Abstract
Osteopontin (OPN) is a bone sialoprotein involved in osteoclast attachment to mineralised bone matrix, as well as being a bone matrix protein, OPN is also a versatile protein that acts on various receptors which are associated with different signalling pathways implicated in cancer. OPN mediates various biological events involving the immune system and the vascular system; the protein plays a role in processes such as immune response, cell adhesion and migration, and tumorigenesis. This review discusses the potential role of OPN in tumour cell proliferation, angiogenesis and metastasis, as well as the molecular mechanisms involved in these processes in different cancers, including brain, lung, kidney, liver, bladder, breast, oesophageal, gastric, colon, pancreatic, prostate and ovarian cancers. The understanding of OPN’s role in tumour development and progression could potentially influence cancer therapy and contribute to the development of novel anti-tumour treatments.
Facts
Osteopontin (OPN) is a versatile protein that acts on various receptors which are associated with different signalling pathways implicated in cancer.
OPN mediates critical processes for cancer progression such as immune response, cell adhesion and migration, and tumorigenesis.
Open questions
What are the precise molecular mechanisms of osteopontin in tumour progression?
How is osteopontin related to the diagnosis and prognosis of cancer?
Which therapeutic strategy targeting osteopontin would be the most effective in treating cancer in the clinical settings?
Introduction
Malignant cancers are described as uncontrolled growths which overcome replicative senescence, resulting in metastatic disease1. There are many factors that have essential functions in the regulation of the survival, proliferation, adhesion and migration of neoplastic cells, such as various growth factors and cytokines1. Recently there has been ongoing research regarding the role of osteopontin in tumour progression. This review provides an insight into the potential role that osteopontin may play in tumour cell proliferation, angiogenesis and metastasis and the molecular mechanisms underlying tumour progression in various organs.
Biology of osteopontin
Molecular pathway of osteopontin
Not only is Osteopontin (OPN) a major non-collagenous bone matrix protein (which gave it its name), it also plays a role in other systems. For example, as an intrinsic component of the immune system, OPN controls cytokine production and regulates cell trafficking 2. OPN is an acidic arginine-glycine-aspartate-containing adhesive glycoprotein with a molecular mass of approximately 44 kDa (Fig. 1)2. The central section of the molecule consists of sequences that interact with seven integrins, such as αvβ3 and β5, as well as a sequence of β1-consisting integrins and a cryptic α9β1 region that is only functional after protease cleavage, thus suggesting a specific role for OPN fragments3. Not only does OPN communicate with cells via integrins, but also via CD44, which involves an intracellular interaction4. OPN has a conserved region that separates both the integrin and CD44 binding domains, which possess distinct signalling functions5. Different isoforms of CD44 are produced by alternate splicing of the pre-mRNA and have been found in different cancer cells6. The presence of these isoforms in different cancers suggests that they can be used as a marker for cancer progression and patient survival.
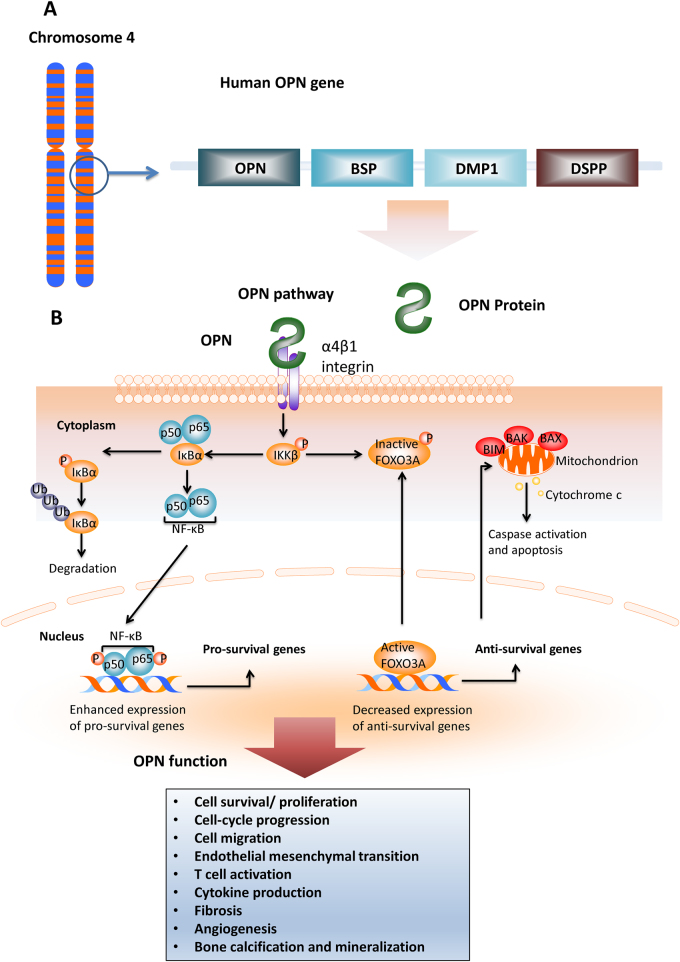
Fig. 1. The OPN gene, protein, signalling pathway and function in normal tissues.
a The schematic representation of the location of OPN on human chromosome 4.OPN is at location 4q22.1. Genes encompassed within a 600 kb region on chromosome 4 encodes several noncollagenous bone and dentin proteins including OPN, bone sialoprotein(BSP), dentin matrix protein I (DMPI) and dentin sialophosphoportin (DSPP). All of them are classified as small intergrin-binding ligand N-linked glycoprotein (SIBLING) family proteins. b The signalling pathway of osteopontin. Osteopontin binds integrin α4β1 which causes degradation of phospharylated inhibitor of nuclear factor kappa-B kinase subunit beta (IKKβ). Inhibitor of nuclear transcription factor kappa-B (IκBα) and nuclear transcription factor kappa-B (NF-κB; p50 and p65) are both freed following this process. IκBα is degraded by the ubiquitination pathway, while NF-κB enters the cell nucleus where it is phosphorylated and it enhances the expression of pro-survival genes. Moreover, upon binding of osteopontin to integrin α4β1, phosphorylated IKKβ causes inactivation of Forkhead box O3 (FOXO3A). Active FOXO3A is important in decreasing the expression of anti-survival genes such as BIM, BAK and BAX which cause caspase activation and cell apoptosis via mitochondrion and release of cytochrome c. Activation of OPN mediate a diverse range of cellular function, including cell survival/ proliferation, cell-cycle progression, cell migration, endothelial mesenchymal transition, T cell activation, cytokine production, fibrosis, angiogenesis and bone calcification and mineralisation2
OPN is mainly synthesised by osteoblasts, osteocytes and other hematopoietic cells7. In addition, OPN is also secreted by neutrophils, dendritic cells, NK cells, T cells and B cells8. The structure of OPN is relatively simple, with about 300 amino acids composing a single chain polypeptide and expressed as a 33-KDa nascent protein8. OPN is actively involved in many physiological functions. It is an important factor in bone remodelling, anchoring osteoclasts to the mineral matrix of bones9. In addition, OPN regulates both the innate and adaptive immune systems. Functioning in a similar manner to T cell helper 1 cytokines, OPN promotes a cell-mediated immune response10. OPN contains an integrin –binding RGD sequence that interacts with CD44v 6/7, which is a cell surface glycoprotein involved in cell-cell interactions. OPN can also activate intracellular pathways to regulate gene expression within the immune system11. The important role of OPN in cell differentiation has been thoroughly investigated and it has been shown to suppress adipogenic differentiation and promote osteogenic differentiation in mesenchymal stem cells by interacting with integrin αvβ112. In addition, OPN regulates cytokine production 2. In summary, OPN is a protein with a diverse array of functions, ranging from the regulation of bone mineralisation, to recruiting macrophages and facilitating cell adhesion and migration.
General role of osteopontin in cancer progression
In addition to its role in mediating normal physiological responses, the role of OPN signalling pathways in cancer progression is becoming increasingly recognised (Fig. 2)13 and it has been shown to be involved in multi-steps of tumour metastasis (Fig. 3).
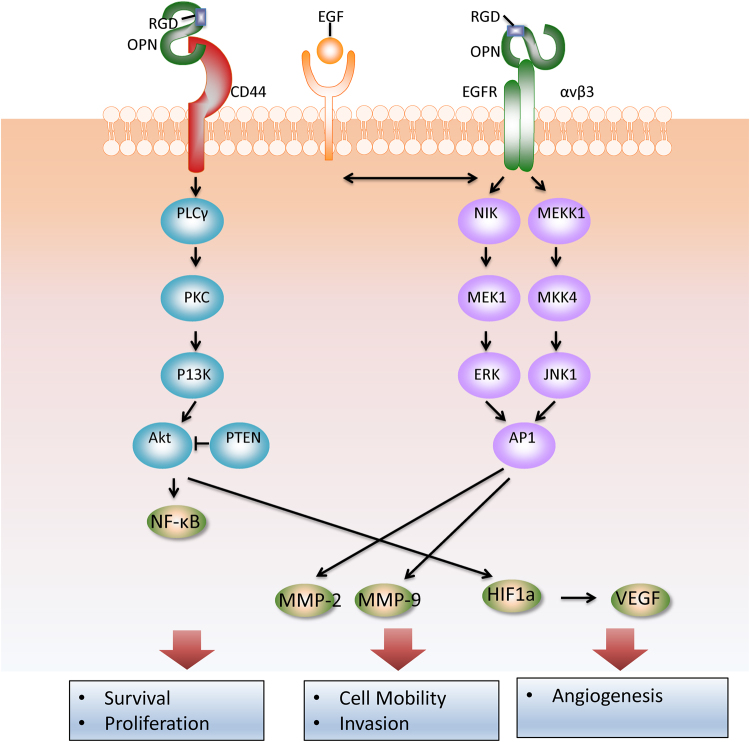
Fig. 2. The signalling pathway of osteopontin in tumour progression.
