Abstract
The immune cytokine tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) has received significant attention as a cancer therapeutic due to its ability to selectively trigger cancer cell apoptosis without causing toxicity in vivo. While TRAIL has demonstrated significant promise in preclinical studies in mice as a cancer therapeutic, challenges including poor circulation half-life, inefficient delivery to target sites, and TRAIL resistance have hindered clinical translation. Recent advances in drug delivery, materials science, and nanotechnology are now being exploited to develop next-generation nanoparticle platforms to overcome barriers to TRAIL therapeutic delivery. Here, we review the design and implementation of nanoparticles to enhance TRAIL-based cancer therapy. The platforms we discuss are diverse in their approaches to the delivery problem and provide valuable insight into guiding the design of future nanoparticle-based TRAIL cancer therapeutics to potentially enable future translation into the clinic.
Keywords: nanotechnology, cancer therapy, oncology, metastasis, biological barriers, drug delivery, gene therapy, immunotherapy, biomaterials, tumor targeting
Graphical abstract
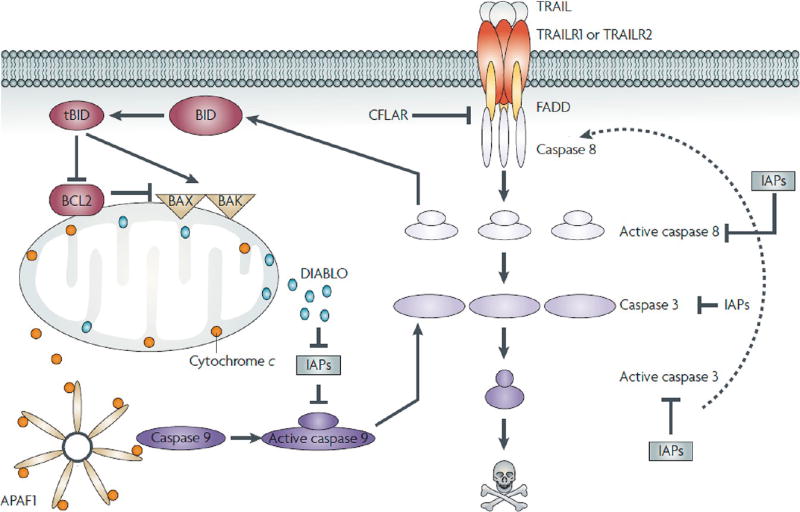
The development of therapeutics that induce apoptosis specifically in tumor cells while sparing normal cells has long been a goal for clinicians and researchers to avoid the debilitating effects of conventional chemotherapies. One protein of heightened interest as a cancer-specific therapeutic is tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), an innate immune cytokine broadly expressed by immune cells including lymphocytes, natural killer cells, and neutrophils (Figure 1).1 TRAIL-expressing immune cells play a role in the clearance of diseased cells in vivo and have also been shown to suppress tumor initiation and metastasis.2,3 TRAIL binds to death receptors 4 and 5 (DR4 and DR5) overexpressed on the surface of a range of cancer cells to trigger apoptosis and also binds to three decoy receptors (DcR1, DcR2, DcR3) expressed on cells that do not trigger apoptosis and act as competitive inhibitors (Figure 1).4,5 TRAIL-based therapeutic approaches have shown high efficacy in numerous preclinical cancer models.
Figure 1.
Apoptotic signaling through the tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) pathway. Binding of TRAIL to TRAIL receptor 1 (TRAILR1 or D4) or TRAILR2/DR5 results in receptor oligomerization and recruitment of FAS-associated protein with death domain (FADD) and caspase 8 to form a functional death-inducing signaling complex (DISC). Upon DISC formation, caspase 8 is cleaved and activated, which in turn can cleave and activate caspase 3 and the BH3-only protein BID. Active, cleaved BID (tBID) can bind to pro-apoptotic Bax and Bak, resulting in mitochondrial membrane permeabilization and release of mitochondrial proteins cytochrome c and DIABLO. Cytochrome c, apoptotic protease-activating factor 1 (APAF1), and caspase 9 combine with ATP to form a functional apoptosome that results in cleavage and activation of caspase 9, which can then cleave caspase 3. DIABLO suppresses the caspase-inhibitory activities of inhibitor of apoptosis proteins. Caspase 3 can cleave a large number of intracellular targets, resulting in the morphological and biochemical hallmarks of apoptosis. Caspase 3 can also cleave and activate caspase 8, thereby amplifying the apoptotic signal. Reprinted with permission from ref 4. Copyright 2016 Nature.
While potent antitumor activity of TRAIL has been observed in vitro and in preclinical in vivo mouse models, these effects have not been broadly reproduced in clinical trials. Intravenous administration of TRAIL-based therapeutics have been well-tolerated in clinical trials, both as a monotherapy or in combination with conventional chemotherapy and immunotherapy.6,7 Minimal adverse effects of TRAIL therapeutics have been reported in patients with non-small-cell lung cancer, metastatic pancreatic cancer, basal-like breast cancer, colorectal carcinoma, non-Hodgkin’s lymphoma, and multiple myeloma (Table 1),6,7 indicating that TRAIL-based therapies do not induce the toxic side effects in patients that are commonly faced during chemotherapeutic regimens. While the lack of toxic side effects is promising, studies in cancer patients have demonstrated little efficacy. For example, monomeric, soluble TRAIL protein and agonistic DR4 and DR5 antibodies, used in the first clinical trials, have relatively low potency in terms of inducing apoptosis and thus limit clinical efficacy. Challenges in translating preclinical success into clinical settings include the short circulation half-life of TRAIL (from several minutes in rodents to approximately 60 min in humans), its lack of accumulation in target tumor sites, and the low potency of TRAIL ligands or antibodies in activating DR4 and DR5 on the surface of tumor cells.7–10 However, the fact that TRAIL has been relatively well-tolerated in clinical trials suggests there is a therapeutic window for increasing tumor exposure and thus augmenting the efficacy of the ligand.9
Table 1.
Examples of TRAIL-Based Therapeutic Approaches in Clinical Trials
| approach | details | tolerability | ref |
|---|---|---|---|
| recombinant human soluble TRAIL protein | |||
| Dulanermin | RhApo2L/TRAIL aa. 114–281 | safe | 7,8,11 |
| CPT | circularly permutated TRAIL; N-terminus of Apo2L/TRAIL aa. 121–135 is lined to C-terminus of the aa. 135–281 of Apo2L/TRAIL | safe | 12 |
| agonistic TRAIL receptor antibodies | |||
| Mapatumumab | fully human agonistic monoclonal antibody against DR4 | safe | 13–17 |
| Lexatumumab | fully human agonistic monoclonal antibody against DR5 | safe | 18 |
| Conatumumab | fully human agonistic monoclonal antibody against DR5 | safe | 19–21 |
| Drozitumab | fully human agonistic monoclonal antibody against DR5 | safe | 22,23 |
| Tigatuzumab | humanized agonistic monoclonal antibody against DR5 | safe | 24–27 |
| TAS266 | tetrameric agonist nanobody against DR5 | induces hepatotoxicity | 28 |
| other | |||
| T-cell-based immunotherapy | autologous T cells transduced with a T cell receptor recognizing TRAIL bound to DR4 | terminated; no results posted | |
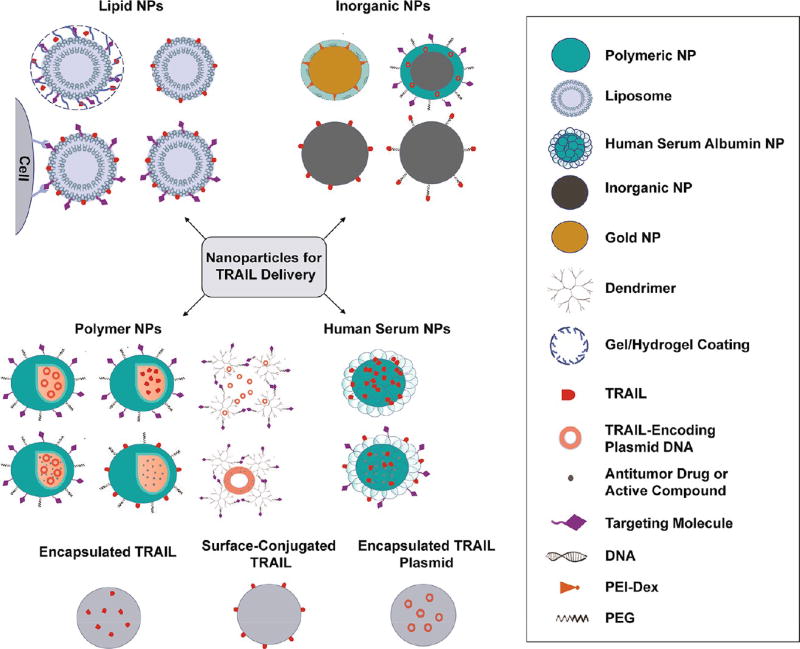
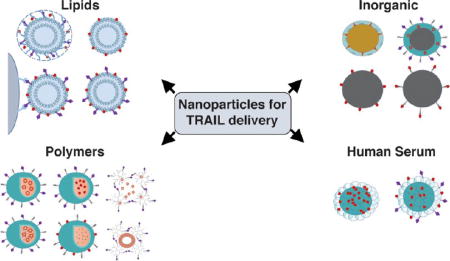
A key challenge to realizing the broad potential of TRAIL-based therapeutics is the need for safe and effective delivery methods. Recent advances in drug delivery, nanotechnology, and materials science hold promise in overcoming the in vivo delivery barriers that hinder TRAIL efficacy in clinical trials. Nanoparticles (NPs) are now utilized to both encapsulate and/or bind TRAIL therapeutics, which can reduce degradation in blood, offer ideal surface presentation of ligands to target cells, and modify the biodistribution and selectivity of drugs to target tissues (Figure 2).29–32 Gene delivery materials, administered in vivo or used to engineer therapeutic cells ex vivo, can be exploited for targeted expression and secretion of TRAIL therapeutics at tumor sites in vivo.33,34 Direct modifications of therapeutics with materials such as serum proteins35 and polymers such as polyethylene glycol (PEG),36 have shown enhanced circulation half-life, improved tumor targeting, and reduced off-target effects.37
Figure 2.
Examples of classes of NPs used for TRAIL-based cancer therapy.
Here, we overview recent advances in the design and implantation of NP platforms for TRAIL-based cancer therapeutics. These systems, with reported in vitro and in vivo efficacy, are diverse in their size, shape, structure, chemistry, and mechanism of action. We highlight the opportunities and challenges in utilizing NPs to advance TRAIL-based cancer therapeutics and provide our analysis of the future outlook for these emerging areas.
Nanoparticle-Based Approaches
The design and implementation of NPs as drug delivery vehicles for cancer therapy has been extensively studied. NPs encapsulate drugs with limited solubility, protect therapeutics from degradation in blood, offer ideal surface presentation of therapeutic ligands to target cells, and modify the biodistribution and selectivity of drugs to target tissues.29–32 The first generation of clinically approved nanomedicines including Doxil, a liposomal NP-encapsulated doxorubicin, and Abraxane, an albumin NP-stabilized paclitaxel, reduced toxic side effects and enabled higher tolerated doses of drug in patients, respectively.38,39 NP-based strategies can exploit either passive or active targeting methods, or a combination of both, to achieve therapeutic delivery to tumors. Through the enhanced permeation and retention (EPR) effect, NPs can passively traverse through large pores in tumor vasculature and accumulate in solid tumors.40–44 However, the use of passive strategies alone has limitations, as permeability and vessel distribution are not uniform across tumors, preventing the deep penetration and uptake of NPs.45–47 Active targeting strategies can address this lack of penetration and enhance delivery to tumors by tethering targeting molecules, such as nucleic acids, peptides, and proteins, to the surface of NPs to enable binding to overexpressed receptors or antigens on tumor cells.40,48–51
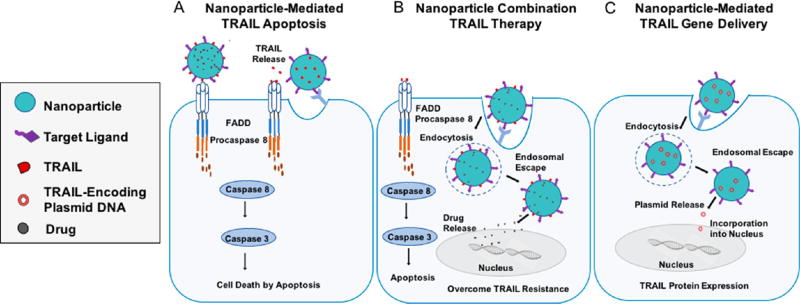
TRAIL is considered to be both a targeting agent and a cell death ligand that triggers apoptosis.52,53 With negligible toxicity to most normal cells, TRAIL triggers apoptosis by selectively binding to death receptors on the surface of cancerous cells (Figure 1).52,53 Although the therapeutic potential of TRAIL as a targeting molecule and therapeutic compound are well-established in preclinical studies, challenges remain to enable translation to the clinic. Recently, the use and development of NP platforms have been explored to improve TRAIL-based therapeutics through (i) enhanced circulation half-life and biodistribution, (ii) overcoming TRAIL resistance, and (iii) improved targeting to diseased tissues.54 NP-based strategies include (i) the encapsulation of soluble TRAIL protein or TRAIL-encoding plasmids, (ii) TRAIL tethered directly to the surface of NPs to engage TRAIL death receptors on tumor cells in vivo, or (iii) combination approaches (Figure 3). Additionally, NP-based approaches utilizing combination therapies of TRAIL with chemotherapeutics or other sensitizers are now being investigated to overcome tumor resistance to TRAIL (Figure 3).55–57
Figure 3.
Approaches to utilize NPs to induce TRAIL-mediated apoptosis in tumor cells via (A) TRAIL-coated NPs binding to the tumor cell surface, (B) a combination NP-based approach that delivers encapsulated small molecule drugs to sensitize tumor cells to TRAIL-mediated apoptosis, or (C) gene delivery to cells to induce secretion of TRAIL.
