Abstract
Background
In adults with dilated cardiomyopathy (DCM), malnutrition is associated with mortality while obesity with survival. We aimed to examine the role of nutrition in pediatric DCM.
Methods
NHLBI-funded Pediatric Cardiomyopathy Registry was used to identify DCM patients and categorized by anthropometric measurements: malnourished (MN, body mass index (BMI) <5% for ≥2 years or weight-for-length <5% for <2 years), obesity (BMI> 95% for age ≥ 2 years or weight-for-length >95% for <2 years), or normal bodyweight (NB). Of 904 DCM patients, 23.7% (214) were MN, 13.3% (120) were obese, and 63.1% (570) were NB.
Results
Obese patients were older (9.0 vs. 5.7 years for NB, P<0.001) and more likely to have a family history of DCM (36.1% vs. 23.5% for NB, P=0.023). MN patients were younger (2.7 years vs. 5.7 years for NB, P<0.001) and more likely to have heart failure (79.9% vs. 69.7% for NB, P=0.012), cardiac dimension z-scores > 2, and higher ventricular mass compared to NB. In multivariable analysis, MN was associated with increased risk of death (HR: 2.06, 95% CI: 1.66-3.65, P<0.001); whereas, obesity was not (HR: 1.49, 95% CI: 0.72, 3.08). Competing outcomes analysis demonstrated increased risk of mortality for MN compared to NB (P=0.03), but no difference in transplant rate (P=0.159).
Conclusions
Malnutrition is associated with increased mortality and other unfavorable echocardiographic and clinical outcomes compared to those with NB. The same effect of obesity on survival was not observed. Further studies are needed investigating the long-term impact of abnormal anthropometric measurements on outcomes in pediatric DCM.
Clinical Trial Registration
NCT00005391; https://clinicaltrials.gov/ct2/show/NCT00005391?term=NCT00005391&rank=1
Keywords: dilated cardiomyopathy, pediatrics, heart failure
Subject Terms: diet and nutrition, obesity, pediatrics, cardiomyopathy, heart failure
INTRODUCTION
Children with heart failure are at increased risk for developing malnutrition given higher than normal energy requirements.1 Poor growth is frequent even in patients receiving supplemental enteral feedings.2 In children undergoing heart transplant, malnutrition has been implicated as a risk factor for mortality before and after transplant.3,4
Obesity is another important consideration in the pediatric population. Adults with obesity are at increased risk for developing heart failure.5,6 Although obesity is a risk factor for mortality after heart transplant in adults, an “obesity paradox” exists where obese adults with systolic dysfunction or clinical symptoms of heart failure survive longer than adults who are malnourished or are of normal weight.7–10 The mechanism of this difference in outcomes is not well understood and has not been investigated in children.11 Because the rate of childhood obesity and metabolic syndrome is now sufficiently high, encountering children with cardiomyopathy and obesity is no longer uncommon.12
The primary aim of this study was to describe the impact of malnourishment and obesity on clinical outcomes (death or heart transplantation) in children with dilated cardiomyopathy (DCM). We hypothesized that there would be an increased frequency of death or transplantation in malnourished patients while obesity would not adversely impact death or transplantation compared to patients with normal anthropometric measurements.
METHODS
The National Heart, Lung, and Blood Institute-funded Pediatric Cardiomyopathy Registry (PCMR) was used to identify patients with DCM. This registry consists of demographic, echocardiographic, and clinical data on over 3,000 children (age <18 years at diagnosis) with cardiomyopathy diagnosed at any of 98 pediatric cardiac centers in the United States and Canada since the registry was established in 1990. Data, including anthropometric and echocardiographic data, are collected within 30 days of diagnosis and enrollment in the study as well as annually until a patient reaches the age of 18 years. Each participating center obtains Institutional Review Board or Ethics Committee approval for the study. Detailed information regarding registry design and conduct are detailed elsewhere.13,14 Data used for this study were frozen on January 14th, 2013.
The PCMR defines DCM based on echocardiographic measurements of ventricular size and function, pathological findings on biopsy or autopsy, or clinical diagnosis.15 Patients with a neuromuscular disorder, malformation syndrome, or inborn error of metabolism were excluded from the current analysis because the underlying disease in these patients was believed to influence outcomes independently of DCM. Patients were also excluded if weight or height measurements were not available in the registry.
Participants were categorized into one of three mutually exclusive nutrition groups based on anthropometric measurement at time of diagnosis. The categories were: Malnourished (MN), Normal Bodyweight (NB), and Obese. Participants two years of age and older were considered MN if their BMI was lower than the 5th percentile for age and gender, and, participants less than two years were considered MN if their weight-for-length was less than the 5th percentile. Participants two years of age or older were considered obese if their BMI for age and gender was above the 95th percentile, and participants younger than two years were obese if their weight-for-length was greater than the 95th percentile.
