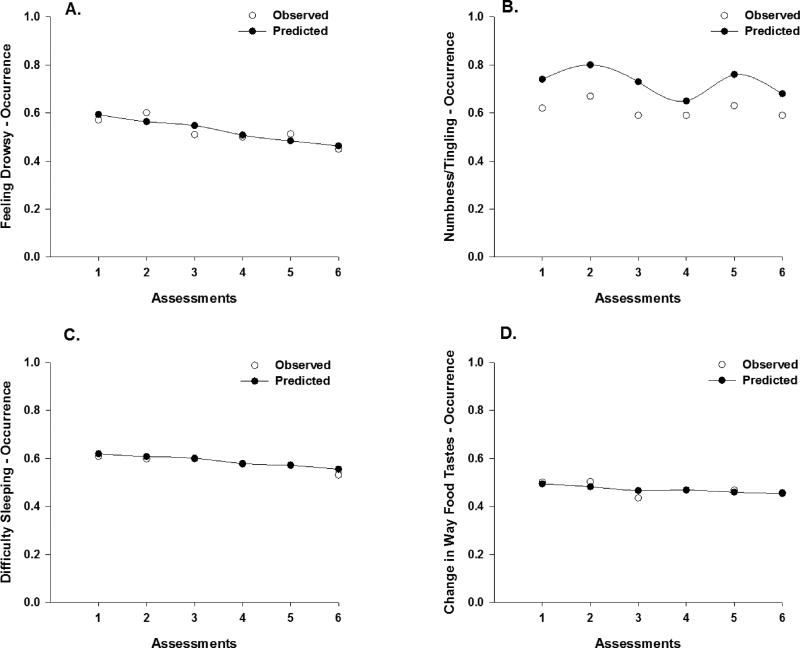
Figure 2.
Observed (open circles) and predicted (filled circles) trajectories for the probability of occurrence of feeling drowsy (a), numbness/tingling in hands/feet (b), difficulty sleeping (c), and change in the way food tastes (d) across the six assessments.
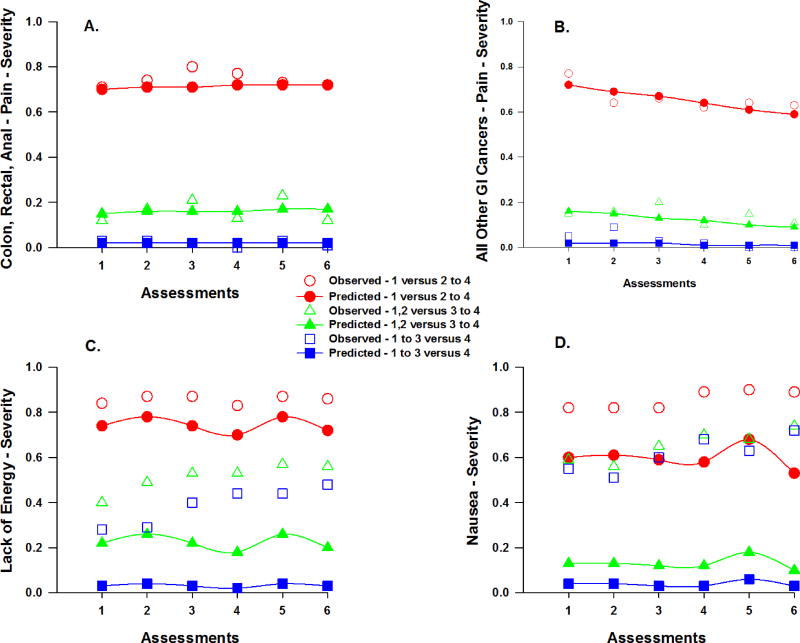
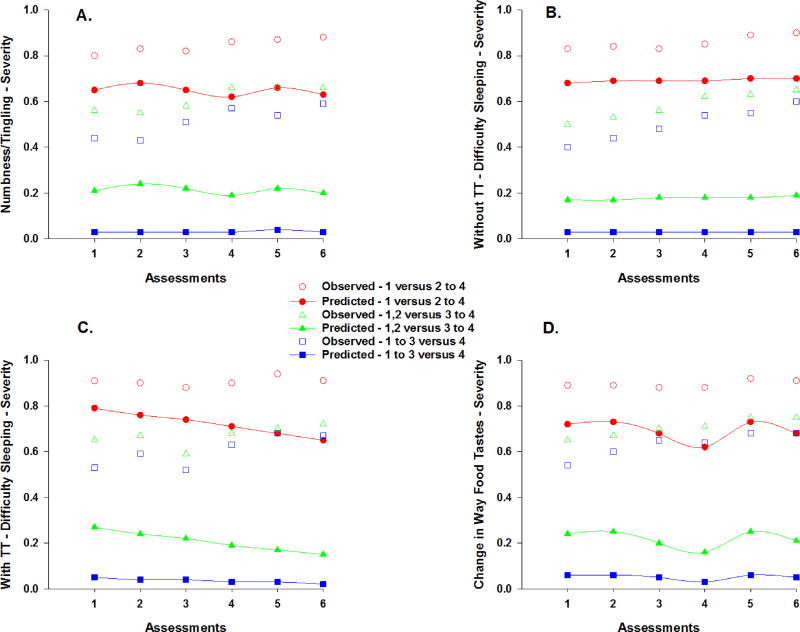
The figures that illustrate the unconditional and conditional models for the severity (Figures 3 and 4) and distress (Figures 5 and 6) ratings for the various symptoms can be interpreted as the probabilities that a patient would give a rating for a higher value or values, compared to the lower value(s) on the ordinal symptom severity or distress scales. For example, for severity, one line displays the predicted probability that a patient would give a rating of 2, 3, or 4, compared to 1. A second line in the plot displays the probability that a patient would respond 3 or 4, compared to 1 or 2, and so forth. For the same figure, the observed plots show the cumulative proportions of responding 1 compared to 2–4, a second line for the cumulative proportions of responding 1 or 2 compared to 3 to 4, and a third line for the cumulative proportions of responding 1, 2, or 3 compared to 4. The computed probabilities for each plot are based on either the linear effect, or the linear and quadratic effects of time, or the piecewise coefficients for the change over time given multiple “bends” in the trajectory due to the two treatment cycles.
Figure 3.
Observed and predicted trajectories for the probability of a reporting a higher versus a lower severity rating for pain across the six assessments for patients with colon, rectal, and anal cancers (a) versus patients with all other gastrointestinal (GI) cancers (b). Observed and predicted trajectories for the probability of a higher versus a lower severity rating for lack of energy (c) and nausea (d) across the six assessments. Severity ratings are plotted as observed (open circles) and predicted (filled symbols) values for slight versus moderate to very severe (1 versus 2 to 4), slight/moderate versus severe/very severe (1,2 versus 3 to 4), and slight to severe versus very severe (1 to 3 versus 4).
Figure 4.
Observed and predicted trajectories for the probability of a higher versus a lower severity rating for numbness/tingling in the hands/feet (a) and change in the way food tastes (d) across the six assessments. Observed and predicted trajectories for the probability of a reporting a higher versus a lower severity rating for difficulty sleeping across the six assessments for patients who did not (b) and did (c) received targeted therapy (TT) with their chemotherapy. Severity ratings are plotted as observed (open circles) and predicted (filled symbols) values for slight versus moderate to very severe (1 versus 2 to 4), slight/moderate versus severe/very severe (1,2 versus 3 to 4), and slight to severe versus very severe (1 to 3 versus 4).
