Significance
Animals, including humans, are surrounded by many objects, only some of which are valuable. To survive, it is critical to efficiently discriminate valuable objects, particularly those that are only occasionally or seasonally available. Here, we use fMRI to show that, in macaques, a network consisting of areas in the temporal and prefrontal cortex and their associated subcortical structures maintained value memories for a large number of objects. This memory representation lasted for many months after the objects were last seen and accordingly the monkeys were able to find valuable objects efficiently. We postulate that this temporal–prefrontal circuit is critical for drawing on learned value memory to guide goal-oriented behavior toward certain objects.
Keywords: object values, temporal–prefrontal circuits, long-term high-capacity memory, fMRI, macaque monkey
Abstract
Remembering and discriminating objects based on their previously learned values are essential for goal-directed behaviors. While the cerebral cortex is known to contribute to object recognition, surprisingly little is known about its role in retaining long-term object–value associations. To address this question, we trained macaques to arbitrarily associate small or large rewards with many random fractal objects (>100) and then used fMRI to study the long-term retention of value-based response selectivity across the brain. We found a pronounced long-term value memory in core subregions of temporal and prefrontal cortex where, several months after training, fractals previously associated with high reward (“good” stimuli) elicited elevated fMRI responses compared with those associated with low reward (“bad” stimuli). Similar long-term value-based modulation was also observed in subregions of the striatum, amygdala, and claustrum, but not in the hippocampus. The value-modulated temporal–prefrontal subregions showed strong resting-state functional connectivity to each other. Moreover, for areas outside this core, the magnitude of long-term value responses was predicted by the strength of resting-state functional connectivity to the core subregions. In separate testing, free-viewing gaze behavior indicated that the monkeys retained stable long-term memory of object value. These results suggest an implicit and high-capacity memory mechanism in the temporal–prefrontal circuitry and its associated subcortical regions for long-term retention of object-value memories that can guide value-oriented behavior.
Accumulated experience shapes the way we perceive and interact with the objects around us. Many animals are able to adapt quickly and respond to stimuli that predict reward, as evidenced by the capacity to condition behavior to initially neutral stimuli in vertebrates (1–3) and invertebrates (4) using food reward. Primates rely strongly on their sense of vision to interact with their surroundings and are adept at forming new object–reward associations, often with limited reward repetitions. For example, rhesus macaques readily learn to discriminate and choose high-value objects with fewer than 10 reward pairings per object (5–7). Such short-term adaptability is thought to be critical for behavioral flexibility when encountering stimuli in a novel or volatile environment.
The short-term learning of value is, however, most advantageous if this information is retained over the longer term, so that learned information can be applied for the rest of the animal’s lifetime. This capacity entails mechanisms that support the formation of stable memories, for example, of objects that deliver consistently positive outcomes (good objects). From an ecological perspective, the persistence of value-based memories is important for activities such as foraging, when cues associated with food are not encountered for protracted periods due to seasonal availability. However, aberrations of such long-term value memories become important when persistent memories turn maladaptive, as observed in drug addiction.
Indeed, recent behavioral evidence demonstrates the existence of long-term object-value memory in nonhuman primates. For example, we recently found that long-term object–reward pairing created a visual pop-out for good objects from surrounding bad objects, which was sustained for many weeks (8). The good object was often detected by the first saccade with a short latency (<150 ms), among objects that were equally familiar to the animals. While there are extensive studies on cortical circuitry involved in long-term memory of familiar objects (9–11), the cortical substrate for long-term retention of value memories for equally familiar objects is not known. Research in our laboratory has shown that the posterior basal ganglia rapidly differentiates good and bad objects (<120 ms) and maintains that discrimination over periods lasting from a few days to several months (7, 12, 13). Since the posterior basal ganglia receives its input mainly from visual cortical areas, there is a possibility that value memory is maintained by changes in sensory representation of good objects in the cortex. While studies of cortical visual processing have shown some evidence for reward-related modulation (14–17), it is unknown to what extent high-level cortical areas can retain the learned responses to arbitrary value associations for extended periods of time after training.
To address this question we exploited the coverage of whole-brain fMRI in macaque monkeys to study the persistence of stimulus reward associations long after training. During a training period of at least 10 d, two animals were repeatedly shown 100 or more complex fractal objects. Each fractal was arbitrarily chosen to be consistently associated with a small or large juice reward. The long-term effects of this training on visual responses across the brain were then evaluated in two rounds of fMRI scanning. The first round was performed within 10 d after completion of the training period (days-old memory). The second round, designed to test the persistence of the long-term memories, took place much later (months-old memory). In this case, testing took place after the monkey had gone 6–13 mo without any exposure to the stimuli. In both rounds, analysis focused on the differential hemodynamic response to the learned good vs. bad visual objects (hereafter GB discrimination or coding). Both rounds of scanning were performed using the contrast agent monocrystalline iron oxide nanoparticles (MION), which, through its isolation and enhancement of local cerebral blood volume, leads to an improved signal-to-noise ratio compared with BOLD (blood-oxygenation-level-dependent), albeit with a somewhat diminished temporal resolution (18).
We found a core of visually responsive regions in the temporal and prefrontal cortex that exhibited a memory for learned value that persisted across months. We assessed several features of this cortical value memory signal, including its retinotopic specificity, the position of the cortical core areas relative to well-described face patches in the same animals, and its network properties relative to resting-state functional connectivity. A similar, albeit weaker, expression of long-term memory was also observed in several subcortical structures, but not in the hippocampus, which is known to be involved in episodic or relational memories (19). Finally, we verified the long-term behavioral biases toward good and bad objects in the context of a free-viewing task (20, 21). We discuss the results in regard to the neural substrates of object-value learning across the brain and long-term value discrimination in posterior basal ganglia.
Results
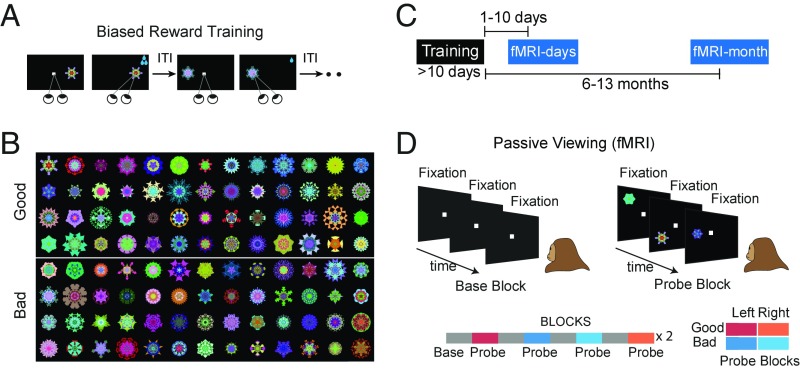
Two rhesus macaques (U and D) viewed computer-generated fractal patterns that were repeatedly paired with low or high rewards (Fig. 1A), dividing the stimuli into good and bad object categories. Previous studies have shown that efficient behavioral discrimination of good and bad objects emerges after a few days of reward pairing (7, 8). In the present study, monkeys underwent this training for at least 10 d (sessions), after which the fMRI responses were evaluated during passive viewing. We employed a large number of random fractals (≥100) for each monkey (Fig. 1B), in part to gauge the capacity of the long-term associations, but also to ensure that any observed fMRI response differences could not be attributed to idiosyncratic features in the stimuli themselves.
Fig. 1.
Stimuli and experimental paradigm. (A) Abstract fractal objects were repeatedly associated with low or high reward (good and bad fractals) over >10 d (long-term biased reward training). (B) Fractals used in the fMRI for monkey U (n = 104), which were randomly assigned to good or bad category during training. (C) Differential cortical activation to good and bad fractals (GB discrimination) were measured using fMRI days and months after last reward training using a passive viewing task and in the absence of reward. (D) Passive viewing task with a block design: In all blocks, subject kept central fixation. In the base blocks, no object was shown. In the probe blocks, good or bad objects were shown on the left or right hemifield at 6° eccentricity.
