Abstract
Drug addiction can be conceptualized on a basic level as maladaptive learning and memory. Addictive substances elicit changes in brain circuitry involved in reward, cognition, and emotional state, leading to the formation and persistence of strong drug-associated memories that lead to craving and relapse. Recently, perineuronal nets (PNNs), extracellular matrix structures surrounding neurons, have emerged as regulators of learning, memory, and addiction behaviors. PNNs do not just provide structural support to neurons, but are dynamically remodeled in an experience-dependent manner by metalloproteinases. They function in various brain regions through constituent proteins such as brevican that are implicated in neural plasticity. Understanding the function of PNN components in memory processes may lead to new therapeutic approaches to treating addiction.
Keywords: Addiction, Alcohol, Brevican, Memory, Metalloproteinase, Perineuronal Nets
Components and Regulators of Perineuronal Nets (PNNs)
PNNs are mesh-like, specialized extracellular matrix (see Glossary) structures that surround specific neurons in the central nervous system. They were first described by Camillo Golgi in 1882 but their functional importance in regulating neural plasticity only became appreciated in this century [1]. Over the past few years, it has become apparent that PNNs, and their individual constituents, are important regulators of memories associated with addiction-related behaviors in animals. Understanding the function of specific components that make up the structure of PNNs and the extracellular proteinases that remodel PNNs will provide potential new therapeutic avenues to reducing relapse to drug and alcohol addiction.
In the cerebral cortex, most neurons enclosed by PNNs produce the inhibitory neurotransmitter GABA and express the calcium-binding protein parvalbumin [2, 3], although PNNs also envelop a smaller number of neurons that produce the excitatory neurotransmitter glutamate in such regions as the cortex and specific areas of the hippocampus [3–6]. In the cerebellum, PNNs surround large excitatory deep cerebellar and inhibitory Golgi interneurons [7]. The structural composition of PNNs has recently been reviewed [1, 8] and will be briefly summarized here (Figure 1). Major constituents of PNNs are the chondroitin sulfate proteoglycans, proteins that are heavily modified by chondroitin sulfate glycosaminoglycan sugar chains. These proteins are aggrecan, brevican, neurocan and versican, and are encoded by the genes ACAN, BCAN, NCAN, and VCAN, respectively. They are collectively referred to as lecticans. Aggrecan is essential to the formation of PNNs, since cultured cortical neurons from Acan knockout mice, although able to differentiate into neurons that produce GABA and express parvalbumin, are unable to form PNNs [9]. Acan gene expression is induced by neuronal depolarization, indicating that it is regulated by neuronal activity [10]
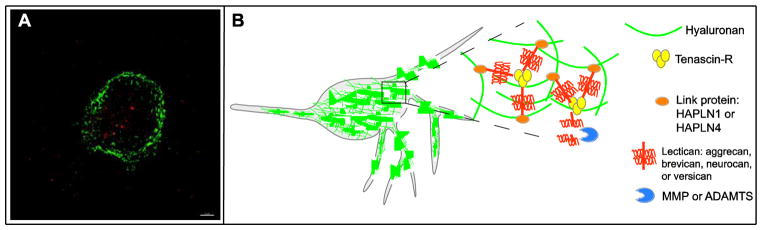
Figure 1. Fluorescent image and conceptual structure of PNNs.
(A) A neuron expressing parvalbumin (red) covered by a PNN as visualized by WFA fluorescent staining. Scale bar, 3 μm. (B) Structural components and regulators of PNN structure: hyaluronan, tenascin-R, hyaluronan and proteoglycan link proteins (HAPLN1, HAPLN4), the lecticans (aggrecan, brevican, neurocan, and versican), and enzymes that degrade the extracellular matrix (MMP and ADAMTS proteins).
The lecticans bind to hyaluronan, a non-sulfated glycosaminoglycan chain produced by hyaluronan synthases located on the plasma membrane of neurons. Hyaluronan is an important structural component of PNNs and also exists in a macromolecular complex with other PNN proteins known as link proteins and tenascins [8, 11]. A schematic of PNN structural components is shown in Figure 1. Different structural components of PNNs are produced by neurons and glia and secreted by these cell types into the extracellular space, where they assemble into PNNs [1, 10]. Link proteins (hyaluronan and proteoglycan link proteins, abbreviated HAPLNs) stabilize PNNs by binding to hyaluronan. Two link proteins that are known to localize to PNNs are HAPLN1 and HAPLN4, which are encoded by the HAPLN1 and HAPLN4 genes, respectively. Brain-specific Hapln1 knockout mice form PNNs, but their structure is abnormal [12], while mice deficient in Hapln4 have decreased brevican localization in PNNs in the cerebellum and brainstem [13]. Tenascin proteins are multimeric proteins that bind to lecticans. Tenascin-R (encoded by the TNR gene) and tenascin-C (encoded by the TNC gene) are expressed in the brain [14]. Tnr knockout mice do not form normal PNNs, demonstrating critical role for tenascin-R in PNN formation [11, 15]. Although tenascin-C is not necessary for PNN formation, it is co-localized with PNNs in the cerebellum and may play a functional role in PNNs [16, 17].
PNNs are formed during brain development at different times depending on the brain region. Their appearance in the cortex roughly correlates with the maturation of GABA neurons into parvalbumin-expressing neurons, with parvalbumin neurons in the somatosensory cortex appearing earlier than in the prefrontal cortex [18, 19]. PNNs are most often detected in the brain using immunohistochemistry (IHC) with labeled Wisteria floribunda agglutinin (WFA). In the mouse cortex, PNNs begin to appear in the somatosensory cortex at postnatal days 7–10 and continue to develop until adolescence at 5 weeks of age [18, 20], whereas in the mouse and rat prefrontal cortex, hippocampus, and amygdala, PNNs do not appear until postnatal day 14 and continue to develop until adulthood [19–21]. The developmental formation of PNNs is regulated by excitatory neural activity [22] and corresponds to the closure of periods of plasticity [23, 24].
PNNs are dynamically altered by experiences such as environmental enrichment, sensory deprivation, social isolation, epileptic neural activity, learning, and memory retrieval and as a result play a role in neural plasticity [6, 16, 23, 25–32]. Decreased intensity of PNNs and/or numbers of neurons enveloped by PNNs as measured by WFA labeling occur in the cerebellum and cortex after environmental enrichment [16, 30–32], although PNN intensity has been demonstrated to increase in the hippocampal CA2 region after enrichment [6]. This may be due to the different neuronal types that have PNNs in these brain regions responding differently to environmental enrichment. Interestingly, the number of neurons with PNNs also decreased in the cortex after sensory deprivation and social isolation [23, 28, 29]. Brevican protein levels, as measured by Western blots, are decreased in the mouse hippocampus after environmental enrichment and in brain tissue from epileptic patients [26]. Decreased PNNs after different sensory experiences may enhance neural plasticity by allowing, for example, new synapse formation [33].
