Significance
Adipocyte differentiation is necessary for metabolic homeostasis. Transcription factors control adipocyte differentiation by regulating expression of genes required for adipocyte function. Here we show that the bromodomain and extraterminal domain (BET) family of bromodomain-containing proteins are important coregulators of adipogenesis. BRD4 binds to enhancers that drive expression of PPARγ, a master transcription factor required for normal adipocyte differentiation. Disrupting BRD4 at these enhancers blocks PPARγ expression and impairs differentiation. Understanding the molecular determinants of PPARγ expression and adipocyte differentiation may provide insights into the pathogenesis of metabolic diseases related to adipose tissue dysfunction and obesity. Furthermore, these data implicate metabolic pathways and their transcriptional regulators as potential targets of BET bromodomain inhibitors, as currently under investigation in human clinical trials of cancer therapy.
Keywords: chromatin, coactivator, BET bromodomain, adipogenesis, transcription
Abstract
Developmental transitions are guided by master regulatory transcription factors. During adipogenesis, a transcriptional cascade culminates in the expression of PPARγ and C/EBPα, which orchestrate activation of the adipocyte gene expression program. However, the coactivators controlling PPARγ and C/EBPα expression are less well characterized. Here, we show the bromodomain-containing protein, BRD4, regulates transcription of PPARγ and C/EBPα. Analysis of BRD4 chromatin occupancy reveals that induction of adipogenesis in 3T3L1 fibroblasts provokes dynamic redistribution of BRD4 to de novo super-enhancers proximal to genes controlling adipocyte differentiation. Inhibition of the bromodomain and extraterminal domain (BET) family of bromodomain-containing proteins impedes BRD4 occupancy at these de novo enhancers and disrupts transcription of Pparg and Cebpa, thereby blocking adipogenesis. Furthermore, silencing of these BRD4-occupied distal regulatory elements at the Pparg locus by CRISPRi demonstrates a critical role for these enhancers in the control of Pparg gene expression and adipogenesis in 3T3L1s. Together, these data establish BET bromodomain proteins as time- and context-dependent coactivators of the adipocyte cell state transition.
Transcription factors (TFs) guide cell state transitions during development (1). Adipocyte differentiation is controlled by the action of peroxisome proliferator-activated receptor gamma (PPARγ) and CCAAT/enhancer binding protein alpha (C/EBPα), master TFs that control the gene expression program of the developing adipocyte (2). In model systems, proadipogenic stimuli induce PPARγ and C/EBPα expression by activating an upstream TF cascade comprised of C/EBPβ, C/EBPδ, and the glucocorticoid receptor (GR) (3). Disruption of this TF network impairs adipocyte differentiation and adipose tissue formation (4).
These master TF networks signal via chromatin to execute the gene expression programs that drive adipogenesis. Comparative analysis of chromatin states in preadipocytes versus adipocytes has demonstrated massive enhancer reorganization during adipogenesis, as marked by genome-wide changes in the acetylation of histone 3 lysine 27 (H3K27ac) or lysine 9 (H3K9ac) (5, 6). Super-enhancers—cis-regulatory domains comprised of dense clusters of DNA-bound TFs and exceptionally high enrichment of chromatin-associated coactivators [e.g., BRD4, Mediator subunit-1 (MED1)]—have recently been identified in models of adipogenesis (7). Super-enhancers, akin to stretch enhancers or locus control regions, contain a disproportionately large amount of coactivator molecules for any given cell state (8, 9) and drive transcription of genes essential for cell identity (10, 11). During adipogenesis, super-enhancers have been identified near genes important for adipocyte differentiation including Pparg (7). However, the specific mechanisms by which dynamically remodeled enhancers signal to RNA polymerase and regulate adipogenesis are not as well characterized.
The bromodomain and extraterminal domain (BET) family of bromodomain-containing coactivator proteins—BRD2, BRD3, BRD4—associate with chromatin (12–14). BETs coactivate transcription by forming scaffolds with other coregulatory proteins, including Mediator subunits and the positive transcription elongation factor-b (PTEF-b) complex at enhancers. These multiprotein complexes activate RNA polymerase II (RNA Pol II) via long-range chromatin interactions (15). Prior studies establish BRD4 as a dynamic constituent of super-enhancers (10, 16, 17). Recently, a distinct role for BETs has been described in transcription control of dynamic, stimulus-coupled cell state transitions in heart failure and inflammation (18, 19). Overall, these data reveal that BETs can transduce dynamic, genome-wide changes in enhancer activity to regulate cell identity.
