Abstract
INTRODUCTION: Gene expression analyses have identified similarities between bladder and breast cancer, where clinical risk stratification is based on Her2, ESR1, PGR and Ki67 expression. The aim of the study was to assess the respective marker gene expression in patients treated with radical cystectomy for muscle-invasive bladder cancer (MIBC) and to evaluate the applicability of breast cancer subtypes for MIBC risk stratification. MATERIALS & METHODS: 102 patients treated with radical cystectomy for MIBC were assessed. Using routine FFPE tissue and an IVD validated kit, mRNA expression was measured by single step RT-qPCR. Partition test were employed to define cut-off values for high or low marker gene expression. Association of expression with outcome was assessed using Kaplan-Meier analysis and multivariate cox regression analysis. Finally, we performed validation of our results in the MD-Anderson cohort (n = 57). RESULTS: Cancer specific survival (CSS) was impaired in patients with high gene expression of Her2 (P = 0.0009) and ESR1 (P = 0.04). In the multivariate regression model Her2 expression remained significant for the prediction of CSS (HR = 2.11, CI 1.11-4.21, P = 0.024). Furthermore, molecular stratification by breast cancer subgroups was significant (P = 0.023) for CSS prediction. Especially the differentiation between Her2-positive and Luminal A (HR = 4.41, CI 1.53-18.71, P = 0.004) and Luminal B (HR = 1.96, CI 0.99-4.08, P = 0.053) respectively was an independent prognostic parameter for CSS. External validation resulted in comparable risk stratification with differences in fractional subgroups distribution. CONCLUSION: Gene expression of Her2, ESR1, PGR, Ki67 and corresponding breast cancer subtypes allow a risk-stratification in MIBC, whereby Her2 overexpressing tumors reveal a particularly poor prognosis.
Introduction
Urothelial carcinoma (UCC) of the bladder is the second most common urogenital neoplasm worldwide. Standard clinical parameters in bladder cancer such as stage, grade or patient’s age have limitations in predicting individual patient's prognosis and response to different treatment options [1]. Therefore, identifying distinct molecular subtypes of UC of the bladder is highly anticipated to improve risk stratification and provide individual therapy regimens in the future [2]. Recently, gene expression profiling of UC has identified molecular subgroups that can help to predict outcome and clinical stage or even select patients for systemic therapies [3], [4], [5], [6], [7]. Up to now at least four different molecular classifications have been proposed [2], [3], [8], [9], [10]. Recently, a comprehensive comparison of the existing models revealed a large overlap between the respective subgroups [11]. Although molecular classification of UC evolve prediction of treatment response towards a more personalized therapy, a lack of standardized assessment of subgroups still prevents their clinical use [11].
Interestingly, the molecular sub-classification of UC resemble the biological situation in breast cancer revealing a comparable clinical outcome prediction [4], [8]. Accordingly some UC classifications identified basal and luminal subtypes [2], [12]. Currently, clinical breast cancer management involves assessment of gene expression of four molecular markers to stratify therapy and predict prognosis. The markers are the oncogene human epidermal growth factor receptor 2 (Her2), estrogen receptor 1 (ESR1), progesterone receptor (PR) and the proliferation marker Ki67 protein (Ki-67) [2]. Assessing the respective marker expression allows an assignment to one of four subgroups Her2 positive, Luminal A, Luminal B and Triple Negative initially defined by gene expression profiling [13], [14]. The subtypes have got a distinct prognosis and are differentially treated [15].
Given the discovered similarities between UC and breast cancer, the aim of this study was to assess the respective marker panel in muscle invasive bladder cancer. Here, Her2 has already been shown to be frequently overexpressed and associated with features of aggressiveness and metastases, whereas finding concerning its prognostic role remain controversy [16], [17]. Moreover targeted therapies against Her2 are available and currently under evaluation [18]. For Ki-67 expression multiple studies haven proven prognostic significance in bladder cancer [19], [20]. The role of the hormone receptors ESR1 and PGR as prognostic markers in MIBC are less intensively studied [21], [22]. Generally, immunohistochemistry (IHC) is the most commonly used method to analyze marker expression in breast cancer. However, IHC is associated with inter-observer variability and varies depending on the antibodies used [23]. Therefore a standardized molecular diagnostic tool was used, that allows objectively assessing expression of Her2, ESR1, PR and Ki-67 in patients with breast cancer. This would possibly allow stratifying UCC in a simple, standardized and valid method on the basis of Her2, ESR1, PGR and Ki-67 expression. The MammaTyper® enables simple and fast measurement of the mRNA transcripts of the corresponding genes (Her2, ESR1, PGR, and Ki-67) in routine FFPE material. Furthermore it is a quantitative, sensitive and objective method, not affected by inter-observer variability and can be performed in a standardized and automated manner [24], [25], [26]. In the current pilot study we thus evaluated the feasibility of mRNA based molecular characterization of bladder cancer with a standardized kit and assessed the predictive efficiency of the marker expression (Her2, ESR1, PGR and Ki-67) and the resulting molecular subgroups (Her2 positive, Luminal A, Luminal B and Triple Negative) in a cohort of patients treated with radical cystectomy (RC) for MIBC.
Patients and Methods
Patient Population
The study was approved by the institutional ethical committee (2013-834R-MA). 102 patients with MIBC were treated with RC (T1-4, Nx) and bilateral lymphadenectomy (obturator fossa, external and internal iliac region) at the Department of Urology of the University Hospital Center Mannheim between 2000 and 2010. Patients with lymph node metastases or ≥ pT3a disease were offered an adjuvant chemotherapy, which was conducted or not depending on shared decision making with the patients. Tumor tissue samples (RC) were obtained retrospectively. Patients, who received neo-adjuvant chemotherapy, were excluded. Clinical records were assessed for clinical and pathological data.
Pathological Evaluation
After intraoperative frozen section of ureteric and urethral resection margins, tissue was fixed in either 10% non-buffered or 10% buffered formaldehyde, followed by paraffin-embedding using standardized protocols. Storage time of the archival samples was up to 15 years at room temperature. Hematoxylin-eosin stained sections were re-evaluated within this study for pathological stage according to the 2002 TNM classification of the American Joint Committee on Cancer and tumor grade according to the 1998 WHO/International Society of Urologic Pathology consensus classification. Tumor samples and respective patients with pure squamous differentiation were excluded.
RNA Isolation
RNA isolation from formalin-fixed paraffin-embedded (FFPE) tissue was performed as described before [26], [27], [28]. From each tumor samples a 3μm section was cut, H&E stained and evaluated for tumor fraction. A corresponding 10-μm-thick section was used for subsequent isolation of RNA. A tumor fraction of more than 20% has been shown to enable the use the entire section for valid RNA isolation [23], [24]. In case of less malignant tissue on the section macro-dissection of malignant tissue was performed. RNA isolation was conducted according to a fully automated, high-throughput extraction workflow on an Xtract XL liquid-handling robot (STRATIFYER Molecular Pathology GmbH, Cologne, Germany). The extraction solutions and chemicals are commercially available in Germany as part of the XTRAKT FFPE kit, which is based on magnetic bead technology (STRATIFYER). In brief, FFPE sections were solubilized and paraffin was melted by incubating with a lysis buffer in a thermo-mixer. Tissue digestion was performed with Proteinase K. The lysates were then admixed with germanium-coated magnetic particles in buffer-controlled conditions which enhance preferential attachment of nucleic acid molecules to the surface of the particles. Purification was carried out by means of 3 consecutive washing cycles involving magnetization, centrifugation, washing and removal of the supernatant. Nucleic acids were eluted with 100 μl elution buffer and treated with DNase I. The DNA-free RNA eluates were stored at -80°C until use. RNA was reversely transcribed using the sequence specific primers and Super Script III reverse transcriptase (Thermo Fisher Scientific, Waltham, USA).
Total RNA from cell lines were extracted using RNeasy Mini Kit (Qiagen, Hilden Germany) according to the manufacturer’s instructions. RNA was reversely transcribed using random hexamer priming and MMLV reverse transcriptase (Thermo Fisher Scientific, Waltham, USA).
Gene Expression by RT-qPCR
The mRNA expression levels of ERBB2, ESR1, PGR, and MKI67 as well as of two reference genes (REF), namely B2M (Beta2 Microglobulin) and CALM2 (Calmodulin2), were determined by RT-qPCR, using the MammaTyper® Kit as described before [25]. The 6 assays (assay = primer pair and probe specific for the respective target sequence) were duplexed into three assay mixes, each using a pair of hydrolysis probes labeled with different fluorophores for separate detection of the duplexed assays.
The experiments were run on the StepOnePlus Real-Time PCR System (Thermo Fisher, Waltham, USA) with 30 min at 50° C, 2 min at 95° C followed by 40 cycles of 15 sec at 95° C and 30 sec at 60° C. according to MammaTyper® instructions for use (140603-90020-EU Rev 2.0).
