Abstract
Mesenchymal stromal cells (MSCs) are used as salvage therapy to treat steroid-refractory acute graft-versus-host disease (aGvHD). We studied the immunological response to MSC treatment in 16 aGvHD patients by assessing lymphocyte profiles and three proposed aGvHD serum markers during the MSC treatment. Surprisingly, there were no obvious differences in the lymphocyte profiles between the responders and non-responders. The numbers of T, B, and NK cells were below the normal reference interval in all patients. CD4+ T helper (Th) cell levels remained particularly low throughout the follow-up period. The relative proportion of Th1 cells decreased, while regulatory T cells remained unaltered, and only very few Th2 and Th17 cells could be detected. Serum concentrations of regenerating islet-derived protein 3-alpha, cytokeratin-18 fragments (CK18F), and elafin were significantly elevated in patient samples compared with healthy controls, but only CK18F showed any potential in the prediction of patients’ response to MSCs. No obvious markers for MSC therapy response were revealed in this study, but the results suggest that allogeneic MSCs do not provoke overt T cell-mediated immune responses at least in immunosuppressed aGvHD patients. The results advocate for the safety of MSC therapy and bring new insights in MSC immunomodulation mechanisms.
Keywords: allogeneic hematopoietic stem cell transplantation, biomarker, graft-versus-host disease, mesenchymal stromal cells, T cell, safety
Graphical Abstract
Introduction
Mesenchymal stromal cells (MSCs) have emerged as a potential salvage therapy for steroid-refractory graft-versus-host disease (GvHD) because of their immunomodulatory properties.1 There is an urgent need for effective second-line therapies for allogeneic hematopoietic stem cell transplantation (allo-HSCT) recipients who develop steroid-resistant acute GvHD (aGvHD), because these patients have a very poor prognosis with overall survival of less than 10%.2 Several studies have presented promising early clinical results when utilizing bone marrow-derived MSCs to treat severe GvHD.3, 4, 5, 6 MSC treatment efficacy, as measured by the two most relevant clinical endpoints, namely response to treatment and overall survival, has been reviewed in two recent meta-analysis studies,7, 8 suggesting that MSCs could be utilized as a treatment option for steroid-refractory GvHD and could improve long-term survival of allo-HSCT recipients. We, among others, have recently shown that some patients treated with bone marrow-derived third-party MSCs respond to MSC therapy with a significant improvement in the aGvHD symptoms of the frequent target organs intestine, liver, and skin.9
The MSCs are usually derived from a third-party donor and are used in a non-HLA-matched manner in the current aGvHD treatment regimens. MSCs have the theoretical ability to influence several immune cell types in vivo, including T, B, and NK cells, which is one of their proposed immunomodulatory mechanisms.10 Simultaneously, they pose a risk of activating allo-immune responses.11 MSCs are, however, typically considered hypo-immunogenic and well tolerated, but detailed molecular knowledge of the immunological safety and the actual mechanism of MSC therapy is still limited.10, 12 It is also becoming evident that albeit some patients with severe aGvHD markedly benefit from MSC treatment, other patients experience no improvement of the symptoms. Biological markers predicting or reflecting MSC treatment response in GvHD would help in distinguishing patients responding to MSCs and in designing more effective treatment protocols. The utilization of serum proteins in prediction of allo-HSCT transplant outcome and GvHD prevalence has been extensively studied.13, 14, 15, 16 While some promising biomarker panels have been introduced to predict GvHD after HSCT, the correlation of serum proteins with MSC treatment response has been addressed only in a few studies.17, 18, 19, 20 Monitoring of serum protein concentrations supplementary to the traditional aGvHD organ grading could be informative in the MSC treatment setting because changes in biomarker concentrations can precede the clinical symptoms of aGvHD.21, 22
In the present study, our aim was to evaluate the immunological response to and safety of MSC therapy in aGvHD, and to study the usability of cell and protein markers in the prediction and follow-up of MSC treatment response. We recently reported the day 28 response and survival of 30 patients treated with MSCs for steroid-refractory GvHD.9 A subgroup of the aforementioned patient cohort, comprising 16 consecutively treated adult patients with aGvHD, was included in this study. We analyzed the lymphocyte profiles, with a special focus on CD4+ T helper (Th) cell subpopulations, and serum concentrations of three proteins proposed to describe the damage of aGvHD target organs, regenerating islet-derived protein 3-alpha (Reg3α), cytokeratin-18 fragments (CK18F), and elafin, to: (1) explore the immunological response, (2) explore any signs of a potential immune activation provoked by MSCs, and (3) retrospectively evaluate the utility of serum markers in prediction of MSC therapy response and in patient follow-up.
Results
Lymphocyte Counts Remained Low in Patients and Did Not Correlate with MSC Treatment Response
We were able to complete the immune cell profiling as planned for 11 patients, including 4 responders (2 with complete response and 2 with partial response) and 7 non-responders (day 28 response; Salmenniemi et al.9). The evaluation of the clinical response is described in more detail in the Materials and Methods. Samples were collected before the first MSC infusion (day 0) and approximately 1 week (range 5–16 days) and 1 month (range 27–32 days) after the first MSC dose (Table 1). We first performed a lymphocyte differential counting. The average absolute numbers of T cells (CD3+) and B cells (CD19+) were generally low before the first MSC dose in all patients and remained unaltered in the follow-up samples (Figure 1A). Also, the average number of NK cells (CD16+CD56+) remained at a constant, moderately low level during the MSC treatment (Figure 1A). The average lymphocyte counts in patients responding and not responding to MSCs were not significantly different (Figure 1B), and no correlation of lymphocyte numbers with the 3-month or 6-month survival was noted (data not shown).
Table 1.
Blood Sampling for Immune Cell and Serum Protein Analysis
| Patient Characteristics during MSC Treatment |
Cell Samples |
Serum Samples |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient No. | Day 28 Response | HSCT |
GvHD |
Pre-MSC | 1 Week | 1 Month | Pre-MSC | 1 Week | 1 Month |
| →MSC (days) | |||||||||
| 1 | CR | 42 | 13 | 0 | 7 | 28 | 0 | 7 | 25 |
| 2 | PR | 234 | 5 | 0 | 5 | 28 | 0 | 8 | 42 |
| 4 | CR | 147 | 7 | 0 | 7 | ns | 0 | 7 | 26 |
| 7 | PR | 129 | 19 | 0 | 7 | 28 | 0 | 7 | 41 |
| 16 | CR | 63 | 8 | ns | ns | ns | 0 | 4 | 31 |
| 17 | CR | 90 | 11 | ns | ns | ns | 0 | 7 | 32 |
| 18 | VGPR | 438 | 278 | ns | ns | ns | 0 | 7 | 28 |
| 19 | VGPR | 39 | 7 | ns | ns | ns | ns | 7 | 28 |
| 3 | NR | 25 | 7 | 0 | 16 | 27 | 0 | 6 | 24 |
| 8 | NR | 41 | 11 | 0 | ns | ns | 0 | ns | ns |
| 9 | NR | 49 | 6 | 0 | 10 | ns | 0 | 7 | ns |
| 10 | NR | 61 | 14 | 0 | 8 | 28 | ns | 6 | 27 |
| 11 | NR | 77 | 22 | 0 | 7 | 27 | 0 | 7 | 26 |
| 12 | NR | 160 | 59 | 0 | 8 | 30 | 0 | 13 | 27 |
| 13 | NR | 59 | 10 | 0 | 11 | 32 | 0 | 7 | 21 |
| 15 | NR | 175 | 77 | ns | ns | ns | 0 | 7 | 27 |
The pre-MSC (day 0) samples were collected immediately before the first MSC dose. Thereafter, samples were collected approximately 1 week and 1 month after the first MSC dose. The exact sample collection time points are indicated in days after the first MSC dose. Day 28 response is presented as complete response (CR), very good partial response (VGPR), partial response (PR) or no response/progression of the disease (NR). Time from HSCT to the first MSC dose and time from GvHD diagnosis to the first MSC dose are also indicated. Time points where planned samples could not be obtained are marked ns (no sample).
