Abstract
Acute inflammation can exacerbate brain injury after ischemic stroke. Beyond its well-characterized role in calcium metabolism, it is becoming increasingly appreciated that the active form of vitamin D, 1,25-dihydroxyvitamin D3 (1,25-VitD3), has potent immunomodulatory properties. Here, we aimed to determine whether 1,25-VitD3 supplementation could reduce subsequent brain injury and associated inflammation after ischemic stroke. Male C57Bl6 mice were randomly assigned to be administered either 1,25-VitD3 (100 ng/kg/day) or vehicle i.p. for 5 day prior to stroke. Stroke was induced via middle cerebral artery occlusion for 1 h followed by 23 h reperfusion. At 24 h post-stroke, we assessed infarct volume, functional deficit, expression of inflammatory mediators and numbers of infiltrating immune cells. Supplementation with 1,25-VitD3 reduced infarct volume by 50% compared to vehicle. Expression of pro-inflammatory mediators IL-6, IL-1β, IL-23a, TGF-β and NADPH oxidase-2 was reduced in brains of mice that received 1,25-VitD3 versus vehicle. Brain expression of the T regulatory cell marker, Foxp3, was higher in mice supplemented with 1,25-VitD3 versus vehicle, while expression of the transcription factor, ROR-γ, was decreased, suggestive of a reduced Th17/γδ T cell response. Immunohistochemistry indicated that similar numbers of neutrophils and T cells were present in the ischemic hemispheres of 1,25-VitD3- and vehicle-supplemented mice. At this early time point, there were also no differences in the impairment of motor function. These data indicate that prior administration of exogenous vitamin D, even to vitamin D-replete mice, can attenuate infarct development and exert acute anti-inflammatory actions in the ischemic and reperfused brain.
Electronic supplementary material
The online version of this article (10.1007/s12017-018-8484-z) contains supplementary material, which is available to authorized users.
Keywords: Vitamin D, Inflammation, Stroke, Middle cerebral artery occlusion, Mouse
Introduction
Stroke is the world’s second leading cause of death, contributing to 6.7 million deaths annually (Mozaffarian et al. 2016). It is also the most frequent cause of permanent disability in adults, with half of all survivors discharged into care (Mozaffarian et al. 2016). Currently, there is only one approved pharmacological agent available to treat stroke, recombinant tissue plasminogen activator (rtPA), which must be administered within a 4.5-h window of stroke onset and only after a CT scan has diagnosed a thrombotic cause (Del Zoppo et al. 2009). Due to these strict limitations, < 10% of stroke patients are eligible to receive rtPA (Reeves et al. 2005; Kleindorfer et al. 2008). Consequently, there is a desperate need to identify modifiable mechanisms capable of limiting the impact of acute stroke.
Secondary brain injury following stroke is driven by local inflammation, production of reactive oxygen species and the infiltration of circulating immune cells (Anrather and Iadecola 2016). Thus, targeting these inflammatory processes has been of intense interest to stroke researchers. However, one immunomodulatory molecule that has received very little attention as a potential stroke therapy is vitamin D, a fat-soluble vitamin that functions as a steroid hormone. Vitamin D is synthesized predominantly from 7-dehydrocholesterol in response to skin exposure to ultraviolet light, but can also be obtained through dietary supplementation (Holick 2007). To become biologically active, vitamin D must first be converted to 1,25-dihydroxyvitamin D3 (1,25-VitD3) via two hydroxylation steps. This occurs firstly in the liver by 25-hydroxylase and then typically in the kidney by 1-α-hydroxylase (CYP27B) (Holick 2007). The latter hydroxylation step can also occur in macrophages, T cells and neurons, which also express 1-α-hydroxylase (Lugg et al. 2015). Once in this active form, vitamin D can engage with the vitamin D receptor (VDR) which is located on a number of cell types including leukocytes, endothelial cells, astrocytes and neurons (Provvedini et al. 1983; Merke et al. 1989; Langub et al. 2001; Lee et al. 2008). Vitamin D is best characterized to promote calcium absorption from the small intestine, but recent findings indicate that it may also control expression of a large number of genes, particularly those involved in inflammatory processes (Lugg et al. 2015).
1,25-VitD3 exerts such immunomodulatory actions through a variety of cellular and molecular mechanisms. Firstly, 1,25-VitD3 can prevent the development of pathogenic T helper (Th) 1, Th17 and γδ T cells, and can promote the formation of anti-inflammatory Th2 and T regulatory cells (Zeitelhofer et al. 2017; Chang et al. 2010a; Gregori et al. 2002; Joshi et al. 2011; Nashold et al. 2013; Sloka et al. 2011; Cantorna et al. 2004; Hart et al. 2011; Chen et al. 2005). Studies have also shown that 1,25-VitD3 promotes the generation of tolerogenic dendritic cells (Takeda et al. 2010; Gorman et al. 2010) and can prevent the release of pro-inflammatory cytokines from monocytes and microglia (Korf et al. 2012; Zhang et al. 2012; Boontanrart et al. 2016; Verma and Kim 2016). Further, 1,25-VitD3 may inhibit the production of reactive oxygen species by decreasing expression of NADPH oxidase (NOX) enzymes (Dong et al. 2012) and enhancing expression of antioxidants such as superoxide dismutase and glutathione (Jain and Micinski 2013; Dong et al. 2012).
Observational studies have documented that patients with lower serum levels of vitamin D experience larger infarct volumes and worse functional outcomes following stroke (Tu et al. 2014; Wang et al. 2014; Turetsky et al. 2015; Daubail et al. 2013; Park et al. 2015), suggesting that vitamin D may play a protective role during cerebral ischemia. We recently reported that low baseline levels of vitamin D, resulting from a vitamin D-deficient diet, had no discernible impact on selected outcome measures within 24 h of large vessel occlusion stroke (Evans et al. 2017). Here, we have instead examined the effect of elevated baseline levels of vitamin D achieved by supraphysiological doses of vitamin D given to vitamin D-replete animals during the 5 days prior to stroke, in an analogous manner to high dose supplementation regimes in humans (Wong et al. 2014; Sotirchos et al. 2016). For this, we adopted a similar supplementation regime that was found to reduce vascular injury in mice following hindlimb ischemia (Wong et al. 2014). Indeed, we report that 1,25-VitD3 supplementation can reduce post-stroke brain injury, reduce expression of pro-inflammatory cytokines, modulate the phenotype of T cells and increase the number of M2-polarized (anti-inflammatory) macrophages/microglia in the brain.
Materials and Methods
Animals
A total of 92 male C57Bl6 mice (7–10 week old; 21–30 g) were used for this study. Mice were housed under a 12-h light/dark cycle and had free access to water and food pellets. Mice were excluded from the study if during the surgical procedure to induce middle cerebral artery occlusion: [1] > 0.2 ml of blood was lost (n = 1); [2] subarachnoid hemorrhage occurred (n = 2); [3] death occurred during ischemia (n = 2); [4] cerebral blood flow failed to reach ≥ 80% pre-ischemic levels upon reperfusion (n = 1) and [5] death occurred after reperfusion and prior to the designated time for euthanasia (n = 7). All animals were randomly assigned to groups, and the investigator performing the surgical procedure or data analysis was, wherever possible, blinded to the treatment group.
Administration of 1-25,Dihydroxyvitamin D3
Vitamin D3 was administered as its active form, 1α, 25-dihydroxyvitamin D3 (1,25-VitD3; Sigma; 100 ng/kg/day) which was dissolved in a solvent mixture of sterile water, propylene glycol and ethanol in a 5:4:1 ratio. Animals were injected i.p. for 5 consecutive days prior to experimental stroke and again on the day of the procedure, as previously described (Wong et al. 2014).
Middle Cerebral Artery Occlusion
Mice underwent either sham surgery or focal cerebral ischemia as previously described (Evans et al. 2017). Cerebral ischemia was produced in anesthetized mice (ketamine: 80 mg/kg plus xylazine: 10 mg/kg i.p.) by occlusion of the middle cerebral artery (MCA) using a 6.0 silicone-coated monofilament (Doccol Corporation). Rectal temperature was monitored and maintained at 37.0 ± 0.5 °C. MCA occlusion (MCAO) was sustained for 60 min, and the filament then retracted to allow reperfusion. Both successful occlusion (> 70% reduction in cerebral blood flow; CBF) and reperfusion (≥ 80% return of CBF to the pre-ischemic level) were confirmed by transcranial laser-Doppler flowmetry (PeriMed). Sham-operated mice were anesthetized and the right carotid bifurcation exposed, but no filament was inserted. Neck wounds were then closed with sutures and covered with Betadine® (Sanofi) and spray dressing. Head wounds were closed with superglue, and mice were returned to their cages after regaining consciousness.
