Abstract
We studied the association between neighborhood socioeconomic status (SES) and incidence of coronary heart disease (CHD) or ischemic stroke in the total population and in full- and half-siblings to determine whether these associations are causal or a result from familial confounding. Data were retrieved from nationwide Swedish registers containing individual clinical data linked to neighborhood of residence. After adjustment for individual SES, the association between neighborhood SES and CHD showed no decrease with increasing genetic resemblance, particularly in women. This indicates that the association between neighborhood SES and CHD incidence is partially causal among women, which represents a novel finding.
Keywords: Myocardial infarction, Ischemic stroke, Neighborhood deprivation, Socioeconomic status
Introduction
Coronary heart disease (CHD) and ischemic stroke are associated with increased mortality rates and disability as well as substantial costs for health care systems worldwide (Mozaffarian et al., 2016). Strong correlations to socioeconomic factors have been established, both at the individual- and neighborhood level (Winkleby et al., 2007, Sundquist et al., 2004b, Sundquist et al., 2004a, Diez Roux et al., 2001, Hamano et al., 2013, Calling et al., 2016).
However, appropriate preventive efforts and interventions, particularly at the neighborhood level, have been hampered because causality in the associations between neighborhood socioeconomic factors and CHD and ischemic stroke has been difficult to prove. Establishing causality is often a challenge in observational studies, including those examining neighborhood effects (Oakes, 2004). This is because it is nearly impossible to perform randomized controlled trials where large numbers of individuals are randomly assigned and adhere to move to different types of neighborhoods (Diez Roux, 2004). However, novel analytic strategies, like quasi-experimental co-relative designs (Lahey and D'Onofrio, 2010, Merlo et al., 2013, Kendler et al., 2014), provide new opportunities for investigating the causal effect of neighborhood exposure on health-related outcomes in individuals who share similar genes and similar family environmental background but differ in exposure. In co-relative designs, the association between a certain exposure and an outcome is examined in the general population as well as in relatives (Kendler et al., 1993).
Previous studies have used co-relative designs to examine the causal nature of neighborhood effects on individual CHD in full brothers (Chaix, 2009, Merlo et al., 2013). However, to the best of our knowledge, no previous study has included both women and men or examined the causal nature of neighborhood effects on individual CHD as well as ischemic stroke in both full-siblings and half-siblings and in different age groups. These knowledge gaps will be addressed in the present study.
The aim of the present study was to examine the association between neighborhood socioeconomic status (SES) and incidence of CHD or ischemic stroke in the total population and in full- and half-siblings in order to determine whether these potential associations are causal or result from familial confounding factors. These associations were examined in men and women and in different age groups separately.
Method
The data used in the present study were retrieved from nationwide registers provided by the National Board of Health and Welfare (health care data) and Statistics Sweden, the Swedish Government-owned statistics bureau (population and family data). All data were linked by the personal Swedish identification number, which was replaced by a serial number in the dataset in order to maintain anonymity for all individuals. Ethical approval was granted by the Ethics Committee of Lund University, Sweden, and was conducted in accordance with the 1975 Declaration of Helsinki. Health care data was retrieved from two nationwide Swedish medical registers: the Hospital Discharge Register containing in-patient data from 1987 and onwards, and the Outpatient Register containing outpatient specialist care data from 2001 and onwards. The Swedish Multi-generation Register, which includes all individuals born in 1932 and onwards (2011), was used to identify all full-sibling and half-sibling pairs.
