Abstract
Objective
To evaluate race/ethnicity influence on BMI and mortality relationship among HFpEF and HFrEF patients.
Background
Prior studies demonstrate an “obesity paradox” among overweight/obese patients, where they have a better HF prognosis compared to normal-weight patients. Less is known about BMI and mortality relationship among diverse HF patients, particularly given disparities in obesity and HF prevalence.
Methods
Utilizing GWTG-HF data, we assessed BMI and in-hospital mortality relationship using logistic regression modeling. We assessed 30-day and 1-year all-cause mortality following discharge using Cox regression modeling.
Results
39,647 HF patients were included[white=32,434(81.8%); black=3,809(9.6%); Hispanic=1,928(4.9%); Asian=544(1.4%); other=932(2.3%)] with 59.7% HFpEF and 30.7% obese. More black and Hispanic patients had class I obese or higher (BMI≥30 kg/m2) than white, Asian or patients of other racial/ethnic groups(P <0.0001). Among HFpEF, higher BMI was associated with lower 30-day mortality, up to 30 kg/m2 with a small risk increase above 30 kg/m2[BMI=30 vs 18.5 kg/m2 hazard ratio(HR)=0.63, 95% confidence interval(CI) 0.54–0.73]. A modest relationship was observed in HFrEF(BMI=30 vs BMI=18.5 kg/m2 HR=0.73, 95% CI 0.60–0.89), with no risk increase above 30 kg/m2. There were no significant BMI by race/ethnicity interactions related to 30-day mortality(p>0.05).
Conclusions
Our work is one of the first suggesting the obesity paradox for 30-day mortality exists at all BMI levels in HFrEF, not HFpEF. Higher BMI was associated with lower 30-day mortality across racial/ethnic groups in a manner inconsistent with the J-shaped relationship noted for CAD. The differential slope of obesity and mortality among HFpEF and HFrEF patients potentially suggests differing mechanistic factors requiring further exploration.
Keywords: heart failure, obesity, race/ethnicity, mortality, GWTG-Heart Failure
INTRODUCTION
Both obesity and heart failure (HF) are unremitting in their rise; in the United States, 35% of Americans are obese (1) and HF afflicts 5.7 million individuals (2). Consequently, these conditions combined contribute to an estimated 246 billion dollars in healthcare expenditure (3,4). Although evidence independently implicates overweight (body mass index [BMI]≥25 kg/m2) and obesity (BMI≥30 kg/m2) with increased HF risk (1), most studies have suggested that increased BMI is associated with lower mortality in HF patients (6,7). However, whether this relationship differs between HF with preserved (HFpEF) and reduced ejection fraction (HFrEF) patients and whether there are important differences among HF patients of different racial/ethnic groups is less clear.
Considering the diverse racial composition of the U.S., the association of race/ethnicity on any BMI-HF mortality relationship becomes increasingly important. For example, African Americans (blacks) have the highest rates of both overweight/obesity and HF compared to other racial/ethnic groups in the U.S. (8) as well as increased HF and HF hospitalization rates (9) - factors likely significantly contributing to gaps in mortality and longevity by race/ethnicity. Additionally, as hospital readmissions are higher in blacks and Hispanics (10–13) and readmissions are closely linked to morbidity and mortality, it is imperative that BMI’s association with HF be characterized both in general and according to race/ethnicity to understand its effect on HF outcomes, and to potentially inform therapeutic interventions. Utilizing Get With The Guidelines Heart Failure (GWTG-HF) registry data for both HFpEF and HFrEF patients, we sought to 1) assess the association between BMI and in-hospital mortality according to race/ethnicity; and 2) determine associations between BMI and 30-day and 1-year all-cause mortality following discharge alive according to race/ethnicity.
METHODS
Data
Data Source
The GWTG-HF is a registry and performance improvement initiative started in 2005 to enhance adherence to practice guidelines for hospitalized HF patients. This voluntary American Heart Association (AHA) program collects data on patient characteristics using web-based information systems. The program’s methods, design, and validity have been published previously (14–17). Hospitals participating in the registry submit clinical information regarding medical history, laboratories, diagnostic testing, hospital care and outcomes of patients hospitalized for HF using an online, interactive case report form and patient management tool (Quintiles, Cambridge MA). To be eligible for GWTG-HF, patients had to be adults hospitalized for a HF episode as the primary cause of admission or with significant HF symptoms that developed during hospitalization with a primary discharge diagnosis of HF. Race/ethnicity data were collected for evaluating subgroup differences in outcomes. Patients, based on self-reported race/ethnicity, were assigned to race/ethnicity categories using options defined by the case report form as follows: race - American Indian or Alaska Native, Asian, Black or African-American, Native Hawaiian or Pacific Islander, White or unable to be determined; ethnicity, Hispanic- yes, no or unable to be determined.
Data Collection Protocols
HF status, including HFpEF vs. HFrEF diagnosis, was determined by point-of-care providers, based on American Heart Association guidelines. HFrEF patients had ejection fraction (EF) ≤ 40% and HFpEF patients had EF≥40% (18). Patient height and weight were collected at time of admission and BMI was imputed by the GWTG Patient Management Tool (PMT) data collection form. Covariates were collected from historical records or during admission, depending on time of presentation. For example, HF clinical characteristics were recorded based on current hospital admission or when the condition was first recognized. Quintiles is the GWTG data collection (through their Patient Management Tool – PMT) and coordination center. Duke Clinical Research Institute (DCRI) serves as the data analysis center and has an agreement to analyze aggregate de-identified data for research purposes. Participating institutions were required to comply with local regulatory guidelines and the local institutional review board.
Study Population
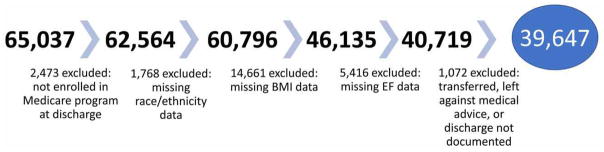
The starting population for this study was 65,037 GWTG-HF patients linked to CMS from 292 sites. The study period was from January 2005 through December 2011. We sequentially excluded patients who were not enrolled in fee-for-service Medicare at discharge (n= 2,473), patients without race/ethnicity data (n= 1,768), BMI missing (n= 14,661), ejection fraction missing (n= 5,416), transfers out or discharge information missing or not documented or left against medical advice (n= 1,072) (Figure 1). A total of 39,647 patients having HFpEF or HFrEF, with BMI and race/ethnicity data were documented. For post discharge outcomes, we excluded the study population patients that died in hospital or discharged to hospice (n=2,086).