Remodeling of PNNs during an experience is controlled by extracellular proteases that cleave the structural components of PNNs. The Matrix Metalloproteinase (MMP) and A Disintegrin and Metalloproteinase with Thrombospondin motifs (ADAMTS) gene families encode proteins that enzymatically cleave extracellular matrix proteins, including those in PNNs. Each of these protein families comprises multiple members with overlapping and distinct protein substrates [34–36]. Like PNNs, the activity of MMP and ADAMTS proteins are regulated by neural activity, sensory experiences, and during learning and memory processes [16, 37–42]. For instance, MMP-9/2 activity as measured by an in situ enzymatic assay increased in the cerebellum after environmental enrichment, which correlated with decreased intensity of PNNs as measured by WFA staining [27], suggesting that high MMP activity triggered by environment enrichment was degrading the PNN components. MMP-9 co-localized with PNNs in the cerebellum and its activity was also associated with decreased PNNs after environmental enrichment, an effect that was absent in Mmp9 knockout mice [16]. Likewise, Mmp9 mRNA levels increased after fear learning, and Mmp3 and Mmp13 mRNA levels were elevated after an epileptic seizure [37, 38]. MMP-3 and MMP-13 both cleave aggrecan and MMP-13 was recently shown to co-localize with PNNs in the dentate gyrus of the hippocampus [37]. In the ADAMTS family, ADAMTS8 and 15 are expressed by parvalbumin-positive neurons [43, 44], whereas ADAMTS4 cleaves brevican and is localized to synapses [45]. Given the complexity of the MMP and ADAMTS protein families and their abilities to act on multiple substrates, it is likely that other members of these families are able to regulate the structure of PNNs in an experience- and activity-dependent manner.
Although decreased PNN intensity and numbers of cells enclosed by PNNs after different sensory experiences may be due to increased activity of MMP and ADAMTS proteins leading to degradation of PNN protein components, this is likely not the only mechanism for altering PNN structure in response to stimuli. Several studies have found that Acan, Bcan and Ncan mRNA expression is altered after different sensory experiences [23, 25–27]. These findings suggest that epigenetic mechanisms occurring at the promoters of genes encoding PNN structural components may also be involved in PNN remodeling in response to various experiences.
Involvement of PNNs in Learning and Memory
Evidence that PNNs contribute to memory storage was first demonstrated in experiments performed by Gogolla et al [46], who showed that long-term memories of a fearful event could be removed by injecting chondroitinase ABC (ChABC, an enzyme that degrades PNNs) into the amygdala of mice. In these experiments, mice treated with ChABC were tested for fear responding one day after conditioning and exhibited a normal fear response (freezing behavior) when presented with a stimulus associated with the foot shock. However, the ChABC-treated mice either more rapidly extinguished or forgot the fear memory. In addition, the fearful memory could be spontaneously reinstated in control mice by presentation of the foot shock-associated stimulus up to one month after extinction training, whereas the ChABC-treated mice did not reinstate the memory. These results indicate that PNNs in the amygdala prevent the removal of a stored fear memory.
In contrast, PNNs in the hippocampus and auditory cortex appear to be important for the consolidation of fear learning [25, 47]. Injection of ChABC into the auditory cortex of mice abolished freezing responses one day after fear conditioning, although ChABC-treated mice still learned the association between the auditory stimulus and the shock immediately after conditioning [25]. Acan, Bcan, and Ncan gene expression was increased four hours after fear conditioning (but not earlier) along with an increased intensity and number of PNN-containing neurons [25]. In the hippocampus, injection of hyaluronidase (another enzyme that destroys PNNs by digesting hyaluronan) attenuated fear responding one day after conditioning [47], and a combination of hyaluronidase plus ChABC injected into the hippocampus also attenuated long-term fear memory [48]. Together, these studies indicate that PNNs are important in the hippocampus, auditory cortex, and amygdala for consolidating or maintaining long-term fear memories.
Interestingly, other types of learning and memory controlled by different brain regions are differentially affected by PNN removal. Digestion of PNNs using ChABC in the perirhinal cortex, a brain region important for object recognition memory, resulted in enhanced memory for the object [49]. Similarly, removing PNNs in the auditory cortex using hyaluronidase led to an increased ability to relearn a task that requires behavioral flexibility, known as reversal learning [50]. Both of these studies indicate that PNNs may restrict the ability to learn new behaviors related to cognitive functioning. Although these results seem to contradict the fear conditioning studies, extinction of a memory is hypothesized to involve learning a new memory that suppresses the old memory, rather than the erasure of the old memory [51]. PNNs may play a role in preventing new learning and/or memory formation during both extinction and reversal learning by restricting plasticity. This is consistent with the well-known role of PNNs in inhibiting plasticity of visual cortex neurons during a critical period of brain development [24].
The studies described above used enzymes to digest PNNs and therefore do not provide information on the specific components of PNNs that might regulate learning and memory. Four genes encoding PNN components are known to modulate learning and memory: Hapln1, Tnr, Tnc, and Bcan. Mice deficient in Hapln1 in the central nervous system exhibited enhanced object recognition, similar to what is observed in mice infused with ChABC in the perirhinal cortex [49]. Mice deficient in Tnr also showed enhanced object recognition and faster reversal learning in several tests [52], although they had subtle deficits in associative and spatial learning [53]. The increased ability of Tnr knockout mice to learn a new behavioral response is reminiscent of mice treated with hyaluronidase in the auditory cortex [50], suggesting that Tnr may be one component of PNNs that restricts flexibility in learning a new behavior. Interestingly, Tnr-deficient mice do not show altered fear learning, although fear extinction and long-term fear memory have not been tested [52]. Tnc-deficient mice learn fear normally, but exhibit impaired extinction of a fear memory [54]. Tnc knockout mice also have deficits in spatial learning [55]. Finally, Bcan knockout mice have impaired short-term working memory, but normal long-term memory when tested one day after conditioning in the novelty preference test [26]. The short-term memory deficit in Bcan-deficient mice is recapitulated by knocking down Bcan in parvalbumin-expressing neurons in the hippocampus using a viral-delivered short-hairpin RNA, indicating that Bcan expression in these specific neurons in the hippocampus is necessary for short-term memory [26]. Interestingly, in another hippocampal-dependent learning and memory test, novel object recognition, Bcan knockout mice exhibit defective short-term memory, but enhanced long-term memory, similar to Hapln1 and Tnr knockout mice [26].
As mentioned above, PNNs are altered by experience, including during learning and memory retrieval and reconsolidation [6, 16, 25–31, 56]. Activity-dependent modulation of PNN structure by extracellular proteases is likely important for the ability of PNNs to modulate learning and memory. MMP-9 is the most-well studied member of the MMP family in the brain and regulates several types of learning and memory [39–42, 57]. It is likely that ADAMTS proteins will play a similar role as MMPs in regulating PNN structure and learning and memory processes, but much remains to be discovered with regard to the remodeling of PNNs by specific MMP and ADAMTS proteins and where these act in the brain during learning and memory processes.