Echocardiographic variables, including LV end-diastolic dimension (EDD), posterior wall thickness, septal thickness, and mass, were expressed as z-scores conditional on body surface area and calculated using best possible fit with BSA as the determinant and were determined using the lambda mu sigma (LMS) method as described by Foster et all (Sluysmans and Foster). Left ventricular fractional shortening (LVFS) and posterior wall thickness to end diastolic diameter ratio were expressed as the z-score conditional on age.16
The New England Research Institutes (Watertown, MA) was the PCMR Data Coordinating Center and performed all data analyses. To describe the patients’ characteristics and baseline echocardiographic parameters, means and standard deviations were used for normally distributed variables and frequencies and percentages were used for categorical variables. Analysis of variance (ANOVA) and Chi-square test were used to assess overall differences across the three nutritional groups. Kaplan-Meier plots of time from diagnosis to death and/or transplantation were generated, and log rank P-values were calculated. Patients were censored at seven years given duration of study follow-up.
Statistical Methods
Analysis of variance (ANOVA) and Chi-square test were used for overall comparison across the nutritional groups. Pair-wise comparisons were conducted when the overall p value is < 0.05. Post-hoc Bonferroni-corrected P values < 0.017 (0.05/3 comparison groups) were considered statistically significant. Cox proportional hazard regression models were used to determine impact of nutritional status on survival and freedom from transplant as the outcome of interest while adjusting for potential confounders. First, univariate Cox regression models were fit for a selection of predictors which were chosen based on prior knowledge that were potentially associated with survival outcomes, then multivariable Cox regression models were fit step by step by adding covariates that were significant in the univariate analyses at the <0.10 level, including age, congestive heart failure, anti-congestive therapy, FS z-score, HAZ, and ratio of LV EDPT/EDD z-score. Finally, backwards model selection was used to determine the final model. To test the proportionality assumption, the Kaplan-Meier plots were used to check the proportional hazards for categorical covariates, while scatterplot smooths were used to examine the relationship between scaled Schoenfeld residuals and follow-up time for continuous covariates. We also assessed proportional hazards assumption by testing the interaction between the covariate and the follow-up time. Estimates of cumulative incidence for each of the three competing outcomes (death, transplantation, or survival and freedom from transplant) were calculated in the competing risk analysis. Statistical significance was established at a P-value <0.05. Analysis was performed using SAS version 9.3 (Cary, NC) and R version 3.1.1.
RESULTS
There were 1,755 DCM patients who did not have neuromuscular disorder, malformation syndrome or an inborn error of metabolism. Of the 1,755 participants who met inclusion criteria, 851 were excluded due to missing height and weight measurements. Demographics of excluded patients are included in Appendix A and revealed younger age and a different race distribution among excluded patients (Appendix A). The study population therefore included 904 participants with documented height and weight information. Of these participants, 23.7% (214) were MN, 63.1% (570) were NB, and 13.3 % (120) were obese. The median follow up time was 1.4 years (mean ± SD: 2.8 ± 3.4 years) for all participants, 1.3 (3.0±3.8), 1.6 (2.9±3.4), and 0.9 (1.9±2.7) years for MN, NB, and obese participants, respectively. MN patients were more likely to be younger, have a lower weight-for-age z-score, have heart failure at time of diagnosis and receive anti-heart failure therapy compared to patients with NB (P<0.05 for all). Compared to NB, obese patients were more likely to be older, have lower height-for-age z-score, higher weight-for-age z-score, and have a family history of cardiomyopathy (P<0.05 for all). These patterns were similar for MN compared to obese with MN being younger, more frequently female, greater height-for-age z-score, lower weight for age z-score, lower BMI, more heart failure at diagnosis and on anti-heart failure therapy. Otherwise, there were no significant differences between the groups by race, etiology, family history of sudden death, or anti-heart failure therapy (Table 1).
Table 1.
Patient Characteristics by Nutrition Group
| Bodyweight | P-Value# | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
|
Characteristic Mean (±SD) or N (%) |
Malnourished (N=214) |
Normal Bodyweight (N=570) |
Obese (N=120) |
Overall |
Malnourished vs. Normal | Normal vs. Obese | Malnourished vs. Obese |
| Age at Diagnosis (years), mean±SD | 2.70±4.50 | 5.70±5.95 | 9.00±6.34 | <0.001 | <0.001 | <0.001 | <0.001 |
| Gender (male) | 101 (47.2%) | 293 (51.4%) | 72 (60%) | 0.080 | |||
| Height-for-age z-score | 0.27±1.98 | −0.20±1.44 | −0.67±2.77 | <0.001 | 0.001 | 0.010 | <0.001 |
| Weight-for-age z-score | −1.77±1.75 | −0.29±1.24 | 1.81±2.21 | <0.001 | <0.001 | <0.001 | <0.001 |
| BMI | 13.25±1.49 | 17.13±3.10 | 29.90±13.20 | <0.001 | <0.001 | <0.001 | <0.001 |
| Race | 0.832 | ||||||
| White | 127 (59.9%) | 328 (58.8%) | 58 (48.7%) | ||||
| Black | 42 (19.8%) | 108 (19.4%) | 39 (32.8%) | ||||
| Hispanic | 23 (10.8%) | 89 (15.9%) | 16 (13.4%) | ||||
| Other | 22 (10.2%) | 45 (5.9%) | 7 (5.0%) | ||||
| Etiology | 0.164 | ||||||
| Idiopathic | 174 (81.3%) | 441 (77.5%) | 100 (83.3%) | ||||
| Myocarditis | 29 (13.6%) | 97(17.0%) | 9 (7.5%) | ||||
| Familial | 11 (5.1%) | 31 (5.4%) | 11 (9.2%) | ||||
| Family History of Cardiomyopathy* | 27 (21.1%) | 80 (23.5%) | 26 (36.1%) | 0.044 | 0.595 | 0.023 | 0.018 |
| Family History of Sudden Death | 12 (8.9%) | 44 (13.1%) | 13 (17.1%) | 0.207 | |||
| HF at Diagnosis | 171 (79.9%) | 396 (69.7%) | 81 (68.6%) | 0.012 | 0.005 | 0.813 | 0.028 |
| Anti-heart Failure Therapy | 193 (91.0%) | 476 (85.6%) | 99 (83.2%) | 0.072 | |||
Family history is defined in this Registry as any family member with a known cardiomyopathy and captured separately from familial dilated cardiomyopathy, which is defined as genetic cause of cardiomyopathy. SD-standard deviation, BMI-body mass index, FamHx-family history, HF-heart failure.
