Summary
Stem cells and tissue-derived stromal cells stimulate the repair of degenerated and injured tissues, motivating a growing number of cell-based interventions in the musculoskeletal field. Recent investigations have indicated that these cells are critical for their trophic and immunomodulatory role in controlling endogenous cells. This review presents recent clinical advances where stem cells and stromal cells have been used to stimulate musculoskeletal tissue repair, including delivery strategies to improve cell viability and retention. Emerging bioengineering strategies are highlighted, particularly towards the development of biomaterials for capturing aspects of the native tissue environment, altering the healing niche, and recruiting endogenous cells.
Keywords: Musculoskeletal tissue repair, clinical trials, mesenchymal stromal cells, stem cells, biomaterials, tissue engineering
Introduction
Musculoskeletal tissue injuries and degeneration are common and debilitating to a high number of patients (Brooks, 2006). Unfortunately, endogenous musculoskeletal tissue regeneration is limited in many cases and may be affected by inflammation and the degree of damage. For example, most fractures of long bones heal spontaneously, whereas large segmental defects fail to heal. Additionally, while articular cartilage has almost no intrinsic reparative potential, tendons and ligaments may heal, but often with inferior properties. The high prevalence of these injuries has led to significant investment in the development of new therapies to enhance healing and augment current surgical interventions. Often, the goal is to mimic and recapitulate the natural healing cascade and developmental process by transplantation of tissue-specific stromal and progenitor cells or by endogenous manipulation to enhance the native repair capacity of cells.
There has been a continuing increase in the number and type of stem and stromal cells being pursued in human clinical trials for treatment of musculoskeletal injuries (Steinert et al., 2012). Most approaches in this area are based on ex vivo expanded mesenchymal stromal cells (MSCs) derived from bone marrow (BM). Originally identified and characterized by their multilineage differentiation potential in vitro, multipotent capabilities of MSCs in vivo have not been clearly demonstrated to date, particularly due to the lack of methods to identify and define differentiated populations (Nombela-Arrieta et al., 2011). Central to recent progress in the field has been the understanding that stem and progenitor functions of MSCs may not be the key attribute that mediates tissue repair. In addition, there is outstanding controversy over the terminology of exogenously supplied MSCs as stromal cells, and various terms, including medicinal signaling cells, have been proposed to more accurately reflect their therapeutic function in vivo (Caplan, 2017). Nevertheless, the therapeutic benefit of these cells has been largely explored. Significant advances have been made in developing strategies that deliver, protect, and recruit stem cells, and the bioengineering field is evolving to improve current surgical techniques.
This review first describes current treatments and reports the recent progress in clinical investigations of stem and stromal cell-based therapies for musculoskeletal repair with a particular focus on bone and fibrocartilaginous tissues. The current understanding of appropriate cell sources and delivery strategies are then illustrated towards endogenous repair of musculoskeletal tissues. Lastly, emerging therapeutic concepts are highlighted in the context of biomaterials as a particularly attractive tool to control stem and stromal cell behavior both ex vivo and in vivo, to recruit endogenous stem cells, and to control the local healing environment. Such approaches have great potential looking forward to future therapies in musculoskeletal repair.
Current surgical interventions and associated limitations
Damage from trauma is a major cause for surgical repair of musculoskeletal structures, and correlates with the increasing prevalence of post-traumatic and degenerative pathologies. Detailed descriptions of the indications, clinical applications and outcomes of current surgical procedures have been described in several excellent reviews (Makris et al., 2015, Grayson et al., 2015, Sakai and Andersson, 2015). A brief understanding of these surgical principles is important here, as many cell-based interventions have been developed that aim to improve - and not substitute - surgical repair (Table 1).
Table 1.
Musculoskeletal repair: surgical techniques and limitations
| Long bone defects | Autologous bone graft | Allogeneic bone graft | Bone substitutes |
| Donor-site morbidity, graft size, bone quality | Slower healing compared to autograft, risk of rejection | Mechanically inferior to bone-grafts | |
|
| |||
| Osteonecrosis of femoral head | Decompression and autologous bone graft | Joint replacement | |
| Palliative treatment | Limited implant life-time, requiring replacement particularly for young patients | ||
|
| |||
| Articular cartilage defects | Microfracture | Autologous chondrocyte implantation | Joint replacement |
| Formation of fibrocartilage with inferior mechanical properties, formation of subchondral bone cysts | Long healing process, ex vivo expansion and de-differentiation of chondrocytes, limited to focal cartilage defects, OA is contra- indication | Risk of complications including aseptic loosening, dislocation and infection | |
|
|
|||
| Meniscal tears | Meniscal suture (peripheral regions) | Partial meniscectomy (in central regions) | Meniscal allograft/ synthetic substitute |
| Limited to small tears | Increased risk of OA | Do not match mechanical complexity | |
|
| |||
| Volumetric muscle loss | Scar tissue debridement | Autologous innervated muscle tissue transfer | |
| Functional deficiency often remains | Donor-site morbidity, complex surgical procedure | ||
|
| |||
| Rotator cuff injuries | Subacromial decompression and tendon debridement | Suture and re-attachment of the tendon to the bone | |
| Creates more space, but does not treat the tear | Risk of re-tear, scar tissue and fibrosis may cause impingement | ||
|
| |||
| IVD degeneration | Resection of protrusions | Segmental fusion | Total disc arthroplasty |
| Often causes imbalance of adjacent segments | Limited motion, increased risk of adjacent segment degeneration | Increased risk of adjacent segment degeneration | |
Abbreviations: IVD, intervertebral disc; OA, osteoarthritis
Bone repair
The intrinsic repair of bone defects mirrors many events of embryonic development and makes fracture healing one of the rare postnatal processes that are regenerative and can ultimately restore damaged tissue to its pre-injury structure, composition and biomechanical function (Figure 1). In spite of the unique capacity of bone to heal, a number of clinical indications remain where therapeutic intervention is required. In the case of complex trauma with multiple fractures, infections, and tumor-associated and endocrine diseases (e.g. Diabetes, osteoporosis), the body’s natural healing response is impaired and non-union can occur in up to 15% of cases (Grayson et al., 2015). Another debilitating disorder is non-traumatic avascular osteonecrosis, which can lead to collapse of the femoral head and accounts for 10.000–20.000 total hip replacement surgeries in the United States per year (Figure 1) (Moya-Angeler et al., 2015). Autologous bone grafting presents the gold-standard for management of bone defects and non-unions, and union rates of more than 90% have been reported using iliac crest bone. However, considerable donor site morbidity and limited volumes must be taken into consideration. Additionally, allogeneic or synthetic bone substitutes, such as ceramics, corals or polymer-based materials, have not reached biological and mechanical properties equivalent to autologous bone (Table 1).
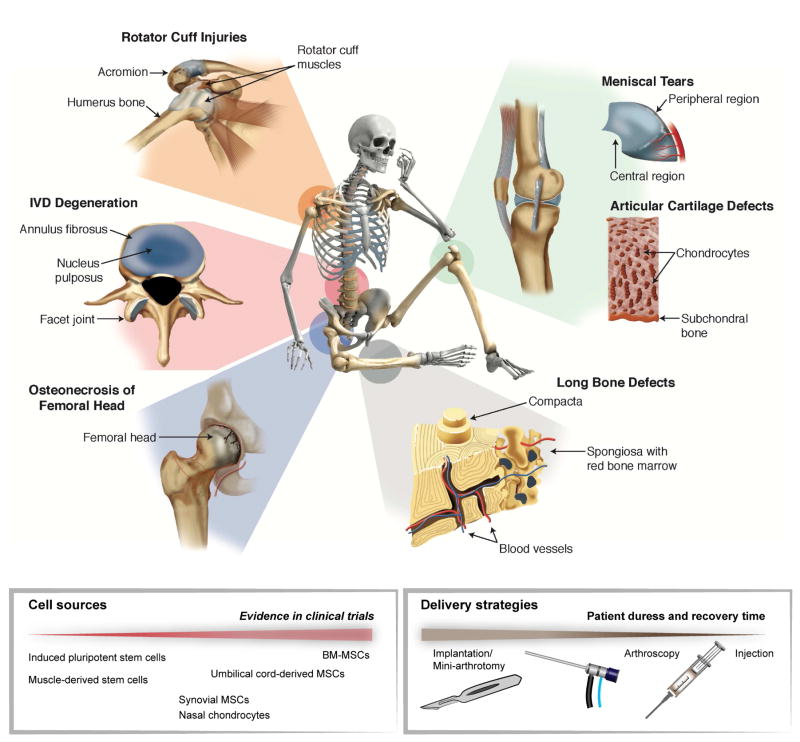
Figure 1. Musculoskeletal tissues with high incidence of injuries and degeneration.
The skeleton, joints, cartilage, intervertebral disc (IVD), tendons, ligaments and muscles are part of the musculoskeletal system, which provides stability and motion. Musculoskeletal diseases due to injuries and degeneration are one of the major causes of pain and disability. Cell therapies for musculoskeletal tissue repair are at different levels of evidence in clinical trials. For implantation of these cells, various delivery approaches are being used to optimize viability and minimize patient duress.
Skeletal muscle
In addition to direct traumatic injury, complex damage of bone tissue (e.g. open fractures, tumor ablations) often results in concomitant soft tissue injury, including adjacent muscles. While skeletal muscle has the inherent ability to regenerate after injuries, the regenerative capacity fails when a large volume of muscle is lost (i.e. volumetric loss). Such severe injuries may lead to fibrosis, atrophy and ischemia if left untreated, accounting for significant socioeconomic costs ($18.5 billion healthcare costs associate with sarcopenia alone) (Janssen et al., 2004). Therapeutic treatment options are limited to physical therapy, scar tissue debridement, and transfer of healthy, innervated, and vascularized autologous muscle tissue. However, outcomes of both surgical reconstructions often remain aesthetically and functional deficient (Grogan et al., 2011) (Table 1).
Articular cartilage and meniscus
In contrast to bone and skeletal muscle tissue, the poor intrinsic healing capacity of articular cartilage and meniscus tissue presents a major challenge in clinics. Lesions from injuries or degeneration often result in gradual tissue erosion, leading to impaired function of the affected joint and degenerative osteoarthritis (OA) (Figure 1). Patients with post-traumatic OA account for more than 10% of the 27 million adults in the United States that have a clinical diagnosis of OA (Johnson and Hunter, 2014). Commonly, the first line treatment of articular injuries includes arthroscopic lavage, partial meniscectomy and BM stimulation techniques to penetrate subchondral bone (Table 1). Microfracture has been considered the gold standard for stimulating endogenous repair; however, it often results in the formation of fibrocartilaginous repair tissue. This vulnerable cartilaginous tissue with altered biomechanics, owing to the penetration of the subchondral bone, raises concerns about the long-term efficacy of microfracture (Solheim et al., 2016). Therefore, secondary and more complex procedures strive to restore the hyaline cartilage, such as osteochondral autografting from the less weight-bearing periphery (mosaicplasty) and autologous chondrocyte implantation (ACI). ACI represents one of the first clinical applications of tissue engineering where a biopsy from a low-weight bearing region is performed and ex vivo expanded chondrocytes are implanted in a second operation. The de-differentiation of monolayer expanded chondrocytes and potential of recovery once implanted has been a matter of debate and matrix-based ACI techniques have been developed, which use absorbable scaffolds (e.g. porcine collagen) to support the implanted cells (Makris et al., 2015). An important limitation of these techniques is the long recovery time (6–12 months) to ensure neotissue formation. The choice of articular injury treatment depends on several factors, including localization and size of the lesion, the level of activity, and the degree of associated damage of menisci and ligaments.