Figure 5.
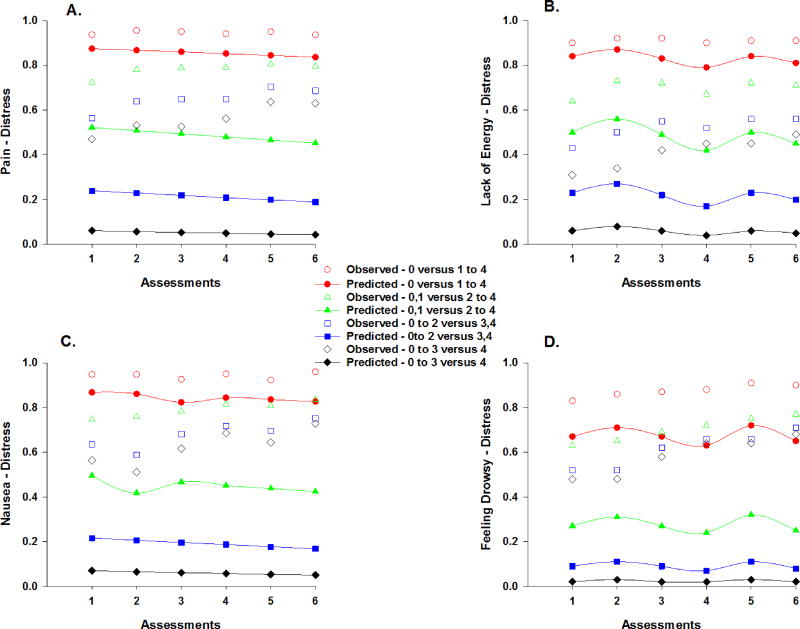
Observed and predicted trajectories for the probability of a higher versus a lower distress rating for pain (a), lack of energy (b), nausea (c), and feeling drowsy (d) across the six assessments. Distress ratings are plotted as observed (open circles) and predicted (filled symbols) values for not at all versus a little bit to very much (0 versus 1 to 4), not at all/a little bit versus somewhat to very much (0,1 versus 2 to 4), not at all to somewhat versus quite a bit/very much (0 to 2 versus 3,4), and not at all to quite a bit versus very much (0 to 3 versus 4).
Figure 6.
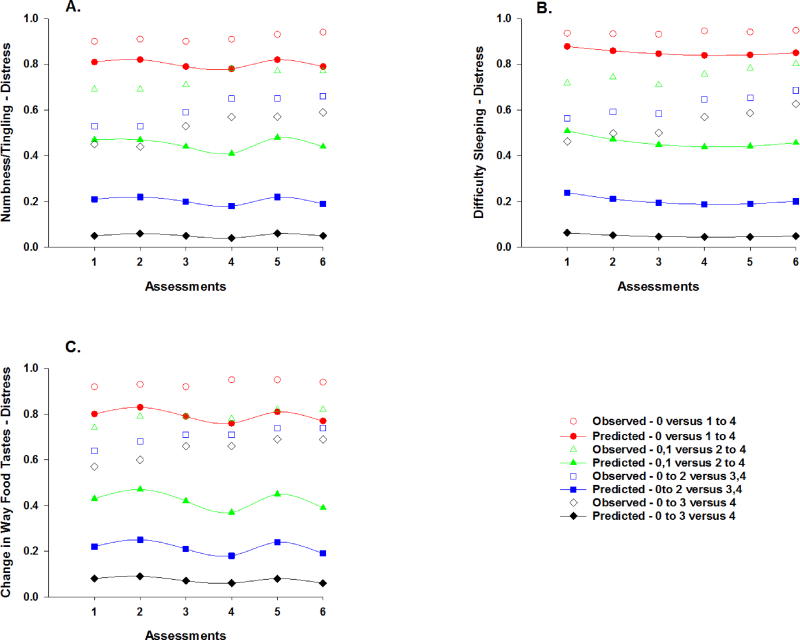
Observed and predicted trajectories for the probability of a higher versus a lower distress rating for numbness/tingling in hands/feet (a), difficulty sleeping (b), and change in the way food tastes (c) across the six assessments. Distress ratings are plotted as observed (open circles) and predicted (filled symbols) values for not at all versus a little bit to very much (0 versus 1 to 4), not at all/a little bit versus somewhat to very much (0,1 versus 2 to 4), not at all to somewhat versus quite a bit/very much (0 to 2 versus 3,4), and not at all to quite a bit versus very much (0 to 3 versus 4).
Pain
Approximately 60% of patients reported pain before their next dose of CTX (i.e., T1). As illustrated in Figure 1a, occurrence rates for pain displayed a linear decrease after T1 through T6 (odds ratio [OR] = 0.91), which was attenuated by a 9% decrease in the odds of reporting the occurrence of pain (Table 2). Age, time from cancer diagnosis, and number of prior cancer treatments were associated with the occurrence of pain at enrollment. For each additional year of age, the odds of reporting pain decreased by 6%. For each additional year from the patients’ cancer diagnosis, the odds of reporting pain were 1.13 times more likely. As the number of prior cancer treatments increased, the odds of reporting pain were 1.80 times more likely. In addition, a cross-level interaction with cancer diagnosis (i.e., colon, rectal, or anal [i.e., CRC group] versus other GI cancers) was significant (Figure 1b). For each additional assessment, the odds of reporting pain decreased by 16% for patients in the CRC group compared to patients with other GI cancers.
Severity ratings for pain did not change over time (Table 3). However, number of prior cancer treatments was associated with the severity of pain at enrollment. As the number of prior cancer treatments increased, the odds of moving one point higher on the pain severity scale were 1.32 times more likely. In addition, the cross-level interaction with cancer diagnosis was significant (Figures 3a and 3b). For each additional assessment, the odds of moving one point higher on the pain severity scale increased by 27% for patients in the CRC group compared to those with other types of GI cancers.
Distress ratings for pain changed over time (Table 4). As illustrated in Figure 5a, for each additional assessment, the odds of moving one point higher on the pain distress scale decreased by 10%. Only number of prior cancer treatments was associated with pain distress at enrollment. As the number of prior cancer treatments increased, the odds of moving one point higher on the pain distress scale were 1.29 times more likely. In addition, the cross-level interaction with time from cancer diagnosis was significant. For each additional assessment, as the year from the patients’ cancer diagnosis increased, the odds of moving one point higher on the pain distress scale were 1.03 times more likely.
Lack of Energy
Approximately, 80% of patients reported lack of energy before their next dose of CTX (i.e., T1). As illustrated on Figure 1c, occurrence rates for lack of energy displayed a linear decrease after T1 through T6 (OR = 0.91), which was attenuated by a 9% decrease in the odds of reporting the occurrence of lack of energy (Table 2). Age and time from cancer diagnosis were associated with the occurrence of lack of energy at enrollment. For each additional year of age, the odds of reporting lack of energy decreased by 3%. For each additional year from the patients’ cancer diagnosis, the odds of reporting lack of energy were 1.17 times more likely. In addition, a cross-level interaction with CTX regimen was significant. For each additional assessment, patients who received FOLFIRINOX were 1.47 times more likely to report lack of energy compared to patients who received FOLFOX.