Figure 1. Flow diagram of the study population selection process.
Participants were excluded systematically from the original GWTG-HF cohort.
Outcome Measures
The study objective was to assess the association between BMI and in-hospital mortality according to race/ethnicity and associations between BMI and 30-day or 1-year all-cause mortality following discharge alive, according to race/ethnicity. The outcomes were in-hospital mortality, 30-day and 1-year all-cause mortality. The 30-day and 1-year mortality were evaluated from discharge date to 30 days and 1 year afterwards.
Statistical Analysis
The study population was stratified by HFpEF and HFrEF and analyses were performed separately for each cohort. For descriptive analyses, baseline patient and hospital characteristics were stratified among Whites, Blacks, Hispanic, Asian and other race/ethnicity groups. For categorical variables, proportions were used and differences assessed by Chi-square test. For continuous/ordinal variables, means and standard deviations were presented across race/ethnicity groups assessed by Kruskal-Wallis tests.
The relationship between BMI, race/ethnicity, and in-hospital mortality was evaluated using logistic regression. We tested the interaction between BMI and race/ethnicity to assess whether the relationship between BMI and odds of mortality were similar among racial/ethnic groups. A multivariable model adjusted for patient and hospital characteristics, including age, sex, medical history (anemia, ischemic history, CVA/TIA, diabetes, hyperlipidemia, hypertension, COPD or asthma, PVD, renal insufficiency, smoking), admission vitals and labs (systolic blood pressure, heart rate, sodium, and blood urea nitrogen), hospital region, academic status and number of beds. Post-discharge outcomes of 30-day and 1-year mortality were similarly evaluated using Cox proportional hazards regression.
For missing adjustment variables, medical history variables were imputed to “no” as data abstractors were likely to skip this section of the data collection form when none applied; multiple imputation with 25 imputations was used for other patient covariates. Hospital characteristics were not imputed. Adjustment covariates were assessed for linearity and proportional hazards assumptions as needed and transformations applied when appropriate. Restricted cubic spline transformations flexibly illustrate relationships between BMI and mortality. To interpret results numerically, we also fit linear splines of BMI. A spline knot was chosen which balanced model-fit by maximizing model likelihood and interpretation of results. In HFrEF, we report results for BMI per 1 kg/m2 increase up to 25 kg/m2 and per 1 kg/m2 increase above 25 kg/m2 for in-hospital mortality. Among HFpEF, the knot point for in-hospital mortality was 30 kg/m2. For 30-day and 1-year mortality, the BMI knot point was 30 kg/m2. Restricted cubic spline relationships are plotted for 30-day mortality.
All tests were two-tailed and a p-value<0.05 was considered statistically significant. All analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC). The authors had full access to all study data and take responsibility for its integrity and the data analysis.
RESULTS
Baseline Characteristics
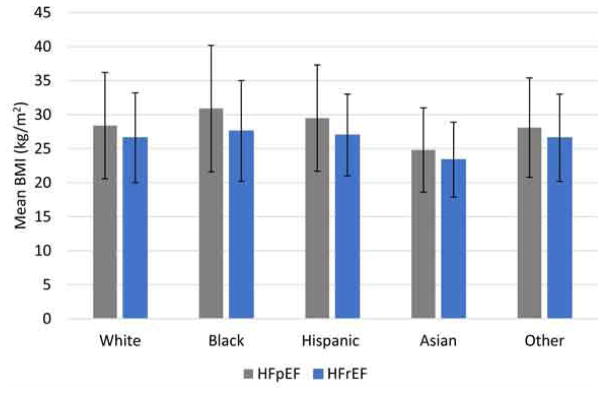
Of the 39,647 patients in the study population, 23,653(59.7%) had HFpEF and 15,994(40.3%) had HFrEF. Baseline patient and hospital characteristics for those with HFpEF are summarized in Table 1 and e-Table 1 in Supplement, while those of patients with HFrEF are summarized in Table 2 and e-Table 2 in Supplement. Figure 2 demonstrates the mean BMI distribution by race and HF status.
Table 1.
Baseline Patient and Hospital Characteristics of Patients with HFpEF, GWTG-HF, 2005–2011
| Variable | Overall (N=23653) | White (N=19575) | Black (N=2085) | Hispanic (N=1088) | Asian (N=368) | Other (N=537) | P-value |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Demographics | |||||||
| Age, yrs, mean(SD*) | 80.8 (8.1) | 81.35 (8.0) | 77.2 (8.3) | 79.1 (8.3) | 80.4 (7.9) | 79.6 (7.9) | <0.0001 |
| Female, No. (%) | 14872 (62.7) | 12188 (62.3) | 1411 (67.7) | 675 (62.0) | 214 (58.2) | 339 (63.1) | <0.0001 |
| Insurance Status, No. (%) | <0.0001 | ||||||
| Medicare | 16806 (74.4) | 14078 (75.2) | 1342 (68.9) | 741 (70.6) | 267 (75.0) | 378 (72.7) | |
| Medicaid | 1301 (5.8) | 688 (3.7) | 326 (16.7) | 186 (17.7) | 55 (15.5) | 46 (8.9) | |
| Other | 4496 (19.9) | 3963 (21.2) | 281 (14.4) | 122 (11.6) | 34 (9.6) | 96 (18.5) | |
| BMI, kg/m2, mean(SD*) | 28.6 (7.9) | 28.4 (7.8) | 30.9 (9.3) | 29.5 (7.8) | 24.8 (6.2) | 28.1 (7.3) | <0.0001 |
| BMI, categories, No. (%) | <0.0001 | ||||||
| Underweight (≤ 18.5 kg/m2) | 1076 (4.6) | 911 (4.7) | 66 (3.2) | 33 (3.0) | 41 (11.1) | 25 (4.7) | |
| Normal (18.5 – 24.9 kg/m2) | 7602 (32.1) | 6438 (32.9) | 508 (24.4) | 292 (26.8) | 182 (49.5) | 182 (33.9) | |