Tears of the fibrocartilaginous menisci require surgical intervention for nearly 1 million patients in the United States annually (Vrancken et al., 2013). For lesions located in the peripheral vascularized region of the meniscus, repair strategies, such as sutures and anchors, allow preservation of the meniscal tissue. However, meniscal lesions often appear in the avascular central regions, which makes them less suitable for healing and usually requires partial or (sub)total meniscectomy (Figure 1, Table 1). In some cases, further treatment with a meniscal substitute, such as an allograft or a synthetic implant is indicated to limit OA (Vrancken et al., 2013).
Other fibrous musculoskeletal tissues
Another large proportion of musculoskeletal injuries in the clinics is represented by other damaged fibrous structures, including tendons, ligaments and the annulus fibrosus. Often, degenerative pathology precedes acute trauma and, like articular cartilage, these tissues have a limited healing capacity. One of the most common tendon injuries presented clinically is tearing of one or more of the interdigitating tendons of the rotator cuff (Figure 1). Failure of initial physical therapy or acute trauma in young patients motivates surgical repair using open or arthroscopic approaches for subacromial decompression, tendon debridement, and suture or anchor supplementation (Table 1). Still, regenerative success is limited, particularly within the complex anatomic arrangement forming the shoulder cuff, and inadequate healing of tendon-bone junctions. The formation of fibrovascular scar tissue frequently leads to significant morbidity, re-ruptures and difficulties in treatment choice.
The intervertebral discs (IVD) are composed of the nucleus pulposus (NP), a hydrophilic proteoglycan-rich gelatinous core, surrounded by a dense fibrocartilage ring - the annulus fibrosus (Figure 1). The gradual progression of IVD degeneration and the extrusion of the NP through defects in the annulus fibrosus is a major cause for lower back pain, a leading cause of global disability (Sakai and Andersson, 2015). Available treatments are mostly symptomatic, and surgical treatments often resect the structural obstruction resulting from herniation or fuse motion segments (Table 1). However, the complex structural features of IVDs surrounded by neural elements and inflammation frequently cause a homeostatic imbalance favoring a catabolic response governed by the loss of the IVD structure, which is often followed by facet joint arthritis and vertebrae deformation, canal stenosis and even deformations. Most importantly, disc replacement with synthetic implants or fusion of the motion segment does not cure the underlying pathology of IVD degeneration (Sakai and Andersson, 2015).
Cell-based interventions in the clinic
Cell therapy approaches have evolved to face challenges associated with restoring tissue homeostasis and to direct endogenous healing of musculoskeletal tissues (Figure 1, Table 2). To date, BM derived MSCs are the most frequently used cell source for these applications. This section focuses primarily on recent developments in the use of BM-MSCs and highlights some newer stem cells sources, which will likely be important in the development of future therapies. There has been extensive clinical activity in examining the benefit of adipose tissue derived stromal cells (ASCs) and unprocessed stromal vascular fractions (SVF) in cartilage repair (Pak et al., 2017). Concerns have been raised on whether these cell populations have been clearly defined and characterized prior clinical application (Keating, 2012). There are also discrepancies among clinical trials regarding isolation and expansion conditions and parameters such as cell dose and preparation of ASCs and SVF. Thus, care must be taken with ASCs and SVF to evaluate their use as a cell product and randomized trials are needed to validate clinical benefit.
Table 2.
Recent clinical interventions in musculoskeletal repair: cell source and delivery strategies
| Cells | Trauma/Disease | Carrier | Delivery | Reference | Comments |
|---|---|---|---|---|---|
| BM-MSCs | Cartilage OA | HA solution | Injection | (Wong et al., 2013) | Microfracture and HTO |
|
| |||||
| Saline | Injection | (Vega et al., 2015) | Allogeneic | ||
|
| |||||
| HA solution | Injection | (Vangsness et al., 2014) | Allogeneic | ||
|
| |||||
| Cartilage Defect | HA solution | Injection | (Gupta et al., 2016) | Allogeneic, placebo-controlled (HA only) | |
|
| |||||
| Osteonecrosis | Saline | Injection (through decompressi on canal) | (Zhao et al., 2012) | BM subtrochanteric from femoral head | |
|
| |||||
| IVD | Saline | Injection | (Noriega et al., 2016) | Allogeneic | |
|
| |||||
| HA solution | Injection | NCT01290367 | Allogeneic | ||
|
| |||||
| Synovial MSCs | Cartilage Defect | Collagen membrane (ChondroGide®) | Mini- arthrotomy | (Akgun et al., 2015) | Cultured for two additional days on collagen membrane |
|
| |||||
| None | Synovial brush | NCT02696876 | Microfracture | ||
|
| |||||
| Umbilical cord derived MSCs | Cartilage Defect | HA solution (Cartistem®) | Arthroscopy | (Park et al., 2017), NCT01626677, NCT01733186 | Allogeneic, microfracture |
|
| |||||
| ACL Defect | HA solution (Cartistem®) | Arthroscopy | NCT02755376 | Allogeneic | |
|
| |||||
| Nasal chondro- cytes | Cartilage Defect | Collagen membrane (ChondroGide®) | Mini- arthrotomy | (Pelttari et al., 2014), NCT01605201, NCT02673905 | Cultured on collagen membrane, autologous serum |
|
|
|||||
Abbreviations: ACL, anterior cruciate ligament; HA, hyaluronic acid; HTO, High-tibial osteotomy; IVD, intervertebral disc; MSC, mesenchymal stromal cells; OA, osteoarthritis
Tissue-derived MSCs
Bone-marrow derived MSCs (BM-MSCs) The harvest of autologous BM is a minimally invasive procedure of percutaneous aspiration of the iliac crest. BM-MSCs are then isolated using density centrifugation to separate the mononuclear fraction from the other marrow constituents and plating onto tissue culture plastic to separate the MSCs from the non-adherent hematopoietic cells using Good Manufacturing Practice (GMP). Given the long expansion time required for autologous BM-MSC expansion (2–3 weeks), off–the-shelf allogeneic BM-MSCs are increasingly being investigated in the field of skeletal repair. These MSCs are generally considered immune-suppressive by virtue of their expression of cytokines and growth factors and their trophic and anti-inflammatory properties render them potentially useful for clinical application.
Clinical trials exploring allogeneic BM-MSC therapy have largely been sponsored by companies with MSC products, such as Mesoblast Ltd and Stempeucel®. Their cell products are often subjected to extensive culture expansion to achieve the desired quantities, which may lead to reduced potency (Ankrum et al., 2014). Nevertheless, randomized controlled trials have shown benefits of both autologous and allogeneic derived BM-MSCs. For implantation, these cells are often suspended in saline and directly delivered into the targeted musculoskeletal tissue via a syringe or arthroscopic port (Figure 1). However, limited cell engraftment has motivated the use of biomaterial carriers (e.g., hyaluronic acid (HA), fibrin, collagen, platelet-rich-plasma (PRP)) that help to retain injected cells and provide a microenvironment that supports cell function (Burdick et al., 2016) (Table 2). This section is not meant to be comprehensive, but rather highlights recent examples of clinical interventions that represent the field of BM-MSC therapy for musculoskeletal tissue repair.
Cell therapies for cartilage repair have predominantly been performed with autologous chondrocytes, such as in ACI, where a small biopsy of cartilage provides a chondrocyte population that is expanded in vitro and implanted into the cartilage defect in a second operation. Functional benefits have been reported in clinical trials and long-term case series, yet many surgeons are still concerned about the clinical efficacy, particularly given the complexity of the procedure and long recovery time (Makris et al., 2015). Thus, to avoid limitation associated with ACI techniques, methods have been developed using BM-MSCs. For example, OA of the knee was improved in clinical parameters in response to microfracture of the cartilage lesion and injection of autologous BM-MSCs as assessed by pain, knee functionality and disability (Wong et al., 2013). Injected MSCs with HA as a cell carrier significantly improved cartilage coverage and integration in 9 of 28 patients (32%) relative to HA injections alone (0%), as evaluated by MRI scans after a one-year follow-up. These are encouraging data and long-term follow-up results will be critical to evaluate the clinical relevance of autologous BM-MSC injections in cartilage repair. However, the un-blinded study design (BM harvest from the iliac crest) and potential placebo effects may make it difficult to show efficacy.
BM-MSC from allogeneic source have also been explored in OA therapy of the knee. In a multicenter study (15 patients per arm), the pain-reducing effect of allogeneic BM-MSCs (40 million cells/knee) relative to HA alone was observed in patients with primary idiopathic OA of the knee (Vega et al., 2015). Although intra-articular injection of MSCs had a minor benefit on cartilage quality after 6 and 12-month follow-up (evaluated by MRI), the analgesic effect was remarkable with 38–40% improvement in pain compared to 10–14% in control groups. This study suggested that there may be anti-inflammatory effects of allogeneic MSC therapy in OA and sustained benefits now need to be demonstrated in trials involving larger numbers of patients. It is of interest to note that the majority of patients (50–60% of both groups) reported local pain in the injected knee and the co-administration of anti-inflammatory agents (ibuprofen) may be a cofounding factor. In another example, a symptom- and pain-reducing effect of allogeneic BM-MSCs (50 million cells) was observed in osteoarthritic knees with subtotal meniscectomy (Vangsness et al., 2014). Although this study also reported indications of meniscus repair in 14% of the patients (evaluated by MRI), the contribution of allogeneic MSCs to tissue repair is unlikely to be the major effect and may arise from a number of confounding factors including inconsistencies of MRI scans and the small number of patients (20 per arm). In this study, administration of higher cell numbers (150 million cells/knee) had no additional benefit and greater incidence of adverse events was reported in a similar randomized trial for Stempeucel®, an allogeneic pooled BM-MSC source (Gupta et al., 2016). Importantly, it has yet to be shown clinically that higher numbers or improved in vivo survival of delivered MSCs leads to enhanced therapeutic efficacy.
A number of safety and feasibility studies have also been undertaken for chronic lower back pain, using intradiscal injection of allogeneic and autologous BM-MSCs (reviewed in (Sakai and Andersson, 2015)). These have shown favorable trends in pain reduction that encourage further randomized and controlled trials to evaluate the contribution of BM-MSCs to pain relief. For example, in a study with 24 patients with lumbar pain, sustained improvement in daily life activities was reported after intradiscal injection of 25 million allogeneic BM-MSCs (one-year follow-up) compared with impaired disability in the control group who received only the local anesthesia injections (Noriega et al., 2016). Of note, all patients reported improvements in pain, and the large placebo effect makes it difficult to evaluate the clinical efficacy of intradiscal MSC injections. A randomized, controlled multicenter study is now evaluating the sustained benefit of intradiscal injection of allogeneic BM-MSCs (6 or 18 million) with HA as a carrier and HA or saline as controls (25 patients per arm) (NCT01290367). Preliminary results have been reported by the company Mesoblast, Ltd on the improved pain relief in patients after 24 months compared with patients who received saline control injections (Trounson and Mcdonald, 2015).
Whereas early case reports and studies of small patient numbers have suggested BM-MSCs have the potential to enhance healing of bone non-unions (reviewed in (Steinert et al., 2012)), clinical benefit from BM-MSCs in controlled large trials remains elusive. In the treatment of early-stage osteonecrosis, injection of BM-MSCs has been investigated in a randomized clinical trial with 93 patients (50 patients in BM-MSC and 43 patients in control group). Comparison of core decompression with and without 2 million BM-MSCs/hip obtained from the subtrochanteric region, showed significant protection of autologous BM-MSC treated hips from collapse (Zhao et al., 2012). Progression to advanced stages of osteonecrosis was reported for 2 of the 53 hips (4%) compared to 10 of the 44 hips (23%) in the control group that required autologous bone grafting or hip arthroplasty. This data suggested clinical benefit of BM-MSCs and core decompression in delaying the need for total hip replacement in early stage osteonecrosis (Zhao et al., 2012).