We hypothesized that BETs are essential coregulators of adipogenesis. Here, we show that BRD4 is a critical enhancer factor that potently coactivates stage-specific expression of PPARγ and C/EBPα during adipogenesis. Induction of adipogenesis in 3T3L1 preadipocytes provokes dynamic redistribution of BRD4 to de novo super-enhancer regions including the cis-regulatory elements controlling transcription of PPARγ and C/EBPα, thereby promoting differentiation. Displacement of BRD4 from enhancer chromatin with a BET bromodomain inhibitor blocks transcription of Pparg resulting in failure of differentiation. These data establish BET bromodomain proteins as essential transcriptional coactivators of the adipocyte cell state transition and implicate BET proteins in the regulation of systemic metabolic processes.
Results
BET Bromodomain Proteins Control Adipocyte Differentiation.
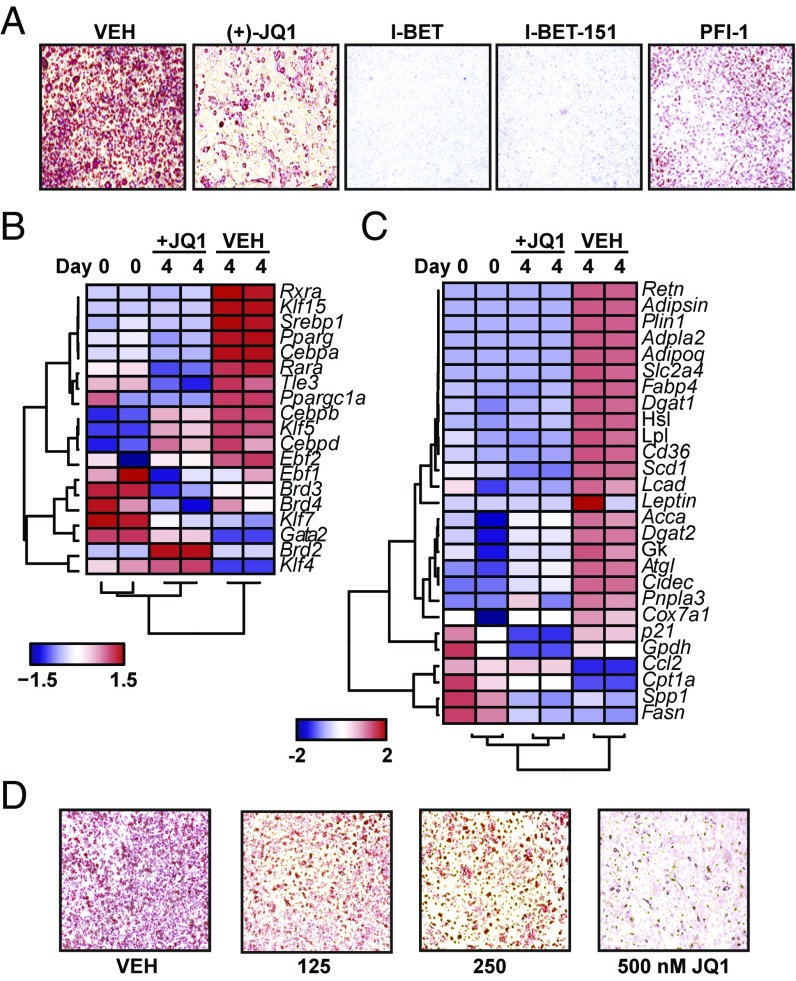
We first tested the effect of BET bromodomain inhibition on adipocyte differentiation using the 3T3L1 murine fibroblast model system (“L1”). Induction of differentiation using dexamethasone, isobutylmethylxanthine (IBMX), and insulin (DMI) resulted in adipocyte differentiation on day 8, as reflected by uniform lipid accumulation measured by oil red O staining (Fig. 1A). Cotreatment of preadipocytes with a panel of structurally dissimilar BET bromodomain inhibitors blocked differentiation (Fig. 1A) (20–24). Effects on adipogenesis correlated with their relative IC50 against BRD4 (25). PFl-1 demonstrated a more modest block in adipogenesis, while RVX-208 demonstrated no difference at the concentration tested (1 μM) (Fig. 1A and Fig. S1A). Inhibition of L1 differentiation by JQ1 was concentration-dependent, occurred without significant cytotoxicity, and resulted in only a modest slowing of cell cycle (Fig. S1 B and C).