The main question of interest was whether learned reward associations lead to changes in cortical processing of objects that persist long after the training period and in the absence of rewards (Fig. 1C). To address this question, good and bad objects were first presented to the monkey in the scanner within 10 d after the last object–reward association (days-later scans). The monkey’s task during the fMRI experiment was to simply fixate a white center dot (Fig. 1D). A scanning run consisted of alternating base and probe blocks, each lasting for 30 s (total of 16 blocks). During the base blocks, only a white fixation dot was shown. During the probe block, the previously experienced fractal objects were presented one at a time in the periphery along with the central fixation. There were four types of probe blocks consisting of good or bad objects presented in the left or right visual hemifields (2 value × 2 hemifield, Fig. 1D, Bottom). These four probe block types were presented in a pseudorandom order (SI Methods).
Since the present study focused on how previously learned associations were expressed and retained over time, all fMRI tests were carried out using passive viewing and in the absence of contingent reward for objects. The animals were rewarded at random time intervals for the successful maintenance of central fixation, with the total number of rewards received being similar between good and bad blocks in both monkeys (Fig. S1A). Both animals had extensive experience with this passive viewing task, both outside and inside the scanner (>3 mo), before the actual scans. As a result, fixation breaks were infrequent during the scans (<12% fix-break/object) and were not significantly different for good and bad blocks for both monkeys (Fig. S1A). The frequency and pattern of fixational saccades during fixation were also similar in good and bad blocks (Fig. S1B). Thus, differences in activation between good and bad blocks were attributed to modulation caused by previously learned object–reward associations. The number of rewards and fixation breaks were also comparable for the left- and right-presentation blocks (Fig. S1C). Thus, the interaction between value and spatial coding were not attributable to differences in reward or fixation breaks between the two visual hemifields.
GB Discrimination After Days and Months.
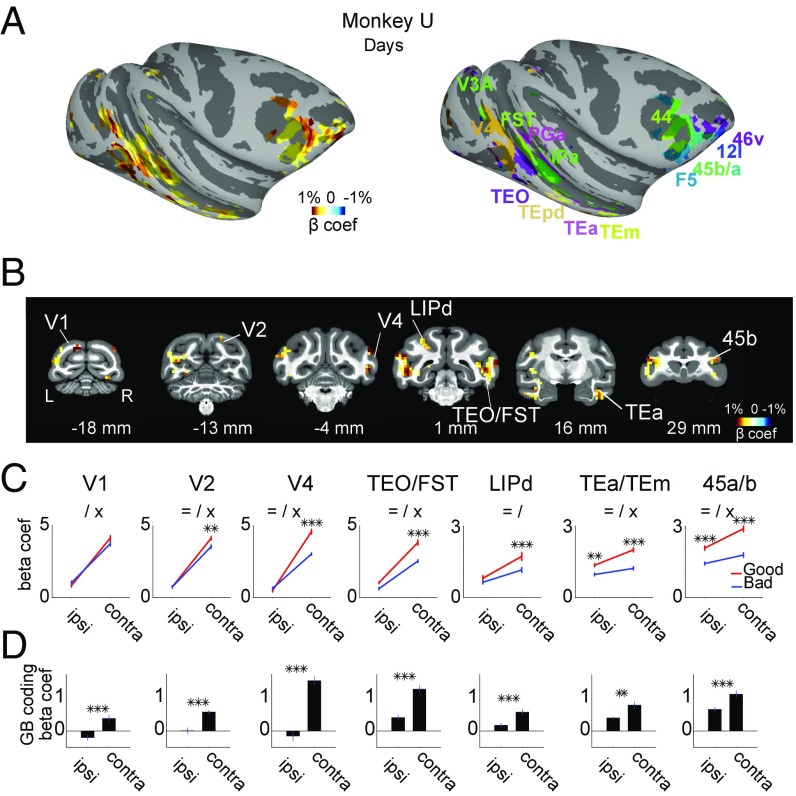
Scans in the days following training showed robust differentiation of good and bad objects in several cortical areas (Fig. 2 A and B and Fig. S2). This was most prominent in the posterior-ventral superior temporal sulcus (pvSTS), including areas TEO, FST, and IPa, and in ventrolateral prefrontal cortex (vlPFC), including areas 45a/b and 46v. Similar effects were found in lateral intraparietal area (LIP), anterior-ventral superior temporal sulcus (avSTS, such as TEa and TEm), and early visual areas V1–4 (Fig. 2 C and D). These areas were more strongly activated by good than bad objects, days after final reward training, and in the absence of rewards.
Fig. 2.
GB discrimination days after reward association. (A) Object discrimination by days-old values across cortical areas: good–bad beta coefficients in areas with significant GB discrimination (Left) and corresponding anatomical areas annotated with different colors (Right) are shown on the right hemisphere (P < 0.001, α < 0.01 cluster corrected). For the beta coefficient map, regions with higher saturation are more strongly modulated by days-old value. Warmer colors mean bigger activation to good compared with bad and cooler colors mean the opposite. Data in Figs. 2 and 3 are from monkey U. (B) Example coronal sections from posterior to anterior (distance to interaural shown): beta coefficients for good–bad contrast for days-old value is shown in voxels with significant GB discrimination (P < 0.001, α < 0.01 cluster-corrected). (C) Average beta coefficient within example anatomical areas (n = top 15 visually responsive voxels in each area) for good and bad objects shown in ipsilateral and contralateral hemifields in days-later scans. (Error bars denote SEM in this and other plots, = main effect of value, / main effect of hemifield, × interaction **P < 0.01, ***P < 0.001.) (D) Same data as in C but showing good–bad coefficients separately for ipsi- and contralateral object presentations (**P < 0.01, ***P < 0.001).
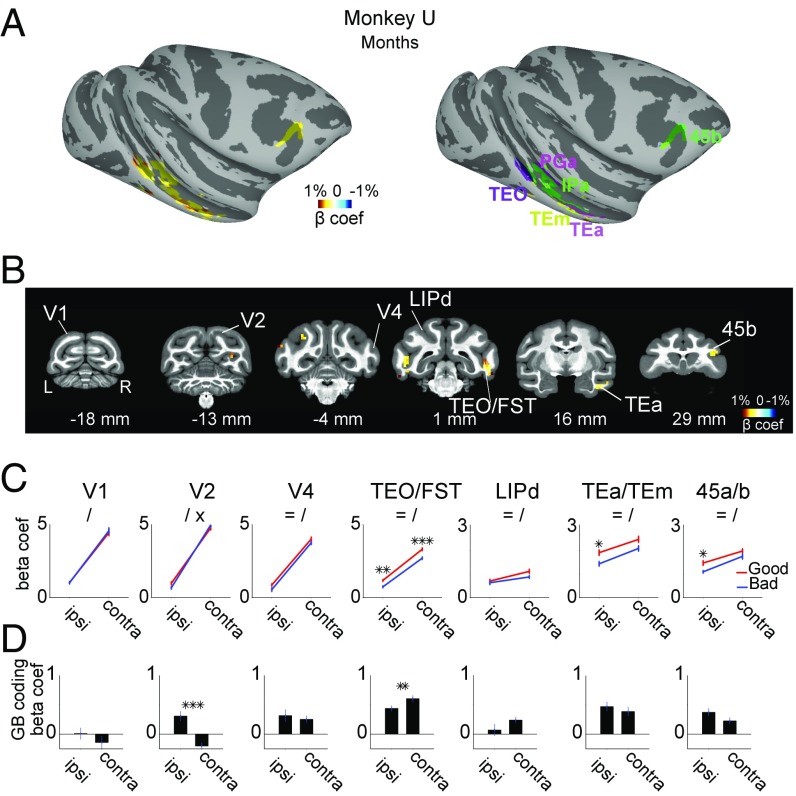
We then performed the same experiment 6–13 mo later (months-later scans) (Fig. 3 A and B and Fig. S2). During the intervening period, the monkeys never saw the trained objects. However, upon passive presentation of the fractals, we found enhanced responses to the good stimuli in several areas, including pvSTS (TEO, FST, and IPa), avSTS (TEa and TEm), and vlPFC (area 45b). Most of these areas had also shown enhanced activity in days-later scans (Fig. 3 vs. Fig. 2). In contrast, many other areas showed diminished (e.g., LIP) or no (e.g., V1–4) value coding (Fig. 3 C and D; for the response time course within example areas in days- and months-later scans see Fig. S3).
Fig. 3.
GB discrimination months after reward association. (A–D) Same format as Fig. 2 but for months-old value discrimination.