Osteopontin (OPN) can interact with several integrins in Arg-Gly-Asp (RGD) dependent and RGD independent manners13. OPN can also interact with the CD44 family of receptors. Upon binding of receptors, OPN can induce cellular reactions include: survival, motility and tumour progression, MMP localisation and complement inhibition. By interacting with CD44 family of receptors, OPN can activate the cell anti-apoptotic signals in tumour cells through hospholipase C-γ(PLCγ–protein kinase C (PKC)–phosphatidylinositol 3-kinase (PI3K)–Akt pathway. Phosphatase and tensin homologue (PTEN) could inhibit Akt phosphorylation. Upon binding to αv β3, OPN activates AP1 through nuclear factor-inducing kinase (NIK)–ERK (extracellular signal-related kinase) and MEKK1 (mitogen-activated protein kinase kinase kinase1)–JNK1 (c-Jun N-terminal kinase 1) signalling pathways. AP1 promotes cancer cells motility and tumour progression. In addition, transactivation of epidermal growth factor receptor (EGFR) by OPN promotes phosphorylation of ERK which ultimately leads to activation of AP1. Activation of both MMP and HIF-1 pathways leads to enhanced tumour cell survival, proliferation, invasion and angiogenesis2
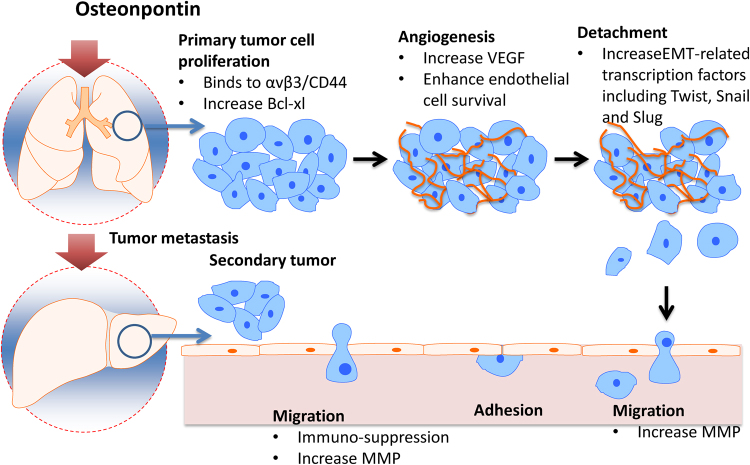
Fig. 3. The role of osteopontin in multi-steps of cancer cell metastasis.
The OPN overexpression induces multi-steps of cancer cell metastasis through activating different protein mediators. A primary tumour undergoes vascularisation by angiogenesis as various growth factors such vascular endothelial growth factor are secreted. Detachment of the cancerous cell then occurs followed by intravasation; the tumour cell enters and circulates the vascular system. The cell eventually attaches to the wall of the blood vessel before undergoing extravasation and leaving the blood vessel. The tumour cell then grows as a secondary tumour causing metastasis
Role of osteopontin in tumour cell proliferation
Several studies have demonstrated the correlation between elevated OPN secretion and various malignancies, such as breast and prostate cancer, squamous cell carcinoma, melanoma, osteosarcoma and gliobastoma1. The high concentration of OPN cDNA in OPN negative breast cancer cells was shown to promote angiogenesis and, therefore, skeletal metastasis, thus enhancing tumour progression14. As well as this, OPN has also been linked to the promotion of tumour cell growth in invasive melanoma, whilst blocking the expression of OPN decreased melanoma cell numbers in vitro. These findings indicate that the recruitment of OPN may be an early feature in the development of melanoma15. A study conducted in nude mice demonstrated that NIK is responsible for the stimulation of MMP-9 by OPN and subsequent melanoma growth1. Furthermore, it has been found that activation of the melanocyte growth factor receptor stimulates the secretion of OPN, thus encouraging anti-apoptotic signalling and increasing the progression of the melanoma15. Overall, these studies suggest that OPN plays a key role in mediating tumour progression by regulating various pathways.
Role of osteopontin in tumour angiogenesis
OPN plays a role in the process of angiogenesis due to its high affinity for αvβ3, an integrin highly expressed on particular endothelial cells1. Signalling via αvβ3 is essential for endothelial cell survival, and it has been found that OPN promotes the survival of these cells1. However, there is no strong evidence to suggest a correlation between OPN and angiogenesis in vivo, and it is more likely that OPN interacts with other pro-angiogenic molecules to stimulate angiogenesis16. More studies are required to fully elucidate the role of OPN in mediating angiogenic processes.
Role of osteopontin in tumour cell metastasis
Cancer metastasis is a complex process which broadly involves the following processes: the detachment of cancer cells from the primary tumour, intravasation, the circulation of cancer cells, adhesion to the blood vessel wall, extravasation and the growth of the secondary tumour17. Although the role of OPN in regulating all of these processes is not completely understood, a meta-analysis published recently reported that overexpression of OPN is closely associated with the metastasis of colorectal cancers, lung cancers and melanomas18.
Epithelia-Mesenchymal Transition (EMT) describes the process in which epithelial cells revert back to their mesenchymal phenotype. EMT is characterised by the loss of apical-basal polarity, increased cellular motility and cytoskeleton reorganisation. There are three major types of EMT: type 1 refers to embryogenesis, type 2 is wound healing, and type 3 is cancer metastases. OPN has been shown to play a crucial role in mediating type 2 and 3 EMT19. In a breast cancer model, OPN caused an increase in EMT-related transcription factors including, Twist, Snail and Slug19. OPN overexpression results in serine phosphorylation of Twist, which then binds to the Bmi-1 promotor, which in turn activates EMT in breast cancer cell lines20. OPN is also able to mediate EMT in hepatocellular carcinoma (HCC) models by regulating Twist. In addition, OPN overexpression is shown to activate the PI3K-AKT-Twist pathway, thus promoting EMT and ultimately resulting in HCC metastases21. In colorectal cancer, metastasis is also mediated by OPN activating Twist22. OPN-mediated Twist activation causes enhanced cell migration, increased invasion and decreased cell-cell adhesion22.
In addition, OPN also exerts its function by inducing the hypoxia-inducible factor-1 alpha (HIF-1α) pathway. Intra-tumour hypoxia stabilises HIF-1 alpha, which regulates the expression of Twist by binding to the Twist promotor, thus inducing EMT. OPN is shown to increase HIF-1α via the PI3k/AKT pathway in ovarian cancer and breast cancer models23, 24.
OPN has been proposed to shape the tumour microenvironment, thus promoting metastasis in different cancer models. OPN within the tumour milieu can either be tumour-derived or host-derived, and its presence within the tumour milieu may result in enhanced metastasis25.
Role of osteopontin in tumour chemoresistance
The role of OPN in chemoresistance is currently under investigation, with pre-clinical evidence suggesting that OPN is involved in inducing chemoresistance. Two theories have been proposed to rationalise the association between OPN and chemoresistance.
Autophagy is an evolutionarily conserved catabolic process where organelles are degraded by lysosomes26 when cells are subject to cellular stress. A growing body of evidence indicates a paradoxical role of autophagy following chemotherapy, with its response either increasing or reducing chemotherapy anti-cancer activity. Autophagy is shown to promote chemoresistance in some cancer cells during chemotherapy. However, autophagy may also induce autophagic cell death, a form of cell death different to apoptosis26. Based on current literature, chemoresistance can be enhanced through the upregulation of autophagy27–29. Recent research demonstrated that OPN-induced autophagy via activation of the OPN/NF- κB pathway contributes to chemoresistance to gemcitabine in human pancreatic cancer cells28. By silencing OPN expression using lentiviral transfection, gemcitabine conferred enhanced chemotherapy-induced cytotoxic effects in human pancreatic cancer cells28. Similar results were obtained from HCC cells29. OPN also has chemoresistant effects in HCC cells by activating autophagy via the integrin alpha v beta 3/MEK/ERK1/2 pathway29. Lentiviral-mediated miRNA against OPN abolishes the pro-authophagic effect on HCC cells when treated with chemotherapeutic agents29.
The second theory proposed to explain the association between OPN and chemoresistance is that OPN may result in anti-apoptotic effects on cancer cells. Yang et al. recently demonstrated that by downregulating OPN expression in breast cancer cell lines with siRNA, the sensitivity of breast cancer cells to doxorubicin was enhanced by increasing cell senescence. The authors concluded that the PI-3K/Akt signalling pathway was potentially responsible for these findings30. In addition, another group demonstrated that OPN-knockout breast cancer cells conferred higher levels of cyclophosphamide-induced apoptosis compared to normal breast cancer cells. Furthermore, the authors found that OPN exerted its anti-apoptotic effects on breast cancer cells by activating the p38 MAPK pathway31.
