Abstract
In this work, the properties of saturated and unsaturated fatty acid acyl chains 16:0, 18:0, 18:1(n-9)cis, 18:2(n-6)cis, 18:3(n-3)cis, 18:4(n-3)cis, 18:5(n-3)cis, 20:4(n-6)cis, 20:5(n-3)cis and 22:6(n-3)cis in a bilayer liquid crystalline state and similar hydrocarbon chains (with CH terminal groups instead of C=O groups) in the unperturbed state characterised by a lack of long-range interaction were investigated. The unperturbed hydrocarbon chains were modelled by Monte Carlo simulations at temperature K; sixteen fully hydrated homogeneous liquid crystalline phosphatidylcholine bilayers containing these chains were studied by molecular dynamics simulations at the same temperature. To eliminate effects of the simulation parameters, the molecular dynamics and Monte Carlo simulations were carried out using the same structural data and force field coefficients. From these computer simulations, the average distances between terminal carbon atoms of the chains (end-to-end distances) were calculated and compared. The trends in the end-to-end distances obtained for the unperturbed chains were found to be qualitatively similar to those obtained for the same lipid chains in the bilayers. So, for understanding of a number of processes in biological membranes (e.g., changes in fatty acid composition caused by environmental changes such as temperature and pressure), it is possible to use, at least as a first approximation, the relationships between the structure and properties for unperturbed or isolated hydrocarbon chains.
Keywords: Lipid bilayers, Biomembranes, Unsaturated hydrocarbon chains, Molecular dynamics, Monte Carlo
Introduction
Biological membranes are very complex heterogeneous systems composed of various molecules such as lipids, sterols, proteins, carbohydrates, etc. Lipid molecules contain different head groups and a wide variety of acyl chains of fatty acids (FAs; Cook and McMaster 2002; Nelson and Cox 2008; Mouritsen and Bagatolli 2016). The FAs are the fundamental building blocks of all lipids in living matter. The most abundant class of lipids in the biological membranes of animals and plants is phosphatidylcholine (PC). FA acyl chains of PC lipids usually contain 12–24 carbon atoms; the most common chain lengths fall between 14 (or 16) and 22. Most of the FA acyl chains are unsaturated, containing 1–6 double bonds of the cis configuration in different positions; the majority of the double bonds in the tails are methylene-interrupted (i.e., one methylene group is localized between each pair of double bonds; Cook and McMaster 2002; Nelson and Cox 2008; Mouritsen and Bagatolli 2016). It is most common to find chains with an even number of carbon atoms, whereas odd ones are found in rare cases.
The FA chains are often denoted in accordance with ‘n-minus’ nomenclature, as , where N refers to the total number of carbon atoms in the chain, d is the number of the methylene-interrupted double bonds and j is the position of the first double bond, counted from the methyl (CH) terminus of the chain, with the methyl carbon as number 1. For brevity, below the term in the notation will be occasionally omitted.
It should be mentioned that unsaturated FA chains and especially polyunsaturated (PU) tails of lipids (for instance, 18:1(n-9)cis FA, 18:2(n-6)cis FA, 18:3(n-3)cis FA, 18:4(n-3)cis FA, 18:5(n-3)cis FA, 20:4(n-6)cis FA, 20:5(n-3)cis FA and 22:6(n-3)cis FA) are of great importance for the structure and function of animal and plant membranes (Table 1).
Table 1.
Occurrence of unsaturated and polyunsaturated (PU) fatty acid (FA) chains of lipids in animal and plant membranes
| Fatty acid chain | Occurrence (findings) | Refs. |
|---|---|---|
| 18:1(n-9)cis FA | The most abundant monoenoic FA in plant and animal tissues | |
| 35–60% of the total FAs of peanut oil acylglycerols | Carrin and Carelli (2010) | |
| 35–69% of the total FAs of peanut oil acylglycerols | Köckritz and Martin (2008) | |
| 60% of the total FAs of the oil from 00-quality oilseed rape | Wittkop et al. (2009) | |
| 40–70% of various vegetable oils | Pinzi et al. (2009) | |
| 91–92% of HO sunflower 90plus oil | Köckritz and Martin (2008) | |
| 30–40% of the total FAs in adipose fats of animals | Nelson and Cox (2008) | |
| 18:2(n-6)cis FA | An ubiquitous component of plant lipids | |
| ~58% in the cold-pressed black cumin seed oil | Lutterodt et al. (2010) | |
| 48–74% of sunflower oil | Köckritz and Martin (2008) | |
| 48–59% of soybean oil | Köckritz and Martin (2008) | |
| 47–58% of cottonseed oil | Köckritz and Martin (2008) | |
| 75% of the total FAs of heart cardiolipin of animals | Minkler and Hoppel (2010) | |
| 18:3(n-3)cis FA | Large amounts in flaxseed and walnuts | |
| 34% of the total FAs in garden cress seed oil | Diwakar et al. (2010) | |
| 56–71% of the total FAs in linseed oil | Köckritz and Martin (2008) | |
| 18:4(n-3)cis FA | Variable amounts in several species of fungi and animals tissues, in seeds of some plant families | Guil-Guerrero (2007) |
| Up to 27% in several species of algae | Guil-Guerrero (2007) | |
| Up to 18% in Echium (Boraginaceae) species | Guil-Guerrero (2007) | |
| 18:5(n-3)cis FA | Certain algal groups in marine phytoplankton | Napolitano et al. (1995) |
| 20:4(n-6)cis FA | The mammalian cell membranes, fish oils, etc. | |
| 30–70% of the total FAs of triacylglycerols produced by the filamentous fungus Mortierella alpina 1S-4 | Sakuradani (2010) | |
| 20:5(n-3)cis FA | One of the most important FAs of the so-called ‘(n-3) family’: animal tissues (especially brain), algae, fish oils | DHA (2009) |
| 22:6(n-3)cis FA | The most unsaturated FA commonly found in nature, it regulates many cell transport and synaptic functions | |
| A major constituent of fish oils, especially from tuna eyeballs | DHA (2009) | |
| 40% of the PU FAs in the brain of animals | DHA (2009) | |
| 60% of the PU FAs in the retina of animals | DHA (2009) |
It should also be noted that all higher plants have the ability to synthesize 18:2(n-6)cis FA and 18:3(n-3)cis FA, and some can also synthesize 18:4(n-3)cis FA (Singh et al. 2005). The chains of 18:2(n-6)cis, 18:3(n-3)cis and 18:4(n-3)cis FAs accumulate in plant tissues as terminal FA metabolites (Rincón-Cervera and Guil-Guerrero 2010). 22:6(n-3)cis FA is usually the end point of 18:3(n-3)cis FA metabolism in animal tissues. On the whole, membranes that are active metabolically, as in rod outer segments, mitochondria, synaptic vesicles, etc., have high levels of PU chains.
Chemical structures of all above-mentioned sn-2 FA acyls and one of the PC molecules [18:0/22:6(n-3)cis PC molecule] are presented in Fig. 1.
Fig. 1.
Structures, from top to bottom, of sn-2 lipid chains (fatty acid acyls) of 18:1(n-9)cis, 18:2(n-6)cis, 18:3(n-3)cis, 18:4(n-3)cis, 18:5(n-3)cis, 20:4(n-6)cis and 20:5(n-3)cis; phosphatidylcholine molecule of 18:0/22:6(n-3)cis PC showing structures of sn-2 22:6(n-3)cis acyl chain and sn-1 18:0 acyl chain; structure of the possible sn-1 16:0 acyl chain
PU FA chains have been linked to a great number of biochemical processes. One of the most notable, observations is that PU FAs play a role in achieving optimal health and in protection against disease. In other words, PU FAs (and their derivatives) have significant clinical implications. The beneficial health effects of PU FAs, particularly, 22:6(n-3)cis FA and 20:5(n-3)cis FA (DHA 2009; Sahena et al. 2009) are related to several tens of human afflictions, such as cancer (Nabavi et al. 2015; D’Eliseo and Velotti 2016; Molfino et al. 2016), cardiovascular disease (Rovere and Christensen 2015; Sperling and Nelson 2016), allergic diseases (Rueter et al. 2015), many skin disorders (McCusker and Grant-Kels 2010), and diabetes (Bhaswant et al. 2015; Wang and Chan 2015), etc.
Thus, it is very important to study physical properties of lipid acyl chains in different conditions to reveal relationships between chemical structure and physical properties. Indeed, such relationships are of great importance for understanding the structure and functioning of biomembranes.
Unfortunately, experimental data for different properties of such hydrocarbons or FA acyl chains are scarce or lacking. Computer simulation is nowadays one of the most powerful tools for studying the properties of different molecular systems (Leach 2001; Berendsen 2007; Gould et al. 2007; Landau and Binder 2009; Binder and Heermann 2010; Brooks et al. 2011; Satoh 2011) including lipid membranes, lipids and lipid chains (see, e.g., several reviews on MD simulations of lipid membranes: Bennett and Tieleman 2013; Rabinovich and Lyubartsev 2013; Pluhackova and Böckmann 2015; Baoukina and Tieleman 2016; Bunker et al. 2016; Kirsch and Böckmann 2016; Lyubartsev and Rabinovich 2016; Pasenkiewicz-Gierula et al. 2016; Pöyry and Vattulainen 2016, and other articles published in Special Issue 10 of BBA-Biomembranes, 2016, v.1858 entitled: Biosimulations) because it allows one to obtain information on an atomistic level. On the other hand, computer simulations of lipid bilayer systems with all possible combinations of chains are still very time-consuming, and, therefore, a different approach to the task would be valuable.
To obtain ‘structure–property’ relationships for different hydrocarbon chains which can be compared with each other, one should use uniform conditions: the same state at the same temperature. The ‘unperturbed’ state of chain molecules (Flory 1969) was used as the uniform state of hydrocarbon chains in this work. The exact definition of this state is presented below, in the ‘2.2 Monte Carlo simulations’ section. It has been proposed (Flory 1969) that properties of chain molecules in this state correspond to the properties in the bulk amorphous state. Neutron scattering experiments were later carried out (Dettenmaier 1978; Yoon and Flory 1978) and the results substantiated this prediction. On the other hand, biological membranes in a physiological form exist in a liquid crystalline (fluid) state having a relatively high degree of disorder and dynamical behaviour; this state is vital for the proper functioning of membranes.
The aim of the present study was to compare properties of a set of hydrocarbon chains in the unperturbed state unaffected by long-range interactions and for comparison in the liquid crystalline state of lipid bilayers, to assess if these properties are similar to each other.
A structural scheme of the chains considered in the present work is as follows: CH–(CH–(CH=CH–CH–(CH–CH,
where a, d, b are integers. The total number of carbons of the chain is . For clear visualization of the connection between structure of the chains and FA acyl chains, these hydrocarbons will be denoted below as , i.e., similar to the ‘n-minus’ nomenclature for FAs.
The chains of alk-16:0, alk-18:0, alk-18:1(n-9)cis, alk-18:2(n-6)cis, alk-18:3(n-3)cis, alk-18:4(n-3)cis, alk-18:5(n-3)cis, alk-20:4(n-6)cis, alk-20:5(n-3)cis and alk-22:6(n-3)cis were studied by Monte Carlo (MC) simulations in an unperturbed state at temperature K. Chemical structures of the 10 studied hydrocarbon chains are presented in Figure 2.
Fig. 2.
Structures, from top to bottom, of hydrocarbon chains alk-16:0, alk-18:0, alk-18:1(n-9)cis, alk-18:2(n-6)cis, alk-18:3(n-3)cis, alk-18:4(n-3)cis, alk-18:5(n-3)cis, alk-20:4(n-6)cis, alk-20:5(n-3)cis and alk-22:6(n-3)cis studied by Monte Carlo simulations. Such names of the hydrocarbon chains are used to stress the chain and corresponding FA acyl (Fig. 1) structural similarity
In addition, 16 fully hydrated homogeneous liquid crystalline PC bilayers containing these chains (as FA acyls) were studied by molecular dynamics (MD) simulations at the same temperature ( K). The MD and MC simulations were carried out using the same force field, to eliminate the effect of the simulation parameters. Both techniques are described below.