Outcome variables
The outcome variables were incidence of CHD and ischemic stroke during follow-up (until 2013). Incidence was defined as the first registered diagnosis of CHD or ischemic stroke during the study period. The CHD outcome diagnoses were collected from the Hospital Discharge Register or Outpatient Register using the International Classification of Diseases (ICD) codes from the following different ICD versions: ICD-8 codes 410–414, ICD-9 codes 410–414 or ICD-10 codes I20–25, which is in accordance with previous studies (Calling et al., 2013). The ischemic stroke outcome diagnoses were collected from the Hospital Discharge Register or Outpatient Register using ICD-8 codes 432–438, ICD-9 codes 433, 434, 435, 437.0, 437.1 or ICD-10 codes I63 (not I63.6), I65, I66, I67.2, I67.8, and G45. For the Hospital Discharge Register we used main and secondary discharge diagnoses encoded in the ICD format (Ludvigsson et al., 2011). The diagnoses in this register have a positive predictive value between 85–95%, and the diagnostic validity of many diseases are even higher; e.g., myocardial infarction (MI) and stroke have a positive predictive value of >95% (Ingelsson et al., 2005, Ludvigsson et al., 2011, Nilsson et al., 1994).
Neighborhood-level socioeconomic status (SES)
The home addresses of all Swedish individuals have been geocoded to small geographical units that have boundaries defined by homogeneous types of buildings. These neighborhood areas, developed by Statistics Sweden, are called small areas for market statistics (SAMS) and have an average of 1000 people each. SAMS were used as proxies for neighborhoods (Sundquist et al., 2006a, Cubbin et al., 2006). A summary index was calculated to characterize neighborhood-level SES (Winkleby et al., 2007). The neighborhood SES index was based on four items: low education level, low income, unemployment and receipt of social welfare. Neighborhood SES was approximately normally distributed with a mean value of 0 and a standard deviation (SD) of 1. Neighborhood SES was used as a continuous variable in the models with the score ranging between −3.2 and 12 with higher values indicating higher levels of neighborhood deprivation. SES was measured at inclusion in the study.
Individual-level variables
Marital status
Individuals were classified as married/cohabitating or widowed/divorced/never married.
Family income
Individualized disposable family income was defined as combined family income minus current taxes divided by the number of people in the family. In order to be able to use the income variable to categorize SES over time, we standardized the variable (mean 0 and SD 1) by sex and year.
Educational attainment
The education variable was primarily based on the number of years of education: less than 9 years; 9 years; 10–11 years; 12 years; 13–15 years; 16 years or more; and having a PhD/ licentiate degree. The education variable was also standardized (mean 0 and SD 1) by sex and year.
Sample, time of inclusion, and follow-up
The dataset included all men and women born in Sweden between 1932 and 1966. Time for inclusion in the study of the men and women was between 1990 and 2006. For the variables neighborhood SES, educational attainment, family income and marital status we had access to yearly information during the entire inclusion period, i.e., 1990 to 2006 for all individuals residing in Sweden. We assessed these variables at time for inclusion in the study in the three different groups, i.e., those who became 40 years (N40y=1,702,541), 50 years (N50y=1,741,835) or 60 years (N60y=1,276,705) somewhere during the inclusion period. The relatively lower number of individuals aged 60 years was because one of the inclusion criteria in this study was to have a registered sibling in the Multi-Generation Register (i.e., brother for the men in the study population or sister for the women in the study population). “Wash-out” was performed in order to secure that all cases were incident cases. For this purpose, we excluded all patients with a CHD diagnosis between 1987 and study start for the CHD analyses and all patients with an ischemic stroke diagnosis between 1987 and study start for the ischemic stroke analyses. The total number of individuals with a CHD registration prior to study start were 97,827 in the three age groups (N40y=3,618, N50y=25,514 and N60y=68,695). Individuals with less than five years of residence in their neighborhood at inclusion were also excluded (N40y=434,337, N50y=320,012 and N60y=175,733). Exclusion of individuals with less than five years of residence was done because these individuals would have had a limited exposure to their current neighborhoods, and, as CHD or stroke most likely develops after longer time exposures. Additionally, all individuals who had lived abroad at some time point (N40y=23,089, N50y=19,366 and N60y=6,408) during the study period were excluded. The follow-up started at time of inclusion and ended at the time of a possible event, death, emigration or at the end of 2013, whichever came first.