| Overweight (25 – 29.9 kg/m2) | 6646 (28.1) | 5518 (28.2) | 555 (26.6) | 332 (30.5) | 89 (24.2) | 152 (28.3) | |
| Obese I (30 – 34.9 kg/m2) | 4147 (17.5) | 3406 (17.4) | 404 (19.4) | 213 (19.6) | 38 (10.3) | 86 (16.0) | |
| Obese II (35 – 39.9 kg/m2) | 2178 (9.2) | 1754 (9.0) | 253 (12.1) | 110 (10.1) | 9 (2.5) | 52 (9.7) | |
| Obese III (≥40 kg/m2) | 2004 (8.5) | 1548 (7.9) | 299 (14.3) | 108 (9.9) | 9 (2.5) | 40 (7.5) | |
| Comorbidities | |||||||
| Diabetes-Insulin Treated, No. (%) | 4087 (17.4) | 3111 (16.0) | 527 (25.4) | 283 (26.2) | 63 (17.2) | 103 (19.4) | <0.0001 |
| Diabetes-Non-insulin Treated, No. (%) | 5511 (23.5) | 4380 (22.6) | 571 (27.6) | 330 (30.5) | 103 (28.1) | 127 (24.0) | <0.0001 |
| Hypertension, No. (%) | 18930 (80.6) | 15436 (79.5) | 1844 (89.0) | 922 (85.3) | 318 (86.9) | 410 (77.4) | <0.0001 |
| Ischemic Etiology, No. (%) | 12858 (54.8) | 10821 (55.7) | 972 (46.9) | 591 (54.7) | 184 (50.3) | 290 (54.7) | <0.0001 |
| CAD, No. (%) | 11434 (48.7) | 9634 (49.6) | 880 (42.5) | 514 (47.6) | 160 (43.7) | 246 (46.4) | <0.0001 |
| CVA/TIA, No. (%) | 4064 (17.3) | 3355 (17.3) | 422 (20.4) | 149 (13.8) | 67 (18.3) | 71 (13.4) | <0.0001 |
| Anemia, No. (%) | 5246 (22.4) | 4287 (22.1) | 587 (28.3) | 200 (18.5) | 65 (17.8) | 107 (20.2) | <0.0001 |
| Smoking, No. (%) | 1827 (7.8) | 1451 (7.5) | 243 (11.7) | 76 (7.0) | 17 (4.7) | 40 (7.5) | <0.0001 |
| Renal Insufficiency, No. (%) | 4451 (19.0) | 3493 (18.0) | 543 (26.2) | 210 (19.4) | 85 (23.2) | 120 (22.6) | <0.0001 |
| Hospital Characteristics | |||||||
| Number of Beds, mean (SD*) | 410.0 (235.6) | 409.2(236.9) | 456.9(234.9) | 358.3(198.4) | 362.0(222.2) | 392.3(234.7) | <0.0001 |
| Teaching Hospital, mean (SD*) | 13589 (57.5) | 11157 (57.0) | 1442 (69.2) | 493 (45.3) | 181 (49.2) | 316 (58.9) | <0.0001 |
SD - standard deviation.
Table 2.
Baseline Patient and Hospital Characteristics of Patients with HFrEF, GWTG-HF 2005–2011
| Variable | Overall (N=15994) | White (N=12859) | Black (N=1724) | Hispanic (N=840) | Asian (N=176) | Other (N=395) | P-value |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Demographics | |||||||
| Age, yrs, mean(SD*) | 78.6 (7.9) | 79.2 (7.8) | 75.7 (7.8) | 76.7 (7.7) | 79.0 (7.6) | 77.3 (8.3) | <0.0001 |
| Female, No. (%) | 6297 (39.4) | 4943 (38.4) | 800 (46.4) | 326 (38.8) | 72 (40.9) | 156 (39.5) | <0.0001 |
| Insurance Status, No. (%) | <0.0001 | ||||||
| Medicare | 11454 (75.9) | 9307 (76.3) | 1161 (72.8) | 570 (74.0) | 132 (79.5) | 156 (39.5) | |
| Medicaid | 728 (4.8) | 387 (3.2) | 208 (13.0) | 106 (13.8) | 16 (9.6) | 284 (77.4) | |
| Other | 2910 (19.3) | 2500 (20.5) | 226 (14.2) | 94 (12.2) | 18 (10.8) | 11 (3.0) | |
| BMI, kg/m2, mean(SD*) | 26.7 (6.6) | 26.6 (6.6) | 27.6 (7.4) | 27.0 (6.0) | 23.4 (5.5) | 26.6 (6.4) | <0.0001 |
| BMI, categories, No. (%) | <0.0001 | ||||||
| Underweight (≤ 18.5 kg/m2) | 903 (5.7) | 734 (5.7) | 89 (5.2) | 37 (4.4) | 24 (13.6) | 19 (4.8) | |
| Normal (18.5 – 24.9 kg/m2) | 6363 (39.8) | 5156 (40.1) | 628 (36.4) | 314 (37.4) | 100 (56.8) | 165 (41.8) | |
| Overweight (25 – 29.9 kg/m2) | 4890 (30.6) | 3972 (30.9) | 489 (28.4) | 275 (32.7) | 40 (22.7) | 114 (28.9) | |
| Obese I (30 – 34.9 kg/m2) | 2344 (14.7) | 1831 (14.2) | 304 (17.6) | 135 (16.1) | 8 (4.6) | 66 (16.7) | |
| Obese II (35 – 39.9 kg/m2) | 899 (5.6) | 709 (5.5) | 120 (7.0) | 53 (6.3) | 2 (1.1) | 15 (3.8) | |
| Obese III (≥40 kg/m2) | 595 (3.7) | 457 (3.6) | 94 (5.5) | 26 (3.1) | 2 (1.1) | 16 (4.1) | |
| Comorbidities | |||||||
| Diabetes-Insulin Treated, No. (%) | 2510 (15.8) | 1899 (14.9) | 329 (19.2) | 196 (23.5) | 23 (13.1) | 63 (16.3) | <0.0001 |
| Diabetes-Non-insulin Treated, No. (%) | 3706 (23.4) | 2894 (22.7) | 426 (24.8) | 245 (29.3) | 60 (34.3) | 81 (20.9) | <0.0001 |
| Hypertension, No. (%) | 11633 (73.4) | 9108 (71.5) | 1423 (83.0) | 695 (83.2) | 132 (75.4) | 275 (71.1) | <0.0001 |
| Ischemic Etiology, No. (%) | 10807 (68.2) | 8981 (70.5) | 915 (53.4) | 553 (66.2) | 114 (65.1) | 244 (63.1) | <0.0001 |
| CAD, No. (%) | 9369 (59.1) | 7812 (61.3) | 783 (45.7) | 482 (57.7) | 89 (50.9) | 203 (52.5) | <0.0001 |
| CVA/TIA, No. (%) | 2487 (15.7) | 2002 (15.7) | 298 (17.4) | 99 (11.9) | 27 (15.4) | 61 (15.8) | 0.0114 |
| Anemia, No. (%) | 2535 (16.0) | 2024 (15.9) | 325 (19.0) | 113 (13.5) | 25 (14.3) | 48 (12.4) | 0.0007 |
| Smoking, No. (%) | 1775 (11.2) | 1345 (10.6) | 285 (16.6) | 86 (10.3) | 6 (3.4) | 53 (13.6) | <0.0001 |
| Renal Insufficiency, No. (%) | 3204 (20.2) | 2497 (19.6) | 414 (24.1) | 165 (19.8) | 49 (28.0) | 79 (20.4) | <0.0001 |
| Hospital Characteristics | |||||||
| Number of Beds, mean (SD*) | 429.9(233.6) | 430.3 (236.2) | 465.0 (233.8) | 386.9(180.6) | 350.5(212.4) | 393.3(229.1) | <0.0001 |
| Teaching Hospital, mean (SD*) | 10142 (63.4) | 8047 (62.6) | 1257 (74.0) | 472 (56.2) | 104 (59.1) | 244 (61.8) | <0.0001 |
SD - standard deviation.