Severity ratings for lack of energy changed over time (Table 3). As illustrated in the piecewise model in Figure 3c, the odds of moving one point higher on the lack of energy severity scale were 1.56 times greater from piecewise segment T1 to T2, decreased by 59% from piecewise segment T2 to T3, were 3.56 times greater from piecewise segment T4 to T5, and decreased by 77% from piecewise segment T5 to T6. Number of prior cancer treatments was associated with the severity of lack of energy at enrollment. As the number of prior cancer treatments increased, the odds of moving one point higher on the lack of energy severity scale were 1.33 times more likely. In addition, the cross level interaction with time from cancer diagnosis was significant. For each additional assessment, as the year from the patients’ cancer diagnosis increased, the odds of moving one point higher on the lack of energy severity scale were 1.28 times greater from piecewise segment T4 to T5.
Distress ratings for lack of energy changed over time (Table 4). As illustrated in the piecewise model in Figure 5b, the odds of moving one point higher on the lack of energy distress scale were 1.47 times greater from piecewise segment T1 to T2, decreased by 59% from piecewise segment T2 to T3, were 2.85 times greater from piecewise segment T4 to T5, and decreased by 57% from piecewise segment T5 to T6. Only number of prior cancer treatments was associated with the distress from lack of energy at enrollment. As the number of prior cancer treatments increased, the odds of moving one point higher on the lack of energy distress scale were 1.27 times more likely.
Nausea
Approximately 51% of the patients reported nausea before their next dose of CTX (i.e., T1). As illustrated in the piecewise model in Figure 1d, occurrence rates for nausea displayed a 1.68 increase in the odds from piecewise segment T1 to T2, a decrease in the odds of 68% from piecewise segment T2 to T3, a 4.47 increase in the odds from piecewise segment T4 to T5, and a decrease in the odds of 85% from piecewise segment T5 to T6 (Table 2). Age was associated with the occurrence of nausea at enrollment. For each additional year of age, the odds of reporting nausea decreased by 6%.
Severity ratings for nausea changed over time (Table 3). As illustrated in the piecewise model in Figure 3d, the odds of moving one point higher on the nausea severity scale were 2.68 times greater from piecewise segment T4 to T5 and decreased by 86% from piecewise segment T5 to T6. Age was associated with the severity of nausea at enrollment. For each additional year of age, the odds of moving one point higher on the nausea severity scale decreased by 4%.
Distress ratings for nausea changed over time (Table 4). As illustrated in Figure 5c, for each additional assessment, the odds of moving one point higher on the nausea distress scale decreased by 10%. None of the covariates were associated with the distress ratings for nausea at enrollment or exhibited cross-level interactions.
Feeling Drowsy
Approximately 58% of the patients reported feeling drowsy before their next dose of CTX (i.e., T1). As illustrated in Figure 2a, occurrence rates for feeling drowsy displayed a linear decrease after T1 through T6 (OR = 0.85) that was attenuated by a 15% decrease in the odds of reporting the occurrence of feeling drowsy (Table 2). Only treatment group was associated with the occurrence of feeling drowsy at enrollment. The odds of reporting feeling drowsy decreased by 53% for patients who received CTX with TT.
Severity ratings for feeling drowsy did not change over time (Table 3). However, age was associated with the severity of feeling drowsy at enrollment. For each additional year of age, the odds of moving one point higher on the feeling drowsy severity scale decreased by 2%.
Distress ratings for feeling drowsy changed over time (Table 4). As illustrated in the piecewise model in Figure 5d, the odds of moving one point higher on the feeling drowsy distress scale decreased by 47% from piecewise segment T2 to T3, were 2.61 times greater from piecewise segment T4 to T5, and decreased by 70% from piecewise segment T5 to T6. None of the covariates were associated with the distress ratings for feeling drowsy at enrollment or exhibited cross-level interactions.
Numbness/Tingling in Hands/Feet
Approximately 63% of the patients reported numbness/tingling in hands/feet before their next dose of CTX (i.e., T1). As illustrated in the piecewise model in Figure 2b, occurrence rates for numbness/tingling in hands/feet displayed a decrease in the odds of 48% from piecewise segment T2 to T3, a 2.45 increase in the odds from piecewise segment T4 to T5, and a decrease in the odds of 59% from piecewise segment T5 to T6 (Table 2). Number of metastatic sites, cancer diagnosis, and CTX regimen were associated with the occurrence of numbness/tingling in hands/feet at enrollment. As the number of metastatic sites increased, the odds of reporting numbness/tingling in hands/feet decreased by 28%. The odds of reporting numbness/tingling in hands/feet were 2.16 times greater for patients in the CRC group. Patients who received FOLFOX were 9.70 times more likely to report numbness/tingling in hands/feet compared to patients who received FOLFIRI. In addition, patients who received other combinations of CTX were 89% less likely to report numbness/tingling in hands/feet compared to patients who received FOLFOX. A cross level interaction was detected for age and the occurrence of numbness and tingling in the hands and feet. For each additional assessment, as the patients’ age increased by one year, the odds of reporting pain decreased by 6% between piecewise segment T4 and T5.
Severity ratings for numbness/tingling in hands/feet changed over time (Table 3). As illustrated in the piecewise model in Figure 4a, the odds of moving one point higher on the numbness/tingling in hands/feet severity scale decreased by 45% from piecewise segment T2 to T3 and were 2.04 times greater from piecewise segment T4 to T5. Number of prior treatments and cancer diagnosis were associated with the severity of numbness/tingling in hands/feet at enrollment. As the number of prior cancer treatments increased, the odds of moving one point higher on the numbness/tingling in hands/feet severity scale were 1.57 times more likely. The odds of moving one point higher on the numbness/tingling in hands/feet severity scale were 2.66 times greater for patients in the CRC group.
Distress ratings for numbness/tingling in hands/feet changed over time (Table 4). As illustrated in the piecewise model in Figure 6a, the odds of moving one point higher on the numbness/tingling in hands/feet distress scale were 2.05 times greater from piecewise segment T4 to T5 and decreased by 55% from piecewise segment T5 to T6. Number of prior cancer treatments and cancer diagnosis were associated with the distress from numbness/tingling in hands/feet at enrollment. As the number of prior cancer treatments increased, the odds of moving one point higher on the numbness/tingling in hands/feet distress scale were 1.36 times more likely. The odds of moving one point higher on the numbness/tingling in hands/feet distress scale were 2.46 times greater for patients in the CRC group. In addition, a cross-level interaction with age was significant. For each additional assessment, as the patients’ age increased by one year, the odds of moving one point higher on the numbness/tingling in hands/feet distress scale were 1.03 times more likely from piecewise segment T1 to T2, decreased by 5% from piecewise segment T2 to T3, were 1.08 times more likely from piecewise segment T4 to T5, and decreased by 8% from T5 to T6.