Effects of Drugs of Abuse on PNNs
Exposure to several drugs of abuse (cocaine, heroin, nicotine, and alcohol) alters PNNs in specific brain regions, similar to what is observed with other types of experience-dependent learning (Table 1). Several studies have focused on the conditioned place preference (CPP) test. In a study by Slaker et al [58], the medial prefrontal cortex was examined for PNNs after the rats had been tested for cocaine CPP and then confined in the cocaine- or non-cocaine (saline)-paired context. The number of cells labeled with WFA in the prelimbic area of the prefrontal cortex was decreased independently of the context. This indicates that PNNs decrease after learning CPP, but not in an environment-specific manner [58]. However, the number of cells that were double-labeled for WFA and c-fos, a marker of neuronal activity, were increased in the prelimbic cortex only in rats that were confined to the cocaine-paired chamber, suggesting that the reactivation of the cocaine-associated memory preferentially increased the activity of neurons surrounded by PNNs in this brain region [58].
Table 1.
Summary of changes in perineuronal nets induced by drugs of abuse.
| Drug | Species/Brain Region | Alteration (Method Used) | Time Point | Reference |
|---|---|---|---|---|
| Cocaine | Rat/prelimbic cortex | Decreased number of WFAa- positive cells (IHCb) | One hour after confinement to a previous cocaine- paired context (3 cocaine injections) | [58] |
| Cocaine | Mouse/cerebellum (glutamatergic medial projection neurons) | Increased intensity of WFA labeling (IHC) | One day after a single cocaine injection given one week after 6 prior injections | [59] |
| Cocaine | Mouse/cerebellum (glutamatergic medial projection neurons) | Decreased intensity of WFA labeling (IHC) | One day after a single cocaine injection, given one month after 6 prior injections | [60] |
| Cocaine | Mouse/cerebellum (glutamatergic medial projection neurons) | Decreased intensity of WFA labeling (IHC) | Seventy minutes after a cocaine CPPc test (8 cocaine injections) | [61] |
| Cocaine | Mouse/cerebellum (Golgi neurons) | Increased intensity of WFA labeling (IHC) | Seventy minutes after a cocaine CPP test (8 cocaine injections) | [61] |
| Ethanol | Mouse/orbitofront al cortex | Increased intensity of WFA, brevican, and neurocan labeling (IHC) | Two months after a binge ethanol procedure (6 injections) | [66] |
| Ethanol | Rat/hippocampus | Increased total chondroitin sulfate glycosamin oglycans (sulfated GAG assay) and neurocan (IHC) | Two hours after last ethanol intubation (10 ethanol exposures) in neonates | [65] |
| Ethanol | Mouse/insular cortex | Increased intensity of WFA labeling (IHC) | One day after last ethanol drinking session (24 drinking sessions) | [67] |
| Heroin | Rat/medial prefrontal cortex | Decreased synaptic tenascin-R and brevican protein (Western blots) | Twenty-one days after last heroin self-administration session (16 self administration sessions) | [62] |
| Heroin | Rat/medial prefrontal cortex | Normalized synaptic tenascin-R and brevican protein (Western blots) | After cue-induced reinstatement of heroin self- administration | [62] |
| Nicotine | Rat/orbitofrontal cortex | Decreased number of WFA- positive parvalbumin cells and WFA intensity (IHC) | Forty-five minutes after 21 days of nicotine self- administration | [63] |
| Nicotine | Rat/ventral tegmental area | Decreased number of WFA- positive parvalbumin cells and WFA intensity (IHC) | Forty-five minutes and seventy-two hours after 21 days of nicotine self- administration | [63] |
Wisteria floribunda agglutinin;
Immunohistochemistry;
Conditioned place preference
Cocaine also alters PNNs in the cerebellum in a temporally- and spatially- specific pattern. Mice injected with six cocaine injections followed by a one-week withdrawal period and a single cocaine challenge had increased intensity of PNNs around glutamatergic medial projection neurons of the cerebellum one day after the cocaine challenge [59]. In contrast, in mice treated with six cocaine injections followed by a one-month withdrawal and cocaine challenge, PNN intensity was decreased around these neurons [60], demonstrating a dynamic regulation of PNNs during withdrawal from chronic cocaine. After cocaine CPP, PNNs surrounding the medial projection neurons were also reduced in intensity independently of whether the mice developed preference for the drug-paired chamber [61]. In contrast, PNN intensity surrounding a different type of cerebellar neuron, the GABAergic Golgi interneuron, was increased following cocaine CPP, but only in mice that developed preference for the cocaine-paired chamber [61]. Increased PNN intensity on Golgi interneurons was also observed in mice that showed preference for the cocaine-paired chamber and were re-exposed to the cocaine-paired context [61], suggesting that the Golgi interneurons encoded the cocaine-associated memory. Together, these results indicate that PNNs in both the prelimbic cortex and the cerebellum are preferentially modified by the activation of a cocaine-associated memory.
Heroin and nicotine can also alter PNNs in another animal model of addiction, known as drug self-administration. Twenty-one days of abstinence from heroin self-administration reduced protein levels of brevican, tenascin-R, and HAPLN1 in synaptic membranes from the medial prefrontal cortex [62]. This reduction was normalized after cue-induced reinstatement of heroin self-administration. For nicotine, the number and intensity of PNNs surrounding parvalbumin-expressing neurons were decreased in the ventral tegmental area (VTA) and the orbitofrontal cortex (a region of the prefrontal cortex) forty-five minutes after a nicotine self-administration session [63]. The reduction in the number of PNNs in the VTA was maintained at seventy-two hours after the last self-administration session [63]. Nicotine also increased protein levels of MMP-2, MMP-3 and MMP-9 in the hippocampus and prefrontal cortex during CPP training [64], suggesting that PNNs could potentially be remodeled in these regions through elevated MMP activity during the learning of a nicotine-environment association.
Finally, alcohol (ethanol) exposure has also been shown to alter the intensity of PNNs in various regions of the brain, although ethanol has generally been shown to increase, rather than decrease, PNN intensity. Neonatal ethanol exposure in rats increased levels of chondroitin sulfate glycosaminoglycans and expression of neurocan in the hippocampus [65]. In adolescent rats, exposure to binge ethanol administration increased the intensity of PNNs and levels of brevican and neurocan immunoreactivity in the orbitofrontal cortex observed in adulthood, changes associated with deficits in reversal learning [66]. In adult mice, six weeks of binge-like drinking resulted in increased intensity of PNNs, expression of aggrecan mRNA and protein, and brevican protein in the insular cortex, a region of the brain involved in compulsive drinking [67, 68]. Ethanol injections decreased MMP-9 activity in the rat prefrontal cortex and hippocampus [69]. This decrease in MMP-9 activity is consistent with increased intensity of PNNs after ethanol exposure, since increased MMP activity is predicted to proteolytically degrade PNN components. In summary, PNNs are modified in various brain regions in response to multiple drugs of abuse (Figure 2A). Of particular interest are changes in PNNs in the context of learning and memory of drug-associated environments that would implicate PNNs in addiction-related behaviors.