Fig. 1.
BET bromodomain proteins control adipocyte differentiation. (A) Photomicrographs of oil red O stained L1 adipocytes after induction of differentiation by DMI (day 8) ± structurally distinct BET bromodomain inhibitors (JQ1 at 500 nM; I-BET, I-BET-151, and PFI-1 at 1 μM). (B and C) Heatmaps of expression of proadipogenic, master regulators (B), and mature adipocyte genes (C) at D0 and D4 of differentiation ± JQ1 (500 nM). (D) Photomicrographs of oil red O staining after differentiation of cells isolated from the stromal vascular fraction of mouse s.c. adipose tissue ± JQ1. Data represent mean ± SEM. The statistical significance of the difference in expression between vehicle (VEH) and JQ1 was determined using a two-tailed t test. (Magnification: 4×.)
Given these potent effects of BET bromodomain inhibition on adipogenesis, we next considered the role of BETs in gene regulation during differentiation. To address this issue, we designed a gene expression probe set (58 probes) for digital mRNA analysis (Nanostring). This curated gene set, containing probes for measuring expression of proadipogenic TFs, key transcriptional coregulators, and mature adipocyte markers, represents a molecular signature that encompasses critical stages of the differentiation process. At day 4 of L1 differentiation, we observed expected induction of master regulatory TFs including Cebpa, Pparg, members of the Klf family, and retinoid-x receptor family (Rxr) as well as downstream target genes involved in adipocyte function including lipid uptake (Cd36; fatty acid binding protein: Fabp4), lipid synthesis (monoacylglyerol lipase: Magl; diacylglycerol O-actyltransferase1: Dgat1), and systemic metabolism (Adipsin; Adipoq) (Fig. 1 B and C). Cotreatment of L1s with JQ1 abrogated induction of the adipocyte gene set (Fig. 1 B and C), and unsupervised hierarchical clustering grouped JQ1-treated L1s more closely with preadipocytes. Both Pparg and Cebpa mRNA levels were quantitatively similar to preadipocytes (Fig. S1 D and E). BET inhibition also suppressed induction of Adipoq and Fabp4, two genes directly regulated by PPARγ (Fig. S1 F and G). Complementary studies using siRNA-mediated knockdown of Brd4 supported a role for this BET family member in L1 differentiation (Fig. S1 H and I). To explore the relevance of these findings in primary cells, we next performed adipocyte differentiation assays using the stromal vascular fraction (SVF) isolated from murine subcutaneous (s.c.) adipose depots. Cells from SVF differentiated into mature adipocytes over 10 d in the presence of a defined hormonal mixture. Concomitant BET bromodomain inhibition resulted in a concentration-dependent block in differentiation (Fig. 1D). In rodents, a short period of visceral adipose tissue expansion occurs in the first weeks of postnatal life, which may, in part, be regulated by adipogenesis (26). To test the role of BET bromodomain proteins in adipose tissue, we treated 2-wk-old pups with JQ1 for 12 d and detected a significant reduction in epididymal white adipose tissue (EWAT) mass (Fig. S1J). In EWAT, Pparg2 mRNA levels were also lower in JQ1-treated animals. Taken together, these data establish a role for BET bromodomain proteins in adipocyte differentiation in both the L1 model system and primary mouse adipogenic precursors.
BETs Control Expression of the Master TFs Pparg and Cebpa.