Difficulty Sleeping
Approximately 61% of the patients reported difficulty sleeping before their next dose of CTX (i.e., T1). As illustrated in Figure 2c, occurrence rates for difficulty sleeping displayed a linear decrease after T1 through T6 (OR = 0.89), which was attenuated by an 11% decrease in the odds of reporting the occurrence of difficulty sleeping (Table 2). Treatment group, age, and number of prior cancer treatments were associated with the occurrence of difficulty sleeping at enrollment. For patients who received TT, the odds of reporting difficulty sleeping decreased by 60%. For each additional year of age, the odds of reporting difficulty sleeping decreased by 6%. As the number of prior cancer treatments increased, the odds of reporting difficulty sleeping were 1.56 times more likely.
Severity ratings for difficulty sleeping did not change over time (Table 3). However, number of prior cancer treatments and CTX regimen were associated with the severity of difficulty sleeping at enrollment. As the number of prior cancer treatments increased, the odds of moving one point higher on the difficulty sleeping severity scale were 1.44 times more likely. In addition, patients who received FOLFIRINOX were 84% less likely to report higher severity scores for difficulty sleeping compared to patients who received FOLFIRI. In addition, a cross-level interaction with treatment group was significant (Figures 4a and 4b). For each additional assessment, the odds of moving one point higher on the difficulty sleeping severity scale decreased by 25% for patients who received CTX with TT.
Distress ratings for difficulty sleeping changed over time (Table 4). As illustrated in the quadratic model in Figure 6b, for each additional assessment, the odds of moving one point higher on the difficulty sleeping distress scale initially decreased by 27%. Then the rate of decrease was attenuated by an increase of 5% in the rate of change for each assessment (i.e., the decreasing trajectory became less steep). Cross level interactions were found for time from cancer diagnosis and number of prior cancer treatments. For each additional year from the patients’ cancer diagnosis, there was a 9% linear increase in the odds of moving one point higher on the difficulty sleeping scale together with a 2% decrease in the rate of change in the distress from difficulty sleeping for each additional assessment. As the number of prior cancer treatments increased, the odds of moving one point higher on the difficulty sleeping scale were 1.27 times more likely. In addition, as the number of prior cancer treatments increased, a 24% linear increase in the odds of moving one point higher on the scale, attenuated by a 4% decrease in the rate of change, was found in reports of difficulty sleeping for each additional assessment.
Change in the Way Food Tastes
Approximately 51% of the patients reported change in the way food tastes before their next dose of CTX (i.e., T1). As illustrated in Figure 2d, occurrence rates for change in the way food tastes displayed a linear decrease after T1 through T6 (OR = 0.91), which was attenuated by a 9% decrease in the odds of reporting the occurrence of change in the way food tastes (Table 2). Except for CTX regimen, none of the covariates were associated with the occurrence of this symptom. However, none of the post hoc contrasts for CTX regimen were significant.
Severity ratings for change in the way food tastes changed over time (Table 3). As illustrated in the piecewise model in Figure 4d, the odds of moving one point higher on the change in the way food tastes severity scale were 3.78 times greater from piecewise segment T4 to T5 and decreased by 72% from piecewise segment T5 to T6. Time from cancer diagnosis was associated with the severity of this symptom at enrollment. For each additional year from the patients’ cancer diagnosis, the odds of moving one point higher on the change in the way food tastes severity scale decreased by 10%.
Distress ratings for change in the way food tastes changed over time (Table 4). As illustrated in the piecewise model in Figure 6c, the odds of moving one point higher on the change in the way food tastes distress scale decreased by 51% from piecewise segment T2 to T3, were 2.94 times greater from piecewise segment T4 to T5, and decreased by 69% from piecewise segment T5 to T6. None of the covariates were associated with distress ratings for change in the way food tastes at enrollment or exhibited cross level interactions.
DISCUSSION
To our knowledge, this study is the first to evaluate for changes over time in the occurrence, severity, and distress of seven common symptoms in GI cancer patients. Depending on the specific symptom, as well as the different dimensions of the symptom experience that were evaluated, the trajectories of these symptoms were highly variable. The various demographic and clinical characteristics, that were included in the regression analyses, provide useful information on phenotypic characteristics that were associated with a higher symptom burden. Findings for each symptom are discussed in detail in the subsequent sections of the discussion.
Pain
Consistent with previous reports,24–26 while 60% of the patients had pain at enrollment, occurrence rates for pain decreased over the two cycles of CTX. In terms of pain severity, while one study found a decrease in pain severity from the first (i.e., 3.00 in 0 to 10 NRS) to the sixth (i.e., 1.91) cycle of CTX,27 pain severity scores in our study did not change over time. This inconsistency may be related to differences in CTX regimens, GI cancer diagnoses, and/or the timing of assessments. Similar to occurrence rates, pain distress ratings decreased over time in our study. In terms of the findings regarding the influence of seven clinically meaningful covariates on the various dimensions of the pain experience, some interesting patterns were found.
Neither the receipt of TT nor the CTX regimen administered, had an effect on any dimension of the pain experience. However, the number of prior cancer treatments, but not the number of metastatic sites was associated with higher occurrence rates, as well as severity and distress ratings for pain at enrollment. While the types of cancer treatments that our patients received were representative of patients with GI cancers,28,29 previous studies in patients with cancer demonstrated that surgery [e.g., post surgical pain30], RT [e.g., enteritis, proctitis31] and CTX [e.g., neuropathy32] can result in chronic pain conditions and may have cumulative effects. While the specific etiologies for pain were not evaluated, the finding that an increased time from their cancer diagnosis, as well as increased number of cancer treatments, were associated with higher occurrence, severity, and distress ratings for pain suggests that our patients may be experiencing multiple chronic pain conditions. This hypothesis warrants investigation in future studies.
In terms of cancer diagnosis, for our analyses, patients were categorized into two groups (i.e., CRC group [colon, rectal, anal cancers] versus other GI cancers). In terms of pain occurrence, compared to patients with other GI cancers, patients in the CRC group were less likely to report the occurrence of pain. This finding is consistent with previous reports of pain in patients with a variety GI cancers,33,34 that found that occurrence rates for pain in patients with liver34 and pancreatic33 cancer ranged from 70.0% to 75.0%, respectively. In contrast, in previous studies, patients with CRC reported pain occurrence rates that ranged from 42.1%35 to 51%,36 respectively. However, in terms of pain severity, over the course of the two cycles of CTX, patients with CRC were more likely to report higher pain severity scores. This finding warrants confirmation in future studies. Finally, and consistent with previous reports,37,38 older patients were less likely to report the occurrence of pain at enrollment. However, age was not associated with differences in severity and distress ratings for pain.
Lack of Energy
Consistent with previous reports,39,40 80% of our patients reported lack of energy (i.e., proxy for fatigue on the MSAS) at enrollment. Of note, while occurrence rates decreased slightly, severity (Figure 3c) and distress (Figure 5b) ratings increased in the week following in the administration of each dose of CTX and then decreased the following week. These fluctuations in fatigue severity are consistent with a previous study of patients with CRC receiving adjuvant CTX.39
The phenotypic characteristics that influenced various dimensions of fatigue were: age, time from cancer diagnosis, number of prior cancer treatments, and the CTX regimen administered. Consistent with previous studies,6,41 younger age was associated with higher occurrence rates for fatigue. This association may be explained by age differences in the doses of CTX administered42 or by a “response shift” in older patients’ perceptions of symptoms.43,44
Similar to pain, a longer time from cancer diagnosis and receipt of a higher number of cancer treatments were associated with higher occurrence rates and/or higher severity and distress ratings for fatigue, respectively. This finding suggests that the cumulative effects of the disease and its treatments influence various dimensions of this symptom.