Models and methods
Molecular dynamics simulations
Sixteen fully hydrated homogeneous phosphatidylcholine (PC) bilayers were studied by MD simulation in an NPT-ensemble at temperature K and pressure bar. The simulation software was the MDynaMix package (Lyubartsev and Laaksonen 2000). The bilayers studied were comprised of one of the PC molecules which contained a saturated sn-1 chain (16:0 or 18:0) and an unsaturated sn-2 chain:
1-palmitoyl-2-oleoyl-sn-glycero-3-PC [16:0/18:1(n-9)cis PC];
1-stearoyl-2-oleoyl-sn-glycero-3-PC [18:0/18:1(n-9)cis PC];
1-palmitoyl-2-linoleoyl-sn-glycero-3-PC [16:0/18:2(n-6)cis PC];
1-stearoyl-2-linoleoyl-sn-glycero-3-PC [18:0/18:2(n-6)cis PC];
1-palmitoyl-2-linolenoyl-sn-glycero-3-PC [16:0/18:3(n-3)cis PC];
1-stearoyl-2-linolenoyl-sn-glycero-3-PC [18:0/18:3(n-3)cis PC];
1-palmitoyl-2-octadecatetraenoyl-sn-glycero-3-PC [16:0/18:4(n-3)cis PC];
1-stearoyl-2-octadecatetraenoyl-sn-glycero-3-PC [18:0/18:4(n-3)cis PC];
1-palmitoyl-2-octadecapentaenoyl-sn-glycero-3-PC [16:0/18:5(n-3)cis PC];
1-stearoyl-2-octadecapentaenoyl-sn-glycero-3-PC [18:0/18:5(n-3)cis PC];
1-palmitoyl-2-arahidonoyl-sn-glycero-3-PC [16:0/20:4(n-6)cis PC];
1-stearoyl-2-arahidonoyl-sn-glycero-3-PC [18:0/20:4(n-6)cis PC];
1-palmitoyl-2-eicosapentaenoyl-sn-glycero-3-PC [16:0/20:5(n-3)cis PC];
1-stearoyl-2-eicosapentaenoyl-sn-glycero-3-PC [18:0/20:5(n-3)cis PC];
1-palmitoyl-2-docosahexaenoyl-sn-glycero-3-PC [16:0/22:6(n-3)cis PC];
1-stearoyl-2-docosahexaenoyl-sn-glycero-3-PC [18:0/22:6(n-3)cis PC].
The choice of the lipid set was motivated by the following considerations: (1) the sixteen bilayers listed contain the most important, biologically meaningful types of PU lipids (see “Introduction” section); (2) An inspection of these bilayers under the same conditions allows one to study:
the ‘double bond number dependence’ (‘d dependence’) of an sn-2 chain of lipid properties over the whole possible range of d from 1 to 5 at fixed in a sequence of 18:1, 18:2, 18:3, 18:4 and 18:5 chains with methylene-interrupted double bonds, all other factors being equal;
the effect of sn-1 chain elongation (from 16 to 18 carbons) on the lipid properties for different fixed sn-2 chains, all other factors being equal;
the effect of sn-2 chain elongation (from 18 to 20 carbons) on the lipid properties for different fixed sn-1 chains and fixed double bond number or 5 of sn-2 chain, all other factors being equal.
The presence of the acyl chain 16:0 in the 8 bilayers 16:0/...PC and acyl chain 18:0 in the 8 bilayers 18:0/...PC is sufficient to study geometrical properties of acyls 16:0 and 18:0 by MD simulation. End-to-end distances (between carbons) calculated for each saturated acyl were averaged over eight corresponding PC bilayers; the influence of the position (sn-1 instead of sn-2) of these chains on their end-to-end distances was neglected.
Experimental melting temperatures of the bilayers were checked to make sure that the temperature K in the MD simulations was appropriate. Available published experimental temperatures for the lamellar gel to liquid-crystalline phase transition of five of eight studied mixed-chain PCs with 16:0 chains in the sn-1 position are gathered in Tables 2 and 3, and six of eight simulated PCs with 18:0 sn-1-chains are gathered in Tables 4 and 5. To the authors’ knowledge, no experimental investigations of the melting temperatures of 16:0/18:4(n-3)cis PC, 16:0/18:5(n-3)cis PC, 16:0/20:5(n-3)cis PC, 18:0/18:4(n-3)cis PC or 18:0/18:5(n-3)cis PC have been published.
Table 2.
Published temperatures of the experimental lamellar gel to liquid-crystalline phase transition of phosphatidylcholines 16:0/18:1(n-9)cis PC
| Lipid | (K) | Method | Refs. |
|---|---|---|---|
| 16:0/18:1(n-9)cis PC | 266.15a | Raman spectr. | Lavialle and Levin (1980) |
| 268.15 | DSC | Kruyff et al. (1973) | |
| 268.15 | 2H NMR | Waespe-Sarcevic (1978 | |
| Perly et al. (1985) and Ghosh (1988) | |||
| 268.15 | SANS | Winter and Pilgrim (1989) | |
| 268.55 ± 0.07b | DSC | Tada et al. (2009) | |
| 268.55 | DSC | Tada et al. (2010) | |
| 268.65 | DSC | Santaren et al. (1982) | |
| 270.15 | DSC | Davis et al. (1980) and Dekker et al. (1983) | |
| Curatolo et al. (1985) | |||
| Lynch and Steponkus (1989) | |||
| 270.55 ± 0.2 | DSC | Davis et al. (1981) and Keough (1986) | |
| 270.65 | DSC | Swaney (1980) | |
| 270.65a | Raman spectr. | Lavialle and Levin (1980) | |
| 270.65 | Raman spectr. | Litman et al. (1991) | |
| 270.65 ± 0.2 | DSC | Hernandez-Borrell and Keough (1993) | |
| 271.15 | DSC | Curatolo (1985) and Curatolo (1986) | |
| 271.55 | DSC | Ichimori et al. (1999) | |
| 271.65c | Raman spectr. | Lavialle and Levin (1980) | |
| 272.95 ± 0.77 | DSC | Bryant et al. (1992) | |
| 276.15 | Hydrol. meas. | Kamp et al. (1975) | |
| 270.65 ± 2.4 | lipid database | Koynova and Caffrey (1998) |
DSC differential scanning calorimetry, H NMR deuterium nuclear magnetic resonance, SANS small-angle neutron scattering, Hydrol. meas. hydrolysis measure. is the temperature averaged over the gel to liquid-crystalline and liquid-crystalline to gel phase transition temperatures, i.e., the heating and cooling transition temperatures, in all cases when a hysteresis was observed. The values for each lipid are presented in order of increasing temperature; values from the lipid database (Koynova and Caffrey 1998) are also presented in the end of the experimental data list.
aThe existence of metastable forms for the pure 16:0/18:1(n-9)cis PC liposomes was detected in Ref. Lavialle and Levin (1980). The / peak height intensity ratio as index was used (interchain disorder-order parameter), where and represent the peak height intensities for 2940- and 2885- transitions.
bAqueous 50 wt% ethylene glycol solution.
cThe / peak height intensity ratio as index was used (intramolecular gauche-trans isomerization parameter), where and represent the peak height intensities for 1100- and 1130- transitions, respectively
Table 3.
Published temperatures of the experimental lamellar gel to liquid-crystalline phase transition of mixed-chain phosphatidylcholines containing an sn-1 palmitoyl (16:0) chain and various sn-2 unsaturated fatty acid chains
| Lipid | (K) | Method | Refs. |
|---|---|---|---|
| 16:0/18:2(n-6)cis PC | 253.15 | DSC | Lynch and Steponkus (1989) |
| 253.65 | DSC | Keough et al. (1989) | |
| 254.45 | DSC | Hernandez-Borrell and Keough (1993) | |
| Lipid database | Koynova and Caffrey (1998) | ||
| 16:0/18:3(n-3)cis PC | H NMR | McCabe et al. (1994) | |
| 16:0/20:4(n-6)cis PC | 250.65 | Raman spectr. | Litman et al. (1991) |
| DSC | Hernandez-Borrell and Keough (1993) | ||
| 250.65 | Lipid database | Koynova and Caffrey (1998) | |
| 16:0/22:6(n-3)cis PC | 261.85 | DSC | Hernandez-Borrell and Keough (1993) |
| H NMR | Barry et al. (1991) | ||
| 266.65 | H NMR | Deese et al. (1981) | |
| 270.15 | Raman spectr. | Litman et al. (1991) | |
| 270.15 | Lipid database | Koynova and Caffrey (1998) |
For abbreviations, see footnote in Table 2
Table 4.
Published temperatures of the experimental lamellar gel to liquid-crystalline phase transition of phosphatidylcholines 16:0/18:1(n-9)cis PC
| Lipid | (K) | Method | Refs. |
|---|---|---|---|
| 18:0/18:1(n-9)cis PC | DSC | Niebylski and Jr. (1994) | |
| 275.15 | Fluor. anis. | Vincent et al. (1985) | |
| 276.15 | DSC | Phillips et al. (1972) | |
| DSC | Sánchez-Migallón et al. (1996) | ||
| H NMR | Holte et al. (1995) | ||
| a | DSC | Tada et al. (2009) | |
| 278.20 | DSC | Lüscher-Mattli (1989) | |
| 278.45 | DSC | Cunningham et al. (1989) | |
| 278.75 | DSC | Wang et al. (1995b, 1995a) and Huang et al. (1996) | |
| 278.85 | DSC | Inoue et al. (1999) | |
| 279.05 | DSC | Stillwell et al. (2000) | |
| 279.15 | DSC | Vilchéze et al. (1996) | |
| 279.35 | DSC | Surewicz and Epand (1986) | |
| DSC | Davis et al. (1980, 1981) and Keough (1986) | ||
| 279.55 | DSC | Davis et al. (1986) | |
| 279.75 | DSC | Boggs and Tümmler (1993) | |
| 279.85 | DSC | Kaneshina et al. (1998) and Ichimori et al. (1999) | |
| Broniec et al. (2009) and Tada et al. (2010) | |||
| b | DSC | Tada et al. (2009) | |
| 280.55 | DSC | Davis and Keough (1983) | |
| 281.35 | DSC | Dai et al. (1991) | |
| 286.15 | Hydrol. meas. | Kamp et al. (1975) | |
| Lipid database | Koynova and Caffrey (1998) |
For abbreviations, see footnote in Table 2; fluor. anis. = fluorescence anisotropy
a Aqueous 50 wt% ethylene glycol solution
b Solvent: water
Table 5.
Published temperatures of the experimental lamellar gel to liquid-crystalline phase transition of mixed-chain phosphatidylcholines containing an sn-1 stearoyl (18:0) chain and various sn-2 unsaturated fatty acid chains
| Lipid | (K) | Method | Ref. |
|---|---|---|---|
| 18:0/18:2(n-6)cis PC | DSC | Sánchez-Migallón et al. (1996) | |
| 256.65 | DSC | Coolbear and Keough (1983) | |
| DSC | Coolbear et al. (1983) | ||
| DSC | Niebylski and Jr. (1994) | ||
| H NMR | Holte et al. (1995) | ||
| 258.15 | DSC | Keough and Parsons (1990) and Tada et al. (2010) | |
| 259.45 | DSC | Keough et al. (1989) | |
| Lipid database | Koynova and Caffrey (1998) | ||
| 18:0/18:3(n-3)cis PC | DSC | Sánchez-Migallón et al. (1996) | |
| 260.15 | DSC | Coolbear et al. (1983) | |
| DSC | Niebylski and Jr. (1994) | ||
| 261.15 | DSC | Coolbear and Keough (1983) | |
| H NMR | Holte et al. (1995) | ||
| Lipid database | Koynova and Caffrey (1998) | ||
| 18:0/20:4(n-6)cis PC | DSC | Sánchez-Migallón et al. (1996) | |
| DSC | Niebylski and Jr. (1994) | ||
| H NMR | Holte et al. (1995) | ||
| 260.15 | DSC | Ichimori et al. (1999) and Tada et al. (2010) | |
| DSC | Coolbear et al. (1983) | ||
| Lipid database | Koynova and Caffrey (1998) | ||
| 18:0/20:5(n-3)cis PC | H NMR | Holte et al. (1995) | |
| DSC | Niebylski and Jr. (1994) | ||
| Lipid database | Koynova and Caffrey (1998) | ||
| 18:0/22:6(n-3)cis PC | 263.95 | DSC | Dumaual et al. (2000) and Stillwell et al. (2000) |
| 264.15 | DSC | Tada et al. (2010) | |
| H NMR | Holte et al. (1995) | ||
| 265.95 | DSC | Ichimori et al. (1999) | |
| DSC | Niebylski and Jr. (1994) | ||
| H NMR | Barry et al. (1991) | ||
| Lipid database | Koynova and Caffrey (1998) |
For abbreviations, see footnote in Table 2
It is seen that the temperature K of the MD computer simulations is higher than experimental gel to liquid-crystalline phase transition temperatures of all the bilayers in Tables 2, 3, 4 and 5. In spite of the fact that values of several lipids, 16:0/18:4(n-3)cis PC, 16:0/18:5(n-3)cis PC, 16:0/20:5(n-3)cis PC, 18:0/18:4(n-3)cis PC and 16:0/18:5(n-3)cis PC, are unknown, there is good reason to believe from the analysis of the noted Tables that missing values of are also less than K. This temperature is acceptable also for MC simulations of hydrocarbon chains because the main phase transition temperature of octadecane (alk-18:0) is 301.2 K and that of hexadecane (alk-16:0) is 291.2 K (Dirand et al. 2002), and phase transition temperatures of unsaturated (alkene) chains are substantially lower than those of n-alkanes.
A description of the MD simulations technique of PC bilayers was presented in a previous paper (Rabinovich and Lyubartsev 2014). The simulation boxes contained 128 PC molecules of one of 16 studied types per bilayer (64 lipids in each leaflet) and 30 HO molecules per lipid that corresponds to a condition of full hydration (overall 3840 water molecules). The two hydrocarbon tails, the glycerol section and the head group of the lipid molecules were treated in accordance with their known chemical structure, all hydrogen atoms were explicitly included in the computations.