Pair-wise comparison P values <0.017 are considered statistically significant, applying post hoc Bonferroni correction
Echocardiographic features differed amongst the anthropometric classifications (Table 2). MN patients had larger left ventricular (LV) end-diastolic and systolic dimension z-scores than those with NB (P<0.001). LV fractional shortening z-scores were lower in MN patients (P<0.001); however, there was no difference in LV posterior wall thickness to end diastolic diameter ratio and ejection fraction z-scores. There was also no difference in percentage of patients with mitral regurgitation or left atrial dilatation between the study groups (Table 2).
Table 2.
Echocardiographic Characteristics by Nutrition Group
| Bodyweight | P-Value# | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Characteristic Mean (±SD) or N (%) |
Malnourished (N=214) |
Normal Bodyweight (N=570) |
Obese (N=120) |
Overall |
Malnourished vs. Normal | Normal vs. Obese | Malnourished vs. Obese |
| Left Ventricular Dimensions, mean±SD | |||||||
| LV EDD* z-score | 5.32±2.40 | 4.19±2.53 | 3.44±2.52 | <0.001 | <0.001 | 0.004 | <0.001 |
| LV ESD† z-score | 7.00±2.92 | 5.79±2.80 | 5.02±2.91 | <0.001 | <0.001 | 0.014 | <0.001 |
| LV EDPT‡ z-score | −0.20±3.09 | −0.56±2.45 | −0.92±2.19 | 0.097 | |||
| LV EDST§ z-score | −0.62±3.09 | −0.87±1.81 | −1.12±1.72 | 0.156 | |||
| Ratio of LV EDPT/EDD | 0.14±0.16 | 0.13±0.04 | 0.14±0.05 | 0.660 | |||
| Ratio of LV EDPT/EDD z-score | −1.02±7.55 | −1.35±2.35 | −1.15±2.32 | 0.685 | |||
| Functional Assessment | |||||||
| LV | −9.29±4.15 | −8.01±3.77 | −7.64±3.39 | <0.001 | <0.001 | 0.363 | <0.001 |
| Fractional Shortening z-score | |||||||
| Mitral Regurgitation | 70 (79.5%) | 146 (68.9%) | 25(73.5%) | 0.169 | |||
| Left Atrial Dilatation | 38 (43.7%) | 66 (31.4%) | 10(30.3%) | 0.113 | |||
LV-EDD- left ventricle end diastolic dimension
LV-ESD- left ventricle end systolic dimension
LV-EDPT- left ventricle end diastolic posterior wall thickness
LV-EDST- left ventricle end diastolic septal thickness
Pair-wise comparison P values <0.017 are considered statistically significant, applying post hoc Bonferroni correction
Logarithmic test from Kaplan-Meier analysis demonstrated that there was decreased survival in both MN and obese patients compared to NB (84.7% transplant-free survival in MN patients and 86.2% in obese patients), compared to 91.8% in NB patients at two years post-diagnosis (P=0.01 and P=0.02, respectively) (Table 3, Figure 1A) with a corresponding increase in event rate (56.3 and 64.5 events/1000 person-years respectively compared to 32.2 in NB participants). There was an increased risk of death or transplant as a composite endpoint in obese vs. NB patients (55.9% 2-year transplant free survival and 248 events/1000 person-years) in obese patients compared to 67.4% and 132.8 events/1000 person-years in NB; p=0.016 (Table 3, Figure 1B), though no difference was seen in MN vs. NB (p=0.191). Conversely, in competing risk analysis, there was increased mortality in MN patients compared to NB (p=0.030), but not between obese and NB (p=0.223). The cumulative incidence rate of death in this analysis was 19.1% in MN patients, significantly higher than the 13.0% in NB patients; however, there was no difference between the groups in rate of transplantation between any of the nutritional groups (p=0.159) (Figure 2).
Table 3.