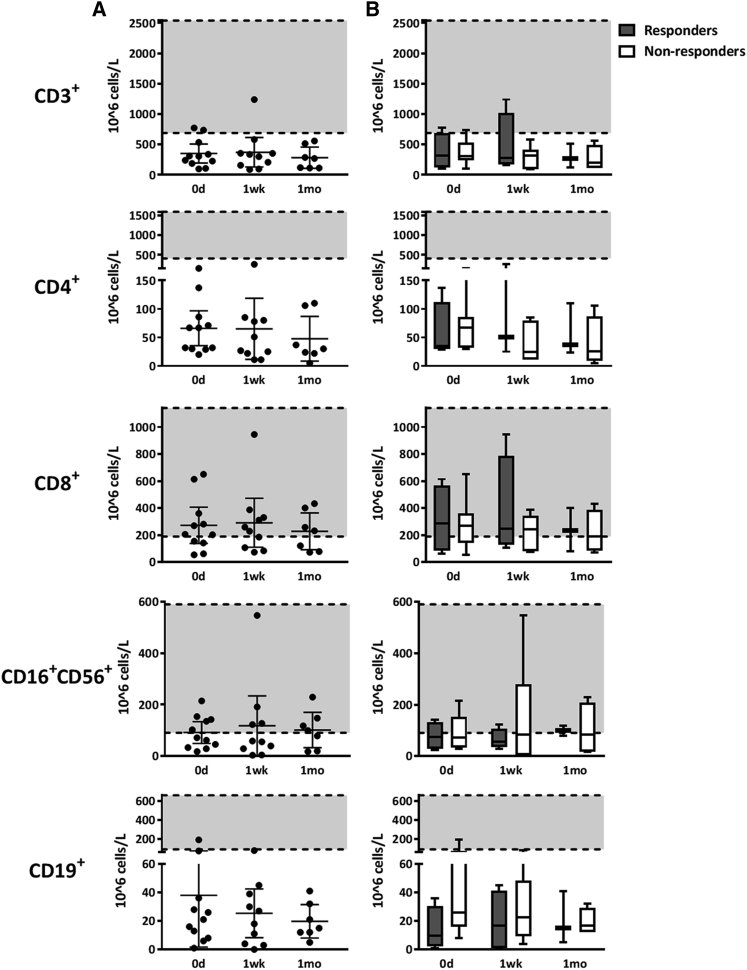
Figure 1.
Lymphocyte Counts in Patients Treated with MSCs
Numbers of T cells (CD3+, CD4+, CD8+), NK cells (CD16+CD56+), and B cells (CD19+) in samples collected from patients before the MSC treatment (day 0) and approximately 1 week and 1 month after the first MSC dose are shown. The shaded area represents the current reference interval (range of cell numbers) for a healthy population. (A) Individual lymphocyte numbers in each patient, with mean values and 95% confidence intervals. The numbers of CD3+, CD4+, and CD19+ lymphocytes were very low throughout the follow-up period. (B) Lymphocyte numbers in patients grouped according to the day 28 response to MSCs. Cell numbers are shown as a whisker plot showing the mean value and range. The Kruskal-Wallis test was used to calculate statistical significance. There was no statistically significant difference between the lymphocyte numbers of responding (n = 4) and non-responding (n = 7) patients or between different sample collection time points.
A more detailed analysis of T cell types revealed that the levels of CD4+ T cells were remarkably low in all patients, only 5–262 × 106 cells/L, which is approximately 6–80 times lower than in healthy individuals (Figure 1). The numbers of CD8+ cytotoxic T cells were also reduced in the patients (55–945 × 106 cells/L in patients and 190–1,140 × 106 cells/L in healthy individuals). Again, no differences in the levels of CD4+ or CD8+ cells were noted with respect to the MSC response documented at day 28 (Figure 1B) or with the 3-month or 6-month survival (data not shown). Altogether, the patient lymphocyte numbers were on a level typical for post-HSCT recovery, and the results indicate that neither significant reduction nor excessive peripheral proliferation of lymphocytes was taking place during MSC therapy.
Relative Proportions of Th Cells Varied Only Moderately during MSC Therapy
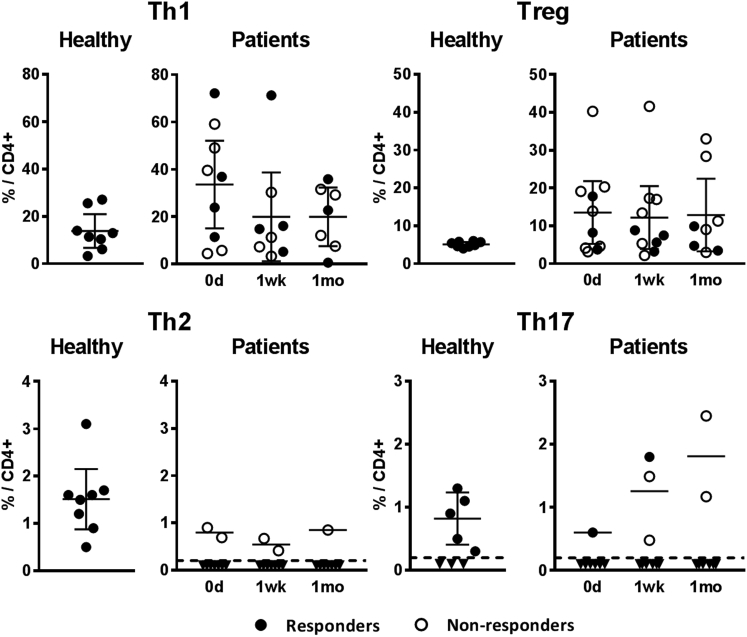
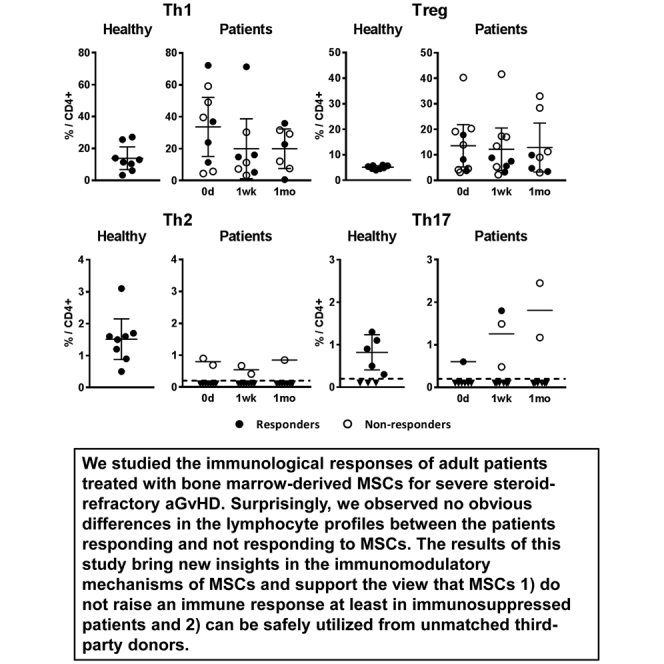
We next analyzed the relative proportions of CD4+ Th cell subpopulations in the same 11 patients and 8 healthy control samples after in vitro stimulation. As shown in Figure 2, the mean relative proportion of Th1 cells in the patient samples before the first MSC dose was clearly higher (34% of CD4+ cells; range 4%–72%; median 37%) than in the samples collected from eight healthy volunteers (14% of CD4+ cells; range 3%–27%; median 12%). Interestingly, the mean proportion of Th1 cells in patients decreased closer to the levels of the healthy controls after initiating the MSC treatment, and although the difference between the sampling points was not statistically significant, the decreased Th1 level could still be observed 1 month after the first MSC dose (Figure 2).