In the starting configuration, the lipids were set parallel to each other, organized in a regular manner in two layers, and water molecules were distributed outside the bilayer. The system was put into a rectangular periodic cell, with the Z axis parallel to the bilayer normal. The size of the box was varied during the simulations under a semianisotropic NPT-ensemble with two degrees of freedom: one in the Z direction and another in the XY direction, so that the box sizes in X and Y direction were equal at each time moment.
To calculate the energy of the lipid molecules in the course of MD simulations, the CHARMM27 force field parameter set (Feller and MacKerell, Jr. 2000) with modifications described in a previous paper (Högberg et al. 2008) was used. It was demonstrated in a number of publications that the original CHARMM27 force field has some disagreements with the experiment (Benz et al. 2005), especially in the tensionless isothermal-isobaric (NPT) ensemble simulations (Hyvönen and Kovanen 2005; Sonne et al. 2007). CHARMM27 force field was recommended to apply only with a fixed surface area; otherwise the simulated bilayer tends to form a gel-like state (Feller et al. 2002; Koubi et al. 2003; Jensen et al. 2004; Siu et al. 2008). For bilayers composed of 14:0/14:0 PC lipids, modifications introduced previously (Högberg et al. 2008) provided perfect agreement with experimental data for the area per lipid, as well as with the X-ray structure factor and NMR order parameters. For lipids considered in this work, we used the same partial charges as in Högberg et al. 2008, the lipid head group including esters (these charges were recalculated on the bases of ab-initio computations), while for tails, we used charges adopted from the original CHARMM27 force field (Feller and MacKerell, Jr. 2000), with scaling of 1–4 electrostatic interactions by factor 0.83 (Högberg et al. 2008). All intramolecular bond and angle parameters, as well as Lennard-Jones interactions, were also borrowed from the original CHARMM27 force field (Feller and MacKerell, Jr. 2000). Water molecules were described by the flexible SPC model (Toukan and Rahman 1985). Use of this water model, in connection with the modified CHARMM27 force field, was verified previously (Högberg et al. 2008).
The double time step algorithm (Tuckerman et al. 1992) was used to treat separately fast forces (covalent bonds, angles, torsions, collision Lennard-Jones forces within 5 Å distance) with time step 0.25 fs, and longer range forces with time step 2.5 fs. The long-range electrostatic interactions were treated by the Ewald summation method (Allen and Tildesley 1987). The reciprocal part of the Ewald sum was cut at the condition that the remaining terms do not contribute more than 0.0001 of the total value. The parameter of the Ewald sum was set to , and cut-off distance Å was optimized for computational performance according to Ref. (Fincham 1994). The dispersion correction from the Lennard-Jones interactions outside the cut-off distance was included in the pressure (Allen and Tildesley 1987).
The systems were firstly simulated 1 ns under constant volume and then 1 ns under constant pressure and isotropic cell fluctuations. The obtained configurations were considered as starting points for longer simulations with independent cell fluctuations in Z and XY directions. The time reversible Nose-Hoover constant-temperature–constant-pressure algorithm (Martyna et al. 1996) was implemented, with the thermostat and barostat relaxation time set to 30 fs and 1 ps, respectively.
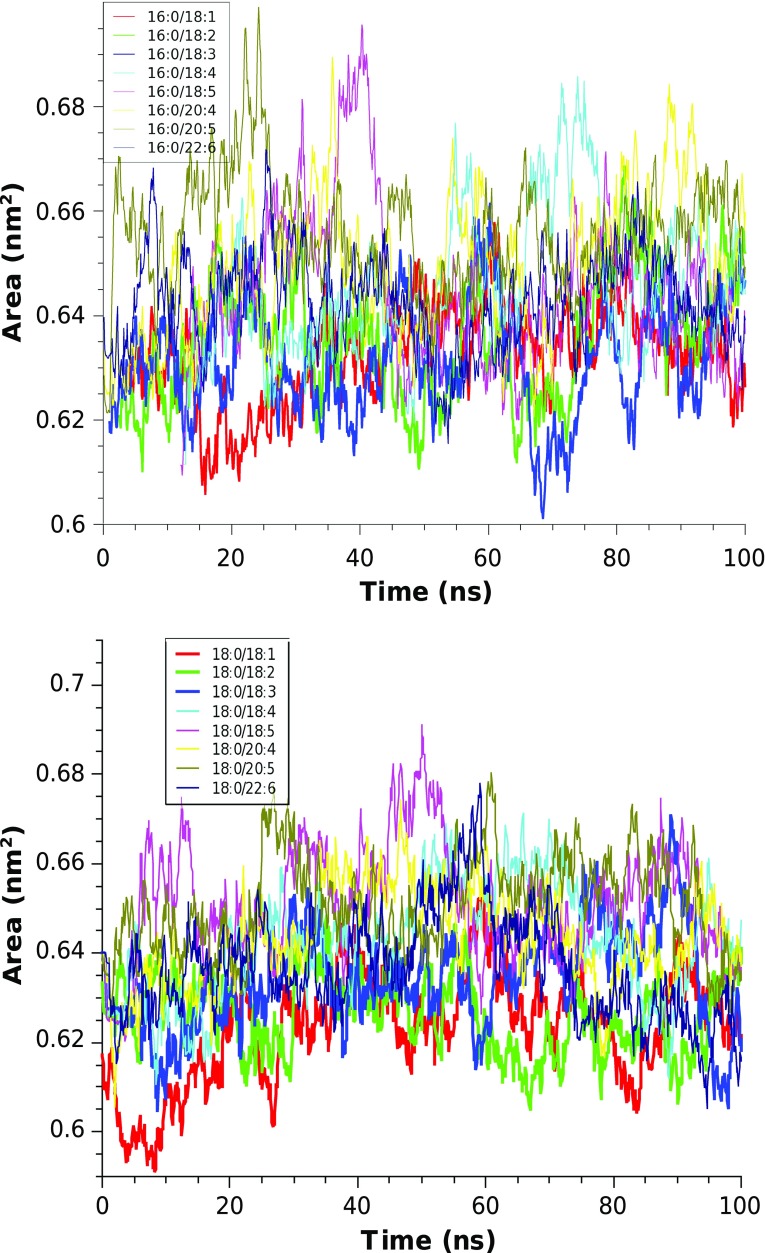
All the PC bilayers were simulated for a total of 100 ns. One of the most fundamental properties of a lipid bilayer and one of the most common ways to determine whether the bilayer system has reached equilibrium is area per lipid . When the area per lipid reaches a stable value, other structural properties (density distributions, NMR order parameters) do not show noticeable trends either. In the present work, area per lipid was calculated as the cross-sectional area of the simulation box divided by the number of lipids per monolayer. The time evolution of the values of the each of 16 PC bilayers are shown in Figs. 3a, b.
Fig. 3.
Time evolution of the average area per lipid of the PC bilayers with sn-1 chain 16:0 (a) and 18:0 (b)
From the observation of the different time evolution traces and calculation of block averages, we concluded that 20 ns of equilibration time is enough for all the bilayer systems considered. Therefore, the first 20 ns of the simulations (from 100 ns) were disregarded in further analysis. Atomic coordinates were saved each 1 ps in the trajectories.
The average areas calculated as a result of our simulations are presented in Table 6.
Table 6.
Average areas per lipid, , and relative fluctuations of the areas obtained for mixed-chain liquid-crystalline phase unsaturated phosphatidylcholine bilayers by MD simulations of the present work; K
| Lipid | ( )a, | Rel. fluct. of areaa |
|---|---|---|
| 16:0/18:1(n-9)cis PC | 0.634 (±0.002) | 0.01436 |
| 16:0/18:2(n-6)cis PC | 0.636 (±0.003) | 0.01839 |
| 16:0/18:3(n-3)cis PC | 0.632 (±0.002) | 0.01835 |
| 16:0/18:4(n-3)cis PC | 0.647 (±0.003) | 0.02087 |
| 16:0/18:5(n-3)cis PC | 0.646 (±0.004) | 0.02369 |
| 16:0/20:4(n-6)cis PC | 0.652 (±0.003) | 0.01932 |
| 16:0/20:5(n-3)cis PC | 0.656 (±0.003) | 0.01602 |
| 16:0/22:6(n-3)cis PC | 0.643 (±0.002) | 0.01446 |
| 18:0/18:1(n-9)cis PC | 0.627 (±0.002) | 0.01515 |
| 18:0/18:2(n-6)cis PC | 0.625 (±0.002) | 0.01503 |
| 18:0/18:3(n-3)cis PC | 0.637 (±0.002) | 0.01790 |
| 18:0/18:4(n-3)cis PC | 0.646 (±0.003) | 0.01702 |
| 18:0/18:5(n-3)cis PC | 0.653 (±0.003) | 0.01915 |
| 18:0/20:4(n-6)cis PC | 0.646 (±0.003) | 0.01516 |
| 18:0/20:5(n-3)cis PC | 0.653 (±0.002) | 0.01685 |
| 18:0/22:6(n-3)cis PC | 0.637 (±0.004) | 0.02024 |
a Statistical error for 20–100 ns is evaluated from the variance of 10-ns block averages
b /
It is possible to compare our data with the available published (experimental) data: in Table 7, available experimental average lipid areas for bilayers formed by PC lipids with one fully saturated and one unsaturated chain are collected. It is seen that our results are in good agreement with the experimentally deduced data.
Table 7.
Published experimental average areas per lipid, , of liquid-crystalline phase mixed-chain PC bilayers (aestimated from a plot)
| Lipid | T (K) | () | Method | Refs. |
|---|---|---|---|---|
| 16:0/18:1(n-9)cis PC | 275 | X-ray scattering | Pabst et al. (2000) | |
| 293 | X-ray scattering | Kučerka et al. (2011) | ||
| 297 | 0.63 | Langmuir film balance | Smaby et al. (1997) | |
| 298 | Isopiestic method | Klose et al. (1992) | ||
| Köenig et al. (1997a) | ||||
| 298 | Fluoresc. reson. energy transf. | Lantzsch et al. (1994) and Lantzsch et al. (1996) | ||
| 298 | X-ray diffraction | König (1992) | ||
| Lantzsch et al. (1996) | ||||
| 301 | H NMR | Leftin et al. (2014) | ||
| 301 | C NMR | Leftin et al. (2014) | ||
| 303 | X-ray scattering | Kučerka et al. (2011) | ||
| 303 | X-ray scattering | Kučerka et al. (2005) | ||
| 310 | 0.66 | Surface-pressure measur. | Hyslop et al. (1990) | |
| 310 | Small-angle X-ray diffraction | Jerabek et al. (2010) | ||
| 321 | H NMR | Leftin et al. (2014) | ||
| 321 | C NMR | Leftin et al. (2014) | ||
| 323 | X-ray scattering | Pabst et al. (2000) | ||
| 323 | X-ray scattering | Kučerka et al. (2011) | ||
| 16:0/18:2(n-6)cis PC | 297 | 0.66 | Langmuir film balance | Smaby et al. (1997) |
| 16:0/20:4(n-6)cis PC | 297 | 0.68 | Langmuir film balance | Smaby et al. (1997) |
| 16:0/22:6(n-3)cis PC | 297 | 0.70 | Langmuir film balance | Smaby et al. (1997) |
| 18:0/18:1(n-9)cis PC | 293 | X-ray scattering | Kučerka et al. (2011) | |
| 303 | H NMR and X-ray | Köenig et al. (1997b) | ||
| 303 | 0.643 | ‘Compressibility’ method | Rand and Parsegian (1989) | |
| 303 | X-ray scattering | Kučerka et al. (2011) | ||
| 303 | 0.66 | Osmotic pressure technique | Rand et al. (1988) | |
| 303 | 0.66 | Gravimetric method | Rand and Parsegian (1989) | |
| 303 | 0.666 | H and P NMR | Separovich and Gawrisch (1996) | |
| Gawrisch and Holte (1996) | ||||
| 303 | 0.67a | Low-angle X-ray scattering | Pan et al. (2009) | |
| 303 | 0.71a | Wide-angle X-ray scattering | Pan et al. (2009) | |
| 323 | 0.681±0.014 | X-ray scattering | Kučerka et al. (2011) | |
| 18:0/18:2(n-6)cis PC | 303 | 0.673 | H and P NMR | Separovich and Gawrisch (1996) |
| Gawrisch and Holte (1996) | ||||
| 18:0/18:3(n-3)cis PC | 303 | 0.666 | H and P NMR | Separovich and Gawrisch (1996) |
| Gawrisch and Holte (1996) | ||||
| 18:0/20:4(n-6)cis PC | 303 | 0.706 | H and P NMR | Separovich and Gawrisch (1996) |
| Gawrisch and Holte (1996) | ||||
| 18:0/20:5(n-3)cis PC | 303 | 0.691 | H and P NMR | Separovich and Gawrisch (1996) |
| Gawrisch and Holte (1996) | ||||
| 18:0/22:6(n-3)cis PC | 303 | X-ray diffraction | Eldho et al. (2003) | |
| 303 | H NMR and X-ray | Köenig et al. (1997b) | ||
| 303 | 0.716 | H and P NMR | Separovich and Gawrisch (1996) | |
| Gawrisch and Holte (1996) |
Monte Carlo simulations
According to concepts developed by Flory (1969), the interpretation of the spatial configuration of a linear chain molecule dispersed in a dilute solution can be resolved into two parts: short-range and long-range interactions. (1) The short-range interactions of the chain are determined by the geometrical parameters (bond lengths and bond angles), together with the potentials affecting rotation about bonds, including the effects of steric interactions between atoms and groups which are near neighbors in sequence along the chain; in other words, the short-range effects are determined by interactions between groups separated by only a few bonds. (2) The long-range interactions are dominated by interactions involving pairs of atoms and groups which are remote in the chain sequence, though near to one another in space when involved in mutual interactions; to put it differently, the long-range interactions are determined by interactions between pairs which are separated by many bonds (Flory 1969).