These value-coding areas also showed spatial biases: stronger responses to contralateral than ipsilateral objects (Figs. 2C and 3C). In both monkeys the contralateral bias was stronger in the early (V1, V2, V4, and TEO/FST) than in the late (LIP, TEa/m, and 45a/b) visual regions [main effect of region F(6, 98) > 55, post hoc t(103) > 13, P < 0.001], consistent with the reduction in the contralaterality and increase in size of the receptive fields along the visual hierarchy (22). The hemifield and GB coding showed an interaction such that in all example areas value-based discrimination was stronger for contralaterally presented objects. This interaction (i.e., contra minus ipsi GB coding) was stronger in early (V1, V2, V4, and TEO/FST) compared with late (LIP, TEa/m, and 45a/b) visual regions (Fig. 2D). Further analysis showed this effect to be significant in both monkeys in the days-old period [main effect of region F(6, 196) > 7.1, post hoc t103 > 4, P < 0.001]. In the months-old period, this difference in interaction between early and late visual areas disappeared in monkey U [Fig. 3D, main effect of days/months period F(1, 196) = 153, P < 0.001] but remained significant in monkey D [t(103) = 2.9, P = 0.004]. Note that this increased symmetry in value coding (Fig. 2D vs. Fig. 3D) in monkey U emerged despite a maintained contralateral dominance of overall visual response selectivity (Fig. 2C vs. Fig. 3C).
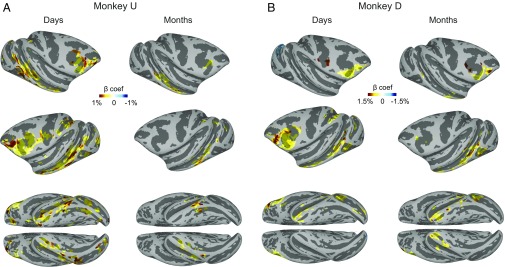
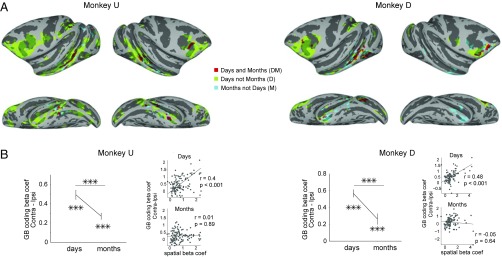
In both monkeys the regions showing long-term value coding were prominent in prefrontal and temporal cortical areas in days-later scans and after a gap of several months became largely restricted to the posterior temporal cortex and the vlPFC (Fig. 4 and Fig. S4). There were also some differences across the monkeys. The value coding in the posterior temporal cortex was lateralized in monkey D (left > right), but was bilateral in monkey U (Fig. 4). Although GB discrimination was mostly positive (good > bad) across cortical areas, some negative coding was also observed (e.g., lunate sulcus in the right hemisphere of monkey D; Fig. 4 and Fig. S2). Table S1 shows the summary of all cortical regions with significant GB discrimination in days-later and months-later scans.
Fig. 4.
Cortical areas with significant GB discrimination in days-later vs. months-later scans. (A) Beta coefficients for days-old value (Left) and months-old value (Right) in areas with significant GB discrimination (P < 0.001, α < 0.01 cluster-corrected) in both hemispheres and on the ventral surface for monkey U. (B) Same as A but for monkey D (P < 0.001, α < 0.01 cluster-corrected).
Fig. 5A further compares the distribution of GB coding voxels in the two memory periods. Most of the value-coding signals in the months-old period were subregions of the days-old period (red DM voxels active in both periods and green D voxels active only in the days-old period). There were some exceptions, where the months-old value coding appeared outside regions with days-old value coding, particularly on the ventral surface of the inferior temporal cortex (blue M voxels; also see Fig. S4). Across all value-coding cortical areas GB discrimination tended to be stronger in more anterior regions in the days-old period and in more ventral regions in the months-old period (Fig. S5).
Fig. 5.
Cortical areas with persistent GB discrimination: anatomical locations and conjoint coding of object value and location. (A) Overlay of days-old and months-old GB coding areas. Regions with significant GB discrimination in days but not months (D: green), in months but not days (emergent, M: blue), and in both days and months (persistent GB discrimination, DM: red) for both monkeys. (B) The contralateral minus ipsilateral GB discrimination in days- and months-later scans within the DM voxels (n = 114 and 107 voxels in monkeys U and D, respectively). Difference from zero and difference between days- and months-later scans are marked [large axes, t(113) > 5 monkey U, t(106) > 5 money D, ***P < 0.001]. The contralateral minus ipsilateral GB discrimination vs. the hemifield discrimination across DM voxels in both monkeys (small axes, Pearson’ correlation “r” and significance “p” are noted in each plot). Correlation was significantly higher in days compared with months in both monkeys (Fisher’s Z test, P < 0.002).
Despite being active in both days- and months-old periods, value coding in DM voxels underwent key changes with respect to the spatial coding. While value coding was stronger in the contralateral visual hemifield in both periods, the spatial asymmetry in value coding decreased in the months-old period in both monkeys (Fig. 5B), consistent with data from the selected areas (Figs. 2D and 3D). Furthermore, we found that the contralateral bias in value coding, which scaled with the strength of contralateral selectivity across the DM voxels in the days-old period, became independent of contralateral selectivity in the months-old period (Fig. 5B; see Fig. S6 for visual hemifield coding map). Thus, the GB discrimination in DM voxels became increasingly independent of retinotopic biases going from days- to months-old period.
To place the cortical value-coding maps into the bigger context, we located the value-coding areas with respect to the face patches, which are known to have stereotypical locations across the temporal and prefrontal cortices (23), using separate localizer scans in the same animals (SI Methods). The face patches were largely nonoverlapping with GB coding patches, except for the ML face patch, which overlapped with the anterior portion of GB coding region in pvSTS. The DM voxels in pvSTS were bordered by the PL face patch posteriorly (Fig. S7) (23). The GB coding areas in vlPFC were more dorsal and posterior to the PL/PO face patches (Fig. S7) (24).
Functional Connectivity Between Value-Coding Areas.
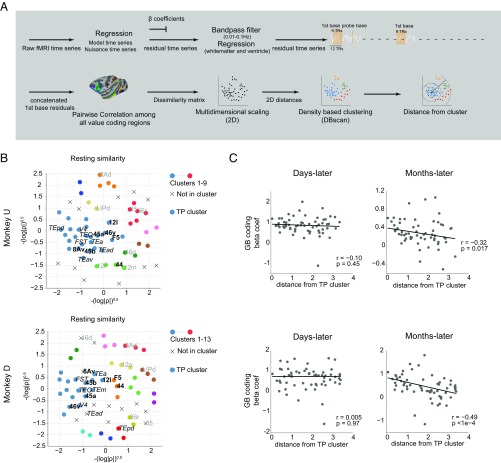
To investigate the network properties of long-term value memory we examined the functional connectivity among value-coding cortical areas based on their spontaneous activity (Fig. 6A and SI Methods, resting correlation analysis). Briefly, the time-course correlations computed across all pairs of areas during rest, which are commonly taken as a measure of functional connectivity, were inverted to provide a distance matrix that allowed for 2D visualization using multidimensional scaling. In the resulting plots (Fig. 6B and Fig. S8) the functional connectivity is approximated by the distance between any two points, each representing a cortical area, where smaller distance indicated stronger functional connectivity. We found that among GB coding areas, regions in vlPFC (45a/b and 46v) had notably high resting similarity to areas in pvSTS (TEO, FST, and IPa) and thus were located close to each other on the 2D graph despite anatomical distance. To aid in visualization, we applied a blind density-based clustering in this 2D space, which separated the value-coding areas into several groups (shown in different colors). In both animals the vlPFC and pvSTS areas fell into the same cluster, which we will refer to as the temporal-prefrontal or “TP” cluster.
Fig. 6.