Lipid Nanoparticles
Nanoscale liposomes (Figure 2) have been investigated extensively as drug carriers for cancer therapy, with clinical trials for liposomes resulting in approved therapeutics such as Doxil58 and DaunoXosome (Kaposi’s sarcoma),59 LipoDox (ovarian and breast cancer and Kaposi’s sarcoma),60,61 Myocet (metastatic breast cancer),62,63 Marqibo (acute lymphoblastic leukemia),64 as well as additional drugs currently in clinical trials.65,66 In addition to their biocompatibility, biodegradability, low toxicity, and low immunogenicity, liposomes can encapsulate both hydrophilic and hydrophobic drugs, protect active compounds from external degradation, and deliver agents into cells. Liposome surface properties, such as membrane fluidity, geometry, hydrophilicity, hydrophobicity, and stability can be optimized to enhance the delivery and presentation of a variety of biomolecules.67 Modulation of liposome surface properties enables manipulation of blood circulation time, pH sensitivity, entrapment efficiency, and ligand attachment, particularly for those that are most potent in their membrane-bound form.68 An example modification strategy using PEG, known as PEGylation, has been shown to enhance the serum stability of numerous NP formulations in circulation.67
Enhancing TRAIL Potency via Lipid NP-Based Membrane Display
Given that clinical trials have focused on the administration of TRAIL in soluble form, it has been suggested that the lack of broad efficacy in patients could be due to a lack of potency of the soluble protein, and thus weak engagement of the extrinsic TRAIL apoptotic pathway. Conjugation of TRAIL to the membrane of lipid NPs, in particular, liposomes, has recently been shown by several groups to augment the apoptotic effect of TRAIL in comparison to soluble protein treatment (Figures 2 and 3).68–73
In one approach, covalent binding of TRAIL to the liposome membrane using cysteine–maleimide bonds widely increased TRAIL receptor clustering, caspase signaling, and apoptosis across a panel of 479 cell lines.68 Compared to soluble protein treatment, systemically administered liposomal TRAIL reduced overall tumor volume in a mouse colon cancer xenograft model. These results indicate that membrane display of TRAIL is essential in enabling TRAIL receptor clustering and subsequently inducing apoptosis. Similar effects have also been observed when TRAIL was bound to membranes using a 1,2-dioleoyl-sn-glycero-3-{[N-(5-amino-1-carboxypentyl)-iminodiacetic acid]succinyl}(nickel salt) (DOGS-Ni-NTA)-containing liposomal system.69,71–73 In terms of the apoptotic mechanism, liposomal TRAIL enhanced caspase 3 activity, an early initiator of apoptosis, and overcame TRAIL resistance in tumor cell lines either overexpressing the antiapoptotic protein Bcl-xL or that which lacked expression of the proapoptotic proteins Bax and Bak. Building upon the mechanism behind this response, it was later shown that liposomes increased receptor clustering of DR5, enhanced recruitment of the death-inducing signaling complex (DISC), and triggered caspase activation more efficiently than soluble TRAIL protein.70 To further enhance targeting for in vivo applications, TRAIL-coated liposomes have been combined with membrane-bound targeting agents such as anti-EGFR antibody fragments, which also enhanced DR4 and DR5 activation.74 Such systems have also been PEGylated to improve pharmacokinetic profiles and in vivo delivery.55
Programmable Liposomal Delivery Systems
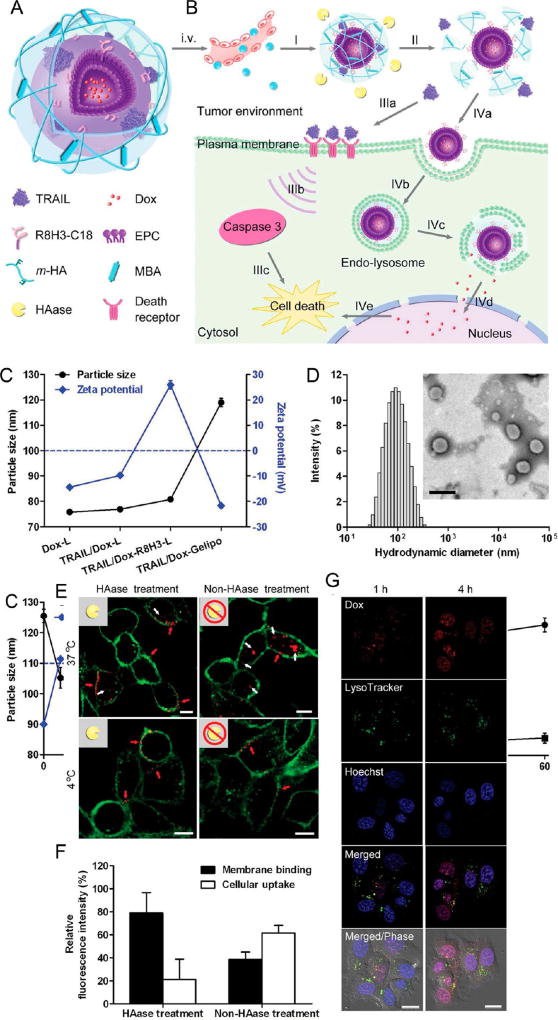
In addition to exploiting membrane display to enhance apoptosis, liposomes complexed with hyaluronic acid gels have been engineered for programmed delivery and release of both TRAIL and doxorubicin to the tumor microenvironment in vivo.56 A cross-linked hyaluronic acid gel shell was used to encapsulate doxorubicin-containing liposomes coated with both TRAIL and the cell-penetrating peptide R8H3 (Figure 4).56 Following NP administration, an extracellular enzyme highly expressed in tumors known as hyaluronidase then degraded the core–shell to trigger the release of TRAIL.56 The elimination of the core–shell exposed R8H3 conjugated to a stearyl chain to anchor the peptide into the liposome membrane via hydrophobic and electrostatic interactions, which enhanced cellular uptake of doxorubicin to further trigger apoptosis.56 In addition to enhanced tumor apoptosis activity in vitro and in vivo, this NP platform also resulted in increased accumulation of doxorubicin in tumor tissue and enhanced drug circulation half-life compared to free doxorubicin alone or R8H3-modified liposomes formulated with doxorubicin.56 Strong antitumor activity and high accumulation in the tumor tissues were demonstrated using this formulation, suggesting that these liposome–gel systems can be utilized for the programmed release of TRAIL at target tumor sites that overexpress certain enzymes.56
Figure 4.
Schematic design of TRAIL/Dox-Gelipo for sequential and site-specific drug delivery. (A) Main components of TRAIL/Dox-Gelipo: R8H3-modified liposomal core loading doxorubicin (Dox) and cross-linked hyaluronic acid (HA) gel-based outer shell encapsulating TRAIL. (B) Sequential delivery of TRAIL to the plasma membrane and Dox to the nuclei by TRAIL/Dox-Gelipo for combination cancer treatment. I, accumulation of Gelipo (blue balls) at the tumor site through the passive and active targeting effects; II, degradation of HA cross-linked shell by HAase; IIIa, released TRAIL binding onto the death receptors on the plasma membrane; IIIb, activation of the caspase 3 signaling pathway; IIIc, induction of the cell death; IVa, exposure of R8H3 facilitating the tumor cellular uptake of Dox-R8H3-L; IVb, internalization of Dox-R8H3-L into the tumor cells; IVc, endolysosomal escape; IVd, accumulation of the released Dox into nucleus; IVe, intercalation of Dox on DNA inducing the cell death. (C) Particle size and ζ-potential of Dox-L, TRAIL/Dox-L, TRAIL/Dox-R8H3-L, and TRAIL/Dox-Gelipo. (D) Hydrodynamic size of TRAIL/Dox-Gelipo measured by dynamic light scattering. Inset: TEM image of TRAIL/Dox-Gelipo. Scale bar is 200 nm. (E) Membrane binding efficiency of r-TRAIL from r-TRAIL-Gelipo with and without 30 min of HAase pretreatment on MDA-MB-231 cells observed by confocal laser scanning microscopy (CLSM). The plasma membranes were stained by Alexa Fluor 488 conjugate of wheat germ agglutinin. Scale bars are 10 µm. (F) Quantitative analysis on the r-TRAIL amount on the plasma membrane and in the cells. (G) Intracellular delivery of TRAIL/Dox-Gelipo after 30 min of HAase pretreatment on MDA-MB-231 cells at different time observed by CLSM. The late endosomes and lysosomes were stained by LysoTracker Green, and the nuclei were stained by Hoechst 33342. Scale bars are 20 µm. Reprinted with permission from ref 56. Copyright 2014 Wiley.
Combination TRAIL Therapies
The ability of liposomes to encapsulate drugs, in addition to conjugation of TRAIL to the liposome membrane, is advantageous for use in combination therapies to either exert synergistic therapeutic effects or overcome TRAIL resistance (Figure 3B). In one approach, a lipid-based NP system was used to exploit both TRAIL-mediated apoptosis as well as intracellular-triggered apoptosis using the small molecule DM-PIT. The particle system consisted of micelles comprised of both PEG derivatives and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine,75 a widely used fusogenic lipid that facilitates endosomal escape of NPs.76 TRAIL was tethered to the surface of micelles, which were loaded with DM-PIT.75 A combination of TRAIL and DM-PIT enhanced TRAIL-resistant U87MG glioblastoma cell apoptosis compared to micelles loaded with DM-PIT.
Although not as widely explored as polymer-based delivery systems, lipid-based NPs have also been utilized for gene delivery, as TRAIL-encoding plasmid DNA has been combined with the antitumor drug paclitaxel to prepare cationic liposomes functionalized with targeting ligands.77,78 Collectively, lipid-based NPs have been shown in several studies to enhance TRAIL potency via membrane display, making them an ideal material for improving the delivery of recombinant TRAIL proteins. Such an approach has high translational potential, given that TRAIL is nontoxic and the lipids used in these formulations are already used in liposomal formulations approved by the U.S. Food and Drug Administration (FDA).
Polymeric Nanoparticles
Polymeric NPs such as micelles, polyplexes, and dendrimers are also being utilized for the delivery of TRAIL-based therapeutics (Figure 2),40,79 as they are particularly advantageous for the release of therapeutics in a controlled manner, stimuli-responsive drug delivery, and gene delivery.40,48,80,81 Polymeric NPs can be formulated from a large variety of polymers and copolymers including synthetic biodegradable polymers (poly(lactic acid) (PLA), poly(lacticco-glycolic acid) (PLGA)), and natural polymers (alginate and chitosan), enabling them to encapsulate both hydrophilic and hydrophobic drugs. Several polymeric NPs are undergoing clinical trials in the United States,79 including (i) PLA and PEG NPs containing Paclitaxel to treat breast cancer, lung cancer, and pancreatic cancer (Genexol-PM);82,83 (ii) PLA and PEG NPs loaded with docetaxel and conjugated to ACUPA (S,S-2-[3-[5-amino-1-carboxypentyl]ureido]pentanedioic acid), an inhibitor for the prostate-specific membrane antigen (PSMA) to target non-small-cell lung cancer and prostate cancer;84 and (iii) a formulation of cyclodextrin polymer, PEG, small interfering RNA (siRNA), and transferrin to target solid tumors (CALAA-01).85 Drug-loaded polymeric micelles, comprised of self-assemblies of amphiphilic block copolymers consisting of a hydrophobic core as a drug reservoir and a PEG hydrophilic shell, have also demonstrated high translational potential for cancer therapy.86 Several micelle formulations, including the paclitaxel micelle formulation NK105, are currently under clinical evaluation.87,88
Polymers for Nonviral TRAIL DNA Delivery in Vivo
While significant efforts have focused on the administration of TRAIL in protein form, clinical translation of such approaches is limited by the failure to deliver sufficient protein into the tumor to generate a therapeutic effect.89–91 As an alternative approach, TRAIL gene therapy can potentially overcome these barriers by delivering TRAIL-encoding plasmid DNA specifically to tumor cells and the surrounding tumor microenvironment, so that they express and secrete TRAIL into tumors locally (Figure 3C).92,93 However, DNA therapeutics face several barriers to successful in vivo delivery. Among those are the degradation of nucleic acids by endonucleases in the bloodstream as well as in tissues.94,95 Plasmid DNA is also cleared from the bloodstream rapidly, as its half-life has been estimated to be 10 min following intravenous injection in mice.96 For this reason, the entrapment of DNA in NPs is a particularly attractive approach both to provide protection from endonuclease degradation and to extend the circulation half-life of DNA. NPs can also aid in avoiding renal clearance from the blood due to size-based effects, and preventing nonspecific interactions via PEGylation of NPs. Additionally, NPs can also extravasate from the bloodstream to reach target tissues via various targeting-based approaches, and mediate cell entry and endosomal escape. Finally, DNA has to be transported to the nucleus to enable access to transcriptional machinery.94 In the context of TRAIL gene therapy, several polymer-based approaches including polyethylenimine (PEI) and cationic dendrimers have been utilized for in vivo delivery.
PEI-Based TRAIL Gene Delivery Systems
PEI is a widely used cationic polymer for DNA and RNA delivery due to its high transfection efficiency and its ability to induce endosomal escape (Figure 3C).80,97 In the context of TRAIL-based cancer therapy, PEI has been complexed with TRAIL-encoding plasmids to enhance delivery to cells, which then secrete the therapeutic locally to induce tumor apoptosis. For example, a peptide–polymer conjugate consisting of an epidermal growth factor receptor (EGFR) targeting peptide (GE11), PEG, and PEI (GE11–PEG–PEI) was developed to form NPs for targeted TRAIL gene delivery.98 PEI and GE11 were bound using a heterobifunctional PEG (maleimide–PEG–NHS), where the amino group of GE11 was coupled to the NHS group to obtain GE11–PEG–maleimide, which was then coupled with PEI using the maleimide amino reaction to obtain GE11–PEG–PEI.98 To formulate NPs, GE11–PEG–PEI was further complexed with a TRAIL-encoding plasmid via electrostatic interactions.98 Both in vitro and in vivo, the modified NPs significantly increased gene transfection efficiency and inhibited Hep-2 cell tumor growth in mice compared to unmodified NPs.98
Alternative strategies have utilized PEG–PEI polymer conjugates functionalized with either RGD peptides targeting integrins expressed by gliomas (RGD–PEG–PEI),99 or small molecules targeting PSMA expressed on prostate cancer cells100 to form NPs for TRAIL gene delivery. In a mouse intracranial glioblastoma xenograft model, RGD–PEG–PEI NPs administered systemically 11 days post-tumor implantation (four injections over days 11–17), in combination with paclitaxel-loaded micelles targeting nicotine acetylcholine receptors that facilitate blood-brain barrier (BBB) crossing (four injections over days 4–16), enhanced the median survival of mice (33.5 days) compared to treatment with paclitaxel micelles alone (25.5 days).99 Similarly, PSMA-targeting NPs administered systemically induced TRAIL expression in a mouse prostate cancer xenograft model.100 In combination with the nontoxic prodrug 5-fluorocytosine (5-FC), which converts to the chemotherapeutic agent 5-fluorouracil (5-FU) in the presence of the prodrug enzyme bacterial cytosine deaminase bound to the NP surface, the combination therapy induced significant inhibition of tumor growth in a mouse prostate cancer xenograft model.100 The conversion of the drug was also measured using noninvasive functional magnetic resonance spectroscopy, indicating that the NPs can also be utilized as a theranostic platform.100 Collectively, PEI has been incorporated into several NP platforms to enhance TRAIL gene delivery in multiple mouse models. However, the toxicity of high molecular weight PEI is a major limitation that must be considered in therapeutic regimens where continuous dosing of NPs are required.97
Dendrimer-Based TRAIL Gene Delivery Systems
Dendrimers have been widely used as scaffolds for drug delivery, due to their highly branched, globular architecture with well-defined molecular weight and arms assembled from a multifunctional core.101 Cationic polyamidoamine (PAMAM) dendrimers have been utilized as TRAIL gene delivery vehicles due to their positive charge, high transfection efficiency, and pH buffering ability, which facilitates cargo release from endosomes.81,102–104 As a means to enable TRAIL gene delivery to gliomas, PAMAM dendrimers complexed with TRAIL-encoding plasmids were conjugated to Angiopep-2, a peptide with high transcytosis capacity capable of crossing the BBB, using a heterobifunctional PEG polymer.102 TRAIL gene therapy using systemically administered, targeted PAMAM NPs extended survival of a mouse intracranial glioma xenograft model compared to treatment with nontargeted NPs or Temozolomide, an FDA-approved chemotherapeutic for the treatment of brain cancer.102 PAMAM NPs have also been functionalized with various ligands targeting transferrin receptors, which are highly expressed on many tumor cell types, to enhance TRAIL gene delivery and extend survival in multiple tumor-bearing mouse models.103,104
Dendrimer-based NPs have also been utilized for the co-delivery of TRAIL-encoding plasmids and chemotherapeutics in tumor-bearing mouse models.104,105 In one approach, doxorubicin was intercalated with a TRAIL-encoding plasmid followed by condensation using dendrigraft poly-L-lysine polymers to form NPs.105 NPs were functionalized with a choline derivate, which has high BBB choline transporter activity and is overexpressed on glioma cells, using a heterobifunctional PEG to facilitate targeting.105 Systemic co-delivery of doxorubicin and the TRAIL-encoding plasmid using NPs extended survival of a mouse intracranial glioma xenograft model, compared to doxorubicin and NP-encapsulated plasmid treatments alone, while also reducing the dosage of doxorubicin required to achieve therapeutic efficacy.105
Collectively, these preclinical studies indicate that dendrimer-based NPs are not only effective in the delivery of TRAIL-encoding plasmids and the transfection of target cells in vivo, but can also be complexed with chemotherapeutics for combination therapies. While considered less toxic than PEI, dendrimers such as PAMAMs are not biodegradable and exhibit cytotoxicity due to their high density of positive charge.106 Looking forward, biodegradable structures such as dendrigraft poly-L-lysine, should they achieve high transfection efficiency, could be investigated further as delivery vehicles with reduced in vivo toxicity to enable continuous dosing. Beyond dendrimers, NP systems consisting of both cationic polypeptides and lipoic acid have been utilized in the co-delivery of doxorubicin and TRAIL-encoding plasmids.107 The polypeptides effectively condensed plasmid DNA, while lipoic acid enabled self-assembly into a micelle-like nanostructure.107 Given that the NP system exerted potent tumor cell apoptosis with minimal systemic toxicity, such systems could also be explored as an alternative to PEI and PAMAM-based gene delivery systems.