Figure 2.
Relative Amounts of CD4+ T Helper Cell Subsets
The relative proportion (out of CD4+ gated cells) of circulating Th1, Th2, Th17, and FOXP3-expressing Tregs, measured from patient samples collected before the MSC treatment (day 0) and approximately 1 week and 1 month after the first MSC dose, as well from healthy volunteer samples, are shown. Closed circles represent samples from patients responding and open circles not responding to MSCs. Dashed line represents the lower limit of quantification of the method; inverted triangles are measured samples that could not be reliably quantified. Results are plotted as individual dots and means with 95% confidence intervals. The Kruskal-Wallis test was used to calculate statistical significance. All differences between the groups were statistically non-significant.
The relative numbers of Th2 and Th17 cells in patient samples were considerably low. The Th2 cell count was below the detection limit in all but five samples, derived from three non-responding patients (Figure 2; Figure S1). Th17 cell count was also below the detection limit in the majority of patient samples. In five patients, however, a transient increase of Th17 cells was noted in either the 1-week or 1-month sample after initiating the MSC treatment, with the Th17 proportion in a similar range or even higher than in the healthy controls (Figure 2; Figure S1).
CD4+ regulatory T cells (Tregs) were detected by intracellular staining of the transcription factor FOXP3. As compared with the healthy controls, the average Treg proportion in the patient samples was 2- to 3-fold higher, and it remained unaltered during the MSC treatment (Figure 2; Figure S1).
The individual variation in proportions of Th subpopulations was considerable, and no consistent differences between the responder and non-responder groups were noted (Figure 2; Figure S1). There was also no correlation with the 3-month or 6-month survival rate (data not shown). Taken together, these results suggest that MSCs did not provoke harmful T cell-mediated immune activation in aGvHD patients.
aGvHD-Associated Serum Markers Were Significantly Elevated in Patient Sera
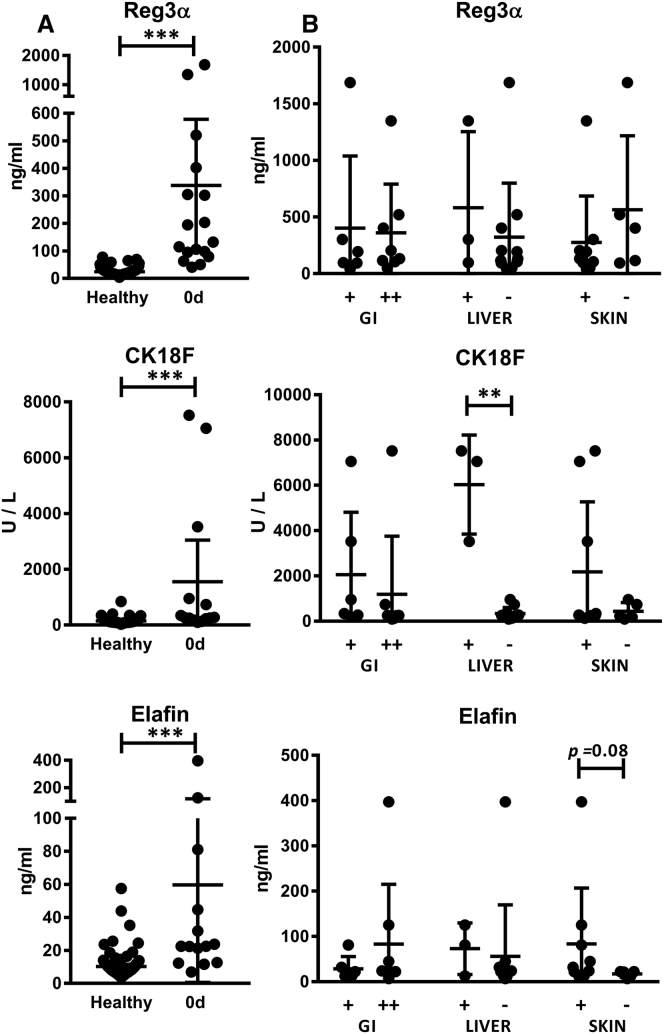
We measured the concentrations of Reg3α, CK18F, and elafin in serum samples collected from all 16 MSC-treated patients (8 responders and 8 non-responders) prior to MSC treatment and at 1-week and 1-month follow-up points. We compared the protein concentrations in the patient samples with control samples collected from 108 healthy blood donors. In the patient samples taken before the initiation of MSC treatment, the mean concentrations of Reg3α, CK18F, and elafin were 365 ng/mL, 1557 U/L, and 60 ng/mL, respectively, while in the healthy controls they were only 25 ng/mL, 144 U/L, and 13 ng/mL, respectively (p < 0.001, for each protein, Mann-Whitney test) (Figure 3A). The results indicate that these three proposed aGvHD markers were associated with aGvHD also in the present study.
Figure 3.
Concentrations of GvHD-Related Serum Protein Markers prior to the MSC Treatment
(A) Concentrations of Reg3α, CK18F, and elafin in patient samples (day 0) shown in comparison with samples collected from healthy blood donors (n = 108). Results are shown as individual dots and mean values, with error bars indicating 95% confidence intervals. (B) Concentrations of the proteins in samples grouped according to the GvHD organ involvement of the patient. Patients are divided into groups based on the GI GvHD severity and liver and skin involvement at the time of GvHD diagnosis (−, no involvement of an organ; +, involvement of an organ; GI +, stage 1–2 gastrointestinal GvHD; GI ++, stage 3–4 gastrointestinal GvHD). Results are shown as individual dots and mean values, with error bars indicating SD. The Mann-Whitney test was used to calculate statistical significance (*p < 0.05; **p < 0.01; ***p < 0.001). Concentrations of all serum proteins were significantly higher in the patients than in the healthy controls; an elevated CK18F concentration was noted in the patients with liver GvHD, and a trend of higher elafin concentrations in the patients with skin GvHD.