Functional connectivity between value-coding areas predicts persistent GB discrimination. (A) Schematic of processing stages in resting correlation analysis. The first base blocks from all of the runs were selected and concatenated after regression of nuisance parameters and band-pass filtering (SI Methods). The pairwise Pearson’s correlation matrix between all GB coding anatomical ROIs was obtained. Pairwise dissimilarity index was made from the correlation matrix. Multidimensional scaling was used to plot the ROIs according to their dissimilarity matrix in the 2D space with every dot representing a single anatomical ROI. Blind density-based clustering was used to cluster points according to their Euclidian distance in 2D space. (B) GB coding anatomical ROIs (n = 60, Table S1) are plotted in 2D space such that smaller distances represent higher similarity in activity during rest (resting similarity). Detected clusters are plotted with different colors. “×” denotes regions that did not fall into any cluster. Several areas in pvSTS and vlPFC fell into the same cluster in both monkeys using density-based clustering (blue; TP cluster). Some regions are annotated: periarcuate areas in bold black, temporal areas in italic black, and others in gray font. For full annotation see Fig. S8. (C) Beta coefficients (n = 60) for days-old (Left) or months-old (Right) values as a function of Euclidian distance from the center of TP cluster in the 2D resting space (blue cluster in B) in both monkeys (Pearson’s correlation “r” and significance “p” are noted in each plot).
We then compared this resting-state data to the value-coding responses obtained in the days-later and months-later testing sessions. We asked whether there was any systematic relationship between the value memory signal and spontaneous network activity, characterized by functional connectivity. We found that not only did regions in the TP cluster had stronger months-long value memory compared with regions outside this cluster in both monkeys (Fig. S9) but that the functional connectivity to the TP cluster predicted the persistence of GB discrimination for areas across the brain. This was shown by computing the Euclidian distance to the center of mass of the TP cluster in the 2D resting space for each region (Fig. 6B) and using that as a measure of functional connectivity to the TP cluster. This analysis showed that GB discrimination after several months (but not before) fell off as a function of distance from the TP cluster (Fig. 6C) and was thus positively related to the resting-state functional connectivity to the TP core. This unexpected finding indicates that the areas showing weaker functional connectivity to the TP core tended to lose their value coding over months.
Subcortical Substrates for GB Discrimination After Days and Months.
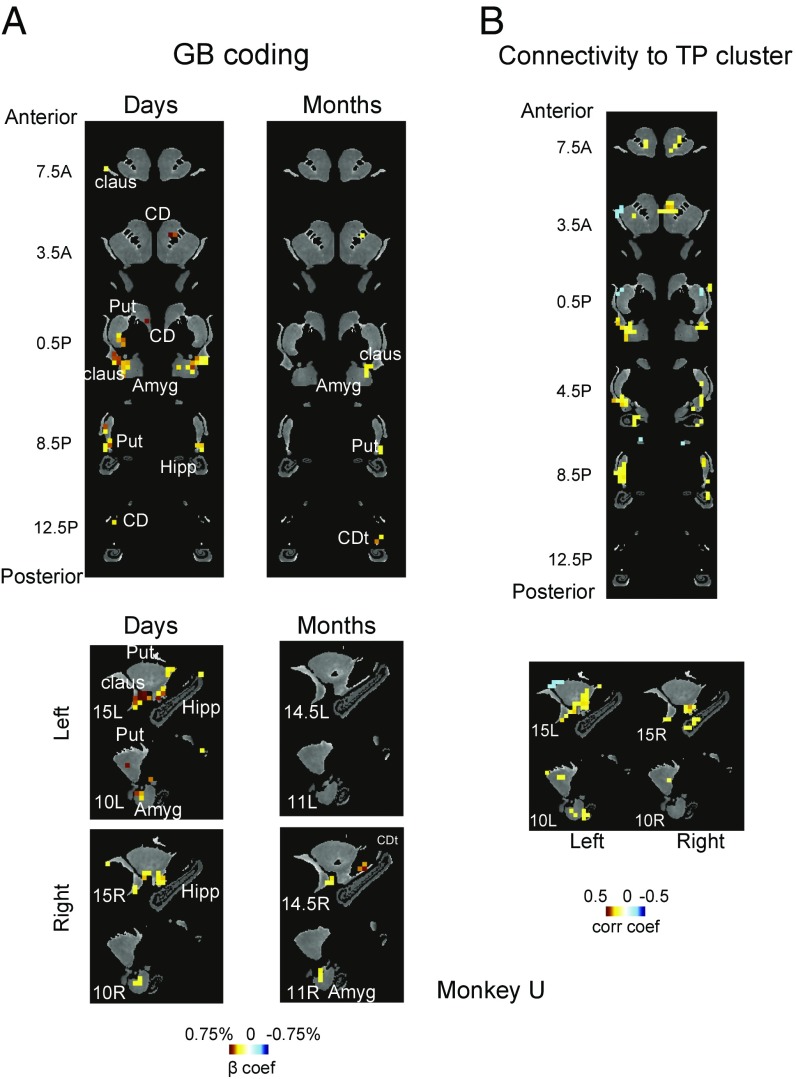
We found similar, albeit weaker, value coding in certain subcortical areas including the striatum, amygdala, and claustrum. Within all three structures we found regions that showed significant GB discrimination days and months after training (Fig. 7A, Fig. S10A, and Table S2; see SI Methods for details). Similar to cortical activation, days-later GB discrimination tended to be stronger than months-later discrimination in all three subcortical areas. In the striatum, GB discrimination was found largely in the caudal-ventral putamen (cvPut) and caudate tail (CDt), consistent with previous electrophysiological findings (12, 18). Parts of cvPut and CDt showed persistent value memory that lasted for many months. Such value memory was largely absent in the dorsal striatum, including the caudate head (CDh) except for some activations close to the internal capsule. In the amygdala, GB discrimination after days was found mostly in dorsal and lateral areas and was maintained to some degree after many months. In the claustrum, days-long value memory was prominent and was largely confined to its caudal-ventral portion. This area also retained GB discrimination after months to some degree. In contrast, the hippocampus showed no consistent GB discrimination in either days- or months-later scans (Fig. 7A), despite sufficient temporal signal-to-noise ratio (SI Methods). Some GB discrimination was also found in cerebellum in monkey U in the most anterior part of posterior lobe close to midline in days-later scans [−1, 34.5, 1.5 RAI DICOM standard atlas (25)].
Fig. 7.
GB discrimination in striatum, amygdala, claustrum, and hippocampus. (A) Voxels with significant GB coding in days- and months-later scans are shown in coronal (Top) and sagittal (Bottom) and color-coded with beta coefficients for good–bad contrast (P < 0.001, α < 0.01 cluster-corrected). Anterior–posterior distance is from anterior commissure (AC) and mediolateral distance is from the midline (15L: 15 mm left of midline, 10R: 10 mm right of midline, etc.). (B) Voxels with significant resting correlation with TP cluster are shown in coronal (Top) and sagittal (Bottom) are shown and color-coded with Pearson’s correlation coefficients (P < 0.001, α < 0.01 cluster-corrected). Data in this figure are from monkey U.
We then asked whether these subcortical areas are functionally connected with the TP cluster (Fig. 6). To this end, the resting signal in the TP cluster was averaged and was correlated with the resting fluctuations in the subcortical areas (SI Methods). We found that subregions within all of the four subcortical areas showed significant correlation to the TP cluster (Fig. 7B and Fig. S10B). Importantly, this correlation was prominent in cvPut, CDt, ventral claustrum, and dorsolateral amygdala, where strong value memory was also observed (Fig. 7A and Fig. S10A). Parts of CDh (close to the internal capsule) also showed positive correlation, but the most dorsal areas showed significant negative correlation. Parts of the hippocampus showed significant correlations, but their locations were not consistent between the two monkeys (anterior and posterior in monkeys U and D, respectively).
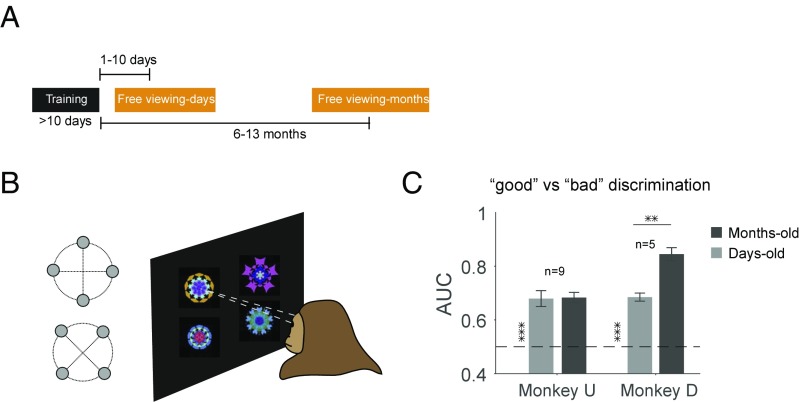
Behavioral Discrimination of Days-Old and Months-Old Values.