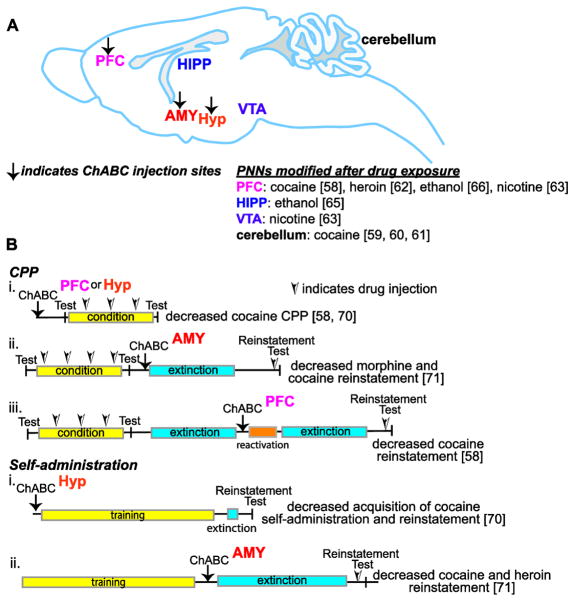
Figure 2. Brain regions in which PNNs are remodeled or regulate addiction behaviors.
(A) Saggital view of rat or mouse brain showing areas in which PNNs have been demonstrated to change in response to drug exposure and/or in which PNNs have been degraded by microinjection of chondroitinase ABC (ChABC) and animals tested for CPP or drug self-administration. Black arrow shows locations in which ChABC has been injected. Note that the insula, a cortical area that shows an increase in PNNs after ethanol exposure, is not shown here. (B) Experimental design of CPP and drug self-administration experiments showing when ChABC was injected relative to testing animals for either CPP or reinstatement of drug-seeking behavior. Abbreviations: PFC, prefrontal cortex (includes prelimbic, medial prefrontal, and orbitofrontal); AMY, amygdala; HIPP, hippocampus; Hyp, hypothalamus; VTA, ventral tegmental area. References are indicated in brackets.
Effects of PNN Digestion on Addiction-Related Behaviors
The ability of drug exposure to regulate the structure of PNNs suggests that PNNs may, in turn, be involved in the adaptive response of the brain to drugs and the learning and/or memory of the drug-associated experience. This is important because strong memories for the environment in which the drug was used can promote relapse to drug use. Evidence that PNNs are involved in the learning and memory of a drug-associated environment has been provided using the CPP and drug self-administration tests.
When ChABC was injected into the prelimbic cortex or a specific region of the hypothalamus of rats prior to conditioning, the development of cocaine CPP was attenuated, indicating that PNNs are important for the learning of the drug-environment association [58, 70]. Interestingly, injection of ChABC into the prelimbic cortex after testing for CPP did not affect the extinction or reinstatement of CPP [58]. However, when ChABC was injected after extinction, just prior to reactivation of the memory, reinstatement to CPP was attenuated compared with control animals, suggesting that PNNs are important for the reconsolidation of the memory [58]. In comparison, ChABC injection into the amygdala after conditioning, but prior to extinction, attenuated the reinstatement of CPP [71]. This is comparable to what has been observed in the fear conditioning experiments, in which reinstatement of a fear memory is reduced after ChABC injection into the amygdala [46]. PNNs in the amygdala are also important for the reinstatement of morphine CPP [71]. Together, these results indicate that PNNs in brain regions involved in addiction are important for the learning and memory of a drug-associated environment (Figure 2B), and indicate that disruption of PNNs might be a novel way to suppress these memories and prevent relapse.
Another behavioral test that models addiction-like behavior in animals is drug self-administration. Digestion of PNNs in a specific region of the hypothalamus (anterior dorsal lateral hypothalamic area) prior to self-administration training decreased acquisition of cocaine self-administration [70]. ChABC injection into the amygdala after self-administration training but prior to extinction enhanced the extinction of cocaine and heroin self-administration, and attenuated reinstatement [71]. Again, this is similar to what has been observed with fear conditioning, in which extinction was enhanced but reactivation of a fear memory was reduced upon disruption of PNNs in the amygdala, and further supports the concept that disruption of PNNs might be a strategy to prevent relapse to drug use.
Brevican Regulates Addiction-Related Behaviors
Currently brevican is the only PNN structural component that has been investigated for its role in addiction-like behavior. Heterozygous Bcan knockout mice, which express ~50% of normal brevican levels, exhibited enhanced cocaine CPP at 21 days, but not one day, after conditioning compared with wild-type Bcan mice [72]. This is analogous to the enhanced long-term novel object recognition memory observed in homozygous Bcan knockout mice [26]. Interestingly, heterozygous Bcan knockout mice did not differ from wild-type mice in cocaine CPP when tested one day after conditioning. Overexpression of Bcan in the hippocampus, but not prefrontal cortex, was able to normalize the enhanced cocaine CPP observed after 21 days [72], suggesting that brevican functions in the hippocampus to suppress a persistent cocaine-associated memory and that enhancing brevican function might allow for a cocaine-associated memory to be forgotten.
MMPs Regulate Addiction-Related Behaviors
The MMPs are also important for the reinstatement of drug-seeking behavior. In an elegant set of experiments by Smith et al [73], microinjection of an MMP-9 or MMP-2 inhibitor into the nucleus accumbens core region of the brain reduced cue-induced reinstatement of cocaine-seeking behavior and injection of an MMP-9 inhibitor decreased cocaine-induced reinstatement. Consistent with a role for MMPs in promoting drug-seeking, MMP activity increased in the nucleus accumbens core after cue-induced reinstatement of cocaine, nicotine, and heroin seeking [73]. MMP-9, in particular, appears to contribute to this increase in activity after reinstatement, because a selective MMP-9 inhibitor attenuated this increase [73]. MMP-9 in the rat medial prefrontal cortex was also higher after cocaine-induced reinstatement of CPP, and inhibition of MMPs in rats with the non-selective MMP inhibitor, FN-439, decreased cocaine-primed reinstatement of CPP [74, 75]. Although MMP-9 has multiple protein substrates and is likely degrading extracellular matrix proteins other than those found in PNNs, it is possible that changes in activity of MMP-9 (or other MMPs) may be responsible for PNN remodeling after drug exposure and subsequent behavioral outcomes.
MMPs also play a role in behaviors related to alcohol, nicotine, and amphetamine addiction. Injection of FN-439 into rat cerebral ventricles attenuated the escalation in lever pressing for ethanol that occurs during ethanol withdrawal [76], suggesting the MMPs are important for the neuroadaptive changes that occur during withdrawal that promote excessive drinking. In addition, Mmp9 knockout mice exhibited decreased motivation to obtain alcohol [77]. MMP-9 activity in the central nucleus of the amygdala promotes alcohol-seeking behavior, since overexpression of MMP-9 in this brain region in Mmp9 knockout mice eliminated the deficit in alcohol seeking [77]. Mmp2 and Mmp9 knockout mice have attenuated amphetamine CPP and administration of a non-specific MMP inhibitor into rat cerebral ventricles decreased amphetamine CPP [78, 79]. Finally, infusion of FN-439 into rat cerebral ventricles attenuated nicotine CPP [64].