Statistical Methods
We used Cox proportional hazards models to assess the risk of CHD as a function of neighborhood SES. In the first model, we estimated hazard ratios (HRs) to assess the risk of CHD from age at inclusion (40, 50 or 60 years) until end of follow-up, death, possible event, or emigration, as a function of neighborhood SES at age at inclusion while controlling for family income, educational attainment, and marital status at inclusion. We then replicated the models for ischemic stroke. All models were stratified by sex. In all models, the proportionality assumption was checked. If not fulfilled, we conducted two additional analyses; in the first, we added a time-dependent variable and, in the second, we divided the follow-up period in three time intervals and modeled each time interval separately.
Next, we sought to assess the degree to which the association between neighborhood SES and our cardiovascular outcomes is a result from confounding by familial risk factors (genetic and/or shared environmental) using a co-relative design for the full- and half-sibling pairs. We used a stratified Cox regression model, in which we refitted all analyses within strata of the defined relative sets (full-sibling pairs and half-sibling pairs). Only pairs in which the members differed in their exposure to neighborhood SES would contribute to the regression estimates.
Within each stratum, the hazard ratio (HR) is adjusted for the familial cluster, and, therefore, accounts for an array of unmeasured genetic and environmental factors shared within the relative set. All statistical analyses were performed using SAS 9.4.
Results
Table 1 shows the total study population for CHD, the number of CHD cases, the proportion of CHD cases in the total population as well as the median age at CHD diagnosis for the three age groups 40, 50 and 60 years and for men and women separately. In the men, 23,479, 66,978 and 72,257 individuals were diagnosed with CHD during follow-up, yielding a proportion of CHD cases of 3.76%, 9.58% and 14.45% for the three age groups 40, 50 and 60 years, respectively. In the women, 10,383, 30,332 and 42,607 individuals were diagnosed with CHD yielding a corresponding proportion of CHD cases of 1.68%, 4.48% and 8.10% for the three age groups 40, 50 and 60 years, respectively. The sizes of the study populations in tables 1 and 2 differ due to different exclusion criteria.
Table 1.
Distribution of study population for coronary heart disease (CHD) for the different age groups.
| Sex | Men | Women | ||||
|---|---|---|---|---|---|---|
| Age group | 40 | 50 | 60 | 40 | 50 | 60 |
| Marital status | ||||||
| married/cohabitating | 53.7% | 65.3% | 70.0% | 61.5% | 68.9% | 67.8% |
| widowed/divorced/never married | 46.3% | 34.7% | 30.0% | 38.5% | 31.1% | 32.2% |
| n Total | 624,010 | 699,161 | 499,975 | 617,487 | 677,782 | 525,894 |
| n CHD | 23,479 | 66,978 | 72,257 | 10,383 | 30,332 | 42,607 |
| % of total | 3.76 | 9.58 | 14.45 | 1.68 | 4.48 | 8.10 |
| Age at CHD (median) | 52 | 60 | 66 | 52 | 60 | 66 |
Table 2.
Distribution of study population for ischemic stroke for the different age groups.
| Sex | Men | Women | ||||
|---|---|---|---|---|---|---|
| Age group | 40 | 50 | 60 | 40 | 50 | 60 |
| Marital status | ||||||
| married/cohabitating | 53.7% | 65.2% | 70.0% | 61.5% | 68.9% | 67.8% |
| widowed/divorced/never married | 46.3% | 34.8% | 30.0% | 38.5% | 31.1% | 32.2% |
| n Total | 624,642 | 709,138 | 528,284 | 617,172 | 679,632 | 533,364 |
| n Stroke | 10,954 | 35,510 | 48,489 | 7,470 | 22,404 | 34,752 |
| % of total | 1.75 | 5.01 | 9.18 | 1.21 | 3.30 | 6.52 |
| Age at CHD (median) | 52 | 61 | 68 | 52 | 61 | 68 |
Table 2 shows the total study population for ischemic stroke, the number of ischemic stroke cases, the proportion of ischemic stroke cases in the total population as well as the median age at ischemic stroke diagnosis for the three age groups 40, 50 and 60 year olds and for men and women separately. In men, 10,954, 35,510, and 48,489 individuals were diagnosed with ischemic stroke during the follow-up, yielding a proportion of stroke cases of 1.75%, 5.01% and 9.18% for the three age groups 40, 50 and 60 years, respectively. In women, 7,470, 22,404 and 34,752 individuals were diagnosed with ischemic stroke during the follow-up, yielding a proportion of stroke cases of 1.21%, 3.30% and 6.52% for the three age groups 40, 50 and 60 years, respectively.
