Abstract
Anthropogenic noise is a pervasive pollutant altering behaviour of wildlife that communicates acoustically. Some species adjust vocalisations to compensate for noise. However, we know little about whether signal adjustments improve communication in noise, the extent to which effectiveness of adjustments varies with noise source, or how individual variation in physiology varies with response capacity. We played noise-adjusted and unadjusted songs to wild Passerculus sandwichensis (Savannah Sparrows) after measurements of adrenocortical responsiveness of individuals. Playbacks using songs adjusted to noisy environments were effective in restoring appropriate conspecific territorial aggression behaviours in some altered acoustic environments. Surprisingly, however, levels of adrenocortical responsiveness that reduced communication errors at some types of infrastructure were correlated with increased errors at others. Song adjustments that were effective in communicating for individuals with lower adrenocortical responsiveness at pumpjacks were not effective at screwpumps and vice versa. Our results demonstrate that vocal adjustments can sometimes allow birds to compensate for disruptions in communication caused by anthropogenic noise, but that physiological variation among receivers may alter effectiveness of these adjustments. Thus mitigation strategies to minimize anthropogenic noise must account for both acoustic and physiological impacts of infrastructure.
Introduction
Anthropogenic noise from industrial activities such as petroleum extraction1 is widespread and alters soundscapes, behaviour and stress responses in many wildlife species2–4. This acoustic pollution could result in extensive impacts to wildlife in critically threatened ecosystems such as grasslands5. Noise can impact fitness by altering physiological costs2 and disrupting behaviours crucial for defending territories and attracting mates6–8 by preventing signals from being detected or recognised9. However, these effects may vary among industrial activities, as spectral characteristics of noise produced by different activities can vary greatly4,10. This suggests that ecological impacts of many different industrial activities might be mitigated by preferentially implementing infrastructure that produces noise at frequencies and amplitudes that produce the least disturbance and allow for the most compensatory behaviours from nearby animals.
Vocalisations can be altered to make signals audible in noisy environments9,11 but this can change signal content6–8 and compromise communication efficacy12. While many studies have demonstrated that the signalling animals can alter vocalisations to compensate for noise10–13, the literature has only recently focused on effects of anthropogenic noise on receivers8,9,14–21. Thus, less is known about whether signal adjustments actually improve communication, if efficacy varies with noise source and how this interacts with intrinsic individual variation to explain capacity for populations to adjust to noise. Thus, why animals show variable behavioural responses to different types of noise2,10,22 is not well understood. Extrinsic characteristics related to sound physics, particularly noise amplitude and frequency overlap between noise and acoustic signals, have been considered in some depth, but this does not always explain why signal alterations are necessary and effective in some systems8 but are ineffective7,16 in others.
Behavioural responses to acoustic signals are mediated not only by the signalling environment, but also by physiological mechanisms23, which can both affect behavioural response patterns to novel stimuli22 and be affected by exposure to chronic human disturbances3. Indeed, noise type can alter the glucocorticoid stress response24. Thus, differential physiological responses to infrastructure with different physical footprints25 and noise spectra might explain why some industrial noises have greater impacts than others. Acute changes in corticosterone levels (hereafter, CORT) can be used as an index of adrenocortical responsiveness. CORT is associated with territorial defence26, interacts with testosterone to regulate levels of territorial aggression during breeding27,28 and is known to increase in response to environmental perturbations23,24. Therefore, spectral characteristics of noise and altered adrenocortical responsiveness may both explain variation in responses to particular noise types and interact to explain why song alterations in the presence of noise improve communication at some types of infrastructure but not others.
We tested whether song adjustments to noise varied in effectiveness with infrastructure and physiology using an experimental design that combined playbacks of noise-adjusted and natural (unadjusted) songs in three environments, with responses of Passerculus sandwichensis (Savannah Sparrows) with naturally varying CORT levels. We colour-banded and sampled CORT using a 12-min standardised stress handling protocol31 from 35 free-living adult male Savannah Sparrows near Brooks, Alberta, Canada, in mixed-grass prairies. Birds held territories within control sites (11 males at 3 sites) and in noisy sites that contained two types of generator-powered oil wells with differing noise spectra: pumpjacks (12 males at 3 sites) and screw pumps (12 males at 3 sites). We predicted that these well types might have different impacts on stress and behaviour because pumpjacks are taller (4.5 m), move rhythmically along a vertical axis and produce noise with a different power spectra (Fig. 1) compared with screw pumps, which are also shorter (2.7 m) and have a horizontal spinning mechanism. Pumpjacks produce noise with significantly lower sound pressure levels than screw pumps, particularly in the frequency range that overlaps with Savannah Sparrow songs (Fig. 1). Savannah sparrows adjust their songs at both infrastructure types29. A mean of 14.0 ± 12.9s.d days (range 0–49.0) after colour-banding and CORT blood sampling, we played noise-adjusted and unadjusted songs to each colour-banded male and summed conspecific territorial aggression behaviours (hereafter “agonistic responses” for brevity) in six categories when the bird approached <20 m of the playback speaker: numbers of songs, calls, attacks (attacking speaker or flying over speaker) and wing flicks (an agitated movement); distance of closest approach; and time to closest approach.
Figure 1.