Polymeric NPs as Mechano-amplifiers of TRAIL-Mediated Apoptosis
Beyond gene delivery, polymeric NPs have also been utilized to sensitize tumor cells to TRAIL-mediated apoptosis in the presence of physiological forces.108 Recent work from our lab has indicated that, in addition to chemical sensitizers, tumor cells become sensitized to TRAIL-mediated apoptosis in the presence of mechanical forces.109 Mechanical force in the form of fluid shear stress, mimicking shear forces of those in the microvasculature in vivo, enhanced cancer cell TRAIL-mediated apoptosis in vitro compared to similar treatments under static conditions.109,110 Inspired by this observation, we developed polymeric NP mechanical amplifiers that exploit in vitro and in vivo physical forces to enhance TRAIL-mediated apoptosis.108 Mechanical amplifiers, consisting of biodegradable, FDA-approved PLGA particles tethered to the tumor cell surface via PEG linkers, increased the apoptotic effect of TRAIL on tumor cells under fluid shear exposure by as much as 50% compared with treatment under static conditions.108 While further in vivo studies will be required to assess if this phenomenon can be exploited therapeutically, initial work in mice has shown that pretreatment of tumor cells with systemically administered, targeted NPs followed by treatment with TRAIL reduced circulating tumor cells in blood and overall tumor cell burden by over 90%.108 Furthermore, the approach reduced solid tumor growth of a mouse prostate cancer xenograft model when treated in combination with the antioxidant resveratrol, potentially opening applications for nanotechnology to enhance TRAIL-mediated signaling and apoptosis in the presence of physical forces in vivo.108
Iron Oxide Nanoparticles
Iron oxide NPs are a class of biocompatible inorganic NPs (Figure 2) with ideal magnetic properties that have been traditionally utilized as contrast agents for magnetic resonance imaging (MRI).111,112 However, recent advances have now opened the door for cellular-specific targeting, drug delivery, and multimodal imaging using iron oxide NPs.113 Iron oxide NPs can induce tumor cell death through hyperthermia through the generation of heat by alternating magnetic fields, improve antitumor drug or gene delivery in the presence of an extracorporeal magnetic field, and serve as MRI contrast agents.114,115 FDA-approved iron oxide NPs currently used in the clinic include iron oxide NPs coated with a carbohydrate shell (Ferumoxytol or Feraheme) to treat iron deficiency anemia.116
Iron Oxide NP-Mediated TRAIL Gene Delivery
In the context of TRAIL therapy, iron oxide NPs have been utilized in combination with polymeric materials for in vivo TRAIL gene delivery to tumors. In a recent study, iron oxide NPs were coated with a chitosan-PEG-grafted PEI copolymer, covalently bound to chlorotoxin, and complexed with a TRAIL-encoding plasmid.112 Chlorotoxin, which binds to matrix metalloproteinase-2 and Annexin A2 on glioblastoma cells, was tethered to the surface of NPs via thiol-specific conjugation.112 Modified iron oxide NPs delivered TRAIL-encoding plasmid DNA to induce apoptosis in glioblastoma cells and significantly reduced tumor growth in a mouse xenograft model of glioblastoma compared to treatment with control plasmid DNA.112 In attempt to exploit their magnetic properties, another study showed that similar PEI-modified NP gene delivery systems, under the presence of a magnetic field, enhanced NP uptake and guided NP transport to tumors in mice. The approach was utilized as a means to induce TRAIL expression and secretion within the local microenvironment to reduce overall tumor burden.117
Magnetic NP Switches for TRAIL-Mediated Apoptosis
Beyond gene delivery, iron oxide NPs have also been utilized to create a “magnetic switch” for tumor cell apoptosis.118 Rather than conjugating TRAIL protein to the NP surface, TRAIL receptor DR4 targeting antibodies were conjugated to the surface of iron oxide NPs to enable binding to tumor cell TRAIL receptors.118 Upon application of a magnetic field, iron oxide NPs bound to the surface caused TRAIL DR4 receptors to cluster together in vitro and in zebrafish,118 which triggered tumor cell apoptosis in a similar fashion to the TRAIL protein itself. Such an approach can be of use when it is necessary to turn on or turn off an apoptotic signaling in vitro or in vivo. In addition to TRAIL receptor antibodies, TRAIL proteins have also been conjugated to the surface of iron oxide NPs,89 which extended the survival of mouse glioma xenograft model compared to treatment with TRAIL protein alone.
Taken together, the magnetic properties of iron oxide can be exploited in TRAIL-based therapies by boosting gene delivery in the presence of a magnetic field, while also promoting the clustering of tumor cell TRAIL receptors as a means to induce apoptosis in the absence of TRAIL protein. Furthermore, their favorable biocompatibility, biodegradability, and use as image contrast agents could open up future applications for TRAIL-based NPs as theranostics.40,48,119
Gold Nanoparticles
Gold NPs are a class of inorganic NPs (Figure 2) that offer an ideal combination of physical, chemical, optical, and electronic properties that have proven useful for drug delivery, as well as for use as imaging agents to diagnose disease and guide surgical procedures.120 Of note, the optical and electronic properties of gold NPs have enabled, in addition to therapeutic delivery, simultaneous photothermal therapy where the irradiation of NPs with near-infrared light at specific frequencies leads to rapid tumor cell death with minimal damage to surrounding tissue.119–122 A notable clinical advantage of gold NPs is their classification by the U.S. FDA as a medical device for photothermal therapy applications, enabling accelerated approval processes with reduced cost.120 Beyond photothermal therapy, several gold NP platforms are currently undergoing clinical trials for drug delivery applications, including PEGylated gold NPs (Aurimmune) surface functionalized with tumor necrosis factor alpha (TNFα) to induce tumor cell death.123 Most recently, a spherical nucleic acid platform consisting of short strands of RNA densely arranged on the surface of spherical gold NPs were approved by the U.S. FDA as an investigational drug, in an early stage clinical trial to treat glioblastoma multiforme.124 These “spherical nucleic acids” were the first to demonstrate nucleic acid delivery by intravenous injection to silence critical oncogenes in mouse models of intracerebral glioblastoma, reducing tumor progression and extending survival significantly.125
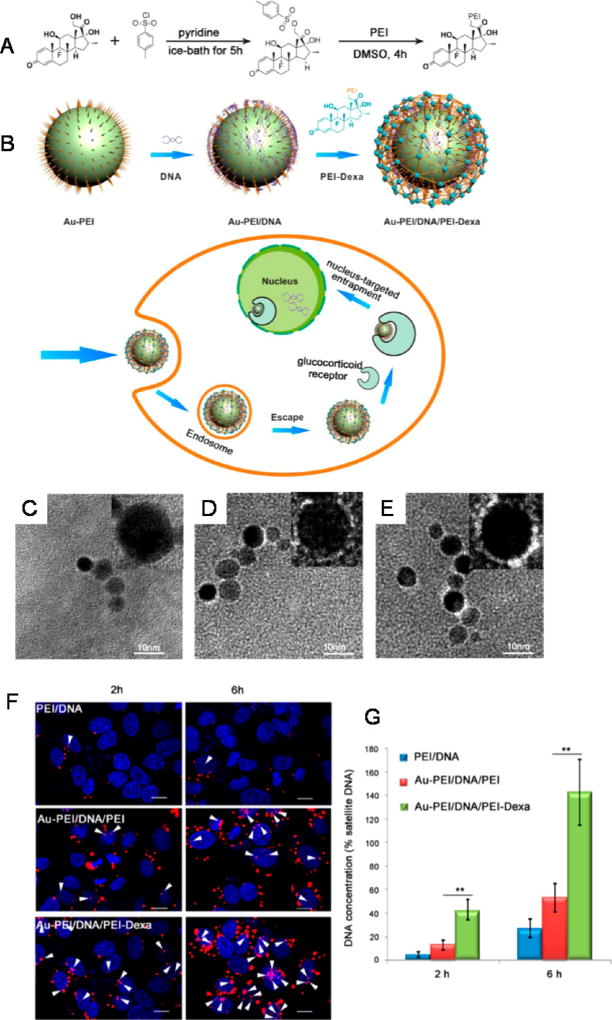
While less studied compared to lipid and polymer-based systems, gold NPs have recently been explored for use as sensitizers to TRAIL-mediated apoptosis as well as for the delivery of TRAIL-encoding plasmids.126,127 A recent study discovered that gold NPs combined with soluble TRAIL protein treatment significantly increased tumor cell apoptosis compared to soluble TRAIL treatment alone, mediated via dynamin related protein 1-based mechanisms.126 In a non-small-cell lung cancer xenograft mouse model, systemic administration of both gold NPs and soluble TRAIL significantly reduced overall tumor burden compared to treatment with TRAIL or gold NPs alone, indicating that solid tumors can be sensitized to TRAIL-mediated apoptosis via simultaneous administration of gold NPs.126 In terms of gene delivery, gold NPs were recently functionalized with TRAIL-encoding plasmids in combination with the nucleustargeting steroid dexamethasone (Figure 5).127 PEI-coated gold NPs were functionalized with plasmid DNA via electrostatic interactions, after which NPs were functionalized with a PEI-dexamethasone conjugate via similar interactions with surface bound DNA to form a sandwich-like NP structure (Figure 5).127 The platform significantly enhanced TRAIL gene delivery to tumor cells and reduced overall tumor burden in a mouse liver cancer xenograft model, compared to nontargeted formulations and PEI-DNA complexes alone.127 Collectively, the use of gold NPs as sensitizers to TRAIL-mediated apoptosis and for use in DNA delivery potentially hold promise, but overall remains an understudied area that requires further investigation. Gold NPs are also nondegradable, and their potential for toxicity upon continuous dosing will require further study. Despite potential limitations, their use in photothermal therapy and as imaging agents could enable future TRAIL combination therapies as well as theranostic-based approaches.
Figure 5.
(A) Schematic illustration of PEI-Dexa synthesis and (B) nucleus-targeted sandwich-type Au-PEI/DNA/PEI-Dexa complex for intercellular DNA delivery. Transmission electron microscopic images of (C) Au-PEI, (D) Au-PEI/DNA, and (E) Au-PEI/DNA/PEI-Dexa. (Insets) Higher magnification micrographs of the assembled NPs. (F) Intracellular localization of PEI/DNA, Au-PEI/DNA/PEI (w/w/w, 0.6/1/2), and Au-PEI/DNA/PEI-Dexa (w/w/w, 0.6/1/2) in Hep3B cells after gene transfection for 2 or 6 h (red, Cy5-labeled pDNA; blue, DAPI stained cell nuclei; pink, colocalization). A dose of 1 µg/well of Cy5-labeled pDNA was used. White arrows show colocalization of nucleus and plasmid. Bars = 15 µm. (G) Intranuclear concentration of pFlag-cmv2 in Hep3B cells treated by PEI/DNA, Au-PEI/DNA/PEI (w/w/w, 0.6/1/2), and Au-PEI/DNA/PEI-Dexa (w/w/w, 0.6/1/2) for 2 or 6 h. Nuclear sections were purified by nucleocytoplasmic separation and examined Flag-cmv2 relative concentration to human satellite DNA, a good control for cellular DNA detection. Reprinted with from ref 127. Copyright 2014 American Chemical Society.
Human Serum Albumin (HSA) Nanoparticles
HSA is a small, abundant protein in blood with desirable properties for NP formulations including biodegradability, biocompatibility and nonimmunogenicity.35,128,129 In addition to reducing systemic toxicity and enhancing the tolerance of high antitumor drug doses (Figure 2), HSA-based NPs have been shown to (i) enhance permeability and retention of macromolecules in tumors, and (ii) induce high intracellular penetration through binding of tumor receptor gp60 and the glycoprotein SPARC (secreted protein acidic and rich in cysteine) in the tumor interstitial space.129 Several HSA products are in clinical trials or are approved for clinical use in diagnostics and treatment of cancer and rheumatoid arthritis, including Abraxane, Albunex, and 99mTc-aggregated albumin (Nanocoll and Albures).130–132
In the context of TRAIL delivery, HSA has been utilized to create negatively charged NPs complexed with TRAIL using a macromolecular cross-linking desolvation process. In vivo, HSA NPs induced a 9-fold increase in TRAIL circulation half-life in a mouse melanoma xenograft model.133 Other approaches using HSA NPs have been utilized to overcome multidrug resistance. One such mechanism for drug resistance is the overexpression of efficient transporter proteins on the surface of tumor cells, which can release therapeutics such as doxorubicin from cells, as a means of reducing the therapeutic effect.57 To overcome drug resistance, TRAIL and the targeting ligand transferrin were conjugated to primary amine-functionalized HSA NPs while simultaneously loaded with doxorubicin.57 Treatment with targeted HSA NPs enhanced apoptosis in a range of multidrug resistant tumor cell lines, and localized within mouse tumor xenograft models.57 While the exact mechanism remains unclear, the synergistic cytotoxic effects of TRAIL and doxorubicin could be due to reduced cellular FLICE-like inhibitory protein (c-FLIP) expression and the upregulation of TRAIL receptors DR4 and DR5.57 In summary, recent work suggests that HSA NPs are particularly advantageous as potential long-acting TRAIL therapeutic agents with extended circulation half-life. However, compared to membrane-bound TRAIL liposomal systems, HSA NPs with covalently bound TRAIL will likely be less potent, due to their inability to induce TRAIL receptor clustering.
Interfacing Nanoparticles with Circulating Cells for Advanced TRAIL Delivery Systems
Leveraging Immune Cells for NP-Based TRAIL Delivery
In an effort to enhance the circulation half-life and targeting of NPs, circulating blood cells have recently been employed in a variety of therapeutic applications to enable the “hitchhiking” of NPs.134–136 The concept of cellular hitchhiking is centered on binding NPs to the cell surface, and leveraging the innate ability of red blood cells and immune cells to (i) transport throughout the circulation while avoiding clearance and uptake by the immune system, (ii) remain relatively inert in the circulation until their function is required, and (iii) perform specific functions, including nutrient delivery to tissues, clearance of pathogens, and immune system surveillance.134
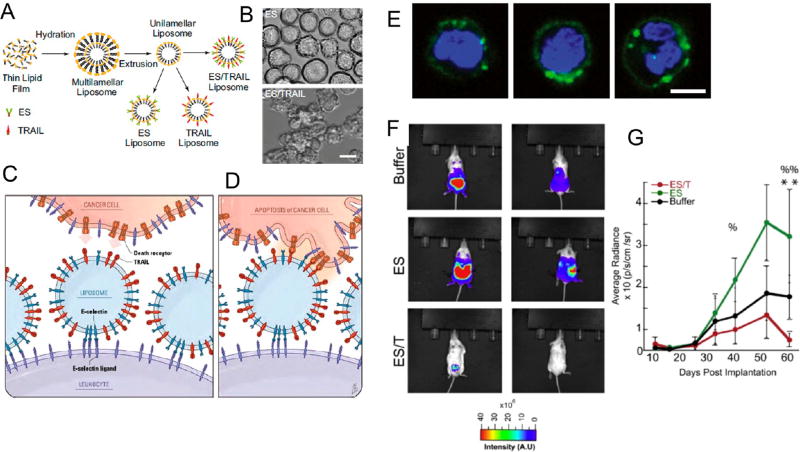
In the context of TRAIL delivery, liposomes functionalized with TRAIL and the vascular adhesion receptor E-selectin137,138 via noncovalent nickel coordination have been used to bind to the surface of circulating immune cells in vivo (Figure 6).139,140 These NP-functionalized immune cells are utilized to target and kill circulating tumor cells (CTCs) in the bloodstream, as a means to reduce metastatic tumor formation.139 In vivo, the delivery system coated circulating immune cells in blood with negligible toxic effects.139 The approach killed most CTCs within 2 h of entering the bloodstream and was more effective than targeting CTCs with liposomes functionalized with TRAIL alone.139 Beyond these benefits, TRAIL displayed a significantly longer circulation half-life due to its tethering to the surface of circulating immune cells.140 Given that CTCs in the bloodstream are rare and difficult to target,141–143 interfacing NP therapeutics with immune cells is a potentially promising approach, as they display similar migration characteristics as CTCs and thus can increase the opportunities for TRAIL to bind and induce apoptosis (Figure 6). Furthermore, the ability to functionalize immune cells in vivo avoids the significant time, cost, and labor burden of conventional ex vivo cellular engineering approaches, which are commonly utilized in most cellular hitchhiking approaches.134
Figure 6.
ES/TRAIL liposomes adhesively interact with and kill cancer cells under uniform shear flow. (A) Synthesis of ES, TRAIL, and ES/TRAIL unilamellar liposomes using a thin film hydration method. Briefly, lipids in chloroform were dried overnight to form a thin lipid film. Lipids were then hydrated and subjected to freeze–thaw cycles to form multilamellar liposomes, which were extruded through membranes to form unilamellar liposomes. ES, TRAIL, or a combination of ES and TRAIL was then conjugated to Ni-NTA on the liposome surface. To assess the ability of ES/TRAIL liposomes to target and kill cancer cells under flow, ES/TRAIL liposomes were added to a suspension of COLO205 cancer cells and exposed to shear flow in a cone-and-plate viscometer at a shear rate of 188 s−1 for 2 h. Cells were then removed, washed, placed into culture for 24 h, and assessed for cell viability. (B) COLO 205 morphology after treatment with ES (top) and ES/TRAIL (bottom) liposomes under shear flow (Scale bar, 20 µm). (C,D) Schematic of the two-step mechanism involving decoration of leukocytes with liposomes (C), which then contact circulating cancer cells and activate the death receptor (D). (E) Confocal images of ES/TRAIL liposomes (green) bound to human leukocytes (blue, cell nuclei) after exposure to shear flow in whole blood in a cone-and-plate viscometer at 188 s−1 for 30 min. Leukocytes have nuclear morphology characteristic of monocytes (left), lymphocytes (center), and neutrophils (right) (scale bar, 5 µm). (F) Whole animal BLI of the ventral (left) and dorsal (right) side of representative animals from each treatment group at the end of the trial (week 9). (G) Average radiance from the primary tumor. Comparisons were made via one-way ANOVA with Tukey’s post-test. Error bars represent the mean ± SD at each time point. ES vs ES/TRAIL: %p < 0.05, %%p < 0.01. Buffer vs ES/TRAIL: **p < 0.05. Reprinted with permission from refs 139 and 140. Copyright 2014 National Academy of Sciences and 2016 Elsevier.