Concluding Remarks and Future Perspectives
Experiments in rodents have provided evidence that PNNs and MMPs may regulate drug-seeking behavior and the long-term persistence of drug-associated memories that promote relapse to drug abuse. Manipulating PNNs during abstinence might be a novel strategy to reduce relapse, since administration of ChABC or MMP inhibitors prior to extinction or reinstatement attenuates drug-seeking behavior for several drugs of abuse. There is genetic evidence that PNNs may be involved in drug abuse in humans. A polymorphism in the human MMP9 gene that increases MMP9 expression was associated with alcoholism and increased motivation to obtain alcohol in alcoholics [77, 80], and a genetic locus containing the TNR gene was associated with high risk for alcohol dependence [81]. In addition, MMP-9 activity was decreased in the hippocampus of cocaine abusers, and ratios of MMP-2 and MMP-9 to their endogenous inhibitors were increased in the serum of heroin abusers, indicating that dynamic alterations in MMP activity may be relevant to cocaine and heroin addiction in humans [82, 83]. Future studies should examine potential genetic associations of other genes encoding PNN components with addiction phenotypes.
It will also be important in the future to understand how specific MMP and ADAMTS proteins in the brain regulate the remodeling of PNNs in response to drug exposure or memory retrieval using genetic mouse models (see Outstanding Questions), because these enzymes could be useful pharmacological targets to treat addiction. Likewise, other components of PNNs such as HAPLN1 and tenascin-R have not been studied for their roles in addiction-like behaviors, despite the fact that they play a role in learning and memory and the gene knockout mice are viable. Region-specific and/or cell-type specific knockout or knockdown of different genes encoding PNN components and enzymatic regulators will allow for a more refined understanding of how PNNs function to regulate drug relapse, facilitating the identification of new target genes for drug discovery efforts for addiction treatment.
Outstanding Questions.
How do individual components of PNNs, such as aggrecan, brevican, tenascin-R, and HAPLN1 regulate reinstatement of drug-seeking behavior?
Which types of neurons surrounded by PNNs in specific brain regions are important for controlling reinstatement to CPP or drug self-administration?
Do PNNs and their individual constituents in particular brain regions regulate alcohol consumption?
How do PNNs control the neuronal firing properties and circuit-level activity in different brain regions and in response to drugs of abuse?
How do individual components of PNNs, the MMPs, and the ADAMTS proteins regulate synaptic plasticity?
Which MMP or ADAMTS proteins are responsible for remodeling PNNs in different brain regions and in response to different drugs of abuse?
Are there genetic associations in humans between genes encoding PNN components, MMPs, or ADAMTS proteins and increased risk for developing addiction?
Are changes in PNNs after drug exposure adaptive (protective) or maladaptive (detrimental)?
How do we explain the paradox that disrupting PNNs enzymatically with ChABC decreases a cocaine-associated memory, but that decreasing brevican function increases a cocaine-associated memory?
Why are some forms of memory disrupted by digesting PNNs while others are enhanced?
Finally, recent appreciation that the molecular composition of PNNs is not uniform throughout the brain indicates that PNNs with different structural components, such as brevican and aggrecan, may have different functions in regulating neuronal activity and, by extension, learning and memory [3, 21, 84, 85]. A greater understanding of the molecular differences in PNNs, the cell types they surround in particular neuroanatomical locations, and the roles of these specific molecules in drug-associated memories is essential for potentially manipulating these fascinating structures as a way to reduce addiction.
Trends Box.
Perineuronal nets (PNNs) are distinct extracellular matrix structures consisting of dense protein and sugar chains that condense around specific neurons in the brain.
PNNs are involved in storing long-term memories and also preventing an animal from learning new behaviors.
PNNs are modified in several brain regions by many types of experiences, including exposure to drugs of abuse such as cocaine, heroin, nicotine and alcohol.
MMP and ADAMTS proteins are extracellular proteases that are activated by exposure to drugs of abuse and remodel the extracellular matrix.
Disruption of PNNs after an animal has learned a drug-environment association can prevent reinstatement, or relapse, of the drug-associated behavior.
Brevican is one component of PNNs that regulates the storage of long-term cocaine-associated memories.
Acknowledgments
We would like to thank Dr. Patrick Mulholland for providing the image of the neuron labeled with fluorescent WFA and parvabumin antibody in Figure 1. A.W.L is supported by grants from the National Institute on Alcohol Abuse and Alcoholism (INIA U01 020912 and the Center for Alcohol Research in Epigenetics P50 AA022538) and the National Institute on Drug Abuse (R01 DA033429).
Glossary
- A Disintegrin and Metalloproteinase with a Thrombospondin motifs (ADAMTS)
Secreted proteases that contain thrombospondin motifs. ADAMTS1, 4, 5, 8, 9, 15, and 20 are also known as aggrecanases because they enzymatically cleave the lecticans such as aggrecan.
- Behavioral flexibility
The ability of an animal to change behavior in response to altered environmental conditions. This adaptive response involves learning a new behavior and is often disrupted in psychiatric and neurological disorders.
- Chondroitin sulfate
A disaccharide (sugar) unit that consists of sulfated glucuronic acid and N-acetylgalactosamine. Chondroitin sulfate is best known for its role in cartilage. Chondroitin sulfate glycosaminoglycans are long chains of repeating chondroitin sulfate units and are attached to chondroitin sulfate proteoglycans.
- Conditioned place preference
An associative learning task in which a drug is administered to an animal in a specific environmental context during conditioning. The animal learns to associate the environment in which it received the drug with the internal state achieved while on the drug. A positive experience results in the animal spending more time in the drug-paired compartment in the absence of drug, known as place preference.
- Extracellular matrix (ECM)
A three-dimensional structure that is present in all tissues on the outside of the cells. The ECM provides structural support to tissues but is also actively remodeled and controls many normal physiological and disease-related processes.
- Hyaluronan
A linear non-sulfated glycosaminoglycan that contains repeating disaccharide units of N-acetylglucosamine. Hyaluronan is a major component of the extracellular matrix and is found throughout the body.
- Consolidation
The process of converting a short-term memory into a long-term memory.
- Extinction
A process by which an animal learns that a conditioned stimulus (CS), such as a light or tone, associated with an unconditioned stimulus (US), such as a foot shock, is no longer associated with the unconditioned stimulus and decreases its behavioral response to the conditioned stimulus. With regard to animal models of drug addiction, extinction occurs when the drug is no longer available and the animal reduces the behavioral response that was previously associated with drug availability, such as place preference or lever pressing for drug.
- Matrix metalloproteinase (MMP)
Enzymes that that proteolytically degrade the extracellular matrix.
- Neural Plasticity
The ability of neurons to change in response to alterations in their environment. Neural plasticity is essential to learning and memory.