It is important to note here that approaches using autologous BM or freshly isolated mononuclear cells, processed in the operating room, are being explored in large numbers of clinical trials (Chahla et al., 2016). Particularly, the availability of BM and whole blood concentration devices has motivated many surgeons to use cell-therapy without the need of GMP facilities. Each of these cell sources is highly heterogeneous between patients and within cell populations and interpretation of its clinical value necessitates a better mechanistic insight.
Synovium-derived stromal cells
Based on studies reporting a resident population of MSCs within the synovia (Karystinou et al., 2009), clinical trials have investigated intraarticular implantation of autologous synovial MSCs for cartilage repair. Randomized comparison of chondrocytes and synovial MSCs for matrix-induced implantation (collagen membrane) into chondral lesions showed little differences at early time-points (synovial MSCs improved pain and disability), and good cartilage quality and integration was reported for both chondrocyte and synovial MSC implantation (Akgun et al., 2015). The better clinical outcomes are believed to be due to the anti-inflammatory effects of synovial MSCs. Another approach targets the stimulation of endogenous MSCs through synovium brushing during microfracture, and a proof-of-concept study is currently recruiting patients (NCT02696876). It is apparent that more information on the mechanism and further clinical trials are needed to determine benefits of synovial MSCs.
Nasal septum-derived chondrocytes
An alternative approach has been taken towards improving the efficacy of ACI. Compared to the mesodermal origin of articular cartilage, chondrocytes from the nasal septum are derived from the neuroectoderm, reflecting a higher self-renewal capacity (Pelttari et al., 2014). Studies in a goat articular cartilage defect model indicated environmental plasticity of nasal chondrocytes, suggesting their contribution to the repair process, similar to what has been described for skeletal progenitor cells. Preliminary results have been reported on a clinical safety study for the implantation of ex vivo engineered constructs for cartilage repair with no adverse events in seven patients ((Pelttari et al., 2014), NCT01605201). A multicenter Phase II clinical trial is now underway to compare the efficacy of such nasal chondrocyte-based tissues and nasal chondrocyte-based cell-therapy (14 days versus 2 days ex vivo culture on collagen I/III membrane) in cartilage defect repair (NCT02673905).
Umbilical cord blood-derived cells
MSCs isolated from allogeneic umbilical cord have also been considered for treatment of cartilage defects and associated OA. In 2011, a clinical safety trial with allogeneic umbilical cord blood derived MSCs in combination with HA (Cartistem®) has received regulatory approval. Early phase studies showed no severe adverse events in seven patients treated with 10–20 million cells/knee (Park et al., 2017). Randomized clinical trials are ongoing for the evaluation of long-term benefit in comparison to microfracture (NCT01626677, NCT01733186). Another controlled trial (30 patients) is currently investigating the benefit of Cartistem® for enhancing the healing response of anterior cruciate ligament (ACL) reconstructions (NCT02755376).
Allogeneic versus autologous
Taken together, ex vivo expanded MSCs have shown promising results in randomized trials and general trends towards allogeneic sources are apparent. However, no definitive clinical advantage of allogeneic MSCs over autologous MSCs has been demonstrated to date and better understanding of the particular MSC mechanisms that contribute to the therapeutic effect is needed. Particularly, recent preclinical and clinical observations of MSC immune responses have raised concerns about the general assumption that allogeneic MSCs are immune-privileged and can represent an off-the-shelf clinical product (Ankrum et al., 2014, Griffin et al., 2013). Although a comprehensive understanding of the therapeutic function of allogeneic MSCs remains elusive, autologous MSCs are not without limitations. For example, generating therapeutic doses generally requires several weeks and the risk of epigenetic changes and senescence is higher when cells are obtained from diseased donors (Alt et al., 2012, Stenderup et al., 2003). Given the significant variations in the trophic and immunomodulatory potency of individual donors, variability between patients is likely to lead to highly variable outcomes. Importantly, the spectrum of regulatory and trophic factors secreted by MSCs and the mechanism of how they impact musculoskeletal repair are beginning to be elucidated (Hofer and Tuan, 2016, Malda et al., 2016). Identifying and characterizing these factors that can promote tissue repair or activate endogenous cells seems essential towards clinical interventions. Ultimately, to realize the potential of MSC therapy, the field is challenged with the validation of potency assays and/or biomarkers that predict whether or not a patient is responsive to treatment (Ankrum et al., 2014).
Clinically emerging stem cell populations
Human pluripotent stem cells
There has been considerable progress in the use of pluripotent stem cells (embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs)) for a variety of applications (Jung et al., 2012). Human ESCs have the potential to differentiate into all types of adult human tissues and possess unlimited capacity to self-renew. Despite optimism for their therapeutic potential, ethical controversy (use of human embryos) and safety concerns (rejection of cells and tissues derived from allogeneic ESCs) have limited their clinical translation. Given these concerns, human iPSCs, somatic cells reprogrammed to become pluripotent cells, have demonstrated great promise to overcome these limitations and to resemble hESCs. Particularly, MSCs derived from iPSCs can be generated in vitro and may exert greater repair potential because of less senescence as compared to BM-MSCs (Lian et al., 2010). Human iPSCs may offer optimism for autologous and allogeneic stem cell therapies in the musculoskeletal field (Craft et al., 2015, Kanke et al., 2014, Chal et al., 2015); however, important safety issues, including the possibility to form tumors and genomic aberrations in the reprogrammed cells, need to be addressed before clinical application (Jung et al., 2012).
Muscle-derived stem and progenitor cells
Muscle satellite cells (MuSCs), the resident stem cells of skeletal muscle, have the capacity to self-renew and generate large numbers of myogenic progenitor cells in response to injury. There has been enthusiasm in using MuSCs as a transplantable cell population to restore the stem cell pool in aged and diseased muscle. In fact, the delivery of freshly isolated MuSCs into the intramuscular space in mice enhanced regenerative outcomes following injury, as shown by new muscle fiber generation (Sacco et al., 2008) and the repopulation of the satellite niche to contribute to future muscle repair (Sacco et al., 2008, Cerletti et al., 2008). While results are promising in rodents, evidence of self-renewal capacity in large animals and humans has yet to be demonstrated. Here, a major obstacle to this approach is that MuSCs are very rare and removal of these cells from their endogenous niche (for in vitro expansion) rapidly alters their cellular state and ultimate functional capacity. Thus, new culture systems that more closely mimic the in vivo niche environment are essential to yield sufficient numbers of functional MuSCs (Cosgrove et al., 2014, Gilbert et al., 2010). As a therapy for volumetric muscle loss, MuSCs are not currently being investigated in clinical trials.
Biomaterials to improve retention of delivered cells
In most clinical interventions, cells are injected directly into the targeted tissue via a syringe or through an arthroscopic port. However, biomaterials may play a role to enhance the viability and engraftment of cells by retaining them at the injection site, as well as to provide tissue-specific microenvironmental niches that play a particular role in encouraging endogenous repair (Wagers, 2012). Natural and synthetic materials have been employed in clinical practice, mostly with materials that have a long history of clinical use (Table 2). Given the diversity of musculoskeletal tissues, the scaffold design depends on the delivery mode and properties of the targeted tissue (Jeon and Elisseeff, 2016). For example, the lack of a supportive structure may account for the variable outcomes/deterioration of ACI; thus, a second generation of ACI addressed this limitation by implanting the cells seeded onto a collagen scaffold.
One of the most established materials in clinical practice has been the injection of HA for treatment of OA. HA is a polysaccharide present in body tissue and when high molecular weight HA is combined with water, it forms a highly viscous solution. Intraarticular injection of HA is well tolerated as a therapeutic modality for the treatment of OA of the knee joint, particularly for patients who are at risk for orthopedic surgery. The effects of HA are suggested to be initially biomechanical, with the viscoelastic solution providing lubrication and shock absorption. Physiologically, HA has been demonstrated as chondroprotective, analgetic and anti-inflammatory. Such properties may prove themselves to be relevant for cell-based interventions in cartilage defects as well. Thus, several clinical trials have used HA as a cell carrier, which may be an effective strategy for increasing the viscosity of the cell-solution and enhancing retention and efficacy of injected cells (Vangsness et al., 2014, Gupta et al., 2016, Park et al., 2017, Wong et al., 2013). Yet, at the same time, the persistence of HA after injection depends on several parameters, such as inflammation and activity, which may reflect the heterogeneity of outcomes (Campbell et al., 2015). Collagen is another clinically available biomaterial that has been used as an implantable construct to deliver cells. One of the commercial products available for orthopedic implantations is ChondroGide®, a collagen type I/III membrane that is obtained from pig collagen. Lately, ChondroGide® matrices have been used for implantation of synovial MSCs (Akgun et al., 2015) and nasal septum-derived chondrocytes (Pelttari et al., 2014) to treat cartilage defects (Table 2). The safety of such matrix-associated therapies encourages further controlled clinical trials with other cell sources, such as culture-expanded BM-MSCs.
Strategies to enhance endogenous repair
Beyond only cell delivery, musculoskeletal tissue repair may benefit from enhancing the recruitment of endogenous stem cells to the damaged tissue to harness their repair response (Ivkovic et al., 2011). Early evidence of stem cell niche therapies, presented by microfracture and tissue debridement, support the concept that cells and matrix factors derived from BM and blood are candidates for endogenous tissue healing. Given the role of blood platelets in wound healing and immune response, platelet-rich plasma (PRP) has taken a prominent place in medical practice and for different areas of musculoskeletal repair (Padilla et al., 2017). PRP contains a cocktail of growth factors released from platelets and endogenous fibrin, and as a minimally manipulated product may be prepared intra-operatively from patients’ whole blood using centrifugation devices. The presence of autologous fibrinogen in PRP results in platelet gel formation upon thrombin or calcium activation. Such niche-directed interventions have been employed to boost recruitment of endogenous cells after arthroscopic microfracture. Given the poor long-term outcomes of this technique, augmentation strategies following microfracture have been developed to improve the quality of the repair tissue (Strauss et al., 2010). For example, injections of autologous PRP following marrow stimulation of articular cartilage defects improved clinical parameters of pain and knee function (Manco et al., 2016). At the same time, two-year follow-up data showed no benefits in cartilage coverage and quality. Still, improvement in pain is an important outcome for the patient and may indicate that recruitment of stem cells may alter the inflammatory response in OA and could aid cartilage repair (Centeno et al., 2014). In addition to PRP augmented marrow stimulation, clinical approaches have also included strategies that use PRP gels as a cell carrier to further enhance homing of endogenous stem cells to the damaged tissue (Liebergall et al., 2013, Koh et al., 2014). Yet, definitions and characterization of PRP vary considerably among studies and the differences in content may account for conflicting clinical results.
An alternate strategy is the use of tissue-specific ECM, which represents a source of various sequestration sites for growth factors and cytokines that can serve as signals to recruit endogenous stem cells. Decellularized ECM materials are fabricated by cell removal and washing out their remnants with various treatments that conserve tissue-specific ECM, a mixture of proteins and proteoglycans, which then can be processed into implants or hydrogels. This ECM closely mimics the native tissue from which the tissue is derived and may provide biological signals important for cellular functions. Such approaches, if successful, could generate ECM-based materials designed to activate endogenous repair processes (Monibi and Cook, 2017). Clinically, decellularized allografts have been used as an injectable paste to augment microfracture (Biocartilage®, (Hirahara and Mueller, 2015)) and as osteochondral scaffolds for full-thickness cartilage defects (Chondrofix®, (Long et al., 2016)); however, generally with minor benefits and high failure rates (Monibi and Cook, 2017). Promising clinical data showed restoration of vascularized and innervated muscle tissue formation in five patients with volumetric muscle loss upon implantation of acellular pig bladder matrix (Sicari et al., 2014). While the connection to the physiological repair response is less clear, similar results were demonstrated in eight patients upon implantation of ECM-derived scaffolds derived from other tissues (porcine dermal, small intestinal submucosa), which suggests the presence of similar signaling mechanism within non-tissue specific ECM derived materials (Dziki et al., 2016).