The long-range interactions introduce alterations (perturbation) in the chain configuration obtained when only the short-range interactions are considered. It is important to note that the long-range effect depends not only on the actual volume of the chain group (fragment, unit) but also on its interaction with the solvent; it is reasonable, therefore, to discuss the effective covolume. The covolume for the chain group can be enhanced by use of a ‘good’ solvent for the chain. It may also be diminished by choice of a ‘poor’ one barely capable of dissolving the chain. Through judicious selection of solvent and temperature, the finite volume of the group can be compensated exactly by the mutual attractions between chain groups when immersed in the poor solvent (Flory 1969). This state was called the ‘Theta () point’; as this takes place, the perturbation of the chain configuration must vanish and the chain become unperturbed (Flory 1969). Further, according to the prediction by Flory, in the bulk amorphous state, perturbation of the chain configuration must vanish. Neutron scattering experiments were later carried out (Dettenmaier 1978; Yoon and Flory 1978), and the results substantiated this prediction.
The ‘unperturbed’ state of chain molecules was used as the uniform state of the different hydrocarbon chains in this work. From the mathematical point of view, to reach this state, the long-range interactions should be excluded.
Thus, the MC simulations were performed here for unperturbed hydrocarbon chains, in which only intramolecular interactions between near neighbours along the chain were included. Let U be the conformational energy of a chain in the unperturbed state, i.e., the short-range interaction energy. The equilibrium properties of all chains were calculated using the classical flexible model (Gö and Scheraga 1976). The average value of the physically observable of an unperturbed molecule in the canonical ensemble for this model is given by
| 1 |
where N is the number of carbons of the main chain; —the Boltzmann constant; T—temperature; and are torsion angles of the main chain. Here, in (1) and below in the MC procedure, we keep bond lengths and bond angles equal to their equilibrium values which correspond to the chosen force field parameters; the torsions for all double bonds arranged cis were also fixed at the equilibrium value.
Assume we generate angles with probability density . Then, an assessment of the value by the MC method (Gould et al. 2007; Landau and Binder 2009; Binder and Heermann 2010; Satoh 2011) is
| 2 |
Here, is the sample size and is the number of the current conformation. The value from expression (2) converges to the value from expression (1).
In previous work (Zhurkin and Rabinovich 2015), an important sampling technique was developed for the efficient generation of chain conformations, with continuous variation of all single C–C bond torsions within [0, ] range. The conformations were generated using the probability density
| 3 |
To calculate the conformational energy U of a hydrocarbon chain in the unperturbed state (the short-range interactions energy), a scheme of interdependence of each of three torsions along the chain was taken into account in our work. The energy U was calculated as the sum of energies of structural units (e.g., = for a saturated chain with N carbons):
| 4 |
where are torsion angles, and m is the structural unit type. The units reproduced precisely the structure of various chain fragments.
The energy U is arranged [according to (4)] in such a way that it is possible to calculate energy of any m unit at the preliminary step, before MC simulation of the chain. To calculate the energies of all units and chains as a whole, the same force field parameters as in the MD simulations were used (CHARMM27; Feller and MacKerell, Jr. 2000) with modifications performed in the paper (Högberg et al. 2008).
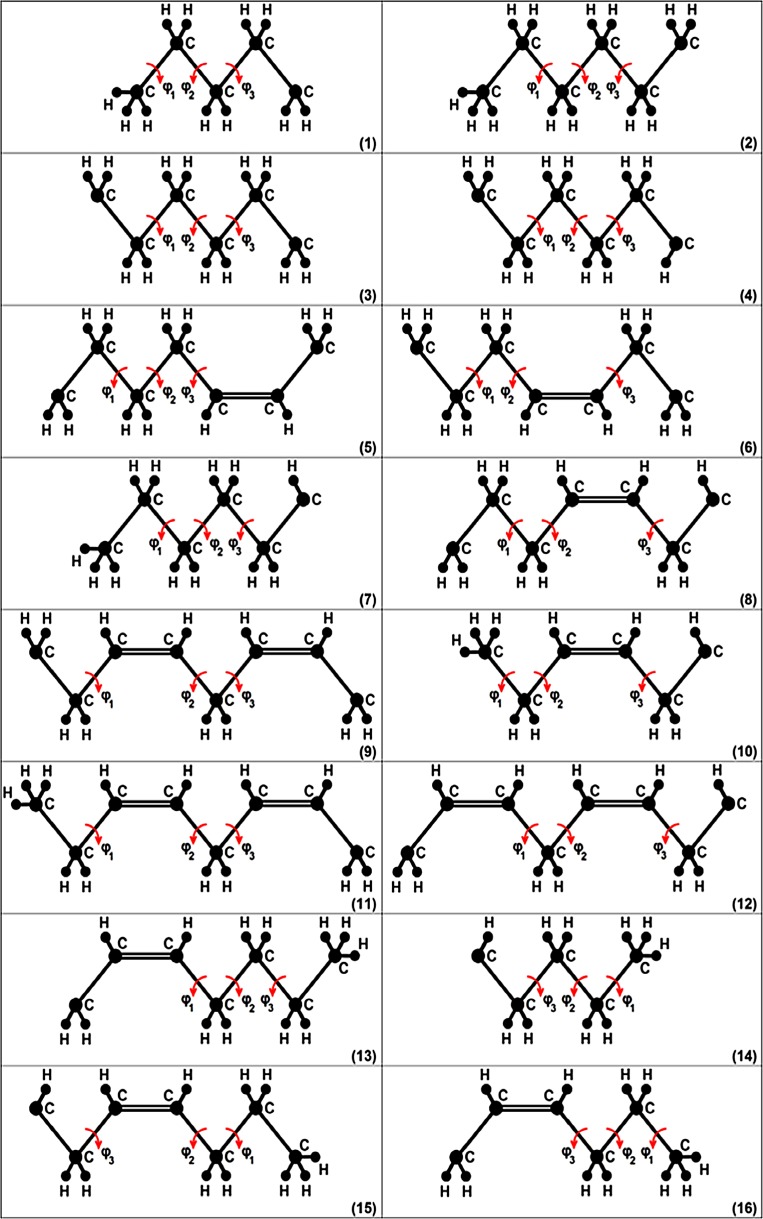
To construct a chain of the above-mentioned structure and then calculate the energy U according to expression (4), several of the 16 structural units presented in Fig. 4 should be properly combined. Three variable torsions in each unit in Fig. 4 are marked by red arrows. It is seen from expression (4) that the structural units in a chain should be connected in such a way that each two consecutive (neighboring) units have two mutually variable torsions.
Fig. 4.
Sixteen structural units; to construct a linear hydrocarbon (n-alkane or n-alkene) chain of the structure, e.g., as in Fig. 2, that is typical for the biomembrane phospholipid chain structure (Fig. 1), and calculate the energy U according to expression (4), several of the presented units should be properly combined. Three variable torsions in each unit are marked by red arrows. The number at the bottom right of the unit is the unit’s number
When calculating of each structural unit, the torsion energy, non-bonded energy and electrostatic energy from the force field were considered; bond lengths and bond angles were fixed at equilibrium values. Therefore bond length energy and bond angle energy were constant. In the calculations, multipliers of 1/2 and 1/3 were used for some energy items to exclude the possibility of double (or triple) summation of any energy items in the final expression (4). The multiplier 1/2 was used for the energy items which are strictly dependent on two variable torsions, and 1/3 was used for items strictly dependent on one variable torsion. The energy items that are strictly dependent on the three variable torsions of the considered structural unit were accounted without multipliers (i.e., a multiplier was equal to 1). It should be recorded that, at this step, it is possible to calculate the short-range energy U according to (4) only approximately; nevertheless, it is quite sufficient for the next step of the algorithm. An exact calculation of the energy U of each conformation is carried out after it has been generated—see below: it will be denoted as in the final assessment of the value .
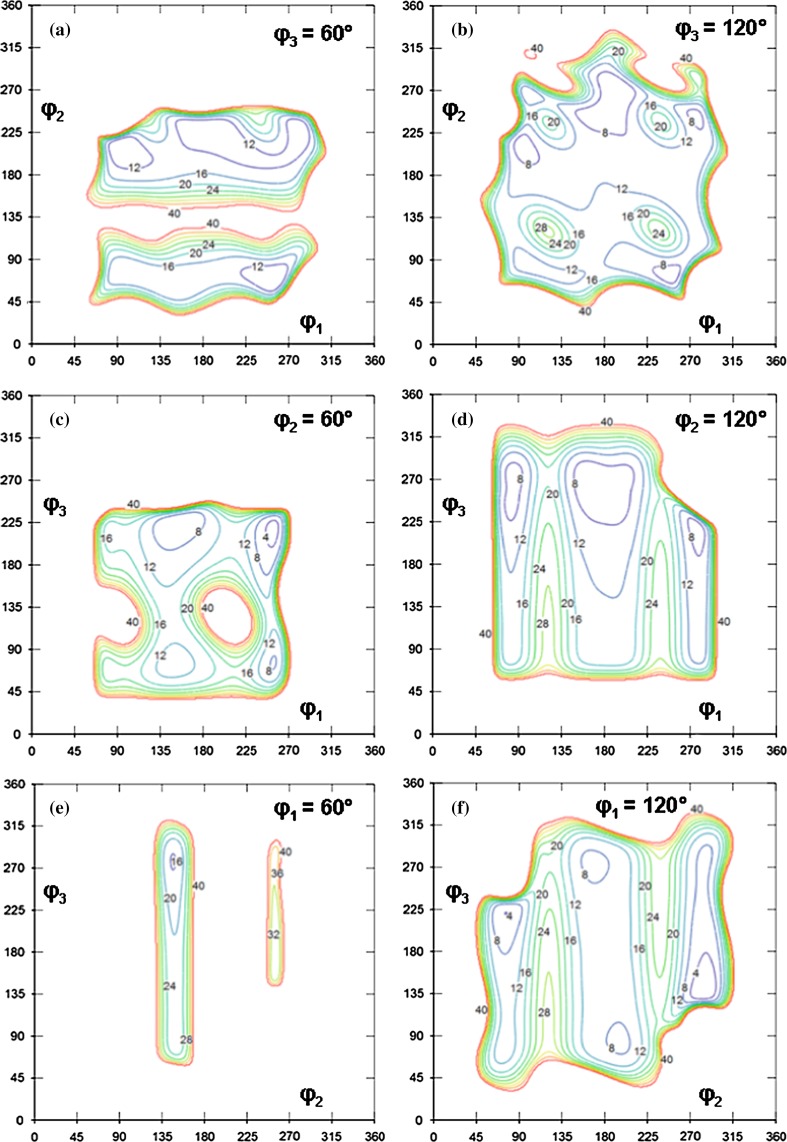
It is possible to demonstrate here an ’interdependence phenomenon’ of three consecutive variable torsions along the chain by the example of structural unit 9 from Fig. 4. Energy of unit 9 was calculated (tabulated) and presented in Fig. 5 in the form of six two-dimensional energy maps containing equienergy contours, i.e., lines connecting the points of equal energies.
Fig. 5.
Six two-dimensional energy maps of structural unit 9 from Fig. 4. The value 0 of any torsions (angles , and ) corresponds to the eclipsed conformation. The numbers near equienergetic contours are energies (kJ/mol). The energy of each map is measured from the global energy minimum of structural unit 9
The variants presented in Fig. 5 contain
Equienergy contours for pair at two fixed values of (, Fig. 5a, and , Fig. 5b),
Equienergy contours for pair at two fixed values of (, Fig. 5c, and , Fig. 5d),
Equienergy contours for pair at two fixed values of (, Fig. 5e, and , Fig. 5f).
Equienergy contours for a pair of torsions can, of course, be demonstrated at any fixed value of the third torsion angle (not only at 60 and 120). It is seen that map (a) of Fig. 5 is significantly different from map (b) of this figure; a large difference is observed for maps (c) and (d), maps (e) and (f), i.e., dependence of energy on torsion angles is pronounced (in other words, interdependence exists between the three torsions).