Functional Assessment
Mice were assessed for functional deficits at approximately 30 min prior to euthanasia. This comprised a 6-point scoring system for neurological deficits: 0 = normal motor function, 1 = flexion of torso and contralateral forelimb when lifted by the tail, 2 = circling to the contralateral side when held by the tail on a flat surface but normal posture at rest, 3 = leaning to the contralateral side at rest, 4 = no spontaneous motor activity, 5 = death. A hanging grip test was performed as a measure of grasping ability and forelimb strength in which mice were suspended by their forelimbs on a wire between 2 posts 60 cm above a soft pillow for up to 60 s. The time until the animal fell was recorded (a score of 0 s was assigned to animals that fell immediately and a score of 60 s was assigned to animals that did not fall), and the average time of 3 trials with 5 min rests in between was calculated. Spontaneous locomotor activity was assessed using a parallel rod floor apparatus using ANY-maze software coupled to an automated video-tracking system as previously described (Lee et al. 2015).
Assessment of Infarct Volume
Cerebral infarct volumes were determined as previously described (Evans et al. 2017). At 24 h post-stroke, mice were killed by inhalation of isoflurane followed by decapitation. Brains were immediately removed, snap-frozen in liquid nitrogen and stored at − 80 °C. Evenly spread (separated by ~ 420 μm) coronal sections (30 μm) spanning the infarct were cut, thaw-mounted onto poly-l-lysine coated glass slides and stained with 0.1% thionin (Sigma) to delineate the infarct area. Infarct volume was quantified using image analysis software (ImageJ, NIH), correcting for brain edema, according to the following formula: CIV = (LHA − (RHA − RIA)) × (thickness of section + distance between sections); where CIV is corrected infarct volume, LHA is left hemisphere area, RHA is right hemisphere area and RIA is right hemisphere infarct area. Edema-corrected infarct volumes of individual brain sections were then added, giving an approximation of the total infarct volume.
Real-Time Polymerase Chain Reaction (rt-PCR)
At 24 h following stroke or sham surgery, mice were euthanized by isoflurane overdose and perfused with RNase-free phosphate-buffered saline (PBS). After removing the cerebellum and olfactory bulbs, the brain was separated into left and right hemispheres and snap-frozen in liquid nitrogen for RNA extraction. Spleens were also removed, cut in half and snap-frozen in liquid nitrogen. Tissues were stored at − 80 °C until required. Total RNA was extracted using Qiazol® reagent (Qiagen) and the RNeasy Mini Kit with on-column DNase step (Qiagen) followed by cDNA conversion using the Quantitect Reverse Transcription kit (for Taqman® gene expression assays; Qiagen). The cDNA was then used as a template in real-time PCR to measure mRNA expression of Vdr, Cyp27b, Cyp24a, Cxcl12, Tbx21, Stat4, Rorc, Gata3, Stat6, Foxp3, Tnfα, Il1β, Il6, Il21, Il23a, Tgfβ1, Ccl2, Ccl5, Gp91phox, Mrc1, Il10 and Icam1. Gapdh and β-actin were assessed as housekeeping genes for brain and spleen tissue, respectively. Assays were performed according to the manufacturer’s instructions using the Bio-Rad CFX96TM real-time PCR machine (Bio-Rad). Data were normalized to the housekeeping gene and calculated as change in fold expression relative to sham using the formula: fold-change = 2−ΔΔCt.
Immunofluorescence
Six serial coronal sections (10 μm thick) per animal were collected at six regions: bregma + 0.06, − 0.78, − 1.2, − 1.62, − 2.04, − 2.46 mm. Frozen brain sections (10 μm) were fixed in 4% paraformaldehyde for 15 min and washed in 0.01 M PBS (3 × 10 min). Sections were then blocked with 10% goat serum (Sigma) for 60 min to block non-specific binding of the secondary antibody. Sections were then incubated overnight at 4 °C with either rabbit anti-CD3 (1:200; Abcam) or rabbit anti-CD206 (1:500; Abcam). On the following day, they were washed (PBS; 3 × 10 min) and incubated for a maximum of 2 h with either goat anti-rabbit Alexa Fluor 594 (1:500; Thermofisher Scientific) or goat anti-rabbit Alexa Fluor 488 (1:500; Thermofisher Scientific). Finally, sections were again washed and then mounted with Vectashield medium containing 4,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories), and a coverslip was applied. All tissue-mounted slides were viewed, analyzed and photographed with an Olympus fluorescence microscope. Numbers of immunoreactive cells were counted manually per whole ischemic hemisphere and then averaged across the six regions, as indicated above.
3,3′-Diaminobenzidine (DAB) Immunohistochemistry
Frozen brain sections (at the regions indicated for immunofluorescence) were fixed in 4% paraformaldehyde for 15 min, washed in PBS and then incubated in peroxidase blocking solution (Dako) for 10 min to block endogenous peroxidases followed by 10% goat serum for 60 min. They were then incubated overnight at room temperature in rabbit anti-myeloperoxidase (1:100; Abcam). The following day, sections were washed and incubated for 2 h in anti-rabbit IgG horse-radish peroxidase conjugate (1:200; Dako), washed again, and DAB (Dako) was then applied for 5–10 min. Sections were then rinsed in dH2O, dehydrated in increasing concentrations of ethanol (70 and 100% vol/vol), cleared in xylene and mounted in DPX. Tissue-mounted slides were viewed, analyzed and photographed using an Olympus light microscope. Numbers of immunoreactive cells were counted manually per whole ischemic hemisphere and then averaged across the six regions, as indicated above.
Statistical Analysis
Data are presented as mean ± standard error of the mean (SEM), with the exception of neurological deficit scores, which are presented as median. Statistical analyses were performed using GraphPad Prism version 6.0 (GraphPad Software Inc. San Diego, CA, USA). Between-group comparisons were compared using one-way ANOVA, or Student’s unpaired t test, as appropriate. If differences were detected by ANOVA, individual groups were compared with Tukey’s multiple comparisons test, where indicated. Neurological deficit scores were compared using a Kruskal–Wallis test followed by Dunn’s multiple comparisons test. If there were two independent variables, data were compared using a two-way ANOVA. Statistical significance was accepted if P < 0.05.
Results
Effect of Cerebral Ischemia on Vitamin D-Associated Genes in Brain and Spleen
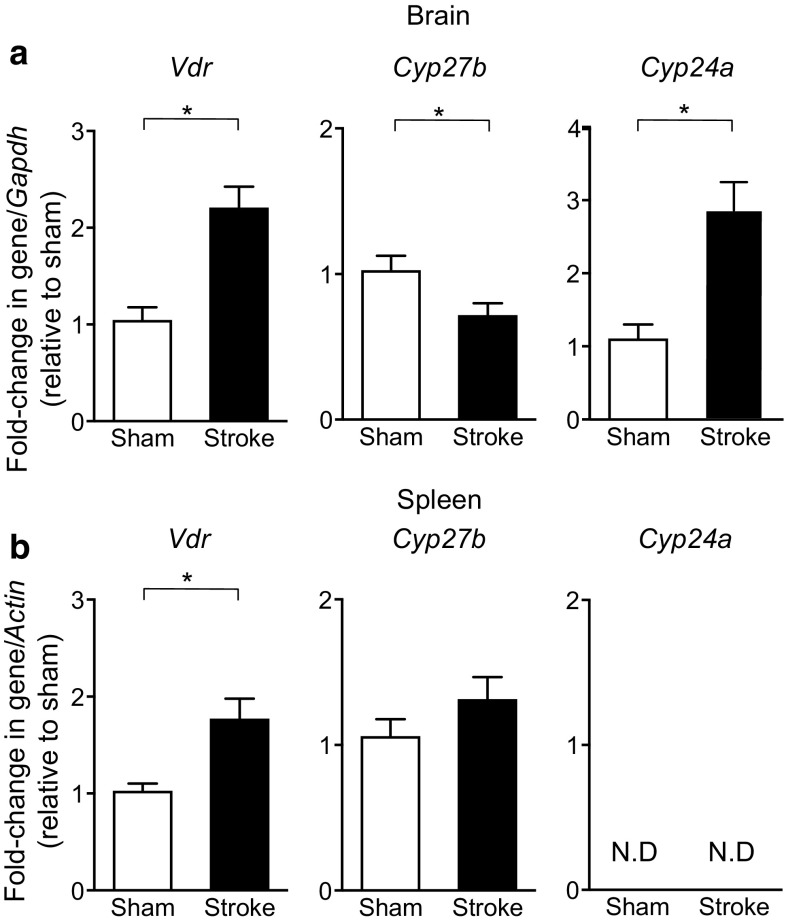
The effect of stroke was first assessed on the expression of VDR and metabolizing enzymes in the brain and spleen at 24 h in otherwise untreated animals. Stroke increased expression of the VDR by ~twofold in both organs (Fig. 1a, b). The vitamin D-activating enzyme, Cyp27b, was reduced by ~ 30% in the brain, but was unchanged in the spleen following stroke (Fig. 1a, b). Expression of the inactivating enzyme, Cyp24a, was increased by ~ 2.5 fold in the brain, but was undetectable in spleen (Fig. 1a, b).