The role of osteopontin on solid organ cancer development
OPN has been shown to play diverse roles in promoting cancer development in different organs (Fig. 4). Recent developments within this area have been summarised as follows:
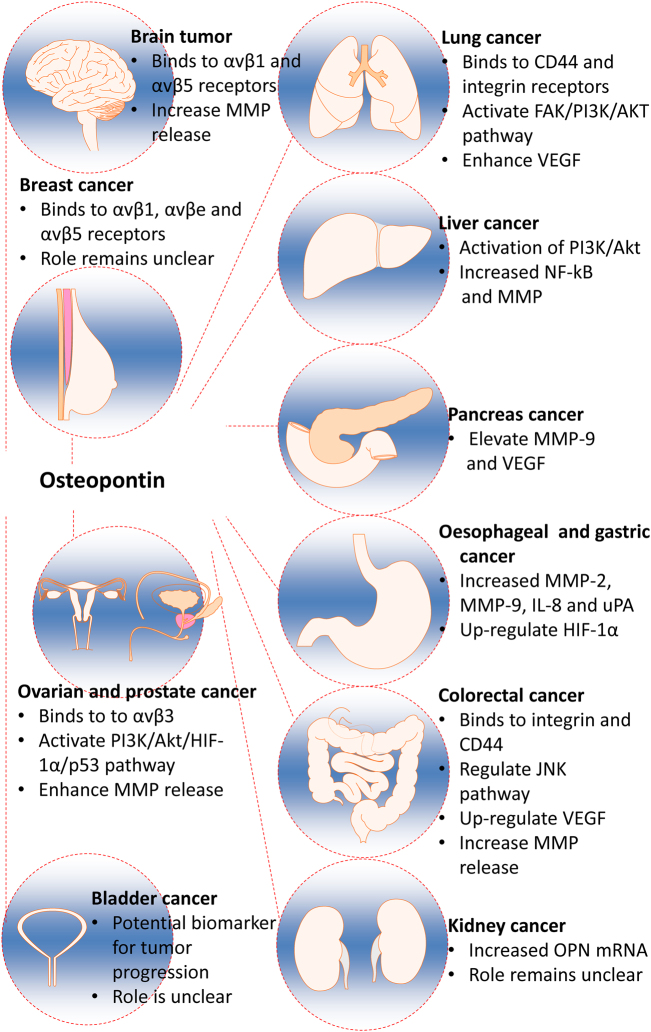
Fig. 4. The role of osteopontin in various solid organ tumours.
Osteopontin (OPN) has demonstrated a role in the development of various solid organ tumours via various differing mechanisms. In breast, brain, ovarian and prostate cancers, OPN has been shown to preferentially bind to a variety of integrins including αvβ1, and αvβ5, αvβe, αvβ3, resulting in an increase in cell adhesion, migration, and invasion, whilst OPN has been shown to bind to both integrin and CD44 receptors in lung cancers. In addition to receptor binding, OPN is involved in enhancing MMP release and thus increasing cell invasiveness and tumour growth in brain, liver, pancreas, colorectal, ovarian and prostate, and oesophageal and gastric cancers. OPN-mediated upregulation of the PI3K/Akt signalling pathway is a common feature of liver, lung, ovarian and prostate tumour progression, thus preferentially regulating cell survival, cell cycle progression and cellular growth in favour of tumour development. Activation of VEGF and its downstream effector, HIF-1α, by OPN may occur dependently or independently of PI3K/Akt activation and promotes tumour angiogenesis, recruitment of endothelial cells and tumour growth, particularly in colorectal, pancreatic, lung, and oesophageal and gastric malignancies. Activation of the JNK pathway by OPN has been shown to be most specific to colorectal cancer, whilst the precise role of OPN in bladder and kidney cancers, particularly, remains to be elucidated. OPN osteopontin, MMP metalloproteinase, PI3K Phosphatidylinositol-3 kinase, VEGF vascular endothelial growth factor, HIF-1α hypoxia-inducible factor 1
Brain Tumour
Glioblastomas are the most invasive type of glioma and are associated with 5-year survival rates of less than 10%. OPN expression has been associated with increased tumour grade and migratory tendency32. Atai et al. (2011) found that in glioblastomas in silico, OPN was one of the 5% most expressed genes in 90% of patients. In situ, the authors also found increased protein levels of OPN in glioblastoma cells compared to normal human brain cells, as well as OPN co-localisation with neutrophils and macrophages. This study suggests that OPN possesses a role in the pathogenesis of glioblastomas, as well demonstrating that OPN in tumours induces the migration of both cancer cells and leucocytes33.
Whilst the extracellular matrix of the normal adult brain lacks the expression of adhesion molecules that promote cell attachment, the malignant invasion of astrocytoma tumours is primarily caused by matrix remodelling and upregulation of cellular attachment. Studies have reported the expression of OPN in astrocytic tumours from patient biopsies4. It is understood that astrocytomas preferentially express αvβ1, and αvβ5 integrins in vitro and in vivo and are markers of astrocytic malignancy. Ding et al. (2002) demonstrated that OPN’s role in promoting cellular attachment is not limited to non-glial cell types, but that the protein is also involved in promoting malignant astrocytoma cell invasion34.
Lung cancer
The mechanisms underlying the aggressive phenotype associated with OPN expression have been extensively investigated in non-small-cell lung carcinomas (NSCLC). Firstly, OPN appears to possess a critical role in mediating the process of tumourigenesis. Lin et al. demonstrated that vascular endothelial growth factor (VEGF) and OPN were both overexpressed in NSCLC patient samples and were both significantly associated with clinical features indicating tumour progression35. Goutam et al. showed that OPN can induce the accumulation of VEGF via autocrine and paracrine mechanisms, and VEGF subsequently promotes tumourigenesis and development36. Secondly, OPN may also play a key role in NSCLC metastasis. EMT is increasingly being recognised as a significant contributor to NSCLC metastasis19. OPN can modulate tumour-specific EMT by generating cancer-associated fibroblasts (CAFs)19. Furthermore, OPN can induce NSCLC tumour cell migration by interacting with integrins and CD4437, 38, and this migration can be blocked by anti-OPN antibodies38. OPN may also activate ROCK signalling via the FAK/PI3K/AKT pathway, thus facilitating the invasion of lung cancer cells through lamellipodia formation and the inactivation of cofilin39, thereby promoting tumour metastasis. Finally, OPN has been proposed to be a therapeutic target for NSCLC. By delivering PSOT/siOPN complexes to NSCLC cell-xenograft mouse models, Cho et al. recently demonstrated that OPN expression was reduced. In addition, tumour volume and weight were reduced in siOPN-treated groups compared to non-treated group, emphasising the therapeutic potential of OPN40.
Kidney cancer
A recent review concluded that OPN, together with various other cytokines (e.g IL-8), were promising prognostic biomarkers for progression-free survival (PFS) for patients with renal cell carcinoma (RCC)41. The authors selected 50 articles assessing the predictive value of biomarkers using the archived specimens from randomised controlled trails41. In fact, Tran et al. reported that IL-8 and OPN were stronger prognostic markers for PFS in the placebo group than standard clinical classifications, such as the Eastern Cooperative Oncology Group performance status, the prognostic models of the Memorial Sloan Kettering Cancer Centre and the International Metastatic Renal Cell Database Consortium42. Another study demonstrated that the mRNA level of OPN is a strong indicator of overall survival (OS) and PFS for patients with clear cell renal carcinoma (ccRCC) in both univariate and multivariate analysis43. The precise underlying pathways of OPN in RCC are currently obscure and require further investigation.
Liver cancer
Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer. Osteopontin is highly expressed in the tumour tissue and serum of patients suffering from many malignancies, including HCC. Studies of surgically resected HCC have demonstrated that osteopontin is highly expressed in tumour tissue and correlates with tumour grade, stage and recurrence44, 45. In addition, the expression of osteopontin has also been shown to correlate with the metastatic disposition of HCC46. Recent literature also proposes that high serum osteopontin concentrations correlate with poor prognosis, reduced liver function, a worse Child-Pugh score and worse disease-free and overall survival47–49. Studies have, therefore, assessed the role of serum osteopontin as a potential tumour biomarker. Evidence exists suggesting that a combination of osteopontin and alpha-fetoprotein enhances the sensitivity and specificity of HCC detection50. However, contradictory results have found that osteopontin is relevant in mediating the general hepatic inflammatory environment, rather carcinogenesis specifically51. The mechanism by which osteopontin enhances tumour development and metastasis is poorly understood. Huang et al. (2006) provided evidence to suggest that the upregulation of osteopontin expression in hepatitis B-associated HCC may be due to amplification of chromosome 4q21, close to the spp (osteopontin) locus47. Furthermore, it has been proposed that osteopontin facilitates tumour progression through activation of downstream signalling, including phosphotidylinositol 3-kinase (PI3K)/Akt, nuclear factor (NF)-kB and matrix metalloproteinases (MMPs)52. Sun et al. (2008) also found that osteopontin-mediated activation of mitogen-activated protein kinases (MAPK), NF-kB and MMP-2 pathways was vital in HCC growth and metastases53. These findings suggest that osteopontin may provide a novel therapeutic avenue in the treatment of malignancies selectively expressing osteopontin, such as HCC. Bhattacharya et al. (2010) found that miRNA-181a resulted in the notable repression of osteopontin in HCC cell lines, suggesting that epigenetic regulation of osteopontin expression may potentially confer resistance against the metastatic characteristics of HCC54.
Bladder cancer
Urothelial carcinoma (UC) is the most common type of primary bladder cancer and causes approximately 150,000 deaths annually worldwide55. Few studies investigating the correlation between OPN and UC of the bladder have been performed. Findings by Coppola et al. (2004) suggest a correlation between OPN expression and pathological tumour staging in bladder UC, as well as a wide range of other tumour histologies56. More recently, Park et al. (2012) conducted a prospective study to determine the value of plasma OPN levels as a predictive factor of disease stage and recurrence in patients with bladder UC57. The prominent findings of the study were the correlation between preoperative plasma OPN levels and muscle invasion, as well as an increase in plasma OPN with disease burden in bladder UC. These results suggest that OPN may be a potential marker for predicting risk and clinical prognosis in patients with bladder UC. Findings by Ke et al. (2011) indicate that OPN may also be a potential biomarker for other urinary tract urothelial carcinomas, including UC of the renal pelvis and ureters58.