Gene expression levels were calculated as described before [24], [25]: In short, cycle quantification threshold (Cq) values of maker genes (MG) for each sample (S) were estimated as the median of the triplicate measurements. To correct for inter-run variations Cq values were normalized against the mean expression of the REF genes (B2M and CALM2) and set off against a calibrator (PC) (ΔΔCq method). By subtracting ΔΔCq from the total number of cycles (40) it was ensured that normalized gene expression is proportional to the corresponding mRNA expression levels. This method facilitates interpretation of data and clinicopathological correlations. The various calculation steps are summarized in the following formula:
Statistical Analysis
Significance in differences of gene expression levels was calculated using an unpaired two-sided t-test. Association between gene expression values and patient characteristics or histopathological data was calculated using the Mann-Whitney test. Cut-Off definitions for gene expression were done by Partitioning tests analysis in regard to CSS. This test partitions data according to a relationship between X and Y values creating a tree of partitions. The partition is performed recursively and thus the optimum splits are identified from a large number of possible splits. The defined cut-off values were: Her2 ≥37.68, ESR1 ≥32.1 , PGR ≥34.27, MKI67 ≥35.85. Log rank analyses were performed stratified by marker gene expression and subgroup assignment in regard to CSS and RFS and illustrated in Kaplan-Meier curves. Univariable Cox proportional hazard models for CSS and RFS assessed clinical and pathological parameters as well marker gene expression and subgroup attribution. Significant parameters from the univariate analysis were analyzed in a multivariate model. All tests were performed two sided and a P< 0.05 was considered to be statistically significant. All statistical analyses were performed using SAS JMP 10.0 and Graph Pad Prism 5.
Molecular Subtyping
To reflect the situation in breast cancer the tumors were stratified into the respective subgroups Her2-positive, Luminal A, Luminal B and Triple negative depending on the expression of the four marker genes [13], [29]. Table 1S shows a simplified subtype classification, which was employed in the current study as described before [29].
External Validation Using the MDACC Cohort
For external validation, array gene expression data of 57 MIBC patients (49 male, 8 female) of the MDACC cohort (GSE48276) were analyzed (median age 67, range 41-89). Analogously, to our cohort partition tests for marker gene expression levels were used to define marker gene expression and subgroup assignment.
Results
Patient Population and Gene Expression
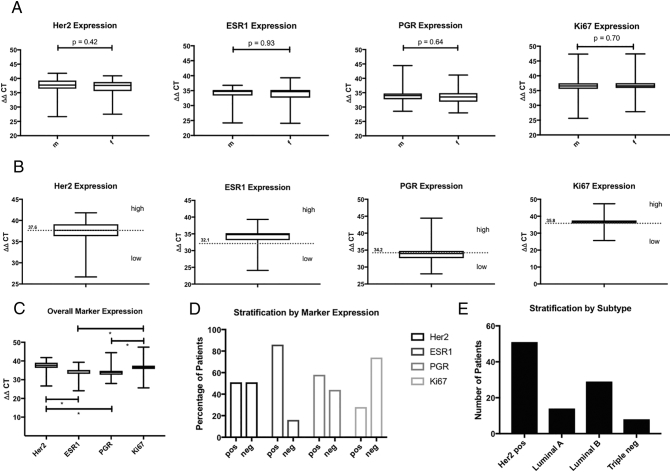
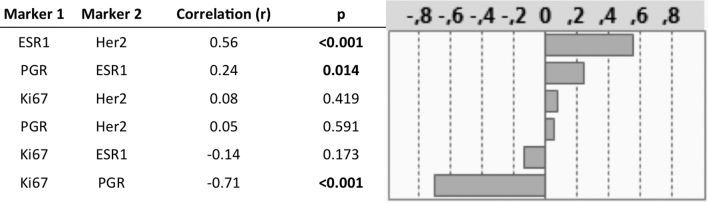
Overall 102 patients (Male 77, female 25) treated with radical cystectomy due to MIBC in a transurethral resection specimen were assessed. The median follow-up of this study population was 20.8 (3.1-179.1) months. None of the patients underwent neo-adjuvant chemotherapy, whereas 11.8% of the (n = 12) patients received an adjuvant systemic treatment (Cis-Platin/Gemcitabine). Clinical and pathological characteristics of the population are listed in Table 1. Pathological reports revealed pT3/4 tumors for 76 (73.8%) cases and 9 (8.8%) patients had a concomitant carcinoma in situ. Overall tumor samples of 11 (10.7) patients revealed micro-papillary differentiation. Median number of removed lymph nodes was 10 (1–28) and 63.7% (n = 65) of the patients had no lymphatic metastasis. Cancer-specific death was observed in 50 cases (49.0%) after a median follow-up of 17.6 (3.1-86.5) months. As shown in Figure 1, C expression of Her2 and Ki67 was significantly higher compared to ESR1 and PGR (P < .0001). There was a high and positive correlation of 0.56 (P < 0.001) between Her2 and ESR1 and a negative correlation between PGR and Ki67 of –0.71 (P < 0.0001). Correlation of the marker gene expression levels is illustrated in Figure 1S.
Table 1.
Patient´s Characteristics of 102 Patients Undergoing Radical Cystectomy
| Mannheim Cohort |
MD Anderson Cohort |
P | |||
|---|---|---|---|---|---|
| n or median | percentage/range | n or median | percentage/range | ||
| Age, y | 66.6 | 45.4-92.1 | 67.4 | 41.0-90.6 | 0.72 |
| ≥75 | 21 | 20.6 | 9 | 15.7 | 0.53 |
| <75 | 81 | 79.4 | 48 | 84.2 | |
| Gender | |||||
| Male | 77 | 75.5 | 49 | 86.0 | 0.29 |
| Female | 25 | 24.5 | 8 | 14.0 | |
| Tumor stage | |||||
| pT1 | 2 | 2.0 | 2 | 3.5 | 0.51 |
| pT2 | 24 | 23.5 | 12 | 21.1 | |
| pT3 | 56 | 53.9 | 35 | 61.4 | |
| pT4 | 20 | 19.6 | 8 | 14.0 | |
| CIS | 0 | 0 | 0 | 0 | |
| pT4 or pN+ | 47 | 46.1 | 39 | 68.4 | 0.001 |
| Nodal stage | |||||
| pN0 | 65 | 63.7 | 22 | 38.6 | 0.002 |
| pN1 | 12 | 11.8 | 9 | 15.8 | |
| pN2 | 25 | 24.5 | 26 | 45.6 | |
| Grading | |||||
| G3 | 82 | 80.5 | n.a. | n.a. | |
| G2 | 19 | 18.6 | n.a. | n.a. | |
| G1 | 1 | 0.9 | n.a. | n.a. | |
| Concomitant carcinoma in situ | 23 | 22.5 | n.a. | n.a. | |
| Chemotherapy | |||||
| Neo-adjuvant | 0 | 0 | 20 | 35.0 | <0.001 |
| Adjuvant | 12 | 14.8 | n.a. | n.a. | |
| Outcome | |||||
| Follow-up | 20.8 | 3.6-179.2 | 38.1 | 3.9-180 | 0.63 |
| Cancer-specific death | 50 | 49.0 | 17 | 30.0 | 0.01 |
| Time to Cancer-specific death, months | 17.6 | 3.1-86.5 | 24.2 | 4.8-79.2 | 0.17 |
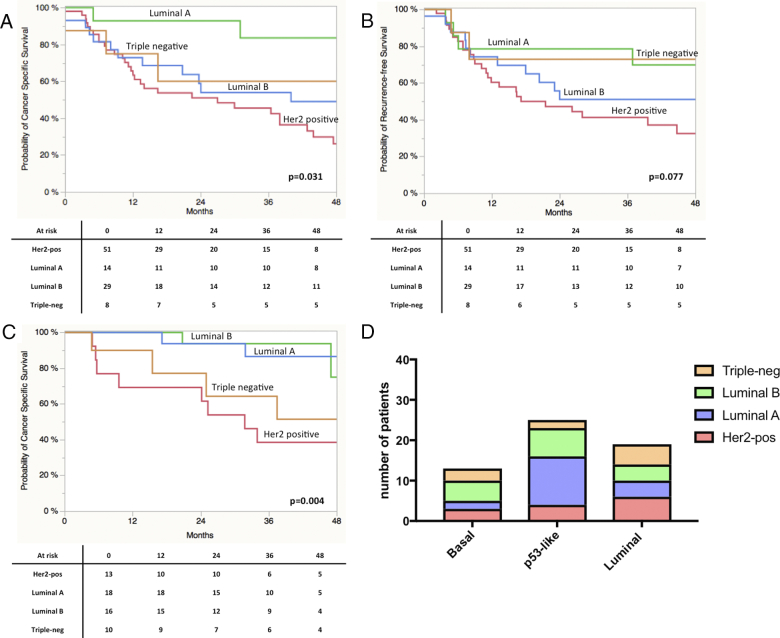
Figure 1.