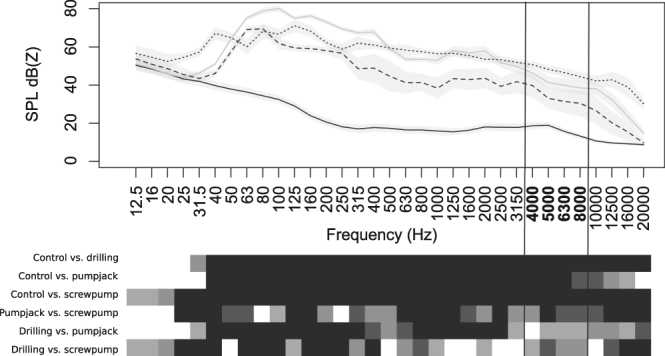
Frequency profiles for acoustic environments. Mean sound pressure levels (error bars 95% c.i.) at 10 m (Z-weighted time-average value in decibels, LZeq) for each 1/3-octave frequency band 12.5–20,000 Hz. Symbols show background (i.e. control environments, black solid line; n = 50 at 46 sites), drilling playback (in which adjusted songs were recorded, grey solid line; n = 36 at 3 sites), pumpjacks (dashed line; n = 15 at 4 sites) and screw pumps (dotted line; n = 17 at 5 sites). Frequencies differ where cells below are black (P ≤ 0.0001), dark grey (P ≤ 0.001), medium grey (P ≤ 0.01) and light grey (P ≤ 0.05). Bold frequencies on the x-axis, bounded by vertical lines, indicate which frequencies are within the range of typical unadjusted Savannah Sparrow songs. Sound pressure levels were measured with a Brüel & Kjær 2250 SPL meter-frequency analyser (Brüel & Kjær, Denmark) along transects away from each site centre. More details on sound measurements are given in Curry et al.57 and Rosa et al.56.
Addressing how agonistic behaviour changes relative to reference baselines (playback of unadjusted songs in natural, quiet environments) adds to our growing knowledge of receiver perception of signals in altered landscape. Our use of CORT as a covariate helps further explain variation in behavioural responses, as CORT may be associated both with potential stress from infrastructure and with the behavioural responses themselves. Finally, associating CORT with behaviours in altered environments can suggest future areas of research into the mechanisms driving differential responses to adjustments made to varying acoustic environments.
Results
At control sites, noise-adjusted songs resulted in different agonistic responses than unadjusted songs, suggesting that receivers interpreted content differently between unadjusted and adjusted songs (Table 1). Adjusted songs in control environments elicited more calls and fewer attacks and wing flicks.
Table 1.
Responses to simulated territorial intrusions with noise-adjusted songs in control environments results in atypical responses for several behaviours.
| Number of songs | Number of calls | Number of attacks | Number of wing flicks | Min approach distance | Ln time to min approach | |
|---|---|---|---|---|---|---|
| Song type (adjusted vs. unadjusted) | −1.16 ± 0.93 (0.214) | 8.27 ± 3.45 (0.017) | −1.44 ± 0.45 (0.001) | −0.94 ± 0.42 (0.025) | 2.76 ± 2.8 (0.351) | −0.04 ± 0.2 (0.833) |
| CORT | −0.41 ± 1.22 (0.739) | 16.16 ± 7.74 (0.037) | 0.66 ± 0.61 (0.277) | 0.05 ± 0.73 (0.943) | 0.69 ± 2.98 (0.819) | −1.15 ± 0.37 (0.009) |
| Song type x CORT | 3.86 ± 1.47 (0.008) | −15.58 ± 7.81 (0.046) | 0.93 ± 0.61 (0.126) | 1.24 ± 0.69 (0.071) | −3.79 ± 3.72 (0.335) | 0.12 ± 0.26 (0.669) |
Results are given as coefficient ±s.e. (P), with P ≤ 0.05 in bold. Categorical variables are shown with 1 vs. 0 (i.e. adjusted = 1 and unadjusted = 0), such that a positive β indicates an increase with category 1.We fit generalised linear mixed models with song played, adrenocortical responsiveness and two-way interactions as fixed effects, with male ID as a random effect, using only data from control sites. Playbacks (n = 19) to individuals (n = 11) were divided by song type: unadjusted songs at control sites (n = 10) and adjusted songs at control sites (n = 9).
In noisy environments, agonistic responses to unadjusted songs were inappropriate (different from unadjusted songs at control sites), but adjusted songs were effective in restoring several appropriate agonistic responses (Table 2; Fig. 2). The response to vocalisations in noisy environments was significantly more similar to the reference response when birds heard adjusted songs than when they heard unadjusted songs for three behaviours (Table 2): number of calls (Fig. 2b), number of attacks (Fig. 2c) and number of wing flicks (screw pumps only; Fig. 2d). Adjusted songs resulted in a more atypical response for minimum approach distance (pumpjacks only; see Supplementary Fig. S2).