Hazard Ratios1 Modeling Time to Death, Time to Transplant, and Time to Death or Transplant by Nutritional Group2, HR (95%CI)
| Normal | Malnourished | Obese | p-value | |
|---|---|---|---|---|
| Death | 1.00 (referent) | 2.06 (1.17, 3.65) | 1.49 (0.72, 3.08) | 0.042 |
| Death or Transplant | 1.00 (referent) | 1.18 (0.84, 1.67) | 1.03 (0.69, 1.54) | 0.630 |
| Transplant | 1.00 (referent) | 0.89 (0.57, 1.38) | 0.87 (0.54, 1.40) | 0.758 |
Figure 1A.
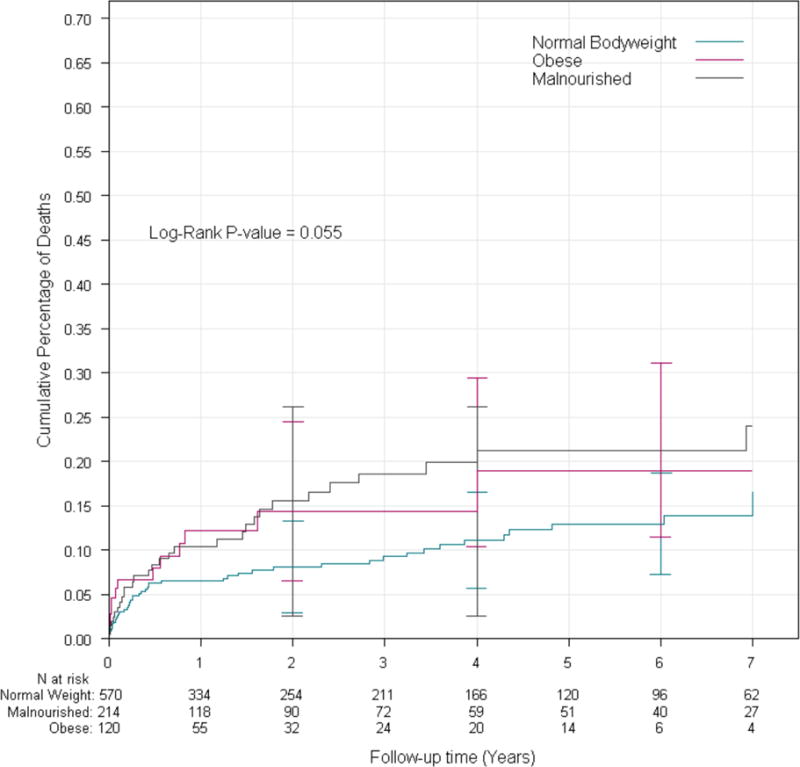
Cumulative incidence of death stratified by nutrition group.
Figure 1B.
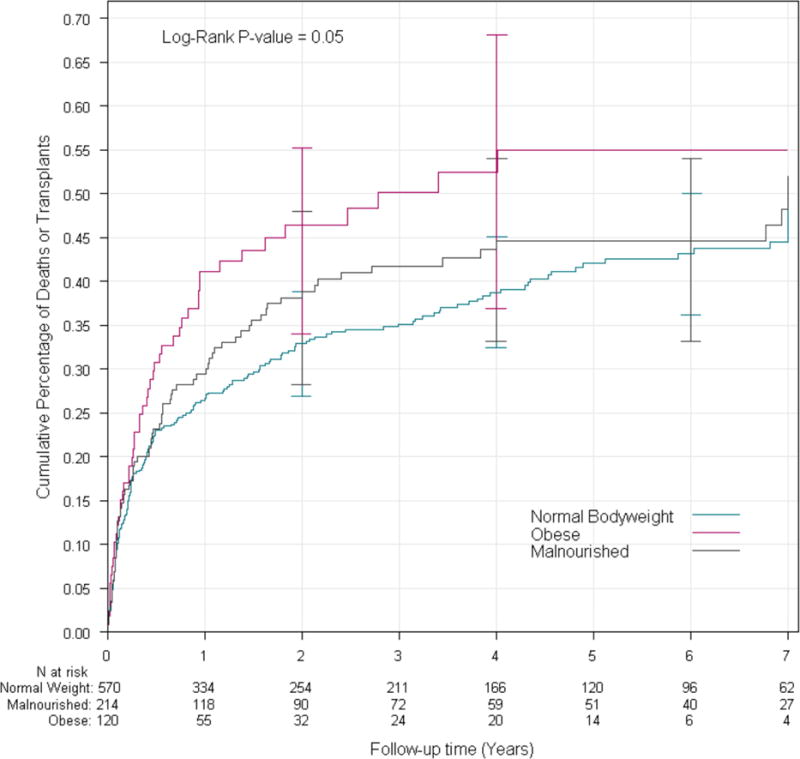
Cumulative incidence of death or transplant stratified by nutrition group. MN = malnourished; NB = normal bodyweight
Figure 2.