- Reinstatement
A behavioral response in which an animal performs an action that was previously associated with drug availability but was extinguished. Reinstatement is a model for relapse and can be elicited by a cue (a stimulus that was previously associated with drug availability) or by an injection of a drug (known as drug-priming).
- Reversal learning
A test of behavioral or cognitive flexibility in which an animal must learn a new response when the outcome has changed.
- Self-administration
A behavioral task in which an animal presses a lever or performs another operant procedure to obtain a drug, typically adminstered by intravenous infusion.
- Wisteria floribunda agglutinin (WFA)
A plant protein (lectin) that binds to sugar chains terminating in N-acetylgalactosamine. WFA is routinely used to visualize PNNs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Oohashi T, et al. The hyaluronan and proteoglycan link proteins: Organizers of the brain extracellular matrix and key molecules for neuronal function and plasticity. Exp Neurol. 2015;274(Pt B):134–44. doi: 10.1016/j.expneurol.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 2.Hartig W, et al. Wisteria floribunda agglutinin-labelled nets surround parvalbumin-containing neurons. Neuroreport. 1992;3(10):869–72. doi: 10.1097/00001756-199210000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Lensjo KK, et al. Differential Expression and Cell-Type Specificity of Perineuronal Nets in Hippocampus, Medial Entorhinal Cortex, and Visual Cortex Examined in the Rat and Mouse. eNeuro. 2017;4(3) doi: 10.1523/ENEURO.0379-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wegner F, et al. Diffuse perineuronal nets and modified pyramidal cells immunoreactive for glutamate and the GABA(A) receptor alpha1 subunit form a unique entity in rat cerebral cortex. Exp Neurol. 2003;184(2):705–14. doi: 10.1016/S0014-4886(03)00313-3. [DOI] [PubMed] [Google Scholar]
- 5.Alpar A, et al. Distribution of pyramidal cells associated with perineuronal nets in the neocortex of rat. Brain Res. 2006;1120(1):13–22. doi: 10.1016/j.brainres.2006.08.069. [DOI] [PubMed] [Google Scholar]
- 6.Carstens KE, et al. Perineuronal Nets Suppress Plasticity of Excitatory Synapses on CA2 Pyramidal Neurons. J Neurosci. 2016;36(23):6312–20. doi: 10.1523/JNEUROSCI.0245-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carulli D, et al. Composition of perineuronal nets in the adult rat cerebellum and the cellular origin of their components. J Comp Neurol. 2006;494(4):559–77. doi: 10.1002/cne.20822. [DOI] [PubMed] [Google Scholar]
- 8.Miyata S, Kitagawa H. Formation and remodeling of the brain extracellular matrix in neural plasticity: Roles of chondroitin sulfate and hyaluronan. Biochim Biophys Acta. 2017;1861(10):2420–2434. doi: 10.1016/j.bbagen.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 9.Giamanco KA, et al. Perineuronal net formation and structure in aggrecan knockout mice. Neuroscience. 2010;170(4):1314–27. doi: 10.1016/j.neuroscience.2010.08.032. [DOI] [PubMed] [Google Scholar]
- 10.Giamanco KA, Matthews RT. Deconstructing the perineuronal net: cellular contributions and molecular composition of the neuronal extracellular matrix. Neuroscience. 2012;218:367–84. doi: 10.1016/j.neuroscience.2012.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruckner G, et al. Postnatal development of perineuronal nets in wild-type mice and in a mutant deficient in tenascin-R. J Comp Neurol. 2000;428(4):616–29. doi: 10.1002/1096-9861(20001225)428:4<616::aid-cne3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 12.Carulli D, et al. Animals lacking link protein have attenuated perineuronal nets and persistent plasticity. Brain. 2010;133(Pt 8):2331–47. doi: 10.1093/brain/awq145. [DOI] [PubMed] [Google Scholar]
- 13.Bekku Y, et al. Bral2 is indispensable for the proper localization of brevican and the structural integrity of the perineuronal net in the brainstem and cerebellum. J Comp Neurol. 2012;520(8):1721–36. doi: 10.1002/cne.23009. [DOI] [PubMed] [Google Scholar]
- 14.Chiquet-Ehrismann R, Tucker RP. Tenascins and the importance of adhesion modulation. Cold Spring Harb Perspect Biol. 2011;3(5) doi: 10.1101/cshperspect.a004960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weber P, et al. Mice deficient for tenascin-R display alterations of the extracellular matrix and decreased axonal conduction velocities in the CNS. J Neurosci. 1999;19(11):4245–62. doi: 10.1523/JNEUROSCI.19-11-04245.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stamenkovic V, et al. The extracellular matrix glycoprotein tenascin-C and matrix metalloproteinases modify cerebellar structural plasticity by exposure to an enriched environment. Brain Struct Funct. 2017;222(1):393–415. doi: 10.1007/s00429-016-1224-y. [DOI] [PubMed] [Google Scholar]
- 17.Irintchev A, et al. Structural and functional aberrations in the cerebral cortex of tenascin-C deficient mice. Cereb Cortex. 2005;15(7):950–62. doi: 10.1093/cercor/bhh195. [DOI] [PubMed] [Google Scholar]
- 18.Nowicka D, et al. Parvalbumin-containing neurons, perineuronal nets and experience-dependent plasticity in murine barrel cortex. Eur J Neurosci. 2009;30(11):2053–63. doi: 10.1111/j.1460-9568.2009.06996.x. [DOI] [PubMed] [Google Scholar]
- 19.Ueno H, et al. Parvalbumin neurons and perineuronal nets in the mouse prefrontal cortex. Neuroscience. 2017;343:115–127. doi: 10.1016/j.neuroscience.2016.11.035. [DOI] [PubMed] [Google Scholar]
- 20.Horii-Hayashi N, et al. Development and Structural Variety of the Chondroitin Sulfate Proteoglycans-Contained Extracellular Matrix in the Mouse Brain. Neural Plast. 2015;2015:256389. doi: 10.1155/2015/256389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baker KD, et al. The development of perineuronal nets around parvalbumin gabaergic neurons in the medial prefrontal cortex and basolateral amygdala of rats. Behav Neurosci. 2017;131(4):289–303. doi: 10.1037/bne0000203. [DOI] [PubMed] [Google Scholar]
- 22.Dityatev A, et al. Activity-dependent formation and functions of chondroitin sulfate-rich extracellular matrix of perineuronal nets. Dev Neurobiol. 2007;67(5):570–88. doi: 10.1002/dneu.20361. [DOI] [PubMed] [Google Scholar]
- 23.McRae PA, et al. Sensory deprivation alters aggrecan and perineuronal net expression in the mouse barrel cortex. J Neurosci. 2007;27(20):5405–13. doi: 10.1523/JNEUROSCI.5425-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pizzorusso T, et al. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298(5596):1248–51. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- 25.Banerjee SB, et al. Perineuronal Nets in the Adult Sensory Cortex Are Necessary for Fear Learning. Neuron. 2017;95(1):169–179. e3. doi: 10.1016/j.neuron.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Favuzzi E, et al. Activity-Dependent Gating of Parvalbumin Interneuron Function by the Perineuronal Net Protein Brevican. Neuron. 2017;95(3):639–655. e10. doi: 10.1016/j.neuron.2017.06.028. [DOI] [PubMed] [Google Scholar]