Progress and concerns for stem and stromal cell therapy
The recent progress of stem and stromal cell clinical trials in the field has been encouraging and published results have demonstrated strikingly positive therapeutic effects of these cells in the musculoskeletal field. However, most clinical reports have been with small numbers of patients and there have been few controlled prospective trials as highlighted in this review. At this point, clinical applications of stromal cells and heterogeneous cell populations, including unprocessed BM and SVF, have often been without significant preclinical evidence or a thorough understanding of mechanism of action (Prockop et al., 2014). This may arise in part from the different perspectives of scientists investigating the basic biology of stem and stromal cells and clinicians facing patients who may benefit from new therapies even prior to the establishment of rigorous scientific evidence. Major challenges for successful clinical trials are the characterization of multipotency and regulatory properties of heterogeneous cell populations and an understanding of their in vivo role during tissue repair. This includes quantitative assays for labeling and monitoring MSCs after implantation in humans to study their persistence and long-term therapeutic effect. Another aspect is the standardization of isolation and culture protocols to reduce variability and to compare MSCs across laboratories (Keating, 2012, Prockop et al., 2014).
With respect to the well-characterized culture-expanded BM-MSCs, the field would greatly benefit from larger placebo-controlled clinical trials that can evaluate the efficacy of these therapies and detect rare side events. One challenge is that there is often a heavy emphasis on subjective outcome measures (e.g. daily activities and pain scales) that are vulnerable to placebo-effects and do not consistently correlate with physiological outcomes (e.g., cartilage thickness and disc height) (Mundi et al., 2014). A second issue is the difficulty of blinding surgeons and patients in a surgical intervention, such as with autologous BM aspiration, which is critical for determining whether isolated cells can be effective in treating tissue injuries or degeneration. Notably, allogeneic bone marrow, adipose and umbilical cord-derived MSCs are in the largest number of blinded clinical trials currently registered. Since these are often industry-sponsored, negative outcomes may rarely be published and require careful evaluation.
As indicated, there are now numerous biomaterials in clinical practice. These materials in combination with MSCs or other cell sources may improve clinical benefits of delivered cells. Presently, there is limited clinical observations regarding cell engraftment and therapeutic outcome in humans, but there are encouraging preclinical data and current developments that suggest the benefit of supportive biomaterials.
Advanced bioengineering concepts
Although stem cell therapies are advancing toward the clinic, there is no standard protocol for the optimal number of cells to be implanted for maximal effect and cell survival has often been observed to be less than 26% (Quintavalla et al., 2002, Emans et al., 2006, Marquardt and Heilshorn, 2016). Given the low engraftment and survival rates of delivered cells, large numbers have been implanted into patients. As such, there is significant effort towards the use of bioengineering principles to further improve these therapies. As described above, biomaterials have been used in the clinical application of stem and stromal cells, such as collagen and HA materials; however, they have mainly been applied as cell carriers without specific focus on their properties and ability to regulate cell behavior. Thus, beyond initial retention, advanced biomaterials are being designed to capture some of the critical biochemical and biophysical ECM signals found in native connective tissues to direct formation of new functional tissue (Figure 2A). Likewise, biomaterials are being developed to deliver biological factors that can play a role in recruiting stem cells for repair, controlling cell behavior, or for immunomodulation to alter the healing environment (Figure 2). Although it is not meant to be comprehensive, this section will highlight exemplary advances in this area towards the repair of musculoskeletal tissues.
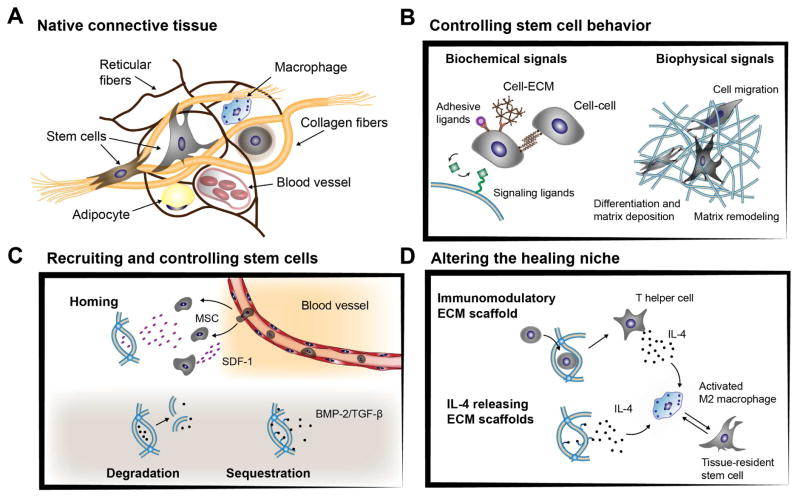
Figure 2. Advanced bioengineering concepts using biomaterials to control cell behavior (A).
The extracellular matrix of native connective tissue is highly dynamic and supports resident cells through presentation of biological and biophysical cues. (B) Biomaterials can recreate aspects of the tissue-specific microenvironment with biochemical signals to mimic cell-ECM and cell-cell interactions or to allow encapsulated cells to actively interact and integrate with their matrix environment. (C) Biomaterials can also be engineered to release chemo-attractive cytokines (e.g. stromal cell-derived factor 1α (SDF- 1α)) that enable migration of resident cells (e.g. mesenchymal stromal cells (MSCs)) or direct cell behavior by controlled release of encapsulated biological factors (e.g. bone-morphogenetic protein (BMP), transforming growth factor-β (TGF-β)). (D) Scaffold microenvironments are further being developed to alter the healing niche, for example by inducing a specific anti-inflammatory immune response or by releasing cytokines (e.g. interleukin 4 (IL-4) that activate M2 macrophages and promote tissue repair.
Control and regulation of stem and stromal cell behavior
Biochemical signals
The spatially and temporally complex interactions of cells directly with their ECM environment and with each other are profound in their ability to regulate stem cell fate and function (Wagers, 2012). With a focus on their biochemical composition, biomaterials fabrication has evolved to present defined adhesive molecules (e.g laminin, fibronectin) or signaling ligands (e.g. transforming growth factor-β (TGF-β), bone-morphogenetic protein (BMP)) to modify the structural environment of cells (Figure 2B). A growing body of data indicates that such microenvironmental cues can be engineered into biomaterials to guide cellular behaviors (Guvendiren and Burdick, 2013). For example, hydrogel-based presentation of specific integrin binding domains, which are cell-adhesive ligands found in the ECM, improved MSC attachment and bone repair upon implantation (Kisiel et al., 2013, Shekaran et al., 2014). Features of native myofiber ECM (e.g. integrins, laminins) can also be engineered into hydrogels to preserve MuSC quiescence and enhance subsequent engraftment in vivo (Quarta et al., 2016). As another example, both TGF-β and BMP are implicated in MSC differentiation, and biomaterials that present these biological cues have shown controlled differentiation of stem cells towards chondrogenic and osteogenic lineages (Re'em et al., 2012).
Biophysical signals
The composition and organization of musculoskeletal tissues that give rise to their biophysical properties also influence the function of resident cells (Figure 2A). Extensive studies have probed these critical mechanical signals in different biomaterial systems (Discher et al., 2009). In bone formation, for example, matrix elasticity is directly related to mechanically induced osteogenesis and biomaterials that allow cells to generate traction forces and reorganize their microenvironment supported osteogenic lineages (Figure 2B) (Khetan et al., 2013). In this regard, hydrogels that can decouple matrix elasticity of the bulk hydrogel from cell confinement, for example by incorporating hydrolytically degrading sacrificial gel porogens, have enabled MSCs to spread and improve bone regeneration in rat cranial defects (Huebsch et al., 2015). Such bioengineered materials are useful to improve survival and differentiation of transplanted cells, which then serve as a source of osteogenic signals and recruitment of endogenous cells for new tissue formation. The importance of matrix reorganization has also been shown in non-covalently crosslinked alginate hydrogels where the rate of visco-elasticity and stress-relaxation determined the degree of new bone tissue formation (Darnell et al., 2017). Thus, biomaterials that facilitate cells to invade and remodel these niches may be effective in inducing functional tissue repair.
Scaffolds can also be fabricated to recreate the architecture of native ECM tissue on a micro- and nanoscale. For example, the nanofibrous and anisotropic structure of musculoskeletal tissue can be mimicked by controlled alignment of polymer fibers using electrospinning (Figure 2B). Toward tissue repair applications, it is important that these fibrous scaffolds recapitulate the dense and organized matrix structure but not impede endogenous cell migration and tissue formation. For example, the hierarchical structure has been fine-tuned in nanofibrous scaffolds by multilayers of aligned fibers to improve ASC migration and tenogenic matrix deposition (Orr et al., 2015). More recently, implantation of polymer fibers mimicking the structural organization of the rotator cuff tendon, together with delivered MSCs, induced repair similar to intact tendon tissue (Peach et al., 2017). In addition to methods like electrospinning, self-assembled peptide hydrogels are an emergent means of recapitulating the microstructural organization of ECM. The use of such self-assembled nanofibers led to greater engraftment of freshly isolated MuSCs upon injection in mice, which was partially attributed to improved cell alignment and proliferation (Sleep et al., 2017) (Figure 3A). Such studies highlight that biomimetic scaffolds may be critical for MuSC engraftment and to instruct MSCs to orchestrate stem cell mobilization and tissue-specific repair. The mimicry of tissue biophysical properties can further be combined with the presentation of biochemical factors, and has been tuned for specific applications, such as the conjugation of connective tissue growth factor (CTGF) for ligament repair (Pauly et al., 2017) and the release of vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) for myofiber repair (Sleep et al., 2017).
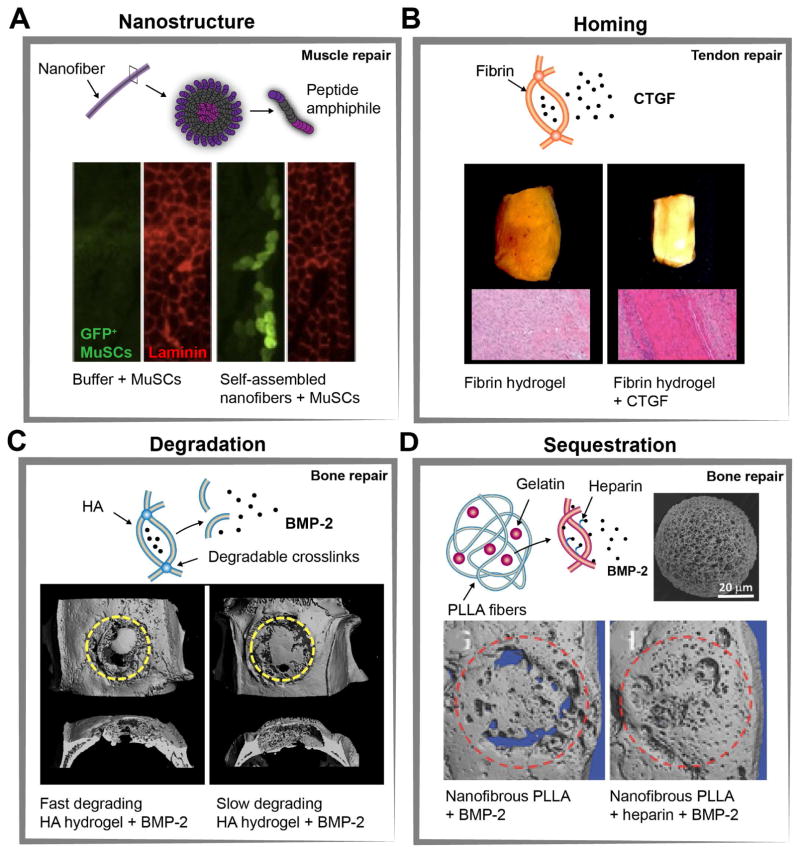
Figure 3. Examples of biomaterials engineered to recruit and control endogenous stem and stromal cell behavior in vivo (A).