To generate the density (3) for a given chain, a special technique was developed. Let for simplicity; then, we can substitute U from (3) by expression (4) and rewrite expression (3) in a different form:
| 5 |
The energy of each structural unit m was tabulated with a step of . Then values under given T were calculated, and integrals
were computed numerically. Then, the configurational space of torsion angles of each chain’s unit m, where (i.e., a ‘cube’), was divided numerically into 100 = 1,000,000 parallelepipeds (’states’) in such a way that they all have equal Boltzmann realization probabilities under given T. As a result, boundaries between the ’states’ along three directions (torsions) in angle units were calculated; to do that, a special mathematical algorithm was developed. The idea of the algorithm is as follows. At first, values of boundaries along axis were calculated using a recurrent relationship, to divide the ‘cube’ onto 100 quadratic () strata (‘layers’) having equal realization probabilities and, as a consequence, different widths. Then, each stratum, using a recurrent relationship, was numerically divided along the second axis, , onto 100 ‘columns’ (‘rods’) in such a way that all ’columns’ have equal realization probabilities (and, because of this, different sizes); the values of boundaries between the ‘columns’ were obtained. Finally, each ‘column’ was similarly divided along the third axis, , onto 100 equiprobable parallelepipeds (‘states’) and, hence, all sizes (edge lengths in angle units) of the parallelepipeds are not equal to each other. It is evident that with this method of splitting, the boundaries of parallelepipeds of each structural unit m gather in the areas where energy minima, i.e. the number of states (parallelepipeds) in the energy minima is much more than those around the maxima.
The calculated boundaries between 1,000,000 equiprobable parallelepipeds for each chain’s unit m are then used in MC simulations of the different hydrocarbon chains. The current chain conformation (a set of values of torsions along the chain) is generated randomly by selection of the stratum, column and parallelepiped numbers and then by selection of exact values of the torsions inside the chosen parallelepipeds. In doing so, the proper sequences of structural units of the given chain and the torsion numbers along the chain are obeyed.
The final assessment of the value within the methodology can be obtained by
| 6 |
where
| 7 |
Here, , and are sizes (edge lengths in angle units) of a parallelepiped randomly chosen for the -th conformation of the chain in the molecular unit number of type m; , and are random numbers of the three edges of the chosen parallelepiped; is the short-range interactions energy of the generated -th chain conformation, properly calculated using all terms of the force field: all bond length and angle energies, all torsion (dihedral) angle energies, out-of-plane energies, Urey-Bradley terms, and non-bonded and electrostatic interactions energies for such pairs of atoms which are included into the sequence of structural units of the given chain. Since all values of the torsions in the generated conformation are already known, the short-range energy is calculated correctly [in contrast to the energy U calculated approximately from expression (4)].
Thus, in the assessment (6), the probability of generation of each chain conformation and probability of its realization are calculated, and hence we obtain . To calculate average characteristics, approximately 10 conformations of each chain were generated in the present work.
Results and discussion
Average distances between terminal carbon atoms of the chains (end-to-end distances) considered in the unperturbed state, , and those of PC lipid chains in liquid crystalline bilayers, , were calculated. The obtained data are presented in Table 8.
Table 8.
Average end-to-end distances, for unperturbed hydrocarbon chains and for the acyl chains in liquid crystalline phosphatidylcholine (PC) bilayers obtained by computer simulations of the present work; K
| Hydrocarbon chain, MC simulation |
()a, [nm] Unperturbed state MC simulation |
Acyl chain, MD simulation |
()b, [nm] 16:0/... PC bilayer MD simulation |
()b, [nm] 18:0/... PC bilayer MD simulation |
Relat. diff. expression (8)) , |
|---|---|---|---|---|---|
| alk-16:0 | 1.395 (±0.001) | 16:0 | 1.503 – 1.520c (±0.001) | 7.2 – 8.2e | |
| alk-18:0 | 1.528 (±0.001) | 18:0 | 1.665 – 1.699d (±0.001) | 8.3 – 10.1f | |
| alk-18:1 | 1.374 (±0.001) | 18:1 | 1.532 (±0.001) | 1.531 (±0.001) | 10.3; 10.3g |
| alk-18:2 | 1.293 (±0.001) | 18:2 | 1.478 (±0.001) | 1.491 (±0.001) | 12.5; 13.3g |
| alk-18:3 | 1.261 (±0.001) | 18:3 | 1.447 (±0.001) | 1.446 (±0.001) | 12.8; 12.8g |
| alk-18:4 | 1.194 (±0.003) | 18:4 | 1.398 (±0.001) | 1.402 (±0.001) | 14.6; 14.8g |
| alk-18:5 | 1.165 (±0.008) | 18:5 | 1.372 (±0.001) | 1.373 (±0.001) | 15.1; 15.1g |
| alk-20:4 | 1.290 (±0.002) | 20:4 | 1.544 (±0.001) | 1.538 (±0.001) | 16.5; 16.1g |
| alk-20:5 | 1.254 (±0.001) | 20:5 | 1.497 (±0.001) | 1.509 (±0.001) | 16.2; 16.9g |
| alk-22:6 | 1.328 (±0.005) | 22:6 | 1.624 (±0.001) | 1.645 (±0.001) | 18.2; 19.3g |
a stat. error evaluated from the variance of 10 conformations
b stat. error for 20–100 ns evaluated from the variance of 10 ns block averages
c range of for 16:0 acyl chain in eight mixed-chain 16:0/... PC bilayers
d range of for 18:0 acyl chain in eight mixed-chain 18:0/... PC bilayers
e range of values for 16:0 acyl chain in eight mixed-chain 16:0/... PC bilayers
f range of values for 18:0 acyl chain in eight mixed-chain 18:0/... PC bilayers
g values of for this unsaturated acyl chain in 16:0/... PC and 18:0/... PC bilayers, respectively
Table 8 shows that values are somewhat less than . This is because only one hydrocarbon chain terminus in the lipid molecule is free in space; the other one is chemically linked to the head group. Due to interactions of all lipid molecules with their neighbors and water molecules the lipids’ head groups are arranged in the vicinity to each other. Therefore, possibilities of rotations around several C–C bonds adjoining the head groups are more restricted than those for the C–C bonds of the opposite end of the chain, so the chain region near the head groups is more stretched, in contrast to the unperturbed chain in which both ends are free.
To compare the results quantitatively, the relative (in percentage) difference between values and for each chain was calculated:
| 8 |
The values of are also presented in Table 8. The calculations showed that the relative difference between both states increases as the number of carbons and/or number of double bonds in the chain increase; is approximately equal to 8–10% (or 9%) for saturated 16:0 and 18:0 chains; to 10–15% for unsaturated chains with N = 18, d = 1–5; to 16–17% for PU chains with N = 20, d = 4 – 5 and maximum 19% for 22:6(n-3)cis chain.
In this connection, a remark should be made. It is possible to divide interactions in the bilayers into three parts: intramolecular short-range, intramolecular long-range interactions (Flory 1969) of the chains, and intermolecular interactions between the chains and the neighboring chains (and PC head groups). Unperturbed hydrocarbon chain properties are fully defined by the short-range interaction energy (Flory 1969). Therefore, the value of can be considered as an assessment of the influence of the long-range interactions inside the chain and interactions with the neighboring chains and PC head groups of the lipid bilayer on the distance , compared with the influence of only short-range interactions inside the chain on the .
While conformations of the unperturbed chains are not the same as those in liquid crystalline bilayers, the relative difference between the end-to-end distances and of the considered typical acyls was found to be comparatively moderate; it is approximately equal to 9–19%.
Therefore, such properties of the listed FA chains of phospholipids in bilayers, as are significantly determined by the short-range interactions in the chains (indeed, it is determined approximately by 81–91%). There is good reason to believe that other geometrical properties of these chains are also determined mainly by their short-range interactions. We mention that the considered saturated and unsaturated chains are widespread, typical constituents of phospholipids, so the obtained result seems to be valuable for biomembranes. In other words, the value seems to be of the same order of magnitude for most of different membrane hydrocarbon chains with methylene-interrupted cis double bonds.
It should be also pointed out, that a relationship between and the number d of double bonds for the unperturbed hydrocarbon chains with constant number N of carbon atoms is the same as for acyl chains of PC molecules in bilayers: both values decrease as d increases (Table 8). In other words, the trends in changes of and in the order 18:0 18:1 18:2 18:3 18:4 18:5 are the same; the trends in the order 20:4 20:5 are also the same.
This decrease is obviously caused mainly by extension of the chain segment with double bonds, in which energy minima are wide and correspond to various collapsed chain conformations, and by shortening of chain’s saturated segments in which the main energy minimum corresponds to the extended chain conformations (Flory 1969).
It is possible to consider also the trends of both discussed values of in the order 18:4 20:4 and the trends in the order 18:5 20:5, i.e., relationships between , and number N of carbon atoms under constant number d of double bonds. Table 8 shows that they are similar to each other, respectively. The discussed trends are shown schematically in Fig. 6. Of course, more rigorous treatment requires consideration also of the value of the parameter j in all trends, i.e., the third structure parameter of the chain in the expression that means the positions of double bonds along the chain. In particular, j differs for the considered chains 18:4 and 20:4, 20:4 and 20:5, and for some chains with .
Fig. 6.
Average end-to-end distances , [nm], for unperturbed hydrocarbon chains obtained by Monte Carlo (MC) simulations, and , [nm], for the same acyl chains in liquid crystalline phosphatidylcholine (PC) bilayers obtained by molecular dynamics (MD) simulations (triangles for the marked chains in 18:0/... PC bilayers, circles for the marked chains in 16:0/... PC bilayers). The ranges for saturated acyl chains 16:0 and 18:0 are obtained for eight appropriate mixed-chain PC bilayers. Computer simulations of the present work, K. Arrows show qualitatively trends in and . To compare the obtained trends, the same names used here both for acyls (ordinate axis) and hydrocarbon chains (abscissa axis)
To our knowledge, a quantitative assessment of the difference between properties of the two chain states (in particular, the difference in values) was obtained for the first time in the present work, while a qualitative similarity of chain properties in the two states was discussed in the literature before. For instance, it has been concluded (Rabinovich et al. 2003) that the bond-order parameters and orientation distribution characteristics of the chains in the lipid monolayer and bilayer ‘liquid’ regions, as found in experiments and in MD computer simulation models (Rabinovich et al. 1999a, b, 2000; Rabinovich and Balabaev 2001), are qualitatively similar to the intramolecular order parameters and the intramolecular bond orientation distributions in single unperturbed (Flory 1969) unsaturated hydrocarbon chains previously investigated with MC simulations (Rabinovich and Ripatti 1999, 2000). Therefore, the behavior of the acyl chains in the liquid region of lipid bilayers (somewhat remote from the membrane–water interface) is dominated by the intramolecular short-range interactions. The long-range interactions of the segments of the lipids in this region of the bilayer and the interactions with the bilayer–water interface may be considered as a disturbance: the intermolecular interactions are largely used to orient the lipid molecules in the direction of the membrane normal.
Thus, from the two above-mentioned facts (about a comparatively moderate quantitative difference in the and values of the chains and the similarity of their trends), a common conclusion can be made: to treat and analyse a number of processes in biological membranes (e.g., changes in FA composition caused by the environmental changes such as temperature and pressure), it is possible to use, at least as the first approximation, the relationships between structure and properties obtained for the unperturbed hydrocarbon chains. This seems not unreasonable: biomembranes are known to contain a wide variety of FA chains; the available ‘bilayer’ relations between their structure and property are incomplete and insufficient for the analysis, whereas the properties of the unperturbed chains and corresponding ‘structure–property’ relationships have been already studied for tens of variants (see, e.g., Zhurkin and Rabinovich 2015).
Conclusions
The average characteristics of hydrocarbon chains calculated in the unperturbed state (which is fully defined by short-range interaction energies) make it possible to estimate the influence of additional energy components on the state of these chains, if they are under other conditions or located in other systems. For the same temperature and force field parameters, it is acceptable to use any characteristic as a criterion. The average end-to-end distance of the chains was chosen as such a criterion in the computer simulations carried out in this work. The relationships between structure and the average end-to-end distances obtained for the considered unperturbed chains were shown to be qualitatively similar to those of lipid chains in bilayers. Such data for the majority of possible lipid acyls in bilayers (as a rule, it is several tens of chains or more) are yet unknown because MD simulations of various lipid bilayers are very time-consuming. On this basis, it is reasonable to investigate the unperturbed hydrocarbon chains instead. As a first approximation of the desired ‘structure–property’ relationships for the lipid chains in bilayers, the corresponding relationships for the unperturbed chains can be used.
Acknowledgements
This work has been supported by the federal budget assignment no. 0221-2014-0033 of the Russian Federation (to AR and DZ), the Swedish Research Council (Vetenskapsrådet, grant 621-2013-4260, to AL), and EU FP7 MembraneNanoPart project (to AR, AL and DZ). The authors thank the Swedish National Infrastructure for Computing (SNIC) for granting computer facilities.