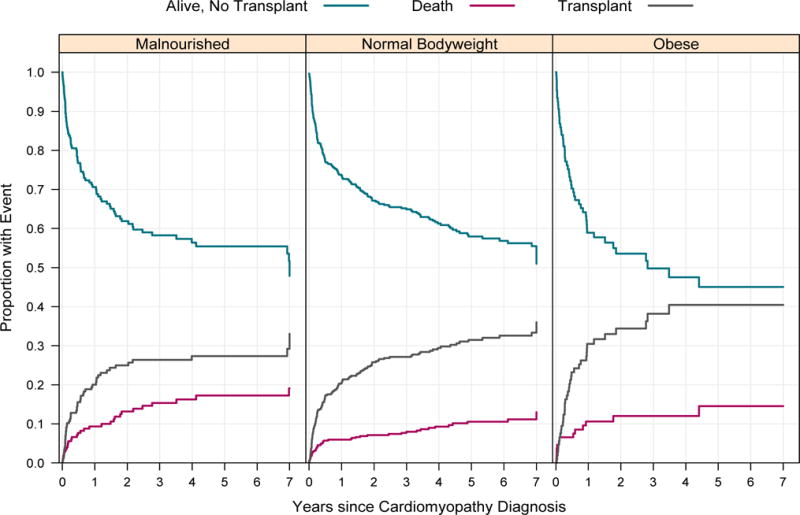
Competing outcomes analysis for patients by nutrition group for years 1 through 7. There was increased risk of mortality in MN patients compared to NB (p=0.030), however, no difference between obese and NB (p=0.223). There were no significant differences between groups in transplant rate (p=0.401).
In multivariable model derived from univariate analysis, there was an increased risk for death in MN patients compared to NB patients (2.06 hazard ratio (HR), 95% CI: 1.17, 3.65; p=0.042). When risk for death or transplant was analyzed, MN was not associated with greater risk for death or transplant compared to NB patients. Likewise, there was no increased risk of listing for transplant in the MN patients. There was also no increased risk of death, death or transplant, or transplant alone in obese patients compared to those who were NB (p>0.05 for all) (Table 3).
Sub-analysis was performed to determine the impact of MN and obesity on survival, stratified by age (< 1 year, 1-10 years, and >10 years). There was no difference in transplant-free survival between patients of different nutritional status based on age (p>0.05 for all) (Figure 3).
Figure 3.
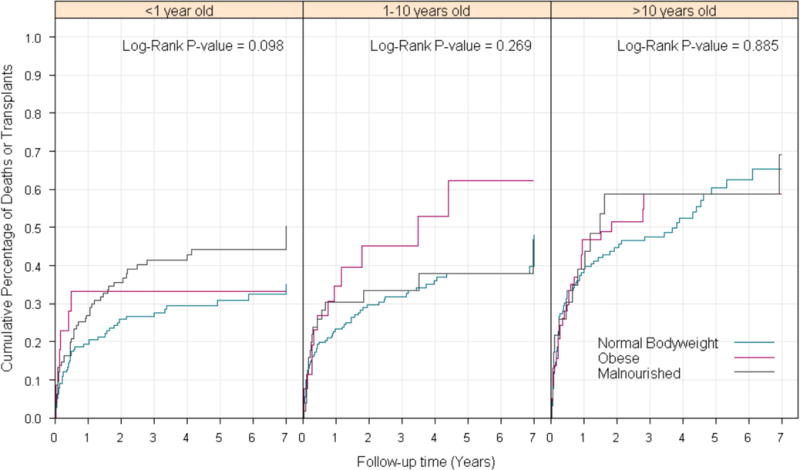
Cumulative incidence of death or transplant or last date of contact by nutrition group stratified by age group. Values are right-censored at seven years.
Number of patients in each category: <1 year old: Total=345; Malnourished =131: Normal Bodyweight =187; Obese =27; 1-10 years old: Total =300; Malnourished =58; Normal Bodyweight= 214; Obese =28; >10 years old: Total =259; Malnourished =25; Normal Bodyweight = 169; Obese =65.
DISCUSSION
In this study from one of the largest registries in pediatric cardiomyopathy with data collection from over 98 pediatric cardiac centers in the United States and Canada that includes patients from the time of diagnosis of DCM, we found that MN was a significant risk factor for death after diagnosis of DCM. Additionally, there was decreased overall survival in obese pediatric patients, though obesity was not associated with increased mortality in multivariable analysis.
Our findings of higher mortality in pediatric DCM with MN have been noted in other pediatric heart failure cohorts. Malnutrition has been associated with increased mortality in pediatric patients listed for transplant.17–20 The higher mortality seen in this population is believed to be secondary to an increase in neurohormonal and inflammatory activation that leads to further increases in metabolic demands while tolerance of feeds diminishes due to gut perfusion.2 Additionally, heart failure leads to maladaptive gastrointestinal responses which produce decreased appetite, poor absorption of nutrients, and increased protein catabolism.20 These patients have higher rates of emergency room visits and hospitalizations, hospital morbidities, and all-cause mortality.21–23
Though malnutrition leads to adverse outcomes in pediatric patients both before and after transplant, it has not been an established risk factor in pediatric patients prior to listing for transplant.17,18 We saw that like patients with end stage heart disease, malnutrition remained a significant risk factor for mortality in all comers with DCM. This suggests that early recognition of malnutrition in this patient population could potentiate outcomes and should be a focus of future study.