Platelet Membrane-Coated NPs for TRAIL Delivery
In addition to whole cells, cellular membranes are now being interfaced with NPs to improve targeting, increase circulation time, and decrease immunogenicity, while retaining and exploiting the biological functions inherent to cells.119,144–147 One recent development is the “cloaking” of NPs with platelet membranes, by fusing human platelet membranes freshly isolated from blood with biodegradable PLGA NPs.144 Platelet membrane-cloaked NPs exhibit several benefits compared to uncoated NPs, including reduced cellular uptake by macrophages and a lack of NP-induced complement activation, while also displaying platelet-mimicking properties including selective adhesion to damaged endothelium as well as enhanced binding to platelet-adhering pathogens.144
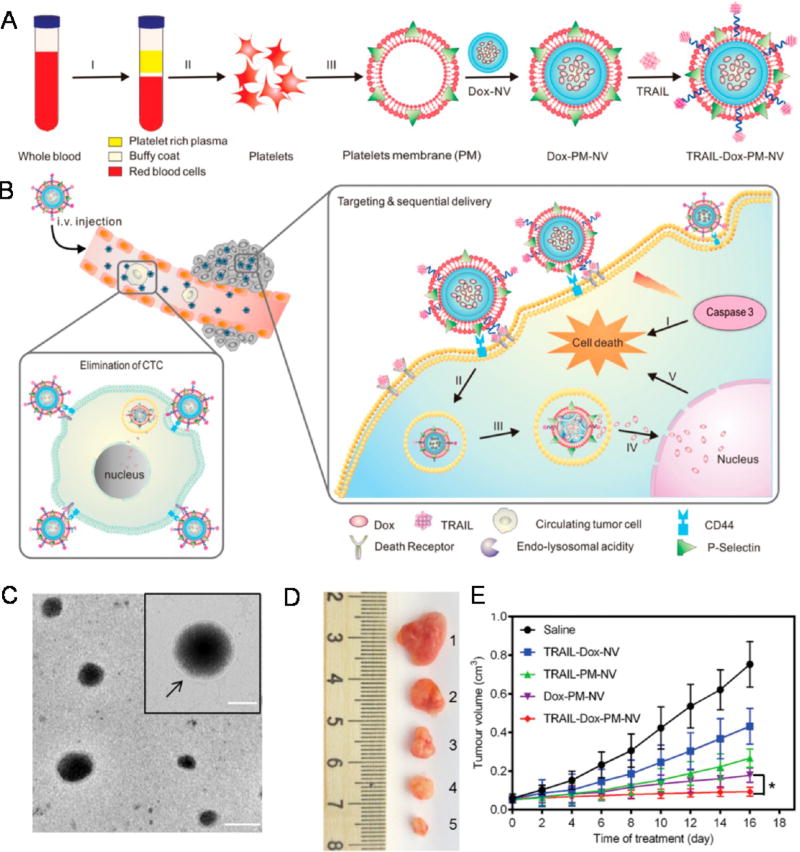
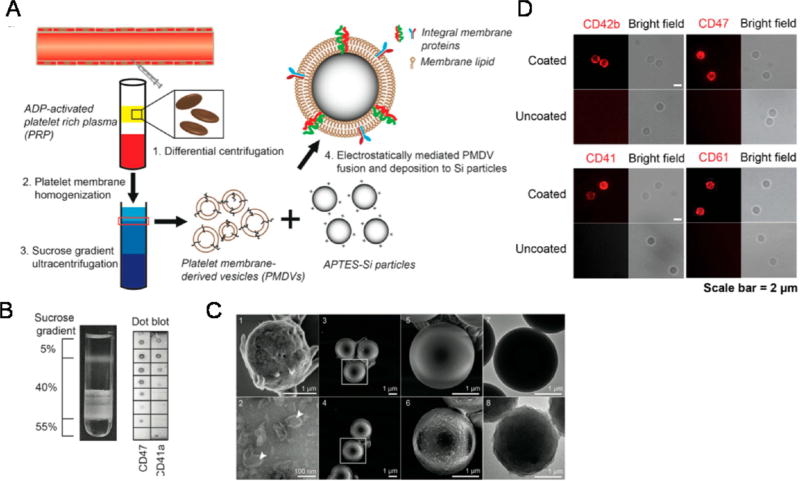
Platelet membrane-coated NPs have recently been employed for in vivo TRAIL delivery.148,149 In a similar approach, doxorubicin-loaded nanogels were cloaked with platelet membranes overexpressing P-selectin, which bind to CD44 receptors upregulated on cancer cells, followed by TRAIL conjugation to the surface (Figure 7).148 Platelet-cloaked NPs exhibited enhanced adhesion and uptake in cancer cells and significantly reduced tumor burden in a mouse breast cancer xenograft model compared to noncoated TRAIL doxorubicin NPs.148 In another study inspired by receptor–ligand interactions between activated platelets and cancer cells in the circulation, biocompatible silica particles were functionalized with platelet membranes and conjugated with TRAIL to target CTCs in blood.149 The silica–platelet membrane approach significantly decreased lung metastases in a mouse breast cancer xenograft model compared to uncoated silica NPs (Figure 8).149 While this approach has demonstrated significant promise in preclinical studies, it must be taken into consideration that tumor cells are remarkably heterogeneous, and may not express adhesion molecules for activated platelet receptors. Thus, further investigation is required to the determine the specific types of metastatic cancer that such a therapeutic approach could target.149 Additionally, the time, cost, and labor burden of isolating platelets, along the reproducibility of such approaches, will need to be further investigated.
Figure 7.
Schematic design of a drug-loaded platelet membrane nanovehicle (PM-NV) for targeting and sequential drug delivery. (A) Main components of TRAIL-Dox-PM-NV: TRAIL-conjugated PM derived from platelets; Dox-NV. (I) centrifugation of whole blood; (II) isolation of platelets; (III) extraction of PM. (B) In vivo elimination of CTCs and sequential delivery of TRAIL and Dox. TRAIL-Dox-PM-NV captured the CTCs via specific affinity of P-selectin and overexpressed CD44 receptors and subsequently triggered TRAIL/Dox-induced apoptosis signaling pathways. (I) Interaction of TRAIL and death receptors (DRs) to trigger the apoptosis signaling; (II) internalization of TRAIL-Dox-PM-NV; (III) dissociation of TRAIL-Dox-PM-NV mediated by the acidity of lyso-endosome; (IV) release and accumulation of Dox in the nuclei; (V) intrinsic apoptosis triggered by Dox. (C) Transmission electron microscope image and hydrodynamic size distribution of PM-NV. Arrow indicates the existence of PM. The scale bar: 100 nm; inset: 50 nm. (D) Representative images of the MDA-MB-231 tumors after treatment with different TRAIL/Dox formulations at day 16 (from top to bottom: (1) saline, (2) TRAIL-Dox-NV, (3) TRAIL-PM-NV, (4) Dox-PM-NV, (5) TRAIL-Dox-PM-NV) at TRAIL dose of 1 mg kg−1 and Dox dose of 2 mg kg−1. (E) MDA-MB-231 tumor growth curves after intravenous injection of different TRAIL/Dox formulations. Error bars indicate SD (n = 5); *p < 0.05 (two-tailed Student’s t-test). Reprinted with permission from ref 148. Copyright 2015 Wiley.
Figure 8.
Functionalization and characterization of PMDV-coated Si particles. (A) Schematic of preparing platelet membrane-coated Si particles. (B) Detection of membrane protein associated lipid layer in discontinuous sucrose gradient solution by dot blot assay. Lipid fractions were identified as translucent layers in between two sucrose concentrations. (C) SEM and TEM characterization. SEM images: (1) activated platelets, (3,5) APTES-Si particles, (4,6) PMDV-coated Si particles. TEM images: (2) PMDVs, (7) APTES-Si particles, (8) PMDV-coated Si particles. Arrow head identifies the hollow structure of PMDVs. (D) Immunofluorescence staining of CD42b, CD47, CD41, and CD61 in PMDV-coated or uncoated Si particles. Far-red and bright-field images are presented. Reprinted with permission from ref 149. Copyright 2015 Elsevier.
Ex Vivo Nanoparticle-Based Genetic Engineering of Cells for TRAIL Therapy
Mesenchymal Stem Cell-Based Therapies
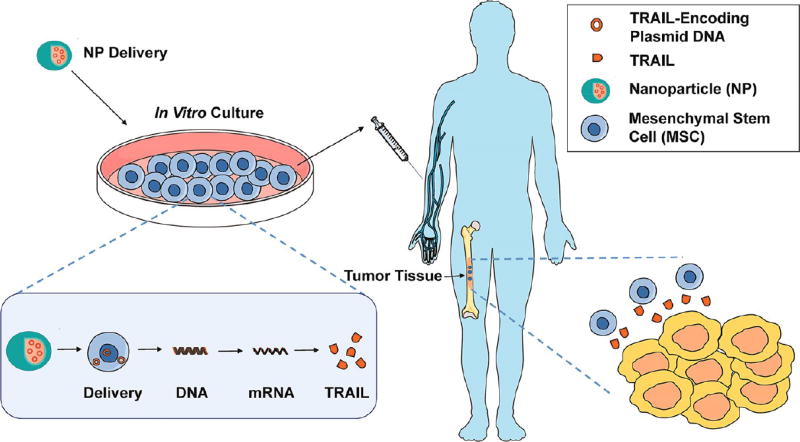
Beyond in vivo delivery of TRAIL-encoding plasmids, which can have significant off-target effects when administered systemically in NP form, cells can also be genetically engineered ex vivo to express and secrete TRAIL (Figure 9). These cells can then be readministered in vivo, where their ability to migrate to diseased tissues is utilized to secrete TRAIL locally (Figure 9). One major cell type used for such approaches are mesenchymal stem cells (MSCs), a form of nonhematopoietic, multipotent stem-like cells currently under investigation as cell-based therapeutics for a range of diseases. In terms of TRAIL delivery, MSCs can be engineered to either secrete or express TRAIL directly on the MSC surface. In contrast to administering TRAIL gene delivery vehicles in vivo, engineered MSCs have two distinct advantages: (i) access to isolated, poorly vascularized tumors and natural migration to sites of inflammation, particularly in the central nervous system, and (ii) potent yet controversial antitumor properties through the downregulation of Akt, NFkB, and Wnt signaling pathways to inhibit tumor growth.34 Supporting this idea, MSCs have been developed as delivery vehicles for interferons, interleukins, prodrugs, oncolytic viruses, antiangiogenic agents, proapoptotic proteins, and growth factor antagonists.34
Figure 9.
Cell-based therapies for TRAIL-based cancer therapeutics. MSCs can be transfected by either viral or nonviral delivery materials, such as NPs, to express and/or secrete TRAIL. MSCs can then cross biological barriers and deliver TRAIL to induce apoptosis in tumor cells.
MSCs may be derived from a range of organs but are primarily obtained from bone marrow.34,150 Bone-marrow-derived MSCs display the largest degree of lineage plasticity, but can only be obtained through invasive procedures at low yields and purities.34 Nonetheless, marrow-derived cells have been shown to be expanded up to 50 passages over a 10 week period.34 Two alternate, less-used sources of MSCs have also gained considerable attention: adipose tissue and umbilical cord blood. MSCs can be reliably extracted from subcutaneous tissue and exhibit a nearly identical expansion potential, differentiation capacity, and immunophenotype to marrow-derived MSCs.34 Genetic stability, easy collection and shipment from the laboratory to the clinic compared to other cell types, and high expansion capabilities enable MSCs to be a potential clinical therapeutic option.150
MSCs that express and secrete TRAIL are a potentially promising therapeutic strategy for cancer treatment, and can be engineered to do so via (i) viral delivery of a TRAIL payload or (ii) nonviral gene delivery using NPs (Figure 9). Viral-based methods have been discussed in detail previously, and vary in terms of their selectivity, immunogenicity, toxicity, and ability to deliver large payloads.151–156 In terms on NP-based delivery, a nonviral vector consisting of a low molecular weight PEI and β-cyclodextrin conjugate was complexed with TRAIL-encoding plasmid DNA for delivery to MSCs.157 Engineered MSCs administered intravenously were shown to migrate into metastatic lung tumors in mice, and induced TRAIL-mediated apoptosis in vivo with negligible lung or liver toxicity.157 In a similar approach, TRAIL-expressing human adipose-derived MSCs were generated through transfection with nucleofector for the treatment of glioma, which extended survival in a rat glioma xenograft model compared to treatment with MSCs alone.158 Other nonviral approaches have shown that pancreas-derived MSCs genetically engineered to express TRAIL are efficient in targeting cells from the same organ, suggesting that MSCs harvested from a particular diseased tissue could be most efficient in targeting that tissue using MSC-based therapies.159
Adipose Tissue-Derived Stem Cell Therapies
In additional to MSCs, human adipose tissue-derived stem cells (ADSCs) have also been genetically engineered for cell-based therapies.160 In contrast of MSCs, ADSCs are a more abundant and easily accessible autologous source of stem cells, have fewer ethical concerns in terms of usage, and have demonstrated more oncogenic resistance than human bone-marrow-derived stem cells.161 In terms of TRAIL delivery, NPs comprised of amino group-ended poly(β-amino ester)s, hydrolytically biodegradable polymers that can condense DNA to form NPs, have been utilized to genetically engineer ADSCs to express TRAIL.160 Engineered ADSCs administered via intracranial injection induced significant inhibition of tumor growth and extended survival in a mouse intracranial xenograft model of glioblastoma multiforme.160 This cell-based approach could represent an alternative therapeutic approach for malignant brain tumors that cannot be removed via surgery.
The translation of stem cell-based therapies into viable clinical strategies is challenged by the fact that large fractions of systemically infused stem cells become entrapped within the lung due to both their large size and via receptor–ligand mediated adhesion to the vessel wall. Such interactions prevent a large number of stem cells from homing and migrating to diseased tissue.162 Furthermore, the site of stem cell administration affects the route by which cells reach target organs. For instance, locally administered stem cells often do not survive as a result of a nutrient and oxygen diffusion limitations.163 In addition to entrapment, stem cells may not survive due to conditions in circulation (i.e., blood vessel diameter, cell deformability).163 Thus, further research to reduce stem cell entrapment and enhance homing is needed to advance cell-mediated TRAIL therapies. Additionally, the heterogeneity of various stem cell populations and mixed results of cell-based therapies in clinical trials for engraftment to tissues have challenged their translational potential.162 Nonetheless, through the optimization of such delivery techniques and a stronger understanding of their homing and migration, tumor-tropic stem cell-based therapies can be leveraged for site-specific delivery and secretion of TRAIL to target tumor cells.