Figure 2. Mean BMI by race/ethnicity among HFpEF and HFrEF patients, GWTG-HF, 2005–2011.
Gray bars represent patients with HFpEF, while blue bars represent patients with HFrEF. Error bars represent standard deviation.
HFpEF Patients
Blacks with HFpEF were significantly younger than other racial/ethnic groups with HFpEF [mean age [(standard deviation(SD)]=77(8.3) years](p < 0.0001) (Table 1). Across all racial/ethnic groups, the majority of HFpEF patients were women. The mean BMI for the overall cohort was 28.6 (7.9) kg/m2; blacks with HFpEF had the highest BMI [mean BMI=30.9(9.3) kg/m2] and highest likelihood of Class III obesity (14.3%, p < 0.0001). Insulin-dependent diabetes was more common among Hispanics (26.2%) with HFpEF compared with other racial/ethnic groups (p< 0.0001). Additionally, hypertension, prior stroke, and renal insufficiency were more prevalent among blacks with HFpEF as compared to other racial/ethnic groups (89.0%, 20.4% and 26.2% respectively, p < 0.0001). Whites (55.7%) were more likely to have an ischemic etiology for HFpEF compared with Hispanics (54.7%), blacks (46.9%), Asians (50.3%) and others (54.7%) (p < 0.0001).
HFrEF Patients
Among HFrEF patients, blacks were younger [mean age 76(7.8) years] and more likely women (46.4%) compared with other racial/ethnic groups with HFrEF (Table 2). Mean BMI for the overall HFrEF population was 26.7(6.6) kg/m2, and blacks with HFrEF had a higher mean BMI than other racial/ethnic groups (p<0.0001); 3.7% of the overall HFrEF population had Class III obesity with blacks (5.5%) having a higher likelihood of Class III obesity as compared to whites (3.6%), Hispanics (3.1%), Asians (1.1%), and others (4.1%) (p<0.0001). Hispanics (23.5%) had the highest rates of insulin-dependent diabetes (p<0.0001), while Asians (34.3%) had the highest rates of non-insulin dependent diabetes (p<0.0001). Hispanics with HFrEF had the highest rates of hypertension (p<0.0001), while blacks had the highest rates of prior CVA (p=0.0114). Asians with HFrEF (28.0%) had the highest rates of renal insufficiency as compared to whites (19.6%), blacks (24.1%), Hispanics (19.8%) and others (20.4%) (p<0.0001). As with HFpEF patients, whites with HFrEF were more likely to have an ischemic etiology compared with other racial/ethnic groups (p<0.0001).
Patient Mortality by BMI Category
HFpEF Patients
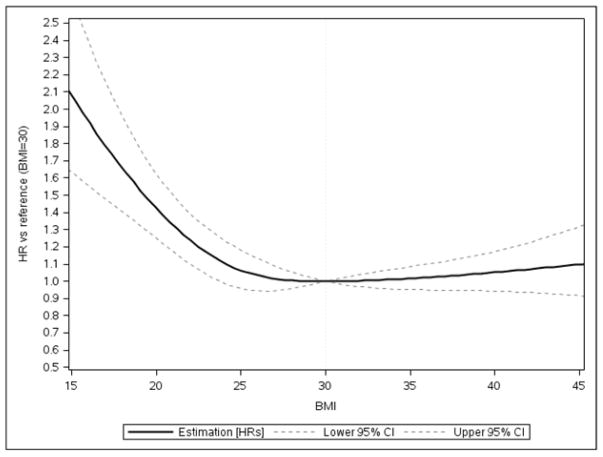
Among HFpEF patients, higher BMI was associated with lower 30-day all-cause mortality up to 30 kg/m2 with a slight increase in risk above 30 kg/m2 (Figure 3; BMI=30 kg/m2 vs. BMI=18.5 kg/m2: hazard ratio (HR) 0.63, 95% CI=0.54 – 0.73). Additionally, hazard of 1-year, all-cause mortality was 4% lower for one-unit BMI increase up to 30 kg/m2 [HR=0.96 (95% CI=0.95–0.96)] (Table 3). There was no significant relationship between BMI and 1-year mortality among those with HFpEF above BMI of 30 kg/m2. With regard to in-hospital mortality, there was no significant relationship between BMI and in-hospital mortality among HFpEF patients up to BMI of 30 kg/m2. For BMI above 30 kg/m2, each one-unit increase in BMI was associated with a 2% greater odds of in-hospital death for HFpEF patients[odds ratio (OR) = 1.02(95% confidence interval (CI)=1.01–1.04)] (e-Table 3 in supplement).
Figure 3. Adjusted Association between Body Mass Index and 30-day Mortality for HFpEF Patients, GWTG-HF, 2005–2011.
Among HFpEF patients, higher BMI was associated with lower 30-day all-cause mortality up to 30 kg/m2 with little change in risk above 30 kg/m2.
Table 3.