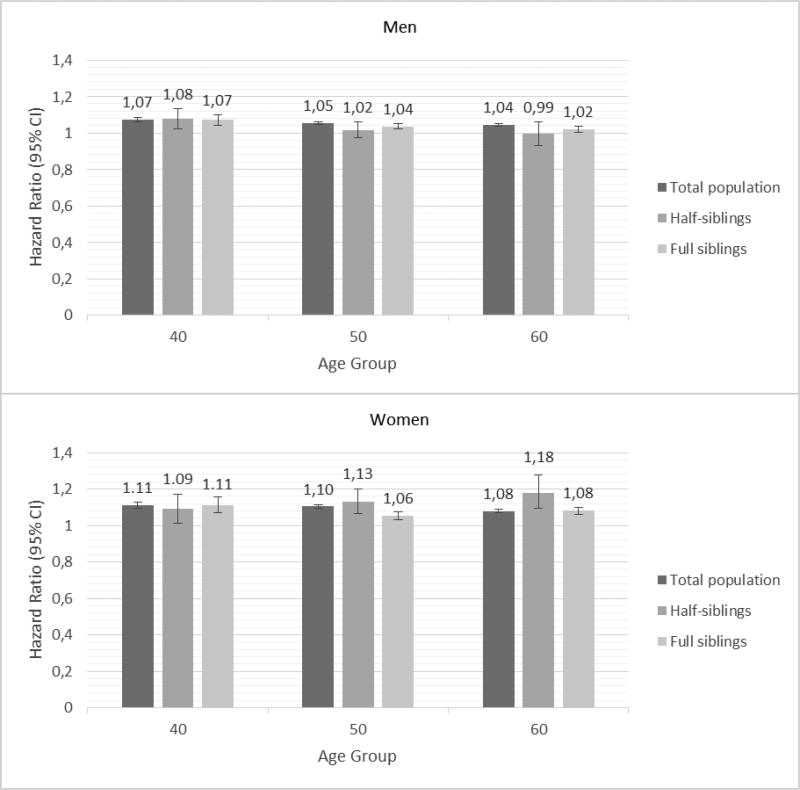
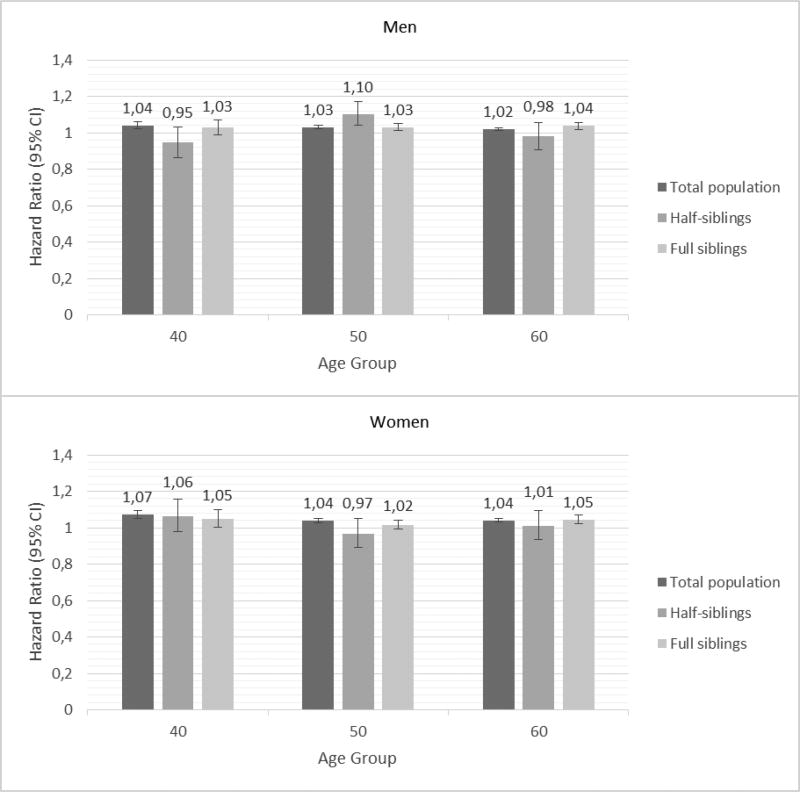
Figures 1 and 2 show the results of the co-relative design that examines the causal nature of neighborhood effects on individual outcomes. If the association between neighborhood SES and incidence of CHD or ischemic stroke is truly causal, one would expect that the association between SES and incidence of CHD or ischemic stroke would be of similar strength in the general population as in relative pairs discordant for their level of neighborhood SES. However, if the association between SES and incidence of CHD or ischemic stroke results partly or entirely from familial confounding, then the association would decrease or disappear entirely in genetically related family members.
Figure 1.
Hazard ratios and 95% confidence intervals for incidence of coronary heart disease (CHD) in men (top) and women (bottom) based on neighborhood socioeconomic status (SES); Results of Cox proportional hazard models.
Figure 2.
Hazard ratios and 95% confidence intervals for incidence of ischemic stroke in men (top) and women (bottom) based on neighborhood socioeconomic status (SES); Results of Cox proportional hazard models.
Figure 1 shows the results of the Cox proportional hazard modeling for the co-relative design, i.e., the hazard ratios and 95% confidence intervals (CI) for incidence of CHD, based on neighborhood SES, used as a continuous variable, for the three age groups 40, 50 and 60 year olds, in men and women, respectively, after controlling for marital status, family income and educational attainment. For example, for 40 year old women, the hazard ratio was 1.11 (95% CI 1.09–1.13), 1.09 (95% CI 1.01–1.17) and 1.11 (95% CI 1.07–1.16) for the total population, half siblings and full siblings, respectively, showing no decrease of the HRs with increasing genetic resemblance. For all age groups, neighborhood SES was associated with incidence of CHD for both sexes, but the association seemed to be somewhat stronger in women. The hazard ratios with 95% confidence intervals are also presented in Supplementary Table 1.