In terms of CTX regimen administered, compared to patients who received FOLFOX, patients who received FOLFIRINOX were approximately 1.5 times more likely to report the occurrence of fatigue. This finding may be related to the addition of irinotecan to the CTX regimen.
Nausea
Consistent with previous reports of acute nausea during CTX,45,46 over 50% of the patients reported the occurrence of nausea prior to their next dose of CTX. While distress from nausea decreased over the two cycles of CTX, occurrence (Figure 1d) and severity (Figure 3d) increased in the week following the administration of each dose of CTX and then decreased the following week. Our findings are consistent with previous studies that found that if nausea is not well controlled its occurrence and severity increases over the course of CTX.47,48 In addition, our findings suggest that a significant number of patients treated for GI cancers experience this symptom and that severity (range, 1 to 4; mean = 1.81, SD = 0.81) and distress (range, 0 to 4; mean = 1.68, SD = 1.12) ratings were at a moderate level. While most of the literature suggests that current antiemetic regimens effectively control vomiting,49,50 additional investigations are warranted on their impact on the various dimensions of nausea during CTX.
Age was the only characteristic associated with nausea. Consistent with previous reports of risk factors for CTX-induced nausea (CIN),51,52 younger patients were more likely to report the occurrence of and higher severity scores for CIN. Potential explanations for this associated risk include the fact that younger patients receive more aggressive treatment regimens, more combinations of CTX, and/or higher doses of CTX than older patients due to the increased toxicity risks.
Feeling Drowsy
Almost 60% of our patients reported the occurrence of feeling drowsy prior to their next dose of CTX. Consistent with other studies that used the MSAS to evaluate symptoms prior to or during CTX,25,26 our occurrence rate decreased over the two cycles of CTX. Possible reasons for the high occurrence of this symptom include the co-occurrence of other symptoms (e.g., pain and difficulty sleeping); medications patients were taking to decrease nausea and vomiting, pain, and/or anxiety; and/or dehydration. While severity ratings did not change over time, in the week following the administration of the first dose of CTX (i.e., T2 to T3), distress ratings for feeling drowsy decreased (Figure 5d). During the second cycle, distress ratings for feeling drowsy increased during the week following the second dose of CTX (i.e., T4 to T5) and then decreased in the subsequent week (i.e., T5 to T6). Future studies need to determine the etiologies for this symptom.
Feeling drowsy was one of two symptoms influenced by treatment regimen. Patients who received TT reported lower occurrence rates for this symptom prior to their next dose of CTX. While no study has reported on treatment group differences in this symptom in patients with GI cancers, in one study of patients with mCRC,53 fatigue scores were lower in the patients who received CTX with TT. This finding is important because previous studies found moderate to high correlations between fatigue and feeling drowsy, daytime sleepiness, and napping.54,55 Again, younger age was associated with higher severity ratings for feeling drowsy. If medications contribute to this symptom, it is known that older oncology patients take lower doses of analgesic medications56 and are less likely to take antiemetics57 and/or anti-anxiety medications.58
Numbness/Tingling in Hands/Feet
Numbness and tingling are symptoms associated with CTX-induced peripheral neuropathy (CIPN).59 While the exact prevalence of CIPN is not known, recent epidemiological studies suggest that between 19% to 85% of patients who receive neurotoxic CTX report this symptom.60,61 In our study, 63% of patients reported this symptom prior their next dose of CTX. This symptom is the only one that had a piecewise model for all three dimensions of the symptom experience. It is interesting to note that for all three symptom dimensions, from the week following the administration of CTX (T2) to the next assessment (T3), the occurrence (Figure 2b), severity (Figure 4a), and distress (Figure 6a) of numbness and tingling decreased. Then from prior to the next dose of CTX (T4) to the week of the next dose (T5), the occurrence, severity, and distress of numbness and tingling increased. While we did not perform an objective assessment of CIPN, the phenomenon of “coasting” is described in the literature. Coasting is defined as neurotoxicity that continues to progress for a period of time after the discontinuation of CTX.62 This piecewise pattern to the three symptom dimensions for numbness/tingling in hands/feet may be associated with this coasting phenomenon observed with neurotoxic CTX.
Except for treatment group and time from cancer diagnosis, every other covariate influenced some dimension of this symptom. Compared to the other four symptoms summarized above, age was not associated with the occurrence or severity of this symptom. Age affected only one piecewise segment of the distress ratings for this symptom. This finding warrants additional investigation given that previous studies found higher occurrence rates for CIPN in older oncology patients.61,63
While a lower number of metastatic sites was associated with higher occurrence rates of numbness/tingling in hands/feet prior to the next cycle of CTX, a higher number of previous treatments was associated with higher severity and distress ratings at enrollment. These associations may be partially explained by the fact that these patients received higher doses of neurotoxic CTX.
In our study compared to patients with other GI cancers, patients in the CRC group had higher occurrence rates, as well as higher severity and distress ratings for numbness/tingling in hands/feet prior to their next dose of CTX. This finding may be partially explained by the fact that in this study, patients in the CRC group were more likely to receive a platinum-based CTX regimen (i.e., FOLFOX [54.4%]). However, the relationship between numbness/tingling in hands/feet and a specific CTX regimen appears to be complex. In terms of occurrence, compared to patients who received other CTX regimens, patients who received FOLFOX reported higher occurrence rates for numbness/tingling in hands/feet at enrollment. None of these CTX regimens were associated with differences in severity and distress ratings associated with this symptom. While a recent review noted that the occurrence and severity of CIPN varies based on the CTX agent administered,11 additional studies are warranted to determine the characteristics and predictors of CIPN in patients with GI cancers who receive neurotoxic CTX.
Difficulty Sleeping
Consistent with previous studies of patients receiving CTX,39,64 61% of our patients reported difficulty sleeping at enrollment. While the occurrence of this symptom decreased over time and severity ratings did not change, distress ratings fit a quadratic model (i.e., decrease followed by an increase). As illustrated in Figure 6b, this slight increase occurs during the second cycle of CTX. Given the high occurrence of this symptom, as well as severity ratings in the moderate range (mean = 1.99, SD = 0.78), it makes sense that persistent sleep problems would be associated with increased levels of distress. Future studies need to determine the causes of sleep disturbance, in order to develop appropriate interventions.
With the exception of number of metastatic sites and cancer diagnoses, the other characteristics influenced some dimension of this symptom. Difficulty sleeping is the other symptom that was influenced by treatment group. For both occurrence and severity, patients who received TT reported less problems with difficulty sleeping. While the reasons for these decreases in occurrence and severity in the patients on TT are not known, one possible explanation could be that fatigue and difficulty sleeping were reported in previous studies to be part of a symptom cluster.65,66 Given that both the occurrence of these two symptoms decreased over two cycles of CTX, it may be that when these two symptoms do occur they interact with each other and display a similar pattern.