Fig. 1.
Post-stroke expression of vitamin D-associated genes. mRNA expression of the vitamin D receptor (Vdr), 1-α-hydroxylase (Cyp27b) and 24-hydroxylase (Cyp24a) was examined in a the brain and b the spleen at 24 h after either stroke or sham surgery. Sham: n = 6–9 per group and stroke: n = 8–12 per group. N.D. denotes not detected. *P < 0.05, Student’s unpaired t test. Data are presented as mean ± SEM
Effects of Vitamin D3 Supplementation on Infarct Volume and Functional Deficits Following Stroke
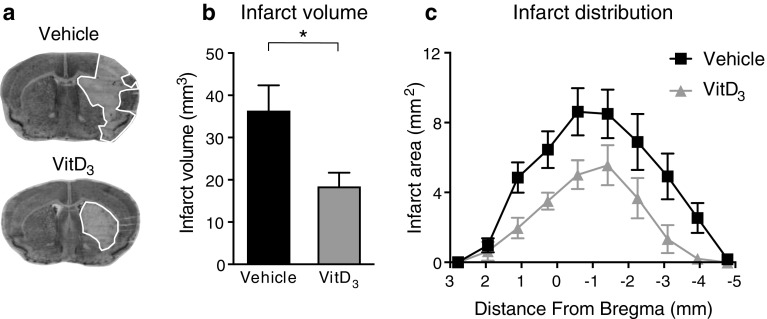
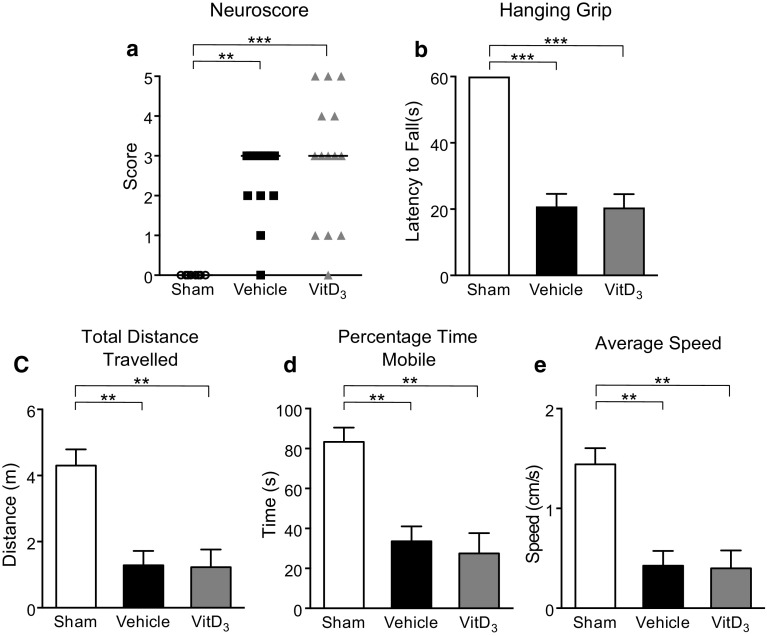
To determine the effect of elevated baseline vitamin D prior to stroke on the extent of subsequent infarct development, mice were treated with 1,25-VitD3 (100 ng/kg/day) for 5 days and then subjected to focal cerebral ischemia. At 24 h post-stroke, we found that mice which received 1,25-VitD3 supplementation had ~ 50% smaller infarct volume than those which received vehicle (Fig. 2a, b). This finding was not associated with any differences in the level of cerebral blood flow during, or immediately after, cerebral ischemia (Fig. S1). Examining the distribution of the infarcts, 1,25-VitD3-supplemented animals tended to have a reduced infarct area in most coronal sections (Fig. 2c). However, mice in both groups displayed similar functional deficits at this early time point (Fig. 3a–e).
Fig. 2.
1,25-Dihydroxyvitamin D3 (VitD3) treatment reduces infarct development. a Representative coronal brain sections from vehicle- and VitD3-treated animals (infarct area is outline in white). b Infarct volume and c distribution of infarct area from vehicle- and VitD3-treated animals. Vehicle: n = 14 per group and VitD3: n = 12 per group. *P < 0.05, Student’s unpaired t test, where appropriate. Data are presented as mean ± SEM
Fig. 3.
1,25-Dihydroxyvitamin D3 (VitD3) treatment does not influence functional outcome at 24 h following stroke. a Neurological deficit scores and b latency to fall on hanging grip test of sham, vehicle- and VitD3-treated animals. Parallel rod floor test examining c total distance travelled, d percentage time mobile and e average speed of sham, vehicle- and VitD3-treated animals. Sham: n = 6–8 per group, vehicle: n = 14 per group and VitD3: n = 11–14 per group. **P < 0.01, ***P < 0.001, Kruskal–Wallis test followed by Dunn’s multiple comparisons test (a) or one-way ANOVA followed by Tukey’s multiple comparison test (b–e). Data are presented as mean ± SEM, with the exception of neurological deficit scores which are presented as median
Effect of Vitamin D3 Supplementation on T Cell Phenotype in the Brain and Spleen After Stroke
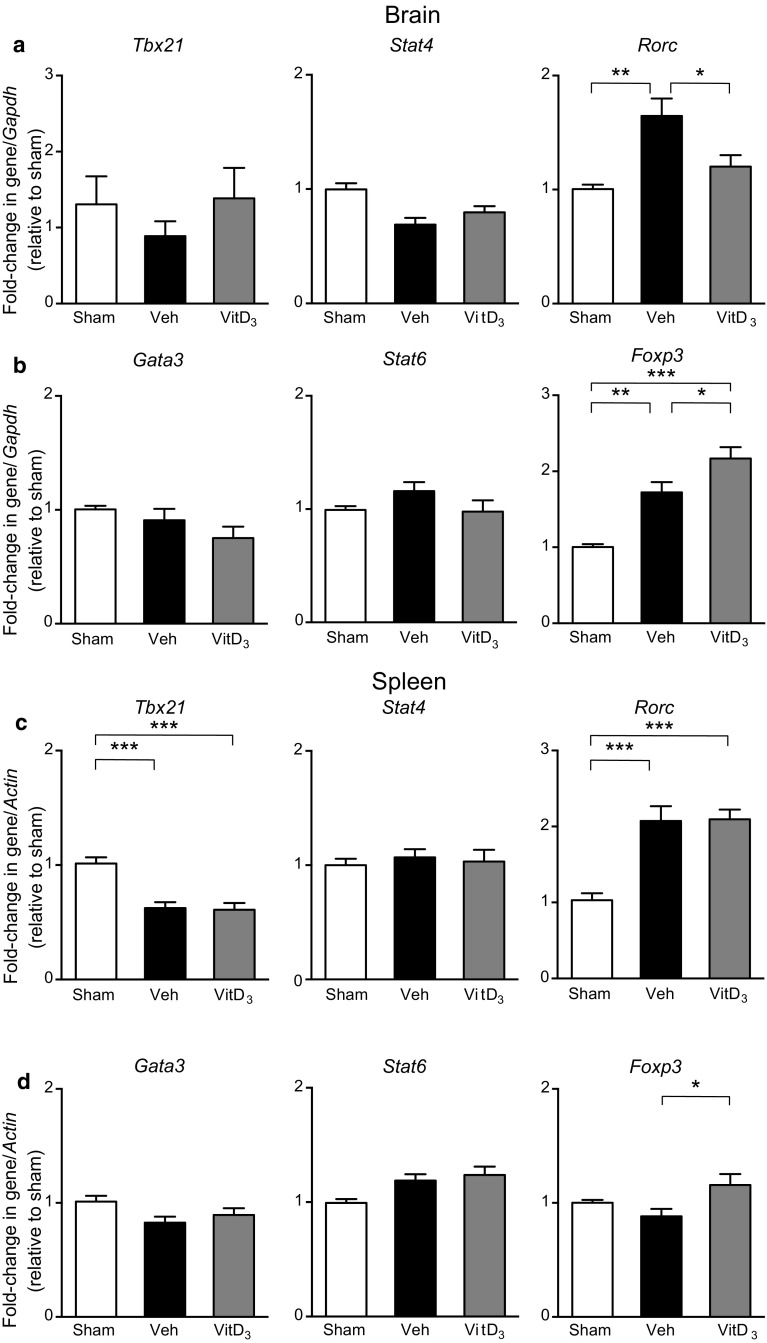
Previous reports suggest that Th1 and γδ T cells exacerbate brain injury following stroke while Th2 and T regulatory cells play a protective role by dampening excessive inflammation (Gu et al. 2012; Gelderblom et al. 2012; Benakis et al. 2016; Liesz et al. 2009). It has been shown that vitamin D can modulate the immune response to injury by polarizing T cells toward an anti-inflammatory phenotype (Hart et al. 2011). We found no effect of stroke or 1,25-VitD3 on mRNA expression of Th1 transcription factors, Tbx21 or Stat4, or Th2 transcription factors, Gata3 or Stat6 in the brain (Fig. 4a, b). However, stroke resulted in an elevation of the Th17/γδ T cell transcription factor, Rorc, and this effect was mitigated by 1,25-VitD3 treatment (Fig. 4a). Moreover, we noted an increase in the T regulatory cell transcription factor, Foxp3, after stroke, and this was augmented in 1,25-VitD3-supplemented animals (Fig. 4b). In the spleen, 1,25-VitD3 had no effect on expression of Tbx21, Stat4, Gata3, Stat6 or Rorc (Fig. 4c, d). However, Foxp3 expression was slightly higher after stroke in 1,25-VitD3-treated mice than in sham mice or in those treated with vehicle (Fig. 4d).