Contributor Information
Alexander L. Rabinovich, Email: rabinov@krc.karelia.ru
Alexander P. Lyubartsev, Email: alexander.lyubartsev@mmk.su.se
Dmitrii V. Zhurkin, Email: ima-2@mail.ru
References
- Allen MP, Tildesley DJ. Computer simulations of liquids. 2. Oxford: Clarendon; 1987. [Google Scholar]
- Baoukina S, Tieleman DP. Computer simulations of lung surfactant. Biochim Biophys Acta. 2016;1858(10):2431–2440. doi: 10.1016/j.bbamem.2016.02.030. [DOI] [PubMed] [Google Scholar]
- Barry JA, Trouard TP, Salmon A, Brown MF. Low-temperature H NMR spectroscopy of phospholipid bilayers containing docosahexaenoyl (22:6) chains. Biochemistry. 1991;30(34):8386–8394. doi: 10.1021/bi00098a016. [DOI] [PubMed] [Google Scholar]
- Bennett WFD, Tieleman DP. Computer simulations of lipid membrane domains. Biochim Biophys Acta. 2013;1828(8):1765–1776. doi: 10.1016/j.bbamem.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Benz RW, Castro-Román F, Tobias DJ, White SH. Experimental validation of molecular dynamics simulations of lipid bilayers: a new approach. Biophys J. 2005;88(2):805–817. doi: 10.1529/biophysj.104.046821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendsen HJC. Simulating the physical world: hierarchical modeling from quantum mechanics to fluid dynamics. Cambridge: Cambridge University Press; 2007. [Google Scholar]
- Bhaswant M, Poudyal H, Brown L. Mechanisms of enhanced insulin secretion and sensitivity with n-3 unsaturated fatty acids. J Nutr Biochem. 2015;26:571–584. doi: 10.1016/j.jnutbio.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Binder K, Heermann DW. Monte Carlo simulations in statistical physics. An introduction. Heidelberg: Springer; 2010. [Google Scholar]
- Boggs JM, Tümmler B. Interdigitated gel phase bilayers formed by unsaturated synthetic and bacterial glycerolipids in the presence of polymyxin B and glycerol. Biochim Biophys Acta. 1993;1145(1):42–50. doi: 10.1016/0005-2736(93)90379-E. [DOI] [PubMed] [Google Scholar]
- Broniec A, Goto M, Matsuki H (2009) A peculiar phase transition of plasmalogen bilayer membrane under high pressure. Langmuir 25(19):11,265–11,268 [DOI] [PubMed]
- Brooks S, Gelman A, Jones GL, Meng X-L. Handbook of Markov Chain Monte Carlo. London: Chapman and Hall/CRC; 2011. [Google Scholar]
- Bryant G, Pope JM, Wolfe J. Low hydration phase properties of phospholipid mixtures. Eur Biophys J. 1992;21(3):223–232. doi: 10.1007/BF00196767. [DOI] [Google Scholar]
- Bunker A, Magarkar A. Rational design of liposomal drug delivery systems, a review: combined experimental and computational studies of lipid membranes, liposomes and their pegylation. Biochim Biophys Acta. 2016;1858(10):2334–2352. doi: 10.1016/j.bbamem.2016.02.025. [DOI] [PubMed] [Google Scholar]
- Carrin ME, Carelli AA. Peanut oil: compositional data. Eur J Lipid Sci Technol. 2010;112:697–707. doi: 10.1002/ejlt.200900176. [DOI] [Google Scholar]
- Cook HW, McMaster CR. Fatty acid desaturation and chain elongation in eukaryotes. In: Vance DE, Vance JE, editors. New comprehensive biochemistry Biochemistry of Lipids, Lipoproteins and Membranes. 4. New York: Elsevier; 2002. pp. 181–204. [Google Scholar]
- Coolbear KP, Berde CB, Keough KMW. Gel to liquid-crystalline phase transitions of aqueous dispersions of polyunsaturated mixed-acid phosphatidylcholines. Biochemistry. 1983;22(6):1466–1473. doi: 10.1021/bi00275a022. [DOI] [PubMed] [Google Scholar]
- Coolbear KP, Keough KMW. Lipid oxidation and gel to liquid-crystalline transition temperatures of synthetic polyunsaturated mixed-acid phosphatidylcholines. Biochim Biophys Acta. 1983;732:531–540. doi: 10.1016/0005-2736(83)90229-8. [DOI] [PubMed] [Google Scholar]
- Cunningham BA, Tsujita T, Brockman H. Enzymatic and physical characterization of diacylglycerol-phosphatidylcholine interactions in bilayers and monolayers. Biochemistry. 1989;28(1):32–40. doi: 10.1021/bi00427a006. [DOI] [PubMed] [Google Scholar]
- Curatolo W. The effects of ethylene glycol and dimethyl sulfoxide on cerebroside metastability. Biochim Biophys Acta. 1985;817(1):134–138. doi: 10.1016/0005-2736(85)90075-6. [DOI] [PubMed] [Google Scholar]
- Curatolo W, Sears B, Neuringer L. A calorimetry and deuterium NMR study of mixed model membranes of 1-palmitoyl-2-oleylphosphatidylcholine and saturated phosphatidylcholines. Biochim Biophys Acta. 1985;817(2):261–270. doi: 10.1016/0005-2736(85)90027-6. [DOI] [PubMed] [Google Scholar]
- Curatolo W. The interactions of 1-palmitoyl-2-oleylphosphatidylcholine and bovine brain cerebroside. Biochim Biophys Acta. 1986;861:373–376. doi: 10.1016/0005-2736(86)90441-4. [DOI] [PubMed] [Google Scholar]
- Dai MC, Chiche HB, Düzgünes N, Ayanoglu E, Djerassi C. Phospholipid studies of marine organisms: 26. interactions of some marine sterols with 1-stearoyl-2-oleoyl phosphatidylcholine (SOPC) in model membranes. Chem Phys Lipids. 1991;59(3):245–253. doi: 10.1016/0009-3084(91)90024-6. [DOI] [PubMed] [Google Scholar]
- Davis PJ, Coolbear KP, Keough KMW. Differential scanning calorimetric studies of the thermotropic phase behaviour of membranes composed of dipalmitoyllecithin and mixed-acid unsaturated lecithins. Can J Biochem. 1980;58(10):851–858. doi: 10.1139/o80-118. [DOI] [PubMed] [Google Scholar]
- Davis PJ, Fleming BD, Coolbear KP, Keough KMW. Gel to liquid-crystalline transition temperatures of water dispersions of two pairs of positional isomers of unsaturated mixed-acid. Biochemistry. 1981;20(12):3633–3636. doi: 10.1021/bi00515a051. [DOI] [PubMed] [Google Scholar]
- Davis PJ, Kariel N, Keough KMW. Gel to liquid-crystalline transitions of aqueous dispersions of positional isomers of a heteroacid unsaturated phosphatidylcholine mixed with epicholesterol and cholesterol. Biochim Biophys Acta. 1986;856(2):395–398. doi: 10.1016/0005-2736(86)90052-0. [DOI] [Google Scholar]
- Davis PJ, Keough KMW. Differential scanning calorimetric studies of aqueous dispersions of mixtures of cholesterol with some mixed-acid and single-acid phosphatidylcholines. Biochemistry. 1983;22(26):6334–6340. doi: 10.1021/bi00295a045. [DOI] [Google Scholar]
- Deese AJ, Dratz EA, Dahlquist FW, Paddy MR. Interaction of rhodopsin with two unsaturated phosphatidylcholines: a deuterium nuclear magnetic resonance study. Biochemistry. 1981;20(22):6420–6427. doi: 10.1021/bi00525a021. [DOI] [PubMed] [Google Scholar]
- Dekker CJ, van Kessel WSMG, Klomp JPG, Pieters J, de Kruijff B. Synthesis and polymorphic phase behaviour of polyunsaturated phosphatidylcholines and phosphatidylethanolamines. Chem Phys Lipids. 1983;33(1):93–106. doi: 10.1016/0009-3084(83)90012-9. [DOI] [PubMed] [Google Scholar]
- D’Eliseo D, Velotti F. Omega-3 fatty acids and cancer cell cytotoxicity: implications for multi-targeted cancer therapy. J Clin Med. 2016;5(2):15. doi: 10.3390/jcm5020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettenmaier M. Conformation of n-alkane molecules in the melt and in cyclohexane solution studied by small-angle neutron scattering. J Chem Phys. 1978;68(5):2319–2322. doi: 10.1063/1.436001. [DOI] [Google Scholar]
- Dirand M, Bouroukba M, Briard AJ, Chevallier V, Petitjean D, Corriou JP. Temperatures and enthalpies of (solid + solid) and (solid + liquid) transitions of n-alkanes. J Chem Thermodyn. 2002;34:1255–1277. doi: 10.1006/jcht.2002.0978. [DOI] [Google Scholar]
- Diwakar BT, Dutta PK, Lokesh BR, Naidu KA. Physicochemical properties of garden cress (Lepidium sativum L.) seed oil. J Am Oil Chem Soc. 2010;87:539–548. doi: 10.1007/s11746-009-1523-z. [DOI] [Google Scholar]
- (2009) Docosahexaenoic acid (DHA). Altern Med Rev 14(4):391–399 [PubMed]
- Dumaual AC, Jenski LJ, Stillwell W. Liquid crystalline/gel state phase separation in docosahexaenoic acid-containing bilayers and monolayers. Biochim Biophys Acta. 2000;1463:395–406. doi: 10.1016/S0005-2736(99)00235-7. [DOI] [PubMed] [Google Scholar]
- Eldho NV, Feller SE, Tristram-Nagle S, Polozov IV, Gawrisch K. Polyunsaturated docosahexaenoic vs docosapentaenoic acid differences in lipid matrix properties from the loss of one double bond. J Am Chem Soc. 2003;125:6409–6421. doi: 10.1021/ja029029o. [DOI] [PubMed] [Google Scholar]
- Feller SE, Gawrisch K, MacKerell AD. Polyunsaturated fatty acids in lipid bilayers: intrinsic and environmental contributions to their unique physical properties. J Am Chem Soc. 2002;124:318–326. doi: 10.1021/ja0118340. [DOI] [PubMed] [Google Scholar]
- Feller SE, MacKerell AD., Jr An improved empirical potential energy function for molecular simulations of phospholipids. J Phys Chem B. 2000;104:7510–7515. doi: 10.1021/jp0007843. [DOI] [Google Scholar]
- Fincham D. Optimization of the Ewald sum for large systems. Mol Simul. 1994;13:1–9. doi: 10.1080/08927029408022180. [DOI] [Google Scholar]
- Flory PJ. Statistical mechanics of chain molecules. New York: Interscience; 1969. [Google Scholar]
- Gawrisch K, Holte LL. NMR investigations of non-lamellar phase promoters in the lamellar phase state. Chem Phys Lipids. 1996;81:105–116. doi: 10.1016/0009-3084(96)02576-5. [DOI] [Google Scholar]
- Ghosh R. P and H NMR studies of structure and motion in bilayers of phosphatidylcholine and phosphatidylethanolamine. Biochemistry. 1988;27(20):7750–7758. doi: 10.1021/bi00420a025. [DOI] [PubMed] [Google Scholar]
- Gö N, Scheraga HA. On the use of classical statistical mechanics in the treatment of polymer chain conformations. Macromolecules. 1976;9(4):535–542. doi: 10.1021/ma60052a001. [DOI] [Google Scholar]
- Gould H, Tobochnik J, Christian W. An introduction to computer simulation methods: applications to physical systems. San Francisco: Pearson; 2007. [Google Scholar]
- Guil-Guerrero JL. Stearidonic acid (18:4n–3): metabolism, nutritional importance, medical uses and natural sources. Eur J Lipid Sci Technol. 2007;109:1226–1236. doi: 10.1002/ejlt.200700207. [DOI] [Google Scholar]
- Hernandez-Borrell J, Keough KMW. Heteroacid phosphatidylcholines with different amounts of unsaturation respond differently to cholesterol. Biochim Biophys Acta. 1993;1153:277–282. doi: 10.1016/0005-2736(93)90416-W. [DOI] [PubMed] [Google Scholar]
- Högberg CJ, Nikitin AM, Lyubartsev AP. Modification of the CHARMM force field for DMPC lipid bilayer. J Comp Chem. 2008;29:2359–2369. doi: 10.1002/jcc.20974. [DOI] [PubMed] [Google Scholar]
- Holte LL, Peter SA, Sinnwell TM, Gawrisch K. H nuclear magnetic resonance order parameter profiles suggest a change of molecular shape for phosphatidylcholines containing a polyunsaturated acyl chain. Biophys J. 1995;68(6):2396–2403. doi: 10.1016/S0006-3495(95)80422-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Li S, Lin H, Wang G. On the bilayer phase transition temperatures for monoenoic phosphatidylcholines and phosphatidylethanolamines and the interconversion between them. Arch Biochem Biophys. 1996;334:135–142. doi: 10.1006/abbi.1996.0438. [DOI] [PubMed] [Google Scholar]