Given persistent value coding within cortical and subcortical areas, we asked whether the monkeys remembered and discriminated good and bad objects behaviorally. To address this question we used a free-viewing task days or months after training (Fig. 8A). Differential gaze bias during free viewing has been previously used as an index of memory strength (10). In each trial, four objects from good and bad categories were chosen randomly and were presented simultaneously on the screen (Fig. 8B). Results showed that after both memory periods the monkeys viewed good objects longer compared with bad objects (Fig. 8C), even though there was no object–reward association during free viewing. This gaze bias is consistent with our previous studies (21, 22). Surprisingly, the level of the free-viewing bias was maintained completely in the months-old compared with days-old period (Fig. 8C and Fig. S11). The months-old bias was even stronger than the days-old bias in monkey D, which may be related to the prevalence of emergent value-coding areas in the TP cortices (M voxels in Fig. 5A: 204 vs. 111 M voxels in monkey D vs. U, respectively).
Fig. 8.
Behavioral GB discrimination days and months after last reward exposure. (A) Objects used in the fMRI scans were tested days or months after reward training in a free-viewing task. (B) Free-viewing task: good and bad objects were randomly selected and shown to the monkey for viewing in the absence of reward. (C) Behavioral discriminability (area under the receiver operating curve, AUC) of objects based on days-old and months-old values as measured by viewing duration [days and months vs. chance: t(8) > 6 monkey U and t(4) > 12 monkey D, P < 1e-3. Days vs. months: t(8) = 0.1 P > 0.9 monkey U and t(4) = 5.4 P < 1e-2 monkey D, **P < 0.01, ***P < 0.001].
Discussion
Our results revealed a robust cortical differentiation of objects based on their old values. Several days after the object–reward association learning the discrimination of good and bad objects was found in multiple, distinct cortical areas, including the vlPFC, the ventral bank of the superior temporal sulcus (vSTS), and the LIP. This discrimination happened during passive viewing of objects in the absence of reward. Importantly, we found that such value-dependent discrimination persisted for many months in core areas within temporal and prefrontal cortices (Figs. 2–4 and Figs. S2–S4). The GB discrimination was maintained for a large number of initially neutral objects, thus revealing a high-capacity long-term memory mechanism in primate brains for objects with biased reward histories. Accordingly, monkeys showed persistent behavioral bias toward good objects days and months after value association (Fig. 8 and Fig. S11).
It is well known that the brain routinely assigns value to objects based on experience. General stimulus reward learning in primates has been studied extensively using a variety of methods such as single unit recording (26), functional imaging (27), or lesions (28). Many electrophysiological studies in monkeys have revealed a distributed network for object-reward learning in visual cortical areas in frontal (29–31), temporal (32, 33), and parietal (34, 35) lobes. fMRI studies in monkeys and humans have also found reward-learning modulations in visual cortical areas (17, 36, 37) and even in early visual cortices (14, 38). However, little work has been done regarding long-term maintenance of value-based discrimination for complex objects outside the value training context. In this fMRI study, we focused on persistent discrimination of objects based on days- and months-old values rather than by values during or shortly after reward learning as was done previously (37, 39). Furthermore, we used many complex objects (>100) to ensure that our data are scalable and relevant to real-life situations. Long-term reward memories were tested using a passive viewing task in the absence of contingent rewards, because otherwise short-term effects of reward (e.g., reward expectation and consummatory behaviors) would be included in fMRI data (37). Such short-term effects would be hard to dissociate from long-term changes in visual processing of objects even when attempts are made to deconvolve their contributions, due to nonlinearities present in hemodynamic (40) and neural responses (41).
Many of the GB coding areas across the cortex appear to conjointly discriminate value and spatial position (Figs. 2–4 and Figs. S2–S4 and S6). The combination of value and position coding may enable the subject not only to identify but also to localize good and bad objects wherever they are located. Indeed, our previous study (8) showed that monkeys can rapidly detect and orient gaze (saccade RT < 150 ms) to a good object among many bad objects. Such rapid gaze bias may be driven by strong value and position coding found in the earlier parts of the ventral stream (e.g., TEO) which are known to have short latency visual responses (42). However, the later parts of the ventral stream (e.g., TEm and TEa) may also contribute to this mechanism because these areas also discriminated object positions, although less strongly (Fig. S6).
Prefrontal cortex is often associated with working memory that has a short-term and low-capacity storage (43, 44). However, our results indicate that vlPFC can also participate in long-term, high-capacity memory for object values (Fig. 4). We found a strong functional connectivity between the vlPFC subregions and the pvSTS subregions that showed strong and persistent value coding (TP cluster, Fig. 6B). An obvious basis for this could be the known reciprocal connections between vlPFC and pvSTS (45, 46). However, it was notable that the strength of functional connectivity to the TP cluster predicted the months-long value memory across the value-coding areas in the cortex. For example, certain areas such as LIP and area 46d, with weak functional connectivity to the TP cluster, showed diminished GB discrimination during the months-later scans (Fig. S2 and Table S1). This relationship to resting-state network connectivity, and its specificity for the months- rather than days-long memory, was unexpected and requires further study. Similarly, we did not observe a persistent GB discrimination in orbitofrontal cortex (OFC) which was out of the TP cluster, despite its known role in learning object values (29, 31, 47) but consistent with reports that the OFC is less important for the retention of object values (39, 48). These data suggest that the vlPFC–pvSTS network plays a key role in retaining object-value memories over long time periods. Our data thus extend the known role of the prefrontal and temporal cortices in object discrimination learning and memory (30, 37, 39, 49–52) to retention over longer time periods. While unlikely given the low-frequency nature of resting correlations, we note that one cannot completely rule out the possibility that the low-frequency filtering caused by MION could have reduced our power to detect further significant resting correlations.
Value coding was also found in early visual areas (V1–3) in the days-old period. This is relevant to recent studies showing that early visual cortical areas are influenced by reward values in rodents (53), monkeys (39), and humans (14). In the monkey, area V2 was activated by a visual stimulus associated with reward, even after the reward association was discontinued (39). These studies together suggest that object-value memories are initially encoded in early visual cortical areas, which may contribute to gaze bias to reward-associated objects (39) (Fig. 8). However, our results showed that the value coding in these early visual areas largely disappeared after several months (Fig. 3). These findings suggest that visual cortical areas may contribute to decision making in different timescales: the early visual cortex for flexible decisions based on shorter-term memory and the TP network for stable decisions based on longer-term memory. This is similar to the hypothesis we proposed for the posterior and anterior parts of the basal ganglia (54).
We also found robust value memory within the striatum, amygdala, and claustrum. Recent studies in our laboratory have shown that posterior basal ganglia circuitry including the CDt is specifically sensitive to an object’s old values (13, 54). Indeed, we found GB discrimination in CDt as well as in cvPut in the days-old period (Fig. 7A and Fig. S10A). The value-coding areas were more localized in the months-old period. Both CDt and cvPut are known to receive direct inputs from the temporal cortex (55) and send signals indirectly to the temporal cortex (56) and the prefrontal cortex (57). Notably, the value-modulated regions in these subcortical areas showed strong functional connectivity with the TP cluster (Fig. 7B and Fig. S10B). Therefore, it is possible that the persistent cortical activation to old values is mediated or trained by the posterior basal ganglia (58). Furthermore, the posterior part of the basal ganglia is implicated in procedural memories (59–61). These data suggest that the long-term memory of object values in the TP network may involve more implicit mechanisms. Indeed, we did not observe significant value memory in the hippocampus, which is known to be involved in relational or episodic but not reward-conditioning memories (62, 63). However, it should be stated that the absence of activity in the hippocampus cannot be used to rule out its involvement, as is the case with any negative fMRI result. Area TEO is also known to project to ventral claustrum (64) and laterodorsal amygdala (65, 66). Interestingly, we found that both of these regions showed GB discrimination in days-later and to some degree in months-later scans. Both of these areas are found to project to GB coding areas in CDt (67). Amygdala is known to be involved in learning object–reward associations (68). While it is generally accepted that amygdala plays a role in long-term fear memory (69), its involvement in long-term reward memory was not previously shown. The role of claustrum in object reward learning is less clear. It is known that the ventral claustrum responds preferentially to visual stimuli (70). Our result showed that such a visual response is strongly modulated by long-term reward memory associated with objects. Whether ventral claustrum is also important for learning the reward associations remains to be tested.