Fig. 4.
1,25-Dihydroxyvitamin D3 (VitD3) supplementation alters mRNA expression of T cell transcription factors in the brain and spleen following stroke. a Expression of Th1 transcription factors, Tbx21 and Stat4, and Th17 transcription factor, Rorc, within the brain of sham, vehicle- (Veh) and VitD3-treated mice at 24 h post-surgery. b Expression of Th2 transcription factors, Gata3 and Stat6, and T regulatory cell transcription factor, Foxp3, within the brain of sham, vehicle- and VitD3-treated mice at 24 h post-surgery. c Expression of Tbx21, Stat4, and Rorc within the spleen of sham, vehicle- and VitD3-treated mice at 24 h post-surgery. d Expression of Gata3, Stat6 and Foxp3 within the spleen of sham, vehicle- and VitD3-treated mice 24 h post-surgery. Sham: n = 6–9 per group, vehicle: n = 11–13 per group, VitD3: n = 10–12 per group. *P < 0.05, **P < 0.01, ***P < 0.001, one-way ANOVA followed by Tukey’s multiple comparisons test. Data are presented as mean ± SEM
Effect of Vitamin D3 Supplementation on Expression of Pro-inflammatory Mediators in the Brain Following Stroke
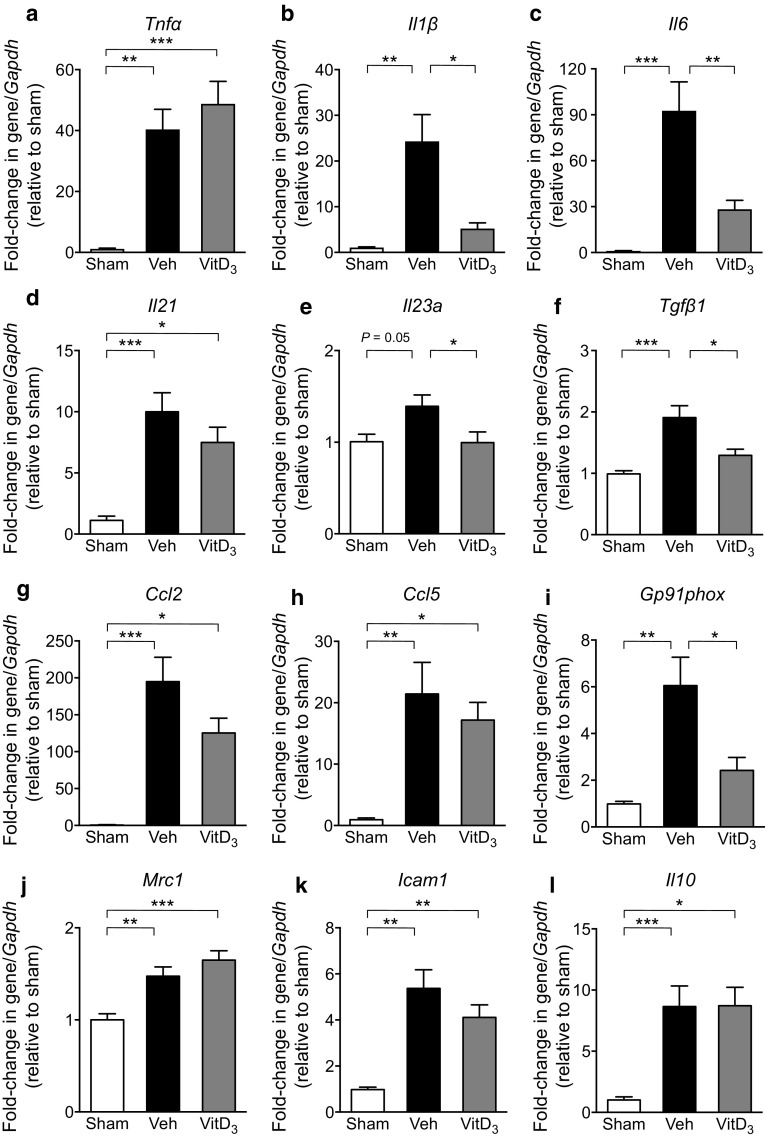
As vitamin D3 has immunomodulatory actions, we also examined mRNA expression of various inflammatory mediators known to be involved in ischemic brain injury. Indeed, 1,25-VitD3-treated animals had lower expression of Ilβ, Il6, Il23a, Tgfβ1 and Gp91phox (NOX-2) than vehicle-treated animals (Fig. 5b, c, e, f, i). However, there was no effect of 1,25-VitD3 on Tnfα, Il21, Ccl2, Ccl5, Mrc1, Icam1 or Il10 (Fig. 5a, d, g, h, j, k, l).
Fig. 5.
1,25-Dihydroxyvitamin D3 (VitD3) supplementation alters mRNA expression of inflammatory mediators in the brain following stroke. mRNA expression of a Tnfα, b Il1β, c Il6, d Il21, e Il23a, f Tgfβ1, g Ccl2, h Ccl5, i Gp91phox, j Mrc1, k Icam1 and l Il10 within the brains of sham, vehicle (Veh)- and VitD3-treated mice at 24 h post-surgery. Sham: n = 6–7 per group, vehicle: n = 12 per group and VitD3: n = 9–11 per group. *P < 0.05, **P < 0.01, ***P < 0.001, one-way ANOVA followed by Tukey’s multiple comparisons test. Data are presented as mean ± SEM
Effect of Vitamin D3 Supplementation on Numbers of Infiltrating Leukocytes and M2-Polarized Macrophages/Microglia in the Brain Following Stroke
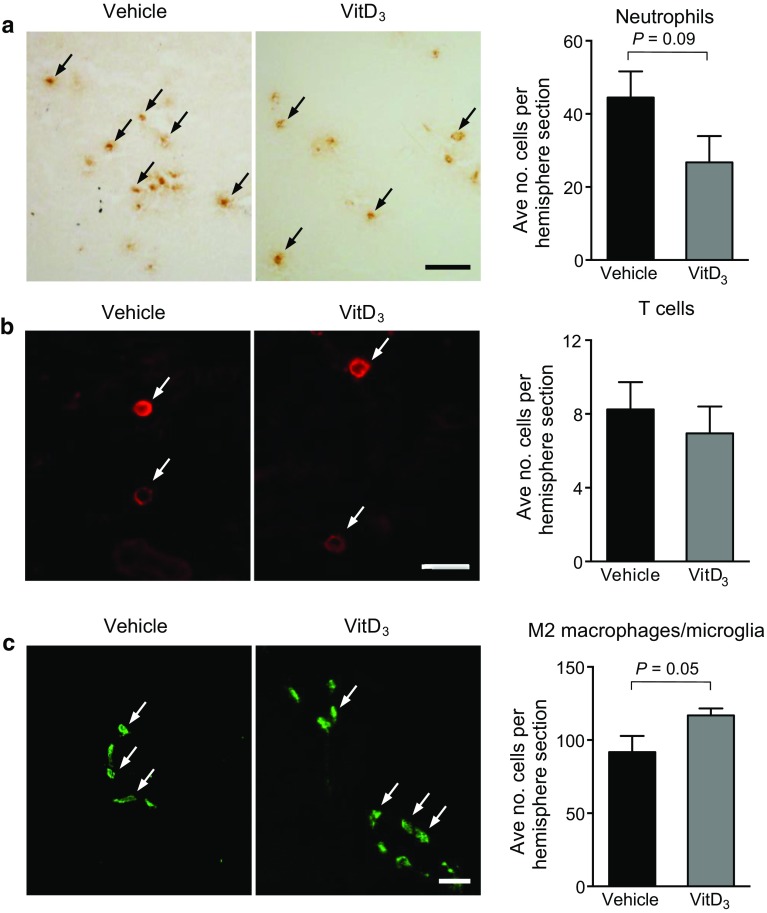
We tested for any effect of vitamin D on migration of immune cells toward the site of injury, by quantifying leukocyte infiltration into the ischemic hemisphere using immunohistochemistry. We noted a tendency for 1,25-VitD3-treated animals to have fewer MPO+ neutrophils in the brain at 24 h post-stroke, whereas there was no effect on CD3+ T cells (Fig. 6a, b). Additionally, there was a trend for greater numbers of “M2” polarized microglia/macrophages (defined as CD206+) after stroke in 1,25-VitD3-treated animals (Fig. 6c).