- 27.Foscarin S, et al. Experience-dependent plasticity and modulation of growth regulatory molecules at central synapses. PLoS One. 2011;6(1):e16666. doi: 10.1371/journal.pone.0016666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ueno H, et al. Region-specific impairments in parvalbumin interneurons in social isolation-reared mice. Neuroscience. 2017;359:196–208. doi: 10.1016/j.neuroscience.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 29.Ueno H, et al. Sensory experience-dependent formation of perineuronal nets and expression of Cat-315 immunoreactive components in the mouse somatosensory cortex. Neuroscience. 2017;355:161–174. doi: 10.1016/j.neuroscience.2017.04.041. [DOI] [PubMed] [Google Scholar]
- 30.Madinier A, et al. Enriched housing enhances recovery of limb placement ability and reduces aggrecan-containing perineuronal nets in the rat somatosensory cortex after experimental stroke. PLoS One. 2014;9(3):e93121. doi: 10.1371/journal.pone.0093121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pollock E, et al. Metalloproteinase inhibition prevents inhibitory synapse reorganization and seizure genesis. Neurobiol Dis. 2014;70:21–31. doi: 10.1016/j.nbd.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 32.Slaker M, et al. Impact of Environmental Enrichment on Perineuronal Nets in the Prefrontal Cortex following Early and Late Abstinence from Sucrose Self-Administration in Rats. PLoS One. 2016;11(12):e0168256. doi: 10.1371/journal.pone.0168256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pyka M, et al. Chondroitin sulfate proteoglycans regulate astrocyte-dependent synaptogenesis and modulate synaptic activity in primary embryonic hippocampal neurons. Eur J Neurosci. 2011;33(12):2187–202. doi: 10.1111/j.1460-9568.2011.07690.x. [DOI] [PubMed] [Google Scholar]
- 34.Bonnans C, et al. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15(12):786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huntley GW. Synaptic circuit remodelling by matrix metalloproteinases in health and disease. Nat Rev Neurosci. 2012;13(11):743–57. doi: 10.1038/nrn3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kelwick R, et al. The ADAMTS (A Disintegrin and Metalloproteinase with Thrombospondin motifs) family. Genome Biol. 2015;16:113. doi: 10.1186/s13059-015-0676-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dubey D, et al. Increased metalloproteinase activity in the hippocampus following status epilepticus. Epilepsy Res. 2017;132:50–58. doi: 10.1016/j.eplepsyres.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ganguly K, et al. Matrix metalloproteinase (MMP) 9 transcription in mouse brain induced by fear learning. J Biol Chem. 2013;288(29):20978–91. doi: 10.1074/jbc.M113.457903. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Knapska E, et al. Reward learning requires activity of matrix metalloproteinase-9 in the central amygdala. J Neurosci. 2013;33(36):14591–600. doi: 10.1523/JNEUROSCI.5239-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagy V, et al. The extracellular protease matrix metalloproteinase-9 is activated by inhibitory avoidance learning and required for long-term memory. Learn Mem. 2007;14(10):655–64. doi: 10.1101/lm.678307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagy V, et al. Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. J Neurosci. 2006;26(7):1923–34. doi: 10.1523/JNEUROSCI.4359-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wright JW, et al. Inhibition of hippocampal matrix metalloproteinase-3 and -9 disrupts spatial memory. Neural Plast. 2007;2007:73813. doi: 10.1155/2007/73813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rossier J, et al. Cortical fast-spiking parvalbumin interneurons enwrapped in the perineuronal net express the metallopeptidases Adamts8, Adamts15 and Neprilysin. Mol Psychiatry. 2015;20(2):154–61. doi: 10.1038/mp.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levy C, et al. Cell-specific and developmental expression of lectican-cleaving proteases in mouse hippocampus and neocortex. J Comp Neurol. 2015;523(4):629–48. doi: 10.1002/cne.23701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valenzuela JC, et al. Hyaluronan-based extracellular matrix under conditions of homeostatic plasticity. Philos Trans R Soc Lond B Biol Sci. 2014;369(1654):20130606. doi: 10.1098/rstb.2013.0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gogolla N, et al. Perineuronal nets protect fear memories from erasure. Science. 2009;325(5945):1258–61. doi: 10.1126/science.1174146. [DOI] [PubMed] [Google Scholar]
- 47.Kochlamazashvili G, et al. The extracellular matrix molecule hyaluronic acid regulates hippocampal synaptic plasticity by modulating postsynaptic L-type Ca(2+) channels. Neuron. 2010;67(1):116–28. doi: 10.1016/j.neuron.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hylin MJ, et al. Disruption of the perineuronal net in the hippocampus or medial prefrontal cortex impairs fear conditioning. Learn Mem. 2013;20(5):267–73. doi: 10.1101/lm.030197.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Romberg C, et al. Depletion of perineuronal nets enhances recognition memory and long-term depression in the perirhinal cortex. J Neurosci. 2013;33(16):7057–65. doi: 10.1523/JNEUROSCI.6267-11.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Happel MF, et al. Enhanced cognitive flexibility in reversal learning induced by removal of the extracellular matrix in auditory cortex. Proc Natl Acad Sci U S A. 2014;111(7):2800–5. doi: 10.1073/pnas.1310272111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bouton ME, et al. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol Psychiatry. 2006;60(4):352–60. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 52.Morellini F, et al. Improved reversal learning and working memory and enhanced reactivity to novelty in mice with enhanced GABAergic innervation in the dentate gyrus. Cereb Cortex. 2010;20(11):2712–27. doi: 10.1093/cercor/bhq017. [DOI] [PubMed] [Google Scholar]
- 53.Montag-Sallaz M, Montag D. Severe cognitive and motor coordination deficits in tenascin-R-deficient mice. Genes Brain Behav. 2003;2(1):20–31. doi: 10.1034/j.1601-183x.2003.00003.x. [DOI] [PubMed] [Google Scholar]
- 54.Morellini F, et al. Impaired Fear Extinction Due to a Deficit in Ca2+ Influx Through L-Type Voltage-Gated Ca2+ Channels in Mice Deficient for Tenascin-C. Front Integr Neurosci. 2017;11:16. doi: 10.3389/fnint.2017.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stamenkovic V, et al. Enriched environment alters the behavioral profile of tenascin-C deficient mice. Behav Brain Res. 2017;331:241–253. doi: 10.1016/j.bbr.2017.05.047. [DOI] [PubMed] [Google Scholar]