Association between BMI and 30-day and 1-year All-Cause Mortality, HFpEF and HFrEF Patients, GWTG-HF 2005–2011
| Outcome | Variable | HR*,† | Lower 95% CI | Upper 95% CI | P-value |
|---|---|---|---|---|---|
| 30-day All-Cause Mortality for HFpEF patients | Overall BMI up to 30 kg/m2 * Race | 0.92 | |||
| BMI per 1 unit increase up to 30 kg/m2 | 0.96 | 0.94 | 0.97 | <0.0001 | |
| Overall BMI above 30 kg/m2* Race | 0.96 | ||||
| BMI per 1 unit increase above 30 kg/m2 | 1.01 | 1.00 | 1.03 | 0.0477 | |
| 30-day All-Cause Mortality for HFrEF patients | Overall BMI up to 30 kg/m2 * Race | 0.08 | |||
| BMI per 1 unit increase up to 30 kg/m2 | 0.97 | 0.95 | 0.99 | 0.0016 | |
| Overall BMI above 30 kg/m2 * Race | 0.92 | ||||
| BMI per 1 unit increase above 30 kg/m2 | 1.01 | 0.99 | 1.03 | 0.51 | |
| 1-year All-Cause Mortality for HFpEF patients | Overall BMI up to 30 kg/m2* Race | 0.27 | |||
| BMI per 1 unit increase up to 30 kg/m2 | 0.96 | 0.95 | 0.96 | <0.0001 | |
| Overall BMI above 30 kg/m2* Race | 0.65 | ||||
| BMI per 1 unit increase above 30 kg/m2 | 1.00 | 1.00 | 1.01 | 0.67 | |
| 1-year All-Cause Mortality for HFrEF patients | Overall BMI up to 30 kg/m2* Race | 0.0246 | |||
| BMI per 1 unit increase up to 30 kg/m2 | 0.96 | 0.95 | 0.97 | <0.0001 | |
| Overall BMI above 30 kg/m2 * Race | 0.96 | ||||
| BMI per 1 unit increase above 30 kg/m2 | 1.01 | 1.00 | 1.02 | 0.0438 |
HR reported per 1 kg/m2 BMI increase
30-day and 1-year all-cause mortality were evaluated from the discharge date to 30 days and 1 year afterward
HFrEF Patients
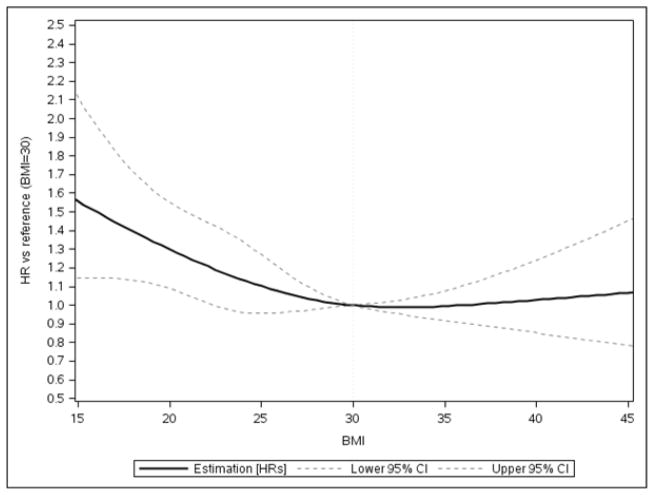
For HFrEF patients, up to a BMI of 30 kg/m2, 30-day all-cause mortality decreased with every BMI unit increase (BMI=30 kg/m2 vs BMI=18.5 kg/m2 HR 0.73, 95% CI:0.60–0.89) (Figure 4). A smaller relationship between BMI and lower 30-day all-cause mortality was observed in HFrEF patients, as shown in Figure 4. Thirty-day mortality rates were similar across obesity classes. Above BMI of 30 kg/m2, each one-unit BMI increase was associated with a 1% higher hazards of 1-year all-cause mortality [HR=1.01(95% CI=1.00–1.02)] (Table 3). Among patients with HFrEF, each BMI unit increase up to 25 kg/m2 was associated with a 5% lower odds of in-hospital death [OR = 0.95(95% CI= 0.91–0.99)]. Above a BMI of 25 kg/m2, each one unit BMI increase was associated with a 4% higher odds of in-hospital death [OR = 1.04(95% CI=1.03–1.05)] (e-Table 3 in supplement).
Figure 4. Adjusted Association between Body Mass Index and 30-day Mortality for HFrEF Patients, GWTG-HF, 2005–2011.
Up to a BMI of 30 kg/m2, 30-day all-cause mortality decreased with every BMI unit increase (BMI=30 kg/m2 vs BMI=18.5 kg/m2 HR 0.73, 95% CI:0.60–0.89).
Interactions Between BMI and Race/Ethnicity
No significant BMI by race/ethnicity interactions related to 30-day mortality were seen among HFpEF or HFrEF patients (all p>0.05, Table 3). The relationship between BMI below 30 kg/m2 and hazards of 1-year all-cause mortality was significantly different among racial/ethnic groups with HFrEF. BMI by race/ethnicity interactions were not statistically significant for 1-year, all-cause mortality among HFpEF and HFrEF patients with a BMI > 30 kg/m2. There were no significant BMI by race/ethnicity interactions related to in-hospital mortality for patients with HFpEF or HFrEF (all p>0.05, e-Table 3 in supplement).
DISCUSSION
Our findings demonstrate that in a nationally representative, racially and ethnically diverse cohort, high BMI was both common (30.7%) and associated with lower 30-day all-cause mortality for older (>65 years) HFpEF and HFrEF patients. While blacks and Hispanics had higher obesity rates than whites, Asians, and other racial/ethnic groups, there was no significant interaction between BMI and race in the relationship between HF and mortality. Unlike the relationship between BMI and mortality for CAD patients, the relationship between BMI and 30-day mortality was not J-shaped for HFpEF and HFrEF patients and 30-day mortality rates remained relatively constant in Class II and Class III obesity in HFrEF patients. However, there was a small, but statistically significant increase in 30-day mortality rates for HFpEF patients with a BMI above 30 kg/m2. Differential slopes in the association between BMI below 30 kg/m2 and 30-day mortality among HFpEF and HFrEF patients likely suggests differences in mechanistic factors that promote early mortality between the two HF phenotypes.