Obesity is associated with heart failure in both the adult and pediatric general populations.5, 6 This is believed to be secondary to an obesity-related increased heart rate and stroke volume that leads to left ventricular concentric remodeling (normal left ventricular mass with elevated mass-to-volume ratio), and subsequently increased LV mass and diastolic dysfunction.24–26 This can progress to LV systolic dysfunction and dilation.26 Length of time with obesity is associated with lower LV systolic function and greater diastolic dysfunction.27,28 These effects are independent of hypertension or other cardiovascular risk factors.29
The impact of obesity on outcomes in patients with heart failure is not well understood. Several large studies have demonstrated that obese patients do better than those with normal weight including decreased mortality and hospitalizations, leading to the theory of the existence of an “obesity paradox.”30,31 This has been confirmed in the meta-analysis by Sharma et al where MN was associated with increased mortality; however, obesity was associated with lower risk of hospitalization and cardiovascular mortality.30 Mechanisms postulated that are believed to be associated with this difference in outcome include greater metabolic reserve, protective cytokines, attenuated response to renin-angiotensin-aldosterone system, or different cause of heart failure among others.30 Recent data, however have challenged the link between obesity and decreased mortality, suggesting that it is explained by a confounding bias within the population.11 Additionally, these patients may be more closely monitored for cardiac complications and have incidental ventricular dilation from obesity related adaptive changes.6 Thus clinical symptoms related to obesity are often unrelated to cardiac causes.32–34 Gustafesson found that heart failure patients with a preserved LV ejection fraction demonstrated better survival and that outcome was worse in patients with obesity and depressed LV systolic function compared to patients with normal anthropometric measurements.35 Although we found that there was decreased event-free survival in obese patients, obesity itself was not a risk factor for death in this pediatric cardiomyopathy population as a whole, including symptomatic and asymptomatic individuals.
Malnutrition and obesity are both significant considerations in transplant suitability given the increased risk of post-transplant morbidity and mortality.36 Because of the impact of obesity in particular on morbidity post-transplant, current International Society for Heart and Lung Transplantation recommendations for adult candidates are to delay listing for transplantation until patients reach a BMI <35 kg/m37 Neither obesity nor malnutrition was found to impact frequency of transplantation in our pediatric cohort.
The impact of malnutrition or obesity may not be the same across all age ranges. For instance, infants with malnutrition may have worse outcomes given their limited reserve.20 Likewise, obesity may have a greater impact in older patients, especially adolescents, given that the chronicity of obesity has been shown to impact outcomes.38 Though there were no differences seen in the age subgroups, there was a trend towards worse survival over time for both malnutrition and obesity across all age groups.
Limitations
Though the patient cohort studied was large, one significant limitation was the amount of missing height and weight data in the population. These patients were also sicker (Appendix B), possibly reflecting the difficulty obtaining accurate height and weight measurements in patients who present in extremis. Also, body composition, including lean or fat mass, as estimates of nutritional status was not collected in the registry and body surface normalization through the use of standardized z-scores for echocardiographic measurements may be misleading at the extremes of the weight spectrums. Information regarding body composition, especially for patients meeting criteria for malnutrition, is largely unknown and not part of the registry. Weight information was collected prospectively at the time of enrollment; however, detailed information regarding presence or absence of edema is also unknown. Information on clinical symptoms, including New York Heart Association and Ross classification of heart failure, was available for too few patients to allow inclusion of symptomatic status in the analysis.
CONCLUSIONS
Malnutrition is associated with an increased risk of death after the diagnosis of DCM. Additionally, obesity was not protective in this population as it is in adults; and there was, in fact, decreased overall survival in the obese population compared to the NB. However, obesity itself was not an explanatory factor. Neither malnutrition nor obesity was associated with a significant difference in rate of transplantation compared to the normal body weight. Prospective studies of nutritional interventions are needed to understand influence of nutritional status on clinical outcomes in children with dilated cardiomyopathy.
Supplementary Material
CLINICAL PERSPECTIVES.
Our study has significant implications for both the pediatric and population. Ongoing emphasis on improving nutrition in patients with dilated cardiomyopathy is important as it has significant prognostic implications, thus providing an avenue to improve clinical outcomes. Likewise, obesity should be taken seriously in this patient population, as there was no association with decreased morbidity. Lifestyle modification in these young patients is most likely an avenue for improving outcomes, as this factor is also potentially modifiable.
TRANSLATIONAL OUTLOOK.
Future studies are needed to understand the impact of standard nutritional interventions addressing malnutrition in pediatric dilated cardiomyopathy. Additionally, understanding the impact of lifestyle modification on pediatric dilated cardiomyopathy patients who are obese may be helpful in management of these patients as well possibly contributing to a better understanding of the obesity paradox in adult heart failure.
Acknowledgments
We thank the participating centers for subject recruitment and follow-up data collection. We also thank the Children’s Cardiomyopathy Foundation for their ongoing support of the Pediatric Cardiomyopathy Registry’s research efforts.
Funding Sources
Supported by grants from the National Heart, Lung, and Blood Institute NHLBI HL 53392 and the Children’s Cardiomyopathy Foundation (CCF). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NHLBI or CCF.