Figure 2 shows the results of Cox proportional hazard modeling: the hazard ratios and 95% confidence intervals for incidence of ischemic stroke, based on neighborhood SES for the three age groups 40, 50 and 60 year olds, in men and women, respectively, after controlling for marital status, family income and educational attainment. The association between neighborhood SES and incidence of ischemic stroke seemed to be somewhat weaker than the association between neighborhood SES and CHD. The hazard ratios with 95% confidence intervals are also presented in Supplementary Table 2.
Neighborhood SES was used as a continuous variable in the models with higher values indicating higher levels of neighborhood deprivation. As an example, a HR of 1.1 for CHD means that the risk of CHD would increase by 10% for one unit’s increase in the neighborhood SES score. An increase in neighborhood SES score by 2.5, roughly corresponding to moving from a neighborhood in the most affluent quintile to a neighborhood in the least affluent quintile, would then give an increased risk of CHD by 21–30% for women and 11–20% for men, depending on age group, after controlling for individual level variables.
Discussion
The main finding of the present co-relative study suggests that the association between neighborhood SES and incidence of CHD is, at least in part, causal, as the HRs in both men and women were of similar size for the total population, half-siblings and full siblings and for the three age groups analyzed. Similar results were found for ischemic stroke in the co-relative analyses, but the associations were somewhat weaker than for CHD.