Table 2.
Responses to simulated territorial intrusions depended on song’s match to acoustic environment and adrenocortical responsiveness of individuals.
| Number of songs | Number of calls | Number of attacks | Number of wing flicks | Min approach distance | Ln time to min approach | |
|---|---|---|---|---|---|---|
| Song type (adjusted vs. unadjusted) | −1.21 ± 0.95 (0.203) |
6.05 ± 1.69 (<0.001) |
−1.45 ± 0.45 (0.001) | −0.94 ± 0.42 (0.025) | 2.77 ± 2.84 (0.34) |
−0.04 ± 0.25 (0.868) |
| Infrastructure (pumpjack vs. control) | 2.17 ± 0.74 (0.003) | 3.21 ± 2.31 (0.164) | −2.04 ± 0.87 (0.019) | −0.84 ± 0.82 (0.31) | 8.14 ± 3.58 (0.028) | 0.36 ± 0.50 (0.479) |
| Infrastructure (screw pump vs. control) | 1.1 ± 0.75 (0.144) |
5.68 ± 1.97 (0.004) | −0.56 ± 0.71 (0.43) | −0.78 ± 0.73 (0.286) | 2.23 ± 3.23 (0.493) | 0.17 ± 0.48 (0.728) |
| CORT | −0.44 ± 0.83 (0.60) |
9.32 ± 2.52 (<0.001) |
0.70 ± 0.66 (0.292) | 0.08 ± 0.68 (0.904) | 0.7 ± 3.12 (0.824) |
−1.15 ± 0.46 (0.016) |
| Song type x Infrastructure (pumpjack vs. control) | 0.66 ± 0.98 (0.502) | −4.37 ± 2.14 (0.041) |
3.31 ± 0.77 (<0.001) |
1.3 ± 0.85 (0.126) |
−8.72 ± 4.24 (0.05) | −0.06 ± 0.39 (0.875) |
| Song type x Infrastructure (screw pump vs. control) | 1.9 ± 1.01 (0.06) |
−7.02 ± 1.78 (<0.001) |
1.41 ± 0.52 (0.006) | 1.37 ± 0.58 (0.017) | −4.64 ± 4.05 (0.264) | −0.22 ± 0.35 (0.535) |
| Song type x CORT | 3.66 ± 1.34 (0.006) | −9.19 ± 2.59 (<0.001) |
0.93 ± 0.61 (0.13) |
1.23 ± 0.68 (0.072) | −3.78 ± 3.77 (0.328) | 0.12 ± 0.33 (0.73) |
| Infrastructure (pumpjack vs. control) x CORT | 0.58 ± 0.90 (0.515) |
−7.79 ± 2.64 (0.003) | 0.43 ± 0.85 (0.607) | −1.16 ± 0.94 (0.218) | −5.81 ± 3.83 (0.136) | 0.57 ± 0.55 (0.302) |
| Infrastructure (screw pump vs control) x CORT | 1.41 ± 1.01 (0.165) | −9.72 ± 2.65 (<0.001) |
−0.62 ± 0.95 (0.516) | −0.3 ± 0.98 (0.756) | 0.02 ± 4.34 (0.996) | 1.52 ± 0.64 (0.023) |
| Song type x Infrastructure (pumpjack vs. control) x CORT | −4.00 ± 1.36 (0.003) | 9.15 ± 2.71 (0.001) | −1.25 ± 0.72 (0.081) | 1.03 ± 0.96 (0.279) | 6.71 ± 4.56 (0.155) | 0.22 ± 0.41 (0.592) |
| Song type x Infrastructure (screw pump vs. control) x CORT | −4.81 ± 1.45 (0.001) | 8.78 ± 2.82 (0.002) | −1.04 ± 0.7 (0.14) |
−1.93 ± 0.88 (0.028) | 5.57 ± 5.80 (0.346) |
−0.13 ± 0.53 (0.812) |
Results are β ±s.e. (P), P ≤ 0.05 in bold. Categorical variables are shown with 1 vs. 0 (i.e. adjusted = 1 and unadjusted = 0), such that a positive β indicates an increase with category 1.We tested whether adjusted songs received appropriate responses in noisy environments with song played, infrastructure type and adrenocortical responsiveness as fixed effects, plus all interactions, with individual as random effect. Playbacks (n = 57) to individuals (n = 35) were divided by song and infrastructure: unadjusted songs at control sites (reference category; playbacks: n = 10), adjusted songs at control sites (playbacks: n = 9), unadjusted songs at infrastructure sites (playbacks: npumpjacks = 10; nscrew pumps = 9) and adjusted songs at infrastructure sites (playbacks: npumpjacks = 8; nscrew pumps = 11). Categorical variables are shown with 1 vs. 0 (i.e. adjusted = 1 and unadjusted = 0), such that a positive β indicates an increase with category 1.
Figure 2.
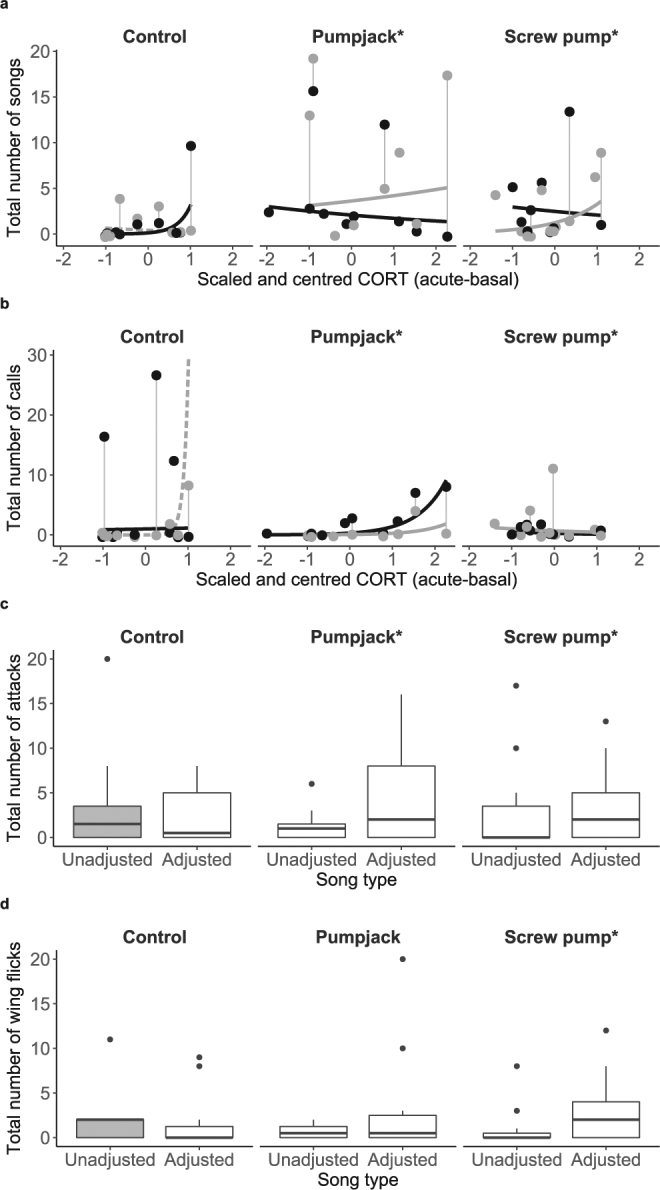
Responses to acoustically appropriate songs varied with treatment and adrenocortical responsiveness. Panels marked *show significant interactions from Table 1: three-way (a,b) or two-way (c,d) interactions relative to reference (unadjusted song at control sites). Reference (unadjusted songs in control sites) shown by grey dashed line and grey-filled box plots. (a,b), Vertical lines connect playbacks for each individual. Remaining lines (model-predicted values for treatments) and symbols (one playback) are responses to unadjusted (grey) or adjusted (black) songs. (a), songs. (b), calls. (c,d), box plots showing quartiles, median and outliers (dots) by song and infrastructure type for (c), attacks. (d), wing flicks.
