Abstract
Objective
Birth diagnosis of HIV-1 infection offers an ideal opportunity for early antiretroviral treatment (ART) to limit HIV-1 reservoir size and limit disease progression. Although data on cellular HIV-1 DNA decay exist for children commencing treatment from 2–3 months of age, data is lacking for starting shortly after birth.
Design
We studied infants who initiated ART within 8 days after birth to assess HIV-1 DNA levels longitudinally.
Methods
Children were recruited from public health clinics in Cape Town where birth diagnosis of HIV-1 coupled with early ART initiation occurred. Total cellular HIV-1 DNA levels were determined using a sensitive quantitative PCR targeting a conserved region in integrase.
Results
Of 11 infants diagnosed and beginning ART within 8 days of birth with detectable pre-ART HIV-1 DNA, 3 subsequently had undetectable HIV-1 DNA after 6 days, 3 months and 4 months on treatment, respectively. In 7 who had virologic suppression (defined as a continuous downward trend in plasma HIV-1 RNA, and <100 copies/mL after 6 months) total HIV-1 DNA continued to decay over 12 months (mean half-life of 64.8 days [95% CI: 47.9–105.7]).
Conclusion
In infants initiated on ART within 8 days of life the combination of maternal ART, and early ART for prophylaxis and treatment contribute to rapid decline of HIV-1 infected cells to low or undetectable levels. However, rapid decline of HIV-1 RNA and DNA may complicate definitive diagnosis when confirmatory testing is delayed.
Introduction
Most intra-uterine HIV-1 infections occur during the last weeks of gestation[1]. Infant diagnosis by sensitive HIV-1 nucleic acid testing at birth offers a unique opportunity to diagnose infection as soon as possible to begin therapy and linkage to care as infant HIV-1 disease is rapidly progressive with high mortality[2–5]. Early antiretroviral therapy (ART) can also limit the HIV-1 reservoir size[6–9]. Low reservoir sizes are associated with a delayed rebound after ART discontinuation, most likely due to stochastic activation of rare infected cells containing intact proviruses[10]. This was evident from a prolonged period without rebound viremia, despite absent detectable immune response in the Mississippi child and adult Boston hematopoietic stem cell transplant patients[11–14]. Early therapy may also provide an opportunity to achieve ART-free remission due to a small reservoir size and intact immune system: in adults, early therapy followed by interruption resulted in post treatment control in about 15% in the Visconti cohort[15], but data from the SPARTAC study suggest that the effect of early treatment may have been inflated by the natural occurrence of transient control early after infection[16,17]. Nevertheless, the proportion remains higher than naturally occurring elite controllers (<1%). Post treatment control was also observed in perinatally infected individuals: in two children beginning ART within the first 3 months of life[18,19] and a young adult who started therapy, after perinatal infection, at 3.5 years of age[20].
In children who initiated ART between 0.5 to 2.6 months of age a study described that HIV-1 DNA concentration decayed to 1.0 to 1.5 log10 copies/million cells at 1–2 years of age[21]. Two other studies described median HIV-1 DNA half-lives of 53-[22] and 107 days[23], in children initiating ART around a median of 2 months or before 3 months, respectively. We have previously shown that therapy before 2 months of age reduces the number of infected cells and their transcriptional activity measured by unspliced cellular RNA[24]. However, information on the early decay of HIV-1 DNA in infants who began ART shortly after birth is limited. Our aim was therefore to investigate changes in total HIV-1 DNA in infants starting ART within 8 days after birth.
Methods
Children were diagnosed through a public health sector birth HIV-1 diagnosis program in Cape Town, South Africa, and initiated ART as soon as feasible. Parents or legal guardians provided informed consent. The study was approved by the Stellenbosch University’s Health Research Ethics Committee (reference: M14/07/029).