This is, to the best of our knowledge, the first co-relative study that examines the causal nature of neighborhood effects on individual risk of CHD and ischemic stroke in both women and men. In addition to including the total population to explore this relationship, both full-siblings and half-siblings were included as well as different age groups.
Previous studies have used co-relative designs to examine the causal nature of neighborhood effects on individual CHD in full brothers (Chaix, 2009, Merlo et al., 2013). However, to the best of our knowledge, no previous study has included both women and men or applied a co-relative design to disentangle the causal nature of neighborhood effects on individual CHD as well as ischemic stroke in both full-siblings and half-siblings and in different age groups, which represents a novel contribution.
Various mechanisms have been suggested to lie behind the causal pathways of neighborhood effects on individual health. One possible mechanism is that the neighborhood affects health-related behaviors such as a healthy diet, physical activity, and non-smoking (Sundquist et al., 2004a, Sundquist et al., 2004b). For example, a lack of access to smoke-free areas, healthy food stores, and safe places to exercise may deny people the opportunity to develop and maintain heart-healthy behaviors. Moreover, access to health care resources and information about the advantages of obtaining treatment for hypertension and diabetes may differ between neighborhoods. Lack of social cohesion, and in particular violent crime, has also been suggested to lie in the direct causal pathway between the neighborhood social environment and health, as well as social disorganization and signs of lacking social control such as vandalism and littering (Sundquist et al., 2006b).
Traditionally, two different possible explanations have been used to explain the well-established inverse association between neighborhood SES and cardiovascular disease. The first is social causation or the stress hypothesis, which attributes the association between neighborhood SES and cardiovascular disease to the adversity and stress associated with low social status, i.e., low neighborhood SES would cause cardiovascular disease (Dohrenwend et al., 1992, Adams, 2003). The second possible explanation is social selection or the drift hypothesis, which proposes that predisposed persons, i.e., already unhealthy individuals, will drift down to or fail to rise out of areas with low neighborhood SES. Here, the neighborhood would not cause poor health; rather, already unhealthy individuals would move to more deprived neighborhoods (Dohrenwend et al., 1992). The present co-relative study suggests that the association between neighborhood SES and cardiovascular health is stronger in women than in men, at least for CHD and ischemic stroke, and indicates that the stress hypothesis rather than the drift hypothesis may be more salient for women than for men.
Speculating, the potential neighborhood effects on men might be confounded by genetics and shared family environmental factors to a higher extent than on women. In addition, women have often spent more time in their home environment, which may result in a greater exposure to their local neighborhoods. Also, men have traditionally been the breadwinners to a higher extent than women whereas women’s SES often has depended on their husband’s.
For ischemic stroke, the association between neighborhood SES and stroke incidence was somewhat weaker than for CHD. This might indicate that different neighborhood mechanisms may be at work affecting the incidence of CHD differently compared to ischemic stroke. Further studies are, however, needed to confirm these findings.
Limitations and strengths
There are several limitations to this study. The present study is limited to Sweden, although it included the entire population. Residual confounding may exist as socioeconomic measures only represent proxies for individual-level status. Additionally, family based designs do not completely eliminate issues of residual confounding because family members may also differ in life-course exposures to non-shared environmental factors. Such exposures may condition both the selection of neighborhood of residence and the risk to develop CHD or ischemic stroke. This means that our results, despite their apparent evidence for a causal relationship between neighborhood SES and incidence of CHD or stroke, may be partly confounded by our inability to completely adjust for confounders that drive self-selection into certain neighborhoods. The co-relative design allows us, however, to greatly reduce the confounding effect of such unknown factors. Additionally, the availability of universal health care in Sweden might mitigate potential negative neighborhood effects on health. In other countries without widespread universal health care, e.g., the United States, neighborhood effects are potentially even more pronounced. The outpatient register is only available from 2001 and onwards, but this would most likely have a small impact on our results. This is because most newly diagnosed CHD and stroke cases are hospitalized and the outpatient register was only used as a complement to collect those cases who had not been registered as in-patients. This study has also, however, several strengths. The very large cohort, including all residents of Sweden during the follow-up period increases the generalizability of our results. The Hospital Discharge register has very high validity and have a positive predictive value of >95% for many diagnoses e.g., MI, angina pectoris and stroke (Ingelsson et al., 2005, Ludvigsson et al., 2011, Nilsson et al., 1994). Both the Hospital Discharge Register and the Swedish Multi-generation Register have almost 100% complete data. Using the Swedish Multi-generation Register allows for analysis of causality through co-sibling design, which otherwise is a great challenge in observational studies.
