Abstract
Purpose
To report on a case of uveitis and scleritis resulting as an immune-mediated side effect of cancer immunotherapy with nivolumab and cabiralizumab.
Observations
Bilateral anterior nongranulomatous anterior uveitis and bilateral diffuse anterior and posterior scleritis occurred following the use of combination cancer immunotherapy. The uveitis and scleritis resolved following temporary discontinuation of nivolumab and cabiralizumab as well as systemic prednisone.
Conclusions and importance
Ophthalmologists should be aware of the possibility of acute ocular inflammation developing with cancer immunotherapy. Systemic corticosteroids play a first-line role in managing such immune-mediated side effects.
Keywords: Uveitis, Scleritis, Cancer immunotherapy, Side effects, Nivolumab, Cabiralizumab
1. Introduction
Cancer immunotherapy has emerged as a novel therapeutic treatment option in a variety of neoplasms. With the advent of monoclonal antibodies that target the checkpoint protein, programmed cell death protein 1 (PD-1), in T-cells, has increased the survival rate for patients with certain lung and renal cancers and melanomas.1 Its use continues to expand to other neoplastic processes. Monoclonal antibodies targeting macrophage colony stimulating factor 1 (CSF-1) have also been developed. However, because PD-1 and CSF-1 are involved in mediating inflammatory responses, immune-mediate side effects are possible. Uveitis has been recently described to occur with the use of the PD-1 inhibitors.2, 3, 4
Nivolumab (BMS-936558) (Opdivo, Bristol-Myers Squibb Co.) is a monoclonal antibody that binds to PD-1 receptors on T-cells thereby preventing the inhibition of T-cell mediated anti-tumor responses. Cabiralizumab (FPA008) is a monoclonal antibody targeting macrophages and monocytes. The combination of cabiralizumab and nivolumab is being used synergistically in phase 1a/1b clinical trials against selected malignancies, including renal cell carcinoma (ClinicalTrial.gov Identifier: NCT02526017). Potential immune-mediated adverse events including pneumonitis, vitiligo, colitis, hepatitis, and thyroiditis have been reported to occur in 44% of patients treated with nivolumab.1 While uveitis has been reported with the use of nivolumab,5 scleritis has not been previously reported to occur with nivolumab or cabiralizumab.
2. Case report
A 66-year-old Caucasian man presented to the Proctor Foundation for complaints of bilateral ocular pain and light sensitivity starting 5 days prior to presentation. His past ocular history was notable for early nuclear sclerotic cataracts. His past medical history included a history of iron deficiency anemia, hypothyroidism, and metastatic renal cell carcinoma, clear cell type with sarcomatoid features requiring left nephrectomy with metastases to the lungs. He was enrolled into a clinical trial and commenced on the combination of cabiralizumab and nivolumab two months prior to presentation. A computed tomographic scan of the chest, abdomen, and pelvis performed on the day of his referral to the Proctor Foundation (per clinical trial protocol) revealed an interval improvement in his metastatic lesions. The Oncology service noted that the patient also exhibited bilateral periorbital edema.
Spectacle-corrected visual acuity measured 20/40 OU. While there was mild periorbital edema (Fig. 1a), ocular motility was full OU, there was no APD, and intraocular pressure measured 12 mm Hg OD and 13 mm Hg OS by applanation. Slit lamp examination revealed a bilateral diffuse anterior scleritis (Fig. 1a). There were fine, nongranulomatous keratic precipitates inferiorly OU, 1 + cell and 1 + flare OU. Both irides exhibited multiple areas of posterior synechiae (Fig. 1b). There was ½+ anterior vitreous cell OU. Dilated funduscopic examination showed no vitreous haze in either eye, flat disc and macula OU, and flat periphery OU. B-scan ultrasonography revealed posterior scleritis characterized by a T-sign (Fig. 2).
Fig. 1.
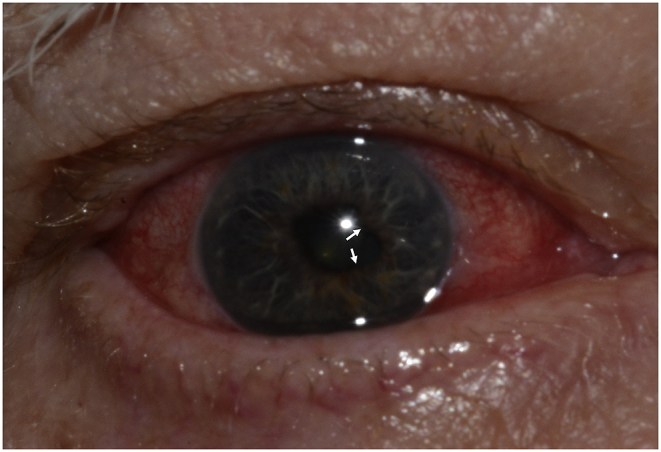
Periorbital edema was more prominent OD (a). Both eyes exhibited diffuse anterior scleritis as represented here in OD (a). Posterior synechiae were present in both eyes as represented here in OD (b).