CK18F and Elafin Concentrations Correlated with Affected Target Organs
Correlation of the pre-MSC serum protein concentrations with the clinical manifestations of GvHD revealed that none of the markers could distinguish the less (stage 1–2) or more (stage 3–4) severe gastrointestinal (GI) GvHD in our patient cohort, where all patients had GI symptoms (Figure 3B). In contrast, CK18F was significantly increased in three patients who had elevated transaminases, indicating hepatic involvement of GvHD (“liver” GvHD; p = 0.005, Mann-Whitney test) (Figure 3B). A trend to increased concentrations of elafin was noted in the patients with skin GvHD (n = 11; p = 0.08, Mann-Whitney test) (Figure 3B). The patient group with skin GvHD also had an increased mean level of CK18F, but the highest concentrations were measured in the same three patients who had concurrent liver GvHD. These results are in line with the reported association of CK18F with liver GvHD and, correspondingly, elafin with skin GvHD.
CK18F Showed Moderate Potential in Prediction of MSC Therapy Response
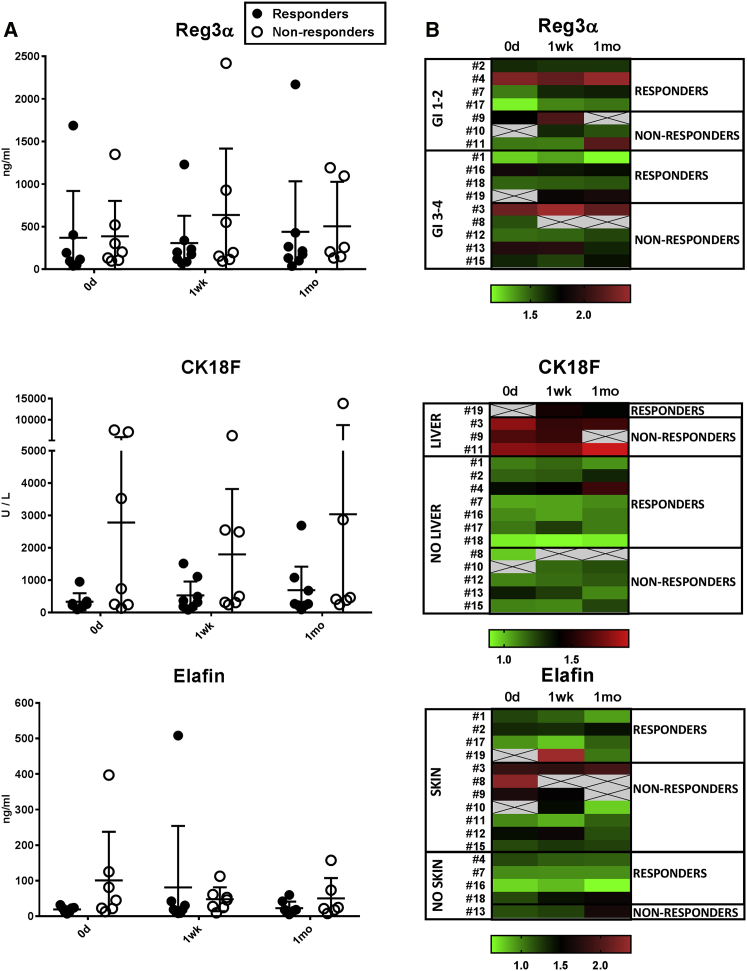
Comparison of protein levels in the patients responding and not responding to MSCs showed that there was a considerable individual variation in the protein concentrations throughout the follow-up period (Figure 4). The mean concentration of Reg3α did not differ considerably between the groups at any time point, while the mean concentration of elafin was 5-fold higher in the non-responders (100 ng/mL) than in the responders (19 ng/mL), and most notably, CK18F was on the average nearly nine times higher in the non-responders (2781 U/L) versus the responders (332 U/L) at day 0 (Figure 4A), albeit the differences were not statistically significant.
Figure 4.
Concentrations of GvHD-Related Serum Protein Markers in the Patients Responding and Not Responding to MSC Therapy
(A) Concentrations of Reg3α, CK18F, and elafin in serum samples collected from patients before the MSC treatment (day 0) and approximately 1 week and 1 month after the first MSC dose are shown. Results are shown as individual dots and mean values, with error bars indicating 95% confidence intervals. (B) Heatmaps showing the individual kinetics of the proteins during the follow-up period were created using log2 transformed concentrations. Patients are grouped according to the day 28 response to MSCs (A and B) and the GvHD target organ (B). The Kruskal-Wallis test was used to calculate statistical significance. All differences between the groups were statistically non-significant.
In the group with liver GvHD, CK18F levels decreased in the sole responding patient, but a decrease was noted also in one of the three non-responding patients (Figure 4B). Elafin levels, in turn, decreased in two and increased in two responders with skin symptoms (Figure 4B). Thus, in the present patient cohort, we found no evident value for the given serum proteins in MSC treatment follow-up. However, the results suggest that CK18F, which was on a particularly high level in the pre-MSC samples from non-responding patients with liver GvHD (Figures 3B and 4), may have potential in the prediction of patients’ response to MSC treatment.
Discussion
We studied the immunological response to MSC treatment in patients with aGvHD by studying blood samples collected immediately before and up to 1 month after the MSC treatment. GvHD is a T cell-driven complication of allo-HSCT, and especially the pro-inflammatory Th1 and Th17 CD4+ cells are considered responsible for the extensive tissue damage characteristic of this disease,23 and Th1 and Th17 cell levels have been reported to increase in peripheral blood during active GvHD.24, 25 MSCs are theoretically able to dampen excessive immune responses and induce tolerance in vitro through inhibition of these pro-inflammatory GvHD-promoting Th subsets and by induction of Tregs.26 Increased Treg levels have, for instance, been reported to be associated with the clinical remission in MSC-treated patients with systemic lupus erythematosus.27 Due to the proposed role of Tregs in the prevention and resolution of aGvHD,28, 29, 30 it should therefore be highly relevant to also study patient-specific levels of Tregs during an MSC treatment.
In line with previous studies, we can conclude that aGvHD patients indeed have elevated proportions of Th1 cells prior to MSC therapy, and a substantial decrease in Th1 cell proportions was observed during the MSC treatment. This, together with the relatively high and stable Treg levels, may reflect the desired immunosuppressive effect of MSCs, which skews the Th1/Treg ratio to a less inflammatory direction. We found no statistically significant differences in the relative proportions of Tregs between the patients responding and not responding to MSCs, which is in line with previous studies of Yin et al.19 and Te Boome et al.20 In the study of Dander et al.,17 where both complete responders were pediatric patients, decreased Th1 cell levels were also observed after MSC treatment, and this was further accompanied by an increase in Tregs. Our patient cohort consisted solely of adult patients and we, among others, have shown that children are more likely to respond to MSC therapy,8, 9 which might partially explain the differing results between the published studies.
