Abstract
Context
Attrition is common in longitudinal observational studies in palliative care. Few studies have examined predictors of attrition.
Objectives
To identify patient characteristics at enrollment associated with attrition in palliative oncology outpatient setting.
Methods
In this longitudinal observational study, advanced cancer patients [ACP] enrolled in an outpatient multicenter study were assessed at baseline and 2–5 weeks later. We compared baseline characteristics between patients who returned for follow-up and those who dropped out.
Results
744 patients were enrolled from Jordan, Brazil, Chile, Korea and India. Attrition rate was 33%, with variation among countries (22%–39%; p=.023). In univariate analysis, baseline predictors for attrition were cognitive failure (odds ratio [OR] 1.23 per point in Memorial Delirium Assessment Scale; p<.01), functional status (OR 1.55 per 10 point decrease in Karnofsky Performance Status; p<.01), Edmonton Symptom Assessment System [ESAS] physical score (OR 1.03 per point; p<.01), ESAS emotional score (OR 1.05 per point; p<.01) and shorter duration between cancer diagnosis and palliative care referral in months (OR .89 per log; p=.028). In multivariate analysis, cognitive failure (OR 1.12 per point; p=.007), ESAS physical score (OR 1.18 per point; p=.027), functional status (OR 1.35 per 10 point decrease; p<.001) and shorter duration from cancer diagnosis (OR .86 per log; p=.01) remained independent predictors of attrition.
Conclusion
ACP with cognitive failure, increased physical symptoms, poorer performance status and shorter duration from cancer diagnosis were more likely to dropout. These results have implications for research design, patient selection and data interpretation in longitudinal observational studies.
Keywords: Palliative care, longitudinal study, outpatient, oncology, attrition
INTRODUCTION
Palliative oncology is a relatively new discipline whose aim is to alleviate patient’s suffering due to cancer (1). Palliative oncology patients experience symptoms and functional decline throughout the course of their disease but particularly, during end-of- life. Longitudinal observational studies in this field describing a variety of phenomena have been published during the last few decades (2–4). However, in this particular type of studies, as no interventions are implemented, we need motivated subjects who are able to continue to participate throughout the study in a context of expected clinical decline. Therefore, there are several methodological challenges in performing these studies, such as patient recruitment, attrition and missing data that need to be addressed in order to maintain high quality standards (5).
Attrition is a common problem in longitudinal observational studies in palliative oncology. There are no systematic reviews of attrition rates in palliative oncology, but reports have documented variability in this population with rates up to 60% (6–8). Factors for attrition have been identified in palliative oncology clinical trials and include death, patient illness, symptom severity, impaired mental status, functional status, increased age, being from a minority group, low socioeconomic status, longer study duration, patient preferences, among others (9–11). Prospective observational studies contribute important knowledge to the developing field of palliative care (4, 12, 13). Although previous studies have identified factors associated with attrition in supportive/palliative oncology trials (8), few studies have specifically examined the rate and predictors of dropout in longitudinal studies in the palliative care setting.
Identification of patient factors associated with attrition in palliative care observational longitudinal studies is important to better estimate sample size, improve patient selection and maximize patient retention over time. Addressing these methodological challenges may be useful in refining study design, interpretation of results and generalizability of the findings. The aim of this research project is to identify patient characteristics at enrollment associated with attrition in an international multisite longitudinal observational study involving patients with advanced cancer.
METHODS
Patients
This is a secondary analysis of an international multisite longitudinal observational study designed to examine novel treatment response criteria for the Edmonton Symptom Assessment Scale (ESAS), one of the most commonly used symptom assessment batteries in oncology and palliative care (14). This study’s primary objective was to identify the minimal clinically important difference for ESAS (15–17). Briefly, we enrolled patients with the diagnosis of advanced cancer who were aged 18 years or older, seen at an outpatient clinic at one of six participating centers (King Hussein Cancer Center in Jordan; Barretos Cancer Hospital in Brazil; Pontificia Universidad Catolica de Chile in Chile; Kangdong Sacred Heart Hospital in Korea; Tata Memorial Center in India; and MD Anderson Cancer Center in USA), and had a scheduled clinic visit 14–34 days after the first study visit. Patients with delirium were excluded. All institutions were tertiary care hospitals with access to cancer care treatment and concurrent supportive care. The study protocol was approved by the institutional review boards at all participating centers. All participants provided written informed consent.
The study was conducted between December 8, 2011 and April 30, 2014. Baseline patient characteristics were collected during the first study visit, including the CAGE questionnaire and the Memorial Delirium Assessment Scale (MDAS) to assess delirium. The ESAS and the Karnofsky performance status (KPS) were assessed both during the first and second visits and the Patient’s Global Impression (PGI) scale during the second visit. We then used the PGI scale as the external criterion against which the ESAS scores were anchored and calibrated. All participants were enrolled at palliative care outpatient clinic consultation with the exception of Korean patients who were enrolled from oncology clinics. For the analysis of this current study, we excluded patients from the USA because recruitment was performed at the follow-up visit and baseline data at consultation were retrieved retrospectively (16).