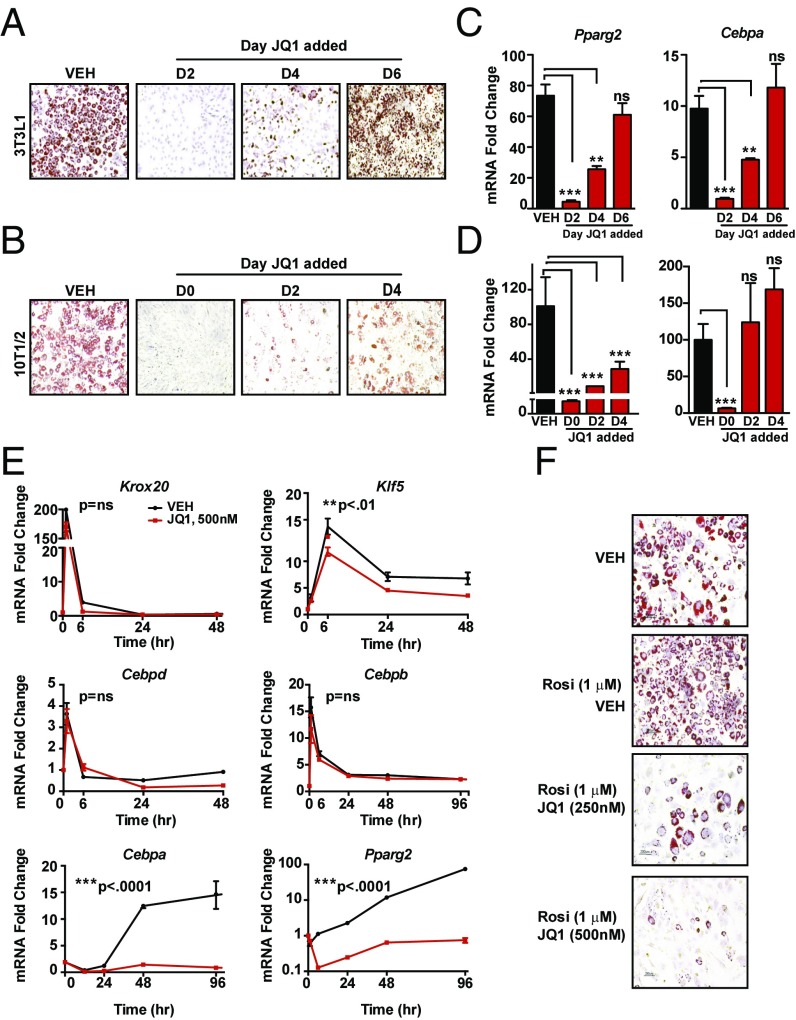
A precise, temporally regulated cascade of TF activity drives adipogenesis. Consistent with this wave of TF function, we observed a time-dependent effect of BET bromodomain inhibition on L1 differentiation. JQ1 cotreatment on day 2 or day 4 after induction of differentiation resulted in significant reductions in lipid accumulation and Pparγ, Cebpα and Adipoq gene expression, with these inhibitory effects diminishing when JQ1 was administered at later time points in the differentiation process (Fig. 2 A and C and Fig. S2A). JQ1 treatment of fully differentiated adipocytes (day 6) had no effect on Pparg and Cebpa gene expression or the mature adipocyte phenotype measured on day 8. We next tested whether JQ1 effects were reversible during this first 96-h window by performing JQ1 washout experiments. As before, JQ1 treatment throughout the 8 d completely blocked L1 differentiation (Fig. S2B). JQ1 exposure during day 0 through day 2 also suppressed differentiation and Pparg expression, but partial recovery of expression did occur. This recovery was more evident in cells exposed to BET inhibition for only 24 h between D1 and D2 (Fig. S2 B and C).
Fig. 2.