The total number of CD4+ cells was extremely low in all patients, which is frequent after allo-HSCT. Due to the low CD4+ T cell numbers, the proportions of Th2 and Th17 cells remained at an undetectable level through the MSC treatment with a few exceptions. In five patients, the proportion of Th17 cells transiently increased to a detectable level. In these samples, the Th17 cell amounts were up to two times higher than the average level in the healthy controls, but within the similar range as previously reported in patients with active GvHD.24, 31 We cannot completely rule out the possibility of Th17 induction by MSCs, but it should be borne in mind that infections and other inflammatory events that are frequently seen in allo-HSCT recipients greatly influence the frequencies of the Th cell subsets. As reported previously, the infections in this patient cohort emerged not until 30–90 days after initiation of the MSC therapy,9 and without matching follow-up samples beyond 1 month we can merely speculate over potential causes for the Th17 induction.
Steroids and other immunosuppressive medications dampen immune responses. The patients in this cohort received a standardized GvHD prophylaxis protocol of either calcineurin inhibitor (CNI)/everolimus + short-course methotrexate (MTX) or CNI/everolimus + MTX + mycophenolate mofetil (MMF). All patients received a standardized protocol of 2 mg/kg methylprednisolone as first-line GvHD treatment as described previously.9 All patients, except one patient in the responder group, had received antithymocyte globulin (ATG; 2 doses of 2.5 mg/kg), which further standardizes the given GvHD prophylaxis in this patient cohort. At the time of the MSC treatment, most of the patients still received GvHD prophylaxis and 2 mg/kg of methylprednisolone and with no major differences between the responders and the non-responders. It is, however, impossible to draw any conclusions of patient-specific differences in GvHD prophylaxis and steroid dosing during the MSC therapy and its potential impact on the outcome with such small patient numbers. Interestingly, however, we can conclude that in this particular subcohort, more patients in the non-responder group had received the CNI/everolimus + MTX + MMF GvHD prophylaxis protocol versus the responder group (63% versus 13%, respectively). A more specific association analysis of GvHD prophylaxis protocols and corticosteroid dosing and outcome of MSC therapy needs to be performed with a substantially larger patient cohort when available. However, the noted overall immunological inertness of the patients, combined with the lack of serious treatment-related adverse effects in our study, suggest that MSC therapy is an immunologically safe salvage treatment option for patients with severe aGvHD.
The three protein markers that we analyzed from patient serum samples were selected because of their reported organ-specific association with aGvHD. Reg3α, which is an anti-microbial peptide secreted by Paneth’s cells in intestinal crypts, is an extensively validated serum marker of GI GvHD.32, 33 Elafin is an epidermal protease inhibitor whose plasma levels have been reported to increase in GvHD patients with skin symptoms.34 CK18F, in turn, is a caspase-cleaved fragment of cytokeratin-18 that is detectable in serum and its levels correlate with the extent of epithelial damage. High CK18F levels have been reported in patients with steroid-refractory GI and liver GvHD.35, 36 The concentrations of all three proteins were significantly higher in the patient than in the healthy blood donor samples in our study, supporting previous reports of their suitability as biological markers of aGvHD. Because Reg3α, CK18F, and elafin all are also indicators of ongoing tissue damage, it is of note that their average serum concentration in patients did not increase during the follow-up period.
The selected proteins appeared not to be optimal candidates for patient follow-up during MSC treatment. This was most evident with Reg3α, whose average concentration remained indistinguishable between the responder and non-responder groups throughout the MSC treatment period. Also, fluctuations in elafin concentrations during MSC treatment were highly individual, and no concordant changes between the responders and non-responders were noted. The most promising serum marker in our study was CK18F. The 9-fold elevated concentration of CK18F in the pre-MSC samples from non-responders suggests that serum CK18F could have potential in prediction of MSC therapy response. The average CK18F concentrations remained stable both in the responders and non-responders at later sampling points, which is in contrast to the results of Jitschin et al.,18 who described a decrease in CK18F levels in six patients treated with MSCs. Together these results give promise for the usefulness of CK18F in the MSC therapy setting and warrant future studies with larger patient cohorts.
Conclusions
We studied the immunological responses of 16 adult patients treated with bone marrow-derived MSCs for severe steroid-refractory aGvHD. We observed that a clinical response to the MSC therapy was not evident in the immune profile of the aGVHD patients. On the other hand, the MSCs did not either provoke any significant T cell-mediated immune activation in patients with aGvHD. The level of lymphocytes was low as frequently seen in aGvHD, especially after initiation of steroids, and remained stable during MSC treatment. The results of this study support the view that MSCs: (1) do not raise an immune response, at least in heavily immunosuppressed patients; and (2) can be safely utilized from unmatched third-party donors. Interestingly, we can conclude that our results are in line with the very recently published work by Galleu and colleagues,37 where a completely new immunomodulatory mechanism based on apoptosis and macrophage polarization was presented for MSCs. Our immune profiling results strongly support this theory, because no obvious differences were seen in the basic lymphocyte profiles between MSC responders and non-responders. We believe that the results from our study will strengthen this new and exciting paradigm shift concerning the immunomodulatory mechanisms of MSCs in aGVHD. Our study is yet limited by the small patient cohort size and the limited possibilities to collect comparable immunoprofiling samples during a longer follow-up period for more patients. Nevertheless, our results also support the immunological safety of utilizing pooled platelet lysate from multiple platelet donors in the production of MSCs. Moreover, our results suggest that serum CK18F could be useful in the prediction of MSC therapy response. Future studies are needed to confirm the potential predictive value of CK18F and the immunomodulatory mechanisms of MSCs in aGVHD.
Materials and Methods
Production of Mesenchymal Stromal Cells
Bone marrow-derived, third-party MSCs (in-house product name LY-MSC) were produced under a national ATMP hospital exemption license authorized by the Finnish Medicines Agency (Fimea; national ATMP manufacturing licenses #6322/20.10.01/2011 and #5103/20.30.01/2013) according to a validated standard operating procedure (SOP) and predetermined quality requirements in the Good Manufacturing Practice (GMP) facility of the Advanced Cell Therapy Centre, Finnish Red Cross Blood Service (FRCBS), Helsinki, Finland. The clinical-grade LY-MSC production method has been described in detail previously.9, 38 In brief, each MSC batch was derived from 40 mL of bone marrow from a healthy voluntary donor after adequate testing and health status evaluation. The donor tests are described in detail in Eichler et al.39 (p.71-72). The MSCs were expanded in pooled platelet lysate. The final LY-MSC product was composed of cells in p2 (detached when 95%–100% confluent), which were washed and frozen in human albumin and 10% DMSO in CryoMACS freezing bags (Miltenyi Biotec, Gladbach, Germany). The LY-MSC batch release was based on predetermined quality criteria covering MSC phenotype, cell morphology, population doubling number, differentiation capacity, in vitro immunosuppression capacity, viability, karyotype, sterility, and endotoxin levels. The release criteria for the LY-MSC batches have been described in more detail previously.39 Each MSC batch was exposed to platelet lysate pools derived from 48 or 96 blood donors and plasma from 12 or 24 donors. An individual patient was treated with cells from one or maximally two individual LY-MSC batches. The cryopreserved cells were delivered frozen to the clinic and used immediately after thawing and diluting bedside. The diluted MSC product contained 2 × 106 cells/mL.