Fig. 6.
Quantification of leukocytes in the brain post-stroke. Immunohistochemistry was used to determine the numbers of a myeloperoxidase (MPO)+ cells, b CD3+ cells and c CD206+ cells per right (ischemic) hemisphere in vehicle- and 1,25-dihydroxyvitamin D3 (VitD3)-treated animals at 24-h post-stroke. Vehicle: n = 9–11 per group and VitD3: n = 9–10 per group. Student’s unpaired t test. Data are presented as mean ± SEM. Arrows on representative images indicate examples of positive cells, and scale bars represent either 20 μm (b) or 50 μm (a, c)
Discussion
Inflammation is a major contributor to secondary brain injury after ischemic stroke and thus represents a potential target for therapy (Anrather and Iadecola 2016). Beyond its well-characterized role in calcium metabolism, vitamin D has potent immunomodulatory properties and can alter the immune response to injury in various disease settings (Nashold et al. 2013; Takeda et al. 2010; Martorell et al. 2016; Schedel et al. 2016). If vitamin D was found to exert such effects in post-stroke brain injury, it could represent a novel direction for acute therapy. Indeed, here we report data supporting this concept. This neuroprotective effect appears to occur in association with reduced expression of pro-inflammatory mediators in the brain. Moreover, our data suggest that 1,25-VitD3 supplementation alters the phenotype of T cells and increases numbers of M2 macrophages/microglia in the ischemic brain, both of which may contribute to the neuroprotection by 1,25-VitD3 treatment.
Previous studies have demonstrated that the VDR and the vitamin D regulatory enzymes, 1-α-hydroxylase and 24-hydroxylase, to be expressed in non-classical tissues such as the brain and activated immune cells, suggesting that vitamin D may exert paracrine functions (Penna et al. 2007; Overbergh et al. 2000; Provvedini et al. 1983; Eyles et al. 2005). Additionally, studies have documented that the expression of the VDR and these enzymes can be altered during inflammation and disease (Luo et al. 2013; Yao et al. 2015; von Essen et al. 2010; Yang et al. 2011; Liu et al. 2006; Spanier et al. 2012). In the current study, we examined expression of the VDR (Vdr), 1-α-hydroxylase (Cyp27b) and 24-hydroxylase (Cyp24a), in both the brain and spleen at 24 h after stroke or sham surgery. We found that expression of the VDR was elevated in both organs after stroke. Interestingly, we observed that expression of the vitamin D-activating enzyme, 1-α-hydroxylase, was reduced in the brain after stroke, while expression of the vitamin D inactivating enzyme, 24-hydroxylase, was increased. However, in the spleen, we observed that expression of 1-α-hydroxylase and 24-hydroxylase was unchanged and undetected, respectively. These findings may imply that local levels of the active form of endogenous vitamin D may be reduced in the brain after stroke, raising the possibility that supplementation with exogenous 1,25-VitD3 may be of benefit.
Indeed, we found that 1,25-VitD3-supplemented animals developed a smaller infarct volume than vehicle-treated controls by 24 h. However, at this time point, there were no apparent differences in functional outcome. While 24 h is a relatively early time point for examining outcomes after stroke, we know from our previous work that infarct size is fully developed within 24 h in this model of stroke (Evans et al. 2018). Therefore, in seeking to test whether vitamin D might exert a neuroprotective effect to limit infarct development potentially by inhibiting inflammation, we chose to examine outcomes at 24 h. However, we do acknowledge the importance of evaluating the effect of 1,25-VitD3 at later time points after stroke, particularly on functional recovery. Our findings are analogous to those reported by two previous studies using rat models of stroke, whereby 1,25-VitD3 pre-treatment reduced infarct volume (Fu et al. 2013; Oermann et al. 2004). However, neither of these studies examined functional outcome. Moreover, the precise mechanisms by which 1,25-VitD3 reduced brain injury in those studies were unclear.
To this end, we tested for evidence that 1,25-VitD3 may modulate the immune response to ischemic stroke. Vitamin D can modulate the phenotype of T cells (Hart et al. 2011; Cantorna et al. 1996). For instance, in mouse models of multiple sclerosis vitamin D can down-regulate signaling pathways essential for development of Th1 and Th17 cells (Zeitelhofer et al. 2017; Mattner et al. 2000; Muthian et al. 2006; Chang et al. 2010b; Joshi et al. 2011). Moreover, vitamin D can promote the formation of Th2 and T regulatory cells (Hart et al. 2011) and limit the development of γδ T cells (Chen et al. 2005). Several studies have revealed that Th1 and γδ T cells can aggravate brain injury after stroke, and that blocking their invasion may be neuroprotective (Gu et al. 2012; Yilmaz et al. 2006; Gelderblom et al. 2012; Shichita et al. 2009). Th2 and T regulatory cells are thought to be injury-limiting in the setting of stroke (Gu et al. 2012; Liesz et al. 2009). We thus examined whether the neuroprotection by 1,25-VitD3 may be associated with modulation of T cell phenotypes. In the brain, we found that neither stroke nor 1,25-VitD3 had any effect on expression of Th1 or Th2 transcription factors. However, 1,25-VitD3 blunted expression of the Th17/γδ T cell transcription factor, Rorc (ROR-γt), and enhanced expression of the T regulatory cell transcription factor, Foxp3. In the spleen 1,25-VitD3 increased expression of Foxp3, but had no effect on Th1, Th2 or Th17/γδ transcription factors. Collectively, these data may indicate that 1,25-VitD3 promotes the formation of T regulatory cells while inhibiting development of Th17/γδ T cells, consistent with a neuroprotective profile.
1,25-VitD3 supplementation reduced mRNA expression of pro-inflammatory cytokines, Il1β (IL-1β), Il6 (IL-6), Tgfβ1 (TGF-β) and Il23a (IL-23a). Interestingly, these cytokines are thought to play key roles in the function of both Th17 and γδ T cells (Vantourout and Hayday 2013). Treatment with 1,25-VitD3 had no effect on expression of Il10 (IL-10), an immunosuppressive cytokine often involved in T regulatory cell function (Taylor et al. 2006); however, T regulatory cells may limit injury and excessive inflammation via other mechanisms (Sakaguchi et al. 2009). We also observed a reduction in gp91phox (NOX2) expression, a key producer of superoxide and mediator cellular damage following ischemic stroke (De Silva et al. 2011).
As 1,25-VitD3 can reduce leukocyte recruitment to injured tissues (Pedersen et al. 2007; Korf et al. 2012; Grishkan et al. 2013), we examined its effect on leukocyte infiltration into the brain following stroke. We observed a trend for 1,25-VitD3-treated animals to have fewer infiltrating neutrophils but no apparent effect on T cells. It is also possible that 1,25-VitD3 reduces recruitment of other types of immune cell subsets or that it modulates their functional phenotype rather than migration to the site of post-stroke injury. It is important to note that 24 h represents a relatively early pathological time point after stroke with significant immune cell infiltration continuing after this time point (Gelderblom et al. 2009; Benakis et al. 2016). We also examined the effect of 1,25-VitD3 on the numbers of M2-polarized macrophages/microglia in the brain after stroke. Studies have reported that M2 macrophages/microglia are likely to be protective in the setting of stroke by reducing inflammation and coordinating repair processes (Benakis et al. 2014; Chu et al. 2015; Hu et al. 2012). We observed a strong trend for 1,25-VitD3 to augment numbers of CD206+ M2 macrophages/microglia in the brain after stroke.