Including individuals in three different age group groups enables a life-course perspective to the causal nature of neighborhood effects on health. The inclusion of women and men allows for disentangling differences in potential neighborhood effects depending on sex. Finally, studying both CHD and ischemic stroke enables the detection of potential differences in neighborhood effects depending on diagnosis - different types of CVD might be differently affected by the neighborhood, and by different mechanisms.
Conclusion
Neighborhood SES was associated with incidence of CHD and ischemic stroke, and the present study suggests that these associations were, at least in part, causal.
These findings raise important clinical and public health concerns, and indicate that policy and practice need to reframe health problems from a sole focus on individual approaches to a broader focus that includes neighborhoods. Future studies should focus on the potential mechanisms behind the neighborhood effect on cardiovascular diseases in order to develop possible intervention strategies targeting these mechanisms.
Supplementary Material
Highlights.
Neighborhood deprivation is associated with coronary heart disease and stroke.
This association is unaffected by increasing genetic resemblance.
A causal association is plausible, particularly for coronary heart disease among women.
Acknowledgments
Acknowledgements of grant support: the Swedish Research Council and the National Heart, Lung, And Blood Institute of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Conflict of interest: None
References
- Multi-generation register 2010: a description of contents and quality. Statistiska centralbyrån. Avdelningen för befolknings- och välfärdsstatistik. 2011 http://www.scb.se/
- Adams P, Hurd M, Mcfadden D, Merrill A, Ribeiro T. Healthy, wealthy, and wise? Tests for direct causal paths between health and socioeconomic status. Journal of Econometrics. 2003;112:3–56. [Google Scholar]
- Calling S, Ji J, Sundquist J, Sundquist K, Zöller B. Shared and non-shared familial susceptibility of coronary heart disease, ischemic stroke, peripheral artery disease and aortic disease. Int J Cardiol. 2013;168:2844–50. doi: 10.1016/j.ijcard.2013.03.149. [DOI] [PubMed] [Google Scholar]
- Calling S, Li X, Kawakami N, Hamano T, Sundquist K. Impact of neighborhood resources on cardiovascular disease: a nationwide six-year follow-up. BMC Public Health. 2016;16:634. doi: 10.1186/s12889-016-3293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaix B. Geographic life environments and coronary heart disease: a literature review, theoretical contributions, methodological updates, and a research agenda. Annu Rev Public Health. 2009;30:81–105. doi: 10.1146/annurev.publhealth.031308.100158. [DOI] [PubMed] [Google Scholar]
- Cubbin C, Sundquist K, Ahlen H, Johansson SE, Winkleby MA, Sundquist J. Neighborhood deprivation and cardiovascular disease risk factors: protective and harmful effects. Scand J Public Health. 2006;34:228–37. doi: 10.1080/14034940500327935. [DOI] [PubMed] [Google Scholar]
- Diez Roux AV. Estimating neighborhood health effects: the challenges of causal inference in a complex world. Soc Sci Med. 2004;58:1953–60. doi: 10.1016/S0277-9536(03)00414-3. [DOI] [PubMed] [Google Scholar]
- Diez Roux AV, Merkin SS, Arnett D, Chambless L, Massing M, Nieto FJ, Sorlie P, Szklo M, Tyroler HA, Watson RL. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345:99–106. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- Dohrenwend BP, Levav I, Shrout PE, Schwartz S, Naveh G, Link BG, Skodol AE, Stueve A. Socioeconomic status and psychiatric disorders: the causation-selection issue. Science. 1992;255:946–52. doi: 10.1126/science.1546291. [DOI] [PubMed] [Google Scholar]