HIV-1 infection was confirmed with at least 2 positive HIV nucleic acid tests on separate samples (qualitative and/or quantitative) with Roche COBAS ® AmpliPrep/COBAS® TaqMan® (CAP/CTM) HIV-1 v2.0 or HIV-1 Qualitative v2 (CAP/CTM) (Roche Molecular Diagnostics, Pleasanton, CA). Subsequently the infants enrolled in a study of HIV-1 reservoirs and neurodevelopment in infants and children. We studied total cell associated HIV-1 DNA kinetics in infants beginning ART within 8 days of birth. Other inclusion criteria were having detectable baseline HIV-1 DNA and at least 2 stored peripheral blood mononuclear cell (PBMC) samples on treatment.
PBMCs and plasma were processed at 3 monthly visits. Samples were processed and stored according to the HANC Cross-Network PBMC processing SOP (https://www.hanc.info/labs/labresources/procedures/Pages/pbmcSop.aspx). HIV-1 total DNA was extracted and measured through a sensitive quantitative PCR adapted for HIV-1 subtype C, targeting a conserved region in HIV-1 integrase (iCAD; limit of detection: 3 copies/million PBMCs; Supplementary Table 1)[25,26]. HIV-1 RNA was quantified with the CAP/CTM v2.0, with a 100 copies/mL limit of detection for a 200 microliter plasma input. We defined virologic suppression as a continuous downward trend in plasma HIV-1 RNA and no HIV-1 RNA > 100 copies/mL at the first measurement after 6 months on ART. Infants not meeting these criteria were classified as viremic.
As pre-treatment PBMCs were unavailable, we assayed pre-treatment dried blood spots (DBS), routinely stored in the diagnostic laboratory, to confirm assay detectability. The PBMC extraction method was adapted for DBS nucleic acid extraction: A solution comprising proteinase K and guanidinium hydrochloride was added to the dried blood spot, sonicated immediately for 10 seconds, incubated at 37°C overnight and followed by extraction and total HIV-1 DNA quantification as described[26].
Statistics: Kinetics were modelled with log-linear regression. To predict total HIV-1 DNA (log copies/mL), mixed effect models (participant as random effect and time-treated as fixed effect) were compared and random intercept models were found to be optimal (adding random slopes did not improve Akaike information criterion (AIC) or Bayesian information criterion (BIC)). Goodness of fit was assessed with a conditional generalized linear least square method and confidence intervals were calculated with bootstrapping[27,28]. Graphics and statistics were performed with R version 3.3.1[29]. We expressed decay rates as half-lives (t½) which allows for an intuitive comparison between different phases of decay and with published studies.
Results
Fifteen infants started ART between 0 and 8 days (median 4 days) after birth (Table 1) and had at least 3 samples collected (a pre-ART DBS and at least two on-treatment PBMC samples). After enrollment, two infants had no detectable plasma HIV-1 RNA and cellular HIV-1 DNA samples, and another two had detectable plasma HIV-1 RNA only. The remaining 11 had at least one baseline assay-detectable HIV-1 DNA sample (pre-ART samples were from DBS) to allow longitudinal HIV-1 DNA observation, 7 of these were suppressed “S” and 4 viremic “V” participants (Figure; Supplementary Table 2).
Table.
Infants included in the study
| Patient ID | Gender | Child PMTCT | Child PMTCT Drugs | Age(days) at ARV Start # | Age (days) recruited* |
|---|---|---|---|---|---|
| S2 | Male | Yes | Immediate cART | 0 | 35 |
| S3 | Female | Yes | NVP, AZT | 3 | 18 |
| S4 | Female | Yes | AZT/3TC | 5# | 7 |
| S5 | Male | Yes | NVP, AZT | 7 | 22 |
| S6 | Female | Yes | Immediate cART | 0 | 30 |
| S7 | Female | Yes | NVP, AZT | 6 | 12 |
| S9 | Male | Yes | NVP, AZT | 8 | 8 |
| V1 | Female | Yes | NVP | 6 | 22 |
| V3 | Male | Yes | NVP, AZT | 8 | 54 |
| V4 | Male | Yes | NVP, AZT | 7 | 20 |
| V5 | Female | Yes | NVP, AZT | 4 | 10 |
A 2–5 day delay between initial dual therapy and triple therapy. The initial combination antiretroviral regimen in all was azidothymidine (AZT), lamivudine (3TC) and nevirapine (NVP). NVP was replaced by lopinavir/ritonavir (LPV/r) at a corrected age of 42 weeks; AZT was replaced by abacavir (ABC) at approximately 3 months of age. “S” indicate virologic suppressed infants and “V” viremic infants.