As mentioned above, there is a strong rationale to gain a deeper understanding of how altered levels of vitamin D—prior and/or subsequent to stroke—might impact on the degree of ensuing brain injury. Following on from our previous finding that low baseline vitamin D levels did not impact on outcome measures at 24 h (Evans et al. 2017), here we have instead assessed the effect of increasing baseline levels achieved by five daily supraphysiological doses of vitamin D prior to stroke. Indeed, the present data suggest that supplementing mice with the active form of vitamin D prior to stroke can reduce the extent of brain injury. With regard to therapeutic relevance for acute clinical stroke, our data are important in terms of proof-of-concept. However, a limitation is that 1,25-VitD3 was administered only prior to stroke induction, and clearly, studies are now required in which post-stroke treatment of 1,25-VitD3 is evaluated. While our data suggest that 1,25-VitD3 can also modulate the immune response to brain injury following stroke, at least part of this protection may occur via non-immune mechanisms, such as inhibiting excitotoxicity (Taniura et al. 2006; Brewer et al. 2001), stimulating production of neurotrophic factors (Neveu et al. 1994; Naveilhan et al. 1996; Landel et al. 2016) or improving blood brain barrier integrity (Won et al. 2015). It is also noteworthy that we administered 1,25-VitD3 to mice that were vitamin D replete. Whether a similar or greater level of neuroprotection might be achieved in vitamin D-deficient animals by 1,25-VitD3 therapy will be important to clarify.
In conclusion, these findings indicate that administration of vitamin D can attenuate infarct development following stroke possibly by modulating the inflammatory response to cerebral ischemia. Therefore, vitamin D supplementation may represent a novel direction for limiting the impact of acute stroke.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplementary material 1 Cerebral blood flow profile. Regional cerebral blood flow was recorded during and after 1 h of middle cerebral artery occlusion in mice, treated either with vehicle (Veh) or 1,25-dihydroxyvitamin D3 (VitD3). Vehicle: n = 20 per group, VitD3: n = 20 per group. Data are presented as mean ± SEM
Funding
We wish to acknowledge support from Monash Faculty Postgraduate Scholarship (MAE), Australian Postgraduate Award (YHL) and NHMRC Senior Research Fellowships (GRD and CGS).
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in these studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. Specifically, the studies were approved by Monash University Animal Ethics Committees and performed in accordance with the National Health and Medical Research Council of Australia guidelines for the care and use of animals in research. This article does not contain any studies with human participants performed by any of the authors.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s12017-018-8484-z) contains supplementary material, which is available to authorized users.
References
- Anrather J, Iadecola C. Inflammation and stroke: An overview. Neurotherapeutics. 2016;13(4):661–670. doi: 10.1007/s13311-016-0483-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benakis C, Brea D, Caballero S, Faraco G, Moore J, Murphy M, et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nature Medicine. 2016;22(5):516–523. doi: 10.1038/nm.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benakis C, Garcia-Bonilla L, Iadecola C, Anrather J. The role of microglia and myeloid immune cells in acute cerebral ischemia. Frontiers in Cellular Neuroscience. 2014;8:461. doi: 10.3389/fncel.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boontanrart M, Hall SD, Spanier JA, Hayes CE, Olson JK. Vitamin D3 alters microglia immune activation by an IL-10 dependent SOCS3 mechanism. Journal of Neuroimmunology. 2016;292:126–136. doi: 10.1016/j.jneuroim.2016.01.015. [DOI] [PubMed] [Google Scholar]
- Brewer LD, Thibault V, Chen K-C, Langub MC, Landfield PW, Porter NM. Vitamin D hormone confers neuroprotection in parallel with downregulation of L-Type calcium channel expression in hippocampal neurons. Journal of Neuroscience. 2001;21(1):98–108. doi: 10.1523/JNEUROSCI.21-01-00098.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantorna MT, Hayes CE, DeLuca HF. 1,25-Dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proceedings of the National Academy of Sciences, USA. 1996 doi: 10.1073/pnas.93.15.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantorna MT, Zhu Y, Froicu M, Wittke A. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. American Journal of Clinical Nutrition. 2004;80(6 Suppl):1717s–1720s. doi: 10.1093/ajcn/80.6.1717S. [DOI] [PubMed] [Google Scholar]
- Chang JH, Cha HR, Lee DS, Seo KY, Kweon MN. 1,25-Dihydroxyvitamin D3 inhibits the differentiation and migration of T(H)17 cells to protect against experimental autoimmune encephalomyelitis. PLoS ONE. 2010;5:e12925. doi: 10.1371/journal.pone.0012925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SH, Chung Y, Dong C. Vitamin D suppresses Th17 cytokine production by inducing C/EBP homologous protein (CHOP) expression. Journal of Biological Chemistry. 2010;285(50):38751–38755. doi: 10.1074/jbc.C110.185777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Cencioni MT, Angelini DF, Borsellino G, Battistini L, Brosnan CF. Transcriptional profiling of γδ T cells identifies a role for vitamin D in the immunoregulation of the Vγ9 Vδ2 response to phosphate-containing ligands. The Journal of Immunology. 2005;174(10):6144–6152. doi: 10.4049/jimmunol.174.10.6144. [DOI] [PubMed] [Google Scholar]
- Chu HX, Broughton BR, Kim HA, Lee S, Drummond GR, Sobey CG. Evidence that Ly6C(hi) monocytes are protective in acute ischemic stroke by promoting M2 macrophage polarization. [Research Support, Non-U.S. Gov’t] Stroke. 2015;46(7):1929–1937. doi: 10.1161/STROKEAHA.115.009426. [DOI] [PubMed] [Google Scholar]
- Daubail B, Jacquin A, Guilland JC, Hervieu M, Osseby GV, Rouaud O, et al. Serum 25-hydroxyvitamin D predicts severity and prognosis in stroke patients. European Journal of Neurology. 2013;20(1):57–61. doi: 10.1111/j.1468-1331.2012.03758.x. [DOI] [PubMed] [Google Scholar]
- De Silva TM, Brait VH, Drummond GR, Sobey CG, Miller AA. Nox2 oxidase activity accounts for the oxidative stress and vasomotor dysfunction in mouse cerebral arteries following ischemic stroke. PLoS ONE. 2011;6(12):e28393. doi: 10.1371/journal.pone.0028393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Zoppo GJ, Saver JL, Jauch EC, Adams HP., Jr Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association. [Practice guideline] Stroke. 2009;40(8):2945–2948. doi: 10.1161/STROKEAHA.109.192535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Wong SL, Lau CW, Lee HK, Ng CF, Zhang L, et al. Calcitriol protects renovascular function in hypertension by down-regulating angiotensin II type 1 receptors and reducing oxidative stress. European Heart Journal. 2012;33(23):2980–2990. doi: 10.1093/eurheartj/ehr459. [DOI] [PubMed] [Google Scholar]
- Evans MA, Kim HA, De Silva TM, Arumugam TV, Clarkson AN, Drummond GR, et al. Diet-induced vitamin D deficiency has no effect on acute post-stroke outcomes in young male mice. Journal of Cerebral Blood Flow and Metabolism. 2017 doi: 10.1177/0271678X17719208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MA, Lim R, Kim HA, Chu HX, Gardiner-Mann CV, Taylor KWE, et al. Acute or delayed systemic administration of human amnion epithelial cells improves outcomes in experimental stroke. Stroke. 2018 doi: 10.1161/STROKEAHA.117.019136. [DOI] [PubMed] [Google Scholar]
- Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. Journal of Chemical Neuroanatomy. 2005;29(1):21–30. doi: 10.1016/j.jchemneu.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Fu J, Xue R, Gu J, Xiao Y, Zhong H, Pan X, et al. Neuroprotective effect of calcitriol on ischemic/reperfusion injury through the NR3A/CREB pathways in the rat hippocampus. Mol Med Rep. 2013;8(6):1708–1714. doi: 10.3892/mmr.2013.1734. [DOI] [PubMed] [Google Scholar]