Nanoconjugates for TRAIL Delivery
Beyond NPs, nanoscale conjugates consisting of a therapeutic protein bound to PEG or HSA have also been designed to enhance drug delivery to tumors. Conjugates offer several benefits to delivery, including enhanced TRAIL pharmacokinetics, are nontoxic, nonimmunogenic, and are highly soluble in water.164 Conjugates are ideal delivery vehicles in terms of their size in that they are generally smaller than most lipid and polymeric NPs, which are taken up by the mononuclear phagocytic system in the liver and spleen.165 They are also typically large enough to avoid clearance by the kidney, which excretes particles smaller than 5.5 nm in hydrodynamic diameter.165,166
PEG-Based TRAIL Conjugates
PEG is widely used to enhance the circulation half-life of therapeutics in the bloodstream.167 Conjugation of PEG to TRAIL through PEGylation techniques prolongs in vivo pharmacokinetics by increasing molecular size and steric hindrance, extending circulation half-life, and delaying renal excretion.91 In a recent study, PEGylated TRAIL conjugates were synthesized with varying PEG molecular weights (2–30 kDa), and increased PEG molecular weight was found to improve circulation halflife to 17.8 h compared to 1.1 h for soluble TRAIL protein.168 Larger PEG-TRAIL conjugates also significantly reduced overall tumor burden in a mouse colon cancer xenograft model compared to smaller conjugates and soluble TRAIL protein.168 In addition to extended circulation half-life, PEG can be further customized to enhance both bioactivity and targeting. Multifunctional TRAIL conjugates have been designed by incorporating a zipper sequence to facilitate TRAIL trimerization and enhance potency, while also adding PEG to the N-terminus of TRAIL to increase circulation half-life.169 Heterobifunctional PEGs have also been employed to conjugate both TRAIL and the tumor-targeting ligand transferrin to enhance delivery.91
While TRAIL-PEG conjugates are potentially promising delivery systems, the steric effect of a larger PEG molecule could impact the bioactivity of PEGylated TRAIL conjguates.168 Thus, while optimized PEGylated TRAIL constructs enhance circulation half-life, effects such as steric hindrance can limit its biological activity and thus should be considered when designing conjugates. PEGylation can also occur at random molecular positions, thus affecting biological activity.167
HSA-Based TRAIL Conjugates
As an alternative to PEG, circulating serum proteins such as HSA have been explored to enhance the half-life of TRAIL. The conjugation of albumin protein to therapeutics is a common strategy for extending circulation half-life and has been applied to interferon alpha, erythropoietin, coagulation factors IX and VIIa, human growth hormone, and TNF.170 Noting that serum albumin is the most abundant protein in blood plasma, the protein can be covalently conjugated to a therapeutic of interest or be allowed to noncovalently interact endogenously.170,171 In the case of TNF ligands, albumin proteins such as HSA reduce clearance from circulation yet do not interfere with biological activity.172 Conjugation of HSA to the N-terminus of TRAIL has been shown to increase circulation half-life from 1 to 15 h, with significantly reduced dosages shown to achieve a significant in vivo antitumoral effect in comparison with unconjugated TRAIL.172 HSA-TRAIL conjugates have also demonstrated preserved bioactivity in addition to an improved pharmacodynamics profile.173 In comparison with PEG, HSA-TRAIL conjugates have been understudied and thus warrant further investigation.
CONCLUSIONS
The ability of TRAIL to induce cell death specifically in cancer cells without inducing normal cell toxicity renders the immune cytokine as a promising therapeutic for a wide range of cancers. Recent innovations in the development of polymers, lipids, and inorganic materials are being used to formulate NPs to overcome the challenges faced by TRAIL-based therapeutics in clinical trials, including the improvement of TRAIL circulation half-life, targeting, and bioactivity. In addition to the delivery of TRAIL therapeutic proteins, advances in NPbased systems have also enabled the delivery of TRAIL-encoding plasmid DNA in vivo, opening up therapeutic strategies to transfect tumor cells and the surrounding microenvironment for TRAIL secretion locally at target tumor sites. NPs have also been employed in cell-based therapies, including ex vivo genetic engineering of mesenchymal stem cells, which can traverse complex biological barriers in the body to migrate into tumors and secrete therapeutic TRAIL proteins. While the range of NP-based approaches reviewed here show promise in overcoming many hurdles, additional challenges remain in the development of effective TRAIL cancer therapeutics.
TRAIL resistance remains a significant barrier to clinical translation, as certain types of tumor cells overexpress intracellular proteins, including cFLIP, inhibitor of apoptosis proteins (IAPs) and antiapoptotic members of the Bcl-2 family.174 Moreover, recent research suggests that TRAIL death receptor expression induces side effects in addition to apoptosis, such as cancer cell invasion, which can pose challenges in the context of cancer therapy.175 Although cancer cells show different levels of sensitivity to TRAIL, a wide range of agents have been reported which sensitize cancer cells, including low doses of conventional chemotherapeutics (doxorubicin, 5-fluorouracil, irinotecan, paclitaxel, camptothecin, cisplatin), Bcl-2 inhibitors, IAP antagonists, proteasome inhibitors, histone deacetylase inhibitors, CD20 antibodies, irradiation, aspirin, as well as natural products including piperlongumine, resveratrol, and curcumin.9,176 Given that the majority of these sensitization agents can be encapsulated or conjugated to TRAIL NPs highlighted in this review, we envision numerous opportunities in future research to design NPs for TRAIL combination therapies to overcome drug resistance issues faced in clinical studies.
Moving forward, these delivery materials must be further assessed in advanced animal models, such genetically engineered mouse models of cancer, which better mimic the heterogeneity of human cancers encountered in the clinic. Major differences exist between mice and humans, as mice only express one death receptor (DR5), which shares 43% and 49% sequence homology with human TRAIL-R1 and TRAIL-R2.177,178 Mice also express two decoy receptors that are distinct from human decoy receptors.177,179 While promising, the optimal delivery process needed to improve TRAIL therapy in the clinic remains unsolved, and there is significant room for innovation and development of NPs to achieve these goals.
Acknowledgments
This work was supported by the Bridge Project, a partnership between the Koch Institute for Integrative Cancer Research at MIT and the Dana-Farber/Harvard Cancer Center (to R.L., M.J.M., and R.C.). This work was supported in part by a Cancer Center Support (core) Grant P30-CA14051 from the National Cancer Institute, and a grant from the Koch Institute’s Marble Center for Cancer Nanomedicine (to R.L.). M.J.M. is supported by a Burroughs Wellcome Fund Career Award at the Scientific Interface, a Ruth L. Kirschstein National Research Service Award (F32CA200351) from the National Institutes of Health (NIH), a fellowship from the Max Planck Society and a grant from the Burroughs Wellcome Fund (No. 1015145). P.P.G.G. was supported by Leslie Misrock Cancer Nanotechnology Postdoctoral Fellowship and Fundação Estudar. S.G. was supported by a Fulbright Canada Killam Fellowship.
Footnotes
The authors declare no competing financial interest.
References
- 1.Smyth MJ, Hayakawa Y, Takeda K, Yagita H. New Aspects of Natural-Killer-Cell Surveillance and Therapy of Cancer. Nat. Rev. Cancer. 2002;2:850–861. doi: 10.1038/nrc928. [DOI] [PubMed] [Google Scholar]
- 2.Takeda K, Hayakawa Y, Smyth MJ, Kayagaki N, Yamaguchi N, Kakuta S, Iwakura Y, Yagita H, Okumura K. Involvement of Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand in Surveillance of Tumor Metastasis by Liver Natural Killer Cells. Nat. Med. 2001;7:94–100. doi: 10.1038/83416. [DOI] [PubMed] [Google Scholar]
- 3.Cretney E, Takeda K, Yagita H, Glaccum M, Peschon JJ, Smyth MJ. Increased Susceptibility to Tumor Initiation and Metastasis in TNF-Related Apoptosis-Inducing Ligand-Deficient Mice. J. Immunol. 2002;168:1356–1361. doi: 10.4049/jimmunol.168.3.1356. [DOI] [PubMed] [Google Scholar]
- 4.Johnstone RW, Frew AJ, Smyth MJ. The TRAIL Apoptotic Pathway in Cancer Onset, Progression and Therapy. Nat. Rev. Cancer. 2008;8:782–798. doi: 10.1038/nrc2465. [DOI] [PubMed] [Google Scholar]
- 5.Plasilova M, Zivny J, Jelinek J, Neuwirtova R, Cermak J, Necas E, Andera L, Stopka T. TRAIL (Apo2L) Suppresses Growth of Primary Human Leukemia and Myelodysplasia Progenitors. Leukemia. 2002;16:67–73. doi: 10.1038/sj.leu.2402338. [DOI] [PubMed] [Google Scholar]
- 6.Subbiah V, Brown RE, Buryanek J, Trent J, Ashkenazi A, Herbst R, Kurzrock R. Targeting the Apoptotic Pathway in Chondrosarcoma Using Recombinant Human Apo2L/TRAIL (Dulanermin), a Dual Proapoptotic Receptor (DR4/DR5) Agonist. Mol. Cancer Ther. 2012;11:2541–2546. doi: 10.1158/1535-7163.MCT-12-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herbst RS, Eckhardt SG, Kurzrock R, Ebbinghaus S, O’Dwyer PJ, Gordon MS, Novotny W, Goldwasser MA, Tohnya TM, Lum BL, Ashkenazi A, Jubb AM, Mendelson DS. Phase I Dose-Escalation Study of Recombinant Human Apo2L/TRAIL, a Dual Proapoptotic Receptor Agonist, in Patients with Advanced Cancer. J. Clin. Oncol. 2010;28:2839–2846. doi: 10.1200/JCO.2009.25.1991. [DOI] [PubMed] [Google Scholar]
- 8.Soria J-C, Márk Z, Zatloukal P, Szima B, Albert I, Juhász E, Pujol J-L, Kozielski J, Baker N, Smethurst D, Hei YJ, Ashkenazi A, Stern H, Amler L, Pan Y, Blackhall F. Randomized Phase II Study of Dulanermin in Combination with Paclitaxel, Carboplatin, and Bevacizumab in Advanced Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2011;29:4442–4451. doi: 10.1200/JCO.2011.37.2623. [DOI] [PubMed] [Google Scholar]
- 9.Ashkenazi A. Targeting the Extrinsic Apoptotic Pathway in Cancer: Lessons Learned and Future Directions. J. Clin. Invest. 2015;125:487–489. doi: 10.1172/JCI80420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelley SK, Harris LA, Xie D, Deforge L, Totpal K, Bussiere J, Fox JA. Preclinical Studies to Predict the Disposition of Apo2L/Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand in Humans: Characterization of in Vivo Efficacy, Pharmacokinetics, and Safety. J. Pharmacol. Exp. Ther. 2001;299:31–38. [PubMed] [Google Scholar]
- 11.Soria J-C, Smit E, Khayat D, Besse B, Yang X, Hsu C-P, Reese D, Wiezorek J, Blackhall F. Phase 1b Study of Dulanermin (Recombinant Human Apo2L/TRAIL) in Combination with Paclitaxel, Carboplatin, and Bevacizumab in Patients with Advanced Non-Squamous Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2010;28:1527–1533. doi: 10.1200/JCO.2009.25.4847. [DOI] [PubMed] [Google Scholar]
- 12.Geng C, Hou J, Zhao Y, Ke X, Wang Z, Qiu L, Xi H, Wang F, Wei N, Liu Y, Yang S, Wei P, Zheng X, Huang Z, Zhu B, Chen WM. A Multicenter, Open-Label Phase II Study of Recombinant CPT (Circularly Permuted TRAIL) Plus Thalidomide in Patients with Relapsed and Refractory Multiple Myeloma. Am. J. Hematol. 2014;89:1037–1042. doi: 10.1002/ajh.23822. [DOI] [PubMed] [Google Scholar]
- 13.Hotte SJ, Hirte HW, Chen EX, Siu LL, Le LH, Corey A, Iacobucci A, MacLean M, Lo L, Fox NL, Oza AM. A Phase 1 Study of Mapatumumab (Fully Human Monoclonal Antibody to TRAIL-R1) in Patients with Advanced Solid Malignancies. Clin. Cancer Res. 2008;14:3450–3455. doi: 10.1158/1078-0432.CCR-07-1416. [DOI] [PubMed] [Google Scholar]
- 14.Greco FA, Bonomi P, Crawford J, Kelly K, Oh Y, Halpern W, Lo L, Gallant G, Klein J. Phase 2 Study of Mapatumumab, a Fully Human Agonistic Monoclonal Antibody Which Targets and Activates the TRAIL Receptor-1, in Patients with Advanced Non-Small Cell Lung Cancer. Lung Cancer. 2008;61:82–90. doi: 10.1016/j.lungcan.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Younes A, Vose JM, Zelenetz AD, Smith MR, Burris HA, Ansell SM, Klein J, Halpern W, Miceli R, Kumm E, Fox NL, Czuczman MS. A Phase 1b/2 Trial of Mapatumumab in Patients with Relapsed/Refractory Non-Hodgkin’s Lymphoma. Br. J. Cancer. 2010;103:1783–1787. doi: 10.1038/sj.bjc.6605987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Pawel J, Harvey JH, Spigel DR, Dediu M, Reck M, Cebotaru CL, Humphreys RC, Gribbin MJ, Fox NL, Camidge DR. Phase II Trial of Mapatumumab, a Fully Human Agonist Monoclonal Antibody to Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand Receptor 1 (TRAIL-R1), in Combination with Paclitaxel and Carboplatin in Patients with Advanced Non–Small-Cell Lung Cancer. Clin. Lung Cancer. 2014;15:188–196.e2. doi: 10.1016/j.cllc.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Trarbach T, Moehler M, Heinemann V, Köhne C-H, Przyborek M, Schulz C, Sneller V, Gallant G, Kanzler S. Phase II Trial of Mapatumumab, a Fully Human Agonistic Monoclonal Antibody That Targets and Activates the Tumour Necrosis Factor Apoptosis-Inducing Ligand Receptor-1 (TRAIL-R1), in Patients with Refractory Colorectal Cancer. Br. J. Cancer. 2010;102:506–512. doi: 10.1038/sj.bjc.6605507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wakelee HA, Patnaik A, Sikic BI, Mita M, Fox NL, Miceli R, Ullrich SJ, Fisher GA, Tolcher AW. Phase I and Pharmacokinetic Study of Lexatumumab (HGS-ETR2) Given Every 2 Weeks in Patients with Advanced Solid Tumors. Annu. Oncol. 2010;21:376–381. doi: 10.1093/annonc/mdp292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herbst RS, Kurzrock R, Hong DS, Valdivieso M, Hsu C-P, Goyal L, Juan G, Hwang YC, Wong S, Hill JS, Friberg G, LoRusso PM. A First-in-Human Study of Conatumumab in Adult Patients with Advanced Solid Tumors. Clin. Cancer Res. 2010;16:5883–5891. doi: 10.1158/1078-0432.CCR-10-0631. [DOI] [PubMed] [Google Scholar]
- 20.Kindler HL, Richards DA, Garbo LE, Garon EB, Stephenson JJ, Rocha-Lima CM, Safran H, Chan D, Kocs DM, Galimi F, McGreivy J, Bray SL, Hei Y, Feigal EG, Loh E, Fuchs CS. A Randomized, Placebo-Controlled Phase 2 Study of Ganitumab (AMG 479) or Conatumumab (AMG 655) in Combination with Gemcitabine in Patients with Metastatic Pancreatic Cancer. Annu. Oncol. 2012;23:2834–2842. doi: 10.1093/annonc/mds142. [DOI] [PubMed] [Google Scholar]
- 21.Paz-Ares L, Bálint B, de Boer RH, van Meerbeeck JP, Wierzbicki R, De Souza P, Galimi F, Haddad V, Sabin T, Hei YJ, Pan Y, Cottrell S, Hsu CP, RamLau R. A Randomized Phase 2 Study of Paclitaxel and Carboplatin with or Without Conatumumab for First-Line Treatment of Advanced Non–Small-Cell Lung Cancer. J. Thorac. Oncol. 2013;8:329–337. doi: 10.1097/JTO.0b013e31827ce554. [DOI] [PubMed] [Google Scholar]
- 22.Camidge DR, Herbst RS, Gordon MS, Eckhardt SG, Kurzrock R, Durbin B, Ing J, Tohnya TM, Sager J, Ashkenazi A, Bray G, Mendelson D. A Phase I Safety and Pharmacokinetic Study of the Death Receptor 5 Agonistic Antibody PRO95780 in Patients with Advanced Malignancies. Clin. Cancer Res. 2010;16:1256–1263. doi: 10.1158/1078-0432.CCR-09-1267. [DOI] [PubMed] [Google Scholar]
- 23.Rocha Lima CS, Baranda JC, Wallmark J, Choi Y, Royer-Joo S, Portera CC. Phase Ib Study of Drozitumab Combined with First-Line FOLFOX Plus Bevacizumab (BV) in Patients (Pts) with Metastatic Colorectal Cancer (mCRC) J. Clin. Oncol. 2011;29:546–546. [Google Scholar]
- 24.Forero-Torres A, Varley KE, Abramson VG, Li Y, Vaklavas C, Lin NU, Liu MC, Rugo HS, Nanda R, Storniolo AM, Traina TA, Patil S, Van Poznak CH, Nangia JR, Irvin WJ, Jr, Krontiras H, De Los Santos JF, Haluska P, Grizzle W, Myers RM, et al. TBCRC 019: a Phase II Trial of Nanoparticle Albumin-Bound Paclitaxel with or Without the Anti-Death Receptor 5 Monoclonal Antibody Tigatuzumab in Patients with Triple-Negative Breast Cancer. Clin. Cancer Res. 2015;21:2722–2729. doi: 10.1158/1078-0432.CCR-14-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forero-Torres A, Shah J, Wood T, Posey J, Carlisle R, Copigneaux C, Luo FR, Wojtowicz-Praga S, Percent I, Saleh M. Phase I Trial of Weekly Tigatuzumab, an Agonistic Humanized Monoclonal Antibody Targeting Death Receptor 5 (DR5) Cancer Biother. Radiopharm. 2010;25:13–19. doi: 10.1089/cbr.2009.0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reck M, Krzakowski M, Chmielowska E, Sebastian M, Hadler D, Fox T, Wang Q, Greenberg J, Beckman RA, von Pawel J. A Randomized, Double-Blind, Placebo-Controlled Phase 2 Study of Tigatuzumab (CS-1008) in Combination with Carboplatin/Paclitaxel in Patients with Chemotherapy-Naïve Metastatic/Unresectable Non-Small Cell Lung Cancer. Lung Cancer. 2013;82:441–448. doi: 10.1016/j.lungcan.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 27.Forero-Torres A, Infante JR, Waterhouse D, Wong L, Vickers S, Arrowsmith E, He AR, Hart L, Trent D, Wade J, Jin X, Wang Q, Austin T, Rosen M, Beckman R, von Roemeling R, Greenberg J, Saleh M. Phase 2, Multicenter, Open-Label Study of Tigatuzumab (CS-1008), a Humanized Monoclonal Antibody Targeting Death Receptor 5, in Combination with Gemcitabine in Chemotherapy-Naive Patients with Unresectable or Metastatic Pancreatic Cancer. Cancer Med. 2013;2:925–932. doi: 10.1002/cam4.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papadopoulos KP, Isaacs R, Bilic S, Kentsch K, Huet HA, Hofmann M, Rasco D, Kundamal N, Tang Z, Cooksey J, Mahipal A. Unexpected Hepatotoxicity in a Phase I Study of TAS266, a Novel Tetravalent Agonistic Nanobody® Targeting the DR5 Receptor. Cancer Chemother. Pharmacol. 2015;75:887–895. doi: 10.1007/s00280-015-2712-0. [DOI] [PubMed] [Google Scholar]