Our findings contribute to the literature on BMI and mortality in HF patients in several important ways. First, this is one of few studies to compare the relationship between BMI and 30-day all-cause mortality across racial/ethnic groups with HFpEF or HFrEF (19), as highlighted in Table 4. Prior GWTG-HF studies have been limited to evaluating in-hospital mortality among those with HFpEF and HFrEF in one racial/ethnic group (20) or have not distinguished outcomes between HFpEF and HFrEF patients when comparing in-hospital or 30-day mortality across racial/ethnic groups (13,21). In GWTG-HF, Hispanics with HFpEF were less likely to have ischemic heart disease compared with Hispanics with HFrEF. Additionally, Hispanics with HFpEF - but not HFrEF - had lower in-hospital mortality compared with whites (21). Thomas and colleagues demonstrated that blacks and Hispanics in the GWTG-HF registry had a lower likelihood of in-hospital death as compared to white patients. One potential explanation for the differences was that HF with a non-ischemic etiology was more common among blacks and Hispanics (20). More recent data suggested that 30-day survival after index admission is greater among blacks compared with whites, even after controlling for co-morbidities, hospital characteristics, and socioeconomic status. Our work extends the findings from these prior GWTG-HF studies to demonstrate that BMI differences do not appear to explain differences in 30-day all-cause mortality across racial and ethnic groups. This conclusion is supported by a recent meta-analysis which demonstrated that greater BMI was associated lower one-year all-cause mortality in racially/ethnically diverse cohorts with HF from different parts of the world (i.e., Asia, North/South America, Europe) (19).
Table 4.
Comparison of Studies Examining the Obesity Paradox in Cardiovascular Disease, 2014 to Present
| Reference | Main Findings |
|---|---|
| Powell-Wiley TM, Ngwa J, Kedebe S et al. 2017. Impact of BMI on Heart Failure with Preserved or Reduced Ejection Fraction: Data by Race/Ethnicity from Get With The Guidelines- Heart Failure Registry. | Race/ethnicity does not appear to modify the relationship between BMI and mortality among HF patients, and the obesity paradox appears to exist across BMI levels for HF patients with preserved or reduced ejection fraction. |
| Wang ZJ, Zhou YJ, Galper BZ, et al. Association of body mass index with mortality and cardiovascular events for patients with coronary artery disease: a systematic review and meta- analysis. Heart. 2015;101(20):1631–8. | There is a J-shaped relationship between BMI categories and risk of mortality among patients with CAD, with underweight patients having highest risk. Survival benefit of obesity was attenuated by 5-year follow up. |
| Vivo RP, Krim SR, Liang L, et al. Short- and long-term rehospitalization and mortality for heart failure in 4 racial/ethnic populations. J Am Heart Assoc. 2014;3:e001134. | Black and Hispanic HF patients had higher 30-day and 1-year hospital readmission rates, but lower 30-day and 1-year mortality compared to white patients, after adjustment for socioeconomic status and patient/hospital characteristics. |
| Shah R, Gayat E, Januzzi JL, Jr., et al. Body mass index and mortality in acutely decompensated heart failure across the world: a global obesity paradox. J Am Coll Cardiol. 2014;63:778–85. | There is an obesity paradox in 1-year mortality of acute decompensated HF patients globally, after adjustment for patient characteristics. However, the association of BMI and outcome is restricted to specific groups, such as non-diabetic, older patients with de novo HF diagnosis or HF with reduced LVEF. |
| Padwal R, McAlister FA, McMurray JJV et al. The obesity paradox in heart failure patients with preserved versus reduced ejection fraction: a meta-analysis of individual patient data. Int J Obesity. 2014;38:1110–1114. | There is a U-shaped mortality curve among HF patients with preserved or reduced LVEF. Lowest mortality was seen in patients with BMI between 30 and 30.4 kg/m2. After adjustment for confounders, high BMI status remained significantly associated with lower mortality risk. |
| Ziaeian B, Heidenreich PA, Xu H, et al. Race/ethnic differences in outcomes among hospitalized Medicare patients with heart failure and preserved ejection fraction. J Am Coll Cardiol HF. 2017;5:483–493. | Lower mortality risk after hospitalization for HFpEF was found in black, Hispanic, and Asian patients after adjusting for SES and patient/hospital characteristics. Black and Hispanic HFpEF patients had higher readmission rates, but lower short- and long-term mortality. |
Second, our findings enhance the body of knowledge about the perplexing repercussions of increasing BMI on HF outcomes. Many prior studies have demonstrated the presence of an “obesity paradox” among overweight and Class I and II obese patients, or that these patients have a better prognosis with HF as compared to patients with a normal BMI (22–25. Our work is one of the first studies to suggest that the BMI paradox for 30-day mortality exists at all BMI levels, including Class II and Class III obesity, among HFrEF patients; our findings appear consistent with a recent meta-analysis of six studies and over 22,000 patients (26). Other cohorts in which the relationship between Class II or III obesity and HF mortality has been investigated have been limited in size (27), in the numbers of those with Class III obesity (28), or in diversity (29). Padwal and colleagues, for example, did not look across race/ethnic groups when evaluating the obesity paradox in HF patients (29), while Ziaeian and colleagues looked across race/ethnic groups without discussing the obesity paradox (30). Differences in the proportion of study participants with HFpEF compared with HFrEF, in the racial/ethnic composition of the populations, and prevalent co-morbidities among study participants may explain the differences between our findings and those of other studies looking at Class II/III obesity and mortality. In particular, further work is needed to differentiate how Class III obesity impacts HF mortality because Class III obesity is strongly associated with adverse cardiac remodeling and the subsequent HF development (31).
Finally, this is one of the first studies to distinguish between HFpEF and HFrEF when evaluating the association of BMI and mortality among HF patients. Unlike prior studies, which have only looked at HFrEF or HFpEF populations (32,33), our findings suggest heterogeneity in mortality risk at lower BMI between the two HF phenotypes, with a greater 30-day mortality risk at higher BMI among HFpEF patients. From a pathophysiologic standpoint, this may represent differences in co-morbidities between those with HFrEF and HFpEF who survive hospitalization. For example, compared with HFpEF patients, HFrEF patients who survive a hospitalization may be more likely to have reductions in ejection fraction due to mechanisms unrelated to cardiovascular risk factors or cardiovascular disease and which may carry a better prognosis (27). Additionally, HFpEF patients at a given BMI likely have higher blood pressures and tolerate higher doses of cardioprotective medications compared with those with HFrEF, leading to differences in mortality risk (34). Recent work suggests that patient characteristics, including age, left ventricular function, and HF chronicity, impact the prognostic association between BMI and all-cause mortality. As compared with our findings, BMI was more strongly associated with all-cause mortality for HF patients with a left ventricular ejection fraction less than 50% as compared to those with an ejection fraction greater than or equal to 50% in this meta-analysis of international cohorts (19). Our study findings highlight the importance of differentiating between HFpEF and HFrEF when evaluating BMI and mortality in HF patients.