Data Collection
Baseline patient characteristics, including age, sex, race, educational level, cancer diagnosis, KPS, CAGE questionnaire (18), MDAS (19), and ESAS, were collected during the first study visit. The CAGE questionnaire consists of 4 questions to assess feelings and attitudes regarding alcohol consumption (Cut down, Annoyed, Guilt, and Eye opener), and has been widely used for alcoholism detection (more than 2 positive responses) (18). The MDAS is a validated 10-item instrument for delirium assessment. The total score ranges from 0 to 30 points, with a score >13 suggesting the presence of delirium (19). The ESAS is a validated symptom assessment instrument that uses a numeric rating scale of 0 (no symptoms) to 10 (worst intensity) to examine the mean intensity of 10 symptoms (ie., pain, fatigue, nausea, depression, anxiety, drowsiness, shortness of breath, lack of appetite, and feelings of well-being, and sleep) over the past 24 hours (20, 21) It has been psychometrically and linguistically validated and is available in the languages of the respective countries in the current study (ie, English, Arabic, Portuguese, Spanish, and Hindi) (22–26). Symptom burden can be estimated with the ESAS using the physical and the emotional scores (27). The physical score ranges between 0 and 60, and represents the sum of ESAS pain, fatigue, nausea, drowsiness, dyspnea, and loss of appetite. The emotional score ranges between 0 and 20 and represents the sum of ESAS anxiety and depression. Higher scores indicate higher symptom burden.
The study questions were translated into the local languages to facilitate data collection and back-translated to ensure accuracy of translation. The site investigators visited Houston to understand the data collection process, and provided training to the local research staff. Teleconferences were held between the principal investigator and each site investigator twice each month to provide data monitoring.
Statistical considerations
We summarized our data using descriptive statistics. Attrition rate was defined as the proportion of patients who did not return for a follow-up visit among all enrolled patients. We calculated the attrition rate for the whole population and for each country. We also registered the cause for attrition, including attrition due to death, due to illness and at random, following international recommendations (6).
We compared baseline characteristics among patients who returned for follow-up visit and those who dropped out from the study using chi-square statistics and t tests for categorical and/or continuous variables respectively. For each baseline characteristic, we performed a univariate analysis estimating the OR (95% CI) for dropping out the study. We also compared baseline characteristics among patients with different reasons for attrition using an ANOVA test, Kruskal-Wallis test or chi-square test depending on the type of variable. A Bonferoni correction was used to specifically identify the direction of the differences among the groups. We further examined baseline characteristics associated with dropping out from the study using multivariate logistic regression analysis. We performed both forward stepwise and backward stepwise selection strategies to define our multivariate logistic regression model with a p-value < 0.1 to either include or remove variables from it. We then compared the obtained model with the full model using a likelihood ratio test. Stata 13.1 was used to perform the statistical analysis. A p-value <0.05 was considered statistically significant.
RESULTS
Patient characteristics
744 patients with advanced cancer were enrolled from the 5 study sites. Demographic characteristics are detailed in Table 1. Briefly, the mean age was 57 years, 47% were females and the most common cancers were gastrointestinal (GI), breast and respiratory. The median (interquartile range [IQR]) time from diagnosis to palliative care referral was 18 (7–46) months. The most intense symptoms at baseline were pain, fatigue, lack of appetite and lack of wellbeing. Most of the patients had a Karnofsky Performance Status (KPS) of 70% or higher.
Table 1.
Demographic characteristics of the population and by dropping out from the study (not attending to the follow-up visit) Univariate analysis
| Dropped out N = 744 |
|||||
|---|---|---|---|---|---|
|
| |||||
| Variable | Total N = 744 N(%) |
Yes N = 248 N (%) |
No N = 496 N (%) |
OR (95%CI) Unadjusted |