- 29.Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Nanoparticles in Medicine: Therapeutic Applications and Developments. Clin. Pharmacol. Ther. 2008;83:761–769. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- 30.Groneberg D, Giersig M, Welte T, Pison U. Nanoparticle-Based Diagnosis and Therapy. Curr. Drug Targets. 2006;7:643–648. doi: 10.2174/138945006777435245. [DOI] [PubMed] [Google Scholar]
- 31.Emerich DF, Thanos CG. Targeted Nanoparticle-Based Drug Delivery and Diagnosis. J. Drug Target. 2007;15:163–183. doi: 10.1080/10611860701231810. [DOI] [PubMed] [Google Scholar]
- 32.Singh R, Lillard JW., Jr Nanoparticle-Based Targeted Drug Delivery. Exp. Mol. Pathol. 2009;86:215–223. doi: 10.1016/j.yexmp.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stuckey DW, Shah K. TRAIL on Trial: Preclinical Advances in Cancer Therapy. Trends Mol. Med. 2013;19:685–694. doi: 10.1016/j.molmed.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shah K. Mesenchymal Stem Cells Engineered for Cancer Therapy. Adv. Drug Delivery Rev. 2012;64:739–748. doi: 10.1016/j.addr.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elsadek B, Kratz F. Impact of Albumin on Drug Delivery-New Applications on the Horizon. J. Controlled Release. 2012;157:4–28. doi: 10.1016/j.jconrel.2011.09.069. [DOI] [PubMed] [Google Scholar]
- 36.Harris JM, Chess RB. Effect of Pegylation on Pharmaceuticals. Nat. Rev. Drug Discovery. 2003;2:214–221. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 37.Holliger P, Hudson PJ. Engineered Antibody Fragments and the Rise of Single Domains. Nat. Biotechnol. 2005;23:1126–1136. doi: 10.1038/nbt1142. [DOI] [PubMed] [Google Scholar]
- 38.O’Brien MER, Wigler N, Inbar M, Rosso R, Grischke E, Santoro A, Catane R, Kieback DG, Tomczak P, Ackland SP, Orlandi F, Mellars L, Tendler C. Reduced Cardiotoxicity and Comparable Efficacy in a Phase III Trial of Pegylated Liposomal Doxorubicin HCl (CAELYX/Doxil®) Versus Conventional Doxorubicin for First-Line Treatment of Metastatic Breast Cancer. Annu. Oncol. 2004;15:440–449. doi: 10.1093/annonc/mdh097. [DOI] [PubMed] [Google Scholar]
- 39.Cortes J, Saura C. Nanoparticle Albumin-Bound (Nab)-Paclitaxel: Improving Efficacy and Tolerability by Targeted Drug Delivery in Metastatic Breast Cancer. EJC Suppl. 2010;8:1–10. [Google Scholar]
- 40.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an Emerging Platform for Cancer Therapy. Nat. Nanotechnol. 2007;2:751. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 41.Matsumura Y, Maeda H. A New Concept for Macromolecular Therapeutics in Cancer Chemotherapy: Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 42.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor Vascular Permeability and the EPR Effect in Macromolecular Therapeutics: a Review. J. Controlled Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 43.Fox ME, Szoka FC, Fréchet JMJ. Soluble Polymer Carriers for the Treatment of Cancer: the Importance of Molecular Architecture. Acc. Chem. Res. 2009;42:1141–1151. doi: 10.1021/ar900035f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hashizume H, Baluk P, Morikawa S, McLean JW, Thurston G, Roberge S, Jain RK, McDonald DM. Openings Between Defective Endothelial Cells Explain Tumor Vessel Leakiness. Am. J. Pathol. 2000;156:1363–1380. doi: 10.1016/S0002-9440(10)65006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitchell MJ, Jain RK, Langer R. Engineering and Physical Sciences in Oncology: Challenges and Opportunities. Nat. Rev. Cancer. 2017;17:659–675. doi: 10.1038/nrc.2017.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perrault SD, Chan WCW. In Vivo Assembly of Nanoparticle Components to Improve Targeted Cancer Imaging. Proc. Natl. Acad. Sci. U. S. A. 2010;107:11194–11199. doi: 10.1073/pnas.1001367107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prabhakar, Maeda H, Jain RK, Sevick-Muraca EM, Zamboni W, Farokhzad OC, Barry ST, Gabizon A, Grodzinski P, Blakey DC. Challenges and Key Considerations of the Enhanced Permeability and Retention Effect for Nanomedicine Drug Delivery in Oncology. Cancer Res. 2013;73:2412–2417. doi: 10.1158/0008-5472.CAN-12-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun T, Zhang YS, Pang B, Hyun DC, Yang M, Xia Y. Engineered Nanoparticles for Drug Delivery in Cancer Therapy. Angew. Chem., Int. Ed. 2014;53:12320–12364. doi: 10.1002/anie.201403036. [DOI] [PubMed] [Google Scholar]
- 49.Swami A, Reagan MR, Basto P, Mishima Y, Kamaly N, Glavey S, Zhang S, Moschetta M, Seevaratnam D, Zhang Y, Liu J, Memarzadeh M, Wu J, Manier S, Shi J, Bertrand N, Lu ZN, Nagano K, Baron R, Sacco A, et al. Engineered Nanomedicine for Myeloma and Bone Microenvironment Targeting. Proc. Natl. Acad. Sci. U. S. A. 2014;111:10287–10292. doi: 10.1073/pnas.1401337111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murphy EA, Majeti BK, Barnes LA, Makale M, Weis SM, Lutu-Fuga K, Wrasidlo W, Cheresh DA. Nanoparticle-Mediated Drug Delivery to Tumor Vasculature Suppresses Metastasis. Proc. Natl. Acad. Sci. U. S. A. 2008;105:9343–9348. doi: 10.1073/pnas.0803728105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dhar S, Gu FX, Langer R, Farokhzad OC, Lippard SJ. Targeted Delivery of Cisplatin to Prostate Cancer Cells by Aptamer Functionalized Pt(IV) Prodrug-PLGA–PEG Nanoparticles. Proc. Natl. Acad. Sci. U. S. A. 2008;105:17356–17361. doi: 10.1073/pnas.0809154105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ashkenazi A, Holland P, Eckhardt SG. Ligand-Based Targeting of Apoptosis in Cancer: the Potential of Recombinant Human Apoptosis Ligand 2/Tumor Necrosis Factor–Related Apoptosis-Inducing Ligand (rhApo2L/TRAIL) J. Clin. Oncol. 2008;26:3621–3630. doi: 10.1200/JCO.2007.15.7198. [DOI] [PubMed] [Google Scholar]
- 53.Wang S. The Promise of Cancer Therapeutics Targeting the TNF-Related Apoptosis-Inducing Ligand and TRAIL Receptor Pathway. Oncogene. 2008;27:6207–6215. doi: 10.1038/onc.2008.298. [DOI] [PubMed] [Google Scholar]
- 54.Micheau O, Shirley S, Dufour F. Death Receptors as Targets in Cancer. Br. J. Pharmacol. 2013;169:1723–1744. doi: 10.1111/bph.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Loi M, Becherini P, Emionite L, Giacomini A, Cossu I, Destefanis E, Brignole C, Di Paolo D, Piaggio F, Perri P, Cilli M, Pastorino F, Ponzoni M. sTRAIL Coupled to Liposomes Improves Its Pharmacokinetic Profile and Overcomes Neuroblastoma Tumour Resistance in Combination with Bortezomib. J. Controlled Release. 2014;192:157–166. doi: 10.1016/j.jconrel.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 56.Jiang T, Mo R, Bellotti A, Zhou J, Gu Z. Drug Delivery: Gel–Liposome-Mediated Co-Delivery of Anticancer Membrane-Associated Proteins and Small-Molecule Drugs for Enhanced Therapeutic Efficacy. Adv. Funct. Mater. 2014;24:2258–2258. [Google Scholar]
- 57.Bae S, Ma K, Kim TH, Lee ES, Oh KT, Park E-S, Lee KC, Youn YS. Doxorubicin-Loaded Human Serum Albumin Nanoparticles Surface-Modified with TNF-Related Apoptosis-Inducing Ligand and Transferrin for Targeting Multiple Tumor Types. Biomaterials. 2012;33:1536–1546. doi: 10.1016/j.biomaterials.2011.10.050. [DOI] [PubMed] [Google Scholar]
- 58.Barenholz YC. Doxil® — the First FDA-Approved Nano-Drug: Lessons Learned. J. Controlled Release. 2012;160:117–134. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 59.Petre CE, Dittmer DP. Liposomal Daunorubicin as Treatment for Kaposi’s Sarcoma. Int. J. Nanomed. 2007;2:277–288. [PMC free article] [PubMed] [Google Scholar]
- 60.Ayen WY, Kumar N. In Vivo Evaluation of Doxorubicin-Loaded (PEG)3-PLA Nanopolymersomes (PolyDoxSome) Using DMBA-Induced Mammary Carcinoma Rat Model and Comparison with Marketed LipoDox. Pharm. Res. 2012;29:2522–2533. doi: 10.1007/s11095-012-0783-8. [DOI] [PubMed] [Google Scholar]
- 61.Chou H-H, Wang K-L, Chen C-A, Wei L-H, Lai C-H, Hsieh C-Y, Yang Y-C, Twu N-F, Chang T-C, Yen M-S. Pegylated Liposomal Doxorubicin (Lipo-Dox®) for Platinum-Resistant or Refractory Epithelial Ovarian Carcinoma: a Taiwanese Gynecologic Oncology Group Study with Long-Term Follow-Up. Gynecol. Oncol. 2006;101:423–428. doi: 10.1016/j.ygyno.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 62.Park JW. Liposome-Based Drug Delivery in Breast Cancer Treatment. Breast Cancer Res. 2002;4:95. doi: 10.1186/bcr432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Batist G, Ramakrishnan G, Rao CS, Chandrasekharan A, Gutheil J, Guthrie T, Shah P, Khojasteh A, Nair MK, Hoelzer K, Tkaczuk K, Park YC, Lee LW. Reduced Cardiotoxicity and Preserved Antitumor Efficacy of Liposome-Encapsulated Doxorubicin and Cyclophosphamide Compared with Conventional Doxorubicin and Cyclophosphamide in a Randomized, Multicenter Trial of Metastatic Breast Cancer. J. Clin. Oncol. 2001;19:1444–1454. doi: 10.1200/JCO.2001.19.5.1444. [DOI] [PubMed] [Google Scholar]
- 64.Silverman JA, Deitcher SR. Marqibo® (Vincristine Sulfate Liposome Injection) Improves the Pharmacokinetics and Pharmacodynamics of Vincristine. Cancer Chemother. Pharmacol. 2013;71:555–564. doi: 10.1007/s00280-012-2042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fenske DB, Cullis PR. Liposomal Nanomedicines. Expert Opin. Drug Delivery. 2008;5:25–44. doi: 10.1517/17425247.5.1.25. [DOI] [PubMed] [Google Scholar]
- 66.Torchilin VP. Multifunctional, Stimuli-Sensitive Nanoparticulate Systems for Drug Delivery. Nat. Rev. Drug Discovery. 2014;13:813–827. doi: 10.1038/nrd4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Allen TM, Cullis PR. Liposomal Drug Delivery Systems: From Concept to Clinical Applications. Adv. Drug Delivery Rev. 2013;65:36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 68.Nair PM, Flores H, Gogineni A, Marsters S, Lawrence DA, Kelley RF, Ngu H, Sagolla M, Komuves L, Bourgon R, Settleman J, Ashkenazi A. Enhancing the Antitumor Efficacy of a Cell-Surface Death Ligand by Covalent Membrane Display. Proc. Natl. Acad. Sci. U. S. A. 2015;112:5679–5684. doi: 10.1073/pnas.1418962112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wajant H, Moosmayer D, Wuest T, Bartke T, Gerlach E, Schonherr U, Peters N, Scheurich P, Pfizenmaier K. Differential Activation of TRAIL-R1 and −2 by Soluble and Membrane TRAIL Allows Selective Surface Antigen-Directed Activation of TRAIL-R2 by a Soluble TRAIL Derivative. Oncogene. 2001;20:4101–4106. doi: 10.1038/sj.onc.1204558. [DOI] [PubMed] [Google Scholar]
- 70.De Miguel D, Gallego-Lleyda A, Anel A, Martinez-Lostao L. Liposome-Bound TRAIL Induces Superior DR5 Clustering and Enhanced DISC Recruitment in Histiocytic Lymphoma U937 Cells. Leuk. Res. 2015;39:657–666. doi: 10.1016/j.leukres.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 71.De Miguel D, Basáñez G, Sánchez D, Malo PG, Marzo I, Larrad L, Naval J, Pardo J, Anel A, Martinez-Lostao L. Liposomes Decorated with Apo2L/TRAIL Overcome Chemoresistance of Human Hematologic Tumor Cells. Mol. Pharmaceutics. 2013;10:893–904. doi: 10.1021/mp300258c. [DOI] [PubMed] [Google Scholar]
- 72.De Miguel D, Gallego-Lleyda A, Ayuso JM, Pejenaute-Ochoa D, Jarauta V, Marzo I, Fernandez LJ, Ochoa I, Conde B, Anel A, Martinez-Lostao L. High-Order TRAIL Oligomer Formation in TRAIL-Coated Lipid Nanoparticles Enhances DR5 Cross-Linking and Increases Antitumour Effect Against Colon Cancer. Cancer Lett. 2016;383:250–260. doi: 10.1016/j.canlet.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 73.De Miguel D, Gallego-Lleyda A, Ayuso JM, Erviti-Ardanaz S, Pazo-Cid R, del Agua C, Fernández LJ, Ochoa I, Anel A, Martinez-Lostao L. TRAIL-Coated Lipid-Nanoparticles Overcome Resistance to Soluble Recombinant TRAIL in Non-Small Cell Lung Cancer Cells. Nanotechnology. 2016;27:185101. doi: 10.1088/0957-4484/27/18/185101. [DOI] [PubMed] [Google Scholar]
- 74.Seifert O, Pollak N, Nusser A, Steiniger F, Rüger R, Pfizenmaier K, Kontermann RE. Immuno-LipoTRAIL: Targeted Delivery of TRAIL-Functionalized Liposomal Nanoparticles. Bioconjugate Chem. 2014;25:879–887. doi: 10.1021/bc400517j. [DOI] [PubMed] [Google Scholar]
- 75.Skidan I, Miao B, Thekkedath RV, Dholakia P, Degterev A, Torchilin V. In Vitro Cytotoxicity of Novel Pro-Apoptotic Agent DM-PIT-1 in PEG-PE-Based Micelles Alone and in Combination with TRAIL. Drug Delivery. 2009;16:45–51. doi: 10.1080/10717540802517951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Farhood H, Serbina N, Huang L. The Role of Dioleoyl Phosphatidylethanolamine in Cationic Liposome Mediated Gene Transfer. Biochim. Biophys. Acta, Biomembr. 1995;1235:289–295. doi: 10.1016/0005-2736(95)80016-9. [DOI] [PubMed] [Google Scholar]
- 77.Sun X, Pang Z, Ye H, Qiu B, Guo L, Li J, Ren J, Qian Y, Zhang Q, Chen J, Jiang X. Co-Delivery of pEGFP-hTRAIL and Paclitaxel to Brain Glioma Mediated by an Angiopep-Conjugated Liposome. Biomaterials. 2012;33:916–924. doi: 10.1016/j.biomaterials.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 78.Luo C, Miao L, Zhao Y, Musetti S, Wang Y, Shi K, Huang L. A Novel Cationic Lipid with Intrinsic Antitumor Activity to Facilitate Gene Therapy of TRAIL DNA. Biomaterials. 2016;102:239–248. doi: 10.1016/j.biomaterials.2016.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cheng CJ, Tietjen GT, Saucier-Sawyer JK, Saltzman WM. A Holistic Approach to Targeting Disease with Polymeric Nanoparticles. Nat. Rev. Drug Discovery. 2015;14:365. doi: 10.1038/nrd4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liechty WB, Kryscio DR, Slaughter BV, Peppas NA. Polymers for Drug Delivery Systems. Annu. Rev. Chem. Biomol. Eng. 2010;1:149–173. doi: 10.1146/annurev-chembioeng-073009-100847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhu L, Mahato RI. Lipid and Polymeric Carrier-Mediated Nucleic Acid Delivery. Expert Opin. Drug Delivery. 2010;7:1209–1226. doi: 10.1517/17425247.2010.513969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Werner ME, Cummings ND, Sethi M, Wang EC, Sukumar R, Moore DT, Wang AZ. Preclinical Evaluation of Genexol-PM, a Nanoparticle Formulation of Paclitaxel, as a Novel Radiosensitizer for the Treatment of Non-Small Cell Lung Cancer. Int. J. Radiat. Oncol., Biol., Phys. 2013;86:463–468. doi: 10.1016/j.ijrobp.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee KS, Chung HC, Im SA, Park YH, Kim CS, Kim S-B, Rha SY, Lee MY, Ro J. Multicenter Phase II Trial of Genexol-PM, a Cremophor-Free, Polymeric Micelle Formulation of Paclitaxel, in Patients with Metastatic Breast Cancer. Breast Cancer Res. Treat. 2008;108:241–250. doi: 10.1007/s10549-007-9591-y. [DOI] [PubMed] [Google Scholar]
- 84.Hrkach J, von Hoff D, Ali MM, Andrianova E, Auer J, Campbell T, De Witt D, Figa M, Figueiredo M, Horhota A, Low S, McDonnell K, Peeke E, Retnarajan B, Sabnis A, Schnipper E, Song JJ, Summa J, Tompsett D, et al. Preclinical Development and Clinical Translation of a PSMA-Targeted Docetaxel Nanoparticle with a Differentiated Pharmacological Profile. Sci. Transl. Med. 2012;4:128ra39. doi: 10.1126/scitranslmed.3003651. [DOI] [PubMed] [Google Scholar]
- 85.Davis ME, Zuckerman JE, Choi CHJ, Seligson D, Tolcher A, Alabi CA, Yen Y, Heidel JD, Ribas A. Evidence of RNAi in Humans From Systemically Administered siRNA via Targeted Nanoparticles. Nature. 2010;464:1067–1070. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cabral H, Kataoka K. Progress of Drug-Loaded Polymeric Micelles Into Clinical Studies. J. Controlled Release. 2014;190:465–476. doi: 10.1016/j.jconrel.2014.06.042. [DOI] [PubMed] [Google Scholar]
- 87.Anselmo AC, Mitragotri S. Nanoparticles in the Clinic. Bioeng. Transl. Med. 2016;1:10–29. doi: 10.1002/btm2.10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.U.S. National Library of Medicine Clinicaltrials.gov. [accessed Nov 18, 2017]; https://clinicaltrials.gov/ct2/show/NCT01644890?cond=nk105&rank=1.