(A) Normalized marker gene expression distributed by gender. (B) Normalized maker gene expression and respective cut-off levels defined by participation analyses. (C) Overall expression of the four marker genes. (D) Percentage of patients/tumors defined as positive or negative by the respective marker gene expression based-stratification. (E) Number of patients allocated to the four breast cancer subtypes.
Figure 1S.
Correlation gives as Spearman´s rho (r) between different marker gene expressions.
Association of Gene Expression with Histopathology
Normalized expression of the four markers was 36.8 ± 0.34 for Her2, 33.6 ± 0.29 for ESR1, 34.2 ± 0.3 for PGR and 36.6 ± 0.38 for Ki67. No difference in marker gene expression between male and female patients was found as shown in Figure 1, A. In addition, normalized marker gene expression did not differ when stratified for tumor stage (T2 vs. T3/4 and T1-3 vs. T4 and/or N+), nodal stage (pN0 vs. pN+), Grading (G1-2 vs. G3-4) and LVI (data not shown). Micropapillary tumors showed a higher Her2 expression, which however did not reach statistical significance (38.11 vs. to 36.7, P = 0.2). Figure 1, B shows the results of the participation tests. Thus the cut-off levels were determined at 37.6 (Her2), 32.1 (ESR1), 34.2 (PGR) and 35.8 (Ki67). This resulted in the respective marker-based stratification as displayed in Figure 1, D. On the basis of the classification illustrated in Table 1S patients were assigned to one of the respective subgroups, namely Her2 positive 50.0% (n = 51), 13.7% Luminal A (n = 14), 28.4% Luminal B (n = 29) and Triple Negative 7.8% (n = 8) (Figure 1, E).
Table 2 illustrates the differences in patients and tumor characteristics between the four subgroups. There were no differences observed between the subgroups expect for the Luminal B group revealing significantly less pT2 (vs. pT3-4) tumors.
Table 2.
Distribution of Patient´s and Tumor Characteristics Between Different Subtypes in the Mannheim and MD Anderson (*) cohort
| Her2-positiv |
Luminal A like |
Luminal B like |
Triple neg |
|||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | |
| Patient | ||||||||
| Age ≥75 (vs. <75) | 23.5 (12) | 17.6 (9) | 14.3 (2) | 21.6(19) | 20.7 (6) | 20.5 (15) | 12.5 (1) | 21.3 (20) |
| Age ≥75 (vs. <75)* | 1.75 (1) | 14.0 (8) | 7.0 (4) | 8.8 (5) | 3.5 (2) | 12.3 (7) | 12.3 (7) | 3.5 (2) |
| Male (vs. female) | 78.4 (40) | 72.5 (37) | 78.5 (11) | 75.0 (66) | 72.4 (21) | 76.7 (56) | 62.5 (5) | 76.6 (72) |
| Male (vs. female)* | 17.5 (10) | 68.4 (39) | 31.6 (18) | 54.4 (31) | 21.1 (12) | 64.9 (37) | 15.8 (9) | 70.2 (40) |
| Tumor | ||||||||
| pT1-2 (vs. pT3/4) | 33.3 (17) | 17.6 (9) | 28.6 (4) | 25.0 (22) | 10.3 (3) | 31.5 (23) | 25.0 (2) | 25.5 (24) |
| pT1-2 (vs. pT3/4) | 3.5 (2) | 21.1 (12) | 12.3 (7) | 12.3 (7) | 5.3 (3) | 19.3 (11) | 3.5 (2) | 21.1 (12) |
| pN0 (vs pN+) | 65.3 (32) | 60.8 (31) | 57.1 (8) | 63.9 (55) | 55.1 (16) | 66.2 (47) | 87.5 (7) | 60.8 (56) |
| pN0 (vs pN+)* | 8.8 (5) | 29.8 (17) | 19.3 (11) | 19.3 (11) | 5.3 (3) | 33.3 (19) | 33.3 (19) | 5.3 (3) |
| pT1-3, N0 (vs pT4 or N+) |
60.8 (31) | 47.1(24) | 50.0 (7) | (54.6) 48 | 37.9 (11) | 60.3 (44) | 75.0 (6) | 52.1 (49) |
| pT1-3, N0 (vs pT4 or N+)* |
7.0 (4) | 24.5 (14) | 15.8 (9) | 15.8 (9) | 3.5 (2) | 28.1 (16) | 5.3 (3) | 26.3 (15) |
| G3 (vs G2) | 74.5 (38) | 86.3 (44) | 78.6 (11) | 80.7 (71) | 89.4 (26) | 76.7 (56) | 87.5 (7) | 79.8 (75) |
| Concomitant carcinoma in situ | 21.5 (11) | 23.5 (12) | 21.4 (3) | 22.7 (20) | 27.5 (8) | 20.5 (15) | 12.5 (1) | 23.4 (22) |
| Lymphovascular invasion | 52.9 (27) | 56.9 (29) | 64.3 (9) | 53.4 (47) | 58.6 (17) | 53.4 (39) | 37.5 (3) | 56.4 (53) |