Agonistic responses also varied with adrenocortical responsiveness. At control sites when song was unadjusted, increased adrenocortical responsiveness was correlated with more calls (Table 1) and reduced time to closest approach (Table 1; see Supplementary Fig. S3). Adrenocortical responsiveness was correlated with proximity to infrastructure (adult males banded in 2015–2016, n = 82; Fig. 3); adrenocortical responsiveness was significantly higher closer to pumpjacks (β = −3.92 ± 1.97, P = 0.05) but not screw pumps (β = 0.46 ± 2.08, P = 0.83). Further, adrenocortical responsiveness interacted with song type, such that birds with higher adrenocortical responsiveness responded more atypically to the adjusted songs at control sites (Fig. 2a; Table 1).
Figure 3.
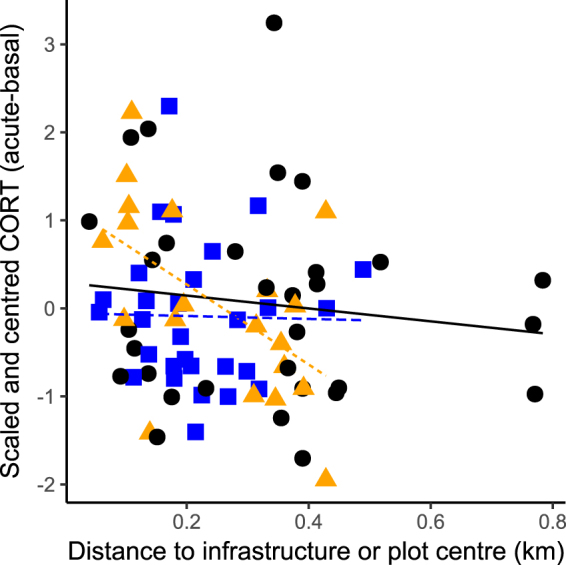
Adrenocortical responsiveness is related to distance from pumpjacks but not distance from screw pumps or distance from control site centre. Each symbol represents one male captured at control sites (black circle), pumpjacks (orange triangle) and screw pumps (blue square). Lines are predictions from linear models for control sites (black solid line), pumpjacks (orange short dashed line) and screw pumps (blue long dashed line).
Although use of acoustically appropriate songs (i.e. adjusted at infrastructure sites) often restored agonistic behaviours to reference levels, the relative effectiveness of appropriate songs varied with individual physiology and infrastructure type (Table 2). Interaction slopes for adrenocortical responsiveness, infrastructure type and song type at pumpjacks were similar to those at screw pumps (Table 2). However, these similar relationships resulted in opposite consequences at the two infrastructure types. Birds with lower adrenocortical responsiveness deviated farther from reference behaviour when presented with acoustically inappropriate songs at screw pumps (number of songs and wing flicks; see Supplementary Fig. S1), whereas birds with higher adrenocortical responsiveness deviated farther from reference agonistic behaviour when presented with acoustically inappropriate songs at controls and pumpjacks (i.e. adjusted songs at control sites for number of songs [Fig. 2a; Table 1] and calls [Fig. 2b; Table 1]; to unadjusted songs at pumpjacks for number of songs [Fig. 2a] and calls [Fig. 2b]).
Discussion
Our results demonstrate that to communicate effectively in noisy environments, Savannah Sparrow songs must be adjusted. Use of adjusted songs in control environments resulted in atypical responses, suggesting that receivers may interpret content differently between unadjusted and adjusted songs. Therefore, adjusting songs could have unintended consequences by altering perception of content7,12,21,30. However, adjusting songs was necessary for communicating vocalisation content and was often effective for restoring successful communication at noisy sites. The fact that birds corrected response to adjusted song in noisy conditions suggests that they were responding to adjusted features of the song, not altering response solely to a poor signal-to-noise ratio recording.
Agonistic responses to vocal cues varied, at least in part, with physiological characteristics of the individual, which in turn varied with some environmental conditions. Adrenocortical responsiveness was higher near pumpjacks than near screwpumps or at controls, suggesting that, despite their lower amplitude, pumpjacks are associated with elevated adrenocortical responsiveness, perhaps because pumpjacks induce stress23. Although pumpjacks are quieter than screw pumps, pumpjacks may present a stronger visual stimulus. Several studies in humans have shown that a stressor occurring in multiple sensory modalities (e.g. acoustic and visual) has a greater potential to reorient attention than a cue occurring in a single modality (i.e. acoustic)31–33, suggesting that pumpjacks might be disruptive because they present both acoustic and visual disruptions.
At pumpjacks, birds with high adrenocortical responsiveness benefitted most strongly from song adjustments, while birds with low adrenocortical responsiveness made fewer errors when presented with acoustically inappropriate songs; the converse was true at screw pumps. Higher overall adrenocortical responsiveness at pumpjacks was correlated with inappropriate conspecific territorial aggression behaviours to acoustically mismatched songs. The importance of vocal adjustments in the presence of noise may become increasingly important under conditions of elevated adrenocortical responsiveness (or other physiological measures correlated with it), such as those resulting from chronic environmental disturbance, which may result in other life history and resource allocation changes34 in addition to territorial aggression behaviours. Surprisingly, our results demonstrate that physiological characteristics that are correlated with beneficial agonistic behavioural responses in the presence of some types of anthropogenic infrastructure may be detrimental at others. Davies et al. (2017) suggested this as well for House Wrens5.
Our study suggests one reason why variation in response to anthropogenic disturbance may occur among studies, species and individuals. Adrenocortical responsiveness can be viewed as a physiological correlate of behavioural plasticity that allows individuals either to successfully cope with a given disturbance, or constrains behavioural responses resulting in detrimental effects due to chronic exposure35,36. Hence, differences in physiology among species37, with age, sex, or body condition38–40 and with previous exposure to disturbance24 may result in different responses to the same anthropogenic stimuli. Indeed, our study agrees with previous findings that different noise types can be associated with glucocorticoid responses in different ways5.