Breast cancer
The relationship between OPN and breast cancer progression was initially studied by Tuck et al. (1997) by extracting tissue samples from a patient with synchronous, bilateral, invasive mammary carcinomas of the same histology that later developed metastatic recurrence59. Subsequently, the same authors investigated OPN protein and mRNA expression in the tumours of 154 women with lymph-node negative breast cancer60. OPN was identified in tumour infiltrating macrophages and lymphocytes in 70% of tumours and also localised specifically to carcinoma cells in 26% of tumours. Overall, the results of this pilot study suggest that tumour aggressiveness and poor prognosis is associated with the ability of breast cancer cells to either synthesise OPN or to bind and sequester OPN from the tumour61 microenvironment. More recently, the potential for OPN to provide diagnostic, prognostic and clinical information for patients with breast cancer has been demonstrated. Serum levels of OPN are increased by up to 10-fold in patients with disseminated breast cancer, with higher concentrations associated with higher tumour grade62–64. A recent study investigating integrin-mediated induction of cellular migration found that the αvβ3 integrin is intrinsically involved in the migration of a highly tumorigenic, metastatic breast cancer cell line towards OPN, whilst non-metastatic cells were found to use αvβ1, and αvβ5 integrins65. Various other studies have supported the notion that tumour cells capable of binding αvβ3 and OPN possess more aggressive tendencies and a significant survival advantage66.
Oesophageal cancer
Studies have found that higher expression of OPN, including all its isoforms, OPNa, OPNb, OPNc, is seen in oesophageal cancer67. The overexpression of different isoforms confers different cancer cell characteristics that promote tumorigenesis. For instance, OPNb promotes cell proliferation and migration, but increased cell adhesion. In contrast, OPNc prevents cell migration but promotes cell detachment. OPN may be used as a prognostic marker in oesophageal cancer. One study demonstrated that more advanced oesophageal cancer is associated with higher expression of OPN, although the higher level of OPN cannot predict patient survival67. Another study, however, demonstrated that patients with oesophageal cancer that express more OPN had poorer survival rates68. The inconsistency between these studies indicate the necessity for further research to elucidate the role of OPN in the prognostic prediction of oesophageal cancer.
Pancreatic cancer
An increased serum level of OPN has been seen in pancreatic cancer patients 69and in vitro experiments have demonstrated that OPN mRNA is upregulated in pancreatic cancer cell lines69. Using anti-sense oligonucleotides to downregulate OPN demonstrates that it is possible to inhibit cell proliferation70. Metastasis is seen frequently in pancreatic cancer patients. The increased expression of OPN mRNA has been seen in mice liver metastatic cell lines (HPC-3H4) that are derived from pancreatic cancer cell lines71. In smoking patients, metastasis of pancreatic cancer cells seems to be associated with the upregulation of OPN. Lazar et al. demonstrated that OPN plays a role in elevating the levels of MMP-9 and VEGF, both of which are important in tumour development and metastasis. The increase in MMP-9 and VEGF is attenuated by inhibiting the expression of OPN72. Hence, OPN may be a therapeutic target for pancreatic cancer.
Since there has been an established association between an elevated level of OPN and pancreatic cancer, it has been suggested that OPN may be used as a biomarker73. Poruk et al. demonstrated that tissue inhibitor of metalloproteinase 1 (TIMP-1) is elevated in pancreatic cancer, but not chronic pancreatitis and, therefore, the combination of TIMP-1 and OPN may help differentiate between the two74.
Gastric cancer
It has been established that Helicobacter Pylori (H. pylori) infection is one of the risk factors for gastric cancer75. An in vivo study involving OPN knockout (KO) mice showed that OPN plays a vital role in the development of gastric cancer from H. Pylori infection76. The study demonstrated that the size of gastric tumours is larger and the incidence of gastric cancer is higher in wild-type (WT) mice expressing OPN, compared with OPN KO mice. It was observed that the loss of OPN is associated with increased levels of inducible nitric oxide synthase (iNOS) and its regulator, signal transducer and activator of transcription 1 (STAT1), both of which have indicated the ability to induce apoptosis and growth arrest76–79.
OPN may also possess anti-apoptotic properties in the tumourigenesis of gastric cancer by modulating the balance between pro-apoptotic and anti-apoptotic factors. It has been observed that increased levels of OPNb and OPNc are associated with an increase in anti-apoptotic Bcl-2, and a decreased production of pro-apoptotic capase-3 and Bax, hence facilitating tumour survival80. In addition to its anti-apoptotic properties, OPN also appears to promote invasion and metastasis in gastric cancer. Overexpression of OPN can cause an increased expression of MMP-2, MMP-9, IL-8 and urokinase plasminogen activator (uPA), as well as inhibition of caspase 3 generation, thus promoting cancer cell invasion and metastasis80, 81. Furthermore, OPN may also promote angiogenesis and tumour growth in gastric cancer by upregulating VEGF82. Although the mechanism for this remains to be fully elucidated, it is believed that OPN promotes the translocation of NF-Κb via the MAPK and PI3K/Akt pathways, which upregulates HIF-1α to promote cancer cell proliferation and survival81, 83.
Colorectal cancer
Although the precise mechanisms underlying OPN’s ability to promote tumourigenesis are not fully understood, studies have demonstrated that OPN may bind to CD44v6 to promote colorectal cancer cell proliferation and survival, possibly via the JNK pathway84. It is also evident that OPN may promote the expression of cancer stem cell markers, such as OCT4 and SOX2, which not only improves cancer cell survival but also enhances chemotherapeutic resistance, namely to oxaliplatin85.
OPN may be an alternative prognostic marker in colorectal cancer. Multiple studies have indicated that high expression of OPN is associated with lymph node metastasis, postoperative metastasis, venous invasion, advanced staging and poorer survival22, 86–88. A recent meta-analysis of 15 studies has demonstrated that overexpression of OPN correlates with more advanced tumour grade, lymph node and distant metastasis, and poorer 2-year, 3-year and 5-year survival rates. However, no significant correlation has been found between increased OPN and depth of tumour invasion89.
Prostate cancer
Evidence has shown that OPN is closely associated with the proliferation and metastasis of prostate cancer90, 91. A study involving genetically engineered mice demonstrated that the expression of OPN occurs at the early stages of prostate neoplasm development, and a high level of OPN is observed in developed adenocarcinomas and metastatic deposits90. The molecular mechanisms underlying OPN-mediated tumourigenesis in prostate cancer has been explored in several studies. It appears that the binding of OPN to integrin αvβ3 may activate multiple signalling cascades, thus promoting tumourigenesis. For instance, OPN, upon binding to αvβ3, can activate Rho GTPase via RANKL, subsequently upregulating the expression of CD44 and MMP-9 and resulting in the increased ability for cancer cell movement and metastasis92. It is also possible that the binding of OPN to αvβ3 may promote the formation of invadopodia via the WASP-Arp2/3 pathway and upregulation of VEGF via the MAPK pathway, which also results in enhanced prostate cancer cell invasion93, 94. Furthermore, OPN may have an important regulatory role in the activation of ERK1/2 in prostate cancer. It has been shown that OPN can phosphorylate c-Raf to activate the ERK1/2, but also indirectly inhibits it by activating Akt95. Additionally, the binding of OPN to αvβ3 also appears to activate the PKCα/c-Src/IKK/NF-kB signalling cascade, resulting in the upregulation of COX-2 expression and PGE2 production, which further promotes cancer cell invasion and angiogenesis96. In addition to binding to αvβ3, an in vitro study further speculated that OPN may also bind to integrin β1 to maintain the activation of EGFR, which allows the proliferation of tumour cells97.
Ovarian cancer
Many studies have been conducted to evaluate the correlation between increased levels of OPN and ovarian cancer. In a meta-analysis of 15 studies, it was found that higher serum levels of OPN were positively associated with ovarian cancer and may be a risk factor in Asian populations98. It was discovered that among the three isoforms, OPNa, OPNb and OPNc, OPNc is the only isoform that is overexpressed in ovarian tumours99. Furthermore, OPNc is thought to be associated with increased tumour cell proliferation, migration and invasion99. In the human ovarian cancer cell line, OvCar-3, overexpression of OPNc has been shown to be associated with the upregulation of multiple genes. These are responsible for angiogenesis, cell cycle control, metastasis and cell adhesion100. It is thought that OPNc may promote these features of tumour development by upregulating HIF-1α via the activation of the PI3K/Akt/HIF-1α/p53 signalling pathway23.
Clear cell carcinoma of the ovary (CCCO) is a subtype of ovarian epithelial carcinoma and is associated with a higher resistance to platinum-based chemotherapy, thus conferring a poorer prognosis101. Overexpression of OPN has also been observed in CCCO102, 103. It is speculated that HNF-1β, a transcription factor, plays a role in the upregulation of OPN in CCCO, and that OPN promotes invasion by binding to several integrins, including αvβ1, αvβ3, αvβ5 and α5β1102, 103. Interestingly, Matsuura et al. demonstrated that the administration of simvastatin downregulates the expression of OPN and integrins, both of which reduce extracellular matrix invasion102. Although the mechanism of this reduction is not fully understood, it is thought that statins may deplete OPN expression by inhibiting HMG-CoA reductase and reducing isoprenoids104.
Osteopontin as therapeutic target
It has been suggested that OPN produced from tumours lacks important domains and is, therefore, structurally different to other types of OPN. As a result, blocking its production could be used to reduce tumour progression105. There are currently two methods to reduce OPN gene expression on an RNA level: ribozyme cleavage and hybridisation with antisense oligonucleotides. However, both methods are limited due to the difficulty in delivering nucleic acid drugs on an intracellular level106. Inhibiting OPN protein has been attempted using antibodies or synthetic peptides105. Several antibodies have been developed that identify specific epitopes of OPN107. Polyclonal antibodies to OPN prevent increased tumour growth in human prostate carcinoma cells, whilst murine anti-human OPN antibody has been shown to prevent the adhesion of MDA-MB-435 breast cancer cells105. As inhibiting adhesion of epithelial cells is linked to increased apoptosis, it may be possible that preventing cancer cell adhesion to OPN may also stimulate apoptosis of tumour cells, further ameliorating cancer treatment. A list of potential therapeutic strategies presented in Table 1
Table 1.