Patients
The patient cohort and the MSC treatment protocol in our prospective single-arm study have been described previously.9 Sixteen consecutive adult aGvHD patients stem cell transplanted at the Turku University Hospital were included in the patient subcohort of this study. The present study cohort consisted of nine male and seven female patients, with the mean age of 46 (range 21–66) years. All patients had undergone allo-HSCT and had steroid-refractory grade II-IV aGvHD.9 Acute GVHD was graded according to the Glucksberg criteria,40 and steroid resistance was defined as a lack of response after 5 days of treatment with methylprednisolone (2 mg/kg), progression of the disease, or recurrent aGVHD while tapering steroids. Average time from HSCT to MSC therapy was 70 (range 25–438, median 114) days. The overall grade and the organ-specific stage of GvHD were recorded throughout the study period. The patient subgroup received twice-a-week MSC doses with an average total amount of 4 (range 1–6) doses and with a mean cell dose of 2 × 106 cells/kg (range 1.4–2.7 × 106 cells/kg). The sampling times and day 28 response status of the patients are described in Table 1.
The response to MSC treatment was assessed on day 28 after the first MSC dose. Organ-specific stage and overall grade were recorded, and the responses were classified as complete response (CR; complete resolution of symptoms), very good partial response (VGPR; decrease in overall grade ≥2 grades), partial response (PR; improvement less than VGPR), and no response (NR). Patients were considered responders if they demonstrated at least PR. The overall response rate in this cohort was 50% (8/16).
The patient groups were not biased for age or the amount of received MSC doses. The mean age was 46.3 (min 21, max 66) years in the responders and 45.6 (min 24, max 60) years in the non-responders (p = 0.9594; Mann-Whitney test). The responder group received in total 3–6 MSC doses (mean 4.8 doses; mean dosing 2.1 × 106 cells/kg), while the non-responders received 1–6 doses (mean 3.8 doses; mean dosing 1.9 × 106 cells/kg). The difference between the groups was not statistically significant either regarding the total amount of doses (p = 0.3587) or the amount of cells per kilogram (p = 0.1201; Mann-Whitney test).
The study was approved by the Ethical committee of Turku University Hospital. A written informed consent, in accordance with Declaration of Helsinki, was received from all patients.
Patient Sample Collection and Lymphocyte Count
Blood samples for immune cell profiling were collected from 11 patients and for serum protein analysis from 16 patients. Samples were collected before the first MSC infusion (day 0) and approximately 1–2 weeks and 1 month after the first MSC dose (Table 1). Cell sample processing was started within the same day, while serum samples were stored at −80°C until use. Lymphocyte differential counting was carried out with flow cytometry using a commercial staining kit for lymphocyte subsets (BD Multitest IMK kit and FACSCanto flow cytometer; Becton Dickinson, San Jose, CA, USA) in Turku University Hospital.
Control Samples from Healthy Individuals
Samples from eight healthy volunteers were used in the Th cell phenotyping as controls and to demonstrate the levels of Th cells in healthy individuals. For serum protein assays, healthy control samples were collected from 108 voluntary, non-remunerated blood donors at the FRCBS. Collection of the samples for determination of blood biological markers was approved by the Coordinating Ethics Committee of the Hospital District of Helsinki and Uusimaa (#221/13/03/00/2015), and written informed consent was obtained from the blood donors.
Th Cell Phenotyping
For Th phenotyping, peripheral blood mononuclear cells (PBMCs) were isolated from fresh blood samples collected in EDTA-tubes by using density gradient centrifugation (Ficoll-Paque PLUS; GE Healthcare, Uppsala, Sweden). After isolation the cells were kept in media consisting of RPMI 1640 with 5% fetal bovine serum (FBS) and 100 U/mL penicillin-streptomycin (all from GIBCO, Paisley, Scotland).
For Th cell determination, PBMCs were stimulated with phorbol 12-myristate 13-acetate (PMA) and ionomycin (both from Sigma-Aldrich, Saint Louis, MO, USA) in the presence of GolgiStop reagent (BD Biosciences, San Diego, CA, USA) for 5 hr in +37°C in 5% CO2. The cells were then fixed with Cytofix buffer (BD Biosciences), suspended in FBS containing 10% DMSO (Sigma-Aldrich), and stored at −80°C until labeling and analysis. The baseline secretion of cytokines was assessed from unstimulated cells treated with GolgiStop only. After thawing, Th cell staining was performed with the Human Th1/Th2/Th17 Phenotyping Cocktail (BD Biosciences) according to the manufacturer’s instructions. Appropriate isotype controls were used to measure background fluorescence.
For Treg analysis, unstimulated cells were fixed with a formaldehyde-containing fixative from the Human FOXP3 Buffer Set (BD Biosciences) according to the manufacturer’s instructions and stored frozen in FBS containing 10% DMSO (Sigma) at −80°C until labeling and analysis. After thawing, the cells were stained with anti-CD4-PerCP-Cy5.5 and anti-FOXP3-Alexa Fluor 647 according to manufacturer’s instructions, and appropriate isotype controls (all from BD Biosciences) were used.
We determined to use cryopreserved cells in this assay after a careful comparison with fresh cells. Fixed and frozen cells allowed us to perform the analysis of each patient’s samples in the same batch in order to minimize the effects of intra-assay variation. This was done in accordance with the manufacturers’ recommendations.
Data acquisition and analysis were carried out using the FACSAria IIu flow cytometer (BD Biosciences) and FlowJo software (version 10.0.7; FlowJo, Ashland, OR, USA).
Serum Protein Analysis
Concentrations of Reg3α (PAP1; Ab-Match Assembly Human PAP1 kit and Ab-Match Universal kit; MBL International, Des Plaines, IL, USA), CK18F (M30 Apoptosense ELISA; Peviva AB, Sundbyberg, Sweden), and elafin (R&D Systems, Minneapolis, MN, USA) were measured from patient and healthy control serum samples by sandwich ELISA according to the manufacturers’ protocols. Serum samples with very high protein concentrations were diluted to match the protocols’ ranges of detection. Absorbance at 450 nm was measured by using the ClarioStar reader (BMG Labtech, Ortenberg, Germany). Samples were run in triplicate. Analyses were carried out with the Mars Data Analysis software (BMG Labtech).
Statistical Analyses
Statistical analyses were performed using the GraphPad Prism (version 7.02) software (GraphPad Software, La Jolla, CA, USA). Kruskal-Wallis test was used in comparison of more than two groups, i.e., patient samples from different sampling points. Mann-Whitney test was used for the comparison of two groups, i.e., patient versus healthy control samples and responder versus non-responder samples. Differences were considered statistically significant when p < 0.05.