While the findings on age-related changes in sleep disturbance in the general population67 and cancer patients68 are inconclusive, consistent with our other symptoms, younger age was associated with higher occurrence rates for sleep disturbance at enrollment. In addition, receipt of a higher number of cancer treatments was associated with increases in all three dimensions of this symptom. This finding is consistent with previous reports that found that sleep problems remained elevated during and following cancer treatments.64,69
Change in the Way Food Tastes
In the limited amount of research on taste changes during CTX,70,71 occurrence rates ranged from 46% to 72%. In our study, over 50% of the patients reported changes in the way food tastes in the moderate range in terms of severity (mean = 2.11, SD = 0.81) and distress (mean = 1.61, SD = 1.20) at enrollment. While the occurrence of taste changes decreased over time, severity (Figure 4D) and distress (Figure 6c) scores during the second cycle displayed an increase in patients in the week following the administration (i.e., T4 to T5) followed by a decrease in this symptom in the following week (i.e., T5 to T6). While findings from a study of patients with breast and gynecological cancers suggest that distress scores for taste changes decrease over time,72 in our study distress scores followed a cyclic pattern. Time from cancer diagnosis was the only covariate associated with the severity of this symptom at enrollment. Additional research is warranted on factors that contribute to inter-individual variability in change in the way food tastes.
Limitations and Conclusions
Limitations of this study need to be acknowledged. Given the tremendous stress that patients experience at the initiation of CTX, patients were recruited during their second or third cycles of CTX. Future studies need to evaluate patients’ symptom experience prior to the initiation of CTX. In addition, if patients were followed for a longer period of time, the trajectories of the symptoms may change.
Despite these limitations, the findings from this study provide a more complete picture of the symptom experience of GI cancer patients receiving CTX. These findings suggest that seven symptoms occur at relatively high rates and are severe and distressing. An important finding is that compared to the occurrence of the five symptoms (i.e., pain, lack of energy, feeling drowsy, difficulty sleeping, change in the way food tastes) that decreased over time; nausea and numbness/tingling in hands/feet had more complex patterns of occurrence. In addition, the various covariates that were evaluated (i.e., treatment group, age, number of metastatic sites, time from cancer diagnosis, number of prior cancer treatments, cancer diagnosis, CTX regimen) had different effects on various dimensions of these patients’ symptom experiences, as well as on their overall symptom burden. Findings from this study can be used to identify patients with GI cancers who are at higher risk for more severe and distressing symptoms during CTX and to enable the initiation of more timely symptom management interventions.
Acknowledgments
This study was funded by a grant from the National Cancer Institute (NCI, CA134900). Dr. Christine Miaskowski is an American Cancer Society Clinical Research Professor and is funded by a K05 award from the NCI (CA168960). Mr. Tantoy was funded by a National Institutes of Health (NIH) T32 grant (NR007088). Dr. Wright was funded by a T32 postdoctoral fellowship (NR008346).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors have no conflicts of interest to disclose.
References
- 1.Tantoy IY, Cataldo JK, Aouizerat BE, Dhruva A, Miaskowski C. A review of the literature on multiple co-occurring symptoms in patients with colorectal cancer who received chemotherapy alone or chemotherapy with targeted therapies. Cancer Nurs. 2016;39:437–445. doi: 10.1097/NCC.0000000000000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tantoy IY, Dhruva A, Cataldo J, Venook A, Cooper BA, Paul SM, Levine JD, Conley YP, Cartwright F, Lee K, Wright F, Miaskowski C. Differences in symptom occurrence, severity, and distress ratings between patients with gastrointestinal cancers who received chemotherapy alone or chemotherapy with targeted therapy. J Gastrointest Oncol. 2017;8:109–126. doi: 10.21037/jgo.2017.01.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miaskowski C, Barsevick A, Berger A, et al. Advancing symptom science through symptom cluster research: Expert panel proceedings and recommendations. J Natl Cancer Inst. 2017:109. doi: 10.1093/jnci/djw253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lo C, Zimmermann C, Rydall A, et al. Longitudinal study of depressive symptoms in patients with metastatic gastrointestinal and lung cancer. J Clin Oncol. 2010;28:3084–3089. doi: 10.1200/JCO.2009.26.9712. [DOI] [PubMed] [Google Scholar]
- 5.Walker MS, Pharm EY, Kerr J, et al. Symptom burden & quality of life among patients receiving second-line treatment of metastatic colorectal cancer. BioMed Central. 2012;20:1–10. doi: 10.1186/1756-0500-5-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dhruva A, Dodd M, Paul SM, et al. Trajectories of fatigue in patients with breast cancer before, during, and after radiation therapy. Cancer Nurs. 2010;33:201–212. doi: 10.1097/NCC.0b013e3181c75f2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miaskowski C, Paul SM, Cooper BA, et al. Trajectories of fatigue in men with prostate cancer before, during, and after radiation therapy. J Pain Symptom Manage. 2008;35:632–643. doi: 10.1016/j.jpainsymman.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilbertson-White S, Aouizerat BE, Jahan T, Miaskowski C. A review of the literature on multiple symptoms, their predictors, and associated outcomes in patients with advanced cancer. Palliat Support Care. 2011;9:81–102. doi: 10.1017/S147895151000057X. [DOI] [PubMed] [Google Scholar]
- 9.Gilbertson-White S, Aouizerat BE, Jahan T, et al. Determination of cutpoints for low and high number of symptoms in patients with advanced cancer. J Palliat Med. 2012;15:1027–1036. doi: 10.1089/jpm.2012.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papachristou N, Barnaghi P, Cooper BA, et al. Congruence between latent class and K-modes analyses in the identification of oncology patients with distinct symptom experiences. J Pain Symptom Manage. 2017 doi: 10.1016/j.jpainsymman.2017.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kerckhove N, Collin A, Conde S, et al. Long-term effects, pathophysiological mechanisms, and risk factors of chemotherapy-induced peripheral neuropathies: A comprehensive literature review. Front Pharmacol. 2017;8:86. doi: 10.3389/fphar.2017.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wright F, D’Eramo Melkus G, Hammer M, et al. Trajectories of evening fatigue in oncology outpatients receiving chemotherapy. J Pain Symptom Manage. 2015;50:163–175. doi: 10.1016/j.jpainsymman.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright F, D’Eramo Melkus G, Hammer M, et al. Predictors and trajectories of morning fatigue are distinct from evening fatigue. J Pain Symptom Manage. 2015;50:176–189. doi: 10.1016/j.jpainsymman.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karnofsky D. Performance scale. New York: Plenum Press; 1977. [Google Scholar]