BETs control expression of the master TFs Pparg and Cebpa. (A and B) Photomicrographs oil red O stained L1s (A) or C3H10T1/2 mesenchymal stem cells (B) after induction of differentiation ± JQ1 (500 nM). JQ1 was added to differentiation medium on indicated days. Staining was on D6 for L1 and D4 for 10T1/2. (Magnification: 4×.) (C and D) Bar plots of fold change in Pparg and Cebpa mRNA levels in 3T3L1 cells and 10T1/2 cells from A and B. ns, not significant. (E) Line plots of fold change in mRNA of early response TFs involved in adipogenesis measured at 1, 6, 24, 48, and 96 h following induction of differentiation ± JQ1 (500 nM). (F) Oil red O staining of L1 adipocytes 8 d after induction of differentiation in the presence or absence of rosiglitazone (1 μM) ± JQ1 (500 nM). (Magnification: 10×.) Data represent mean ± SEM. The statistical significance of the difference in expression between vehicle (VEH) and JQ1 at each time point in C and D was determined using one-way ANOVA with post hoc (Dunnett’s) multiple comparison tests of the means. **P < 0.01, ***P < 0.001. For E, two-way ANOVA was performed with ***P < 0.001 for interaction comparing time x treatment groups.
Adipogenesis proceeds in two phases: (i) early fate specification, involving commitment of stem cells to the adipocyte lineage, and (ii) terminal differentiation, in which committed cells progressively acquire features of mature adipocyte phenotype (3). As L1 cells are already committed to the adipocyte lineage (3), we asked whether BETs also played a role in the earlier events of lineage specification. Therefore, we performed an additional series of differentiation experiments with C3H10T1/2 cells (10T1/2), a cell line derived from the C3H mouse embryo that possesses mesenchymal stem cell-like properties and can be induced to differentiate into adipocytes using a hormonal mixture (27). In the 10T1/2 model, mature adipocytes are evident by day 4. Similar to our L1 experiments, BET inhibition blocked differentiation in 10T1/2 cells in a time-dependent manner (Fig. 2 B and D and Fig. S2D). Reductions in Pparg expression persisted in the presence of BET inhibition whether initiated on day 0 or day 2 of differentiation, while Cebpa mRNA levels were similar to vehicle in day 2-treated cells (Fig. 2D). In contrast to BRD4 loss-of-function experiments, enforced expression of BRD4 in NIH3T3s—a nonadipogenic cell line—did not induce differentiation (Fig. S2E), consistent with a role for BRD4 as a coactivaor that transduces signals from enhancer-bound TFs to the transcriptional machinery. Collectively, these data establish that BETs are stage-specific regulators of adipocyte differentiation and Pparg expression in committed preadipocytes (L1) and mesenchymal stem cells (10T1/2), but BRD4 is not sufficient to drive differentiation in cells lacking intrinsic adipogenic potential.
L1 differentiation proceeds by the coordinated action of a TF cascade that converges on Pparg and Cebpa expression. We next tested whether BET bromodomain proteins regulate the first wave of master TF expression during adipogenesis. In time-course experiments, expression of Krox20, Klf5, Cebpb, and Cebpd increased 1 h after starting L1 differentiation, peaked by 6 h, and returned to baseline by 24–48 h (Fig. 2E). Cotreatment of L1 cells with JQ1 had no effect on the magnitude or kinetics of Krox20, Cebpb, or Cepbd regulation, with a modest effect on Klf5 mRNA levels. Despite no significant effects on the expression of these early TFs, BET inhibition completely abolished induction of both Cebpa and Pparg. In addition, treatment of L1 cells with a high-affinity, synthetic PPARγ ligand (rosiglitazone, 1 μM) that further drives adipogenesis also failed to rescue the block in differentiation by BET bromodomain inhibition (Fig. 2F and Fig. S2F). Overall, these data support a model in which BET bromodomain proteins promote adipocyte differentiation by coactivating expression of Pparg and Cebpa in a cell type-specific manner.
BRD4 Is Dynamically Redistributed to Proadipogenic Super Enhancers.