Our results showed that the discrimination of good and bad objects during the free-viewing task remained equally strong or even stronger after several months (Fig. 8C), even though the number of voxels with GB discrimination decreased (Figs. 4 and 5A). One possibility is that voxels exhibiting long-term value coding were sufficient to maintain the behavioral discrimination. As for the broad distribution of value-coding voxels across the cortex in the initial days after training, they may not have been important for guiding behavior or may have had a critical role in consolidation of value memories. It is also possible that, after the memory consolidation, less synaptic or metabolic activity is required to support the neural activity which is reflected in the diminished fMRI responses (71). One notable finding related to consolidation is the emergence of value-coding in a few areas only after a several months. Whether the areas marked by emergent value coding indeed reflect consolidation and are critical for long-term value memory awaits further study. Future experiments with faster dynamics may partially address these issues by examining the neural correlates that explain variability of behavioral memory between individual objects which was not currently possible with MION in a block-design paradigm.
Some studies have shown that while both high- and low-value stimuli are remembered well above chance, high-value objects tend to be remembered better (refs. 72 and 73, but also see ref. 74 for lack of a recognition difference between high- and low-value objects). Thus, the effect of value in GB discrimination in our study may be partially mediated by selective forgetting of bad objects, especially after months. While we cannot completely rule out this possibility, we note that we did not observe differential activation in hippocampus, known to be important for recognition memory, to good and bad objects. Also, it is known that viewing is biased toward novel objects during free viewing (10, 21). Thus, the presumed novelty of bad objects should have reduced (or reversed) the free-viewing preference for good objects after months, which we did not observe either (Fig. 8). One possibility is that long-term exposure to objects during training (>10 d, >100 trials per object) prevented forgetting of objects themselves in our experiment. Nevertheless, a direct examination of recognition memory after months awaits further behavioral testing.
We speculate that various physiological and psychological phenomena, such as enhanced attention or positive emotional responses to good objects, will be contingent upon value-dependent activations observed across the brain in this study. Indeed, many of the activated areas in the current study in prefrontal, parietal, and temporal areas are known to be implicated in visual attention (75). Determining the functional role of the observed activations requires causal manipulations in each activated region in tasks designed to test specific behaviors that rely on value memory of objects. Nevertheless, a dissociation between attention and valuation signals in a given brain region may be elusive (76, 77). For instance, we observed a reduction of activated voxels in months-later scans in regions that are thought to be involved in visual attention (Fig. 5A) without observing a behavioral reduction in attentional bias toward good objects (Fig. 8). This warns against a simple equivalence between value-dependent activation in the brain and attention.
In summary, our results revealed a long-term high-capacity memory mechanism in the primate cerebral cortex for discrimination of objects based on their old values. Repeated reward association created differential object selectivity in the ventral stream as early as areas V4 and TEO that was persistent for many months (Table S1). The stability of object discrimination for many months in temporal and prefrontal cortical areas as well as several subcortical regions explains the stability of behavioral memory of object values reported here and in previous studies (7, 8, 78, 79). Maintaining memory of an object’s old value is important in real life, where many objects are experienced and must be efficiently detected in future encounters. This system allows animals and humans to robustly adapt to their environments to find previously rewarding objects accurately and quickly. However, this long-term high-capacity memory could also be relevant for maladaptive behaviors. For example, one can speculate that in drug addiction a large number of reward cues can be easily remembered and persistently activate selective temporal and prefrontal cortical areas several months after drug exposure. Our results thus reveal a key cortical network that can be targeted by novel interventional methods proposed for drugs of addiction (80).
Methods
All procedures followed National Institutes of Health guidelines and were approved by the Animal Care and Use Committee of the National Eye Institute. A detailed explanation of experimental procedures and data analysis is provided in SI Methods. Briefly, two rhesus macaques (U: female, D: male) participated in awake tests of value-based object discrimination for values trained days or months before. fMRI data were preprocessed and multiple linear regression using MION hemodynamics was performed to quantify differential activation as percentage change from mean to good vs. bad objects and to contra- vs. ipsilateral hemifields across the whole brain using AFNI and custom written MATLAB code. Visualization on the inflated surface of a standard brain was made by SUMA. A summary of all cortical areas with GB coding in days or months in both monkeys is provided in Table S1. Face-patch localizers were done in separate runs using conspecific monkey faces vs. ordinary objects or unrewarded fractals. Resting-state similarity of cortical areas with GB coding was determined from pairwise Pearson’s correlation obtained from first base block from all runs for each monkey. Multidimensional scaling was done using MATLAB mdscale with Sammon criteria and density-based clustering was done with a MATLAB function provided by Yarpiz Project. A summary of all subcortical regions of interest (ROIs) with GB coding in days or months in both monkeys is provided in Table S2.
Supplementary Material
Acknowledgments
We thank David Yu, Charles Zhu, and Frank Ye for assistance with fMRI scanning and Daniel Glen, Richard Reynolds, Jakob Seidlitz, and Brian Russ for discussions on the analysis. This work was supported by the Intramural Research Program at the National Eye Institute. Functional and anatomical MRI scanning was carried out in the Neurophysiology Imaging Facility Core (National Institute of Mental Health, National Institute of Neurological Disorders and Stroke, and National Eye Institute).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. R.J.D. is a guest editor invited by the Editorial Board.
See Commentary on page 1956.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1707695115/-/DCSupplemental.
References
- 1.Pavlov IP, Anrep GV. Conditioned Reflexes. Courier Corp.; North Chelmsford, MA: 2003. [Google Scholar]
- 2.Skinner B. The Behavior of Organisms. Appleton-Century-Crofts; New York: 1938. [Google Scholar]
- 3.Pribram KH, Mishkin M. Simultaneous and successive visual discrimination by monkeys with inferotemporal lesions. J Comp Physiol Psychol. 1955;48:198–202. doi: 10.1037/h0049140. [DOI] [PubMed] [Google Scholar]
- 4.Carew TJ, Sahley CL. Invertebrate learning and memory: From behavior to molecules. Annu Rev Neurosci. 1986;9:435–487. doi: 10.1146/annurev.ne.09.030186.002251. [DOI] [PubMed] [Google Scholar]
- 5.Rolls ET, Critchley HD, Mason R, Wakeman EA. Orbitofrontal cortex neurons: Role in olfactory and visual association learning. J Neurophysiol. 1996;75:1970–1981. doi: 10.1152/jn.1996.75.5.1970. [DOI] [PubMed] [Google Scholar]