On the recruitment day the first PBMC samples to investigate HIV-1 reservoirs were collected.
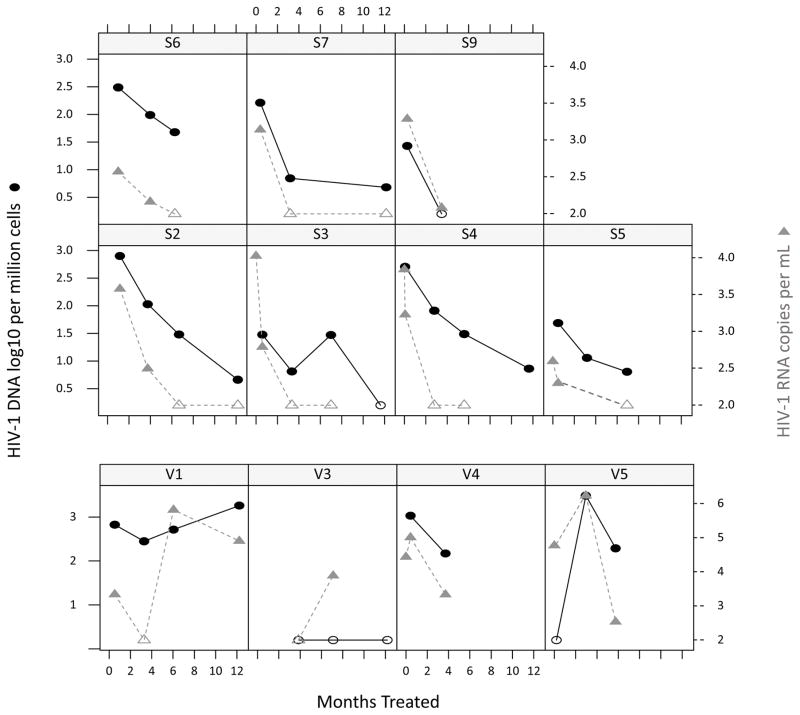
Figure.
Infants who had assay detectable cell associated HIV-1 DNA before or at treatment initiation were included. As only dried blood samples (DBS) were available from pre-treatment, which contain cells other than peripheral blood mononuclear cells (PBMC), HIV-1 cell associated DNA results shown in this figure were all from PBMCs collected after treatment initiation. HIV-1 DNA (black circles) copies/million cells and plasma HIV-1 RNA (grey triangles) copies/mL were assessed in 11 patients. “S” denotes those suppressed and “V” those viremic. Open icons indicate HIV-1 DNA or plasma RNA below the limit of detection: plasma HIV-1 RNA was censored at < 100 copies per mL and HIV-1 DNA loads at < 3 copies/million peripheral blood mononuclear cells (PBMCs). All suppressed infants except S6 reached an HIV-1 DNA level < 10 copies/million PBMCs. S9 had HIV-1 DNA declining to undetectable at 3.5 months. The decay slope of suppressed individuals had a conditional R2 (95% CI) of 0.77(0.53–0.91); Mean t ½ (95% CI): 64.8 (47.9–105.7) days. V3 had a visit at 1.5 months on ART for which no blood sample was received, thereafter at 3.8 months HIV-1 DNA was undetectable. V5 had undetectable HIV-1 DNA at the first on-ART visit but had viremia detected subsequently. HIV-1 DNA levels increased in V1 and V5.