- 89.Perlstein B, Finniss SA, Miller C, Okhrimenko H, Kazimirsky G, Cazacu S, Lee HK, Lemke N, Brodie S, Umansky F, Rempel SA, Rosenblum M, Mikklesen T, Margel S, Brodie C. TRAIL Conjugated to Nanoparticles Exhibits Increased Anti-Tumor Activities in Glioma Cells and Glioma Stem Cells in Vitro and in Vivo. Neuro Oncol. 2013;15:29–40. doi: 10.1093/neuonc/nos248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim H, Jeong D, Kang HE, Lee KC, Na K. A Sulfate Polysaccharide/TNF-Related Apoptosis-Inducing Ligand (TRAIL) Complex for the Long-Term Delivery of TRAIL in Poly(Lactic-Co-Glycolic Acid) (PLGA) Microspheres. J. Pharm. Pharmacol. 2013;65:11–21. doi: 10.1111/j.2042-7158.2012.01564.x. [DOI] [PubMed] [Google Scholar]
- 91.Kim TH, Jo YG, Jiang HH, Lim SM, Youn YS, Lee S, Chen X, Byun Y, Lee KC. PEG-Transferrin Conjugated TRAIL (TNF-Related Apoptosis-Inducing Ligand) for Therapeutic Tumor Targeting. J. Controlled Release. 2012;162:422–428. doi: 10.1016/j.jconrel.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jaganathan J, Petit JH, Lazio BE, Singh SK, Chin LS. Tumor Necrosis Factor–Related Apoptosis-Inducing Ligand–Mediated Apoptosis in Established and Primary Glioma Cell Lines. Neurosurg. Focus. 2002;13:1–11. doi: 10.3171/foc.2002.13.3.6. [DOI] [PubMed] [Google Scholar]
- 93.Wohlfahrt ME, Beard BC, Lieber A, Kiem H-P. A Capsid-Modified, Conditionally Replicating Oncolytic Adenovirus Vector Expressing TRAIL Leads to Enhanced Cancer Cell Killing in Human Glioblastoma Models. Cancer Res. 2007;67:8783–8790. doi: 10.1158/0008-5472.CAN-07-0357. [DOI] [PubMed] [Google Scholar]
- 94.Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG. Non-Viral Vectors for Gene-Based Therapy. Nat. Rev. Genet. 2014;15:541. doi: 10.1038/nrg3763. [DOI] [PubMed] [Google Scholar]
- 95.Oberli MA, Reichmuth AM, Dorkin JR, Mitchell MJ, Fenton OS, Jaklenec A, Anderson DG, Langer R, Blankschtein D. Lipid Nanoparticle Assisted mRNA Delivery for Potent Cancer Immunotherapy. Nano Lett. 2017;17:1326–1335. doi: 10.1021/acs.nanolett.6b03329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kawabata K, Takakura Y, Hashida M. The Fate of Plasmid DNA After Intravenous Injection in Mice: Involvement of Scavenger Receptors in Its Hepatic Uptake. Pharm. Res. 1995;12:825–830. doi: 10.1023/a:1016248701505. [DOI] [PubMed] [Google Scholar]
- 97.Creixell M, Peppas NA. Co-Delivery of siRNA and Therapeutic Agents Using Nanocarriers to Overcome Cancer Resistance. Nano Today. 2012;7:367–379. doi: 10.1016/j.nantod.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ren H, Zhou L, Liu M, Lu W, Gao C. Peptide GE11–Polyethylene Glycol–Polyethylenimine for Targeted Gene Delivery in Laryngeal Cancer. Med. Oncol. 2015;32:293. doi: 10.1007/s12032-015-0624-9. [DOI] [PubMed] [Google Scholar]
- 99.Zhan C, Wei X, Qian J, Feng L, Zhu J, Lu W. Co-Delivery of TRAIL Gene Enhances the Anti-Glioblastoma Effect of Paclitaxel in Vitro and in Vivo. J. Controlled Release. 2012;160:630–636. doi: 10.1016/j.jconrel.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 100.Chen Z, Penet M-F, Krishnamachary B, Banerjee SR, Pomper MG, Bhujwalla ZM. PSMA-Specific Theranostic Nanoplex for Combination of TRAIL Gene and 5-FC Prodrug Therapy of Prostate Cancer. Biomaterials. 2016;80:57–67. doi: 10.1016/j.biomaterials.2015.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gillies ER, Fréchet JMJ. Dendrimers and Dendritic Polymers in Drug Delivery. Drug Discovery Today. 2005;10:35–43. doi: 10.1016/S1359-6446(04)03276-3. [DOI] [PubMed] [Google Scholar]
- 102.Huang S, Li J, Han L, Liu S, Ma H, Huang R, Jiang C. Dual Targeting Effect of Angiopep-2-Modified, DNA-Loaded Nanoparticles for Glioma. Biomaterials. 2011;32:6832–6838. doi: 10.1016/j.biomaterials.2011.05.064. [DOI] [PubMed] [Google Scholar]
- 103.Gao S, Li J, Jiang C, Hong B, Hao B. Plasmid pORF-hTRAIL Targeting to Glioma Using Transferrin-Modified Polyamidoamine Dendrimer. Drug Des., Dev. Ther. 2015;10:1–11. doi: 10.2147/DDDT.S95843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Han L, Huang R, Li J, Liu S, Huang S, Jiang C. Plasmid pORF-hTRAIL and Doxorubicin Co-Delivery Targeting to Tumor Using Peptide-Conjugated Polyamidoamine Dendrimer. Biomaterials. 2011;32:1242–1252. doi: 10.1016/j.biomaterials.2010.09.070. [DOI] [PubMed] [Google Scholar]
- 105.Li J, Guo Y, Kuang Y, An S, Ma H, Jiang C. Choline Transporter-Targeting and Co-Delivery System for Glioma Therapy. Biomaterials. 2013;34:9142–9148. doi: 10.1016/j.biomaterials.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 106.Duncan R, Izzo L. Dendrimer Biocompatibility and Toxicity. Adv. Drug Delivery Rev. 2005;57:2215–2237. doi: 10.1016/j.addr.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 107.Hu C, Gu F, Tai Z, Yao C, Gong C, Xia Q, Gao Y, Gao S. Synergistic Effect of Reduced Polypeptide Micelle for Co-Delivery of Doxorubicin and TRAIL Against Drug-Resistance in Breast Cancer. Oncotarget. 2016;7:61832–61844. doi: 10.18632/oncotarget.11451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mitchell MJ, Webster J, Chung A, Guimarães PPG, Khan OF, Langer R. Polymeric Mechanical Amplifiers of Immune Cytokine-Mediated Apoptosis. Nat. Commun. 2017;8:14179. doi: 10.1038/ncomms14179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mitchell MJ, King MR. Fluid Shear Stress Sensitizes Cancer Cells to Receptor-Mediated Apoptosis via Trimeric Death Receptors. New J. Phys. 2013;15:015008. doi: 10.1088/1367-2630/15/1/015008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mitchell MJ, King MR. Computational and Experimental Models of Cancer Cell Response to Fluid Shear Stress. Front. Oncol. 2013;3:44. doi: 10.3389/fonc.2013.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sun C, Lee J, Zhang M. Magnetic Nanoparticles in MR Imaging and Drug Delivery. Adv. Drug Delivery Rev. 2008;60:1252–1265. doi: 10.1016/j.addr.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang K, Kievit FM, Jeon M, Silber JR, Ellenbogen RG, Zhang M. Nanoparticle-Mediated Target Delivery of TRAIL as Gene Therapy for Glioblastoma. Adv. Healthcare Mater. 2015;4:2719–2726. doi: 10.1002/adhm.201500563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Veiseh O, Gunn JW, Zhang M. Design and Fabrication of Magnetic Nanoparticles for Targeted Drug Delivery and Imaging. Adv. Drug Delivery Rev. 2010;62:284–304. doi: 10.1016/j.addr.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Soenen SJ, Parak WJ, Rejman J, Manshian B. Intra Cellular Stability of Inorganic Nanoparticles: Effects on Cytotoxicity, Particle Functionality, and Biomedical Applications. Chem. Rev. 2015;115:2109–2135. doi: 10.1021/cr400714j. [DOI] [PubMed] [Google Scholar]
- 115.Duan L, Yang F, He W, Song L, Qiu F, Xu N, Xu L, Zhang Y, Hua Z, Gu N. A Multi-Gradient Targeting Drug Delivery System Based on RGD- L-TRAIL-Labeled Magnetic Microbubbles for Cancer Theranostics. Adv. Funct. Mater. 2016;26:8313–8324. [Google Scholar]
- 116.Spinowitz BS, Kausz AT, Baptista J, Noble SD, Sothinathan R, Bernardo MV, Brenner L, Pereira BJG. Ferumoxytol for Treating Iron Deficiency Anemia in CKD. J. Am. Soc. Nephrol. 2008;19:1599–1605. doi: 10.1681/ASN.2007101156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Miao L, Liu C, Ge J, Yang W, Liu J, Sun W, Yang B, Zheng C, Sun H, Hu Q. Antitumor Effect of TRAIL on Oral Squamous Cell Carcinoma Using Magnetic Nanoparticle-Mediated Gene Expression. Cell Biochem. Biophys. 2014;69:663–672. doi: 10.1007/s12013-014-9849-z. [DOI] [PubMed] [Google Scholar]
- 118.Cho MH, Lee EJ, Son M, Lee J-H, Yoo D, Kim J-W, Park SW, Shin J-S, Cheon J. A Magnetic Switch for the Control of Cell Death Signalling in in Vitro and in Vivo Systems. Nat. Mater. 2012;11:1038–1043. doi: 10.1038/nmat3430. [DOI] [PubMed] [Google Scholar]
- 119.Nie Z, Petukhova A, Kumacheva E. Properties and Emerging Applications of Self-Assembled Structures Made From Inorganic Nanoparticles. Nat. Nanotechnol. 2010;5:15. doi: 10.1038/nnano.2009.453. [DOI] [PubMed] [Google Scholar]
- 120.Dreaden EC, Austin LA, Mackey MA, El-Sayed MA. Size Matters: Gold Nanoparticles in Targeted Cancer Drug Delivery. Ther. Delivery. 2012;3:457. doi: 10.4155/tde.12.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Giljohann DA, Seferos DS, Daniel WL, Massich MD, Patel PC, Mirkin CA. Gold Nanoparticles for Biology and Medicine. Angew. Chem., Int. Ed. 2010;49:3280–3294. doi: 10.1002/anie.200904359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Auffan M, Rose J, Bottero J-Y, Lowry GV, Jolivet J-P, Wiesner MR. Towards a Definition of Inorganic Nanoparticles From an Environmental, Health and Safety Perspective. Nat. Nanotechnol. 2009;4:634. doi: 10.1038/nnano.2009.242. [DOI] [PubMed] [Google Scholar]
- 123.Tsai D-H, Elzey S, DelRio FW, Keene AM, Tyner KM, Clogston JD, MacCuspie RI, Guha S, Zachariah MR, Hackley VA. Tumor Necrosis Factor Interaction with Gold Nanoparticles. Nanoscale. 2012;4:3208–3217. doi: 10.1039/c2nr30415e. [DOI] [PubMed] [Google Scholar]
- 124.U.S. National Library of Medicine Clinicaltrials.gov. [accessed Nov 18, 2017]; https://clinicaltrials.gov/ct2/show/NCT03020017?cond=NU-0129&rank=1.