p-value |
|
| |||||
| Age (mean; SD) | 57; 13 | 57.8 | 56.9 | 1.006 (.99–1.02) | .40 |
|
| |||||
| Gender | |||||
| Female | 347 (47) | 111 (45) | 236 (48) | .89 (.68–1.21) | .47 |
|
| |||||
| Site | .02 | ||||
| Jordan 39% | 298 (40) | 116 (47) | 182 (37) | --------- | |
| Brazil 29% | 184 (25) | 53 (21) | 131 (26) | .63 (.43–.94) | .02 |
| Chile 33% | 106 (14) | 35 (14) | 71 (14) | .77 (.48–1.23) | .28 |
| Korea 22% | 87 (12) | 19 (8) | 68 (14) | .43 (.25–.77) | .004 |
| India 36% | 69 (9) | 25 (10) | 44 (9) | .89 (.52–1.53) | .68 |
|
| |||||
| Ethnicity | .03 | ||||
| Hispanic | 290 (39) | 88 (35) | 202 (41) | --------- | |
| Asian | 156 (21) | 44 (18) | 112 (22) | .90 (.59–1.39) | .64 |
| Other | 298 (40) | 116 (47) | 182 (37) | 1.46 (1.04–2.06) | .03 |
|
| |||||
| Marital Status | .49 | ||||
| Single | 83 (11) | 25 (10) | 58 (12) | --------- | |
| Married | 530 (71) | 174 (70) | 356 (72) | 1.13 (.69–1.87) | .62 |
| Divorced/Widowed | 131 (18) | 49 (20) | 82 (16) | 1.39 (.77–2.49) | .28 |
|
| |||||
| Cancer type | .05 | ||||
| GI | 193 (26) | 71 (29) | 122 (25) | --------- | |
| Breast | 128 (17) | 42 (17) | 86 (17) | .84 (.52–1.34) | .47 |
| Respiratory | 121 (16) | 45 (18) | 76 (15) | 1.02 (.64–1.63) | .94 |
| GU | 65 (9) | 11 (4) | 54 (11) | .35 (.17–.71) | .004 |
| H&N | 63 (9) | 26 (10) | 37 (7) | 1.21 (.68–2.16) | .53 |
| GYN | 62 (8) | 17 (7) | 45 (9) | .65 (.35–1.22) | .18 |
| Hematological | 40 (5) | 11 (4) | 29 (6) | .65 (.31–1.38) | .27 |
| Other | 72 (10) | 25 (10) | 47 (9) | .91 (.52–1.61) | .76 |
|
| |||||
| Stage | .16 | ||||
| Advanced | 24 (3) | 12 (5) | 12 (2) | --------- | |
| Locally advanced | 70 (10) | 20 (8) | 50 (10) | .4 (.15–1.04) | .06 |
| Metastatic | 650 (87) | 216 (87) | 434 (88) | .5 (.22–1.13) | .09 |
|
| |||||
| MDAS (median; IQR)* | 2;(1–4) | 3; (2–4) | 2;(1–3) | 1.23 (1.14–1.33) | < .001 |
|
| |||||
| CAGE | .98 | ||||
| 0 | 569 (77) | 190 (76) | 379 (76) | --------- | |
| 1 | 74 (10) | 24 (10) | 50 (10) | .96 (.57–1.61) | .87 |
| 2 ≤ | 101 (13) | 34 (14) | 67 (14) | 1.01 (.65–1.58) | .96 |
|
| |||||
| ESAS (median; IQR)* | |||||
| Pain (n=743) | 5; (2–7) | 5; (3–8) | 5;(2–7) | 1.07 (1.02–1.12) | .009 |
| Fatigue | 5; (3–7.5) | 6; (4–8) | 5;(2–7) | 1.16 (1.10–1.22) | <.001 |
| Nausea | 0; (0–3) | 0; (0–4) | 0;(0–3) | 1.09 (1.03–1.15) | 003 |
| Depression | 2; (0–5) | 3;(0–6) | 1;(0–5) | 1.11 (.85–.95) | <.001 |
| Anxiety | 3; (0–6) | 3.5;(0–7) | 3;(0–6) | 1.05 (1.00–1.10) | .036 |
| Drowsiness | 4; (0–6) | 4;(1–7) | 3;(0–5) | 1.09 (1.04–1.15) | <.001 |
| Anorexia (n=743) | 5; (1–7) | 5;(2–8) | 4;(0–6) | 1.11 (1.06–1.17) | <.001 |
| Wellbeing | 5; (3–7) | 5;(4–8) | 5;(2–6) | 1.17 (1.11–1.24) | <.001 |
| Dyspnea | 1; (0–5) | 2;(0–6) | 0;(0–5) | 1.07 (1.02–1.12) | .007 |
| Sleep | 4; (0–6) | 4;(1–7) | 3;(0–6) | 1.06 (1.01–1.11) | .026 |
|
| |||||
| ESAS physical score (mean; SD) (n = 742) | 22; 12 | 25; 11 | 20; 12 | 1.04 (1.02–1.05) | <.001 |
|
| |||||
| ESAS emotional score (mean; SD) | 6; 6 | 7; 6 | 6; 6 | 1.05 (1.02–1.07) | .001 |
|
| |||||
| Time from cancer diagnosis in months (median; IQR) (n = 737)^ | 18; (7–46) | 15;(6–34) | 20; (7–52) | .89 (.80–.99) | .028 |
|
| |||||
| Karnofsky Performance Status (n = 737) | <.001 | ||||
| 90 ≤ | 97 (13) | 15 (15) | 82 (85) | ------- | |
| 80 | 175 (24) | 42 (24) | 133 (76) | 1.73 (.90–3.31) | .1 |
| 70 | 206 (28) | 69 (33) | 137 (67) | 2.75 (1.48–5.13) | .001 |
| 60 | 144 (19) | 49 (34) | 95 (66) | 2.82 (1.48–5.40) | .002 |
| ≤ 50 | 115 (16) | 66 (57) | 49 (43) | 7.36 (3.80–14.29) | <.001 |
Karnofsky Performance Status (KPS)
SD = Standard deviation
IQR = Interquartile Range
OR and p-value assumed normal distribution of symptom score
OR and p-value were calculated using log (time from cancer diagnosis)
Attrition rates
248 (33%) patients did not return for the second visit and dropped out of the study, with significant variation among the different countries (Jordan 39%, Brazil 29%, Chile 33%, Korea 22% and India 36%; p=.02; Table 1). Reasons for dropping out were available for 107 subjects who came from Brazil, Chile and Korea. Reasons for dropping out were not available for subjects enrolled in India and Jordan. Reasons for attrition included 29 (27%) patients who died before the follow-up visit, 30 (28%) patients were unable to participate due to a medical condition (e.g. delirium, hospital admission) and 48 (45%) patients did not show up to the second visit. For 141 patients reason for dropping out was not recorded.