Plasma HIV-1 RNA decay kinetics: Average baseline HIV-1 RNA was 3.9 (range 2.6–4.7) log 10 copies/mL in the 11 infants. In the 7 suppressed infants, HIV-1 RNA was undetectable (< 100 copies mL for 200 microliter input) in 3 children within 3.4 months, and in one child it was 120 copies at 3.5 months. In the remaining 3 patients, the first undetectable plasma HIV-1 RNA loads were recorded between 6 and 7 months.
Cellular HIV-1 DNA decay kinetics: HIV-1 DNA decay slopes were characterized in the 7 suppressed infants. As pre-ART DBS samples contained cells other than PBMCs we used it only to assess that HIV-1 DNA could be detected by our assay but could not to characterize the decay between pre-ART and the first on-ART time point. Thereafter HIV-1 DNA time slopes showed a good log-linear correlation (mean t ½ (95% CI): 64.8 (47.9–105.7) days; conditional R2 (95% CI): 0.77(0.53–0.91); Figure). At the end of the observation period at a median of 6.9 months (95% CI 3.5–11.6 months), in 6 of 7 suppressed infants, HIV-1 DNA loads dropped to < 10 copies/million PBMCs. In one of these 6 HIV-1 DNA was undetectable at the second visit, 3.5 months on ART (participant S9) in another HIV-1 DNA remained detectable, but unquantifiable at < 3 copies/million PMBCs, at 11.6 months (participant S3). The “suppressed” child whose HIV-1 DNA remained > 10 copies/million PBMCs(participant S6), had delayed viremia suppression (145 HIV-1 RNA copies at 4 months on treatment) with HIV-1 DNA load reaching 47.8 HIV-1 DNA copies/million PBMCs at 6 months. Two “viremic” infants had early undetectable HIV-1 DNA at their first visits, respectively, 6 days (participant V5) and 4 months (participant V3) with subsequent viremia; participant V5 had continued viremia with accompanied HIV-1 DNA increase whereas participant V3 had a single episode of viremia with HIV-1 DNA remaining undetectable.
Discussion
In infants with a rapid plasma HIV-1 RNA load suppression, here defined as HIV-1 RNA < 100 copies/mL within 3.5 months, who had assay detectable HIV-1 DNA, total DNA became undetectable in 3 of 11 infants, respectively at 6 days, 3.5 months and 4 months on treatment. The rate of initial HIV-1 DNA decay from treatment initiation to study enrolment could not be accurately assessed since only DBS were available pre-treatment. Thereafter total HIV-1 DNA in PBMC cells decayed with a t½ of 65 days during the remainder of the first year of life with all but one of seven suppressed infants reaching a total HIV-1 DNA load of <10 copies/million PBMCs within 13 months of birth. Rapid decay of HIV-1 DNA was observed in adults treated in Fiebig stage I and II (t½ of 21 days in the first 2 weeks). Thereafter the second phase decay was much slower (t½ of 198 days)[30]. In children treated around 2 months of life the total HIV-1 DNA t½ in the first 24 weeks of life was 53 days, where after it was 124 days between 24 and 48 weeks[22]. Another study that defined early treatment as before 3 months of life found a t½ of 107 days in the first year of life[23].
The mechanism of rapid HIV-1 DNA decline to low or undetectable levels in infants treated shortly after birth is unknown and requires further investigation. Mechanisms could include the following: 1) Low numbers of pre-treatment infected cells due to maternal ART and infant prophylaxis, 2) Possible rapid loss of cells with unintegrated virus[30]; 3) high CD4 turnover with a large proportion of short-living infected CD4 cells that decay rapidly[31]; or 4) a large proportion of surviving cells with integrated HIV-1 DNA that may have defective genomes harboring deletions, and as our assay detects HIV-1 integrase, these genomes may not be detectable[32].