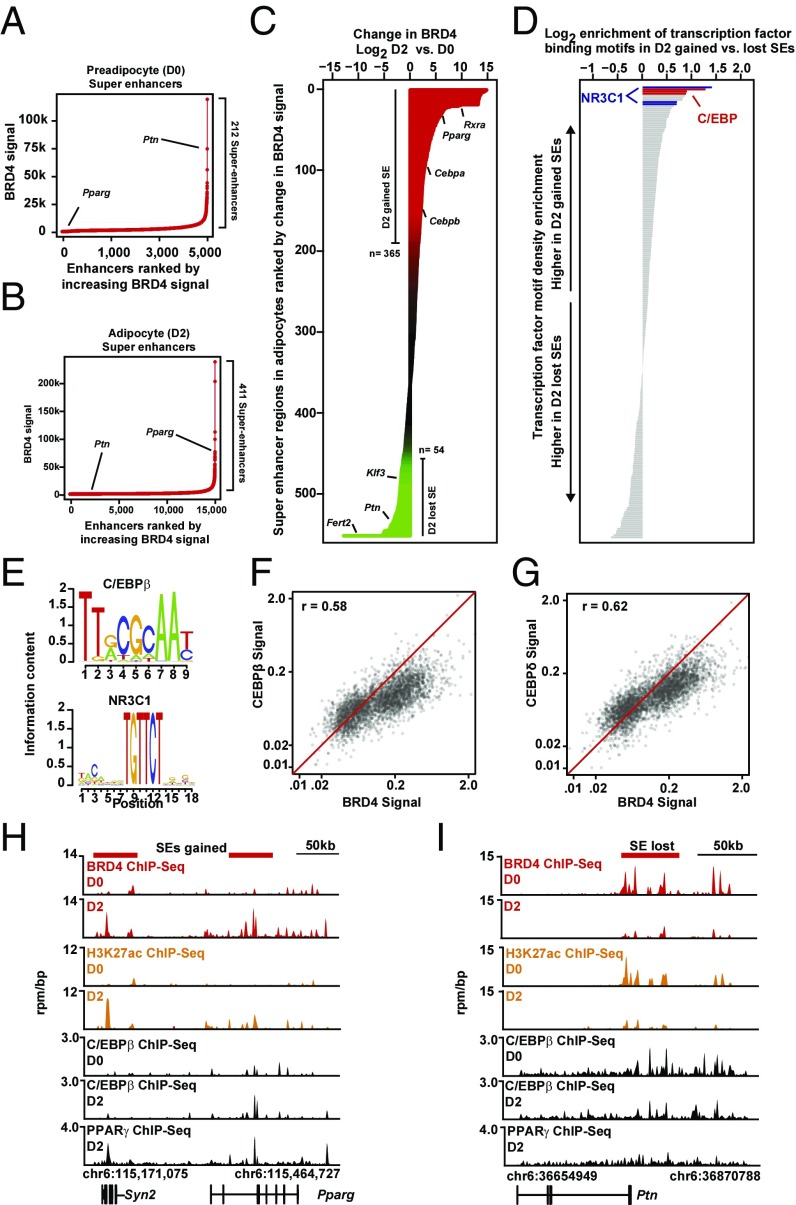
To determine the role of BETs in adipocyte enhancer function, we first investigated genome-wide BRD4 binding to chromatin in L1 preadipocytes (day 0, “D0”) and differentiating adipocytes (day 2, “D2”) using chromatin immunoprecipitation coupled with high-throughput deep sequencing (ChIP-seq). Experimental data obtained for BRD4 were integrated with publicly available datasets that provide high-resolution epigenomic maps of euchromatin during adipocyte differentiation (5). First, we identified BRD4 to be enriched at preadipocyte and adipocyte promoters (13% and 4%, respectively, and as follows) and intergenic (39% and 38%) and intragenic (47% and 57%) regulatory sequences (Fig. S3A). Alignment of BRD4 ChIP-seq data with published chromatin state maps of enhancers (H3K27ac) and promoters (H3K4me3) in L1 cells demonstrated a strong correlation between H3K27ac and BRD4 at both D0 and D2 (Fig. S3B). Global alignment revealed BRD4 localized to chromatin at promoters and enhancers in both preadipocytes and adipocytes (Fig. S3C).