Therapeutic strategy targeting osteopontin in cancer
| Targeting strategy | Inhibitors | Target molecules (s) | Targeting cancer/tissue | Effect/outcome |
|---|---|---|---|---|
| RNAi | siRNA117 | OPN, CD44 | Breast cancer | OPN silencing causes reduced growth, migration and invasion. CD44 silencing abolishes OPN induced signalling |
| siRNA118 | OPN | Mammary cancer | Impairs cancer cells proliferation, survival and migration | |
| siRNA119 | OPN | Breast cancer | Inhibits migration and invasion. Knockdown affects PI3K/Akt/mTOR pathway and promotes expression of autophagy-related gene products LC3 and Beclin 1 | |
| siRNA40 | OPN | Non-small lung cancer | siRNA was delivered by polysorbitol-based transporter (POST). POST-delivered animals demonstrated reduced tumour volume and weight. | |
| miRNA54 | OPN | Hepatocellular carcinoma | Reduced metastatic potential in HCC | |
| Aptamers | OPN-R3116 | OPN | Brest cancer | Decreases cellular adhesion, migration and invasion in breast cancer cells. |
| Small-molecule inhibitors | Parecoxib120 | NR4A2 and Wnt Signalling | Intestinal polyps | Reduces disease burden |
| Simvastatin102 | 3-Hydroxy-3-methylgluaryl coenzyme A reductase | Ovarian cancer | Induces apoptosis and cell growth arrest by reducing OPN expression | |
| Andrographolide115 | OPN | Breast cancer | Reduces cancer growth | |
| Trichostatin A121 | HDAC inhibitor | Cervical cancer | Inhibits PMA-induced tumour growth | |
| Curcumin + BPs122 | OPN, CD44 and MMP-9 | Ovarian cancer | Reduces invasions of ovarian cancer and dendritic cells | |
| Agelastatin A123 | b-Catenin and Tcf-4 signalling | Breast cancer | Inhibits cell invasion and adhesion | |
| Blocking antibody | Anti-OPN Antibody124 | OPN | Lung and breast cancer | Inhibits cell migration adhesion, invasion and metastatic potential of cancer cells |
| Anti-OPN antibody118 | OPN | Mammary cancer | Impairs cancer cells proliferation, survival and migration | |
| Anti-αv3β integrin antibody125 | αvβ3-integrin | Breast cancer | Inhibits OPN-induced tumour growth and angiogenesis | |
| Anti-CD44 antibody126 | CD44 receptor | Fibroblasts | Reduces tumour progression induced by OPN |
As evidenced above, OPN is a promising therapeutic target in the management of various malignancies in the future, due to its role in enhancing tumour-mediated necrosis, cell proliferation, survival, invasion, angiogenesis, metastatic potential and drug resistance25, 108. Various pre-clinical studies have recently investigated the role of OPN inhibition in managing metastatic disease. For instance, Bandopadhyay et al. produced a review indicating the ability for small interfering RNA and short hairpin RNA against OPN to inhibit tumour progression and metastasis109. In addition, Wu et al. demonstrated that OPN knockout mice experienced slower tumour growth, whilst OPN knockout B16 melanoma cells had a reduction in metastasis to bone and soft tissues110, 111. Furthermore, Wu conducted a study investigating the effects of OPN knockout in colon cancer cells using four siRNA molecules of a target OPN gene112. The team’s findings indicate that OPN silencing results in the inhibition of various downstream effector cascades, involving a reduced expression of urokinase plasminogen activator, VEGF, MMP-2 and MMP-9, which in turn may clinically translate into a reduction in colon cancer invasion, angiogenesis and metastasis. In addition, attenuation of OPN expression is associated with downregulation of HIF-1 and VEGF in MDA-MB-343 breast cancer cells, whilst stable OPN-silencing results in a reduction in cell invasion, an increase in cell apoptosis and senescence, as well as a reduction in clonogenic survival113. Together, these findings indicate that OPN silencing results in enhanced radiosensitivity and mediation of cellular apoptosis in breast cancer cells.
The use of blocking antibodies to directly target OPN and its receptors, CD44 and αvβ3-integrin, has also demonstrated promising results. It has been shown that targeting CD44 on mesenchymal stromal cells results in a reduction in OPN-mediated tumour growth, whilst blocking antibodies to αvβ3-integrin and targeting of 4T1 cell-surface integrins in murine mammary epithelial cancer cells are both associated with an attenuation of ILK, MMP-2 and uroplasminogen activator expression114.
Small molecule inhibitors have also been shown to attenuate OPN expression. For instance, Andrographolide, a diterpenoid lactone sequestered from Andrographis paniculata, inhibits breast cancer cell proliferation via the attenuation of the PI3kinase/Akt signalling pathway115.
Finally, aptamers have been classically considered as promising therapeutic options, primarily due to their biological stability, capacity for immunogenic resistance and low-dose efficacy. A study designed to characterise the critical sequence of an RNA aptamer, OPN-R3, directed against human OPN in MDA-MB231 human breast cancer cells116 found that exposure to OPN-R3 was associated with significant downregulation of OPN signal transduction pathways, PI3K, JNK1/2, Src and Akt, as well as extracellular matrix degradation pathways involving matrix metalloproteinases. An in vitro model found that OPN-R3 inhibits MDA-MB231 adhesion by 60%, migration by 50% and invasion by 65%, whilst an in vivo xenograft model of breast cancer indicated that the aptamer significantly decreased local progression and distant metastases116.
Conclusion
There is well-documented evidence to suggest that OPN contributes to tumour progression. However, further research is necessary to improve the current understanding of these various molecular pathways and elucidate the precise role of OPN in mediating solid cancer progression and metastasis. OPN has the potential to be a novel biomarker and anti-cancer therapeutic target to manage tumour progression in various malignancies, thus heralding the possibility for earlier detection and treatment of cancer and metastasis.
Acknowledgements
We thank Dr Hina Jamall for her critical comments on the manuscript. This work was supported by the BOC Chair grant, RCoA, UK and the Basic and Frontier Research Fund (No.cstc2015jcyjA10055), Chongqing, China.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
These authors contributed equally: Hailin Zhao, Qian Chen
Edited by Y. Wang
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jianteng Gu, Phone: +0086 23 68765366, Email: jiantenggu@hotmail.com.
Daqing Ma, Phone: +0044 020 3315 8495, Email: d.ma@imperial.ac.uk.
References
- 1.Rangaswami H, Bulbule A, Kundu GC. Osteopontin: role in cell signaling and cancer progression. Trends Cell. Biol. 2006;16:79–87. doi: 10.1016/j.tcb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Steinman L. A molecular trio in relapse and remission in multiple sclerosis. Cancer Nat Rev Immunol. 2009;9:440–7. doi: 10.1038/nri2548. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto N, et al. Essential role of the cryptic epitope SLAYGLR within osteopontin in a murine model of rheumatoid arthritis. J. Clin. Invest. 2003;112:181–188. doi: 10.1172/JCI17778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sodek J, Ganss B, McKee MD. Osteopontin. Crit. Rev. Oral. Biol. Med. 2000;11:279–303. doi: 10.1177/10454411000110030101. [DOI] [PubMed] [Google Scholar]
- 5.Senger DR, Perruzzi CA, Papadopoulos-Sergiou A, Van de Water L. Adhesive properties of osteopontin: regulation by a naturally occurring thrombin-cleavage in close proximity to the GRGDS cell-binding domain. Mol. Biol. Cell. 1994;5:565–574. doi: 10.1091/mbc.5.5.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prochazka L, Tesarik R, Turanek J. Regulation of alternative splicing of CD44 in cancer. Cell Signal. 2014;26:2234–9. doi: 10.1016/j.cellsig.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Wang KX, Denhardt DT. Osteopontin: role in immune regulation and stress responses. Cytokine Growth Factor. Rev. 2008;19:333–345. doi: 10.1016/j.cytogfr.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Haylock DN, Nilsson SK. Osteopontin: a bridge between bone and blood. Br. J. Haematol. 2006;134:467–474. doi: 10.1111/j.1365-2141.2006.06218.x. [DOI] [PubMed] [Google Scholar]
- 9.Reinholt FP, Hultenby K, Oldberg A, Heinegard D. Osteopontin--a possible anchor of osteoclasts to bone. Proc. Natl Acad. Sci. USA. 1990;87:4473–4475. doi: 10.1073/pnas.87.12.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higashi A, et al. The potential role of inflammation associated with interaction between osteopontin and CD44 in a case of pulmonary tumor thrombotic microangiopathy caused by breast cancer. Intern. Med. 2015;54:2877–2880. doi: 10.2169/internalmedicine.54.4749. [DOI] [PubMed] [Google Scholar]
- 11.Ramaiah SK, Rittling S. Pathophysiological role of osteopontin in hepatic inflammation, toxicity, and cancer. Toxicol. Sci. 2008;103:4–13. doi: 10.1093/toxsci/kfm246. [DOI] [PubMed] [Google Scholar]