- 125.Jensen SA, Day ES, Ko CH, Hurley LA, Luciano JP, Kouri FM, Merkel TJ, Luthi AJ, Patel PC, Cutler JI, Daniel WL, Scott AW, Rotz MW, Meade TJ, Giljohann DA, Mirkin CA, Stegh AH. Spherical Nucleic Acid Nanoparticle Conjugates as an RNAi-Based Therapy for Glioblastoma. Sci. Transl. Med. 2013;5:209ra152. doi: 10.1126/scitranslmed.3006839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ke S, Zhou T, Yang P, Wang Y, Zhang P, Cheng K, Ren L, Ye S. Gold Nanoparticles Enhance TRAIL Sensitivity Through Drp1-Mediated Apoptotic and Autophagic Mitochondrial Fission in NSCLC Cells. Int. J. Nanomed. 2017;12:2531–2551. doi: 10.2147/IJN.S129274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chen Z, Zhang L, He Y, Li Y. Sandwich-Type Au-PEI/DNA/PEI-Dexa Nanocomplex for Nucleus-Targeted Gene Delivery in Vitro and in Vivo. ACS Appl. Mater. Interfaces. 2014;6:14196–14206. doi: 10.1021/am503483w. [DOI] [PubMed] [Google Scholar]
- 128.Kratz F. Albumin as a Drug Carrier: Design of Prodrugs, Drug Conjugates and Nanoparticles. J. Controlled Release. 2008;132:171–183. doi: 10.1016/j.jconrel.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 129.Hawkins MJ, Soon-Shiong P, Desai N. Protein Nanoparticles as Drug Carriers in Clinical Medicine. Adv. Drug Delivery Rev. 2008;60:876–885. doi: 10.1016/j.addr.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 130.Feinstein SB, Cheirif J, Ten Cate FJ, Silverman PR, Heidenreich PA, Dick C, Desir RM, Armstrong WF, Quinones MA, Shah PM. Safety and Efficacy of a New Transpulmonary Ultrasound Contrast Agent: Initial Multicenter Clinical Results. J. Am. Coll. Cardiol. 1990;16:316–324. doi: 10.1016/0735-1097(90)90580-i. [DOI] [PubMed] [Google Scholar]
- 131.Ibrahim NK, Desai N, Legha S, Soon-Shiong P, Theriault RL, Rivera E, Esmaeli B, Ring SE, Bedikian A, Hortobagyi GN, Ellerhorst JA. Phase I and Pharmacokinetic Study of ABI-007, a Cremophor-Free, Protein-Stabilized, Nanoparticle Formulation of Paclitaxel. Clin. Cancer Res. 2002;8:1038–1044. [PubMed] [Google Scholar]
- 132.Ren K, An, Dusad, Dong R, Quan L. Albumin as a Delivery Carrier for Rheumatoid Arthritis. J. Nanomed. Nanotechnol. 2013;4:176. [Google Scholar]
- 133.Kim TH, Jiang HH, Youn YS, Park CW, Lim SM, Jin C-H, Tak KK, Lee HS, Lee KC. Preparation and Characterization of Apo2L/TNF-Related Apoptosis-Inducing Ligand–Loaded Human Serum Albumin Nanoparticles with Improved Stability and Tumor Distribution. J. Pharm. Sci. 2011;100:482–491. doi: 10.1002/jps.22298. [DOI] [PubMed] [Google Scholar]
- 134.Anselmo AC, Mitragotri S. Cell-Mediated Delivery of Nanoparticles: Taking Advantage of Circulatory Cells to Target Nanoparticles. J. Controlled Release. 2014;190:531–541. doi: 10.1016/j.jconrel.2014.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mitchell MJ, King MR. Leukocytes as Carriers for Targeted Cancer Drug Delivery. Expert Opin. Drug Delivery. 2015;12:375–392. doi: 10.1517/17425247.2015.966684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mitchell MJ, King MR. Unnatural Killer Cells to Prevent Bloodborne Metastasis: Inspiration From Biology and Engineering. Expert Rev. Anticancer Ther. 2014;14:641–644. doi: 10.1586/14737140.2014.916619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cao T, Mitchell MJ, Liesveld J, King MK. Stem Cell Enrichment with Selectin Receptors: Mimicking the pH Environment of Trauma. Sensors. 2013;13:12516–12526. doi: 10.3390/s130912516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mitchell MJ, Chen CS, Ponmudi V, Hughes AD, King MR. E-Selectin Liposomal and Nanotube-Targeted Delivery of Doxorubicin to Circulating Tumor Cells. J. Controlled Release. 2012;160:609–617. doi: 10.1016/j.jconrel.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mitchell MJ, Wayne E, Rana K, Schaffer CB, King MR. TRAIL-Coated Leukocytes That Kill Cancer Cells in the Circulation. Proc. Natl. Acad. Sci. U. S. A. 2014;111:930–935. doi: 10.1073/pnas.1316312111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wayne EC, Chandrasekaran S, Mitchell MJ, Chan MF, Lee RE, Schaffer CB, King MR. TRAIL-Coated Leukocytes That Prevent the Bloodborne Metastasis of Prostate Cancer. J. Controlled Release. 2016;223:215–223. doi: 10.1016/j.jconrel.2015.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mitchell MJ, King MR. Physical Biology in Cancer. 3. the Role of Cell Glycocalyx in Vascular Transport of Circulating Tumor Cells. Am. J. Physiol., Cell Physiol. 2014;306:C89–C97. doi: 10.1152/ajpcell.00285.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mitchell MJ, Castellanos CA, King MR. Surfactant Functionalization Induces Robust, Differential Adhesion of Tumor Cells and Blood Cells to Charged Nanotube-Coated Biomaterials Under Flow. Biomaterials. 2015;56:179–186. doi: 10.1016/j.biomaterials.2015.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mitchell MJ, Castellanos CA, King MR. Immobilized Surfactant-Nanotube Complexes Support Selectin-Mediated Capture of Viable Circulating Tumor Cells in the Absence of Capture Antibodies. J. Biomed. Mater. Res., Part A. 2015;103:3407–3418. doi: 10.1002/jbm.a.35445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hu C-MJ, Fang RH, Wang K-C, Luk BT, Thamphiwatana S, Dehaini D, Nguyen P, Angsantikul P, Wen CH, Kroll AV, Carpenter C, Ramesh M, Qu V, Patel SH, Zhu J, Shi W, Hofman FM, Chen TC, Gao W, Zhang K, et al. Nanoparticle Biointerfacing by Platelet Membrane Cloaking. Nature. 2015;526:118–121. doi: 10.1038/nature15373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fang RH, Hu C-MJ, Luk BT, Gao W, Copp JA, Tai Y, O’Connor DE, Zhang L. Cancer Cell Membrane-Coated Nanoparticles for Anticancer Vaccination and Drug Delivery. Nano Lett. 2014;14:2181–2188. doi: 10.1021/nl500618u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Copp JA, Fang RH, Luk BT, Hu C-MJ, Gao W, Zhang K, Zhang L. Clearance of Pathological Antibodies Using Biomimetic Nanoparticles. Proc. Natl. Acad. Sci. U. S. A. 2014;111:13481–13486. doi: 10.1073/pnas.1412420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Parodi A, Quattrocchi N, van de Ven AL, Chiappini C, Evangelopoulos M, Martinez JO, Brown BS, Khaled SZ, Yazdi IK, Enzo MV, Isenhart L, Ferrari M, Tasciotti E. Synthetic Nanoparticles Functionalized with Biomimetic Leukocyte Membranes Possess Cell-Like Functions. Nat. Nanotechnol. 2013;8:61. doi: 10.1038/nnano.2012.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hu Q, Sun W, Qian C, Wang C, Bomba HN, Gu Z. Anticancer Platelet-Mimicking Nanovehicles. Adv. Mater. 2015;27:7043–7050. doi: 10.1002/adma.201503323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li J, Ai Y, Wang L, Bu P, Sharkey CC, Wu Q, Wun B, Roy S, Shen X, King MR. Targeted Drug Delivery to Circulating Tumor Cells via Platelet Membrane-Functionalized Particles. Biomaterials. 2016;76:52–65. doi: 10.1016/j.biomaterials.2015.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Giordano A, Galderisi U, Marino IR. From the laboratory bench to the patient's bedside: An update on clinical trials with mesenchymal stem cells. J. Cell. Physiol. 2007;211:27–35. doi: 10.1002/jcp.20959. [DOI] [PubMed] [Google Scholar]
- 151.Loebinger MR, Eddaoudi A, Davies D, Janes SM. Mesenchymal Stem Cell Delivery of TRAIL Can Eliminate Metastatic Cancer. Cancer Res. 2009;69:4134–4142. doi: 10.1158/0008-5472.CAN-08-4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sasportas LS, Kasmieh R, Wakimoto H, Hingtgen S, van de Water JAJM, Mohapatra G, Figueiredo JL, Martuza RL, Weissleder R, Shah K. Assessment of Therapeutic Efficacy and Fate of Engineered Human Mesenchymal Stem Cells for Cancer Therapy. Proc. Natl. Acad. Sci. U. S. A. 2009;106:4822–4827. doi: 10.1073/pnas.0806647106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kim SM, Lim JY, Park SI, Jeong CH, Oh JH, Jeong M, Oh W, Park S-H, Sung Y-C, Jeun S-S. Gene Therapy Using TRAIL-Secreting Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells Against Intracranial Glioma. Cancer Res. 2008;68:9614–9623. doi: 10.1158/0008-5472.CAN-08-0451. [DOI] [PubMed] [Google Scholar]
- 154.Menon LG, Kelly K, Yang HW, Kim SK, Black PM, Carroll RS. Human Bone Marrow-Derived Mesenchymal Stromal Cells Expressing S-TRAIL as a Cellular Delivery Vehicle for Human Glioma Therapy. Stem Cells. 2009;27:2320–2330. doi: 10.1002/stem.136. [DOI] [PubMed] [Google Scholar]
- 155.Luetzkendorf J, Mueller LP, Mueller T, Caysa H, Nerger K, Schmoll HJ. Growth Inhibition of Colorectal Carcinoma by Lentiviral TRAIL-Transgenic Human Mesenchymal Stem Cells Requires Their Substantial Intratumoral Presence. J. Cell. Mol. Med. 2010;14:2292–2304. doi: 10.1111/j.1582-4934.2009.00794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kim SW, Kim SJ, Park S-H, Yang HG, Kang MC, Choi YW, Kim SM, Jeun S-S, Sung Y-C. Complete Regression of Metastatic Renal Cell Carcinoma by Multiple Injections of Engineered Mesenchymal Stem Cells Expressing Dodecameric TRAIL and HSV-TK. Clin. Cancer Res. 2013;19:415–427. doi: 10.1158/1078-0432.CCR-12-1568. [DOI] [PubMed] [Google Scholar]
- 157.Hu Y-L, Huang B, Zhang T-Y, Miao P-H, Tang G-P, Tabata Y, Gao J-Q. Mesenchymal Stem Cells as a Novel Carrier for Targeted Delivery of Gene in Cancer Therapy Based on Nonviral Transfection. Mol. Pharmaceutics. 2012;9:2698–2709. doi: 10.1021/mp300254s. [DOI] [PubMed] [Google Scholar]
- 158.Choi SA, Hwang S-K, Wang K-C, Cho B-K, Phi JH, Lee JY, Jung HW, Lee D-H, Kim SK. Therapeutic Efficacy and Safety of TRAIL-Producing Human Adipose Tissue–Derived Mesenchymal Stem Cells Against Experimental Brainstem Glioma. Neuro Oncol. 2011;13:61–69. doi: 10.1093/neuonc/noq147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Moniri MR, Sun X-Y, Rayat J, Dai D, Ao Z, He Z, Verchere CB, Dai L-J, Warnock GL. TRAIL-Engineered Pancreas-Derived Mesenchymal Stem Cells: Characterization and Cytotoxic Effects on Pancreatic Cancer Cells. Cancer Gene Ther. 2012;19:652–658. doi: 10.1038/cgt.2012.46. [DOI] [PubMed] [Google Scholar]
- 160.Jiang X, Fitch S, Wang C, Wilson C, Li J, Grant GA, Yang F. Nanoparticle Engineered TRAIL-Overexpressing Adipose-Derived Stem Cells Target and Eradicate Glioblastoma via Intracranial Delivery. Proc. Natl. Acad. Sci. U. S. A. 2016;113:13857–13862. doi: 10.1073/pnas.1615396113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Vilalta M, Dégano IR, Bagó J, Gould D, Santos M, García-Arranz M, Ayats R, Fuster C, Chernajovsky Y, García-Olmo D, Rubio N, Blanco J. Biodistribution, Long-Term Survival, and Safety of Human Adipose Tissue-Derived Mesenchymal Stem Cells Transplanted in Nude Mice by High Sensitivity Non-Invasive Bioluminescence Imaging. Stem Cells Dev. 2008;17:993–1004. doi: 10.1089/scd.2007.0201. [DOI] [PubMed] [Google Scholar]
- 162.Ankrum J, Karp JM. Mesenchymal Stem Cell Therapy: Two Steps Forward, One Step Back. Trends Mol. Med. 2010;16:203–209. doi: 10.1016/j.molmed.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Karp JM, Leng Teo GS. Mesenchymal Stem Cell Homing: the Devil Is in the Details. Cell Stem Cell. 2009;4:206–216. doi: 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 164.Pan L-Q, Zhao W-B, Lai J, Ding D, Wei X-Y, Li Y-Y, Liu W-H, Yang X-Y, Xu Y-C, Chen S-Q. Hetero-Modification of TRAIL Trimer for Improved Drug Delivery and in Vivo Antitumor Activities. Sci. Rep. 2015;5:14872. doi: 10.1038/srep14872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, Chan WCW. Analysis of Nanoparticle Delivery to Tumours. Nat. Rev. Mater. 2016;1:16014. [Google Scholar]
- 166.Yu M, Zheng J. Clearance Pathways and Tumor Targeting of Imaging Nanoparticles. ACS Nano. 2015;9:6655–6674. doi: 10.1021/acsnano.5b01320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Caliceti P. Pharmacokinetic and Biodistribution Properties of Poly(Ethylene Glycol)–Protein Conjugates. Adv. Drug Delivery Rev. 2003;55:1261–1277. doi: 10.1016/s0169-409x(03)00108-x. [DOI] [PubMed] [Google Scholar]
- 168.Kim TH, Youn YS, Jiang HH, Lee S, Chen X, Lee KC. PEGylated TNF-Related Apoptosis-Inducing Ligand (TRAIL) Analogues: Pharmacokinetics and Antitumor Effects. Bioconjugate Chem. 2011;22:1631–1637. doi: 10.1021/bc200187k. [DOI] [PubMed] [Google Scholar]
- 169.Chae SY, Kim TH, Park K, Jin C-H, Son S, Lee S, Youn YS, Kim K, Jo D-G, Kwon IC, Chen X, Lee KC. Improved Antitumor Activity and Tumor Targeting of NH(2)-Terminal-Specific PEGylated Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand. Mol. Cancer Ther. 2010;9:1719–1729. doi: 10.1158/1535-7163.MCT-09-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kontermann RE. Strategies for Extended Serum Half-Life of Protein Therapeutics. Curr. Opin. Biotechnol. 2011;22:868–876. doi: 10.1016/j.copbio.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 171.Sleep D, Cameron J, Evans LR. Albumin as a Versatile Platform for Drug Half-Life Extension. Biochim. Biophys. Acta, Gen. Subj. 2013;1830:5526–5534. doi: 10.1016/j.bbagen.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 172.Müller N, Schneider B, Pfizenmaier K, Wajant H. Superior Serum Half Life of Albumin Tagged TNF Ligands. Biochem. Biophys. Res. Commun. 2010;396:793–799. doi: 10.1016/j.bbrc.2010.04.134. [DOI] [PubMed] [Google Scholar]
- 173.Byeon HJ, Min SY, Kim I, Lee ES, Oh KT, Shin BS, Lee KC, Youn YS. Human Serum Albumin-TRAIL Conjugate for the Treatment of Rheumatoid Arthritis. Bioconjugate Chem. 2014;25:2212–2221. doi: 10.1021/bc500427g. [DOI] [PubMed] [Google Scholar]
- 174.Zhang L, Fang B. Mechanisms of Resistance to TRAIL-Induced Apoptosis in Cancer. Cancer Gene Ther. 2005;12:228–237. doi: 10.1038/sj.cgt.7700792. [DOI] [PubMed] [Google Scholar]
- 175.von Karstedt S, Conti A, Nobis M, Montinaro A, Hartwig T, Lemke J, Legler K, Annewanter F, Campbell AD, Taraborrelli L, Grosse-Wilde A, Coy JF, El-Bahrawy MA, Bergmann F, Koschny R, Werner J, Ganten TM, Schweiger T, Hoetzenecker K, Kenessey I, et al. Cancer Cell-Autonomous TRAIL-R Signaling Promotes KRAS-Driven Cancer Progression, Invasion, and Metastasis. Cancer Cell. 2015;27:561–573. doi: 10.1016/j.ccell.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ashkenazi A, Herbst RS. To Kill a Tumor Cell: the Potential of Proapoptotic Receptor Agonists. J. Clin. Invest. 2008;118:1979–1990. doi: 10.1172/JCI34359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lemke J, von Karstedt S, Zinngrebe J, Walczak H. Getting TRAIL Back on Track for Cancer Therapy. Cell Death Differ. 2014;21:1350–1364. doi: 10.1038/cdd.2014.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wu GS, Burns TF, Zhan Y, Alnemri ES, El-Deiry WS. Molecular Cloning and Functional Analysis of the Mouse Homologue of the KILLER/DR5 Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL) Death Receptor. Cancer Res. 1999;59:2770–2775. [PubMed] [Google Scholar]
- 179.Schneider P, Olson D, Tardivel A, Browning B, Lugovskoy A, Gong D, Dobles M, Hertig S, Hofmann K, Van Vlijmen H, Hsu YM, Burkly LC, Tschopp J, Zheng TS. Identification of a New Murine Tumor Necrosis Factor Receptor Locus That Contains Two Novel Murine Receptors for Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL) J. Biol. Chem. 2003;278:5444–5454. doi: 10.1074/jbc.M210783200. [DOI] [PubMed] [Google Scholar]