Bold font indicates significant (P < 0.05) differences in the respective clinical or pathological parameters between subgroups.
Univariable and Multivariable Data Analysis
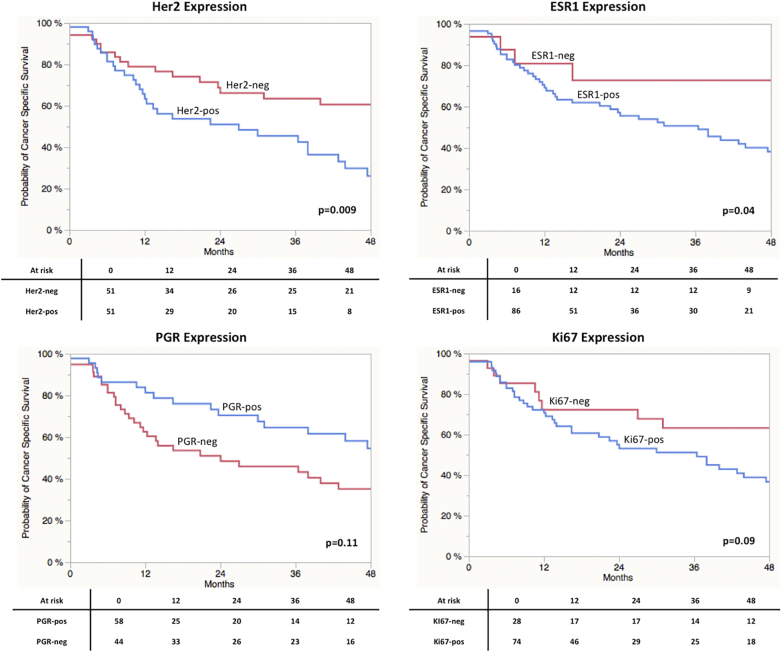
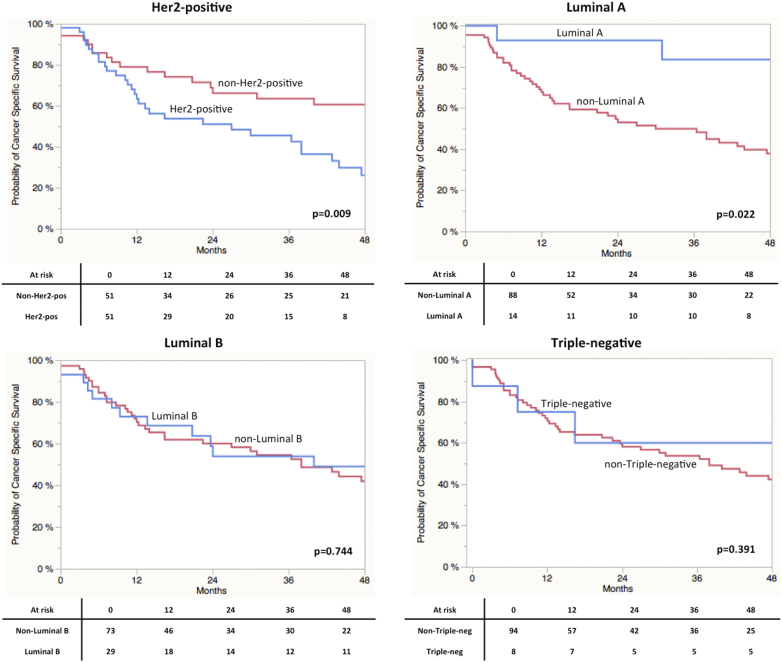
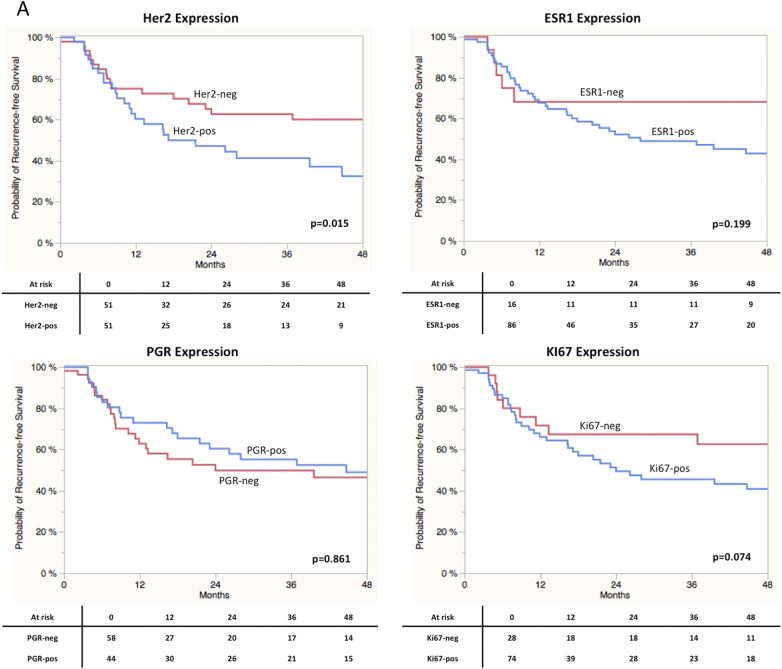
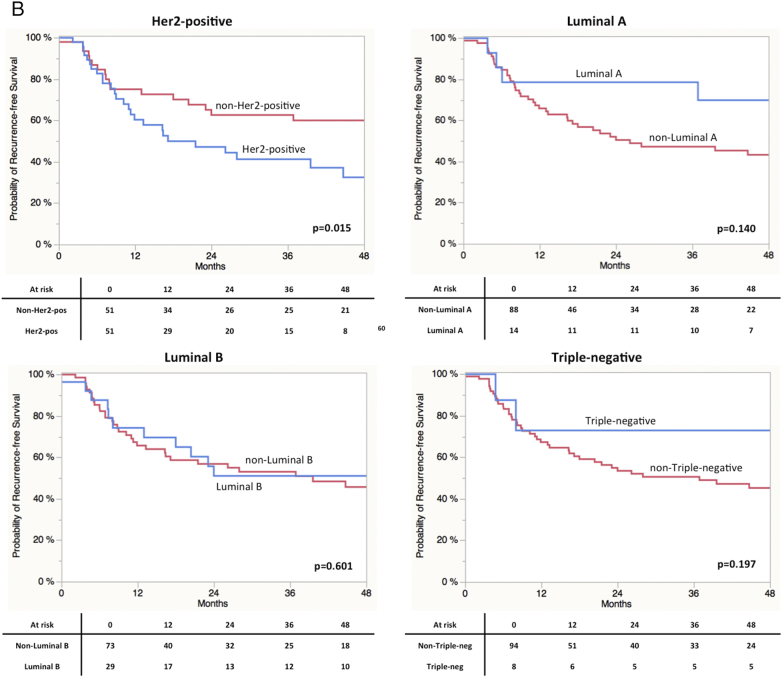
As displayed in Figure 2, A Log-Rank analyses found a significant shorter CSS in patients with high gene expression of Her2 (P = 0.0009) and ESR1 (P = 0.04). A trend towards significance in RFS was found in terms of Ki67 (P = 0.09) expression. Concerning RFS, only stratification by Her2 expression resulted in a significant difference in prognosis (P = 0.015; Figure 2SA). Ki67 expression revealed a trend towards significance (P = 0.074). Stratification by the different subtypes revealed a significantly impaired CSS in Her2 positive vs. non-Her2 positive (P = 0.0009) and Luminal A vs. non-Luminal A (P = 0.0022) cases (Figure 2S). Results for RFS are shown in Figure 3SB. Kaplan-Meier curves for all four subgroups are displayed in Figure 3. A clear stratification was archived by the subgroups for CSS (P = 0.032) and a strong trend towards significance in case of RFS (P = 0.077). In addition uni- and multivariate analysis were performed for CSS and RFS regarding single marker gene expression first and subgroups stratification second. The results are shown in Table 3, Table 4 for CSS and Tables 2S and 3S for RFS. In the first multivariate regression model only Her2 expression remained significant for the prediction of CSS (HR = 2.11, CI 1.11-4.21, P = 0.024). In the second model age (HR = 2.02, CI 1.01-3.93, P = 0.049) and subgroup stratification (P = 0.023) were significant. Especially the differentiation between Her2-positive and Luminal A (HR = 4.41, CI 1.53-18.71, P = 0.004) and Luminal B (HR = 1.96, CI 0.99-4.08, P = 0.053) respectively turned out to be an independent prognostic parameter. The regression models for RFS revealed similar results (Tables 2S und 3S).
Figure 2.
(A) Kaplan-Meier curves of CSS stratified by maker gene expression.
Figure 2S.
Kaplan-Meier curves of CSS stratified by molecular subclasses.
Figure 3.
(A) Kaplan-Meier curves of CSS stratified by molecular subclasses. (B) Kaplan-Meier curves of RFS stratified by molecular subclasses. (C) Kaplan-Meier curves of CSS stratified by molecular subclasses derived from the analysis of the MD Anderson cohort. (D) Allocation of breast cancer subtypes (defined by the four marker genes) to the molecular subclasses defined by Choi and colleagues.8
Table 3.
Uni- and Multivariate Analysis for the Prediction of CSS
| Univariate Analysis |
Multivariate Analysis |
|||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P | HR | 95%CI | P | |
| Cancer-specific survival | ||||||
| Age (≤75 y vs. >75 y) | 2.50 | 1.30-4.57 | 0.007 | 1.96 | 0.97-3.80 | 0.058 |
| Sex (male vs. female) | 1.19 | 0.59-2.22 | 0.591 | |||
| T-Stage (pT2 vs. p3-4) | 1.76 | 0.91-3.73 | 0.009 | 1.73 | 0.84-3.84 | 0.136 |
| Grade (G2 vs. 3-4) | 1.22 | 0.62-2.69 | 0.574 | |||
| Nodal-status (N0 vs. N+) | 2.32 | 1.29-4.15 | 0.006 | 1.97 | 0.96-4.14 | 0.065 |
| LVI | 1.84 | 1.05-3.344 | 0.033 | 1.22 | 0.58-2.53 | 0.587 |
| Concomitant carcinoma in situ | 1.34 | 0.68-2.95 | 0.408 | |||
| Adjuvant chemotherapy | 0.79 | 0.36-1.97 | 0.587 | |||
| Micropapillary | 1.50 | 0.61-3.13 | 0.343 | |||
| Her2 | 2.11 | 1.19-3.82 | 0.009 | 2.11 | 1.11-4.21 | 0.024 |
| ESR | 2.81 | 1.38-9.32 | 0.023 | 1.25 | 0.43-4.53 | 0.697 |
| PGR | 0.63 | 0.35-1.10 | 0.110 | |||
| Ki67 | 1.80 | 0.93-3.82 | 0.079 | 1.59 | 0.79-3.51 | 0.197 |
Table 4.