Univariate analysis
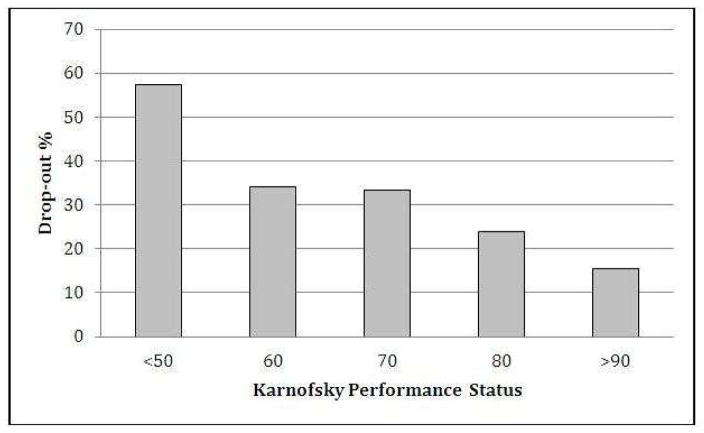
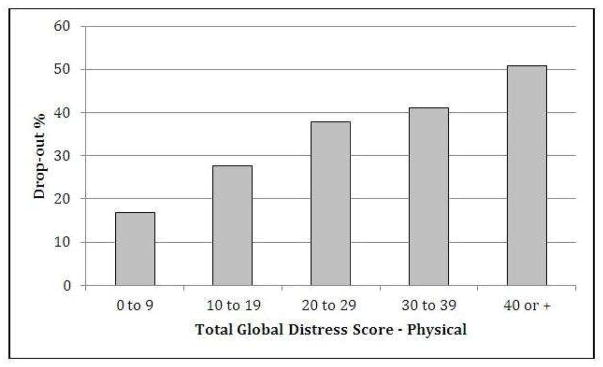
We compared baseline characteristics of patients who dropped out from the study with those who attended to the second visit (Table 1). Baseline predictors for patient attrition were cancer type, MDAS score, ESAS physical score, ESAS emotional score and (log) time from cancer diagnosis to PC referral in months. Also, the attrition rates significantly differed among participating countries (Table 1) with ranges between 22% in Korea and 39% in Jordan. We found that lower KPS was also a predictor of not attending to a second visit considering KPS both as a categorical variable and as a linear variable (Figure 1). We tested whether the categorical KPS variable was nested within the continuous KPS variable and it was. (LR test between the two models: LR chisq: 5.12; p = 0.1629). Therefore we used the KPS as a continuous variable in our multivariate model, as it is more parsimonious and easier to understand. We also observed a gradient effect with higher attrition rates as the physical symptom burden increases (Figure 2).
Figure 1. Association between Karnofsky Performance Status and Attrition.
Considering KPS as a categorical variable, we estimated the odds of dropping out from the study using KPS >90 as baseline. The OR (95% CI; p-value) for each category were: KPS=80: 1.73 (.90–3.31; .1) / KPS=70: 2.75 (1.48–5.13; .001) / KPS=60: 2.82 (1.48–5.40; .002) / KPS<50: 7.36 (3.80–14.29; <.001). We also estimated the odds of dropping out from the study by KPS, assuming that KPS was a continuous variable: OR 1.55 (1.35–1.76;p <.001) per 10 points decrease in the KPS score.
Figure 2.
Drop-out percentage by Global Distress Score – Physical.
We also compared patient characteristics among patients who dropped out according to reasons for attrition recorded (Table 2). We found that there were no differences between groups according to demographic characteristics. However, we found significant differences between the groups in total physical symptom burden, specific symptoms (fatigue, nausea, depression, drowsiness, wellbeing and dyspnea), and KPS. We found that patients with attrition due to death compared to patients with attrition at random had increased overall symptom burden, fatigue, depression, dyspnea and wellbeing and worse KPS. We also found that patients with attrition due to death compared to patients with attrition due to illness had increased fatigue, depression and drowsiness. There were no differences in patient characteristics between patients with attrition due to illness and at random.
Table 2.