- Hyslop PA, Morel B, Sauerheber RD. Organization and interaction of cholesterol and phosphatidylcholine in model bilayer membranes. Biochemistry. 1990;29(4):1025–1038. doi: 10.1021/bi00456a027. [DOI] [PubMed] [Google Scholar]
- Hyvönen MT, Kovanen PT. Molecular dynamics simulations of unsaturated lipid bilayers: effects of varying the number of double bonds. Eur Biophys J. 2005;34:294–305. doi: 10.1007/s00249-004-0455-7. [DOI] [PubMed] [Google Scholar]
- Ichimori H, Hata T, Matsuki H, Kaneshina S. Effect of unsaturated acyl chains on the thermotropic and barotropic phase transitions of phospholipid bilayer membranes. Chem Phys Lipids. 1999;100:151–164. doi: 10.1016/S0009-3084(99)00050-X. [DOI] [Google Scholar]
- Inoue T, Kitahashi T, Nibu Y. Phase behavior of hydrated bilayer of binary phospholipid mixtures composed of 1,2-distearoylphosphatidylcholine and 1-stearoyl-2-oleoylphosphatidylcholine or 1-oleoyl-2-stearoylphosphatidylcholine. Chem Phys Lipids. 1999;99(1):103–109. doi: 10.1016/S0009-3084(99)00007-9. [DOI] [Google Scholar]
- Jensen MØ, Mouritsen OG, Peters GH. Simulations of a membrane-anchored peptide: structure, dynamics, and influence on bilayer properties. Biophys J. 2004;86:3556–3575. doi: 10.1529/biophysj.103.029140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerabek H, Pabst G, Rappolt M, Stockner T. Membrane-mediated effect on ion channels induced by the anesthetic drug ketamine. J Am Chem Soc. 2010;132(23):7990–7997. doi: 10.1021/ja910843d. [DOI] [PubMed] [Google Scholar]
- Kamp JAFOD, Kauerz MT, Deenen LLMV. Action of pancreatic phospholipase on phosphatidylcholine bilayers in different physical states. Biochim Biophys Acta. 1975;406(2):169–177. doi: 10.1016/0005-2736(75)90001-2. [DOI] [PubMed] [Google Scholar]
- Kaneshina S, Ichimori H, Hata T, Matsuki H. Barotropic phase transitions of dioleoylphosphatidylcholine and stearoyl-oleoylphosphatidylcholine bilayer membranes. Biochim Biophys Acta. 1998;1374:1–8. doi: 10.1016/S0005-2736(98)00122-9. [DOI] [PubMed] [Google Scholar]
- Keough KMW. Modifications of lipid structure and their influence on mesomorphism in model membranes: the influence of hydrocarbon chains. Biochem Cell Biol. 1986;64(1):44–49. doi: 10.1139/o86-007. [DOI] [PubMed] [Google Scholar]
- Keough KMW, Giffin B, Matthews PLJ. Phosphatidylcholine-cholesterol interactions: bilayers of heteroacid lipids containing linoleate lose calorimetric transitions at low cholesterol concentration. Biochim Biophys Acta Biomembr. 1989;983(1):51–55. doi: 10.1016/0005-2736(89)90379-9. [DOI] [PubMed] [Google Scholar]
- Keough KMW, Parsons CS. Differential scanning calorimetry of dispersions of products of oxidation of 1-stearoyl-2-linoleoyl-sn-glycero-3-phosphocholine. Biochem Cell Biol. 1990;68(1):300–307. doi: 10.1139/o90-041. [DOI] [PubMed] [Google Scholar]
- Kirsch SA, Böckmann RA. Membrane pore formation in atomistic and coarse-grained simulations. Biochim Biophys Acta. 2016;1858(10):2266–2277. doi: 10.1016/j.bbamem.2015.12.031. [DOI] [PubMed] [Google Scholar]
- Klose G, Köenig B, Paltauf F. Sorption isotherms and swelling of POPC in H20 and 2H20. Chem Phys Lipids. 1992;61(3):265–270. doi: 10.1016/0009-3084(92)90106-Y. [DOI] [Google Scholar]
- Köckritz A, Martin A. Oxidation of unsaturated fatty acid derivatives and vegetable oils. Eur J Lipid Sci Technol. 2008;110:812–824. doi: 10.1002/ejlt.200800042. [DOI] [Google Scholar]
- Köenig BW, Dietrich U, Klose G. Hydration and structural properties of mixed lipid/surfactant model membranes. Langmuir. 1997;13(3):525–532. doi: 10.1021/la960571y. [DOI] [Google Scholar]
- Köenig BW, Strey HH, Gawrisch K. Membrane lateral compressibility determined by NMR and X-ray diffraction: effect of acyc chain polyunsaturation. Biophys J. 1997;73(4):1954–1966. doi: 10.1016/S0006-3495(97)78226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König B (1992) Hydration in Phospholipidmembranen. Ph.D. Thesis, University of Leipzig, Leipzig
- Koubi L, Saiz L, Tarek M, Scharf D, Klein ML. Influence of anesthetic and nonimmobilizer molecules on the physical properties of a polyunsaturated lipid bilayer. J Phys Chem B. 2003;107(51):14500–14508. doi: 10.1021/jp035169o. [DOI] [Google Scholar]
- Koynova R, Caffrey M. Phases and phase transitions of the phosphatidylcholines. Biochim Biophys Acta. 1998;1376:91–145. doi: 10.1016/S0304-4157(98)00006-9. [DOI] [PubMed] [Google Scholar]
- Kruyff BD, Demel RA, Slotboom AJ, van Deenen LLM, Rosenthal AF. The effect of the polar headgroup on the lipid-cholesterol interaction: monolayer and differential scanning calorimetry study. Biochim Biophys Acta. 1973;307(1):1–19. doi: 10.1016/0005-2736(73)90020-5. [DOI] [PubMed] [Google Scholar]
- Kučerka N, Tristram-Nagle S, Nagle JF. Structure of fully hydrated fluid phase lipid bilayers with monounsaturated chains. J Membr Biol. 2005;208(3):193–202. doi: 10.1007/s00232-005-7006-8. [DOI] [PubMed] [Google Scholar]
- Kučerka N, Nieh MP, Katsaras J. Fluid phase lipid areas and bilayer thicknesses of commonly used phosphatidylcholines as a function of temperature. Biochim Biophys Acta. 2011;1808:2761–2771. doi: 10.1016/j.bbamem.2011.07.022. [DOI] [PubMed] [Google Scholar]
- Landau DP, Binder K. A guide to Monte Carlo simulations in statistical physics. Cambridge: Cambridge University Press; 2009. [Google Scholar]
- Lantzsch G, Binder H, Heerklotz H. Surface area per molecule in lipid/C12En membranes as seen by fluorescence resonance energy transfer. J Fluorescence. 1994;4(4):339–343. doi: 10.1007/BF01881452. [DOI] [PubMed] [Google Scholar]
- Lantzsch G, Binder H, Heerklotz H, Wendling M, Klose G. Surface areas and packing constraints in POPC/C12EOn membranes. A time-resolved fluorescence study. Biophys Chem. 1996;58:289–302. doi: 10.1016/0301-4622(95)00108-5. [DOI] [PubMed] [Google Scholar]
- Lavialle F, Levin IW. Raman spectroscopic study of the interactions of dimyristoyl- and 1-palmitoyl-2-oleoylphosphatidylcholine liposomes with myelin proteolipid apoprotein. Biochemistry. 1980;19(26):6044–6050. doi: 10.1021/bi00567a015. [DOI] [PubMed] [Google Scholar]
- Leach AR. Molecular modelling. Principles and applications. London: Pearson Education Ltd; 2001. [Google Scholar]
- Leftin A, Molugu TR, Job C, Beyer K, Brown MF. Area per lipid and cholesterol interactions in membranes from separated local-field NMR spectroscopy. Biophys J. 2014;107(11):2274–2286. doi: 10.1016/j.bpj.2014.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litman BJ, Lewis EN, Levin IW. Packing characteristics of highly unsaturated bilayer lipids: Raman spectroscopic studies of multilamellar phosphatidylcholine dispersions. Biochemistry. 1991;30(2):313–319. doi: 10.1021/bi00216a001. [DOI] [PubMed] [Google Scholar]
- Lüscher-Mattli M. Effect of the mitogenic lectin concanavalin A on the thermotropic behavior of glycosyl-free cationic lipids and their mixtures with zwitterionic lipids. Biopolymers. 1989;28(4):799–817. doi: 10.1002/bip.360280403. [DOI] [PubMed] [Google Scholar]
- Lutterodt H, Luther M, Slavin M, Yin J, Parry J, Gao J, Yu L. Fatty acid profile, thymoquinone content, oxidative stability, and antioxidant properties of cold-pressed black cumin seed oils. LWT Food Sci Technol. 2010;43:1409–1413. doi: 10.1016/j.lwt.2010.04.009. [DOI] [Google Scholar]
- Lynch DV, Steponkus PL. Lyotropic phase behavior of unsaturated phosphatidylcholine species: relevance to the mechanism of plasma membrane destabilization and freezing injury. Biochim Biophys Acta Biomembr. 1989;984(3):267–272. doi: 10.1016/0005-2736(89)90292-7. [DOI] [Google Scholar]
- Lyubartsev AP. Rabinovich AL (2016) Force field development for lipid membrane simulations. Biochim Biophys Acta. 1858;10:2483–2497. doi: 10.1016/j.bbamem.2015.12.033. [DOI] [PubMed] [Google Scholar]
- Lyubartsev AP, Laaksonen A. A general and scalable parallel software package for arbitrary mixtures of molecules. Comput Phys Commun. 2000;128:565–589. doi: 10.1016/S0010-4655(99)00529-9. [DOI] [Google Scholar]
- Martyna GJ, Tuckerman ME, Tobias DJ, Klein ML. Explicit reversible integrators for extended systems dynamics. Mol Phys. 1996;87(5):1117–1157. doi: 10.1080/00268979600100761. [DOI] [Google Scholar]
- McCabe MA, Griffith GL, Ehringer WD, Stillwell W, Wassall SR. H NMR studies of isomeric 3 and 6 polyunsaturated phospholipid membranes. Biochemistry. 1994;33(23):7203–7210. doi: 10.1021/bi00189a024. [DOI] [PubMed] [Google Scholar]
- McCusker MM, Grant-Kels JM. Healing fats of the skin: the structural and immunologic roles of the -6 and -3 fatty acids. Clin Dermatol. 2010;28:440–451. doi: 10.1016/j.clindermatol.2010.03.020. [DOI] [PubMed] [Google Scholar]
- Minkler PE, Hoppel CL. Separation and characterization of cardiolipin molecular species by reverse-phase ion pair high-performance liquid chromatography-mass spectrometry. J Lipid Res. 2010;51:856–865. doi: 10.1194/jlr.D002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molfino A, Amabile M, Monti M, Arcieri S, Fanelli F, Muscaritoli M. The role of docosahexaenoic acid (DHA) in the control of obesity and metabolic derangements in breast cancer. Int J Mol Sci. 2016;17(4):505. doi: 10.3390/ijms17040505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouritsen OG, Bagatolli LA. Life-as a matter of fat: lipids in a membrane biophysics perspective (The Frontiers Collection) New York, Heidelberg, Dordrecht, London: Springer; 2016. [Google Scholar]
- Nabavi S, Bilotto S, Russo G, Orhan I, Habtemariam S, Daglia M, Devi K, Loizzo M, Tundis R, Nabavi S. Omega-3 polyunsaturated fatty acids and cancer: lessons learned from clinical trials. Cancer Metastasis Rev. 2015;34(3):359–380. doi: 10.1007/s10555-015-9572-2. [DOI] [PubMed] [Google Scholar]
- Napolitano GE, Heras H, Stewart AJ. Fatty acid composition of freshwater phytoplankton during a red tide event. Biochem Syst Ecol. 1995;23(1):65–69. doi: 10.1016/0305-1978(95)93659-Q. [DOI] [Google Scholar]
- Nelson DL, Cox MM. Lehninger principles of biochemistry. 5. New York: Freeman W.H. and Co; 2008. [Google Scholar]
- Niebylski CD, Jr, Salem N. A calorimetric investigation of a series of mixed-chain polyunsaturated phosphatidylcholines: effect of -2 chain length and degree of unsaturation. Biophys J. 1994;67(6):2387–2393. doi: 10.1016/S0006-3495(94)80725-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst G, Rappolt M, Amenitsch H, Laggner P. Structural information from multilamellar liposomes at full hydration: full -range fitting with high quality X-ray data. Phys Rev E. 2000;62:4000–4009. doi: 10.1103/PhysRevE.62.4000. [DOI] [PubMed] [Google Scholar]
- Pan J, Tristram-Nagle S, Nagle JF. Effect of cholesterol on structural and mechanical properties of membranes depends on lipid chain saturation. Phys Rev E. 2009;80(021):931. doi: 10.1103/PhysRevE.80.021931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasenkiewicz-Gierula M, Baczynski K, Markiewicz M, Murzyn K. Computer modelling studies of the bilayer/water interface. Biochim Biophys Acta. 2016;1858(10):2305–2321. doi: 10.1016/j.bbamem.2016.01.024. [DOI] [PubMed] [Google Scholar]