- Hamano T, Kawakami N, Li X, Sundquist K. Neighbourhood environment and stroke: a follow-up study in Sweden. PLoS One. 2013;8:e56680. doi: 10.1371/journal.pone.0056680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingelsson E, Arnlov J, Sundstrom J, Lind L. The validity of a diagnosis of heart failure in a hospital discharge register. Eur J Heart Fail. 2005;7:787–91. doi: 10.1016/j.ejheart.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Maclean CJ, Heath AC, Eaves LJ, Kessler RC. Smoking and major depression. A causal analysis. Arch Gen Psychiatry. 1993;50:36–43. doi: 10.1001/archpsyc.1993.01820130038007. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Ohlsson H, Sundquist K, Sundquist J. The causal nature of the association between neighborhood deprivation and drug abuse: a prospective national Swedish co-relative control study. Psychol Med. 2014;44:2537–46. doi: 10.1017/S0033291713003048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, D'Onofrio BM. All in the Family: Comparing Siblings to Test Causal Hypotheses Regarding Environmental Influences on Behavior. Curr Dir Psychol Sci. 2010;19:319–323. doi: 10.1177/0963721410383977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, Heurgren M, Olausson PO. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. doi: 10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo J, Ohlsson H, Chaix B, Lichtenstein P, Kawachi I, Subramanian SV. Revisiting causal neighborhood effects on individual ischemic heart disease risk: a quasi-experimental multilevel analysis among Swedish siblings. Soc Sci Med. 2013;76:39–46. doi: 10.1016/j.socscimed.2012.08.034. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, De Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, Mcguire DK, Mohler ER, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB, Members WG, Committee AHAS, Subcommittee SS. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- Nilsson AC, Spetz CL, Carsjo K, Nightingale R, Smedby B. Reliability of the hospital registry. The diagnostic data are better than their reputation. Lakartidningen. 1994;91:598, 603–5. [PubMed] [Google Scholar]
- Oakes JM. The (mis)estimation of neighborhood effects: causal inference for a practicable social epidemiology. Soc Sci Med. 2004;58:1929–52. doi: 10.1016/j.socscimed.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Sundquist J, Johansson SE, Yang M, Sundquist K. Low linking social capital as a predictor of coronary heart disease in Sweden: a cohort study of 2.8 million people. Soc Sci Med. 2006a;62:954–63. doi: 10.1016/j.socscimed.2005.06.049. [DOI] [PubMed] [Google Scholar]
- Sundquist K, Malmstrom M, Johansson SE. Neighbourhood deprivation and incidence of coronary heart disease: a multilevel study of 2.6 million women and men in Sweden. J Epidemiol Community Health. 2004a;58:71–7. doi: 10.1136/jech.58.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundquist K, Theobald H, Yang M, Li X, Johansson SE, Sundquist J. Neighborhood violent crime and unemployment increase the risk of coronary heart disease: a multilevel study in an urban setting. Soc Sci Med. 2006b;62:2061–71. doi: 10.1016/j.socscimed.2005.08.051. [DOI] [PubMed] [Google Scholar]
- Sundquist K, Winkleby M, Ahlen H, Johansson SE. Neighborhood socioeconomic environment and incidence of coronary heart disease: a follow-up study of 25,319 women and men in Sweden. Am J Epidemiol. 2004b;159:655–62. doi: 10.1093/aje/kwh096. [DOI] [PubMed] [Google Scholar]
- Winkleby M, Sundquist K, Cubbin C. Inequities in CHD incidence and case fatality by neighborhood deprivation. Am J Prev Med. 2007;32:97–106. doi: 10.1016/j.amepre.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.