Nonetheless, regardless of adrenocortical responsiveness, acoustically matched songs received more appropriate agonistic behavioural responses, emphasising the importance of behavioural plasticity in anthropogenically modified environments. Intermediate levels of behavioural plasticity are considered optimal to cope with human-induced rapid environmental change41,42 and species or individuals that are unable to alter behaviour or respond inappropriately to novel disturbances are likely to be at a disadvantage, which may ultimately result in population declines37. Because different types of infrastructure have different impacts on agonistic behaviour and CORT levels24, land management decisions could inadvertently select for certain behavioural response patterns43 but these selected behaviours may not be beneficial to offspring that disperse to disparate industrial landscapes. When combined with changes in adrenocortical responsiveness due to exposure to chronic human disturbance3, this will result in complex selection pressures among behavioural syndromes regulated by CORT43 both within and among species. More study is needed to confirm causative links between physiological changes in birds in anthropogenically disturbed environments and how it affects their behaviours.
Methods
We studied free-living adult male Passerculus sandwichensis (Savannah Sparrows), a grassland passerine bird. Our study (both recording of playback stimuli and playback to banded birds) was conducted in mixed grass prairies southeast of Brooks, Alberta, Canada (49° 0′ 0.004″ to 50° 53′ 56.475″ N; 110° 0′ 2.757″ W to 112° 28′ 44.473″ W).
To colour-band and take blood samples from the 35 territorial males used in the playback experiments in 2015, we lured birds to mist-nets using playbacks (www.xeno-canto.org XC153324, XC186835 and XC206187) and a decoy. Blood samples (<70 uL) were collected by brachial venipuncture in heparinised microcapillary tubes and kept iced <6 h until centrifuged and stored at −20 °C. Samples were collected in under 3 min after capture to reflect baseline circulating levels of CORT44 and again after a 12-min standardised stress handling protocol45, which reflects the ability of an individual to respond physiologically to a novel disturbance and is associated with stable behavioural traits46. CORT measures have been found to be repeatable within individuals in the lab47 and in wild populations48,49. The increase in CORT levels in response to handling can be more repeatable within an individual50, as well as having a stronger heritable component51, than baseline CORT. While not all studies found measures of CORT were repeatable within individuals52, the temporal separation in our study between capture and playback was relatively short (and within the breeding season) and therefore should be more reflective of an individual’s state at the time of playback. Plasma CORT was determined by radioimmunoassay (inter-assay variation: 14.5%, intra-assay variation: 13.4%, extraction efficiency: 113.2%) after extraction with 100% ethanol. Samples were run in duplicate tubes with a 1:6000 dilution of CORT antibody (ABIN343319; antibodies-online) and a known amount of labelled CORT (Perkin Elmer). Assay specific CORT values were determined via interpolation from a curve of serial diluted CORT standards (100–0.01 ng/mL; Steraloids) and corrected for sample volume. Adrenocortical responsiveness was not correlated with playback duration before capture (β = −0.003 ± 0.01, P = 0.80) or date (β = −0.009 ± 0.006, P = 0.14) (linear mixed model, random effect: year; n = 80; adult males, 2015–2016). Year was included as a random effect in the linear mixed model53–55 to account for potential differences in intercept between years.
We recorded the playback song stimuli as spontaneous songs May – July 2014 at control and noisy [receiving high-fidelity playback56 of oil well drilling noise57 sites using Zoom H4N Digital Recorders with built-in stereo microphones angled at 90° at maximal recording volume in uncompressed audio (WAV fles at 48 kHz sampling rate, 16-bit resolution). All recordings were made in the same region as the experiment to ensure that regional variation could not impact comparison of adjusted vs. unadjusted songs, but recordings were spatially and temporally segregated from song playback sites to ensure that receivers never heard recordings of familiar individuals. Infrastructure-free control sites, where unadjusted songs were recorded, contained only naturally occurring background noise, such as avian vocalisations and wind. Noisy sites, where adjusted songs were recorded, contained high-fidelity56, high-amplitude [88 dB(C) at 10 m (C-weighted time average sound pressure level for broadband sound; LCeq] broadcasts of oil well drilling57. The drilling produces frequencies that are significantly different from screw pumps and pumpjacks, but intermediate to both, in the sparrow song range (Fig. 1). The drilling noise sites had more energy in frequency bands audible to birds58 than ambient background noise (Fig. 1). Sparrows recorded at these drilling sites sing more loudly and at higher frequencies57. We chose this intermediate environment so that we could compare responses in both pumpjack and screw pump sites without favouring either treatment and expect that songs produced in an intermediate environment should be applicable to both screw pumps and pumpjacks. We created each playback stimulus with 3 songs from one individual (repeated 5 min, with natural spacing ca. 10s). We created 5 stimuli per song treatment (i.e., 5 adjusted song stimuli and 5 unadjusted song stimuli), for 10 total playback stimuli containing 30 songs from 10 individuals. Songs from both adjusted and unadjusted treatments were chosen to be typical of their category based on 5% and 95% frequency, 90% frequency bandwidth, peak frequency, aggregate entropy and average power29,57. Background noise was filtered below 1,500 Hz and above 12,000 Hz with a rolloff of 12 dB in Audacity59 and all were played at a standardised amplitude. We did not remove background noise from the adjusted songs frequencies themselves, because (1) we did not want to risk removal of song components that could not be distinguished from background noise and (2) amplitudes of background noise in song recordings were generally very low, as we used directional microphones for song recordings. This made our study more conservative, because if noise in the recordings interfered with song reception, it should result in increasingly inappropriate responses to the adjusted songs, in contrast to our prediction, that adjusting songs improves abilities of birds to communicate in noisy environments.