- 6.Gaffan D, Murray EA. Amygdalar interaction with the mediodorsal nucleus of the thalamus and the ventromedial prefrontal cortex in stimulus-reward associative learning in the monkey. J Neurosci. 1990;10:3479–3493. doi: 10.1523/JNEUROSCI.10-11-03479.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yasuda M, Yamamoto S, Hikosaka O. Robust representation of stable object values in the oculomotor basal ganglia. J Neurosci. 2012;32:16917–16932. doi: 10.1523/JNEUROSCI.3438-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghazizadeh A, Griggs W, Hikosaka O. Object-finding skill created by repeated reward experience. J Vis. 2016;16:17. doi: 10.1167/16.10.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winocur G, Moscovitch M, Bontempi B. Memory formation and long-term retention in humans and animals: Convergence towards a transformation account of hippocampal-neocortical interactions. Neuropsychologia. 2010;48:2339–2356. doi: 10.1016/j.neuropsychologia.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 10.Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dudai Y. The neurobiology of consolidations, or, how stable is the engram? Annu Rev Psychol. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. [DOI] [PubMed] [Google Scholar]
- 12.Kim HF, Hikosaka O. Distinct basal ganglia circuits controlling behaviors guided by flexible and stable values. Neuron. 2013;79:1001–1010. doi: 10.1016/j.neuron.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim HF, Ghazizadeh A, Hikosaka O. Dopamine neurons encoding long-term memory of object value for habitual behavior. Cell. 2015;163:1165–1175. doi: 10.1016/j.cell.2015.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serences JT. Value-based modulations in human visual cortex. Neuron. 2008;60:1169–1181. doi: 10.1016/j.neuron.2008.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seitz AR, Kim D, Watanabe T. Rewards evoke learning of unconsciously processed visual stimuli in adult humans. Neuron. 2009;61:700–707. doi: 10.1016/j.neuron.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frankó E, Seitz AR, Vogels R. Dissociable neural effects of long-term stimulus-reward pairing in macaque visual cortex. J Cogn Neurosci. 2010;22:1425–1439. doi: 10.1162/jocn.2009.21288. [DOI] [PubMed] [Google Scholar]
- 17.Anderson BA, Laurent PA, Yantis S. Value-driven attentional priority signals in human basal ganglia and visual cortex. Brain Res. 2014;1587:88–96. doi: 10.1016/j.brainres.2014.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vanduffel W, et al. Visual motion processing investigated using contrast agent-enhanced fMRI in awake behaving monkeys. Neuron. 2001;32:565–577. doi: 10.1016/s0896-6273(01)00502-5. [DOI] [PubMed] [Google Scholar]
- 19.Eichenbaum H, Cohen NJ. From Conditioning to Conscious Recollection: Memory Systems of the Brain. Oxford Univ Press; Oxford: 2004. [Google Scholar]
- 20.Yamamoto S, Kim HF, Hikosaka O. Reward value-contingent changes of visual responses in the primate caudate tail associated with a visuomotor skill. J Neurosci. 2013;33:11227–11238. doi: 10.1523/JNEUROSCI.0318-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghazizadeh A, Griggs W, Hikosaka O. Ecological origins of object salience: Reward, uncertainty, aversiveness, and novelty. Front Neurosci. 2016;10:378. doi: 10.3389/fnins.2016.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boussaoud D, Desimone R, Ungerleider LG. Visual topography of area TEO in the macaque. J Comp Neurol. 1991;306:554–575. doi: 10.1002/cne.903060403. [DOI] [PubMed] [Google Scholar]
- 23.Tsao DY, Moeller S, Freiwald WA. Comparing face patch systems in macaques and humans. Proc Natl Acad Sci USA. 2008;105:19514–19519. doi: 10.1073/pnas.0809662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsao DY, Schweers N, Moeller S, Freiwald WA. Patches of face-selective cortex in the macaque frontal lobe. Nat Neurosci. 2008;11:877–879. doi: 10.1038/nn.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reveley C, et al. Three-dimensional digital template atlas of the macaque brain. Cereb Cortex. 2017;27:4463–4477. doi: 10.1093/cercor/bhw248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schultz W. Multiple reward signals in the brain. Nat Rev Neurosci. 2000;1:199–207. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- 27.O’Doherty JP. Reward representations and reward-related learning in the human brain: Insights from neuroimaging. Curr Opin Neurobiol. 2004;14:769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 28.Iversen SD, Mishkin M. Perseverative interference in monkeys following selective lesions of the inferior prefrontal convexity. Exp Brain Res. 1970;11:376–386. doi: 10.1007/BF00237911. [DOI] [PubMed] [Google Scholar]
- 29.Rolls ET. The orbitofrontal cortex and reward. Cereb Cortex. 2000;10:284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi S, Lauwereyns J, Koizumi M, Sakagami M, Hikosaka O. Influence of reward expectation on visuospatial processing in macaque lateral prefrontal cortex. J Neurophysiol. 2002;87:1488–1498. doi: 10.1152/jn.00472.2001. [DOI] [PubMed] [Google Scholar]
- 31.Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex. Nature. 1999;398:704–708. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- 32.Mogami T, Tanaka K. Reward association affects neuronal responses to visual stimuli in macaque te and perirhinal cortices. J Neurosci. 2006;26:6761–6770. doi: 10.1523/JNEUROSCI.4924-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Z, Richmond BJ. Response differences in monkey TE and perirhinal cortex: Stimulus association related to reward schedules. J Neurophysiol. 2000;83:1677–1692. doi: 10.1152/jn.2000.83.3.1677. [DOI] [PubMed] [Google Scholar]
- 34.Peck CJ, Jangraw DC, Suzuki M, Efem R, Gottlieb J. Reward modulates attention independently of action value in posterior parietal cortex. J Neurosci. 2009;29:11182–11191. doi: 10.1523/JNEUROSCI.1929-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Platt ML, Glimcher PW. Neural correlates of decision variables in parietal cortex. Nature. 1999;400:233–238. doi: 10.1038/22268. [DOI] [PubMed] [Google Scholar]
- 36.Bartra O, McGuire JT, Kable JW. The valuation system: A coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. NeuroImage. 2013;76:412–427. doi: 10.1016/j.neuroimage.2013.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaskan P, et al. Learned value shapes responses to objects in frontal and ventral stream networks in macaque monkeys. Cereb Cortex. 2017;27:2739–2757. doi: 10.1093/cercor/bhw113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arsenault JT, Nelissen K, Jarraya B, Vanduffel W. Dopaminergic reward signals selectively decrease fMRI activity in primate visual cortex. Neuron. 2013;77:1174–1186. doi: 10.1016/j.neuron.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nelissen K, et al. Neural correlates of the formation and retention of cocaine-induced stimulus-reward associations. Biol Psychiatry. 2012;72:422–428. doi: 10.1016/j.biopsych.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 40.Friston KJ, Josephs O, Rees G, Turner R. Nonlinear event-related responses in fMRI. Magn Reson Med. 1998;39:41–52. doi: 10.1002/mrm.1910390109. [DOI] [PubMed] [Google Scholar]
- 41.Ghazizadeh A, Fields HL, Ambroggi F. Isolating event-related neuronal responses by deconvolution. J Neurophysiol. 2010;104:1790–1802. doi: 10.1152/jn.00389.2010. [DOI] [PubMed] [Google Scholar]
- 42.Schmolesky MT, et al. Signal timing across the macaque visual system. J Neurophysiol. 1998;79:3272–3278. doi: 10.1152/jn.1998.79.6.3272. [DOI] [PubMed] [Google Scholar]
- 43.Goldman‐Rakic PS. Comprehensive Physiology. Wiley; New York: 1987. Circuitry of primate prefrontal cortex and regulation of behavior by representational memory; pp. 373–417. [Google Scholar]
- 44.Miller EK, Erickson CA, Desimone R. Neural mechanisms of visual working memory in prefrontal cortex of the macaque. J Neurosci. 1996;16:5154–5167. doi: 10.1523/JNEUROSCI.16-16-05154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gerbella M, Borra E, Tonelli S, Rozzi S, Luppino G. Connectional heterogeneity of the ventral part of the macaque area 46. Cereb Cortex. 2013;23:967–987. doi: 10.1093/cercor/bhs096. [DOI] [PubMed] [Google Scholar]
- 46.Webster MJ, Bachevalier J, Ungerleider LG. Connections of inferior temporal areas TEO and TE with parietal and frontal cortex in macaque monkeys. Cereb Cortex. 1994;4:470–483. doi: 10.1093/cercor/4.5.470. [DOI] [PubMed] [Google Scholar]
- 47.Rudebeck PH, Saunders RC, Prescott AT, Chau LS, Murray EA. Prefrontal mechanisms of behavioral flexibility, emotion regulation and value updating. Nat Neurosci. 2013;16:1140–1145. doi: 10.1038/nn.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Delamater AR. The role of the orbitofrontal cortex in sensory-specific encoding of associations in pavlovian and instrumental conditioning. Ann N Y Acad Sci. 2007;1121:152–173. doi: 10.1196/annals.1401.030. [DOI] [PubMed] [Google Scholar]