Our study had the following limitations: First, infants diagnosed at birth and who initiated on early ART were recruited from the public health sector and often only enrolled later into our study. Second, having only a DBS sample pre-ART, we could not accurately assess decay up to the first on-treatment visit, as although HIV-1 DBS DNA levels were normalized for amplifiable cell equivalents, it contained multiple cell types and there was a limited recovery of total nucleic acid, precluding a comparison with subsequent HIV-1 DNA levels measured in PBMC. This combined with not having more frequent sampling prevented us from determining whether the earliest HIV-1 DNA decay was log-linear or biphasic. Third, adherence in young infants is challenging, evident from the high number of viremic children and some having had delayed HIV-1 RNA suppression, limiting our ability to study HIV-1 DNA decay under optimal conditions.
Despite the difficulty of treating infants, HIV-1 DNA decayed very rapidly in most infants and reached very low levels in the first year of life. As HIV is rapidly progressive in infants, it requires early diagnosis and treatment[5]. Moreover, very early ART could result in infants with small HIV reservoirs, who may be good candidates for future immunological interventions aimed at remission or cure. Rapid HIV-1 RNA and DNA decay poses a diagnostic challenge. The combined result of maternal ART (effectively intra-uterine treatment of infected babies), extensive neonatal prophylaxis and possibly some ARV absorption through breastfeeding[33], may together contribute to suppression of viral replication and result in undetectable HIV-1 DNA and RNA levels early in life and false negative diagnostic tests. It is therefore crucial that a definitive diagnosis is established as soon as possible after birth before HIV-1 DNA or plasma HIV-1 RNA become undetectable.
Supplementary Material
Acknowledgments
We thank the participants, their parents and the clinical Study Team. Author contributions: GvZ and KV planned the investigation. AJvR, BL and MC oversaw patient recruitment and data collection. JM oversaw the DBS extraction method. KV, SI and MGK processed samples. KV performed the assays for HIV-1 DNA. JWM and MC provided scientific and technical advice. GvZ drafted the manuscript with input from KV, JWM and MC. All authors approved of the final manuscript.
Funding: NIMH: 1R01MH105134-01; NCI: 1U01CA200441-01; Poliomyelitis Research Foundation, University of Pittsburgh: Centre for Global Health; SAMRC Collaborating Centre for HIV Laboratory Research.
References
- 1.Kourtis AP, Bulterys M, Nesheim SR, Lee FK, LM, ML N, et al. Understanding the Timing of HIV Transmission From Mother to Infant. JAMA. 2001;285:709. doi: 10.1001/jama.285.6.709. [DOI] [PubMed] [Google Scholar]
- 2.Newell M-L, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364:1236–1243. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 3.Lilian RR, Kalk E, Technau K-G, Sherman GG. Birth Diagnosis of HIV Infection in Infants to Reduce Infant Mortality and Monitor for Elimination of Mother-to-child Transmission. Pediatr Infect Dis J. 2013;32:1080–1085. doi: 10.1097/INF.0b013e318290622e. [DOI] [PubMed] [Google Scholar]
- 4.Cotton M, Holgate SL, Nelson A, Rabie H, Wedderburn C, Mirochnik M. The last and first frontier - emerging challenges for HIV treatment and prevention in the first week of life with emphasis on premature and low birth weight infants. J Int AIDS Soc. 2015;18:20271. doi: 10.7448/IAS.18.7.20271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Innes S, Lazarus E, Otwombe K, Liberty A, Germanus R, Van Rensburg AJ, et al. Early severe HIV disease precedes early antiretroviral therapy in infants: Are we too late? J Int AIDS Soc. 2014:17. doi: 10.7448/IAS.17.1.18914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bitnun