Author Contributions
Conceptualization and Methodology, A.H., M.I.-R., T.K., J.K., M.K., K.L., J.N., J.P., and U.S.; Investigation and Formal Analysis, T.K., J.K., and K.L.; Resources, J.C., M.K., J.N., J.P., M.I.-R., and U.S.; Writing – Original Draft, J.K., K.L., and J.N.; Writing – Review & Editing, J.C., A.H., M.I.-R., T.K., J.K., M.K., K.L., J.N., J.P., and U.S.; Visualization, J.K. and K.L.; Supervision, K.L. and J.N.; Project Administration, J.N.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgments
The authors sincerely thank the technical personnel at the FRCBS Advanced Cell Therapy Centre and Lotta Andersson for excellent technical assistance. The personnel at the FRCBS Espoo Blood Service Centre are acknowledged for professional help in collection of serum samples from healthy blood donors. The study was partially funded by the state VTR research funding system in Finland and by the SalWe Research Programs for IMO and GetItDone (Tekes—the Finnish Funding Agency for Technology and Innovation grants 648/10 and 3986/31/2013).
Footnotes
Supplemental Information includes one figure and can be found with this article online at https://doi.org/10.1016/j.omtm.2018.02.001.
Supplemental Information
References
- 1.Introna M., Rambaldi A. Mesenchymal stromal cells for prevention and treatment of graft-versus-host disease: successes and hurdles. Curr. Opin. Organ Transplant. 2015;20:72–78. doi: 10.1097/MOT.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 2.Cahn J.Y., Klein J.P., Lee S.J., Milpied N., Blaise D., Antin J.H., Leblond V., Ifrah N., Jouet J.P., Loberiza F., Société Française de Greffe de Moëlle et Thérapie Cellulaire. Dana Farber Cancer Institute. International Bone Marrow Transplant Registry Prospective evaluation of 2 acute graft-versus-host (GVHD) grading systems: a joint Société Française de Greffe de Moëlle et Thérapie Cellulaire (SFGM-TC), Dana Farber Cancer Institute (DFCI), and International Bone Marrow Transplant Registry (IBMTR) prospective study. Blood. 2005;106:1495–1500. doi: 10.1182/blood-2004-11-4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Le Blanc K., Frassoni F., Ball L., Locatelli F., Roelofs H., Lewis I., Lanino E., Sundberg B., Bernardo M.E., Remberger M., Developmental Committee of the European Group for Blood and Marrow Transplantation Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 4.Kebriaei P., Isola L., Bahceci E., Holland K., Rowley S., McGuirk J., Devetten M., Jansen J., Herzig R., Schuster M. Adult human mesenchymal stem cells added to corticosteroid therapy for the treatment of acute graft-versus-host disease. Biol. Blood Marrow Transplant. 2009;15:804–811. doi: 10.1016/j.bbmt.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Ball L.M., Bernardo M.E., Roelofs H., van Tol M.J., Contoli B., Zwaginga J.J., Avanzini M.A., Conforti A., Bertaina A., Giorgiani G. Multiple infusions of mesenchymal stromal cells induce sustained remission in children with steroid-refractory, grade III-IV acute graft-versus-host disease. Br. J. Haematol. 2013;163:501–509. doi: 10.1111/bjh.12545. [DOI] [PubMed] [Google Scholar]
- 6.Resnick I.B., Barkats C., Shapira M.Y., Stepensky P., Bloom A.I., Shimoni A., Mankuta D., Varda-Bloom N., Rheingold L., Yeshurun M. Treatment of severe steroid resistant acute GVHD with mesenchymal stromal cells (MSC) Am. J. Blood Res. 2013;3:225–238. [PMC free article] [PubMed] [Google Scholar]
- 7.Hashmi S., Ahmed M., Murad M.H., Litzow M.R., Adams R.H., Ball L.M., Prasad V.K., Kebriaei P., Ringden O. Survival after mesenchymal stromal cell therapy in steroid-refractory acute graft-versus-host disease: systematic review and meta-analysis. Lancet Haematol. 2016;3:e45–e52. doi: 10.1016/S2352-3026(15)00224-0. [DOI] [PubMed] [Google Scholar]
- 8.Chen X., Wang C., Yin J., Xu J., Wei J., Zhang Y. Efficacy of mesenchymal stem cell therapy for steroid-refractory acute graft-versus-host disease following allogeneic hematopoietic stem cell transplantation: a systematic review and meta-analysis. PLoS ONE. 2015;10:e0136991. doi: 10.1371/journal.pone.0136991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salmenniemi U., Itälä-Remes M., Nystedt J., Putkonen M., Niittyvuopio R., Vettenranta K., Korhonen M. Good responses but high TRM in adult patients after MSC therapy for GvHD. Bone Marrow Transplant. 2017;52:606–608. doi: 10.1038/bmt.2016.317. [DOI] [PubMed] [Google Scholar]
- 10.Zhao K., Lou R., Huang F., Peng Y., Jiang Z., Huang K., Wu X., Zhang Y., Fan Z., Zhou H. Immunomodulation effects of mesenchymal stromal cells on acute graft-versus-host disease after hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 2015;21:97–104. doi: 10.1016/j.bbmt.2014.09.030. [DOI] [PubMed] [Google Scholar]
- 11.Ankrum J.A., Ong J.F., Karp J.M. Mesenchymal stem cells: immune evasive, not immune privileged. Nat. Biotechnol. 2014;32:252–260. doi: 10.1038/nbt.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Najar M., Raicevic G., Fayyad-Kazan H., Bron D., Toungouz M., Lagneaux L. Mesenchymal stromal cells and immunomodulation: a gathering of regulatory immune cells. Cytotherapy. 2016;18:160–171. doi: 10.1016/j.jcyt.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 13.Paczesny S., Levine J.E., Braun T.M., Ferrara J.L. Plasma biomarkers in graft-versus-host disease: a new era? Biol. Blood Marrow Transplant. 2009;15(Suppl 1):33–38. doi: 10.1016/j.bbmt.2008.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paczesny S., Krijanovski O.I., Braun T.M., Choi S.W., Clouthier S.G., Kuick R., Misek D.E., Cooke K.R., Kitko C.L., Weyand A. A biomarker panel for acute graft-versus-host disease. Blood. 2009;113:273–278. doi: 10.1182/blood-2008-07-167098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paczesny S., Raiker N., Brooks S., Mumaw C. Graft-versus-host disease biomarkers: omics and personalized medicine. Int. J. Hematol. 2013;98:275–292. doi: 10.1007/s12185-013-1406-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ali A.M., DiPersio J.F., Schroeder M.A. The role of biomarkers in the diagnosis and risk stratification of acute graft-versus-host disease: a systematic review. Biol. Blood Marrow Transplant. 2016;22:1552–1564. doi: 10.1016/j.bbmt.2016.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dander E., Lucchini G., Vinci P., Introna M., Masciocchi F., Perseghin P., Balduzzi A., Bonanomi S., Longoni D., Gaipa G. Mesenchymal stromal cells for the treatment of graft-versus-host disease: understanding the in vivo biological effect through patient immune monitoring. Leukemia. 2012;26:1681–1684. doi: 10.1038/leu.2011.384. [DOI] [PubMed] [Google Scholar]