Characteristics of patients who dropped-out by reason for attrition
| Variable | Attrition due to death | Attrition due to illness | Attrition at random (loss to follow-up) | p |
|---|---|---|---|---|
| N=29 (27%) | N=30 (28%) | N=48 (45%) | ||
|
| ||||
| Age (mean; SD) | 60 (13) | 62 (13) | 60 (14) | .82† |
|
| ||||
| Gender | .22~ | |||
| Female | 11 (38) | 11 (38) | 26 (54) | |
|
| ||||
| Marital Status | .54~ | |||
| Single | 6 (21) | 4 (13) | 6 (13) | |
| Married | 18 (62) | 19 (64) | 26 (54) | |
| Divorced/Widowed | 5 (17) | 7 (23) | 16 (33) | |
|
| ||||
| Cancer type | .74+ | |||
| GI | 10 (34) | 10 (33) | 18 (38) | |
| Breast | 2 (17) | 3 (17) | 4 (17) | |
| Respiratory | 5 (17) | 4 (13) | 7 (16) | |
| GU | 0 (0) | 3 (10) | 4 (8) | |
| H&N | 5 (17) | 4 (13) | 5 (10) | |
| GYN | 3 (10) | 1 (3) | 7 (15) | |
| Hematological | 1 (3) | 1 (3) | 2 (4) | |
| Other | 3 (10) | 4 (13) | 1 (2) | |
|
| ||||
| MDAS (median; IQR)* | 2;(1–4) | 2; (1–4) | 2,5;(1–4) | .85‡ |
|
| ||||
| ESAS (median; IQR)* | ||||
| Pain (n=743) | 4.5; (.5–8) | 5; (2–7) | 5.5;(3–7) | .30‡ |
| Fatigue | 6; (5–8) | 4; (2–6) | 4.5;(1–6.5) | <.001‡ |
| Nausea | 0; (0–5) | 2.5; (0–5) | 0;(0–3) | .01‡ |
| Depression | 5; (2–7) | 2;(0–5) | 2;(0–4.5) | .01‡ |
| Anxiety | 0; (0–5) | 2;(0–5) | 3;(0–6.5) | .14‡ |
| Drowsiness | 6; (3–8) | 2.5;(0–5) | 3;(0–6.5) | .01‡ |
| Anorexia (n=743) | 5; (3–6) | 5.5;(3–8) | 4.5;(1–7) | .34‡ |
| Wellbeing | 6; (5–8) | 5;(3–7) | 3.5;(1–5) | <.001‡ |
| Dyspnea | 4; (0–7) | 1;(0–4) | 0;(0–4) | .04‡ |
| Sleep | 3; (2–6) | 4;(0–6) | 2.5;(0–4.5) | .18‡ |
|
| ||||
| ESAS physical score (mean; SD) (n = 742) | 28 (12) | 23; (11) | 19 (10) | .003† |
|
| ||||
| ESAS emotional score (mean; SD) | 7 (5) | 5 (5) | 6 (5) | .41† |
|
| ||||
| Time from cancer diagnosis in months (median; IQR) (n = 737) | 12; (4–21) | 16;(8–36) | 17; (5–41) | .19†∫ |
|
| ||||
| KPS (n = 737) | .03~ | |||
| 90≤ | 4 (15) | 0 (0) | 8 (19) | |
| 80 | 1 (4) | 4 (13) | 11 (24) | |
| 70 | 5 (19) | 13 (44) | 10 (22) | |
| 60 | 7 (27) | 7 (23) | 10 (22) | |
| ≤50 | 9 (35) | 6 (20) | 6 (13) | |
Abbreviations: SD: standard deviation. IQR: inter-quartile range; ESAS: Edmonton Symptom Assessment Scale; MDAS: memorial Delirium Assessment Scale; KPS: Karnofsky performance status; GI: gastrointestinal; GU: genitourinary; GYN: gynecological; H&N: head and neck.
Anova
Chi-square
Kruskal Wallis
ischer exact
For variable Time from cancer diagnosis, the comparison was made with log(time) and therefore Anova test statistic was used.
Multivariate analysis
Using both forward and backward stepwise selection strategies we obtained a unique model with four variables that were independently associated with dropping out from the study. Variables included in the final model were MDAS score, ESAS physical score, KPS and log time from cancer diagnosis (Table 3). In the multivariate analysis, no differences in the attrition rates were found between the different participating centers from five different countries. A likelihood ratio test to compare the full model with the four variable model showed no differences between them (p =.405).
Table 3.
Multivariate Analysis. N= 728.
| Variable | OR (95%CI) | p-value |
|---|---|---|
| MDAS* | 1.12 (1.03–1.22) | .007 |
| ESAS physical score* | 1.18 (1.02–1.38) | .027 |
| Log Time from cancer diagnosis in months^ | .86 (.77–.97) | .01 |
| KPS (10 point decrease in KPS) | 1.35 (1.16–1.56) | <.001 |
Abbreviations: OR: odds ratio; ESAS: Edmonton Symptom Assessment Scale; MDAS: memorial Delirium Assessment Scale; KPS: Karnofsky performance status;
OR and p-value assumed normal distribution of symptom score
OR and p-value were calculated using log (time from cancer diagnosis)
DISCUSSION
Advanced cancer patients with cognitive failure, increased physical symptom burden poorer performance status and shorter time from cancer diagnosis were more likely to dropout in this prospective observational study. No differences in the attrition rates were found between the different participating centers from five different countries. These results have implications for research design, patient selection and data interpretation in longitudinal observational palliative care studies.