We played stimuli songs to resighted colour-banded males in May-July 2015, during Savannah sparrow breeding season; birds in the region were laying eggs, incubating and feeding nestlings throughout the study period. First playbacks were mean 14.0 ± 12.9s.d days (range 0–49.0; two individuals received first playback immediately before banding) from capture and CORT blood sampling, to ensure territorial response behaviour was not influenced by the stress handling protocol. When possible, each resighted male was exposed to two playbacks in randomized order: one each of adjusted and unadjusted song. However, not all males received both stimuli types, because we were unable to resight some individuals a second time. Mean 4.3 ± 6.4s.d. hours elapsed between playbacks (range 1.0–21.8) (i.e., after the bird resumed normal behaviours such as foraging and moving about its territory with no focus on the playback site). Each playback was 5 min, starting when the focal bird was heard or seen ≤50 m from the playback site. The observer estimated the focal male’s distance to the speaker in 1-m intervals and tallied agonistic behaviours in 10 s intervals. Playbacks occurred <5 h after sunrise under standardised conditions (wind <15 km/h, temp. >0 °C). Playback locations were ≤400 m of wells for noisy treatments (241 ± 103s.d. m to pumpjacks; 194 ± 79s.d. m to screw pumps; two-sided Welch’s t = 1.25, df = 20.6, P = 0.22) and ≥ 800 m from wells for control sites. These distances were chosen to correspond with quarter sections (800 × 800 m squares) that contain wells at their centre, as this is the scale at which many management decisions are made in this rural study region.
We analysed behavioural responses using generalised linear mixed-models46–48,53, comparing other treatments with unadjusted songs in control sites (considered the reference, or appropriate, behavioural responses). To ensure differences among treatments could not be attributed to observer, only one observer (hidden at 20 m) collected data for a given male. Required sample size was estimated a priori (power = 0.8); birds were sampled as logistically feasible to near that count. We fit models with Poisson distributions for behaviour counts after examining residual plots to confirm equal variance and meeting assumptions for dispersion. We met assumptions for normality and equal variance for other response variables and thus used a Gaussian distribution for those analyses. We compared sound pressure levels for noise using two-sided Satterthwaite t-tests for unequal and pooled t-tests for equal variances.
All methods were carried out in accordance with relevant guidelines and regulations under Canadian bird banding subpermits 10840A and 10840B, Canadian Wildlife Service permit #11-MB/SKL/AB-SC007 and Alberta Environment and Sustainable Research Development Research Permit #56016 and Collection Licence #56017. The experimental protocols were approved by the University of Manitoba animal care protocol F15-005.
Data availability
The datasets generated during and/or analysed during the current study are available in the electronic supplementary information that accompanies this paper.
Electronic supplementary material
Acknowledgements
We thank Cenovus Energy for funding and access to lease sites; Eastern Irrigation District for land access; G. Anderson, E. Bridge, K. Ellison, A. Horn, M.A. Patten and M. Warrington for assistance with experimental design, permitting, hormone assays and organisation; field technicians L. Burns, M.- È. Cyr, M. Fenton, E. Geurts, S. Orue and M. Pilon; and funding sources to NK: NSERC, Canadian Foundation for Innovation, Manitoba Research and Innovation Fund and University of Manitoba Clayton Riddell Faculty of Environment, Earth and Resources. CMC was additionally supported by U.S. Department of Agriculture grant USDA-NIFA 2013-67009-20369 and National Science Foundation grants IDBR 1014891 and NSF-ABI Award 1458402 to E.S. Bridge.
Author Contributions
C.M.C., P.D.B. and N.K. designed experiments; C.M.C. and P.D.B. collected and analysed playback and hormone data; P.R. designed, collected and analysed sound profile data; C.M.C. led the writing of the manuscript; N.K. supervised all research. All authors contributed to drafts and gave final approval for publication.
Competing Interests
Some funding for the research was provided by Cenovus Energy to N.K. (supporting all other co-authors). The funding from industry had no effect on the research or our conclusions. Cenovus Energy contributed safety training and helped identify potential research sites, but all research was designed, analyzed, written and submitted for review by the co-authors without industry input, to maintain integrity.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-22253-5.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Northrup JM, Wittemyer G. Characterising the impacts of emerging energy development on wildlife, with an eye towards mitigation. Ecol. Lett. 2013;16:112–125. doi: 10.1111/ele.12009. [DOI] [PubMed] [Google Scholar]
- 2.Kight CR. & Swaddle, J. P. How and why environmental noise impacts animals: an integrative, mechanistic review. Ecol. Lett. 2011;14:1052–1061. doi: 10.1111/j.1461-0248.2011.01664.x. [DOI] [PubMed] [Google Scholar]
- 3.Blickley JL, et al. Experimental Chronic Noise Is Related to Elevated Fecal Corticosteroid Metabolites in Lekking Male Greater Sage-Grouse (Centrocercus urophasianus) PLOS ONE. 2012;7:e50462. doi: 10.1371/journal.pone.0050462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Francis CD, Barber JR. A framework for understanding noise impacts on wildlife: an urgent conservation priority. Front. Ecol. Environ. 2013;11:305–313. doi: 10.1890/120183. [DOI] [Google Scholar]
- 5.Askins RA, et al. Conservation of grassland birds in North America: understanding ecological processes in different regions. Ornithol. Monogr. 2007;64:iii–46. doi: 10.2307/40166905. [DOI] [Google Scholar]