Uni- and Multivariate Analysis for the Prediction of CSS
| Univariate Analysis |
Multivariate Analysis |
|||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P | HR | 95%CI | P | |
| Cancer-specific survival | ||||||
| Age (≤75 y vs. >75 y) | 2.50 | 1.30-4.57 | 0.007 | 2.02 | 1.01-3.93 | 0.049 |
| Sex (male vs. female) | 1.19 | 0.59-2.22 | 0.591 | |||
| T-Stage (pT2 vs. p3-4) | 1.76 | 0.91-3.73 | 0.009 | 1.68 | 0.82-3.73 | 0.158 |
| Grade (G2 vs. 3-4) | 1.22 | 0.62-2.69 | 0.574 | |||
| Nodal-status (N0 vs. N+) | 2.32 | 1.29-4.15 | 0.006 | 1.80 | 0.89-3.70 | 0.105 |
| LVI | 1.84 | 1.05-3.344 | 0.033 | 1.38 | 0.66-2.74 | 0.401 |
| Concomitant carcinoma in situ | 1.34 | 0.68-2.95 | 0.408 | |||
| Adjuvant chemotherapy | 0.79 | 0.36-1.97 | 0.587 | |||
| Micropapillary | 1.50 | 0.61-3.13 | 0.343 | |||
| Subtype | 0.003 | 0.023 | ||||
| Separate multivariate models each with inclusion of the above values and a single a pair of subtypes | ||||||
| Her2 vs. Luminal A | 4.43 | 1.57-18.55 | 0.0208 | 4.41 | 1.53-18.71 | 0.004 |
| Her2 vs. Luminal B | 1.54 | 0.82-3.06 | 0.180 | 1.96 | 0.99-4.08 | 0.053 |
| Her2 vs. Triple neg. | 2.28 | 0.81-9.53 | 0.123 | |||
| Luminal A vs. Luminal B | 0.34 | 0.07-1.07 | 0.062 | 0.44 | 0.10-1.39 | 0.174 |
| Luminal A vs. Triple neg | 0.51 | 0.09-2.77 | 0.419 | |||
| Luminal B vs. Triple neg | 1.47 | 0.48-6.45 | 0.523 | |||
Validation of the Marker Gene Expression in the MD Anderson Cohort
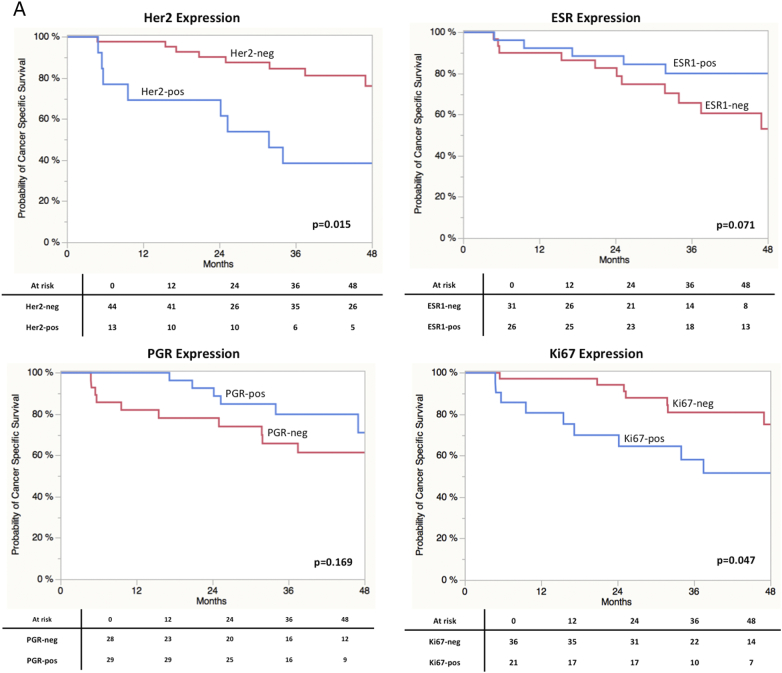
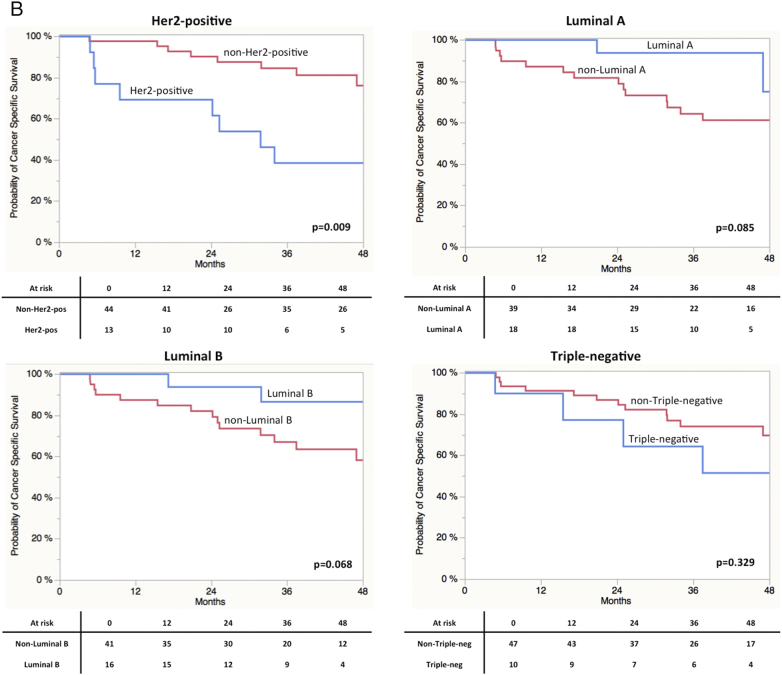
Finally, findings were externally validated using expression data of the MD Anderson cohort (n = 57, GSE48276).8 As shown in Table 1 clinical parameters between groups did not differ significantly. The MD Anderson cohort contained more advanced tumors (T4 or N+) (46% vs, 68.4%, P = 0.001) and neo- 35% (20) of the cases received adjuvant treatment. Data on adjuvant treatment was not available. Concerning the distribution of clinical and pathological parameter in the respective Mamma-Typer subgroups a similar picture as in the finding cohort was obtained. Accordingly Luminal B tumors revealed less advanced tumors (Table 2). The distribution of the four subtypes was: 22.8% (n = 13) Her2 positive, 31.1% (n = 18) Luminal A, 28.1% (16) Luminal B and 17.6% (n = 10) Triple negative. Log-Rank analyses and the respective Kaplan-Meier curves are shown in Figure 3S. Appropriately to our findings stratification for CSS by Her2 (P = 0.0015) was significant. Moreover, patients with high Ki67 expression had a significant reduced CSS (P = 0.047). ESR1 expression revealed a strong trend towards significance (P = 0.071). However in the MD Anderson cohort patients with high ESR1 expression had an improved survival, whereas in our cohort the situation was vice versa. In terms of subgroup stratification similar results as in our cohort were found in terms of the Her2-positive group (Figure 4S). In addition in the MD Anderson cohort patients in Luminal A (P = 0.085) and Luminal B (P = 0.068) subgroups showed a trend towards significance in terms of improved CSS, while in our cohort this applied only for Luminal A vs. non-Lumina A (P = 0.022). The share of Luminal B and Triple negative did rather reflect the situation in breast cancer.
Figure 3S.
(A) Kaplan-Meier curves of RFS stratified by maker gene expression. (B) Kaplan-Meier curves of RFS stratified by molecular subclasses.
Figure 4S.
Kaplan-Meier curves of CSS derived from the in silico analysis of the MD Anderson cohort. (A) Kaplan-Meier curves of CSS stratified by maker gene expression. (B) Kaplan-Meier curves of CSS stratified by molecular subclasses.
Discussion
Molecular markers and subclasses are highly anticipated to improve risk stratification and selection towards individual and targeted therapy in UC, where decisions are based on conventional clinical and pathological parameters. With the given heterogeneity of molecular bladder cancer molecular phenotypes, robust and sensitive methods are requested for subtype and target gene validation, which should be transferrable into the routine use [18], [30], [31], [32]. Therefore, in the present study mRNA expression of Her2, PGR, ESR1 and Ki67 were assed in a cohort of patients treated with RC due to MIBC using a standardized protocol based on an IVD validated kit which allows measuring marker expression at mRNA level using routine FFPE samples [24], [25], [26].
Marker gene expression did not differ when stratified for different patients’ characteristics such as sex or age. In particular no difference between PGR or ESR1 expression levels and gender was found. Here, our results are in accordance to findings of immunohistochemistry based studies [33]. Distinct expression could have been a potential explanation for gender differences in UCC rates and biology [16], [20], [34]. Furthermore, Her2, PGR, ESR1 and Ki67 expression did not vary as a function of pathological findings e.g. tumor stage or LVI. This seems to be in contrast to the situation in non-muscle invasive situation, where our group found a strong correlation between Ki67, Her2 and ESR1 and tumor stage and grading using the same IVD kit [25]. In MIBC Ki67 expression is considered to be a strong predictor of stage, grading and LVI in studies assessing cystectomy specimens [20], [35]. The comparably high percentage of T3/4 tumors (>70%) in our cohort could have offset the association. For Her2 expression a relation to tumor stage is less clear, but it seems to be associated to features of tumor aggressiveness such as lymph node invasion [16], [36]. Whereas for ESR1 differences according to tumor stage have been reported, this has not been observed for PGR, although most studies assessed inhomogeneous cohorts of NMIBC and MIBC [21], [37], [38].
Analyzing outcome prediction and association with RFS and CSS, it could be demonstrated that Her2 expression correlated with both endpoints. In long-rank test patients with high expression of Her2 revealed a significantly impaired RFS and CSS. The uni- and multivariate analyses identified high Her2 expression as independent predictor of RFS and CSS. Generally, Her2 expression levels in MIBC are known to be high and it has been identified as prognostic maker for poor prognosis in several imunhistochemical studies [3], [9], [16], [17], [36], [39], [40]. Astonishingly, in UCC Her2 targeted therapy could not come up to the expectations arising from successes in other Her2 overexpressing tumors such as breast or gastric cancer [41], [42], [43], [44]. Variances and a lack of standardization in protein based assessments with a subsequent inaccurate patients stratification might be one explanations for the absent therapeutic success [18]. Here, a sensitive and quantitative mRNA-based approach, with a broad dynamic range using routine FFPE tissue could improve patient selection and prognostic models [23], [45].