- 12.Chen Q, et al. Fate decision of mesenchymal stem cells: adipocytes or osteoblasts? Cell. Death. Differ. 2016;23:1128–1139. doi: 10.1038/cdd.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bellahcène A, Castronovo V, Ogbureke KU, Fisher LW, Fedarko NS, et al. Small integrin-binding ligand N-linked glycoproteins (SIBLINGs): multifunctional proteins in cancer. Nat Rev Cancer. 2008;8:212–26. doi: 10.1038/nrc2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wai PY, Kuo PC. The role of osteopontin in tumor metastasis. J. Surg. Res. 2004;121:228–241. doi: 10.1016/j.jss.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Y, et al. Osteopontin expression correlates with melanoma invasion. J. Invest. Dermatol. 2005;124:1044–1052. doi: 10.1111/j.0022-202X.2005.23680.x. [DOI] [PubMed] [Google Scholar]
- 16.Leali D, et al. Osteopontin (Eta-1) and fibroblast growth factor-2 cross-talk in angiogenesis. J. Immunol. 2003;171:1085–1093. doi: 10.4049/jimmunol.171.2.1085. [DOI] [PubMed] [Google Scholar]
- 17.Wirtz D, Konstantopoulos K, Searson PC. The physics of cancer: the role of physical interactions and mechanical forces in metastasis. Nat. Rev. Cancer. 2011;11:512–522. doi: 10.1038/nrc3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weber GF, Lett GS, Haubein NC. Categorical meta-analysis of osteopontin as aclinical cancer marker. Oncol. Rep. 2011;25:433–441. doi: 10.3892/or.2010.1106. [DOI] [PubMed] [Google Scholar]
- 19.Kothari AN, et al. Osteopontin—a master regulator of epithelial-mesenchymal transition. J. Clin. Med. 2016;5:1–16. doi: 10.3390/jcm5040039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li NY, et al. Osteopontin up-regulates critical epithelial-mesenchymal transition transcription factors to induce an aggressive breast cancer phenotype. J. Am. Coll. Surg. 2013;217:17–26. doi: 10.1016/j.jamcollsurg.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 21.Dong Q, et al. Osteopontin regulated epithelial-mesenchymal transition via PI3K/AKT signaling pathway in hepatocellular carcinoma. Cancer Res. 2013;73:2695–2695. doi: 10.1158/1538-7445.AM2013-2695. [DOI] [Google Scholar]
- 22.Ng L, et al. Post-operative plasma osteopontin predicts distant metastasis in human colorectal cancer. PLoS. One. 2015;10:e0126219. doi: 10.1371/journal.pone.0126219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song G, et al. Osteopontin promotes ovarian cancer progression and cell survival and increases HIF-1alpha expression through the PI3-K/Akt pathway. Cancer Sci. 2008;99:1901–1907. doi: 10.1111/j.1349-7006.2008.00911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raja R, et al. Hypoxia-driven osteopontin contributes to breast tumor growth through modulation of HIF1 alpha-mediated VEGF-dependent angiogenesis. Oncogene. 2014;33:2053–2064. doi: 10.1038/onc.2013.171. [DOI] [PubMed] [Google Scholar]
- 25.Shevde LA, Samant RS. Role of osteopontin in the pathophysiology of cancer. Matrix Biol. 2014;37:131–141. doi: 10.1016/j.matbio.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sui X, et al. Autophagy and chemotherapy resistance: a promising therapeutic target for cancer treatment. Cell Death Dis. 2013;4:e838. doi: 10.1038/cddis.2013.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang SH, Yu KN, Lee KA, Cho MH. Autophagy execution protein beclin1 regulates radiation-induced osteopontin in human lung cancer cell. Lung. Cancer. 2012;77:S29–S29. doi: 10.1016/j.lungcan.2012.05.051. [DOI] [Google Scholar]
- 28.Yang MC, et al. Blockade of autophagy reduces pancreatic cancer stem cell activity and potentiates the tumoricidal effect of gemcitabine. Mol. Cancer. 2015;14:179. doi: 10.1186/s12943-015-0449-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu G, et al. Osteopontin induces autophagy to promote chemo-resistance in human hepatocellular carcinoma cells. Cancer Lett. 2016;383:171–182. doi: 10.1016/j.canlet.2016.09.033. [DOI] [PubMed] [Google Scholar]
- 30.Yang L, et al. Down-regulation of osteopontin expression by RNA interference affects cell proliferation and chemotherapy sensitivity of breast cancer MDA-MB-231 cells. Mol. Med. Rep. 2012;5:373–376. doi: 10.3892/mmr.2011.679. [DOI] [PubMed] [Google Scholar]
- 31.Pang H, et al. Knockdown of osteopontin chemosensitizes MDA-MB-231 cells to cyclophosphamide by enhancing apoptosis through activating p38 MAPK pathway. Cancer Biother Radio. 2011;26:165–173. doi: 10.1089/cbr.2010.0838. [DOI] [PubMed] [Google Scholar]
- 32.Jan HJ, et al. Osteopontin regulates human glioma cell invasiveness and tumor growth in mice. Neuro. Oncol. 2010;12:58–70. doi: 10.1093/neuonc/nop013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Atai NA, et al. Osteopontin is up-regulated and associated with neutrophil and macrophage infiltration in glioblastoma. Immunology. 2011;132:39–48. doi: 10.1111/j.1365-2567.2010.03335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding Q, et al. Promotion of malignant astrocytoma cell migration by osteopontin expressed in the normal brain: differences in integrin signaling during cell adhesion to osteopontin versus vitronectin. Cancer Res. 2002;62:5336–5343. doi: 10.1100/tsw.2002.247. [DOI] [PubMed] [Google Scholar]
- 35.Lin Q, et al. Clinical and prognostic significance of OPN and VEGF expression in patients with non-small-cell lung cancer. Cancer Epidemiol. 2015;39:539–544. doi: 10.1016/j.canep.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 36.Chakraborty G, Jain S, Kundu GC. Osteopontin promotes vascular endothelial growth factor-dependent breast tumor growth and angiogenesis via autocrine and paracrine mechanisms. Cancer Res. 2008;68:152–161. doi: 10.1158/0008-5472.CAN-07-2126. [DOI] [PubMed] [Google Scholar]
- 37.Shi L, Wang X. Role of osteopontin in lung cancer evolution and heterogeneity. Semin. Cell. Dev. Biol. 2016;64:40–47. doi: 10.1016/j.semcdb.2016.08.032. [DOI] [PubMed] [Google Scholar]
- 38.Shojaei F, et al. Osteopontin induces growth of metastatic tumors in a preclinical model of non-small lung cancer. J. Exp. Clin. Cancer Res. 2012;31:26. doi: 10.1186/1756-9966-31-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kang CG, Han HJ, Lee HJ, Kim SH, Lee EO. Rho-associated kinase signaling is required for osteopontin-induced cell invasion through inactivating cofilin in human non-small cell lung cancer cell lines. Bioorg. Med. Chem. Lett. 2015;25:1956–1960. doi: 10.1016/j.bmcl.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 40.Cho WY, et al. Suppression of tumor growth in lung cancer xenograft model mice by poly(sorbitol-co-PEI)-mediated delivery of osteopontin siRNA. Eur. J. Pharm. Biopharm. 2015;94:450–462. doi: 10.1016/j.ejpb.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 41.Funakoshi T, Lee CH, Hsieh JJ. A systematic review of predictive and prognostic biomarkers for VEGF-targeted therapy in renal cell carcinoma. Cancer Treat. Rev. 2014;40:533–547. doi: 10.1016/j.ctrv.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 42.Tran HT, et al. Prognostic or predictive plasma cytokines and angiogenic factors for patients treated with pazopanib for metastatic renal-cell cancer: a retrospective analysis of phase 2 and phase 3 trials. Lancet Oncol. 2012;13:827–837. doi: 10.1016/S1470-2045(12)70241-3. [DOI] [PubMed] [Google Scholar]
- 43.Rabjerg M, et al. Molecular characterization of clear cell renal cell carcinoma identifies CSNK2A1, SPP1 and DEFB1 as promising novel prognostic markers. APMIS. 2016;124:372–383. doi: 10.1111/apm.12519. [DOI] [PubMed] [Google Scholar]
- 44.Gotoh M, Sakamoto M, Kanetaka K, Chuuma M, Hirohashi S. Overexpression of osteopontin in hepatocellular carcinoma. Pathol. Int. 2002;52:19–24. doi: 10.1046/j.1440-1827.2002.01316.x. [DOI] [PubMed] [Google Scholar]
- 45.Pan HW, et al. Overexpression of osteopontin is associated with intrahepatic metastasis, early recurrence, and poorer prognosis of surgically resected hepatocellular carcinoma. Cancer. 2003;98:119–127. doi: 10.1002/cncr.11487. [DOI] [PubMed] [Google Scholar]
- 46.Ye QH, et al. Predicting hepatitis B virus-positive metastatic hepatocellular carcinomas using gene expression profiling and supervised machine learning. Nat. Med. 2003;9:416–423. doi: 10.1038/nm843. [DOI] [PubMed] [Google Scholar]
- 47.Huang J, et al. Correlation between genomic DNA copy number alterations and transcriptional expression in hepatitis B virus-associated hepatocellular carcinoma. FEBS Lett. 2006;580:3571–3581. doi: 10.1016/j.febslet.2006.05.032. [DOI] [PubMed] [Google Scholar]
- 48.Kim J, et al. Elevated plasma osteopontin levels in patients with hepatocellular carcinoma. Am. J. Gastroenterol. 2006;101:2051–2059. doi: 10.1111/j.1572-0241.2006.00679.x. [DOI] [PubMed] [Google Scholar]
- 49.Zhang H, et al. The prognostic significance of preoperative plasma levels of osteopontin in patients with hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2006;132:709–717. doi: 10.1007/s00432-006-0119-3. [DOI] [PubMed] [Google Scholar]