- Gelderblom M, Leypoldt F, Steinbach K, Behrens D, Choe CU, Siler DA, et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40(5):1849–1857. doi: 10.1161/STROKEAHA.108.534503. [DOI] [PubMed] [Google Scholar]
- Gelderblom M, Weymar A, Bernreuther C, Velden J, Arunachalam P, Steinbach K, et al. Neutralization of the IL-17 axis diminishes neutrophil invasion and protects from ischemic stroke. Blood. 2012;120(18):3793–3802. doi: 10.1182/blood-2012-02-412726. [DOI] [PubMed] [Google Scholar]
- Gorman S, Judge MA, Hart PH. Topical 1,25-dihydroxyvitamin D3 subverts the priming ability of draining lymph node dendritic cells. Immunology. 2010;131(3):415–425. doi: 10.1111/j.1365-2567.2010.03315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregori S, Giarratana N, Smiroldo S, Uskokovic M, Adorini L. A 1α,25-dihydroxyvitamin D analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes. 2002;51(5):1367–1374. doi: 10.2337/diabetes.51.5.1367. [DOI] [PubMed] [Google Scholar]
- Grishkan IV, Fairchild AN, Calabresi PA, Gocke AR. 1,25-dihydroxyvitamin D3 selectively and reversibly impairs T helper-cell CNS localization. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(52):21101–21106. doi: 10.1073/pnas.1306072110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu L, Xiong X, Zhang H, Xu B, Steinberg GK, Zhao H. Distinctive effects of T cell subsets in neuronal injury induced by cocultured splenocytes in vitro and by in vivo stroke in mice. Stroke. 2012;43(7):1941–1946. doi: 10.1161/STROKEAHA.112.656611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart PH, Gorman S, Finlay-Jones JJ. Modulation of the immune system by UV radiation: more than just the effects of vitamin D? Nature Reviews Immunology. 2011;11(9):584–596. doi: 10.1038/nri3045. [DOI] [PubMed] [Google Scholar]
- Holick MF. Vitamin D deficiency. New England Journal of Medicine. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43(11):3063–3070. doi: 10.1161/STROKEAHA.112.659656. [DOI] [PubMed] [Google Scholar]
- Jain SK, Micinski D. Vitamin D upregulates glutamate cysteine ligase and glutathione reductase, and GSH formation, and decreases ROS and MCP-1 and IL-8 secretion in high-glucose exposed U937 monocytes. Biochemical and Biophysical Research Communications. 2013;437(1):7–11. doi: 10.1016/j.bbrc.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, Pantalena LC, Liu XK, Gaffen SL, Liu H, Rohowsky-Kochan C, et al. 1,25-Dihydroxyvitamin D(3) ameliorates Th17 autoimmunity via transcriptional modulation of interleukin-17A. Molecular and Cellular Biology. 2011;31(17):3653–3669. doi: 10.1128/MCB.05020-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleindorfer D, Lindsell CJ, Brass L, Koroshetz W, Broderick JP. National US estimates of recombinant tissue plasminogen activator use: ICD-9 codes substantially underestimate. Stroke. 2008;39(3):924–928. doi: 10.1161/STROKEAHA.107.490375. [DOI] [PubMed] [Google Scholar]
- Korf H, Wenes M, Stijlemans B, Takiishi T, Robert S, Miani M, et al. 1,25-Dihydroxyvitamin D3 curtails the inflammatory and T cell stimulatory capacity of macrophages through an IL-10-dependent mechanism. Immunobiology. 2012;217(12):1292–1300. doi: 10.1016/j.imbio.2012.07.018. [DOI] [PubMed] [Google Scholar]
- Landel V, Millet P, Baranger K, Loriod B, Feron F. Vitamin D interacts with Esr1 and Igf1 to regulate molecular pathways relevant to Alzheimer’s disease. Molecular Neurodegeneration. 2016;11:22. doi: 10.1186/s13024-016-0087-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langub MC, Herman JP, Malluche HH, Koszewski NJ. Evidence of functional vitamin D receptors in rat hippocampus. Neuroscience. 2001;104(1):49–56. doi: 10.1016/S0306-4522(01)00049-5. [DOI] [PubMed] [Google Scholar]
- Lee S, Evans MA, Chu HX, Kim HA, Widdop RE, Drummond GR, et al. Effect of a selective Mas receptor agonist in cerebral ischemia in vitro and in vivo. PLoS ONE. 2015;10(11):e0142087. doi: 10.1371/journal.pone.0142087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, O’Keefe JH, Bell D, Hensrud DD, Holick MF. Vitamin D deficiency an important, common, and easily treatable cardiovascular risk factor? Journal of the American College of Cardiology. 2008;52(24):1949–1956. doi: 10.1016/j.jacc.2008.08.050. [DOI] [PubMed] [Google Scholar]
- Liesz A, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S, et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nature Medicine. 2009;15(2):192–199. doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- Lugg ST, Howells PA, Thickett DR. Optimal vitamin D supplementation levels for cardiovascular disease protection. Disease Markers. 2015;2015:1–10. doi: 10.1155/2015/864370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Hershberger PA, Trump DL, Johnson CS. 24-Hydroxylase in cancer: impact on vitamin D-based anticancer therapeutics. Journal of Steroid Biochemistry and Molecular Biology. 2013;136:252–257. doi: 10.1016/j.jsbmb.2012.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martorell S, Hueso L, Gonzalez-Navarro H, Collado A, Sanz MJ, Piqueras L. Vitamin D receptor activation reduces angiotensin-II-induced dissecting abdominal aortic aneurysm in apolipoprotein E-knockout mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 2016;36(8):1587–1597. doi: 10.1161/ATVBAHA.116.307530. [DOI] [PubMed] [Google Scholar]
- Mattner F, Smiroldo S, Galbiati F, Muller M, Di Lucia P, Poliani PL, et al. Inhibition of Th1 development and treatment of chronic-relapsing experimental allergic encephalomyelitis by a non-hypercalcemic analogue of 1,25-dihydroxyvitamin D3. European Journal of Immunology. 2000;30(2):498–508. doi: 10.1002/1521-4141(200002)30:2<498::AID-IMMU498>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Merke J, Milde P, Lewicka S, Hugel U, Klaus G, Mangelsdorf DJ, et al. Identification and regulation of 1,25-dihydroxyvitamin D3 receptor activity and biosynthesis of 1,25-dihydroxyvitamin D3. Studies in cultured bovine aortic endothelial cells and human dermal capillaries. Journal of Clinical Investigation. 1989;83(6):1903–1915. doi: 10.1172/JCI114097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics-2016 update: A report from the american heart association. Circulation. 2016;133(4):e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- Muthian G, Raikwar HP, Rajasingh J, Bright JJ. 1,25 Dihydroxyvitamin-D3 modulates JAK–STAT pathway in IL-12/IFNγ axis leading to Th1 response in experimental allergic encephalomyelitis. Journal of Neuroscience Research. 2006;83(7):1299–1309. doi: 10.1002/jnr.20826. [DOI] [PubMed] [Google Scholar]
- Nashold FE, Nelson CD, Brown LM, Hayes CE. One calcitriol dose transiently increases Helios + FoxP3 + T cells and ameliorates autoimmune demyelinating disease. Journal of Neuroimmunology. 2013;263(1–2):64–74. doi: 10.1016/j.jneuroim.2013.07.016. [DOI] [PubMed] [Google Scholar]
- Naveilhan P, Neveu I, Wion D, Brachet P. 1,25-Dihydroxyvitamin D3, an inducer of glial cell line-derived neurotrophic factor. NeuroReport. 1996;7(13):2171–2175. doi: 10.1097/00001756-199609020-00023. [DOI] [PubMed] [Google Scholar]
- Neveu I, Naveilhan P, Jehan Fdr, Baudet C, Wion D, De Luca HF, et al. 1,25-Dihydroxyvitamin D3 regulates the synthesis of nerve growth factor in primary cultures of glial cells. Molecular Brain Research. 1994;24(1–4):70–76. doi: 10.1016/0169-328X(94)90119-8. [DOI] [PubMed] [Google Scholar]