- 15.Schnadig ID, Fromme EK, Loprinzi CL, et al. Patient-physician disagreement regarding performance status is associated with worse survivorship in patients with advanced cancer. Cancer. 2008;113:2205–22014. doi: 10.1002/cncr.23856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ando M, Ando Y, Hasegawa Y, et al. Prognostic value of performance status assessed by patients themselves, nurses, and oncologists in advanced non-small cell lung cancer. Br J Cancer. 2001;85:1634–1639. doi: 10.1054/bjoc.2001.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49:156–163. doi: 10.1002/art.10993. [DOI] [PubMed] [Google Scholar]
- 18.Hung H-C, Chien T-W, Tsay S-L, Hang H-M, Liang S-Y. Patient and clinical variables account for changes in health-related quality of life and symptom burden as treatment outcomes in colorectal cancer: A longitudinal study. Asian Pac J Cancer Prev. 2013;14:1905–1909. doi: 10.7314/apjcp.2013.14.3.1905. [DOI] [PubMed] [Google Scholar]
- 19.Browall M, Kenne Sarenmalm E, Nasic S, Wengstrom Y, Gaston-Johansson F. Validity and reliability of the Swedish version of the Memorial Symptom Assessment Scale (MSAS): An instrument for the evaluation of symptom prevalence, characteristics, and distress. J Pain Symptom Manage. 2013;46:131–141. doi: 10.1016/j.jpainsymman.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 20.Portenoy RK, Thaler HT, Kornblith AB, et al. The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer. 1994;30A:1326–1336. doi: 10.1016/0959-8049(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 21.Rabe-Hesketh S, Skrondal A. Multilevel and Longitudinal Modeling Using STATA. 3. College Station, TX: Stata Press; 2012. [Google Scholar]
- 22.Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurence. 1. New York, NY: Oxford University Press; 2003. [Google Scholar]
- 23.Hox JJ. Multilevel analysis: Techniques and application. New York: Routledge Academic; 2010. [Google Scholar]
- 24.Zhang M, Peng L, Liu W, et al. Physical and psychological predictors of quality of life in chinese colorectal cancer patients during chemotherapy. Cancer Nurs. 2015;38:312–321. doi: 10.1097/NCC.0000000000000190. [DOI] [PubMed] [Google Scholar]
- 25.Deshields TL, Potter P, Olsen S, Liu J. The persistence of symptom burden: symptom experience and quality of life of cancer patients across one year. Support Care Cancer. 2014;22:1089–1096. doi: 10.1007/s00520-013-2049-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molassiotis A, Zheng Y, Denton-Cardew L, Swindell R, Brunton L. Symptoms experienced by cancer patients during the first year from diagnosis: patient and informal caregiver ratings and agreement. Palliat Support Care. 2010;8:313–324. doi: 10.1017/S1478951510000118. [DOI] [PubMed] [Google Scholar]
- 27.Brant JM, Beck SL, Dudley WN, et al. Symptom trajectories during chemotherapy in outpatients with lung cancer colorectal cancer, or lymphoma. Eur J Oncol Nurs. 2011;15:470–477. doi: 10.1016/j.ejon.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Williams VS, Smith MY, Fehnel SE. The validity and utility of the BPI interference measures for evaluating the impact of osteoarthritic pain. J Pain Symptom Manage. 2006;31:48–57. doi: 10.1016/j.jpainsymman.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 29.McDermott AM, Toelle TR, Rowbotham DJ, Schaefer CP, Dukes EM. The burden of neuropathic pain: results from a cross-sectional survey. Eur J Pain. 2006;10:127–135. doi: 10.1016/j.ejpain.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 30.Guillou PJ, Quirke P, Thorpe H, et al. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASSIC trial): multicentre, randomised controlled trial. The Lancet. 2005;365:1718–1726. doi: 10.1016/S0140-6736(05)66545-2. [DOI] [PubMed] [Google Scholar]
- 31.Andreyev HJ, Vlavianos P, Blake P, et al. Gastrointestinal symptoms after pelvic radiotherapy: role for the gastroenterologist? Int J Radiat Oncol Biol Phys. 2005;62:1464–1471. doi: 10.1016/j.ijrobp.2004.12.087. [DOI] [PubMed] [Google Scholar]
- 32.Miaskowski C, Mastick J, Paul SM, et al. Chemotherapy-induced neuropathy in cancer survivors. J Pain Symptom Manage. 2017;54:204–218. doi: 10.1016/j.jpainsymman.2016.12.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan BM, Myers RP. Neurolytic celiac plexus block for pain control in unresectable pancreatic cancer. Am J Gastroenterol. 2007;102:430–438. doi: 10.1111/j.1572-0241.2006.00967.x. [DOI] [PubMed] [Google Scholar]
- 34.Ye X, Lu D, Chen X, et al. A multicenter, randomized, double-blind, placebo-controlled trial of shuangbai san for treating primary liver cancer patients with cancer pain. J Pain Symptom Manage. 2016;51:979–986. doi: 10.1016/j.jpainsymman.2015.12.330. [DOI] [PubMed] [Google Scholar]
- 35.Yan H, Sellick K. Symptoms, psychological distress, social support, and quality of life of Chinese patients newly dianosed with gastrointestinal cancer. Cancer Nurs. 2004;27:389–399. doi: 10.1097/00002820-200409000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Rohrl K, Guren MG, Miaskowski C, et al. No differences in symptom burden between colorectal cancer patients receiving curative versus palliative chemotherapy. J Pain Symptom Manage. 2016;52:539–547. doi: 10.1016/j.jpainsymman.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 37.Baker TA, O’Connor ML, Krok JL. Experience and knowledge of pain management in patients receiving outpatient cancer treatment: what do older adults really know about their cancer pain? Pain Med. 2014;15:52–60. doi: 10.1111/pme.12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baker TA, O’Connor ML, Roker R, Krok JL. Satisfaction with pain treatment in older cancer patients: Identifying variants of discrimination, trust, communication, and self-efficacy. J Hosp Palliat Nurs. 2013:15. doi: 10.1097/NJH.0b013e3182a12c24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berger AM, Grem JL, Visovsky C, Marunda HA, Yurkovich JM. Fatigue and other variables during adjuvant chemotherapy for colon and rectal cancer. Oncology Nursing Forum. 2010;37:E359–E369. doi: 10.1188/10.ONF.E359-E369. [DOI] [PubMed] [Google Scholar]
- 40.Vardy JL, Dhillon HM, Pond GR, et al. Fatigue in people with localized colorectal cancer who do and do not receive chemotherapy: a longitudinal prospective study. Ann Oncol. 2016;27:1761–1767. doi: 10.1093/annonc/mdw252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miaskowski C, Paul SM, Cooper BA, et al. Trajectories of fatigue in men with prostate cancer before, during, and after radiation therapy. J Pain Symptom Manage. 2008;35:632–643. doi: 10.1016/j.jpainsymman.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kahn KL, Adams JL, Weeks JC, et al. Adjuvant chemotherapy use and adverse events among older patients with stage III colon cancer. JAMA. 2010;303:1037–1045. doi: 10.1001/jama.2010.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwartz CE, Sprangers MAG. Methodological approahces for assessing response shift in longitudinal health-related quality-of-life research. Soc Science Med. 1999;48:1531–1548. doi: 10.1016/s0277-9536(99)00047-7. [DOI] [PubMed] [Google Scholar]