- 50.Shang S, et al. Identification of osteopontin as a novel marker for early hepatocellular carcinoma. Hepatology. 2012;55:483–490. doi: 10.1002/hep.24703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee YJ, Jang BK. Can combination of osteopontin and peritumor-infiltrating macrophages be a prognostic marker of early-stage hepatocellular carcinoma? Hepatobiliary Surg. Nutr. 2014;3:57–59. doi: 10.3978/j.issn.2304-3881.2014.02.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen RX, Xia YH, Xue TC, Zhang H, Ye SL. Down-regulation of osteopontin inhibits metastasis of hepatocellular carcinoma cells via a mechanism involving MMP-2 and uPA. Oncol. Rep. 2011;25:803–808. doi: 10.3892/or.2010.1116. [DOI] [PubMed] [Google Scholar]
- 53.Sun BS, et al. Lentiviral-mediated miRNA against osteopontin suppresses tumor growth and metastasis of human hepatocellular carcinoma. Hepatology. 2008;48:1834–1842. doi: 10.1002/hep.22531. [DOI] [PubMed] [Google Scholar]
- 54.Bhattacharya SD, et al. Micro-RNA-181a regulates osteopontin-dependent metastatic function in hepatocellular cancer cell lines. Surgery. 2010;148:291–297. doi: 10.1016/j.surg.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jemal A, et al. Global cancer statistics. Cancer J. Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 56.Coppola D, et al. Correlation of osteopontin protein expression and pathological stage across a wide variety of tumor histologies. Clin. Cancer Res. 2004;10:184–190. doi: 10.1158/1078-0432.CCR-1405-2. [DOI] [PubMed] [Google Scholar]
- 57.Park MG, et al. The value of plasma osteopontin levels as a predictive factor of disease stage and recurrence in patients with bladder urothelial carcinoma: a prospective study. Kaohsiung. J. Med. Sci. 2012;28:526–530. doi: 10.1016/j.kjms.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 58.Ke HL, et al. Osteopontin overexpression predicts poor prognosis of upper urinary tract urothelial carcinoma. Urol. Oncol. 2011;29:703–709. doi: 10.1016/j.urolonc.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 59.Tuck AB, et al. Osteopontin and p53 expression are associated with tumor progression in a case of synchronous, bilateral, invasive mammary carcinomas. Arch. Pathol. Lab. Med. 1997;121:578–584. [PubMed] [Google Scholar]
- 60.Tuck AB, et al. Osteopontin expression in a group of lymph node negative breast cancer patients. Int. J. Cancer. 1998;79:502–508. doi: 10.1002/(SICI)1097-0215(19981023)79:5<502::AID-IJC10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 61.Mitas M, et al. Quantitative real-time RT-PCR detection of breast cancer micrometastasis using a multigene marker panel. Int. J. Cancer. 2001;93:162–171. doi: 10.1002/ijc.1312. [DOI] [PubMed] [Google Scholar]
- 62.Rudland PS, et al. Prognostic significance of the metastasis-associated protein osteopontin in human breast cancer. Cancer Res. 2002;62:3417–3427. [PubMed] [Google Scholar]
- 63.Brown LF, et al. Osteopontin expression and distribution in human carcinomas. Am. J. Pathol. 1994;145:610–623. [PMC free article] [PubMed] [Google Scholar]
- 64.Tuck AB, Chambers AF. The role of osteopontin in breast cancer: clinical and experimental studies. J. Mammary Gland. Biol. Neoplasia. 2001;6:419–429. doi: 10.1023/A:1014734930781. [DOI] [PubMed] [Google Scholar]
- 65.Tuck AB, Elliott BE, Hota C, Tremblay E, Chambers AF. Osteopontin-induced, integrin-dependent migration of human mammary epithelial cells involves activation of the hepatocyte growth factor receptor (Met) J. Cell. Biochem. 2000;78:465–475. doi: 10.1002/1097-4644(20000901)78:3<465::AID-JCB11>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 66.Noti JD. Adherence to osteopontin via alphavbeta3 suppresses phorbol ester-mediated apoptosis in MCF-7 breast cancer cells that overexpress protein kinase C-alpha. Int. J. Oncol. 2000;17:1237–1243. doi: 10.3892/ijo.17.6.1237. [DOI] [PubMed] [Google Scholar]
- 67.Wu IC, et al. Osteopontin expression in squamous cell cancer of the esophagus. World J. Surg. 2008;32:1989–1995. doi: 10.1007/s00268-008-9609-6. [DOI] [PubMed] [Google Scholar]
- 68.Ito T, et al. An inducible short-hairpin RNA vector against osteopontin reduces metastatic potential of human esophageal squamous cell carcinoma in vitro and in vivo. Clin. Cancer Res. 2006;12:1308–1316. doi: 10.1158/1078-0432.CCR-05-1611. [DOI] [PubMed] [Google Scholar]
- 69.Kolb A, et al. Osteopontin influences the invasiveness of pancreatic cancer cells and is increased in neoplastic and inflammatory conditions. Cancer Biol. Ther. 2005;4:740–746. doi: 10.4161/cbt.4.7.1821. [DOI] [PubMed] [Google Scholar]
- 70.Zhivkova-Galunska M, et al. Osteopontin but not osteonectin favors the metastatic growth of pancreatic cancer cell lines. Cancer Biol. Ther. 2010;10:54–64. doi: 10.4161/cbt.10.1.12161. [DOI] [PubMed] [Google Scholar]
- 71.Ohno K, et al. Inhibition of osteopontin reduces liver metastasis of human pancreatic cancer xenografts injected into the spleen in a mouse model. Surg. Today. 2010;40:347–356. doi: 10.1007/s00595-009-4082-x. [DOI] [PubMed] [Google Scholar]
- 72.Lazar M, et al. Involvement of osteopontin in the matrix-degrading and proangiogenic changes mediated by nicotine in pancreatic cancer cells. J. Gastrointest. Surg. 2010;14:1566–1577. doi: 10.1007/s11605-010-1338-0. [DOI] [PubMed] [Google Scholar]
- 73.Koopmann J, et al. Evaluation of osteopontin as biomarker for pancreatic adenocarcinoma. Cancer Epidemiol. Biomark. Prev. 2004;13:487–491. [PubMed] [Google Scholar]
- 74.Poruk KE, et al. Serum osteopontin and tissue inhibitor of metalloproteinase 1 as diagnostic and prognostic biomarkers for pancreatic adenocarcinoma. Pancreas. 2013;42:193–197. doi: 10.1097/MPA.0b013e31825e354d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Parsonnet J, et al. Helicobacter pylori infection and the risk of gastric carcinoma. N. Engl. J. Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 76.Lee SH, et al. Ablation of osteopontin suppresses N-methyl-N-nitrosourea and Helicobacter pylori-induced gastric cancer development in mice. Carcinogenesis. 2015;36:1550–1560. doi: 10.1093/carcin/bgv144. [DOI] [PubMed] [Google Scholar]
- 77.Bromberg J, Darnell JE., Jr. The role of STATs in transcriptional control and their impact on cellular function. Oncogene. 2000;19:2468–2473. doi: 10.1038/sj.onc.1203476. [DOI] [PubMed] [Google Scholar]
- 78.Xu W, Liu LZ, Loizidou M, Ahmed M, Charles IG. The role of nitric oxide in cancer. Cell. Res. 2002;12:311–320. doi: 10.1038/sj.cr.7290133. [DOI] [PubMed] [Google Scholar]
- 79.Vakkala M, et al. Inducible nitric oxide synthase expression, apoptosis, and angiogenesis in in situ and invasive breast carcinomas. Clin. Cancer Res. 2000;6:2408–2416. [PubMed] [Google Scholar]
- 80.Tang X, et al. Osteopontin splice variants differentially exert clinicopathological features and biological functions in gastric cancer. Int. J. Biol. Sci. 2013;9:55–66. doi: 10.7150/ijbs.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu J, et al. Osteopontin promotes the progression of gastric cancer through the NF-kappaB pathway regulated by the MAPK and PI3K. Int. J. Oncol. 2014;45:282–290. doi: 10.3892/ijo.2014.2393. [DOI] [PubMed] [Google Scholar]
- 82.Wang ZM, Cui YH, Li W, Chen SY, Liu TS. Lentiviral-mediated siRNA targeted against osteopontin suppresses the growth and metastasis of gastric cancer cells. Oncol. Rep. 2011;25:997–1003. doi: 10.3892/or.2011.1168. [DOI] [PubMed] [Google Scholar]
- 83.Song G, et al. Osteopontin promotes gastric cancer metastasis by augmenting cell survival and invasion through Akt-mediated HIF-1alpha up-regulation and MMP9 activation. J. Cell. Mol. Med. 2009;13:1706–1718. doi: 10.1111/j.1582-4934.2008.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rao G, et al. Reciprocal interactions between tumor-associated macrophages and CD44-positive cancer cells via osteopontin/CD44 promote tumorigenicity in colorectal cancer. Clin. Cancer Res. 2013;19:785–797. doi: 10.1158/1078-0432.CCR-12-2788. [DOI] [PubMed] [Google Scholar]
- 85.Ng L, et al. Osteopontin overexpression induced tumor progression and chemoresistance to oxaliplatin through induction of stem-like properties in human colorectal cancer. Stem Cells Int. 2015;2015:247892. doi: 10.1155/2015/247892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Likui W, Hong W, Shuwen Z. Clinical significance of the upregulated osteopontin mRNA expression in human colorectal cancer. J. Gastrointest. Surg. 2010;14:74–81. doi: 10.1007/s11605-009-1035-z. [DOI] [PubMed] [Google Scholar]
- 87.Li J, Yang GZ, Zhu ZM, Zhou ZY, Li L. Osteopontin is overexpressed in colorectal carcinoma and is correlated with P53 by immunohistochemistry. Exp. Ther. Med. 2012;3:621–624. doi: 10.3892/etm.2012.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sun L, et al. Combination of haptoglobin and osteopontin could predict colorectal cancer hepatic metastasis. Ann. Surg. Oncol. 2012;19:2411–2419. doi: 10.1245/s10434-011-2177-2. [DOI] [PubMed] [Google Scholar]