Besides high Her2 expression, patients with increased ESR1 levels revealed an impaired CSS in log-rank and univariate analysis. In multivariate analysis ESR1 status did however not reach significance. The discovered strong and positive correlation between Her2 and ESR1 expression might explain this finding. Unfortunately, further studies focusing on the prognostic value of ESR1 expression in UC of the bladder are missing [21], [33]. This prevents further interpretation of the opposite findings on ESR1 expression between our cohort and die MD Anderson cohort. Similar to ESR1, little is known in terms of PGR expression. Two immunochemistry-based studies failed to detect PGR signals and another found its expression in only 2% of the tumors [22], [46], [47]. Accordingly, our mRNA based analyses showed PGR expression to be significantly lower than Her2 and Ki67 expression. There was a negative correlation between PGR and Ki67 and no association between PGR expression with RFS and CSS. This fits to the arguably inconsiderable role of PGR expression in UCC proposed by existing literature [21].
For Ki67 many studies could prove a prognostic role especially in patients with MIBC treated with radical cystectomy [20], [35], [48]. Our results confirm these findings, although the association between Ki67 and survival is not as strong as previously indicated, presumably being alleviated the sample size [49].
In respect to the similarities between UCC and breast cancer, we assessed the prognostic role of molecular subtypes of breast cancer defined by the marker gene expression of Her2, ESR1, PGR and Ki67 [2], [13]. Notably, the distribution of the four subtypes did not reflect the situation in breast cancer. In breast cancer there are approximately 30-35% Luminal A and B, 10-15% Her2 positive and Triple negative[27]. We discovered 50% of the tumors to be allocated to the Her2 positive group and only 13.7% to the Luminal A group with comparable distribution of Luminal B and Triple negative. Our group recently assessed the situation in NMIBC using the same methods. Most interestingly, here only 12.9% of 302 NMIBC were found to be in the Her2 positive subgroup [29]. At the same time we have previously identified high Her2 expression to be associated with higher stage and grade in NMIBC and associated with progression in a subset of patients with high-risk NMIBC [25], [50]. Hence the shift and differences from non-invasive to invasive forms might well be accompanied by the Her2 receptor status in bladder cancer. This is in accordance to in vitro experiments, where Her2 overexpression drives epithelial to mesenchymal transition resulting in poor prognosis [51], [52]. Furthermore some histopathological subtypes with worse prognosis such as the micropapillary carcinomas exhibit high rates of Her2 alterations which can contribute to the described discrepancies [53].
In breast cancer Her2-positve tumors (Her2 enriched) represent a separate group, which differs from Luminal and triple-negative (basal) tumors. Molecular subtyping in BC has classified Her2 as Luminal marker. Hence direct assignment and comparison of the classification used in this study to the molecular subtypes (basal vs. luminal) of BC is not feasible. Importantly, the purpose of the used marker panel is not to replace or mimic molecular subtyping of bladder cancer subtypes. Besides marker evaluation, this study however was in fact to prove the application of a simple standardized kit measuring characteristic marker expression in bladder cancer. Considering data from breast and bladder cancer, it was to be expected that Luminal A and B as well as Her2-positve tumors have an improved prognosis compared to triple-negative tumors. These best refer to the basal subtype introduced by the landmark papers on molecular subclassification, which is in simplified terms associated with an impaired prognosis compared to luminal tumors [2], [8], [9], [54]. According to this assumption we found Luminal A and B tumors with a good prognosis. Remarkably, in this cohort and the MD Anderson cohort Her2 positive tumors revealed a poor prognosis compared to Luminal but also to triple negative tumors. The low numbers of patients in the triple negative group in our data set and the cohort of the MD Anderson prevent profound assertion of the significance of this finding. However, the findings concerning the Her2-positve type as well as the known association of Her2 with aggressiveness and invasiveness of BC suggest, that the role of Her2 as Luminal marker in BC should interpreted with caution regards to prognosis. Most interestingly, recent studies found Her2 expression across different molecular subtypes [18], [55]. For instance Eriksson and colleagues identified high Her2 mRNA expression also in the genetically unstable subtype and Robertson and colleagues introduced a Luminal-infiltrated type with Her2-expression and impaired prognosis [56]. Accordingly, we identified Her-2 positive tumors in all bladder cancer subtypes in the validation cohort. Additional comprehensive studies are required to dissect the meaning of Her2 expression in the distinct subtypes. Difference in methods used for detection should be taken into account [18]. Nevertheless, the current data underlines that high mRNA Her2 expression might be a marker for impaired prognosis.
The analysis in the MD Anderson cohort resulted in a different subgroup distribution. However risk stratification was comparable especially with regard to the Her2 positive tumors revealing the worst prognosis. In contrast to our tissue samples were obtained from TUR-B material in the MD Anderson cohort [8]. Furthermore almost 20% of the tumors assessed by Choi and colleagues had squamous differentiation, whereas we excluded the respective tumors samples. These differences might at least partly explain the alteration in subgroup distribution and prognosis of the Luminal B subtype between the MD Anderson and our cohort.
There are several important limitations to our study. First and foremost are the limitations inherent to the retrospective analyses and the relative short median follow-up of 20.8 months. Furthermore data is derived from a single-tertiary center. Only one sample per patient was assessed and the normalization controls have been validated for breast cancer only. For confirmation of our finding a validation in a multi-center study is required. Finally, we only assessed patients without neoadjuvant chemotherapy. Admittedly, this contributes to a homogenous cohort, but prevents analyses of response rates in the identified subgroups.
Conclusion
In the current study marker gene expression of Her2, ESR1, PGR and Ki67 in MIBC and the resulting distribution into breast cancer subclasses were assessed. We could show that in MIBC similar hormone receptor expression and subtypes with prognostic association could be detected. This was most relevant for the Her2 positive subtype. The usage of a simple IVD validated kit allows easy transferring of our results to other cohorts and may serve as risk-stratification in future studies. Further studies are required to assess if it may assist in predicting response to current and novel systemic therapies.
The following are the supplementary data related to this article.
Supplementary material
Acknowledgments
Acknowledgement
The authors like to thank Stefanie Herlein, Elke Veltrup, Annette Steidler and Silke Claas for excellent technical support.
Authors Contribution
M.C.K. and P.E. Concept, Study Design, Data Analyses, Manuscript Writing.
R.M.W. Concept, Study Design, Data Analyses, Experiments.
C.B. Clinical Data Acquisition, Experiments.
T.S.W., J. B., M. R., Study Design, Clinical Data Acquisition, Manuscript Revision.
M.E., C.A.W., A.H. Pathological Re-Assessment, Concept.
B. K., W.O., C.B. Concept, Study Design, Supervision.
Statement of Competing Interests
RMW is founder and employee STRATIFYER Molecular Pathology GmbH.
All other authors declare that they have no conflict of interest.