- 56.Morikawa S, et al. Activation of perineuronal net-expressing excitatory neurons during associative memory encoding and retrieval. Sci Rep. 2017;7:46024. doi: 10.1038/srep46024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meighan SE, et al. Effects of extracellular matrix-degrading proteases matrix metalloproteinases 3 and 9 on spatial learning and synaptic plasticity. J Neurochem. 2006;96(5):1227–41. doi: 10.1111/j.1471-4159.2005.03565.x. [DOI] [PubMed] [Google Scholar]
- 58.Slaker M, et al. Removal of perineuronal nets in the medial prefrontal cortex impairs the acquisition and reconsolidation of a cocaine-induced conditioned place preference memory. J Neurosci. 2015;35(10):4190–202. doi: 10.1523/JNEUROSCI.3592-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vazquez-Sanroman D, et al. The cerebellum on cocaine: plasticity and metaplasticity. Addict Biol. 2015;20(5):941–55. doi: 10.1111/adb.12223. [DOI] [PubMed] [Google Scholar]
- 60.Vazquez-Sanroman D, et al. Cocaine-induced plasticity in the cerebellum of sensitised mice. Psychopharmacology (Berl) 2015;232(24):4455–67. doi: 10.1007/s00213-015-4072-1. [DOI] [PubMed] [Google Scholar]
- 61.Carbo-Gas M, et al. Cerebellar perineuronal nets in cocaine-induced pavlovian memory: Site matters. Neuropharmacology. 2017;125:166–180. doi: 10.1016/j.neuropharm.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 62.Van den Oever MC, et al. Extracellular matrix plasticity and GABAergic inhibition of prefrontal cortex pyramidal cells facilitates relapse to heroin seeking. Neuropsychopharmacology. 2010;35(10):2120–33. doi: 10.1038/npp.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vazquez-Sanroman DB, et al. Nicotine self-administration remodels perineuronal nets in ventral tegmental area and orbitofrontal cortex in adult male rats. Addict Biol. 2016 doi: 10.1111/adb.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Natarajan R, et al. A role for matrix metalloproteinases in nicotine-induced conditioned place preference and relapse in adolescent female rats. J Exp Neurosci. 2013;7:1–14. doi: 10.4137/JEN.S11381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang X, et al. Arylsulfatase B modulates neurite outgrowth via astrocyte chondroitin-4-sulfate: dysregulation by ethanol. Glia. 2014;62(2):259–71. doi: 10.1002/glia.22604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Coleman LG, Jr, et al. Adolescent binge ethanol treatment alters adult brain regional volumes, cortical extracellular matrix protein and behavioral flexibility. Pharmacol Biochem Behav. 2014;116:142–51. doi: 10.1016/j.pbb.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen H, et al. Repeated Binge Drinking Increases Perineuronal Nets in the Insular Cortex. Alcohol Clin Exp Res. 2015;39(10):1930–8. doi: 10.1111/acer.12847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seif T, et al. Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nat Neurosci. 2013;16(8):1094–100. doi: 10.1038/nn.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wright JW, et al. Ethanol-induced impairment of spatial memory and brain matrix metalloproteinases. Brain Res. 2003;963(1–2):252–61. doi: 10.1016/s0006-8993(02)04036-2. [DOI] [PubMed] [Google Scholar]
- 70.Blacktop JM, et al. Role of perineuronal nets in the anterior dorsal lateral hypothalamic area in the acquisition of cocaine-induced conditioned place preference and self-administration. Neuropharmacology. 2017;118:124–136. doi: 10.1016/j.neuropharm.2017.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xue YX, et al. Depletion of perineuronal nets in the amygdala to enhance the erasure of drug memories. J Neurosci. 2014;34(19):6647–58. doi: 10.1523/JNEUROSCI.5390-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lubbers BR, et al. The Extracellular Matrix Protein Brevican Limits Time-Dependent Enhancement of Cocaine Conditioned Place Preference. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smith AC, et al. Synaptic plasticity mediating cocaine relapse requires matrix metalloproteinases. Nat Neurosci. 2014;17(12):1655–7. doi: 10.1038/nn.3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brown TE, et al. Role of matrix metalloproteinases in the acquisition and reconsolidation of cocaine-induced conditioned place preference. Learn Mem. 2007;14(3):214–23. doi: 10.1101/lm.476207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brown TE, et al. Increase in matrix metalloproteinase-9 levels in the rat medial prefrontal cortex after cocaine reinstatement of conditioned place preference. Synapse. 2008;62(12):886–9. doi: 10.1002/syn.20562. [DOI] [PubMed] [Google Scholar]
- 76.Smith AW, et al. Plasticity associated with escalated operant ethanol self-administration during acute withdrawal in ethanol-dependent rats requires intact matrix metalloproteinase systems. Neurobiol Learn Mem. 2011;96(2):199–206. doi: 10.1016/j.nlm.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stefaniuk M, et al. Matrix Metalloproteinase-9 and Synaptic Plasticity in the Central Amygdala in Control of Alcohol-Seeking Behavior. Biol Psychiatry. 2017;81(11):907–917. doi: 10.1016/j.biopsych.2016.12.026. [DOI] [PubMed] [Google Scholar]
- 78.Mizoguchi H, et al. Reduction of methamphetamine-induced sensitization and reward in matrix metalloproteinase-2 and -9-deficient mice. J Neurochem. 2007;100(6):1579–88. doi: 10.1111/j.1471-4159.2006.04288.x. [DOI] [PubMed] [Google Scholar]
- 79.Mizoguchi H, et al. Role of matrix metalloproteinase and tissue inhibitor of MMP in methamphetamine-induced behavioral sensitization and reward: implications for dopamine receptor down-regulation and dopamine release. J Neurochem. 2007;102(5):1548–60. doi: 10.1111/j.1471-4159.2007.04623.x. [DOI] [PubMed] [Google Scholar]
- 80.Samochowiec A, et al. Functional polymorphism of matrix metalloproteinase-9 (MMP-9) gene in alcohol dependence: family and case control study. Brain Res. 2010;1327:103–6. doi: 10.1016/j.brainres.2010.02.072. [DOI] [PubMed] [Google Scholar]
- 81.Zuo L, et al. Genome-wide association study of alcohol dependence implicates KIAA0040 on chromosome 1q. Neuropsychopharmacology. 2012;37(2):557–66. doi: 10.1038/npp.2011.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mash DC, et al. Gene expression in human hippocampus from cocaine abusers identifies genes which regulate extracellular matrix remodeling. PLoS One. 2007;2(11):e1187. doi: 10.1371/journal.pone.0001187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kovatsi L, et al. Alterations in serum MMP and TIMP concentrations following chronic heroin abuse. Toxicol Mech Methods. 2013;23(5):377–81. doi: 10.3109/15376516.2012.758681. [DOI] [PubMed] [Google Scholar]
- 84.Yamada J, Jinno S. Molecular heterogeneity of aggrecan-based perineuronal nets around five subclasses of parvalbumin-expressing neurons in the mouse hippocampus. J Comp Neurol. 2017;525(5):1234–1249. doi: 10.1002/cne.24132. [DOI] [PubMed] [Google Scholar]
- 85.Dauth S, et al. Extracellular matrix protein expression is brain region dependent. J Comp Neurol. 2016;524(7):1309–36. doi: 10.1002/cne.23965. [DOI] [PMC free article] [PubMed] [Google Scholar]