Implantation of muscle satellite cells (MuSCs) in self-assembled nanofibers enhanced donor cell mediated repair of myofibers. Representative immunostaining of muscle tissue sections 5 weeks after implantation shows enhanced engraftment of GFP+ MuSCs compared with cells injected in buffer only. (B) Connective tissue growth factor (CTGF) released from fibrin hydrogels improved repair of transected rat patellar tendons. Gross images and representative histological images 4 weeks after implantation showed dense alignment of collagen fibers for fibrin gels with CTGF by stimulating proliferation and differentiation of endogenous tendon progenitor cells. (C) Bone-morphogenetic protein (BMP-2) incorporated into matrix metalloproteinases (MMP)-degradable hyaluronic acid (HA) hydrogels sustained release of BMP-2 through cell-mediated hydrogel degradation. Representative μ-CT images of rat calvarial defects 6 weeks after implantation demonstrate increased bone volume for faster degrading HA-hydrogels. (D) New bone tissue formation can also be increased through implantation of nanofibrous poly(l-lactic acid) (PLLA) microspheres that contain gelatin-heparin/BMP-2 microspheres (scanning electron microscope (SEM) image of a typical nanofibrous PLLA microsphere). Representative μ-CT images of rat calvarial defects 6 weeks after implantation show improved bone regeneration for PLLA microspheres with heparin-conjugated gelatin due to sustained release of BMP-2. Figures are adapted with permission from the following: (A) (Sleep et al., 2017) (B) American Society For Clinical Investigation Ref: (Lee et al., 2015). (C) Elsevier Ref: (Holloway et al., 2014). (D) Wiley Ref: (Ma et al., 2015).
Recruitment of endogenous stem and progenitor cells
Advances in our understanding of the fundamentals of healing and the inherent repair capacity of many musculoskeletal tissues has led to the design of biomaterials that specifically recruit endogenous cells by delivering soluble signals (Figure 2C). As an example, it has been described that stromal cell-derived factor 1α (SDF-1) plays a critical role in regulating stem progenitor cell recruitment and engraftment at the injury site. For example, SDF-1 acts as a chemoattractant for CXCR4 presenting cells (e.g. MSCs and endothelial progenitor cells) from the BM, where it regulates cell mobilization into the targeted tissue. Studies in a rabbit model of calvarial defects indicated that electrospun PCL/gelatin fiber scaffolds improve bone formation when SDF-1 is physically adsorbed, in part by providing a proangiogenic environment in the defect area through recruiting hematopoietic stem cells (Ji et al., 2013). SDF-1 has also been suggested to decrease the effective BMP-2 dose for calvarial bone repair when co-delivered from proteolytically degradable HA hydrogels (Holloway et al., 2015). Similarly, SDF-1 containing collagen-hydroxyapatite gels enhanced osteoinductive properties of decellularized bone scaffolds in a rabbit large bone defect model (Chen and Lv, 2017). Combinations of SDF-1 with collagen (Chen et al., 2015) and fibrin (Yu et al., 2015) scaffolds have been optimized to treat osteochondral and full cartilage defects, aiming to enhance migration of stem and chondroprogenitor cells from the underlying subchondral BM. Targeting SDF-1 mediated recruitment of MSCs from the surrounding cartilage and synovia, particularly in partial defects, may also be an effective strategy for repairing cartilage (Zhang et al., 2013). Finally, SDF-1 release from hydrogels has potential as an effective strategy for reactivating endogenous repair of fibrocartilaginous tissue (Shen et al., 2010, Pereira et al., 2014). Given the relatively low number of SDF-1 responsive MSCs, often less than 5% express CXCR4 on the cell membrane, increasing receptor expression with specific cytokines may present a potential strategy to augment these bioengineering approaches (Shi et al., 2007, Wynn et al., 2004). Tissue-specific growth factors may also be employed to boost endogenous stem cells and orchestrate healing. For example, preclinical data in a rat tendon model suggest that activation of tendon-resident stem/progenitor cells by CTGF, encapsulated in a fibrin gel, enhances homing, proliferation and tenogenic differentiation of this rare stem cell population (Figure 3B) (Lee et al., 2015). Targeting such endogenous niches, particularly in poor healing tissues, may be an effective strategy for circumventing ex vivo manipulation of transplanted stem and stromal cells.
Control of endogenous stem cell behavior
In addition to recruiting endogenous cells to damaged tissues, advances in biomaterial design can further be tuned towards the delivery of appropriate cues such that they allow spatiotemporal control of the microenvironment of these cells (Figure 2C).
Controlled molecule delivery
The controlled delivery of biological factors is one approach where biomaterials can alter cell behavior and fate. Release profiles of these factors can be actively controlled through biomaterial degradation and its affinity or binding to the released molecules. The bioengineering strategies are diverse in how they bind the molecules, ranging from covalent conjugation to electrostatic interactions and hydrophobic associations (Li and Mooney, 2016). One of the most known clinical applications of biomaterial carriers in musculoskeletal repair has been the INFUSE bone graft device, which consists of a recombinant BMP-2-soaked collagen sponge, as BMP-2 can alter local cell behavior. The INFUSE system provides an affinity and diffusion-controlled BMP-2 release for use in lumbar spinal fusion and open tibial fracture (Carragee et al., 2011). As such, the collagen carrier does not provide tight control over the release and high doses of BMP are implanted, causing initially supraphysiological drug levels.
To better control release of biological factors, one approach is the use of biomaterials that respond to environmental stimuli, such as enzymatic and proteolytic activity of migrating tissue-resident cells. For example, biomaterials have been engineered to degrade via proteases, such as matrix metalloproteinases (MMPs) that are associated with cellular migration and have a key role in ECM remodeling and angiogenesis during bone regeneration. This mode of degradation allowed for release of entrapped biomolecules during cell-mediated material remodeling (Lutolf et al., 2003). With this, hydrogels were tuned for BMP-2 release, supporting new bone tissue formation in rat calvarial defects (Holloway et al., 2014, Shekaran et al., 2014) (Figure 3C). Although these examples have focused on cell-mediated material remodeling, hydrolytic degradation can also be used to control molecule delivery and thus cell behavior (Patterson et al., 2010). When hydrolysis governs delivery of biomolecules, release profiles depend on the biomaterial degradation kinetics, influenced by factors such as crosslinking density, hydrophobicity of the polymers, as well as molecular weight and concentration.
Complementary to sustained diffusion-based release, the interaction between biomaterials and molecules can mediate their sequestration, presentation, and release behavior (Figure 2C). Previous studies have reported successful incorporation of heparin and heparin mimetics to exploit the natural affinity between native ECM and heparin binding domains on growth factors. For example, BMP-4 and TGF-β have been physically entrapped during the crosslinking of two-layer alginate hydrogels containing sulfate groups as heparin mimetics (Re'em et al., 2012). Prolonged presentation of BMP4 and TGF-β upon implantation in osteochondral defects in rabbits resulted in cartilaginous tissue formation with subchondral bone underneath. Such effects demonstrate the complex interplay between growth factors (i.e. TGF-β and BMPs) and ECM proteins in differentiation of migrated cells (Martino et al., 2011, Wagers, 2012).
To more closely mimic the native ECM structure, nanofibers can be engineered to mimic the function of growth factor sequestering microfibrils. For example, nanofibers self-assemble upon mixing of heparin-binding amphiphiles composed of a self-assembling domain and a bioactive TGF-β binding domain. These nanofibers specifically sequestered and enhanced activity of supplemented and endogenous TGF-β resulting in improved cartilage repair in a rabbit model following microfracture (Shah et al., 2010). For bone regeneration, nanofibers have also been engineered to sequester and enhance activity of BMP-2 through a heparin binding domain (Lee et al., 2013). When these nanofibers were incorporated into a collagen scaffold and implanted into rat femoral defects, a reduced concentration of supplemented BMP-2 was needed to elicit the therapeutic effect. Similarly, BMP-2 cooperates synergistically with the integrin-binding regions of fibronectin or engineered recombinant proteins (Martino et al., 2011), improving cell migration and bone formation when incorporated into covalently crosslinked HA hydrogels (Kisiel et al., 2013).
Although these methods allow mimicking of the fibrous ECM, they are often limited toward minimally invasive filling of complex 3D shaped skeletal tissue defects. To address this, complementary microscale hydrogel constructs have been engineered to deliver bioactive molecules as injectable carriers (Tai et al., 2013, Liang et al., 2013) or encapsulated within hydrogel scaffolds (Spiller et al., 2012, Bian et al., 2013) to add functionality that stimulated differentiation of delivered and migrated cells. These microspheres can be modified in similar ways to macroscale hydrogels to alter growth factor interactions. By using covalently crosslinked heparin microspheres, BMP-2 signaling can be enhanced by sequestering encapsulated BMP-2 and cell-secreted growth factors (Hettiaratchi et al., 2014). To combine fibrous structures with the advantages of microspheres, BMP-2 loaded heparin-gelatin nanoparticles can also be encapsulated into nanofibrous poly(l-lactic acid) (PLLA) microspheres (Ma et al., 2015) (Figure 3D). Because these PLLA microspheres had a high porosity, they enabled greater ECM deposition and in combination with sustained BMP-2 release, improved bone regeneration in a rat calvarial bone defect model. Many of the principle here are also used for the delivery of genes that can act on delivered and recruited cells (Evans and Huard, 2015). Similarly, biomaterials can be useful for effective and controlled delivery of extracellular vesicles (EVs), for example for restoration of joint homeostasis and repair (Malda et al., 2016).
Altering the healing niche: immunomodulatory considerations
Although providing the biochemical and biophysical signals of native ECM in a biomaterial is important for stem and stromal cell delivery and homing, variability in patient response is likely to also extend to heterogeneity in patient physiology. This is reflected by an increased understanding that the body’s immune response is critical in tissue repair (Sadtler et al., 2016). The close association of many stem cell types and immune cells within the tissue-specific stem cell niche allows for modulation of stem cell responses by actively triggering the immune response. Specifically, macrophages as a heterogeneous population of the innate immune system exhibit multiple phenotypes in response to the external environment with a spectrum ranging from classically inflammatory M1-like to less inflammatory M2-like phenotypes. These effects can partially be explained by an array of soluble mediators that induce macrophage polarization, some of which induce a specific macrophage subtype: for example, interleukin (IL) 1β, 4, 10 and 13; interferon-gamma (IFN-γ) and TGF-β. The plasticity of these cells and their diverse role in tissue repair therapies have been reviewed elsewhere (Spiller and Koh, 2017). Therefore, bioengineering strategies that harness the regenerative potential of residual immune cells, either by controlling macrophage polarization through designing microenvironmental cues or controlling the release of anti- or pro-inflammatory cytokines, may activate and increase the repair potential of endogenous stem/progenitor cells (Sridharan et al., 2015) (Figure 2D). Indeed, such an immunomodulatory approach has been effectively employed in tissue regeneration, wherein muscle injuries in a mouse were treated with decellularized ECM scaffolds (Sadtler et al., 2016). In this example, the immune response induced by the scaffold microenvironmental niche involved IL-4 releasing T helper cells that released anti-inflammatory IL-4 and activated macrophages towards an M2-like phenotype, which supported the healing response. These data indicate that inflammatory signals may be essential for initiating the crosstalk between macrophages and endogenous stem cells, and the transition into tissue repair.