- 44.Sprangers MA, Schwartz CE. The challenge of response shift for quality-of-life-based clinical oncology research. Ann Oncol. 1999;10:747–749. doi: 10.1023/a:1008305523548. [DOI] [PubMed] [Google Scholar]
- 45.Shelke AR, Roscoe JA, Morrow GR, et al. Effect of a nausea expectancy manipulation on chemotherapy-induced nausea: a university of Rochester cancer center community clinical oncology program study. J Pain Symptom Manage. 2008;35:381–387. doi: 10.1016/j.jpainsymman.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bovbjerg DH. The continuing problem of post chemotherapy nausea and vomiting: contributions of classical conditioning. Auton Neurosci. 2006;129:92–98. doi: 10.1016/j.autneu.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 47.Schnell FM. Chemotherapy induced nausea and vomiting: the importance of acute antiemetic control. Oncologist. 2003;8:187–198. doi: 10.1634/theoncologist.8-2-187. [DOI] [PubMed] [Google Scholar]
- 48.Warr DG, Hesketh PJ, Gralla RJ, et al. Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer after moderately emetogenic chemotherapy. J Clin Oncol. 2005;23:2822–2830. doi: 10.1200/JCO.2005.09.050. [DOI] [PubMed] [Google Scholar]
- 49.Warr DG, Street JC, Carides AD. Evaluation of risk factors predictive of nausea and vomiting with current standard-of-care antiemetic treatment: analysis of phase 3 trial of aprepitant in patients receiving adriamycin-cyclophosphamide-based chemotherapy. Support Care Cancer. 2011;19:807–813. doi: 10.1007/s00520-010-0899-5. [DOI] [PubMed] [Google Scholar]
- 50.Jones JM, Qin R, Bardia A, et al. Antiemetics for chemotherapy-induced nausea and vomiting occurring despite prophylactic antiemetic therapy. J Palliat Med. 2011;14:810–814. doi: 10.1089/jpm.2011.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoffman BM, Zevon MA, D’Arrigo MC, Cecchini TB. Screening for distress in cancer patients: the NCCN rapid-screening measure. Psychooncology. 2004;13:792–799. doi: 10.1002/pon.796. [DOI] [PubMed] [Google Scholar]
- 52.Sekine I, Segawa Y, Kubota K, Saeki T. Risk factors of chemotherapy-induced nausea and vomiting: index for personalized antiemetic prophylaxis. Cancer Sci. 2013;104:711–717. doi: 10.1111/cas.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sobrero AF, Maurel J, Fehrenbacher L, et al. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:2311–2319. doi: 10.1200/JCO.2007.13.1193. [DOI] [PubMed] [Google Scholar]
- 54.Kuo HH, Chiu MJ, Hwang SL. Quality of sleep and related factors during chemotherapy in patients with stage I/II breast cancer. J Formos Med Assoc. 2006;105:64–69. doi: 10.1016/S0929-6646(09)60110-8. [DOI] [PubMed] [Google Scholar]
- 55.Chou FY, Dodd M, Abrams D, Padilla G. Symptoms, self-care, and quality of life of Chinese American patients with cancer. Oncol Nurs Forum. 2007;34:1162–1167. doi: 10.1188/07.ONF.1162-1167. [DOI] [PubMed] [Google Scholar]
- 56.Bernaberi R, Gambassi G, Lapane K, et al. Management of pain in elderly patients with cancer. JAMA. 1999;279:1877–1882. doi: 10.1001/jama.279.23.1877. [DOI] [PubMed] [Google Scholar]
- 57.Chapell R, Aapro MS. Efficacy of aprepitant among patients aged 65 and over receiving moderately to highly emetogenic chemotherapy: a meta-analysis of unpublished data from previously published studies. J Geriatr Oncol. 2013;4:78–83. doi: 10.1016/j.jgo.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 58.Atlantis E, Sullivan T, Sartorius N, Almeida OP. Changes in the prevalence of psychological distress and use of antidepressants or anti-anxiety medications associated with comorbid chronic diseases in the adult Australian population, 2001–2008. Aust N Z J Psychiatry. 2012;46:445–456. doi: 10.1177/0004867411433218. [DOI] [PubMed] [Google Scholar]
- 59.Argyriou AA, Bruna J, Marmiroli P, Cavaletti G. Chemotherapy-induced peripheral neurotoxicity (CIPN): an update. Crit Rev Oncol Hematol. 2012;82:51–77. doi: 10.1016/j.critrevonc.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 60.Fallon MT. Neuropathic pain in cancer. Br J Anaesth. 2013;111:105–111. doi: 10.1093/bja/aet208. [DOI] [PubMed] [Google Scholar]
- 61.Seretny M, Currie GL, Sena ES, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain. 2014;155:2461–2470. doi: 10.1016/j.pain.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 62.Grisold W, Cavaletti G, Windebank AJ. Peripheral neuropathies from chemotherapeutics and targeted agents: diagnosis, treatment, and prevention. Neuro Oncol. 2012;14(Suppl 4):iv45–54. doi: 10.1093/neuonc/nos203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brewer JR, Morrison G, Dolan ME, Fleming GF. Chemotherapy-induced peripheral neuropathy: Current status and progress. Gynecol Oncol. 2016;140:176–183. doi: 10.1016/j.ygyno.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roscoe JA, Kaufman ME, Matterson-Rusby SE, et al. Cancer-related fatigue and sleep disorders. Oncologist. 2007;12:35–42. doi: 10.1634/theoncologist.12-S1-35. [DOI] [PubMed] [Google Scholar]
- 65.Beck SL, Dudley WN, Barsevick A. Pain, sleep disturbance, and fatigue in patients with cancer-using a mediation model to test a symptom cluster. Oncology Nursing Forum. 2005;32:E48–E55. doi: 10.1188/04.ONF.E48-E55. [DOI] [PubMed] [Google Scholar]
- 66.Given BA, Given CW, Sikorskii A, Hadar N. Symptom clusters and physical function for patients receiving chemotherapy. Semin Oncol Nurs. 2007;23:121–126. doi: 10.1016/j.soncn.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 67.Leger D, Poursain B, Neubauer D, Uchiyama M. An international survey of sleeping problems in the general population. Curr Med Res Opin. 2008;24:307–317. doi: 10.1185/030079907x253771. [DOI] [PubMed] [Google Scholar]
- 68.Davidson JR, MacLean AW, Brundage MD, Schulze K. Sleep disturbance in cancer patients. Soc Science Med. 2002;54:1309–1321. doi: 10.1016/s0277-9536(01)00043-0. [DOI] [PubMed] [Google Scholar]
- 69.Otte JL, Carpenter JS, Manchanda S, et al. Systematic review of sleep disorders in cancer patients: can the prevalence of sleep disorders be ascertained? Cancer Med. 2015;4:183–200. doi: 10.1002/cam4.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bernhardson BM, Tishelman C, Rutqvist LE. Self-reported taste and smell changes during cancer chemotherapy. Support Care Cancer. 2008;16:275–83. doi: 10.1007/s00520-007-0319-7. [DOI] [PubMed] [Google Scholar]
- 71.Zabernigg A, Gamper EM, Giesinger JM, et al. Taste alterations in cancer patients receiving chemotherapy: a neglected side effect? Oncologist. 2010;15:913–20. doi: 10.1634/theoncologist.2009-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gamper EM, Giesinger JM, Oberguggenberger A, et al. Taste alterations in breast and gynaecological cancer patients receiving chemotherapy: prevalence, course of severity, and quality of life correlates. Acta Oncol. 2012;51:490–496. doi: 10.3109/0284186X.2011.633554. [DOI] [PubMed] [Google Scholar]