- 49.Eradath MK, Mogami T, Wang G, Tanaka K. Time context of cue-outcome associations represented by neurons in perirhinal cortex. J Neurosci. 2015;35:4350–4365. doi: 10.1523/JNEUROSCI.4730-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brush ES, Rosvold HE, Mishkin M. Effects of object preferences and aversions on discrimination learning in monkeys with frontal lesions. J Comp Physiol Psychol. 1961;54:319–325. [Google Scholar]
- 51.Tomita H, Ohbayashi M, Nakahara K, Hasegawa I, Miyashita Y. Top-down signal from prefrontal cortex in executive control of memory retrieval. Nature. 1999;401:699–703. doi: 10.1038/44372. [DOI] [PubMed] [Google Scholar]
- 52.Browning PGF, Easton A, Gaffan D. Frontal-temporal disconnection abolishes object discrimination learning set in macaque monkeys. Cereb Cortex. 2007;17:859–864. doi: 10.1093/cercor/bhk039. [DOI] [PubMed] [Google Scholar]
- 53.Shuler MG, Bear MF. Reward timing in the primary visual cortex. Science. 2006;311:1606–1609. doi: 10.1126/science.1123513. [DOI] [PubMed] [Google Scholar]
- 54.Hikosaka O, Kim HF, Yasuda M, Yamamoto S. Basal ganglia circuits for reward value-guided behavior. Annu Rev Neurosci. 2014;37:289–306. doi: 10.1146/annurev-neuro-071013-013924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saint-Cyr JA, Ungerleider LG, Desimone R. Organization of visual cortical inputs to the striatum and subsequent outputs to the pallido-nigral complex in the monkey. J Comp Neurol. 1990;298:129–156. doi: 10.1002/cne.902980202. [DOI] [PubMed] [Google Scholar]
- 56.Middleton FA, Strick PL. The temporal lobe is a target of output from the basal ganglia. Proc Natl Acad Sci USA. 1996;93:8683–8687. doi: 10.1073/pnas.93.16.8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Middleton FA, Strick PL. Basal-ganglia ‘projections’ to the prefrontal cortex of the primate. Cereb Cortex. 2002;12:926–935. doi: 10.1093/cercor/12.9.926. [DOI] [PubMed] [Google Scholar]
- 58.Hélie S, Ell SW, Ashby FG. Learning robust cortico-cortical associations with the basal ganglia: An integrative review. Cortex. 2015;64:123–135. doi: 10.1016/j.cortex.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 59.Hikosaka O, Ghazizadeh A, Griggs W, Amita H. Parallel basal ganglia circuits for decision making. J Neural Transm (Vienna) February 2, 2017 doi: 10.1007/s00702-017-1691-1. [DOI] [PubMed] [Google Scholar]
- 60.Squire LR. Memory systems of the brain: A brief history and current perspective. Neurobiol Learn Mem. 2004;82:171–177. doi: 10.1016/j.nlm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 61.Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans. Science. 1996;273:1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- 62.Hannula DE, Ranganath C. The eyes have it: Hippocampal activity predicts expression of memory in eye movements. Neuron. 2009;63:592–599. doi: 10.1016/j.neuron.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Henke K. A model for memory systems based on processing modes rather than consciousness. Nat Rev Neurosci. 2010;11:523–532. doi: 10.1038/nrn2850. [DOI] [PubMed] [Google Scholar]
- 64.Webster MJ, Bachevalier J, Ungerleider LG. Subcortical connections of inferior temporal areas TE and TEO in macaque monkeys. J Comp Neurol. 1993;335:73–91. doi: 10.1002/cne.903350106. [DOI] [PubMed] [Google Scholar]
- 65.Webster MJ, Ungerleider LG, Bachevalier J. Connections of inferior temporal areas TE and TEO with medial temporal-lobe structures in infant and adult monkeys. J Neurosci. 1991;11:1095–1116. doi: 10.1523/JNEUROSCI.11-04-01095.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stefanacci L, Amaral DG. Some observations on cortical inputs to the macaque monkey amygdala: An anterograde tracing study. J Comp Neurol. 2002;451:301–323. doi: 10.1002/cne.10339. [DOI] [PubMed] [Google Scholar]
- 67.Griggs WS, et al. Flexible and stable value coding areas in caudate head and tail receive anatomically distinct cortical and subcortical inputs. Front Neuroanat. 2017;11:106. doi: 10.3389/fnana.2017.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baxter MG, Murray EA. The amygdala and reward. Nat Rev Neurosci. 2002;3:563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- 69.Fanselow MS, Gale GD. The amygdala, fear, and memory. Ann N Y Acad Sci. 2003;985:125–134. doi: 10.1111/j.1749-6632.2003.tb07077.x. [DOI] [PubMed] [Google Scholar]
- 70.Remedios R, Logothetis NK, Kayser C. Unimodal responses prevail within the multisensory claustrum. J Neurosci. 2010;30:12902–12907. doi: 10.1523/JNEUROSCI.2937-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Picard N, Matsuzaka Y, Strick PL. Extended practice of a motor skill is associated with reduced metabolic activity in M1. Nat Neurosci. 2013;16:1340–1347. doi: 10.1038/nn.3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wittmann BC, et al. Reward-related FMRI activation of dopaminergic midbrain is associated with enhanced hippocampus-dependent long-term memory formation. Neuron. 2005;45:459–467. doi: 10.1016/j.neuron.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 73.Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JDE. Reward-motivated learning: Mesolimbic activation precedes memory formation. Neuron. 2006;50:507–517. doi: 10.1016/j.neuron.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 74.Canli T, Zhao Z, Desmond JE, Glover G, Gabrieli JDE. fMRI identifies a network of structures correlated with retention of positive and negative emotional memory. Psychobiology (Austin Tex) 1999;27:441–452. [Google Scholar]
- 75.Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- 76.Maunsell JHR. Neuronal representations of cognitive state: Reward or attention? Trends Cogn Sci. 2004;8:261–265. doi: 10.1016/j.tics.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 77.O’Doherty JP. The problem with value. Neurosci Biobehav Rev. 2014;43:259–268. doi: 10.1016/j.neubiorev.2014.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Anderson BA, Yantis S. Persistence of value-driven attentional capture. J Exp Psychol Hum Percept Perform. 2013;39:6–9. doi: 10.1037/a0030860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chelazzi L, Perlato A, Santandrea E, Della Libera C. Rewards teach visual selective attention. Vision Res. 2013;85:58–72. doi: 10.1016/j.visres.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 80.Terraneo A, et al. Transcranial magnetic stimulation of dorsolateral prefrontal cortex reduces cocaine use: A pilot study. Eur Neuropsychopharmacol. 2016;26:37–44. doi: 10.1016/j.euroneuro.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Leite FP, et al. Repeated fMRI using iron oxide contrast agent in awake, behaving macaques at 3 Tesla. NeuroImage. 2002;16:283–294. doi: 10.1006/nimg.2002.1110. [DOI] [PubMed] [Google Scholar]
- 82.Miyashita Y, Higuchi S, Sakai K, Masui N. Generation of fractal patterns for probing the visual memory. Neurosci Res. 1991;12:307–311. doi: 10.1016/0168-0102(91)90121-e. [DOI] [PubMed] [Google Scholar]
- 83.Yamamoto S, Monosov IE, Yasuda M, Hikosaka O. What and where information in the caudate tail guides saccades to visual objects. J Neurosci. 2012;32:11005–11016. doi: 10.1523/JNEUROSCI.0828-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 85.Saad ZS, Reynolds RC, Argall B, Japee S, Cox RW. 2004 Second IEEE International Symposium on Biomedical Imaging: Macro to Nano. IEEE; Piscataway, NJ: 2004. Suma: An interface for surface-based intra- and inter-subject analysis with AFNI; pp. 1510–1513. [Google Scholar]
- 86.Xiang QS, Ye FQ. Correction for geometric distortion and N/2 ghosting in EPI by phase labeling for additional coordinate encoding (PLACE) Magn Reson Med. 2007;57:731–741. doi: 10.1002/mrm.21187. [DOI] [PubMed] [Google Scholar]
- 87.Murphy K, Bodurka J, Bandettini PA. How long to scan? The relationship between fMRI temporal signal to noise ratio and necessary scan duration. NeuroImage. 2007;34:565–574. doi: 10.1016/j.neuroimage.2006.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fair DA, et al. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. NeuroImage. 2007;35:396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ester M, Kriegel H-P, Sander J, Xu X. A density-based algorithm for discovering clusters in large spatial databases with noise. KDD. 1996;96:226–231. [Google Scholar]
- 90.Saleem KS, Logothetis NK. A Combined MRI and Histology Atlas of the Rhesus Monkey Brain in Stereotaxic Coordinates. Academic; New York: 2012. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.