- 18.Jitschin R., Mougiakakos D., Von Bahr L., Völkl S., Moll G., Ringden O., Kiessling R., Linder S., Le Blanc K. Alterations in the cellular immune compartment of patients treated with third-party mesenchymal stromal cells following allogeneic hematopoietic stem cell transplantation. Stem Cells. 2013;31:1715–1725. doi: 10.1002/stem.1386. [DOI] [PubMed] [Google Scholar]
- 19.Yin F., Battiwalla M., Ito S., Feng X., Chinian F., Melenhorst J.J., Koklanaris E., Sabatino M., Stroncek D., Samsel L. Bone marrow mesenchymal stromal cells to treat tissue damage in allogeneic stem cell transplant recipients: correlation of biological markers with clinical responses. Stem Cells. 2014;32:1278–1288. doi: 10.1002/stem.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Te Boome L.C., Mansilla C., van der Wagen L.E., Lindemans C.A., Petersen E.J., Spierings E., Thus K.A., Westinga K., Plantinga M., Bierings M. Biomarker profiling of steroid-resistant acute GVHD in patients after infusion of mesenchymal stromal cells. Leukemia. 2015;29:1839–1846. doi: 10.1038/leu.2015.89. [DOI] [PubMed] [Google Scholar]
- 21.McDonald G.B., Tabellini L., Storer B.E., Lawler R.L., Martin P.J., Hansen J.A. Plasma biomarkers of acute GVHD and nonrelapse mortality: predictive value of measurements before GVHD onset and treatment. Blood. 2015;126:113–120. doi: 10.1182/blood-2015-03-636753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y.B., Cutler C.S. Biomarkers for acute GVHD: can we predict the unpredictable? Bone Marrow Transplant. 2013;48:755–760. doi: 10.1038/bmt.2012.143. [DOI] [PubMed] [Google Scholar]
- 23.Fu J., Heinrichs J., Yu X.Z. Helper T-cell differentiation in graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Arch. Immunol. Ther. Exp. (Warsz.) 2014;62:277–301. doi: 10.1007/s00005-014-0284-z. [DOI] [PubMed] [Google Scholar]
- 24.Dander E., Balduzzi A., Zappa G., Lucchini G., Perseghin P., Andrè V., Todisco E., Rahal D., Migliavacca M., Longoni D. Interleukin-17-producing T-helper cells as new potential player mediating graft-versus-host disease in patients undergoing allogeneic stem-cell transplantation. Transplantation. 2009;88:1261–1272. doi: 10.1097/TP.0b013e3181bc267e. [DOI] [PubMed] [Google Scholar]
- 25.Yeh S.P., Liao Y.M., Lo W.J., Lin C.L., Bai L.Y., Lin C.Y., Hsieh C.Y., Chang Y.C., Huang Y.T., Chiu C.F. Kinetics of T helper subsets and associated cytokines correlate well with the clinical activity of graft-versus-host disease. PLoS ONE. 2012;7:e44416. doi: 10.1371/journal.pone.0044416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aggarwal S., Pittenger M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 27.Sun L., Wang D., Liang J., Zhang H., Feng X., Wang H., Hua B., Liu B., Ye S., Hu X. Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum. 2010;62:2467–2475. doi: 10.1002/art.27548. [DOI] [PubMed] [Google Scholar]
- 28.Di Ianni M., Falzetti F., Carotti A., Terenzi A., Castellino F., Bonifacio E., Del Papa B., Zei T., Ostini R.I., Cecchini D. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117:3921–3928. doi: 10.1182/blood-2010-10-311894. [DOI] [PubMed] [Google Scholar]
- 29.Edinger M., Hoffmann P., Ermann J., Drago K., Fathman C.G., Strober S., Negrin R.S. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat. Med. 2003;9:1144–1150. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 30.Heinrichs J., Bastian D., Veerapathran A., Anasetti C., Betts B., Yu X.Z. Regulatory T-cell therapy for graft-versus-host disease. J. Immunol. Res. Ther. 2016;1:1–14. [PMC free article] [PubMed] [Google Scholar]
- 31.Dlubek D., Turlej E., Sedzimirska M., Lange J., Lange A. Interleukin-17-producing cells increase among CD4+ lymphocytes before overt manifestation of acute graft-versus-host disease. Transplant. Proc. 2010;42:3277–3279. doi: 10.1016/j.transproceed.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 32.Gironella M., Iovanna J.L., Sans M., Gil F., Peñalva M., Closa D., Miquel R., Piqué J.M., Panés J. Anti-inflammatory effects of pancreatitis associated protein in inflammatory bowel disease. Gut. 2005;54:1244–1253. doi: 10.1136/gut.2004.056309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferrara J.L., Harris A.C., Greenson J.K., Braun T.M., Holler E., Teshima T., Levine J.E., Choi S.W., Huber E., Landfried K. Regenerating islet-derived 3-alpha is a biomarker of gastrointestinal graft-versus-host disease. Blood. 2011;118:6702–6708. doi: 10.1182/blood-2011-08-375006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paczesny S., Braun T.M., Levine J.E., Hogan J., Crawford J., Coffing B., Olsen S., Choi S.W., Wang H., Faca V. Elafin is a biomarker of graft-versus-host disease of the skin. Sci. Transl. Med. 2010;2:13ra2. doi: 10.1126/scitranslmed.3000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luft T., Conzelmann M., Benner A., Rieger M., Hess M., Strohhaecker U., Görner M., Hegenbart U., Ho A.D., Dreger P. Serum cytokeratin-18 fragments as quantitative markers of epithelial apoptosis in liver and intestinal graft-versus-host disease. Blood. 2007;110:4535–4542. doi: 10.1182/blood-2006-10-049817. [DOI] [PubMed] [Google Scholar]
- 36.Luft T., Dietrich S., Falk C., Conzelmann M., Hess M., Benner A., Neumann F., Isermann B., Hegenbart U., Ho A.D., Dreger P. Steroid-refractory GVHD: T-cell attack within a vulnerable endothelial system. Blood. 2011;118:1685–1692. doi: 10.1182/blood-2011-02-334821. [DOI] [PubMed] [Google Scholar]
- 37.Galleu A., Riffo-Vasquez Y., Trento C., Lomas C., Dolcetti L., Cheung T.S., von Bonin M., Barbieri L., Halai K., Ward S. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci. Transl. Med. 2017;9:eaam7828. doi: 10.1126/scitranslmed.aam7828. [DOI] [PubMed] [Google Scholar]
- 38.Laitinen A., Oja S., Kilpinen L., Kaartinen T., Möller J., Laitinen S., Korhonen M., Nystedt J. A robust and reproducible animal serum-free culture method for clinical-grade bone marrow-derived mesenchymal stromal cells. Cytotechnology. 2016;68:891–906. doi: 10.1007/s10616-014-9841-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eichler H., Schrezenmeier H., Schallmoser K., Strunk D., Nystedt J., Kaartinen T., Korhonen M., Fleury-Cappellesso S., Sensebé L., Bönig H. Donor selection and release criteria of cellular therapy products. Vox Sang. 2013;104:67–91. doi: 10.1111/j.1423-0410.2012.01619.x. [DOI] [PubMed] [Google Scholar]
- 40.Glucksberg H., Storb R., Fefer A., Buckner C.D., Neiman P.E., Clift R.A., Lerner K.G., Thomas E.D. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.