The strengths of the current study include data from a nationally representative, multiethnic cohort with well-established protocols for data collection and analyses. The GWTG-HF data was also linked to high-quality, standardized data from the Centers for Medicare and Medicaid Services to determine 30-day mortality for study participants, further enhancing the reliability of the study data. However, limitations of the study must be acknowledged. There are significant differences in demographics, clinical characteristics and treatments by BMI categories and we cannot exclude residual measured and unmeasured confounding factors contributing to these findings. We were unable to compare mortality across measures of body fat distribution or more accurate measures of adiposity, such as waist circumference in this cohort. Alternative measures of body fat distribution instead of BMI may be of particular importance when attempting to differentiate cardiovascular risk relative to adiposity among racially and ethnically diverse populations (35). Patients’ weights for determining BMI were obtained during hospitalization for HF and thus may not represent the patients’ weight at a time they are well compensated. However, relatively few patients would be expected to change BMI categories on the basis of wet versus dry weight. Another potential limitation is that GWTG-HF program hospitals are voluntary participants and may be more motivated for quality improvement, which may lead to better patient outcomes as compared to other hospitals around the country, limiting study generalizability. Additionally, the proportion of racial/ethnic minority patients seen in GWTG-HF hospitals may differ from the proportion in those hospitals not represented in the program. Hospitals outside of the GWTG-HF program may disproportionately care of racial/ethnic minority patients and may have differ from GWTG-HF hospitals in quality of care provided. Finally, method of recording race and ethnicity by patient self-designation as recorded by administrative staff or admitting providers is likely not as reliable as direct patient reporting.
In conclusion, black and Hispanic patients in the GWTG-HF registry were more likely to be obese than white, Asian or other patients. Higher BMI was associated with lower 30-day all-cause mortality in each racial/ethnic group in a manner not consistent with the J-shaped obesity paradox noted in prior studies. Additionally, the differential slope of the BMI and 30-day all-cause mortality association below BMI of 30 kg/m2 in HFpEF and HFrEF and higher mortality risk above BMI of 30 kg/m2 in HFpEF possibly suggests differing mechanistic factors which require further exploration.
Supplementary Material
CLINICAL PERSPECTIVE.
Race/ethnicity does not appear to modify the relationship between BMI and mortality among HF patients, and the obesity paradox appears to exist across BMI levels for HF patients with preserved or reduced ejection fraction.
TRANSLATIONAL OUTLOOK.
Further research is needed to investigate mechanisms by which the obesity paradox exists across BMI levels for patients with preserved or reduced ejection fraction HF.
Acknowledgments
Sources of Funding: The Get With The Guidelines®–Heart Failure (GWTG-HF) program is provided by the American Heart Association. GWTG-HF is sponsored, in part, by Amgen Cardiovascular and has been funded in the past through support from Medtronic, GlaxoSmithKline, Ortho-McNeil, and the American Heart Association Pharmaceutical Roundtable. Dr. Powell-Wiley is funded by the Division of Intramural Research of the National, Heart, Lung, and Blood Institute of the NIH.
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services. The authors would like to acknowledge Samantha Thomas and Sophie Claudel for their assistance in manuscript preparation.
Abbreviations
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- CAD
coronary artery disease
- GWTG-HF
Get With The Guidelines-Heart Failure
- BMI
body mass index
- HR
hazard ratio
- OR
odds ratio
- SD
standard deviation
- CI
confidence interval
Footnotes
Disclosures: Dr. Deepak L. Bhatt discloses the following relationships - Advisory Board: Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Duke Clinical Research Institute, Harvard Clinical Research Institute, Mayo Clinic, Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), Harvard Clinical Research Institute (clinical trial steering committee), HMP Communications (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Population Health Research Institute (clinical trial steering committee), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Amarin, Amgen, AstraZeneca, Bristol-Myers Squibb, Eisai, Ethicon, Forest Laboratories, Ischemix, Medtronic, Pfizer, Roche, Sanofi Aventis, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co-Investigator: Biotronik, Boston Scientific, St. Jude Medical; Trustee: American College of Cardiology; Unfunded Research: FlowCo, PLx Pharma, Takeda.
Dr. Gregg C. Fonarow discloses the following relationships - Research: Agency for Healthcare Research and Quality, National Institutes of Health; Consulting: Amgen, Janssen, Novartis, Medtronic, and St Jude.
The remaining authors have no relevant disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mandviwala T, Khalid U, Deswal A. Obesity and Cardiovascular Disease: a Risk Factor or a Risk Marker? Curr Atheroscler Rep. 2016;18:21. doi: 10.1007/s11883-016-0575-4. [DOI] [PubMed] [Google Scholar]
- 2.Department of Health and Human Services, Centers for Disease Control and Prevention. Heart Failure Fact Sheet. 2016 https://www.cdc.gov/dhdsp/data_statistics/fact_sheets/docs/fs_heart_failure.pdf.