- 6.Mockford EJ, Marshall RC. Effects of urban noise on song and response behaviour in great tits. Proc. R. Soc. B Biol. Sci. 2009;276:2979–2985. doi: 10.1098/rspb.2009.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halfwerk W, et al. Low-frequency songs lose their potency in noisy urban conditions. Proc. Natl. Acad. Sci. 2011;108:14549–14554. doi: 10.1073/pnas.1109091108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunnington GM, Fahrig L. Mate attraction by male anurans in the presence of traffic noise: Anuran mate attraction and traffic noise. Anim. Conserv. 2013;16:275–285. doi: 10.1111/j.1469-1795.2012.00598.x. [DOI] [Google Scholar]
- 9.Pohl NU, Leadbeater E, Slabbekoorn H, Klump GM, Langemann U. Great tits in urban noise benefit from high frequencies in song detection and discrimination. Anim. Behav. 2012;83:711–721. doi: 10.1016/j.anbehav.2011.12.019. [DOI] [Google Scholar]
- 10.Brumm H, Todt D. Noise-dependent song amplitude regulation in a territorial songbird. Anim. Behav. 2002;63:891–897. doi: 10.1006/anbe.2001.1968. [DOI] [Google Scholar]
- 11.Slabbekoorn H, Peet M. Ecology: Birds sing at a higher pitch in urban noise. Nature. 2003;424:267–267. doi: 10.1038/424267a. [DOI] [PubMed] [Google Scholar]
- 12.Luther DA, Phillips J, Derryberry EP. Not so sexy in the city: urban birds adjust songs to noise but compromise vocal performance. Behav. Ecol. 2016;27:332–340. doi: 10.1093/beheco/arv162. [DOI] [Google Scholar]
- 13.Francis CD. Vocal traits and diet explain avian sensitivities to anthropogenic noise. Glob. Change Biol. 2015;21:1809–1820. doi: 10.1111/gcb.12862. [DOI] [PubMed] [Google Scholar]
- 14.Bee MA, Swanson EM. Auditory masking of anuran advertisement calls by road traffic noise. Anim. Behav. 2007;74:1765–1776. doi: 10.1016/j.anbehav.2007.03.019. [DOI] [Google Scholar]
- 15.Leonard ML, Horn AG. Ambient noise increases missed detections in nestling birds. Biol. Lett. 2012;8:530–532. doi: 10.1098/rsbl.2012.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luther D, Magnotti J. Can animals detect differences in vocalizations adjusted for anthropogenic noise? Anim. Behav. 2014;92:111–116. doi: 10.1016/j.anbehav.2014.03.033. [DOI] [Google Scholar]
- 17.McMullen H, Schmidt R, Kunc HP. Anthropogenic noise affects vocal interactions. Behav. Processes. 2014;103:125–128. doi: 10.1016/j.beproc.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 18.LaZerte SE, Otter KA, Slabbekoorn H. Relative effects of ambient noise and habitat openness on signal transfer for chickadee vocalizations in rural and urban green-spaces. Bioacoustics. 2015;24:233–252. doi: 10.1080/09524622.2015.1060531. [DOI] [Google Scholar]
- 19.LaZerte SE, Slabbekoorn H, Otter KA. Territorial black-capped chickadee males respond faster to high- than to low-frequency songs in experimentally elevated noise conditions. PeerJ. 2017;5:e3257. doi: 10.7717/peerj.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleist NJ, Guralnick RP, Cruz A, Francis CD. Anthropogenic noise weakens territorial response to intruder’s songs. Ecosphere. 2016;7:e01259. doi: 10.1002/ecs2.1259. [DOI] [Google Scholar]
- 21.Luther DA, Danner R, Danner J, Gentry K, Derryberry EP. The relative response of songbirds to shifts in song amplitude and song minimum frequency. Behav. Ecol. 2016;28:391–397. [Google Scholar]
- 22.Naguib M, et al. Noise annoys: effects of noise on breeding great tits depend on personality but not on noise characteristics. Anim. Behav. 2013;85:949–956. doi: 10.1016/j.anbehav.2013.02.015. [DOI] [Google Scholar]
- 23.Busch DS, Hayward LS. Stress in a conservation context: A discussion of glucocorticoid actions and how levels change with conservation-relevant variables. Biol. Conserv. 2009;142:2844–2853. doi: 10.1016/j.biocon.2009.08.013. [DOI] [Google Scholar]
- 24.Davies S, Haddad N, Ouyang JQ. Stressful city sounds: glucocorticoid responses to experimental traffic noise are environmentally dependent. Biol. Lett. 2017;13:20170276. doi: 10.1098/rsbl.2017.0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernath-Plaisted, J. & Koper, N. Physical footprint of oil and gas infrastructure, not anthropogenic noise, reduces nesting success of some grassland songbirds. Biol. Conserv. 204, Part B, 434–441 (2016).
- 26.Van Duyse E, Pinxten R, Darras VM, Arckens L, Eens M. Opposite changes in plasma testosterone and corticosterone levels following a simulated territorial challenge in male great tits. Behaviour. 2004;141:451–467. doi: 10.1163/156853904323066739. [DOI] [Google Scholar]
- 27.Lynn SE, Hahn TP, Breuner CW. Free-living male mountain white-crowned sparrows exhibit territorial aggression without modulating total or free plasma testosterone. Condor. 2007;109:173–180. doi: 10.1650/0010-5422(2007)109[173:FMMWSE]2.0.CO;2. [DOI] [Google Scholar]
- 28.Deviche P, Breuner C, Orchinik M. Testosterone, corticosterone and photoperiod interact to regulate plasma levels of Bbinding globulin and free steroid hormone in Dark-Eyed Juncos. Junco hyemalis. Gen. Comp. Endocrinol. 2001;122:67–77. doi: 10.1006/gcen.2001.7613. [DOI] [PubMed] [Google Scholar]
- 29.Warrington MH, Curry CM, Antze B, Koper N. Noise from four types of extractive energy infrastructure affects song features of Savannah Sparrows. Condor. 2018;120:1–15. doi: 10.1650/CONDOR-17-69.1. [DOI] [Google Scholar]
- 30.Huet des Aunay G, et al. Urban noise undermines female sexual preferences for low-frequency songs in domestic canaries. Anim. Behav. 2014;87:67–75. doi: 10.1016/j.anbehav.2013.10.010. [DOI] [Google Scholar]
- 31.Santangelo V, Spence C. Multisensory cues capture spatial attention regardless of perceptual load. J. Exp. Psychol. Hum. Percept. Perform. 2007;33:1311–1321. doi: 10.1037/0096-1523.33.6.1311. [DOI] [PubMed] [Google Scholar]
- 32.Santangelo V, Ho C, Spence C. Capturing spatial attention with multisensory cues. Psychon. Bull. Rev. 2008;15:398–403. doi: 10.3758/PBR.15.2.398. [DOI] [PubMed] [Google Scholar]
- 33.Spence C. Crossmodal spatial attention. Ann. N. Y. Acad. Sci. 2010;1191:182–200. doi: 10.1111/j.1749-6632.2010.05440.x. [DOI] [PubMed] [Google Scholar]