The attrition rate found in this study is consistent with data observed in prior longitudinal observational studies (7, 8). In these studies, baseline factors associated with attrition included patient symptom intensity, older age, reduced functionality (KPS) and increased help from local authorities.
In our study, the strongest baseline predictor of dropout from the study was performance status. Specifically, patients with KPS ≥90% had a 15% attrition rate, whereas patients with KPS ≤50% had an attrition rate of 57%. Our study clearly demonstrates the linear relationship between worse KPS and increased probability of dropout. As described elsewhere, performance status is an independent factor for clinical deterioration and death (28), which is consistent with the findings of this study. These results suggest that even for studies designed with short periods of clinical observation (between 2 and 5 weeks in this research project) performance status is an important issue to consider as criteria for enrollment.
Similarly to what has been published in other reports, overall physical symptom burden and cognitive failure were also predictors of patient drop-out. These results are relevant regarding the generalizability of findings obtained from longitudinal observational studies as it is likely that patients with these characteristics, or with poorer PS, are underrepresented during follow-up assessments due to attrition. In other words, the population of patients with increased symptom burden, altered mental status or lower functionality at baseline, are less likely to provide information during follow-up, limiting the representativeness of follow-up observations.
Our findings have important implications for palliative care researchers conducting longitudinal observational studies. Because palliative care studies by nature often enroll patients who have a poorer performance status and are more symptomatic, it should be expected that patients have a higher rate of dropout. Our study explains why there are few prospective studies involving patients in the last weeks of life (29) and investigating prevalent symptoms at the end-of-life such as delirium (30). This group of patients often has high level of distress and carefully designed studies are needed to improve their care while minimizing attrition. Reviewers and editors need to consider that attrition does not necessary mean sub-optimal science, and grants need to budget for greater number of participants knowing that a certain proportion would dropout even for non-interventional studies with limited study burden.
Another interesting result was that a shorter interval from cancer diagnosis was associated with increased attrition. Several hypotheses can be proposed to explain this finding. For example, it is possible that patients with shorter time from diagnosis are still pursuing cancer treatments that require efforts to complete, which could impair patient participation due to increased burden. Another potential explanation is that these patients with more aggressive disease were referred to palliative care closer to diagnosis. Further studies are needed to distinguish between these possibilities.
To our knowledge, this is the first study characterizing the attrition rates in an outpatient longitudinal study including a variety of different countries from the developing world. Interestingly, after multivariate analysis, there were no differences between the countries, suggesting that the reasons for attrition in palliative oncology longitudinal studies may be fundamentally similar regardless of geographic differences.
Furthermore, although limited information regarding specific reasons to dropping-out was obtained, we found that patients with attrition due to death had increased physical symptom burden and worse functionality than patients with attrition due to illness and attrition at random. When analyzing specific symptoms, patients with attrition due to death had both increased fatigue and drowsiness, which have been recognized as early signs of impending death in cancer (4). These two clinical signs could be of particular relevance to predict the likelihood that a patient will drop out in a longitudinal study. Although these results provide novel and interesting information regarding differences in patient characteristics according to reasons for attrition, the fact that reasons for attrition were not available for subjects from two countries, limits the generalizability of these results. However, regardless of this limitation, this study was able to recognize specific baseline patient characteristics that increase patients’ likelihood of dropping out from longitudinal observational studies due to death.
Several strategies can be implemented to overcome the problem of attrition in longitudinal observational studies. At the patient selection level, including patients with higher KPS, lower symptom burden and no cognitive failure, could be associated with decreased attrition rates. However, using these criteria affects patient selection, raising some issues with the generalizability of findings, given that patients with worse performance status and shorter survival are often excluded from research (29). To address this issue, shortening the follow-up time and increasing the recruitment of patients with increased symptom burden or lower functional status would be plausible solutions. Assessment of patients via mail or by phone call interview or in the home setting during follow-up could also be strategies to facilitate patients’ response and participation in longitudinal research (31).
This study has several limitations. First, it included only academic tertiary care centers from developing countries, which may limit the generalizability of the findings. Second, the follow-up period in this study was short, with only a one time follow-up, limiting the applicability of these findings to studies of longer duration and patients were not consecutively approached/enrolled. Third, we did not include other variables to specifically address attrition issues such as distance from cancer center or desire to participate. Also, reasons for refusal were not systematically collected for all participants. Fourth, we did not include questions regarding acceptance of cancer diagnosis or cancer treatments received, reasons that could affect adherence to longitudinal observational studies. Fifth, we did not record survival data to estimate time to death at enrollment, variable that could also influence patient participation and attrition in longitudinal research. Finally, this study was performed in an outpatient setting. It is unclear whether these findings can be extended to other settings such as palliative oncology inpatient units or home care patients.
Advanced cancer patients with cognitive failure, increased physical symptom burden poorer performance status and shorter time from cancer diagnosis were more likely to dropout in this study. No differences in the attrition rates were found between the different participating centers from five developing countries. Future research should identify the role of novel factors that could influence patient participation in longitudinal research such as acceptance of cancer diagnosis, cancer treatments received, interest for participation, and distance from cancer centers. Also, research should assess the effectiveness of interventions to decrease attrition in this population.