References
- 1.Kaufman DS, Shipley WU, Feldman AS. Bladder cancer. Lancet. 2013;374:239–249. doi: 10.1016/S0140-6736(09)60491-8. [DOI] [PubMed] [Google Scholar]
- 2.Damrauer JS, Hoadley KA, Chism DD, Fan C, Tiganelli CJ, Wobker SE, Yeh JJ, Milowsky MI, Iyer G, Parker JS. Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proc Natl Acad Sci U S A. 2014;111:3110–3115. doi: 10.1073/pnas.1318376111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sjödahl G, Lövgren K, Lauss M, Patschan O, Gudjonsson S, Chebil G, Aine M, Eriksson P, Månsson W, Lindgren D. Toward a Molecular Pathologic Classification of Urothelial Carcinoma. Am J Pathol. 2013;183:681–691. doi: 10.1016/j.ajpath.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Lindgren D, Frigyesi A, Gudjonsson S, Sjödahl G, Hallden C, Chebil G, Veerla S, Ryden T, Månsson W, Liedberg F. Combined Gene Expression and Genomic Profiling Define Two Intrinsic Molecular Subtypes of Urothelial Carcinoma and Gene Signatures for Molecular Grading and Outcome. Cancer Res. 2010;70:3463–3472. doi: 10.1158/0008-5472.CAN-09-4213. [DOI] [PubMed] [Google Scholar]
- 5.McConkey DJ, Choi W, Shen Y, Lee IL, Porten S, Matin SF, Kamat AM, Corn P, Millikan RE, Dinney C. A Prognostic Gene Expression Signature in the Molecular Classification of Chemotherapy-naïve Urothelial Cancer is Predictive of Clinical Outcomes from Neoadjuvant Chemotherapy: A Phase 2 Trial of Dose-dense Methotrexate, Vinblastine, Doxorubicin, and Cisplatin with Bevacizumab in Urothelial Cancer. Eur Urol. 2016;69:855–862. doi: 10.1016/j.eururo.2015.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, Dawson N, O'Donnell PH, Balmanoukian A, Loriot Y. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–1920. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma P, Callahan MK, Bono P, Kim J, Spiliopoulou P, Calvo E, Pillai RN, Ott PA, de Braud F, Morse M. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol. 2016;17:1590–1598. doi: 10.1016/S1470-2045(16)30496-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi W, Porten S, Kim S, Willis D, Plimack ER, Hoffman-Censits J, Roth B, Cheng T, Tran M, Lee IL. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell. 2014;25:152–165. doi: 10.1016/j.ccr.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The Cancer Genome Atlas Research Network Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507:315–322. doi: 10.1038/nature12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McConkey DJ, Choi W, Dinney CPN. Genetic subtypes of invasive bladder cancer. Curr Opin Urol. 2015;25:449–458. doi: 10.1097/MOU.0000000000000200. [DOI] [PubMed] [Google Scholar]
- 11.Aine M, Eriksson P, Liedberg F, Höglund M, Sjödahl G. On Molecular Classification of Bladder Cancer: Out of One, Many. Eur Urol. 2015;68:921–923. doi: 10.1016/j.eururo.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 12.Choi W, Porten S, Kim S, Willis D, Plimack ER, Hoffman-Censits J, Roth B, Cheng T, Tran M, Lee IL. Intrinsic basal and luminal subtypes of muscle-invasive bladder cancer. Nat Rev Urol. 2014;11:400–410. doi: 10.1038/nrurol.2014.129. [DOI] [PubMed] [Google Scholar]
- 13.Choi W, Czerniak B, Ochoa A, Su X, Siefker-Radtke A, Dinney C, McConkey DJ. Molecular subclasses of breast cancer: how do we define them? The IMPAKT 2012 Working Group Statement. Ann Oncol. 2012;23:2997–3006. doi: 10.1093/annonc/mds586. [DOI] [PubMed] [Google Scholar]
- 14.Guiu S, Michiels S, André F, Cortes J, Denkert C, Di Leo A, Hennessy BT, Sorlie T, Sotiriou C, Turner N. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sonnenblick A, Fumagalli D, Azim HA, Sotiriou C, Piccart M. New strategies in breast cancer: the significance of molecular subtypes in systemic adjuvant treatment for small T1a,bN0M0 tumors. Clin Cancer Res. 2014;20:6242–6246. doi: 10.1158/1078-0432.CCR-14-1086. [DOI] [PubMed] [Google Scholar]
- 16.Soria F, Moschini M, Haitel A, Wirth GJ, Gust KM, Briganti A, Rouprêt M, Klatte T, Hassler MR, Karakiewicz PI. The effect of HER2 status on oncological outcomes of patients with invasive bladder cancer. Urol Oncol. 2016;34:533.e1–533.e10. doi: 10.1016/j.urolonc.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Nedjadi T, Al-Maghrabi J, Assidi M, Dallol A, Al-Kattabi H, Chaudhary A, Al-Sayyad A, Al-Ammari A, Abuzenadah A, Buhmeida A. Prognostic value of HER2 status in bladder transitional cell carcinoma revealed by both IHC and BDISH techniques. BMC Cancer. 2016;16:653. doi: 10.1186/s12885-016-2703-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiss B, Wyatt AW, Douglas J, Skuginna V, Mo F, Anderson S, Rotzer D, Fleischmann A, Genitsch V, Hayashi T. Her2 alterations in muscle-invasive bladder cancer: Patient selection beyond protein expression for targeted therapy. Sci Rep. 2017;7:42713. doi: 10.1038/srep42713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo Y, Zhang X, Mo M, Tan Z, Huang L, Zhou H, Wang C, Wei F, Qiu X, He R. High Ki-67 Immunohistochemical Reactivity Correlates With Poor Prognosis in Bladder Carcinoma: A Comprehensive Meta-Analysis with 13,053 Patients Involved. Medicine (Baltimore) 2016;95 doi: 10.1097/MD.0000000000003337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Margulis V, Shariat SF, Ashfaq R, Sagalowsky AI, Lotan Y. Ki-67 is an independent predictor of bladder cancer outcome in patients treated with radical cystectomy for organ-confined disease. Clin Cancer Res. 2006;12:7369–7373. doi: 10.1158/1078-0432.CCR-06-1472. [DOI] [PubMed] [Google Scholar]
- 21.Ide H, Miyamoto H. Steroid Hormone Receptor Signals as Prognosticators for Urothelial Tumor. Dis Markers. 2015;2015:1–12. doi: 10.1155/2015/840640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bolenz C, Lotan Y, Ashfaq R, Shariat SF. Estrogen and Progesterone Hormonal Receptor Expression in Urothelial Carcinoma of the Bladder. Eur Urol. 2009;56:1093–1095. doi: 10.1016/j.eururo.2009.06.032. [DOI] [PubMed] [Google Scholar]
- 23.Wirtz RM, Sihto H, Isola J, Heikkilä P, Kellokumpu-Lehtinen PL, Auvinen P, Turpeenniemi-Hujanen T, Jyrkkiö S, Lakis S, Schlombs K. Biological subtyping of early breast cancer: a study comparing RT-qPCR with immunohistochemistry. Breast Cancer Res Treat. 2016;157:437–446. doi: 10.1007/s10549-016-3835-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laible M, Schlombs K, Kaiser K, Veltrup E, Herlein S, Lakis S, Stöhr R, Eidt S, Hartmann A, Wirtz RM. Technical validation of an RT-qPCR in vitro diagnostic test system for the determination of breast cancer molecular subtypes by quantification of ERBB2, ESR1, PGR and MKI67 mRNA levels from formalin-fixed paraffin-embedded breast tumor specimens. BMC Cancer. 2016;16:432. doi: 10.1186/s12885-016-2476-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breyer J, Wirtz RM, Laible M, Schlombs K, Erben P, Kriegmair MC, Stoehr R, Eidt S, Denzinger S, Burger M. ESR1, ERBB2, and Ki67 mRNA expression predicts stage and grade of non-muscle-invasive bladder carcinoma (NMIBC) Virchows Arch. 2016;469:547–552. doi: 10.1007/s00428-016-2002-1. [DOI] [PubMed] [Google Scholar]
- 26.Bohmann K, Hennig G, Rogel U, Poremba C, Mueller BM, Fritz P, Stoerkel S, Schaefer KL. RNA extraction from archival formalin-fixed paraffin-embedded tissue: a comparison of manual, semiautomated, and fully automated purification methods. Clin Chem. 2009;55:1719–1727. doi: 10.1373/clinchem.2008.122572. [DOI] [PubMed] [Google Scholar]
- 27.Koutras A, Kalogeras KT, Wirtz RM, Alexopoulou Z, Bobos M, Zagouri F, Veltrup E, Timotheadou E, Gogas H, Pentheroudakis G. Evaluation of the prognostic significance of HER family mRNA expression in high-risk early breast cancer: a Hellenic Cooperative Oncology Group (HeCOG) validation study. J Transl Med. 2015;13:171. doi: 10.1186/s12967-015-0530-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kriegmair MC, Balk M, Wirtz R, Steidler A, Weis CA, Breyer J, Hartmann A, Bolenz C, Erben P. Expression of the p53 Inhibitors MDM2 and MDM4 as Outcome Predictor in Muscle-invasive Bladder Cancer. Anticancer Res. 2016;36:5205–5213. doi: 10.21873/anticanres.11091. [DOI] [PubMed] [Google Scholar]