ABBREVIATIONS
- DCM
dilated cardiomyopathy
- MN
malnourished
- BMI
body mass index
- NB
normal bodyweight
- PCMR
pediatric cardiomyopathy registry
- EDD
end-diastolic dimension
- LVFS
left ventricular fractional shortening
- LV
left ventricular
- HR
hazard ratio
- HR
heart rate
- NHLBI
national heart lung and blood institute
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None
References
- 1.Avitzur Y, Singer P, Dagan O, Kozer E, Abramovitch D, Dinari G, Shamir R. Resting energy expenditure in children with cyanotic and noncyanotic congenital heart disease before and after open heart surgery. J Parenter Enteral Nutr. 2003;27:47–51. doi: 10.1177/014860710302700147. [DOI] [PubMed] [Google Scholar]
- 2.Bannister L, Manlhiot C, Pollock-BarZiv S, Stone T, McCrindle BW, Dipchand AI. Anthropometric growth and utilization of enteral feeding support in pediatric heart transplant recipients. Pediatr Transplant. 2010;14:879–886. doi: 10.1111/j.1399-3046.2010.01361.x. [DOI] [PubMed] [Google Scholar]
- 3.Davies RR, Haldeman S, McCulloch MA, Gidding SS, Pizarro C. Low body mass index is associated with increased waitlist mortality among children listed for heart transplant. J Heart Lung Transplant. 2015;34:1462–1470. doi: 10.1016/j.healun.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 4.Castleberry C, White-Williams C, Naftel D, et al. Hypoalbuminemia and poor growth predict worse outcomes in pediatric heart transplant recipients. Pediatr Transplant. 2014;18:280–7. doi: 10.1111/petr.12239. [DOI] [PubMed] [Google Scholar]
- 5.Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 6.Chinali M, de Simone G, Roman MJ, et al. Impact of obesity on cardiac geometry and function in a population of adolescents: the Strong Heart Study. J Am Coll Cardiol. 2006;47:2267–2273. doi: 10.1016/j.jacc.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Grady KL, White-Williams C, Naftel D, et al. Are preoperative obesity and cachexia risk factors for post heart transplant morbidity and mortality: a multi-institutional study of preoperative weight-height indices. Cardiac Transplant Research Database (CTRD) Group. J Heart Lung Transplant. 1999;18:750–763. doi: 10.1016/s1053-2498(99)00035-2. [DOI] [PubMed] [Google Scholar]
- 8.Kobashigawa JA, Starling RC, Mehra MR, et al. Multicenter retrospective analysis of cardiovascular risk factors affecting long-term outcome of de novo cardiac transplant recipients. J Heart Lung Transplant. 2006;25:1063–9. doi: 10.1016/j.healun.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Clark AL, Chyu J, Horwich TB. The obesity paradox in men versus women with systolic heart failure. Am J Cardiol. 2012;110:77–82. doi: 10.1016/j.amjcard.2012.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fonarow GC, Srikanthan P, Costanzo MR, Cintron GB, Lopatin M, ADHERE Scientific Advisory Committee and Investigators An obesity paradox in acute heart failure: analysis of body mass index and inhospital mortality for 108,927 patients in the Acute Decompensated Heart Failure National Registry. Am Heart J. 2007;153:74–81. doi: 10.1016/j.ahj.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Banack HR, Kaufman JS. Does selection bias explain the obesity paradox among individuals with cardiovascular disease? Ann Epidemiol. 2015;25:342–349. doi: 10.1016/j.annepidem.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 12.Li C, Ford ES, McGuire LC, Mokdad AH. Increasing trends in waist circumference and abdominal obesity among US adults. Obesity (Silver Spring) 2007;15:216–224. doi: 10.1038/oby.2007.505. [DOI] [PubMed] [Google Scholar]
- 13.Grenier MA, Osganian SK, Cox GF, et al. Design and implementation of the North American Pediatric Cardiomyopathy Registry. Am Heart J. 2000;139:S86–95. doi: 10.1067/mhj.2000.103933. [DOI] [PubMed] [Google Scholar]
- 14.Lipshultz SE, Sleeper LA, Towbin JA, et al. The incidence of pediatric cardiomyopathy in two regions of the United States. N Engl J Med. 2003;348:1647–1655. doi: 10.1056/NEJMoa021715. [DOI] [PubMed] [Google Scholar]
- 15.Colan SD, Parness IA, Spevak PJ, Sanders SP. Deverlopmental modulation of myocardial mechanics:age- and growth-related alterations in afterload and contractility. J Am Coll Cardiol. 1992;19:619–629. doi: 10.1016/s0735-1097(10)80282-7. [DOI] [PubMed] [Google Scholar]
- 16.Sluysmans T, Colan SD. Theoretical and empirical derivation of cardiovascular allometric relationships in children. J Appl Physiol (1985) 2005;99:445–457. doi: 10.1152/japplphysiol.01144.2004. [DOI] [PubMed] [Google Scholar]