- 89.Zhao M, Liang F, Zhang B, Yan W, Zhang J. The impact of osteopontin on prognosis and clinicopathology of colorectal cancer patients: a systematic meta-analysis. Sci. Rep. 2015;5:12713. doi: 10.1038/srep12713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Khodavirdi AC, et al. Increased expression of osteopontin contributes to the progression of prostate cancer. Cancer Res. 2006;66:883–888. doi: 10.1158/0008-5472.CAN-05-2816. [DOI] [PubMed] [Google Scholar]
- 91.Thalmann GN, et al. Osteopontin: possible role in prostate cancer progression. Clin. Cancer Res. 1999;5:2271–2277. [PubMed] [Google Scholar]
- 92.Desai B, Rogers MJ, Chellaiah MA. Mechanisms of osteopontin and CD44 as metastatic principles in prostate cancer cells. Mol. Cancer. 2007;6:18. doi: 10.1186/1476-4598-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Desai B, Ma T, Chellaiah MA. Invadopodia and matrix degradation, a new property of prostate cancer cells during migration and invasion. J. Biol. Chem. 2008;283:13856–13866. doi: 10.1074/jbc.M709401200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gupta A, Zhou CQ, Chellaiah MA. Osteopontin and MMP9: Associations with VEGF Expression/Secretion and Angiogenesis in PC3 Prostate Cancer Cells. Cancers. 2013;5:617–638. doi: 10.3390/cancers5020617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Robertson BW, Bonsal L, Chellaiah MA. Regulation of Erk1/2 activation by osteopontin in PC3 human prostate cancer cells. Mol. Cancer. 2010;9:260. doi: 10.1186/1476-4598-9-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jain S, Chakraborty G, Kundu GC. The crucial role of cyclooxygenase-2 in osteopontin-induced protein kinase C alpha/c-Src/IkappaB kinase alpha/beta-dependent prostate tumor progression and angiogenesis. Cancer Res. 2006;66:6638–6648. doi: 10.1158/0008-5472.CAN-06-0661. [DOI] [PubMed] [Google Scholar]
- 97.Angelucci A, et al. Osteopontin enhances the cell proliferation induced by the epidermal growth factor in human prostate cancer cells. Prostate. 2004;59:157–166. doi: 10.1002/pros.20008. [DOI] [PubMed] [Google Scholar]
- 98.Wang YD, Chen H, Liu HQ, Hao M. Correlation between ovarian neoplasm and serum levels of osteopontin: a meta-analysis. Tumour Biol. 2014;35:11799–11808. doi: 10.1007/s13277-014-2314-1. [DOI] [PubMed] [Google Scholar]
- 99.Tilli TM, et al. Osteopontin-c splicing isoform contributes to ovarian cancer progression. Mol. Cancer Res. 2011;9:280–293. doi: 10.1158/1541-7786.MCR-10-0463. [DOI] [PubMed] [Google Scholar]
- 100.Tilli TM, Bellahcene A, Castronovo V, Gimba ER. Changes in the transcriptional profile in response to overexpression of the osteopontin-c splice isoform in ovarian (OvCar-3) and prostate (PC-3) cancer cell lines. BMC Cancer. 2014;14:433. doi: 10.1186/1471-2407-14-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sugiyama T, et al. Clinical characteristics of clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy. Cancer. 2000;88:2584–2589. doi: 10.1002/1097-0142(20000601)88:11<2584::AID-CNCR22>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 102.Matsuura M, Suzuki T, Saito T. Osteopontin is a new target molecule for ovarian clear cell carcinoma therapy. Cancer Sci. 2010;101:1828–1833. doi: 10.1111/j.1349-7006.2010.01615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kato N, Motoyama T. Overexpression of osteopontin in clear cell carcinoma of the ovary: close association with HNF-1beta expression. Histopathology. 2008;52:682–688. doi: 10.1111/j.1365-2559.2008.03006.x. [DOI] [PubMed] [Google Scholar]
- 104.Matsuura M, et al. Statin-mediated reduction of osteopontin expression induces apoptosis and cell growth arrest in ovarian clear cell carcinoma. Oncol. Rep. 2011;25:41–47. [PubMed] [Google Scholar]
- 105.Johnston NI, et al. Osteopontin as a target for cancer therapy. Front. Biosci. 2008;13:4361–4372. doi: 10.2741/3009. [DOI] [PubMed] [Google Scholar]
- 106.Weber GF. The metastasis gene osteopontin: a candidate target for cancer therapy. Biochim. Biophys. Acta. 2001;1552:61–85. doi: 10.1016/S0005-2728(01)00198-0. [DOI] [PubMed] [Google Scholar]
- 107.Kon S, et al. Antibodies to different peptides in osteopontin reveal complexities in the various secreted forms. J. Cell. Biochem. 2000;77:487–498. doi: 10.1002/(SICI)1097-4644(20000601)77:3<487::AID-JCB13>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 108.Likui W, Hong W, Shuwen Z, Yuangang Y, Yan W. The potential of osteopontin as a therapeutic target for human colorectal cancer. J. Gastrointest. Surg. 2011;15:652–659. doi: 10.1007/s11605-011-1445-6. [DOI] [PubMed] [Google Scholar]
- 109.Bandopadhyay M, et al. Osteopontin as a therapeutic target for cancer. Expert. Opin. Ther. Targets. 2014;18:883–895. doi: 10.1517/14728222.2014.925447. [DOI] [PubMed] [Google Scholar]
- 110.Wu Y, Denhardt DT, Rittling SR. Osteopontin is required for full expression of the transformed phenotype by the ras oncogene. Br. J. Cancer. 2000;83:156–163. doi: 10.1054/bjoc.2000.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nemoto H, et al. Osteopontin deficiency reduces experimental tumor cell metastasis to bone and soft tissues. J. Bone Miner. Res. 2001;16:652–659. doi: 10.1359/jbmr.2001.16.4.652. [DOI] [PubMed] [Google Scholar]
- 112.Wu XL, et al. Osteopontin knockdown suppresses the growth and angiogenesis of colon cancer cells. World J. Gastroenterol. 2014;20:10440–10448. doi: 10.3748/wjg.v20.i30.10440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yang L, et al. Silencing of osteopontin promotes the radiosensitivity of breast cancer cells by reducing the expression of hypoxia inducible factor 1 and vascular endothelial growth factor. Chin. Med. J. 2012;125:293–299. [PubMed] [Google Scholar]
- 114.Mi Z, et al. Osteopontin promotes CCL5-mesenchymal stromal cell-mediated breast cancer metastasis. Carcinogenesis. 2011;32:477–487. doi: 10.1093/carcin/bgr009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kumar S, et al. Andrographolide inhibits osteopontin expression and breast tumor growth through down regulation of PI3 kinase/Akt signaling pathway. Curr. Mol. Med. 2012;12:952–966. doi: 10.2174/156652412802480826. [DOI] [PubMed] [Google Scholar]
- 116.Mi Z, et al. RNA aptamer blockade of osteopontin inhibits growth and metastasis of MDA-MB231 breast cancer cells. Mol. Ther. 2009;17:153–161. doi: 10.1038/mt.2008.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kumar V, Behera R, Lohite K, Karnik S, Kundu GC. p38 Kinase is crucial for osteopontin-induced furin expression that supports cervical cancer progression. Cancer Res. 2010;70:10381–10391. doi: 10.1158/0008-5472.CAN-10-1470. [DOI] [PubMed] [Google Scholar]
- 118.Saleh S, Thompson DE, McConkey J, Murray P, Moorehead RA. Osteopontin regulates proliferation, apoptosis, and migration of murine claudin-low mammary tumor cells. BMC Cancer. 2016;16:359. doi: 10.1186/s12885-016-2396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang H, et al. Osteopontin knockdown inhibits αv, β3 integrin-induced cell migration and invasion and promotes apoptosis of breast cancer cells by inducing autophagy and inactivating the PI3K/Akt/mTOR pathway. Cell. Physiol. Biochem. 2014;33:991–1002. doi: 10.1159/000358670. [DOI] [PubMed] [Google Scholar]
- 120.Zagani R, et al. Cyclooxygenase-2 inhibitors down-regulate osteopontin and Nr4A2-new therapeutic targets for colorectal cancers. Gastroenterology. 2009;137:1358–1366. doi: 10.1053/j.gastro.2009.06.039. [DOI] [PubMed] [Google Scholar]
- 121.Sharma P, Kumar S, Kundu GC. Transcriptional regulation of human osteopontin promoter by histone deacetylase inhibitor, trichostatin A in cervical cancer cells. Mol. Cancer. 2010;9:178. doi: 10.1186/1476-4598-9-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lv J, et al. Effects and mechanisms of curcumin and basil polysaccharide on the invasion of SKOV3 cells and dendritic cells. Mol. Med. Rep. 2013;8:1580–1586. doi: 10.3892/mmr.2013.1695. [DOI] [PubMed] [Google Scholar]
- 123.Mason CK, et al. Agelastatin A: a novel inhibitor of osteopontin-mediated adhesion, invasion, and colony formation. Mol. Cancer Ther. 2008;7:548–558. doi: 10.1158/1535-7163.MCT-07-2251. [DOI] [PubMed] [Google Scholar]
- 124.Dai J, et al. A humanized anti-osteopontin antibody inhibits breast cancer growth and metastasis in vivo. Cancer Immunol. Immunother. 2010;59:355–366. doi: 10.1007/s00262-009-0754-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ahmed M, Kundu GC. Osteopontin selectively regulates p70S6K/mTOR phosphorylation leading to NF-kappaB dependent AP-1-mediated ICAM-1 expression in breast cancer cells. Mol. Cancer. 2010;9:101. doi: 10.1186/1476-4598-9-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Teramoto H, et al. Autocrine activation of an osteopontin-CD44-Rac pathway enhances invasion and transformation by H-RasV12. Oncogene. 2005;24:489–501. doi: 10.1038/sj.onc.1208209. [DOI] [PubMed] [Google Scholar]