This conceptual strategy is particularly attractive for the complex but highly regulated sequence of inflammatory and anti-inflammatory signals in bone healing. More specifically, decellularized bone scaffolds have been designed to physically and covalently bind cytokines for rapid release of interferon-gamma, an inflammatory cytokine to promote inflammation, and sustained release of IL-4, to promote vascularization (Spiller et al., 2015). This immunomodulatory bone scaffold resulted in sequential actions of M1 and M2-like macrophages in vitro and exemplified that specific immunological cues can be embedded into biomaterials. These recent advances merit further research towards harnessing the complex immunomodulatory properties of macrophages. Yet, at the same time, non-specific protein adsorption and the accompanying foreign body response once implanted present major hurdles towards clinical translation. As such, mechanistic understanding of how biomaterials interact with the immune system both locally and systematically will help develop materials that can actively alter the physiological healing niche.
Conclusion and future outlook
Within the field of musculoskeletal tissue repair and regeneration, particularly towards tissues such as bone, cartilage, intervertebral disc, tendons, ligaments and skeletal muscle there is a large amount of preclinical and clinical data that support cell-based interventions. The majority of clinical trials to date have used BM-MSCs with success in contributing to tissue repair and reduction of pain. There is notable heterogeneity in cells described as MSCs and the isolation methods being used, which are likely to affect in vivo function and therapeutic potential. While such problems can probably be addressed by using animal models in which donor and endogenous cell response can be better quantified (e.g. implantation of GFP+ cells in bone defects (Zeitouni et al., 2012) and for muscle repair (Sleep et al., 2017)), there has been limited availability of valid assays to elucidate the effects on inflammation and pain reduction that are thought to support the clinical observations. In addition, these functions are regulated in the context of host tissue physiology and the nature of repair, and animal models need to be developed to identify the role of exogenous MSCs. At the same time, the establishment of MSC-based therapies in the musculoskeletal field requires evidence-based clinical trials with appropriate follow-up of clinical parameters that may also investigate the mechanism behind therapeutic benefit. A limitation is the current recognition of MSCs as a human cell product that requires approval by the US Food and Drug Administration (FDA) and cells must be cultured under defined GMP conditions, which have continued to increase the cost and slow the development of therapies. Further success of the field will provide avenues for clinicians to work closely with scientists to improve stem and stromal cell-based therapies. Bioengineering principles, such as in the development of engineered biomaterials, will play a role in these advances. Targeting the complexity of physiological healing requires fundamental understanding of the cellular and biological signals that constitute the healing niche under degenerative and repair conditions and the design of therapies with this in consideration. Our knowledge is continuously increasing and ex vivo models are being developed to better understand stem and stromal cell responses in musculoskeletal tissues. Likewise, the capabilities of biomaterials presenting specific chemical, biological and physical cues is expanding and will define new avenues to provide instructional microenvironments for stem and stromal cell induced tissue repair. An important aspect of future cell-based therapies in musculoskeletal tissue repair is the better understanding of distinct healing mechanisms, whether through recapitulating developmental processes or by providing signals for endogenous repair.
Acknowledgments
C.L. acknowledges support from the Swiss National Foundation through an SNF Early Postdoc Mobility Fellowship and J.A.B. acknowledges funding from the National Institutes of Health (R01EB008722, R01AR056624) and the National Science Foundation (DMR Award 1610525). We are grateful to our colleagues whose work we could not cite due to space limitations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akgun I, Unlu MC, Erdal OA, Ogut T, Erturk M, Ovali E, Kantarci F, Caliskan G, Akgun Y. Matrix-induced autologous mesenchymal stem cell implantation versus matrix-induced autologous chondrocyte implantation in the treatment of chondral defects of the knee: a 2-year randomized study. Arch Orthop Trauma Surg. 2015;135:251–63. doi: 10.1007/s00402-014-2136-z. [DOI] [PubMed] [Google Scholar]
- Alt EU, Senst C, Murthy SN, Slakey DP, Dupin CL, Chaffin AE, Kadowitz PJ, Izadpanah R. Aging alters tissue resident mesenchymal stem cell properties. Stem Cell Res. 2012;8:215–25. doi: 10.1016/j.scr.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014;32:252–60. doi: 10.1038/nbt.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian L, Guvendiren M, Mauck RL, Burdick JA. Hydrogels that mimic developmentally relevant matrix and N-cadherin interactions enhance MSC chondrogenesis. Proc Natl Acad Sci U S A. 2013;110:10117–22. doi: 10.1073/pnas.1214100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks PM. The burden of musculoskeletal disease--a global perspective. Clin Rheumatol. 2006;25:778–81. doi: 10.1007/s10067-006-0240-3. [DOI] [PubMed] [Google Scholar]
- Burdick JA, Mauck RL, Gerecht S. To Serve and Protect: Hydrogels to Improve Stem Cell-Based Therapies. Cell Stem Cell. 2016;18:13–5. doi: 10.1016/j.stem.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Campbell KA, Erickson BJ, Saltzman BM, Mascarenhas R, Bach BR, Jr, Cole BJ, Verma NN. Is Local Viscosupplementation Injection Clinically Superior to Other Therapies in the Treatment of Osteoarthritis of the Knee: A Systematic Review of Overlapping Meta-analyses. Arthroscopy. 2015;31:2036–45. e14. doi: 10.1016/j.arthro.2015.03.030. [DOI] [PubMed] [Google Scholar]
- Caplan AI. Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Transl Med. 2017;6:1445–1451. doi: 10.1002/sctm.17-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11:471–91. doi: 10.1016/j.spinee.2011.04.023. [DOI] [PubMed] [Google Scholar]
- Centeno C, Pitts J, Al-Sayegh H, Freeman M. Efficacy of autologous bone marrow concentrate for knee osteoarthritis with and without adipose graft. Biomed Res Int. 2014;2014:370621. doi: 10.1155/2014/370621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerletti M, Jurga S, Witczak CA, Hirshman MF, Shadrach JL, Goodyear LJ, Wagers AJ. Highly efficient, functional engraftment of skeletal muscle stem cells in dystrophic muscles. Cell. 2008;134:37–47. doi: 10.1016/j.cell.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahla J, Cinque ME, Shon JM, Liechti DJ, Matheny LM, Laprade RF, Clanton TO. Bone marrow aspirate concentrate for the treatment of osteochondral lesions of the talus: a systematic review of outcomes. J Exp Orthop. 2016;3:33. doi: 10.1186/s40634-016-0069-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chal J, Oginuma M, Al Tanoury Z, Gobert B, Sumara O, Hick A, Bousson F, Zidouni Y, Mursch C, Moncuquet P, Tassy O, Vincent S, Miyanari A, Bera A, Garnier JM, Guevara G, Hestin M, Kennedy L, Hayashi S, Drayton B, Cherrier T, Gayraud-Morel B, Gussoni E, Relaix F, Tajbakhsh S, Pourquie O. Differentiation of pluripotent stem cells to muscle fiber to model Duchenne muscular dystrophy. Nat Biotechnol. 2015;33:962–9. doi: 10.1038/nbt.3297. [DOI] [PubMed] [Google Scholar]
- Chen G, Lv Y. Matrix elasticity-modified scaffold loaded with SDF-1alpha improves the in situ regeneration of segmental bone defect in rabbit radius. Sci Rep. 2017;7:1672. doi: 10.1038/s41598-017-01938-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Tao J, Zhu S, Cai Y, Mao Q, Yu D, Dai J, Ouyang H. Radially oriented collagen scaffold with SDF-1 promotes osteochondral repair by facilitating cell homing. Biomaterials. 2015;39:114–23. doi: 10.1016/j.biomaterials.2014.10.049. [DOI] [PubMed] [Google Scholar]
- Cosgrove BD, Gilbert PM, Porpiglia E, Mourkioti F, Lee SP, Corbel SY, Llewellyn ME, Delp SL, Blau HM. Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat Med. 2014;20:255–64. doi: 10.1038/nm.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft AM, Rockel JS, Nartiss Y, Kandel RA, Alman BA, Keller GM. Generation of articular chondrocytes from human pluripotent stem cells. Nat Biotechnol. 2015;33:638–45. doi: 10.1038/nbt.3210. [DOI] [PubMed] [Google Scholar]
- Darnell M, Young S, Gu L, Shah N, Lippens E, Weaver J, Duda G, Mooney D. Substrate Stress-Relaxation Regulates Scaffold Remodeling and Bone Formation In Vivo. Adv Healthc Mater. 2017:6. doi: 10.1002/adhm.201601185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673–7. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziki J, Badylak S, Yabroudi M, Sicari B, Ambrosio F, Stearns K, Turner N, Wyse A, Boninger ML, Brown EHP, Rubin JP. An acellular biologic scaffold treatment for volumetric muscle loss: results of a 13-patient cohort study. 2016;1:16008. doi: 10.1038/npjregenmed.2016.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emans PJ, Pieper J, Hulsbosch MM, Koenders M, Kreijveld E, Surtel DA, Van Blitterswijk CA, Bulstra SK, Kuijer R, Riesle J. Differential cell viability of chondrocytes and progenitor cells in tissue-engineered constructs following implantation into osteochondral defects. Tissue Eng. 2006;12:1699–709. doi: 10.1089/ten.2006.12.1699. [DOI] [PubMed] [Google Scholar]
- Evans CH, Huard J. Gene therapy approaches to regenerating the musculoskeletal system. Nat Rev Rheumatol. 2015;11:234–42. doi: 10.1038/nrrheum.2015.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078–81. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson WL, Bunnell BA, Martin E, Frazier T, Hung BP, Gimble JM. Stromal cells and stem cells in clinical bone regeneration. Nat Rev Endocrinol. 2015;11:140–50. doi: 10.1038/nrendo.2014.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin MD, Ryan AE, Alagesan S, Lohan P, Treacy O, Ritter T. Anti-donor immune responses elicited by allogeneic mesenchymal stem cells: what have we learned so far? Immunol Cell Biol. 2013;91:40–51. doi: 10.1038/icb.2012.67. [DOI] [PubMed] [Google Scholar]
- Grogan BF, Hsu JR Skeletal Trauma Research C. Volumetric muscle loss. J Am Acad Orthop Surg. 2011;19(Suppl 1):S35–7. doi: 10.5435/00124635-201102001-00007. [DOI] [PubMed] [Google Scholar]