- 29.Breyer J, Wirtz RM, Otto W, Laible M, Schlombs K, Erben P, Kriegmair MC, Stoehr R, Eidt S, Denzinger S. Predictive value of molecular subtyping in NMIBC by RT-qPCR of ERBB2, ESR1, PGR and MKI67 from formalin fixed TUR biopsies. Oncotarget. 2017;5 doi: 10.18632/oncotarget.18804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehta S, Shelling A, Muthukaruppan A, Lasham A, Blenkiron C, Laking G, Print C. Predictive and prognostic molecular markers for cancer medicine. Ther Adv Med Oncol. 2010;2:125–148. doi: 10.1177/1758834009360519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ross JS, Symmans WF, Pusztai L, Hortobagyi GN. Standardizing Slide-Based Assays in Breast Cancer: Hormone Receptors, HER2, and Sentinel Lymph Nodes. Clin Cancer Res. 2007;13:2831–2835. doi: 10.1158/1078-0432.CCR-06-2522. [DOI] [PubMed] [Google Scholar]
- 32.Ross JS, Wang K, Al-Rohil RN, Nazeer T, Sheehan CE, Otto GA, He J, Palmer G, Yelensky R, Lipson D. Advanced urothelial carcinoma: next-generation sequencing reveals diverse genomic alterations and targets of therapy. Mod Pathol. 2013;27:271–280. doi: 10.1038/modpathol.2013.135. [DOI] [PubMed] [Google Scholar]
- 33.Ide H, Inoue S, Miyamoto H. Histopathological and prognostic significance of the expression of sex hormone receptors in bladder cancer: A meta-analysis of immunohistochemical studies. PLoS One. 2017;12 doi: 10.1371/journal.pone.0174746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tuygun C, Kankaya D, Imamoglu A, Sertcelik A, Zengin K, Oktay M, Sertcelik N. Sex-specific hormone receptors in urothelial carcinomas of the human urinary bladder: a comparative analysis of clinicopathological features and survival outcomes according to receptor expression. Urol Oncol. 2011;29:43–51. doi: 10.1016/j.urolonc.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 35.Wang L, Zhou M, Feng C, Gao P, Ding G, Zhou Z, Jiang H, Wu Z, Ding Q. Prognostic value of Ki67 and p63 expressions in bladder cancer patients who underwent radical cystectomy. Int Urol Nephrol. 2016;48:495–501. doi: 10.1007/s11255-015-1197-4. [DOI] [PubMed] [Google Scholar]
- 36.Zhao J, Xu W, Zhang Z, Song R, Zeng S, Sun Y, Xu C. Prognostic role of HER2 expression in bladder cancer: a systematic review and meta-analysis. Int Urol Nephrol. 2015;47:87–94. doi: 10.1007/s11255-014-0866-z. [DOI] [PubMed] [Google Scholar]
- 37.Kontos S, Kominea A, Melachrinou M, Balampani E, Sotiropoulou Bonikou G. Inverse expression of estrogen receptor-β and nuclear factor-κB in urinary bladder carcinogenesis. Int J Urol. 2010;17:801–809. doi: 10.1111/j.1442-2042.2010.02603.x. [DOI] [PubMed] [Google Scholar]
- 38.Miyamoto H, Yao JL, Chaux A, Zheng Y, Hsu I, Izumi K, Chang C, Messing EM, Netto GJ, Yeh S. Expression of androgen and oestrogen receptors and its prognostic significance in urothelial neoplasm of the urinary bladder. BJU Int. 2012;109:1716–1726. doi: 10.1111/j.1464-410X.2011.10706.x. [DOI] [PubMed] [Google Scholar]
- 39.Krüger S, Weitsch G, Büttner H, Matthiensen A, Böhmer T, Marquardt T, Sayk F, Feller AC, Böhle A. HER2 overexpression in muscle-invasive urothelial carcinoma of the bladder: Prognostic implications. Int J Cancer. 2002;102:514–518. doi: 10.1002/ijc.10731. [DOI] [PubMed] [Google Scholar]
- 40.Bolenz C, Shariat SF, Karakiewicz PI, Ashfaq R, Ho R, Sagalowsky AI, Lotan Y. Human epidermal growth factor receptor 2 expression status provides independent prognostic information in patients with urothelial carcinoma of the urinary bladder. BJU Int. 2010;106:1216–1222. doi: 10.1111/j.1464-410X.2009.09190.x. [DOI] [PubMed] [Google Scholar]
- 41.Begnami MD, Fukuda E, Fregnani JH, Nonogaki S, Montagnini AL, da Costa WL, Jr., Soares FA. Prognostic Implications of Altered Human Epidermal Growth Factor Receptors (HERs) in Gastric Carcinomas: HER2 and HER3 Are Predictors of Poor Outcome. J Clin Oncol. 2011;29:3030–3036. doi: 10.1200/JCO.2010.33.6313. [DOI] [PubMed] [Google Scholar]
- 42.Witzel I, Müller V. Targeted Therapies in Breast Cancer: New Approaches and Old Challenges. Breast Care. 2015;10:157–158. doi: 10.1159/000431067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Powles T, Huddart RA, Elliott T, Sarker SJ, Ackerman C, Jones R, Hussain S, Crabb S, Jagdev S, Chester J. Phase III, Double-Blind, Randomized Trial That Compared Maintenance Lapatinib Versus Placebo After First-Line Chemotherapy in Patients With Human Epidermal Growth Factor Receptor 1/2–Positive Metastatic Bladder Cancer. J Clin Oncol. 2017;35:48–55. doi: 10.1200/JCO.2015.66.3468. [DOI] [PubMed] [Google Scholar]
- 44.Hussain MH, MacVicar GR, Petrylak DP, Dunn RL, Vaishampayan U, Lara PN, Jr., Chatta GS, Nanus DM, Glode LM, Trump DL. Trastuzumab, paclitaxel, carboplatin, and gemcitabine in advanced human epidermal growth factor receptor-2/neu-positive urothelial carcinoma: results of a multicenter phase II National Cancer Institute trial. J Clin Oncol. 2007;25:2218–2224. doi: 10.1200/JCO.2006.08.0994. [DOI] [PubMed] [Google Scholar]
- 45.Susini T, Bussani C, Marini G, Nori J, Olivieri S, Molino C, Bianchi S, Vezzosi V, Paglierani M, Giachi M. Preoperative assessment of HER-2/neu status in breast carcinoma: the role of quantitative real-time PCR on core-biopsy specimens. Gynecol Oncol. 2010;116:234–239. doi: 10.1016/j.ygyno.2009.10.067. [DOI] [PubMed] [Google Scholar]
- 46.Tan W, Boorjian S, Advani P, Farmer S, Lohse C, Cheville J, Kwon E, Leibovich B. The Estrogen Pathway: Estrogen Receptor-α, Progesterone Receptor, and Estrogen Receptor-β Expression in Radical Cystectomy Urothelial Cell Carcinoma Specimens. Clin Genitourin Cancer. 2015;13:476–484. doi: 10.1016/j.clgc.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 47.Mashhadi R, Pourmand G, Kosari F, Mehrsai A. Role of Steroid Hormone Receptors in Formation and Progression of Bladder Carcinoma: A Case-Control Study - ProQuest. Urol J. 2014;11:1968–1973. [PubMed] [Google Scholar]
- 48.Margulis V, Lotan Y, Karakiewicz PI, Fradet Y, Ashfaq R, Capitanio U, Montorsi F, Bastian PJ, Nielsen ME, Müller SC. Multi-institutional validation of the predictive value of Ki-67 labeling index in patients with urinary bladder cancer. J Natl Cancer Inst. 2009;101:114–119. doi: 10.1093/jnci/djn451. [DOI] [PubMed] [Google Scholar]
- 49.Liedberg F, Anderson H, Chebil G, Gudjonsson S, Höglund M, Lindgren D, Lundberg LM, Lövgren K, Fernö M, Månsson W. Tissue microarray based analysis of prognostic markers in invasive bladder cancer: much effort to no avail? URO. 2008;26:17–24. doi: 10.1016/j.urolonc.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 50.Breyer J, Otto W, Wirtz RM, Wullich B, Keck B, Erben P, Kriegmair MC, Stoehr R, Eckstein M, Laible M. ERBB2 Expression as Potential Risk-Stratification for Early Cystectomy in Patients with pT1 Bladder Cancer and Concomitant Carcinoma in situ. Urol Int. 2017;98:282–289. doi: 10.1159/000453670. [DOI] [PubMed] [Google Scholar]
- 51.Ingthorsson S, Andersen K, Hilmarsdottir B, Maelandsmo GM, Magnusson MK, Gudjonsson T. HER2 induced EMT and tumorigenicity in breast epithelial progenitor cells is inhibited by coexpression of EGFR. Oncogene. 2016;35:4244–4255. doi: 10.1038/onc.2015.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nair R, Roden DL, Teo WS, McFarland A, Junankar S, Ye S, Nguyen A, Yang J, Nikolic I, Hui M. c-Myc and Her2 cooperate to drive a stem-like phenotype with poor prognosis in breast cancer. Oncogene. 2014;33:3992–4002. doi: 10.1038/onc.2013.368. [DOI] [PubMed] [Google Scholar]
- 53.Ross JS, Wang K, Gay LM, Al-Rohil RN, Nazeer T, Sheehan CE, Jennings TA, Otto GA, Donahue A, He J. A high frequency of activating extracellular domain ERBB2 (HER2) mutation in micropapillary urothelial carcinoma. Clin Cancer Res. 2014;20:68–75. doi: 10.1158/1078-0432.CCR-13-1992. [DOI] [PubMed] [Google Scholar]
- 54.Sjödahl G, Lauss M, Lövgren K, Chebil G, Gudjonsson S, Veerla S, Patschan O, Aine M, Fernö M, Ringnér M. A Molecular Taxonomy for Urothelial Carcinoma. Clin Cancer Res. 2012;18:3377–3386. doi: 10.1158/1078-0432.CCR-12-0077-T. [DOI] [PubMed] [Google Scholar]
- 55.Eriksson P, Sjödahl G, Chebil G, Liedberg F, Höglund M. HER2 and EGFR amplification and expression in urothelial carcinoma occurs in distinct biological and molecular contexts. Oncotarget. 2017;8:48905–48914. doi: 10.18632/oncotarget.16554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Robertson AG, Kim J, Al-Ahmadie H, Bellmunt J, Guo G, Cherniack AD, Hinoue T, Laird PW, Hoadley KA, Akbani R. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell. 2017;171:540–556.e25. doi: 10.1016/j.cell.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material