A, Samson L, Chun T-W, Kakkar F, Brophy J, Murray D, et al. Early Initiation of Combination Antiretroviral Therapy in HIV-1-Infected Newborn Infants can Achieve Sustained Virologic Suppression with Low Frequency of CD4+ T-cells carrying HIV in Peripheral Blood. Clin Infect Dis. doi: 10.1093/cid/ciu432. Published Online First: 9 June 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luzuriaga K. Early Combination Antiretroviral Therapy Limits HIV-1 Persistence in Children. Annu Rev Med. 2016;67:201–213. doi: 10.1146/annurev-med-091114-111159. [DOI] [PubMed] [Google Scholar]
- 8.Ananworanich J, Puthanakit T, Suntarattiwong P, Chokephaibulkit K, Kerr SJ, Fromentin R, et al. Reduced markers of HIV persistence and restricted HIV-specific immune responses after early antiretroviral therapy in children. AIDS. 2014;28:1015–20. doi: 10.1097/QAD.0000000000000178. [DOI] [PubMed] [Google Scholar]
- 9.Luzuriaga K, Tabak B, Garber M, Chen YH, Ziemniak C, McManus MM, et al. Reduced HIV Reservoirs After Early Treatment HIV-1 Proviral Reservoirs Decay Continously Under Sustained Virologic Control in Early-Treated HIV-1- Infected Children. J Infect Dis. doi: 10.1093/infdis/jiu297. Published Online First: 21 May 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill AL, Rosenbloom DIS, Fu F, Nowak MA, Siliciano RF. Predicting the outcomes of treatment to eradicate the latent reservoir for HIV-1. Proc Natl Acad Sci U S A. 2014;111:13475–80. doi: 10.1073/pnas.1406663111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rouzine IM, Razooky BS, Weinberger LS. Stochastic variability in HIV affects viral eradication. Proc Natl Acad Sci U S A. 2014;111:13251–2. doi: 10.1073/pnas.1413362111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henrich TJ, Hanhauser E, Marty FM, Sirignano MN, Keating S, Lee T-H, et al. Antiretroviral-Free HIV-1 Remission and Viral Rebound After Allogeneic Stem Cell Transplantation. Ann Intern Med. 2014;161:319. doi: 10.7326/M14-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luzuriaga K, Gay H, Ziemniak C, Sanborn KB, Somasundaran M, Rainwater-Lovett K, et al. Viremic Relapse after HIV-1 Remission in a Perinatally Infected Child. N Engl J Med. 2015;372:786–788. doi: 10.1056/NEJMc1413931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hill AL, Rosenbloom DIS, Fu F, Nowak Ma, Siliciano RF. Predicting the outcomes of treatment to eradicate the latent reservoir for HIV-1. Proc Natl Acad Sci U S A. 2014;111:13475–80. doi: 10.1073/pnas.1406663111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sáez-Cirión A, Bacchus C, Hocqueloux L, Avettand-Fenoel V, Girault I, Lecuroux C, et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog. 2013;9:e1003211. doi: 10.1371/journal.ppat.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin GE, Gossez M, Williams JP, Stöhr W, Meyerowitz J, Leitman EM, et al. Post-treatment control or treated controllers? Viral remission in treated and untreated primary HIV infection. AIDS. 2017;31:477–484. doi: 10.1097/QAD.0000000000001382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gossez M, Ramjee G, Hurst J, Kaleebu P, Rees H, Porter K, et al. Virological Remission After ART Interruption in African HIV-1 Seroconverters. CROI 2016 Conference on Retroviruses and Opportunistic Infections; February 22–25; Boston, MA. 2016. [Google Scholar]
- 18.Frange P, Faye A, Avettand-Fenoël V, Bellaton E, Descamps D, Angin M, et al. HIV-1 virological remission lasting more than 12 years after interruption of early antiretroviral therapy in a perinatally infected teenager enrolled in the French ANRS EPF-CO10 paediatric cohort: a case report. Lancet HIV. 2016;3:e49–e54. doi: 10.1016/S2352-3018(15)00232-5. [DOI] [PubMed] [Google Scholar]