- Perly B, Smith ICP, Jarrell HC. Effects of the replacement of a double bond by a cyclopropane ring in phosphatidylethanolamines: a H NMR study of phase transitions and molecular organization. Biochemistry. 1985;24(4):1055–1063. doi: 10.1021/bi00325a038. [DOI] [PubMed] [Google Scholar]
- Phillips MC, Hauser H, Paltauf F. The inter- and intra-molecular mixing of hydrocarbon chains in lecithin/water systems. Chem Phys Lipids. 1972;8(1):127–133. doi: 10.1016/0009-3084(72)90024-2. [DOI] [PubMed] [Google Scholar]
- Pinzi S, Garcia IL, Lopez-Gimenez FJ, de Castro MDL, Dorado G, Dorado MP. The ideal vegetable oil-based biodiesel composition: a review of social, economical and technical implications. Energy Fuels. 2009;23:2325–2341. doi: 10.1021/ef801098a. [DOI] [Google Scholar]
- Pluhackova K, Böckmann RA. Biomembranes in atomistic and coarse-grained simulations. J Phys Condens Matter. 2015;27:323103. doi: 10.1088/0953-8984/27/32/323103. [DOI] [PubMed] [Google Scholar]
- Pöyry S, Vattulainen I. Role of charged lipids in membrane structures—insight given by simulations. Biochim Biophys Acta. 2016;1858(10):2322–2333. doi: 10.1016/j.bbamem.2016.03.016. [DOI] [PubMed] [Google Scholar]
- Rabinovich AL, Ripatti PO, Balabaev N. Molecular dynamics simulations of unsaturated lipid in monolayers: an investigation of bond ordering. Proc SPIE. 1999;3687:175–181. doi: 10.1117/12.347418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovich AL, Ripatti PO, Balabaev NK. Molecular dynamics investigation of bond ordering of unsaturated lipids in monolayers. J Biol Phys. 1999;25(2):245–262. doi: 10.1023/A:1005180027451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovich AL, Ripatti PO, Balabaev N. Molecular dynamics investigation of polar diacylglycerolipid monolayers: bond ordering properties. Proc SPIE. 2000;4064:144–155. doi: 10.1117/12.375418. [DOI] [Google Scholar]
- Rabinovich AL, Ripatti PO, Balabaev NK, Leermakers FAM. Molecular dynamics simulations of hydrated unsaturated lipid bilayers in the liquid-crystal phase and comparison to self-consistent field modeling. Phys Rev E. 2003;67(1):011909. doi: 10.1103/PhysRevE.67.011909. [DOI] [PubMed] [Google Scholar]
- Rabinovich AL, Balabaev NK. Molecular dynamics simulations of unsaturated lipid bilayers. Proc SPIE. 2001;4348:215–224. doi: 10.1117/12.417651. [DOI] [Google Scholar]
- Rabinovich AL, Lyubartsev AP. Computer simulation of lipid membranes: methodology and achievements. Polym Sci Ser C. 2013;55:162–180. doi: 10.1134/S1811238213070060. [DOI] [Google Scholar]
- Rabinovich AL, Lyubartsev AP. Bond orientation properties in lipid molecules of membranes: molecular dynamics simulations. J Phys Conf Ser. 2014;510:012022. doi: 10.1088/1742-6596/510/1/012022. [DOI] [Google Scholar]
- Rabinovich AL, Ripatti PO. Intramolecular ordering of bonds in the cis-4,7,10,13,16,19-docosahexaenic chain. Dokl Biophys. 1999;364–366:6–9. [Google Scholar]
- Rabinovich AL, Ripatti PO. A computer investigation of intramolecular bond ordering: unsaturated chains of natural lipids. Membr Cell Biol. 2000;13(5):697–714. [PubMed] [Google Scholar]
- Rand RP, Fuller N, Parsegian VA, Rau DC. Variation in hydration forces between neutral phospholipid bilayers: evidence for hydration attraction. Biochemistry. 1988;27(20):7711–7722. doi: 10.1021/bi00420a021. [DOI] [PubMed] [Google Scholar]
- Rand RP, Parsegian VA. Hydration forces between phospholipid bilayers. Biochim Biophys Acta. 1989;988:351–376. doi: 10.1016/0304-4157(89)90010-5. [DOI] [Google Scholar]
- Rincón-Cervera MÁ, Guil-Guerrero JL. Preparation of stearidonic acid-enriched triacylglycerols from Echium plantagineum seed oil. Eur J Lipid Sci Technol. 2010;112:227–232. doi: 10.1002/ejlt.200900160. [DOI] [Google Scholar]
- Rovere MTL, Christensen JH. The autonomic nervous system and cardiovascular disease: role of n-3 PUFAs. Vascul Pharmacol. 2015;71:1–10. doi: 10.1016/j.vph.2015.02.005. [DOI] [PubMed] [Google Scholar]
- Rueter K, Haynes A, Prescott S. Developing primary intervention strategies to prevent allergic disease. Curr Allergy Asthma Rep. 2015;15(7):40. doi: 10.1007/s11882-015-0537-x. [DOI] [PubMed] [Google Scholar]
- Sahena F, Zaidul ISM, Jinap S, Saari N, Jahurul HA, Abbas KA, Norulaini NA. PUFAs in fish: extraction, fractionation, importance in health. Compr Rev Food Sci Food Saf. 2009;8:59–74. doi: 10.1111/j.1541-4337.2009.00069.x. [DOI] [Google Scholar]
- Sakuradani E. Advances in the production of various polyunsaturated fatty acids through oleaginous fungus Mortierella alpine breeding. Biosci Biotechnol Biochem. 2010;74:908–917. doi: 10.1271/bbb.100001. [DOI] [PubMed] [Google Scholar]
- Sánchez-Migallón MP, Aranda FJ, Gómez-Fernández JC. Interaction between -tocopherol and heteroacid phosphatidylcholines with different amounts of unsaturation. Biochim Biophys Acta. 1996;1279:251–258. doi: 10.1016/0005-2736(95)00276-6. [DOI] [PubMed] [Google Scholar]
- Santaren JF, Rico M, Guilleme J, Ribera A. Thermal and C NMR study of dynamic structure of 1-palmitoyl-2-oleyl-sn-glycero-3-phosphocholine and 1-oleyl-2-palmitoyl-sn-glycero-3-phosphocholine in aqueous dispersions. Biochim Biophys Acta. 1982;687:231–237. doi: 10.1016/0005-2736(82)90551-X. [DOI] [PubMed] [Google Scholar]
- Satoh A. Introduction to practice of molecular simulation: molecular dynamics, Monte Carlo, Brownian dynamics, Lattice Boltzmann, dissipative particle dynamics. Amsterdam: Elsevier; 2011. [Google Scholar]
- Seelig J, Waespe-Sarcevic N. Molecular order in cis and trans unsaturated phospholipid bilayers. Biochemistry. 1978;17(16):3310–3315. doi: 10.1021/bi00609a021. [DOI] [PubMed] [Google Scholar]
- Separovich F, Gawrisch K. Effect of unsaturation on the chain order of phosphatidylcholines in a dioleoylphosphatidylethanolamine matrix. Biophys J. 1996;71:274–282. doi: 10.1016/S0006-3495(96)79223-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SP, Zhou X, Liu Q, Stymne S, Green AG. Metabolic engineering of new fatty acids in plants. Curr Opin Plant Biol. 2005;8:197–203. doi: 10.1016/j.pbi.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Siu SWI, Vácha R, Jungwirth P, Böckmann RA. Biomolecular simulations of membranes: physical properties from different force fields. J Chem Phys. 2008;128(125):103. doi: 10.1063/1.2897760. [DOI] [PubMed] [Google Scholar]
- Smaby JM, Momsen MM, Brockman HL, Brown RE. Phosphatidylcholine acyl unsaturation modulates the decrease in interfacial elasticity induced by cholesterol. Biophys J. 1997;73:1492–1505. doi: 10.1016/S0006-3495(97)78181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonne J, Jensen MØ, Hansen FY, Hemmingsen L, Peters GH. Reparametrization of all-atom dipalmitoylphosphatidylcholine lipid parameters enables simulation of fluid bilayers at zero tension. Biophys J. 2007;92:4157–4167. doi: 10.1529/biophysj.106.087130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling LS, Nelson JR. History and future of omega-3 fatty acids in cardiovascular disease. Curr Med Res Opin. 2016;32(2):301–311. doi: 10.1185/03007995.2015.1120190. [DOI] [PubMed] [Google Scholar]
- Stillwell W, Jenski LJ, Zerouga M, Dumaual AC. Detection of lipid domains in docosahexaenoic acid-rich bilayers by acyl chain-specific fret probes. Chem Phys Lipids. 2000;104:113–132. doi: 10.1016/S0009-3084(99)00122-X. [DOI] [PubMed] [Google Scholar]
- Surewicz WK, Epand RM. Phospholipid structure determines the effects of peptides on membranes. Differential scanning calorimetry studies with pentagastrin-related peptides. Biochim Biophys Acta. 1986;856(2):290–300. doi: 10.1016/0005-2736(86)90039-8. [DOI] [PubMed] [Google Scholar]
- Swaney JB. Mechanisms of protein-lipid interaction. Association of apolipoproteins A-I and A-II with binary phospholipid mixtures. J Biol Chem. 1980;255(18):8791–8797. [PubMed] [Google Scholar]
- Tada K, Miyazaki E, Goto M, Tamai N, Matsuki H, Kaneshina S. Barotropic and thermotropic bilayer phase behavior of positional isomers of unsaturated mixed-chain phosphatidylcholines. Biochim Biophys Acta. 2009;1788:1056–1063. doi: 10.1016/j.bbamem.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Tada K, Goto M, Tamai N, Matsuki H, Kaneshina S. Pressure effect on the bilayer phase transition of asymmetric lipids with an unsaturated acyl chain. Ann N Y Acad Sci. 2010;1189:77–85. doi: 10.1111/j.1749-6632.2009.05203.x. [DOI] [PubMed] [Google Scholar]
- Toukan K, Rahman A. Molecular dynamics study of atomic motions in water. Phys Rev B. 1985;31:2643–2648. doi: 10.1103/PhysRevB.31.2643. [DOI] [PubMed] [Google Scholar]
- Tuckerman M, Berne BJ, Martyna GJ. Reversible multiple time scale molecular dynamics. J Chem Phys. 1992;97:1990–2001. doi: 10.1063/1.463137. [DOI] [Google Scholar]
- Vilchéze C, McMullen TPW, McElhaney RN, Bittman R. The effect of side-chain analogues of cholesterol on the thermotropic phase behavior of 1-stearoyl-2-oleoylphosphatidylcholine bilayers: a differential scanning calorimetric study. Biochim Biophys Acta. 1996;1279:235–242. doi: 10.1016/0005-2736(95)00258-8. [DOI] [PubMed] [Google Scholar]
- Vincent M, Gallay J, Bony JD, Tocanne J. Steady-state and time-resolved fluorescence anisotropy study of phospholipid molecular motion in the gel phase using l-palmitoyl-2-[9-(2-anthryl)-nonanoyl]--glycero-3-phosphocholine as probe. Eur J Biochem. 1985;150:341–347. doi: 10.1111/j.1432-1033.1985.tb09026.x. [DOI] [PubMed] [Google Scholar]
- Wang G, Lin H, Li S, Huang C. Phosphatidylcholines with -1 saturated and -2 cis-monounsaturated acyl chains. J Biol Chem. 1995;270(39):22738–22746. doi: 10.1074/jbc.270.39.22738. [DOI] [PubMed] [Google Scholar]
- Wang Z, Lin H, Li S, Huang C. Phase transition behavior and molecular structures of monounsaturated phosphatidylcholines. J Biol Chem. 1995;270(5):2014–2023. doi: 10.1074/jbc.270.5.2014. [DOI] [PubMed] [Google Scholar]
- Wang X, Chan CB. n-3 polyunsaturated fatty acids and insulin secretion. J Endocrinol. 2015;224(3):R97–R106. doi: 10.1530/JOE-14-0581. [DOI] [PubMed] [Google Scholar]
- Winter R, Pilgrim WC. A SANS study of high pressure phase transitions in model biomembranes. Ber Bunsen Ges Phys Chem. 1989;93(6):708–717. doi: 10.1002/bbpc.19890930611. [DOI] [Google Scholar]
- Wittkop B, Snowdon RJ, Friedt W. Status and perspectives of breeding for enhanced yield and quality of oilseed crops for Europe. Euphytica. 2009;170:131–140. doi: 10.1007/s10681-009-9940-5. [DOI] [Google Scholar]
- Yoon DY, Flory PJ. Small angle neutron scattering by n-alkane chains. J Chem Phys. 1978;69(6):2536–2538. doi: 10.1063/1.436896. [DOI] [Google Scholar]
- Zhurkin DV, Rabinovich AL. Monte carlo properties of the hydrocarbon chains of phospholipid molecules. Russ J Phys Chem A. 2015;89(2):242–249. doi: 10.1134/S0036024415020387. [DOI] [Google Scholar]