- 34.Crossin GT, et al. Corticosterone predicts foraging behavior and parental care in macaroni penguins. Am. Nat. 2012;180:E31–E41. doi: 10.1086/666001. [DOI] [PubMed] [Google Scholar]
- 35.Koolhaas JM, et al. Coping styles in animals: current status in behavior and stress-physiology. Neurosci. Biobehav. Rev. 1999;23:925–935. doi: 10.1016/S0149-7634(99)00026-3. [DOI] [PubMed] [Google Scholar]
- 36.Groothuis TGG, Carere C. Avian personalities: characterization and epigenesis. Neurosci. Biobehav. Rev. 2005;29:137–150. doi: 10.1016/j.neubiorev.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 37.Sih A, Ferrari MCO, Harris DJ. Evolution and behavioural responses to human-induced rapid environmental change. Evol. Appl. 2011;4:367–387. doi: 10.1111/j.1752-4571.2010.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wingfield JC, O’Reilly KM, Astheimer LB. Modulation of the adrenocortical responses to acute stress in arctic birds: a possible ecological basis. Am. Zool. 1995;35:285–294. doi: 10.1093/icb/35.3.285. [DOI] [Google Scholar]
- 39.Dantzer, B., Fletcher, Q. E., Boonstra, R. & Sheriff, M. J. Measures of physiological stress: a transparent or opaque window into the status, management and conservation of species? Conserv. Physiol. 2 (2014). [DOI] [PMC free article] [PubMed]
- 40.Romero LM, Dickens MJ, Cyr NE. The reactive scope model — A new model integrating homeostasis, allostasis and stress. Horm. Behav. 2009;55:375–389. doi: 10.1016/j.yhbeh.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 41.Ghalambor CK, McKAY JK, Carroll SP, Reznick DN. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol. 2007;21:394–407. doi: 10.1111/j.1365-2435.2007.01283.x. [DOI] [Google Scholar]
- 42.Price TD, Qvarnström A, Irwin DE. The role of phenotypic plasticity in driving genetic evolution. Proc. R. Soc. Lond. B Biol. Sci. 2003;270:1433–1440. doi: 10.1098/rspb.2003.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baugh AT, et al. Corticosterone responses differ between lines of great tits (Parus major) selected for divergent personalities. Gen. Comp. Endocrinol. 2012;175:488–494. doi: 10.1016/j.ygcen.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 44.Romero LM, Reed JM. Collecting baseline corticosterone samples in the field: is under 3 min good enough? Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2005;140:73–79. doi: 10.1016/j.cbpb.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 45.Breuner C, C. Wingfield J, Romero L. Diel rhythms of basal and stress-induced corticosterone in a wild, seasonal vertebrate, Gambel’s White-crowned Sparrow. J. Exp. Zool. 1999;284:334–42. doi: 10.1002/(SICI)1097-010X(19990801)284:3<334::AID-JEZ11>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 46.Baugh AT, van Oers K, Naguib M, Hau M. Initial reactivity and magnitude of the acute stress response associated with personality in wild great tits (Parus major) Gen. Comp. Endocrinol. 2013;189:96–104. doi: 10.1016/j.ygcen.2013.04.030. [DOI] [PubMed] [Google Scholar]
- 47.Wada H, et al. Adrenocortical responses in zebra finches (Taeniopygia guttata): Individual variation, repeatability and relationship to phenotypic quality. Horm. Behav. 2008;53:472–480. doi: 10.1016/j.yhbeh.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 48.Cockrem JF, Candy EJ, Barrett DP, Agnew P, Potter MA. Individual variation and repeatability of corticosterone responses of little penguins (Eudyptula minor) sampled in two successive years at Oamaru, New Zealand. Gen. Comp. Endocrinol. 2017;244:86–92. doi: 10.1016/j.ygcen.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 49.Small TW, Schoech SJ. Sex differences in the long-term repeatability of the acute stress response in long-lived, free-living Florida scrub-jays (<Emphasis Type = ‘Italic’> Aphelocoma coerulescens </Emphasis>) J. Comp. Physiol. B. 2015;185:119–133. doi: 10.1007/s00360-014-0866-4. [DOI] [PubMed] [Google Scholar]
- 50.Cockrem JF, Silverin B. Variation within and between birds in corticosterone responses of Great Tits (Parus major) Gen. Comp. Endocrinol. 2002;125:197–206. doi: 10.1006/gcen.2001.7750. [DOI] [PubMed] [Google Scholar]
- 51.Jenkins, B. R., Vitousek, M. N., Hubbard, J. K. & Safran, R. J. An experimental analysis of the heritability of variation in glucocorticoid concentrations in a wild avian population. Proc. R. Soc. B Biol. Sci. 281 (2014). [DOI] [PMC free article] [PubMed]
- 52.Baugh AT, Oers K, van, Dingemanse NJ, Hau M. Baseline and stress-induced glucocorticoid concentrations are not repeatable but covary within individual great tits (Parus major) Gen. Comp. Endocrinol. 2014;208:154–163. doi: 10.1016/j.ygcen.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 53.Bates, D., Maechler, M. & Bolker, B. lme4: Linear mixed-effects models using S4 classes. (2011).
- 54.Kuznetsova, A., Brockhoff, P. B. & Christensen, R. H. B. lmerTest: tests in linear mixed effects models. (2015).
- 55.R Core Team. R: A language and environment for statistical computing. (R Foundation for Statistical Computing, 2015).
- 56.Rosa P, Swider CR, Leston L, Koper N. Disentangling effects of noise from presence of anthropogenic infrastructure: Design and testing of system for large-scale playback experiments. Wildl. Soc. Bull. 2015;39:364–372. doi: 10.1002/wsb.546. [DOI] [Google Scholar]
- 57.Curry, C. M. et al. Ability to alter song in two grassland songbirds exposed to simulated anthropogenic noise is not related to pre-existing variability. Bioacoustics (2017).
- 58.Dooling, R. J. & Popper, A. N. Technical guidance for assessment and mitigation of the effects of highway and road construction noise on birds. 96 (The California Department of Transportation, Division of Environmental Analysis, 2016).
- 59.Audacity Team. Audacity(R): Free Audio Editor and Recorder. (Audacity Team, 2015).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available in the electronic supplementary information that accompanies this paper.