- 3.Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hammond RA, Levine R. The economic impact of obesity in the United States. Diabetes Metab Syndro Obes. 2010;3:285–295. doi: 10.2147/DMSOTT.S7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff (Millwood) 2009;28:w822–31. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- 6.Fonarow GC, Srikanthan P, Costanzo MR, Cintron GB, Lopatin M Committee ASA and Investigators. An obesity paradox in acute heart failure: Analysis of body mass index and inhospital mortality for 108927 patients in the Acute Decompensated Heart Failure National Registry. American heart journal. 2007;153:74–81. doi: 10.1016/j.ahj.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Gupta PP, Fonarow GC, Horwich TB. Obesity and the obesity paradox in heart failure. Canadian Journal of Cardiology. 2015;31:195–202. doi: 10.1016/j.cjca.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Sharma A, Colvin-Adams M, Yancy CW. Heart failure in African Americas: Disparities can be overcome. Cleveland Clinic J Med. 2014;81(5):301–311. doi: 10.3949/ccjm.81a.13045. [DOI] [PubMed] [Google Scholar]
- 9.Krishnamoorthy A, Greiner MA, Bertoni AG, et al. The Obesity and Heart Failure Epidemics among African Americans: Insights From the Jackson Heart Study. J Card Fail. 2016 doi: 10.1016/j.cardfail.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das SR, Alexander KP, Chen AY, et al. Impact of body weight and extreme obesity on the presentation, treatment, and in-hospital outcomes of 50,149 patients with ST-Segment elevation myocardial infarction results from the NCDR (National Cardiovascular Data Registry) J Am Coll Cardiol. 2011;58:2642–50. doi: 10.1016/j.jacc.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madala MC, Franklin BA, Chen AY, et al. Obesity and age of first non-ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2008;52:979–85. doi: 10.1016/j.jacc.2008.04.067. [DOI] [PubMed] [Google Scholar]
- 12.Quiroz R, Doros G, Shaw P, Liang CS, Gauthier DF, Sam F. Comparison of characteristics and outcomes of patients with heart failure preserved ejection fraction versus reduced left ventricular ejection fraction in an urban cohort. Am J Cardiol. 2014;113:691–6. doi: 10.1016/j.amjcard.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 13.Vivo RP, Krim SR, Liang L, et al. Short- and long-term rehospitalization and mortality for heart failure in 4 racial/ethnic populations. J Am Heart Assoc. 2014;3:e001134. doi: 10.1161/JAHA.114.001134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fonarow GC, Abraham WT, Albert NM, et al. Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF): rationale and design. Am Heart J. 2004;148:43–51. doi: 10.1016/j.ahj.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 15.LaBresh KA, Ellrodt AG, Gliklich R, Liljestrand J, Peto R. Get with the guidelines for cardiovascular secondary prevention: pilot results. Arch Intern Med. 2004;164:203–9. doi: 10.1001/archinte.164.2.203. [DOI] [PubMed] [Google Scholar]
- 16.LaBresh KA, Gliklich R, Liljestrand J, Peto R, Ellrodt AG. Using “get with the guidelines” to improve cardiovascular secondary prevention. Jt Comm J Qual Saf. 2003;29:539–50. doi: 10.1016/s1549-3741(03)29064-x. [DOI] [PubMed] [Google Scholar]
- 17.Smaha LA. The American Heart Association Get With The Guidelines program. Am Heart J. 2004;148:S46–8. doi: 10.1016/j.ahj.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 18.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for management of HF: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. J Am College Cardiology. 2013;62(16):e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 19.Shah R, Gayat E, Januzzi JL, Jr, et al. Body mass index and mortality in acutely decompensated heart failure across the world: a global obesity paradox. J Am Coll Cardiol. 2014;63:778–85. doi: 10.1016/j.jacc.2013.09.072. [DOI] [PubMed] [Google Scholar]
- 20.Thomas KL, Hernandez AF, Dai D, et al. Association of race/ethnicity with clinical risk factors, quality of care, and acute outcomes in patients hospitalized with heart failure. Am Heart J. 2011;161:746–54. doi: 10.1016/j.ahj.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 21.Vivo RP, Krim SR, Krim NR, et al. Care and outcomes of Hispanic patients admitted with heart failure with preserved or reduced ejection fraction: findings from get with the guidelines-heart failure. Circ Heart Fail. 2012;5:167–75. doi: 10.1161/CIRCHEARTFAILURE.111.963546. [DOI] [PubMed] [Google Scholar]
- 22.Clark AL, Fonarow GC, Horwich TB. Obesity and the obesity paradox in heart failure. Prog Cardiovasc Dis. 2014;56:409–14. doi: 10.1016/j.pcad.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Lavie CJ, Alpert MA, Arena R, Mehra MR, Milani RV, Ventura HO. Impact of obesity and the obesity paradox on prevalence and prognosis in heart failure. JACC Heart Fail. 2013;1:93–102. doi: 10.1016/j.jchf.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Lavie CJ, Sharma A, Alpert MA, et al. Update on Obesity and Obesity Paradox in Heart Failure. Prog Cardiovasc Dis. 2016;58:393–400. doi: 10.1016/j.pcad.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Wang ZJ, Zhou YJ, Galper BZ, Gao F, Yeh RW, Mauri L. Association of body mass index with mortality and cardiovascular events for patients with coronary artery disease: a systematic review and meta-analysis. Heart. 2015;101:1631–1638. doi: 10.1136/heartjnl-2014-307119. [DOI] [PubMed] [Google Scholar]
- 26.Sharma A, Lavie CJ, Borer JS, et al. Meta-analysis of the relation of body mass index to all-cause and cardiovascular mortality and hospitalization in patients with chronic heart failure. Am J Cardiol. 2015;115:1428–34. doi: 10.1016/j.amjcard.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 27.Nagarajan V, Cauthen CA, Starling RC, Tang WH. Prognosis of morbid obesity patients with advanced heart failure. Congest Heart Fail. 2013;19:160–4. doi: 10.1111/chf.12038. [DOI] [PubMed] [Google Scholar]
- 28.Kenchaiah S, Pocock SJ, Wang D, et al. Body mass index and prognosis in patients with chronic heart failure: insights from the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation. 2007;116:627–36. doi: 10.1161/CIRCULATIONAHA.106.679779. [DOI] [PubMed] [Google Scholar]
- 29.Padwal R, McAlister FA, McMurray JJV, et al. The obesity paradox in heart failure patients with preserved versus reduced ejection fraction: a meta-analysis of individual patient data. Int J Obesity. 2014;38:1110–1114. doi: 10.1038/ijo.2013.203. [DOI] [PubMed] [Google Scholar]
- 30.Ziaeian B, Heidenreich PA, Xu H, et al. Race/ethnic differences in outcomes among hospitalized medicare patients with heart failure and preserved ejection fraction. J Am Coll Cardiol HF. 2017;5:483–493. doi: 10.1016/j.jchf.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alpert MA, Omran J, Mehra A, Ardhanari S. Impact of obesity and weight loss on cardiac performance and morphology in adults. Prog Cardiovasc Dis. 2014;56:391–400. doi: 10.1016/j.pcad.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Clark AL, Chyu J, Horwich TB. The obesity paradox in men versus women with systolic heart failure. Am J Cardiol. 2012;110:77–82. doi: 10.1016/j.amjcard.2012.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haass M, Kitzman DW, Anand IS, et al. Body mass index and adverse cardiovascular outcomes in heart failure patients with preserved ejection fraction: results from the Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial. Circ Heart Fail. 2011;4:324–31. doi: 10.1161/CIRCHEARTFAILURE.110.959890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lavie CJ, Ventura HO. Analyzing the weight of evidence on the obesity paradox and heart failure--is there a limit to the madness? Congest Heart Fail. 2013;19:158–9. doi: 10.1111/chf.12040. [DOI] [PubMed] [Google Scholar]
- 35.Rao G, Powell-Wiley TM, Ancheta I, et al. Identification of Obesity and Cardiovascular Risk in Ethnically and Racially Diverse Populations: A Scientific Statement From the American Heart Association. Circulation. 2015;132:457–72. doi: 10.1161/CIR.0000000000000223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.