Acknowledgments
Funding sources: Pedro Perez-Cruz is supported in part by funds awarded by the National Commission for Scientific and Technological Research, Santiago Chile (FONDECYT INICIO 11130533; REDES1440024). DH is supported in part by National Institutes of Health Grants (1R01CA214960-01A1, R21NR016736), an American Cancer Society Mentored Research Scholar Grant in Applied and Clinical Research (MRSG-14-1418-01-CCE) and the Andrew Sabin Family Fellowship Award.
We would like to thank to all the patients who participated in the study, as well as to all our research teams in Brazil, Chile, India, Jordan, Korea and in Houston, USA.
Footnotes
Conflict of interest disclosures: The authors made no disclosures.
DISCLOSURE
All authors declare no financial or other conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Epstein AS, Morrison RS. Palliative oncology: identity, progress, and the path ahead. Ann Oncol. 2012;23:43–8. doi: 10.1093/annonc/mds087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bausewein C, Booth S, Gysels M, Kuhnbach R, Haberland B, Higginson IJ. Individual breathlessness trajectories do not match summary trajectories in advanced cancer and chronic obstructive pulmonary disease: results from a longitudinal study. Palliative Med. 2010;24(8):777–86. doi: 10.1177/0269216310378785. [DOI] [PubMed] [Google Scholar]
- 3.Phelps AC, Maciejewski PK, Nilsson M, Balboni TA, Wright AA, Paulk ME, et al. Religious Coping and Use of Intensive Life-Prolonging Care Near Death in Patients With Advanced Cancer. Jama-J Am Med Assoc. 2009;301(11):1140–7. doi: 10.1001/jama.2009.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hui D, dos Santos R, Chisholm G, Bansal S, Silva TB, Kilgore K, et al. Clinical Signs of Impending Death in Cancer Patients. Oncologist. 2014;19(6):681–7. doi: 10.1634/theoncologist.2013-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen EK, Riffin C, Reid MC, Adelman R, Warmington M, Mehta SS, et al. Why is high-quality research on palliative care so hard to do? Barriers to improved research from a survey of palliative care researchers. J Palliat Med. 2014;17(7):782–7. doi: 10.1089/jpm.2013.0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Preston NJ, Fayers P, Walters SJ, Pilling M, Grande GE, Short V, et al. Recommendations for managing missing data, attrition and response shift in palliative and end-of-life care research: part of the MORECare research method guidance on statistical issues. Palliat Med. 2013;27(10):899–907. doi: 10.1177/0269216313486952. [DOI] [PubMed] [Google Scholar]
- 7.Stromgren AS, Sjogren P, Goldschmidt D, Petersen MA, Pedersen L, Hoermann L, et al. A longitudinal study of palliative care - Patient-evaluated outcome and impact of attrition. Cancer. 2005;103(8):1747–55. doi: 10.1002/cncr.20958. [DOI] [PubMed] [Google Scholar]
- 8.Ahlner-Elmqvist M, Bjordal K, Jordhoy MS, Kaasa S, Jannert M. Characteristics and implications of attrition in health-related quality of life studies in palliative care. Palliat Med. 2009;23(5):432–40. doi: 10.1177/0269216309104057. [DOI] [PubMed] [Google Scholar]
- 9.Applebaum AJ, Lichtenthal WG, Pessin HA, Radomski JN, Simay Gokbayrak N, Katz AM, et al. Factors associated with attrition from a randomized controlled trial of meaning-centered group psychotherapy for patients with advanced cancer. Psychooncology. 2012;21(11):1195–204. doi: 10.1002/pon.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hui D, Glitza I, Chisholm G, Yennu S, Bruera E. Attrition rates, reasons, and predictive factors in supportive care and palliative oncology clinical trials. Cancer. 2013;119(5):1098–105. doi: 10.1002/cncr.27854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jordhoy MS, Kaasa S, Fayers P, Ovreness T, Underland G, Ahlner-Elmqvist M. Challenges in palliative care research; recruitment, attrition and compliance: experience from a randomized controlled trial. Palliative Med. 1999;13(4):299–310. doi: 10.1191/026921699668963873. [DOI] [PubMed] [Google Scholar]
- 12.Wright AA, Mack JW, Trice ED, Balboni TA, Block SD, Prigerson HG. Personalized end-of-life care: Associations between patient preferences and treatment intensity near death. J Clin Oncol. 2009;27(15) [Google Scholar]