Fig. 2.
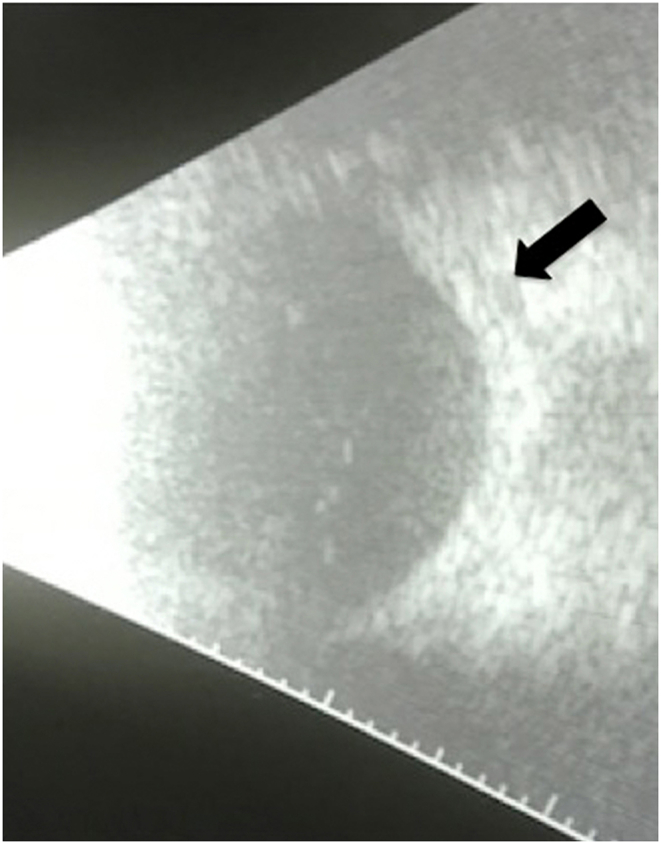
B scan ultrasonography demonstrated T-sign (black arrow) in both eyes (OD shown).
After discussion with the patient's oncologists, we commenced prednisone 60 mg PO daily and prednisolone acetate 1% QID OU. Within one week, both anterior and posterior scleritis became inactive clinically and on B scan ultrasonography. Anterior chamber cell improved to 0.5 + OU. His prednisone was decreased by 10 mg each week. Oncology held further administration of his cancer immunotherapy while the patient was taking systemic prednisone per their protocol.
3. Discussion
While ocular side effects of PD-1 and CSF-1 inhibitors are considered rare, immune-mediated inflammatory processes affecting various organs not involved with the target neoplasm do occur. This case demonstrates that diffuse anterior scleritis and nongranulomatous anterior uveitis can occur in the setting of synergistic cancer immunotherapy. Moreover, B-scan ultrasonography should be considered in such cases as it is effective in identifying posterior scleritis even when then clinical examination of the posterior segment is relatively unremarkable.
4. Conclusions
Prednisone can play an important role in managing the ocular inflammation that can manifest with cancer immunotherapy. While recurrences of inflammation are known to occur2 a balance between managing ocular inflammation while treating the underlying cancer must be struck.
Patient consent
The patient consented in writing to publication of medical record details and photographs.
Funding
Dr. Gonzales is supported by a National Institutes of Health - National Eye Institute Career Development Award grant no. NEI K23EY026998.
The Department of Ophthalmology at UCSF is supported by a core grant from the National Eye Institute, EY02162, and an unrestricted grant from Research to Prevent Blindnessew York, NY.
Conflicts of interest
The following authors have no financial disclosures: JAG, JS.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Acknowledgements
None.
References
- 1.Topalian S.L., Hodi F.S., Brahmer J.R. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abu Samra K., Valdes-Navarro M., Lee S., Swan R., Foster C.S., Anesi S.D. A case of bilateral uveitis and papillitis in a patient treated with pembrolizumab. Eur J Ophthalmol. 2016;26(3):e46–48. doi: 10.5301/ejo.5000724. [DOI] [PubMed] [Google Scholar]
- 3.Basilious A., Lloyd J.C. Posterior subcapsular cataracts and hypotony secondary to severe pembrolizumab induced uveitis: case report. Can J Ophthalmol. 2016;51(1):e4–6. doi: 10.1016/j.jcjo.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Hanna K.S. A rare case of pembrolizumab-induced uveitis in a patient with metastatic melanoma. Pharmacotherapy. 2016;36(11):e183–e188. doi: 10.1002/phar.1839. [DOI] [PubMed] [Google Scholar]
- 5.Karlin J., Gentzler R., Golen J. Bilateral anterior uveitis associated with nivolumab therapy. Ocul Immunol Inflamm. 2016:1–3. doi: 10.1080/09273948.2016.1215473. [DOI] [PubMed] [Google Scholar]