- 19.Violari A, Cotton MF, Kuhn L, Schramm D, Paximadis M, Loubser S, et al. Viral and host characteristics of a child with perinatal HIV-1 following a prolonged period after ART cessation in the CHER trial. In. 9th IAS Conference on HIV Science; Paris. 2017. http://programme.ias2017.org/Abstract/Abstract/5836. [Google Scholar]
- 20.McMahon JH, Chang J, Tennakoon S, Dantanarayana A, Solomon A, Cherry C, et al. Post-treatment control in an adult with perinatally acquired HIV following cessation of antiretroviral therapy. AIDS. 2017;31:1344–1346. doi: 10.1097/QAD.0000000000001472. [DOI] [PubMed] [Google Scholar]
- 21.Luzuriaga K, Tabak B, Garber M, Chen YH, Mcmanus MM, Murray D, et al. HIV type 1 (HIV-1) proviral reservoirs decay continuously under sustained virologic control in HIV-1-infected children who received early treatment. J Infect Dis. 2014;210:1529–38. doi: 10.1093/infdis/jiu297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uprety P, Chadwick EG, Rainwater-Lovett K, Ziemniak C, Luzuriaga K, Capparelli EV, et al. Cell-Associated HIV-1 DNA and RNA Decay Dynamics During Early Combination Antiretroviral Therapy in HIV-1-Infected Infants. Clin Infect Dis. 2015;61:1862–1870. doi: 10.1093/cid/civ688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McManus M, Mick E, Hudson R, Mofenson LM, Sullivan JL, Somasundaran M, et al. Early Combination Antiretroviral Therapy Limits Exposure to HIV-1 Replication and Cell-Associated HIV-1 DNA Levels in Infants. PLoS One. 2016;11:e0154391. doi: 10.1371/journal.pone.0154391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Zyl G, Bedison M, Janse van Rensburg A, Laughton B, Cotton M, Mellors J. Early Antiretroviral Therapy in South African Children Reduces HIV-1-Infected Cells and Cell-Associated HIV-1 RNA in Blood Mononuclear Cells. J Infect Dis. 2015;212:39–43. doi: 10.1093/infdis/jiu827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hong F, Aga E, Cillo AR, Yates AL, Besson G, Fyne E, et al. Novel Assays for Measurement of Total Cell-Associated HIV-1 DNA and RNA. J Clin Microbiol. 2016;54:902–11. doi: 10.1128/JCM.02904-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong F, Spindler J, Musick A, Cillo AR, Bale M, Shao W, et al. ART Reduces Cellular HIV RNA but Not the Fraction of Proviruses Transcribing RNA. Conference on Retroviruses and Opportunistic Infections (CROI 2016); February 22–25; Boston, MA, USA. 2016. [Google Scholar]
- 27.Johnson PCD. Extension of Nakagawa & Schielzeth’s R 2 GLMM to random slopes models. Methods Ecol Evol. 2014;5:944–946. doi: 10.1111/2041-210X.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakagawa S, Schielzeth H. A general and simple method for obtaining R 2 from generalized linear mixed-effects models. Methods Ecol Evol. 2013;4:133–142. [Google Scholar]
- 29.R Core Team. R: A Language and Environment for Statistical Computing. 2016 https://www.r-project.org/
- 30.Ananworanich J, Chomont N, Eller LA, Kroon E, Tovanabutra S, Bose M, et al. HIV DNA Set Point is Rapidly Established in Acute HIV Infection and Dramatically Reduced by Early ART. EBioMedicine. 2016;11:68–72. doi: 10.1016/j.ebiom.2016.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schönland SO, Zimmer JK, Lopez-Benitez CM, Widmann T, Ramin KD, Goronzy JJ, et al. Homeostatic control of T-cell generation in neonates. Blood. 2003:102. doi: 10.1182/blood-2002-11-3591. [DOI] [PubMed] [Google Scholar]
- 32.Bruner KM, Murray AJ, Pollack RA, Soliman MG, Laskey SB, Capoferri AA, et al. Defective proviruses rapidly accumulate during acute HIV-1 infection. Nat Med. 2016;22:1043–1049. doi: 10.1038/nm.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waitt CJ, Garner P, Bonnett LJ, Khoo SH, Else LJ. Is infant exposure to antiretroviral drugs during breastfeeding quantitatively important? A systematic review and meta-analysis of pharmacokinetic studies. J Antimicrob Chemother. 2015;70:1928–1941. doi: 10.1093/jac/dkv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.