- 13.Phelps AC, Maciejewski PK, Nilsson M, Balboni TA, Wright AA, Trice E, et al. Coping with cancer: Associations between coping methods and use of intensive life-prolonging care near death. J Clin Oncol. 2009;27(15) doi: 10.1001/jama.2009.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hui D, Bruera E. The Edmonton Symptom Assessment System 25 Years Later: Past, Present, and Future Developments. Journal of pain and symptom management. 2017;53(3):630–43. doi: 10.1016/j.jpainsymman.2016.10.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hui D, Shamieh O, Paiva CE, Khamash O, Perez-Cruz PE, Kwon JH, et al. Minimal Clinically Important Difference in the Physical, Emotional, and Total Symptom Distress Scores of the Edmonton Symptom Assessment System. Journal of pain and symptom management. 2016;51(2):262–9. doi: 10.1016/j.jpainsymman.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hui D, Shamieh O, Paiva CE, Perez-Cruz PE, Kwon JH, Muckaden MA, et al. Minimal clinically important differences in the Edmonton Symptom Assessment Scale in cancer patients: A prospective, multicenter study. Cancer. 2015;121(17):3027–35. doi: 10.1002/cncr.29437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hui D, Park M, Shamieh O, Paiva CE, Perez-Cruz PE, Muckaden MA, et al. Personalized symptom goals and response in patients with advanced cancer. Cancer. 2016;122(11):1774–81. doi: 10.1002/cncr.29970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ewing JA. Detecting alcoholism. The CAGE questionnaire. JAMA. 1984;252(14):1905–7. doi: 10.1001/jama.252.14.1905. [DOI] [PubMed] [Google Scholar]
- 19.Breitbart W, Rosenfeld B, Roth A, Smith MJ, Cohen K, Passik S. The Memorial Delirium Assessment Scale. Journal of pain and symptom management. 1997;13(3):128–37. doi: 10.1016/s0885-3924(96)00316-8. [DOI] [PubMed] [Google Scholar]
- 20.Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care. 1991;7(2):6–9. [PubMed] [Google Scholar]
- 21.Hannon B, Dyck M, Pope A, Swami N, Banerjee S, Mak E, et al. Modified Edmonton Symptom Assessment System including constipation and sleep: validation in outpatients with cancer. Journal of pain and symptom management. 2015;49(5):945–52. doi: 10.1016/j.jpainsymman.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Chang VT, Hwang SS, Feuerman M. Validation of the Edmonton Symptom Assessment Scale. Cancer. 2000;88(9):2164–71. doi: 10.1002/(sici)1097-0142(20000501)88:9<2164::aid-cncr24>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 23.Carvajal A, Centeno C, Watson R, Bruera E. A comprehensive study of psychometric properties of the Edmonton Symptom Assessment System (ESAS) in Spanish advanced cancer patients. Eur J Cancer. 2011;47(12):1863–72. doi: 10.1016/j.ejca.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 24.Kwon JH, Nam SH, Koh S, Hong YS, Lee KH, Shin SW, et al. Validation of the Edmonton Symptom Assessment System in Korean patients with cancer. Journal of pain and symptom management. 2013;46(6):947–56. doi: 10.1016/j.jpainsymman.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nekolaichuk C, Watanabe S, Beaumont C. The Edmonton Symptom Assessment System: a 15-year retrospective review of validation studies (1991–2006) Palliat Med. 2008;22(2):111–22. doi: 10.1177/0269216307087659. [DOI] [PubMed] [Google Scholar]
- 26.Paiva CE, Manfredini LL, Paiva BS, Hui D, Bruera E. The Brazilian Version of the Edmonton Symptom Assessment System (ESAS) Is a Feasible, Valid and Reliable Instrument for the Measurement of Symptoms in Advanced Cancer Patients. PLoS One. 2015;10(7):e0132073. doi: 10.1371/journal.pone.0132073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zimmermann C, Burman D, Bandukwala S, Seccareccia D, Kaya E, Bryson J, et al. Nurse and physician inter-rater agreement of three performance status measures in palliative care outpatients. Support Care Cancer. 2010;18(5):609–16. doi: 10.1007/s00520-009-0700-9. [DOI] [PubMed] [Google Scholar]
- 28.Downing M, Lau F, Lesperance M, Karlson N, Shaw J, Kuziemsky C, et al. Meta-analysis of survival prediction with Palliative Performance Scale. J Palliat Care. 2007;23(4):245–52. discussion 52–4. [PubMed] [Google Scholar]
- 29.Tayjasanant S, Bruera E, Hui D. How far along the disease trajectory? An examination of the time-related patient characteristics in the palliative oncology literature. Supportive Care in Cancer. 2016;24(9):3997–4004. doi: 10.1007/s00520-016-3225-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hui D, Frisbee-Hume S, Wilson A, Dibaj SS, Nguyen T, De La Cruz M, et al. Effect of Lorazepam With Haloperidol vs Haloperidol Alone on Agitated Delirium in Patients With Advanced Cancer Receiving Palliative Care A Randomized Clinical Trial. Jama-J Am Med Assoc. 2017;318(11):1047–56. doi: 10.1001/jama.2017.11468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bruera E, Hui D, Dalal S, Torres-Vigil I, Trumble J, Roosth J, et al. Parenteral Hydration in Patients With Advanced Cancer: A Multicenter, Double-Blind, Placebo- Controlled Randomized Trial. J Clin Oncol. 2013;31(1):111–8. doi: 10.1200/JCO.2012.44.6518. [DOI] [PMC free article] [PubMed] [Google Scholar]