- Oermann E, Bidmon HJ, Witte OW, Zilles K. Effects of 1alpha,25 dihydroxyvitamin D3 on the expression of HO-1 and GFAP in glial cells of the photothrombotically lesioned cerebral cortex. Journal of Chemical Neuroanatomy. 2004;28(4):225–238. doi: 10.1016/j.jchemneu.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Overbergh L, Decallonne B, Valckx D, Verstuyf A, Depovere J, Laureys J, et al. Identification and immune regulation of 25-hydroxyvitamin D-1-α-hydroxylase in murine macrophages. Clinical and Experimental Immunology. 2000;120(1):139–146. doi: 10.1046/j.1365-2249.2000.01204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K-Y, Chung P-W, Kim YB, Moon H-S, Suh B-C, Won YS, et al. Serum vitamin D status as a predictor of prognosis in patients with acute ischemic stroke. Cerebrovascular Diseases. 2015;40(1–2):73–80. doi: 10.1159/000434691. [DOI] [PubMed] [Google Scholar]
- Pedersen LB, Nashold FE, Spach KM, Hayes CE. 1,25-dihydroxyvitamin D3 reverses experimental autoimmune encephalomyelitis by inhibiting chemokine synthesis and monocyte trafficking. Journal of Neuroscience Research. 2007;85(11):2480–2490. doi: 10.1002/jnr.21382. [DOI] [PubMed] [Google Scholar]
- Penna G, Amuchastegui S, Giarratana N, Daniel KC, Vulcano M, Sozzani S, et al. 1,25-Dihydroxyvitamin D3 selectively modulates tolerogenic properties in myeloid but not plasmacytoid dendritic cells. Journal of Immunology. 2007;178(1):145–153. doi: 10.4049/jimmunol.178.1.145. [DOI] [PubMed] [Google Scholar]
- Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25-Dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221(4616):1181–1183. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- Reeves MJ, Arora S, Broderick JP, Frankel M, Heinrich JP, Hickenbottom S, et al. Acute stroke care in the US: results from 4 pilot prototypes of the Paul Coverdell National Acute Stroke Registry. Stroke. 2005;36(6):1232–1240. doi: 10.1161/01.STR.0000165902.18021.5b. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T. Regulatory T cells: how do they suppress immune responses? International Immunology. 2009;21(10):1105–1111. doi: 10.1093/intimm/dxp095. [DOI] [PubMed] [Google Scholar]
- Schedel M, Jia Y, Michel S, Takeda K, Domenico J, Joetham A, et al. 1,25D3 prevents CD8(+)Tc2 skewing and asthma development through VDR binding changes to the Cyp11a1 promoter. Nature Communication. 2016;7:10213. doi: 10.1038/ncomms10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shichita T, Sugiyama Y, Ooboshi H, Sugimori H, Nakagawa R, Takada I, et al. Pivotal role of cerebral interleukin-17-producing gammadeltaT cells in the delayed phase of ischemic brain injury. Nature Medicine. 2009;15(8):946–950. doi: 10.1038/nm.1999. [DOI] [PubMed] [Google Scholar]
- Sloka S, Silva C, Wang J, Yong VW. Predominance of Th2 polarization by vitamin D through a STAT6-dependent mechanism. Journal of Neuroinflammation. 2011;8(1):56. doi: 10.1186/1742-2094-8-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotirchos ES, Bhargava P, Eckstein C, Van Haren K, Baynes M, Ntranos A, et al. Safety and immunologic effects of high- vs low-dose cholecalciferol in multiple sclerosis. Neurology. 2016;86(4):382–390. doi: 10.1212/WNL.0000000000002316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanier JA, Nashold FE, Olson JK, Hayes CE. The Ifng gene is essential for Vdr gene expression and vitamin D(3)-mediated reduction of the pathogenic T cell burden in the central nervous system in experimental autoimmune encephalomyelitis, a multiple sclerosis model. Journal of Immunology. 2012;189(6):3188–3197. doi: 10.4049/jimmunol.1102925. [DOI] [PubMed] [Google Scholar]
- Takeda M, Yamashita T, Sasaki N, Nakajima K, Kita T, Shinohara M, et al. Oral administration of an active form of vitamin D3 (calcitriol) decreases atherosclerosis in mice by inducing regulatory T cells and immature dendritic cells with tolerogenic functions. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30(12):2495–2503. doi: 10.1161/ATVBAHA.110.215459. [DOI] [PubMed] [Google Scholar]
- Taniura H, Ito M, Sanada N, Kuramoto N, Ohno Y, Nakamichi N, et al. Chronic vitamin D3 treatment protects against neurotoxicity by glutamate in association with upregulation of vitamin D receptor mRNA expression in cultured rat cortical neurons. Journal of Neuroscience Research. 2006;83(7):1179–1189. doi: 10.1002/jnr.20824. [DOI] [PubMed] [Google Scholar]
- Taylor A, Verhagen J, Blaser K, Akdis M, Akdis CA. Mechanisms of immune suppression by interleukin-10 and transforming growth factor-β: the role of T regulatory cells. Immunology. 2006;117(4):433–442. doi: 10.1111/j.1365-2567.2006.02321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu WJ, Zhao SJ, Xu DJ, Chen H. Serum 25-hydroxyvitamin D predicts the short-term outcomes of Chinese patients with acute ischaemic stroke. Clinical Science (London, England: 1979) 2014;126(5):339–346. doi: 10.1042/CS20130284. [DOI] [PubMed] [Google Scholar]
- Turetsky A, Goddeau RP, Jr, Henninger N. Low serum vitamin D is independently associated with larger lesion volumes after ischemic stroke. Journal of Stroke and Cerebrovascular Diseases. 2015;24(7):1555–1563. doi: 10.1016/j.jstrokecerebrovasdis.2015.03.051. [DOI] [PubMed] [Google Scholar]
- Vantourout P, Hayday A. Six-of-the-best: unique contributions of [gamma][delta] T cells to immunology. Nature Reviews: Immunology. 2013;13(2):88–100. doi: 10.1038/nri3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma R, Kim JY. 1,25-Dihydroxyvitamin D3 facilitates M2 polarization and upregulates TLR10 expression on human microglial cells. Neuroimmunomodulation. 2016;23(2):75–80. doi: 10.1159/000444300. [DOI] [PubMed] [Google Scholar]
- von Essen MR, Kongsbak M, Schjerling P, Olgaard K, Odum N, Geisler C. Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nature Immunology. 2010;11(4):344–349. doi: 10.1038/ni.1851. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ji H, Tong Y, Zhang ZB. Prognostic value of serum 25-hydroxyvitamin D in patients with stroke. Neurochemical Research. 2014;39(7):1332–1337. doi: 10.1007/s11064-014-1316-0. [DOI] [PubMed] [Google Scholar]
- Won S, Sayeed I, Peterson BL, Wali B, Kahn JS, Stein DG. Vitamin D prevents hypoxia/reoxygenation-induced blood-brain barrier disruption via vitamin D receptor-mediated NF-kB signaling pathways. PLoS ONE. 2015;10(3):e0122821. doi: 10.1371/journal.pone.0122821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MS, Leisegang MS, Kruse C, Vogel J, Schurmann C, Dehne N, et al. Vitamin D promotes vascular regeneration. Circulation. 2014;130(12):976–986. doi: 10.1161/CIRCULATIONAHA.114.010650. [DOI] [PubMed] [Google Scholar]
- Yang L, Brozovic S, Xu J, Long Y, Kralik PM, Waigel S, et al. Inflammatory gene expression in OVE26 diabetic kidney during the development of nephropathy. Nephron Experimental Nephrology. 2011;119(1):e8–e20. doi: 10.1159/000324407. [DOI] [PubMed] [Google Scholar]
- Yao T, Ying X, Zhao Y, Yuan A, He Q, Tong H, et al. Vitamin D receptor activation protects against myocardial reperfusion injury through inhibition of apoptosis and modulation of autophagy. Antioxidants & Redox Signaling. 2015;22(8):633–650. doi: 10.1089/ars.2014.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz G, Arumugam TV, Stokes KY, Granger DN. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation. 2006;113(17):2105–2112. doi: 10.1161/CIRCULATIONAHA.105.593046. [DOI] [PubMed] [Google Scholar]
- Zeitelhofer M, Adzemovic MZ, Gomez-Cabrero D, Bergman P, Hochmeister S, N’Diaye M, et al. Functional genomics analysis of vitamin D effects on CD4+ T cells in vivo in experimental autoimmune encephalomyelitis. Proceedings of the National Academy of Sciences of the United States of America. 2017 doi: 10.1073/pnas.1615783114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Leung DY, Richers BN, Liu Y, Remigio LK, Riches DW, et al. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. Journal of Immunology. 2012;188(5):2127–2135. doi: 10.4049/jimmunol.1102412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1 Cerebral blood flow profile. Regional cerebral blood flow was recorded during and after 1 h of middle cerebral artery occlusion in mice, treated either with vehicle (Veh) or 1,25-dihydroxyvitamin D3 (VitD3). Vehicle: n = 20 per group, VitD3: n = 20 per group. Data are presented as mean ± SEM