- Gupta PK, Chullikana A, Rengasamy M, Shetty N, Pandey V, Agarwal V, Wagh SY, Vellotare PK, Damodaran D, Viswanathan P, Thej C, Balasubramanian S, Majumdar AS. Efficacy and safety of adult human bone marrow-derived, cultured, pooled, allogeneic mesenchymal stromal cells (Stempeucel(R)): preclinical and clinical trial in osteoarthritis of the knee joint. Arthritis Res Ther. 2016;18:301. doi: 10.1186/s13075-016-1195-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guvendiren M, Burdick JA. Engineering synthetic hydrogel microenvironments to instruct stem cells. Curr Opin Biotechnol. 2013;24:841–6. doi: 10.1016/j.copbio.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettiaratchi MH, Miller T, Temenoff JS, Guldberg RE, Mcdevitt TC. Heparin microparticle effects on presentation and bioactivity of bone morphogenetic protein-2. Biomaterials. 2014;35:7228–38. doi: 10.1016/j.biomaterials.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirahara AM, Mueller KW., Jr BioCartilage: A New Biomaterial to Treat Chondral Lesions. Sports Med Arthrosc. 2015;23:143–8. doi: 10.1097/JSA.0000000000000071. [DOI] [PubMed] [Google Scholar]
- Hofer HR, Tuan RS. Secreted trophic factors of mesenchymal stem cells support neurovascular and musculoskeletal therapies. Stem Cell Res Ther. 2016;7:131. doi: 10.1186/s13287-016-0394-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway JL, Ma H, Rai R, Burdick JA. Modulating hydrogel crosslink density and degradation to control bone morphogenetic protein delivery and in vivo bone formation. J Control Release. 2014;191:63–70. doi: 10.1016/j.jconrel.2014.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway JL, Ma H, Rai R, Hankenson KD, Burdick JA. Synergistic Effects of SDF-1alpha and BMP-2 Delivery from Proteolytically Degradable Hyaluronic Acid Hydrogels for Bone Repair. Macromol Biosci. 2015;15:1218–23. doi: 10.1002/mabi.201500178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebsch N, Lippens E, Lee K, Mehta M, Koshy ST, Darnell MC, Desai RM, Madl CM, Xu M, Zhao X, Chaudhuri O, Verbeke C, Kim WS, Alim K, Mammoto A, Ingber DE, Duda GN, Mooney DJ. Matrix elasticity of void-forming hydrogels controls transplanted-stem-cell-mediated bone formation. Nat Mater. 2015;14:1269–77. doi: 10.1038/nmat4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivkovic A, Marijanovic I, Hudetz D, Porter RM, Pecina M, Evans CH. Regenerative medicine and tissue engineering in orthopaedic surgery. Front Biosci (Elite Ed) 2011;3:923–44. doi: 10.2741/e299. [DOI] [PubMed] [Google Scholar]
- Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52:80–5. doi: 10.1111/j.1532-5415.2004.52014.x. [DOI] [PubMed] [Google Scholar]
- Jeon OH, Elisseeff J. Orthopedic tissue regeneration: cells, scaffolds, and small molecules. Drug Deliv Transl Res. 2016;6:105–20. doi: 10.1007/s13346-015-0266-7. [DOI] [PubMed] [Google Scholar]
- Ji W, Yang F, Ma J, Bouma MJ, Boerman OC, Chen Z, Van Den Beucken JJ, Jansen JA. Incorporation of stromal cell-derived factor-1alpha in PCL/gelatin electrospun membranes for guided bone regeneration. Biomaterials. 2013;34:735–45. doi: 10.1016/j.biomaterials.2012.10.016. [DOI] [PubMed] [Google Scholar]
- Johnson VL, Hunter DJ. The epidemiology of osteoarthritis. Best Pract Res Clin Rheumatol. 2014;28:5–15. doi: 10.1016/j.berh.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Jung Y, Bauer G, Nolta JA. Concise review: Induced pluripotent stem cell-derived mesenchymal stem cells: progress toward safe clinical products. Stem Cells. 2012;30:42–7. doi: 10.1002/stem.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanke K, Masaki H, Saito T, Komiyama Y, Hojo H, Nakauchi H, Lichtler AC, Takato T, Chung UI, Ohba S. Stepwise differentiation of pluripotent stem cells into osteoblasts using four small molecules under serum-free and feeder-free conditions. Stem Cell Reports. 2014;2:751–60. doi: 10.1016/j.stemcr.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karystinou A, Dell'accio F, Kurth TB, Wackerhage H, Khan IM, Archer CW, Jones EA, Mitsiadis TA, De Bari C. Distinct mesenchymal progenitor cell subsets in the adult human synovium. Rheumatology (Oxford) 2009;48:1057–64. doi: 10.1093/rheumatology/kep192. [DOI] [PubMed] [Google Scholar]
- Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell. 2012;10:709–16. doi: 10.1016/j.stem.2012.05.015. [DOI] [PubMed] [Google Scholar]
- Khetan S, Guvendiren M, Legant WR, Cohen DM, Chen CS, Burdick JA. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat Mater. 2013;12:458–65. doi: 10.1038/nmat3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisiel M, Martino MM, Ventura M, Hubbell JA, Hilborn J, Ossipov DA. Improving the osteogenic potential of BMP-2 with hyaluronic acid hydrogel modified with integrin-specific fibronectin fragment. Biomaterials. 2013;34:704–12. doi: 10.1016/j.biomaterials.2012.10.015. [DOI] [PubMed] [Google Scholar]
- Koh YG, Kwon OR, Kim YS, Choi YJ. Comparative outcomes of open-wedge high tibial osteotomy with platelet-rich plasma alone or in combination with mesenchymal stem cell treatment: a prospective study. Arthroscopy. 2014;30:1453–60. doi: 10.1016/j.arthro.2014.05.036. [DOI] [PubMed] [Google Scholar]
- Lee CH, Lee FY, Tarafder S, Kao K, Jun Y, Yang G, Mao JJ. Harnessing endogenous stem/progenitor cells for tendon regeneration. J Clin Invest. 2015;125:2690–701. doi: 10.1172/JCI81589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Huang BJ, Kaltz SR, Sur S, Newcomb CJ, Stock SR, Shah RN, Stupp SI. Bone regeneration with low dose BMP-2 amplified by biomimetic supramolecular nanofibers within collagen scaffolds. Biomaterials. 2013;34:452–9. doi: 10.1016/j.biomaterials.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Mooney DJ. Designing hydrogels for controlled drug delivery. Nature Reviews Materials. 2016;1:16071. doi: 10.1038/natrevmats.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian Q, Zhang Y, Zhang J, Zhang HK, Wu X, Zhang Y, Lam FF, Kang S, Xia JC, Lai WH, Au KW, Chow YY, Siu CW, Lee CN, Tse HF. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation. 2010;121:1113–23. doi: 10.1161/CIRCULATIONAHA.109.898312. [DOI] [PubMed] [Google Scholar]
- Liang CZ, Li H, Tao YQ, Peng LH, Gao JQ, Wu JJ, Li FC, Hua JM, Chen QX. Dual release of dexamethasone and TGF-beta3 from polymeric microspheres for stem cell matrix accumulation in a rat disc degeneration model. Acta Biomater. 2013;9:9423–33. doi: 10.1016/j.actbio.2013.08.019. [DOI] [PubMed] [Google Scholar]
- Liebergall M, Schroeder J, Mosheiff R, Gazit Z, Yoram Z, Rasooly L, Daskal A, Khoury A, Weil Y, Beyth S. Stem cell-based therapy for prevention of delayed fracture union: a randomized and prospective preliminary study. Mol Ther. 2013;21:1631–8. doi: 10.1038/mt.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long WJ, Greene JW, Cushner FD. Early Clinical Outcomes Associated with a Novel Osteochondral Allograft Transplantation System in the Knee. Advances in Orthopedic Surgery. 2016;2016:6. [Google Scholar]
- Lutolf MP, Lauer-Fields JL, Schmoekel HG, Metters AT, Weber FE, Fields GB, Hubbell JA. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: engineering cell-invasion characteristics. Proc Natl Acad Sci U S A. 2003;100:5413–8. doi: 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Jing Y, Sun H, Liu X. Hierarchical Nanofibrous Microspheres with Controlled Growth Factor Delivery for Bone Regeneration. Adv Healthc Mater. 2015;4:2699–708. doi: 10.1002/adhm.201500531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris EA, Gomoll AH, Malizos KN, Hu JC, Athanasiou KA. Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol. 2015;11:21–34. doi: 10.1038/nrrheum.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malda J, Boere J, Van De Lest CH, Van Weeren P, Wauben MH. Extracellular vesicles - new tool for joint repair and regeneration. Nat Rev Rheumatol. 2016;12:243–9. doi: 10.1038/nrrheum.2015.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manco A, Goderecci R, Rughetti A, S DEG, Necozione S, Bernardi A, Calvisi V. Microfracture versus microfracture and platelet-rich plasma: arthroscopic treatment of knee chondral lesions. A two-year follow-up study. Joints. 2016;4:142–147. doi: 10.11138/jts/2016.4.3.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt LM, Heilshorn SC. Design of Injectable Materials to Improve Stem Cell Transplantation. Curr Stem Cell Rep. 2016;2:207–220. doi: 10.1007/s40778-016-0058-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino MM, Tortelli F, Mochizuki M, Traub S, Ben-David D, Kuhn GA, Muller R, Livne E, Eming SA, Hubbell JA. Engineering the growth factor microenvironment with fibronectin domains to promote wound and bone tissue healing. Sci Transl Med. 2011;3:100ra89. doi: 10.1126/scitranslmed.3002614. [DOI] [PubMed] [Google Scholar]
- Monibi FA, Cook JL. Tissue-Derived Extracellular Matrix Bioscaffolds: Emerging Applications in Cartilage and Meniscus Repair. Tissue Eng Part B Rev. 2017 doi: 10.1089/ten.TEB.2016.0431. [DOI] [PubMed] [Google Scholar]
- Moya-Angeler J, Gianakos AL, Villa JC, Ni A, Lane JM. Current concepts on osteonecrosis of the femoral head. World J Orthop. 2015;6:590–601. doi: 10.5312/wjo.v6.i8.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundi R, Chaudhry H, Mundi S, Godin K, Bhandari M. Design and execution of clinical trials in orthopaedic surgery. Bone Joint Res. 2014;3:161–8. doi: 10.1302/2046-3758.35.2000280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nombela-Arrieta C, Ritz J, Silberstein LE. The elusive nature and function of mesenchymal stem cells. Nat Rev Mol Cell Biol. 2011;12:126–31. doi: 10.1038/nrm3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noriega DC, Ardura F, Hernandez-Ramajo R, Martin-Ferrero MA, Sanchez-Lite I, Toribio B, Alberca M, Garcia V, Moraleda JM, Sanchez A, Garcia-Sancho J. Intervertebral disc repair by allogeneic mesenchymal bone marrow cells: a randomized controlled trial. Transplantation. 2016 doi: 10.1097/TP.0000000000001484. [DOI] [PubMed] [Google Scholar]
- Orr SB, Chainani A, Hippensteel KJ, Kishan A, Gilchrist C, Garrigues NW, Ruch DS, Guilak F, Little D. Aligned multilayered electrospun scaffolds for rotator cuff tendon tissue engineering. Acta Biomater. 2015;24:117–26. doi: 10.1016/j.actbio.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla S, Sanchez M, Orive G, Anitua E. Human-Based Biological and Biomimetic Autologous Therapies for Musculoskeletal Tissue Regeneration. Trends Biotechnol. 2017;35:192–202. doi: 10.1016/j.tibtech.2016.09.008. [DOI] [PubMed] [Google Scholar]