- 17.Kaufman BD, Nagle ML, Levine SR, et al. Too fat or too thin? Body habitus assessment in children listed for heart transplant and impact on outcome. J Heart Lung Transplant. 2008;27:508–513. doi: 10.1016/j.healun.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 18.Rossano JW, Grenier MA, Dreyer WJ, et al. Effect of body mass index on outcome in pediatric heart transplant patients. J Heart Lung Transplant. 2007;26:718–723. doi: 10.1016/j.healun.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Godown J, Donohue JE, Yu S, Friedland-Little JM, Gajarski RJ, Schumacher KR. Differential effect of body mass index on pediatric heart transplant outcomes based on diagnosis. Pediatr Transplant. 2014;18:771–776. doi: 10.1111/petr.12352. [DOI] [PubMed] [Google Scholar]
- 20.Godown J, Friedland-Little JM, Gajarski RJ, Yu S, Donohue JE, Schumacher KR. Abnormal nutrition affects waitlist mortality in infants awaiting heart transplant. J Heart Lung Transplant. 2014;33:235–240. doi: 10.1016/j.healun.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 21.Pureza V, Florea VG. Mechanisms for cachexia in heart failure. Curr Heart Fail Rep. 2013;10:307–314. doi: 10.1007/s11897-013-0153-9. [DOI] [PubMed] [Google Scholar]
- 22.McNallan SM, Singh M, Chamberlain AM, et al. Frailty and healthcare utilization among patients with heart failure in the community. JACC Heart Fail. 2013;1:135–141. doi: 10.1016/j.jchf.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kenchaiah S, Pocock SJ, Wang D, et al. Body mass index and prognosis in patients with chronic heart failure: insights from the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation. 2007;116:627–636. doi: 10.1161/CIRCULATIONAHA.106.679779. [DOI] [PubMed] [Google Scholar]
- 24.Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Physiol Rev. 2008;88:389–419. doi: 10.1152/physrev.00017.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aljaroudi W, Halley C, Houghtaling P, et al. Impact of body mass index on diastolic function in patients with normal left ventricular ejection fraction. Nutr Diabetes. 2012;2:e39. doi: 10.1038/nutd.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Batalli-Kepuska A, Bajraktari G, Zejnullahu M, et al. Abnormal systolic and diastolic myocardial function in obese asymptomatic adolescents. Int J Cardiol. 2013;168:2347–2351. doi: 10.1016/j.ijcard.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 27.Messerli FH. Cardiopathy of obesity a not-so-Victorian disease. N Engl J Med. 1986;314:378–380. doi: 10.1056/NEJM198602063140608. [DOI] [PubMed] [Google Scholar]
- 28.Ku CS, Lin SL, Wang DJ, Chang SK, Lee WJ. Left ventricular filling in young normotensive obese adults. Am J Cardiol. 1994;73:613–615. doi: 10.1016/0002-9149(94)90347-6. [DOI] [PubMed] [Google Scholar]
- 29.Alpert MA. Obesity cardiomyopathy: pathophysiology and evolution of the clinical syndrome. Am J Med Sci. 2001;321:225–236. doi: 10.1097/00000441-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Sharma A, Lavie CJ, Borer JS, et al. Meta-analysis of the relation of body mass index to all-cause and cardiovascular mortality and hospitalization in patients with chronic heart failure. Am J Cardiol. 2015;115:1428–1434. doi: 10.1016/j.amjcard.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 31.Lavie CJ, Osman AE, Milani RV, Mehra MR. Body composition and prognosis in chronic systolic heart failure: the obesity paradox. Am J Cardiol. 2003;91:891–894. doi: 10.1016/s0002-9149(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 32.Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Woo MA, Tillisch JH. The relationship between obesity and mortality in patients with heart failure. J Am Coll Cardiol. 2001;38:789–795. doi: 10.1016/s0735-1097(01)01448-6. [DOI] [PubMed] [Google Scholar]
- 33.Caruana L, Petrie MC, Davie AP, McMurray JJ. Do patients with suspected heart failure and preserved left ventricular systolic function suffer from “diastolic heart failure” or from misdiagnosis? A prospective descriptive study. BMJ. 2000;321:215–218. doi: 10.1136/bmj.321.7255.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Habbu A, Lakkis NM, Dokainish H. The obesity paradox: fact or fiction? Am J Cardiol. 2006;98:944–948. doi: 10.1016/j.amjcard.2006.04.039. [DOI] [PubMed] [Google Scholar]
- 35.Gustafsson F, Kragelund CB, Torp-Pedersen C, et al. Effect of obesity and being overweight on long-term mortality in congestive heart failure: influence of left ventricular systolic function. Eur Heart J. 2005;26:58–64. doi: 10.1093/eurheartj/ehi022. [DOI] [PubMed] [Google Scholar]
- 36.Russo MJ, Hong KN, Davies RR, et al. The effect of body mass index on survival following heart transplantation: do outcomes support consensus guidelines? Ann Surg. 2010;251:144–152. doi: 10.1097/SLA.0b013e3181b5db3c. [DOI] [PubMed] [Google Scholar]
- 37.Mehra MR, Canter CE, Hannan MM, et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: A 10-year update. J Heart Lung Transplant. 2016;35:1–23. doi: 10.1016/j.healun.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 38.Charakida M, Khan T, Johnson W, et al. Lancet Diabetes Endocrinol. 2014;2(8):648–654. doi: 10.1016/S2213-8587(14)70103-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