- Pak J, Lee JH, Park KS, Park M, Kang LW, Lee SH. Current use of autologous adipose tissue-derived stromal vascular fraction cells for orthopedic applications. J Biomed Sci. 2017;24:9. doi: 10.1186/s12929-017-0318-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YB, Ha CW, Lee CH, Yoon YC, Park YG. Cartilage Regeneration in Osteoarthritic Patients by a Composite of Allogeneic Umbilical Cord Blood-Derived Mesenchymal Stem Cells and Hyaluronate Hydrogel: Results from a Clinical Trial for Safety and Proof-of-Concept with 7 Years of Extended Follow-Up. Stem Cells Transl Med. 2017;6:613–621. doi: 10.5966/sctm.2016-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson J, Siew R, Herring SW, Lin AS, Guldberg R, Stayton PS. Hyaluronic acid hydrogels with controlled degradation properties for oriented bone regeneration. Biomaterials. 2010;31:6772–81. doi: 10.1016/j.biomaterials.2010.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly HM, Sathy BN, Olvera D, Mccarthy HO, Kelly DJ, Popat KC, Dunne NJ, Haut Donahue TL. Hierarchically Structured Electrospun Scaffolds with Chemically Conjugated Growth Factor for Ligament Tissue Engineering. Tissue Eng Part A. 2017 doi: 10.1089/ten.tea.2016.0480. [DOI] [PubMed] [Google Scholar]
- Peach MS, Ramos DM, James R, Morozowich NL, Mazzocca AD, Doty SB, Allcock HR, Kumbar SG, Laurencin CT. Engineered stem cell niche matrices for rotator cuff tendon regenerative engineering. PLoS One. 2017;12:e0174789. doi: 10.1371/journal.pone.0174789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelttari K, Pippenger B, Mumme M, Feliciano S, Scotti C, Mainil-Varlet P, Procino A, Von Rechenberg B, Schwamborn T, Jakob M, Cillo C, Barbero A, Martin I. Adult human neural crest-derived cells for articular cartilage repair. Sci Transl Med. 2014;6:251ra119. doi: 10.1126/scitranslmed.3009688. [DOI] [PubMed] [Google Scholar]
- Pereira CL, Goncalves RM, Peroglio M, Pattappa G, D'este M, Eglin D, Barbosa MA, Alini M, Grad S. The effect of hyaluronan-based delivery of stromal cell-derived factor-1 on the recruitment of MSCs in degenerating intervertebral discs. Biomaterials. 2014;35:8144–53. doi: 10.1016/j.biomaterials.2014.06.017. [DOI] [PubMed] [Google Scholar]
- Prockop DJ, Prockop SE, Bertoncello I. Are clinical trials with mesenchymal stem/progenitor cells too far ahead of the science? Lessons from experimental hematology. Stem Cells. 2014;32:3055–61. doi: 10.1002/stem.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarta M, Brett JO, Dimarco R, De Morree A, Boutet SC, Chacon R, Gibbons MC, Garcia VA, Su J, Shrager JB, Heilshorn S, Rando TA. An artificial niche preserves the quiescence of muscle stem cells and enhances their therapeutic efficacy. Nat Biotechnol. 2016;34:752–9. doi: 10.1038/nbt.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintavalla J, Uziel-Fusi S, Yin J, Boehnlein E, Pastor G, Blancuzzi V, Singh HN, Kraus KH, O'byrne E, Pellas TC. Fluorescently labeled mesenchymal stem cells (MSCs) maintain multilineage potential and can be detected following implantation into articular cartilage defects. Biomaterials. 2002;23:109–19. doi: 10.1016/s0142-9612(01)00086-2. [DOI] [PubMed] [Google Scholar]
- Re'em T, Witte F, Willbold E, Ruvinov E, Cohen S. Simultaneous regeneration of articular cartilage and subchondral bone induced by spatially presented TGF-beta and BMP-4 in a bilayer affinity binding system. Acta Biomater. 2012;8:3283–93. doi: 10.1016/j.actbio.2012.05.014. [DOI] [PubMed] [Google Scholar]
- Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM. Self-renewal and expansion of single transplanted muscle stem cells. Nature. 2008;456:502–6. doi: 10.1038/nature07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadtler K, Estrellas K, Allen BW, Wolf MT, Fan H, Tam AJ, Patel CH, Luber BS, Wang H, Wagner KR, Powell JD, Housseau F, Pardoll DM, Elisseeff JH. Developing a pro-regenerative biomaterial scaffold microenvironment requires T helper 2 cells. Science. 2016;352:366–70. doi: 10.1126/science.aad9272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai D, Andersson GB. Stem cell therapy for intervertebral disc regeneration: obstacles and solutions. Nat Rev Rheumatol. 2015;11:243–56. doi: 10.1038/nrrheum.2015.13. [DOI] [PubMed] [Google Scholar]
- Shah RN, Shah NA, Del Rosario Lim MM, Hsieh C, Nuber G, Stupp SI. Supramolecular design of self-assembling nanofibers for cartilage regeneration. Proc Natl Acad Sci U S A. 2010;107:3293–8. doi: 10.1073/pnas.0906501107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekaran A, Garcia JR, Clark AY, Kavanaugh TE, Lin AS, Guldberg RE, Garcia AJ. Bone regeneration using an alpha 2 beta 1 integrin-specific hydrogel as a BMP-2 delivery vehicle. Biomaterials. 2014;35:5453–61. doi: 10.1016/j.biomaterials.2014.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Chen X, Chen J, Yin Z, Heng BC, Chen W, Ouyang HW. The effect of incorporation of exogenous stromal cell-derived factor-1 alpha within a knitted silk-collagen sponge scaffold on tendon regeneration. Biomaterials. 2010;31:7239–49. doi: 10.1016/j.biomaterials.2010.05.040. [DOI] [PubMed] [Google Scholar]
- Shi M, Li J, Liao L, Chen B, Li B, Chen L, Jia H, Zhao RC. Regulation of CXCR4 expression in human mesenchymal stem cells by cytokine treatment: role in homing efficiency in NOD/SCID mice. Haematologica. 2007;92:897–904. doi: 10.3324/haematol.10669. [DOI] [PubMed] [Google Scholar]
- Sicari BM, Rubin JP, Dearth CL, Wolf MT, Ambrosio F, Boninger M, Turner NJ, Weber DJ, Simpson TW, Wyse A, Brown EH, Dziki JL, Fisher LE, Brown S, Badylak SF. An acellular biologic scaffold promotes skeletal muscle formation in mice and humans with volumetric muscle loss. Sci Transl Med. 2014;6:234ra58. doi: 10.1126/scitranslmed.3008085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleep E, Cosgrove BD, Mcclendon MT, Preslar AT, Chen CH, Sangji MH, Perez CMR, Haynes RD, Meade TJ, Blau HM, Stupp SI. Injectable biomimetic liquid crystalline scaffolds enhance muscle stem cell transplantation. Proc Natl Acad Sci U S A. 2017;114:E7919–E7928. doi: 10.1073/pnas.1708142114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solheim E, Hegna J, Inderhaug E, Øyen J, Harlem T, Strand T. Results at 10–14 years after microfracture treatment of articular cartilage defects in the knee. Knee Surgery, Sports Traumatology, Arthroscopy. 2016;24:1587–1593. doi: 10.1007/s00167-014-3443-1. [DOI] [PubMed] [Google Scholar]
- Spiller KL, Koh TJ. Macrophage-based therapeutic strategies in regenerative medicine. Adv Drug Deliv Rev. 2017 doi: 10.1016/j.addr.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller KL, Liu Y, Holloway JL, Maher SA, Cao Y, Liu W, Zhou G, Lowman AM. A novel method for the direct fabrication of growth factor-loaded microspheres within porous nondegradable hydrogels: controlled release for cartilage tissue engineering. J Control Release. 2012;157:39–45. doi: 10.1016/j.jconrel.2011.09.057. [DOI] [PubMed] [Google Scholar]
- Spiller KL, Nassiri S, Witherel CE, Anfang RR, Ng J, Nakazawa KR, Yu T, Vunjak-Novakovic G. Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials. 2015;37:194–207. doi: 10.1016/j.biomaterials.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan R, Cameron AR, Kelly DJ, Kearney CJ, O’brien FJ. Biomaterial based modulation of macrophage polarization: a review and suggested design principles. Materials Today. 2015;18:313–325. [Google Scholar]
- Steinert AF, Rackwitz L, Gilbert F, Noth U, Tuan RS. Concise review: the clinical application of mesenchymal stem cells for musculoskeletal regeneration: current status and perspectives. Stem Cells Transl Med. 2012;1:237–47. doi: 10.5966/sctm.2011-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenderup K, Justesen J, Clausen C, Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003;33:919–26. doi: 10.1016/j.bone.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Strauss EJ, Barker JU, Kercher JS, Cole BJ, Mithoefer K. Augmentation Strategies following the Microfracture Technique for Repair of Focal Chondral Defects. Cartilage. 2010;1:145–52. doi: 10.1177/1947603510366718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai IC, Fu YC, Wang CK, Chang JK, Ho ML. Local delivery of controlled-release simvastatin/PLGA/HAp microspheres enhances bone repair. Int J Nanomedicine. 2013;8:3895–904. doi: 10.2147/IJN.S48694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trounson A, Mcdonald C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell. 2015;17:11–22. doi: 10.1016/j.stem.2015.06.007. [DOI] [PubMed] [Google Scholar]
- Vangsness CT, Jr, Farr J, 2nd, Boyd J, Dellaero DT, Mills CR, Leroux-Williams M. Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy: a randomized, double-blind, controlled study. J Bone Joint Surg Am. 2014;96:90–8. doi: 10.2106/JBJS.M.00058. [DOI] [PubMed] [Google Scholar]
- Vega A, Martin-Ferrero MA, Del Canto F, Alberca M, Garcia V, Munar A, Orozco L, Soler R, Fuertes JJ, Huguet M, Sanchez A, Garcia-Sancho J. Treatment of Knee Osteoarthritis With Allogeneic Bone Marrow Mesenchymal Stem Cells: A Randomized Controlled Trial. Transplantation. 2015;99:1681–90. doi: 10.1097/TP.0000000000000678. [DOI] [PubMed] [Google Scholar]
- Vrancken AC, Buma P, Van Tienen TG. Synthetic meniscus replacement: a review. Int Orthop. 2013;37:291–9. doi: 10.1007/s00264-012-1682-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagers AJ. The stem cell niche in regenerative medicine. Cell Stem Cell. 2012;10:362–9. doi: 10.1016/j.stem.2012.02.018. [DOI] [PubMed] [Google Scholar]
- Wong KL, Lee KB, Tai BC, Law P, Lee EH, Hui JH. Injectable cultured bone marrow-derived mesenchymal stem cells in varus knees with cartilage defects undergoing high tibial osteotomy: a prospective, randomized controlled clinical trial with 2 years' follow-up. Arthroscopy. 2013;29:2020–8. doi: 10.1016/j.arthro.2013.09.074. [DOI] [PubMed] [Google Scholar]
- Wynn RF, Hart CA, Corradi-Perini C, O'neill L, Evans CA, Wraith JE, Fairbairn LJ, Bellantuono I. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood. 2004;104:2643–5. doi: 10.1182/blood-2004-02-0526. [DOI] [PubMed] [Google Scholar]
- Yu Y, Brouillette MJ, Seol D, Zheng H, Buckwalter JA, Martin JA. Use of recombinant human stromal cell-derived factor 1alpha-loaded fibrin/hyaluronic acid hydrogel networks to achieve functional repair of full-thickness bovine articular cartilage via homing of chondrogenic progenitor cells. Arthritis Rheumatol. 2015;67:1274–85. doi: 10.1002/art.39049. [DOI] [PubMed] [Google Scholar]
- Zeitouni S, Krause U, Clough BH, Halderman H, Falster A, Blalock DT, Chaput CD, Sampson HW, Gregory CA. Human mesenchymal stem cell-derived matrices for enhanced osteoregeneration. Sci Transl Med. 2012;4:132ra55. doi: 10.1126/scitranslmed.3003396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Chen J, Tao J, Jiang Y, Hu C, Huang L, Ji J, Ouyang HW. The use of type 1 collagen scaffold containing stromal cell-derived factor-1 to create a matrix environment conducive to partial-thickness cartilage defects repair. Biomaterials. 2013;34:713–23. doi: 10.1016/j.biomaterials.2012.10.027. [DOI] [PubMed] [Google Scholar]
- Zhao D, Cui D, Wang B, Tian F, Guo L, Yang L, Liu B, Yu X. Treatment of early stage osteonecrosis of the femoral head with autologous implantation of bone marrow-derived and cultured mesenchymal stem cells. Bone. 2012;50:325–30. doi: 10.1016/j.bone.2011.11.002. [DOI] [PubMed] [Google Scholar]