We next ranked BRD4 enhancers by increasing ChIP-seq signal and identified 212 and 411 super-enhancers in preadipocytes and adipocytes, respectively (Fig. 3 A and B). Differences in super-enhancer regions between preadipocytes and adipocytes (Fig. 3 A and B) prompted a systematic evaluation of the dynamic changes in super-enhancer organization. We observed significant redistribution of BRD4 to new enhancers in the D2-differentiating adipocytes. Differential enhancer analysis reclassified a significant subset of enhancers as transitioning from typical to super-enhancer (“adipocyte-gained,” n = 365) and from super to typical enhancer (“preadipocyte-lost,” n = 54) (Fig. 3C). Genes associated with adipocyte-gained super-enhancers were enriched for TFs that positively regulate adipocyte differentiation, including Pparg, Cebpa, and Cebpb (Fig. 3C). In contrast, other genes associated with preadipocyte-lost super-enhancers, such as Klf3 and Pleotrophin (Ptn), are known to interfere with differentiation and are down-regulated during adipogenesis; furthermore, prior work has revealed that enforced expression of these factors suppresses differentiation (28, 29). Super-enhancers feature high levels of coactivator protein enrichment and are also densely occupied by master regulatory TFs (8). Motif analysis revealed the GR (NR3C1) and C/EBP motifs were found at much higher density at adipocyte-gained super-enhancer regions compared with preadipocyte-lost super-enhancer regions (Fig. 3 D and E). Comparison of binding enrichment between BRD4 and previously generated datasets for C/EBPβ, CEBPδ, GR, and PPARγ ChIP-seq also demonstrated strong statistical correlation at D2 of L1 differentiation between each of these TFs and BRD4 (R2 = 0.58, 0.62, 0.43, 0.57, respectively) (Fig. 3 F and G and Fig. S3D), suggesting genomic colocalization of these factors. In addition, coimmunoprecipitation experiments revealed a modest interaction between CEBPβ and BRD4 during differentiation (Fig. S3E), as previously identified in other cell types (13, 14). At the Pparg locus, preadipocytes feature minimal BRD4 or H3K27ac enrichment (Fig. 3H). Following induction of differentiation, L1 cells on D2 acquired massive increases in both BRD4 and H3K27ac levels at a 5′ distal regulatory element and the promoter region of Pparg, consistent with formation of super-enhancers (Fig. 3H). By contrast, the Ptn locus contained high levels of BRD4 and H3K27ac at the 5′ enhancer region in preadipocytes, which were lost on D2 of differentiation (Fig. 3I). To test the signals that control recruitment of BRD4 to chromatin, we omitted individual components of the adipocyte differentiation mixture. The absence of insulin in this mixture resulted in a reduction of BRD4 recruitment to the Pparg locus after 24 h of differentiation (Fig. 4 and Fig. S3F). Overall, these results establish that BRD4 dynamically redistributes away from preadipocyte genes to new super-enhancers associated with stage-specific expression of master regulatory factors including proadipogenic TFs such as Pparg.
Fig. 3.
Brd4 is dynamically redistributed to proadipogenic enhancers. (A and B) Plots of enhancers in preadipocytes (D0; A) and adipocytes (D2; B) ranked by increasing BRD4 signal in units of reads per million (rpm). Enhancers are defined as regions of BRD4 ChIP-Seq binding not contained in promoters. The cutoff discriminating typical enhancers from super-enhancers is shown as a dashed line. (C) Horizontal bar plot of all genomic regions containing a SE in preadipocytes (D0) or differentiating adipocytes (D2) ranked by log2 change in BRD4 signal. The x axis shows the log2 fold change in BRD4 signal. Change in BRD4 levels at SEs are colored by intensity of change (green to red). (D) Horizontal bar plot showing the ratio of TF motif density between gained (D2) and lost (D0) super-enhancers. Twenty-one TFs are displayed whose motifs occur more frequently than expected based on dinucleotide background model. The motifs are ranked by log2 fold change in density between D2 gained and D0 lost super-enhancers. (E) Consensus sequences of C/EBPβ (Top) and NR3C1 (Bottom) DNA binding motifs. (F and G) Scatter plot of C/EBPβ versus BRD4 binding signals (F) or C/EBPδ versus BRD4 signals (G) in L1 cells on D2 of differentiation. (H and I) Gene tracks of ChIP-Seq signal (rpm/bp) for BRD4, H3K27ac, C/EBPβ, and PPARγ at the Pparg locus (H) or Ptn (I) locus in 3T3L1 cells on D0 (Upper) or D2 (Lower) of differentiation.
Fig. 4.
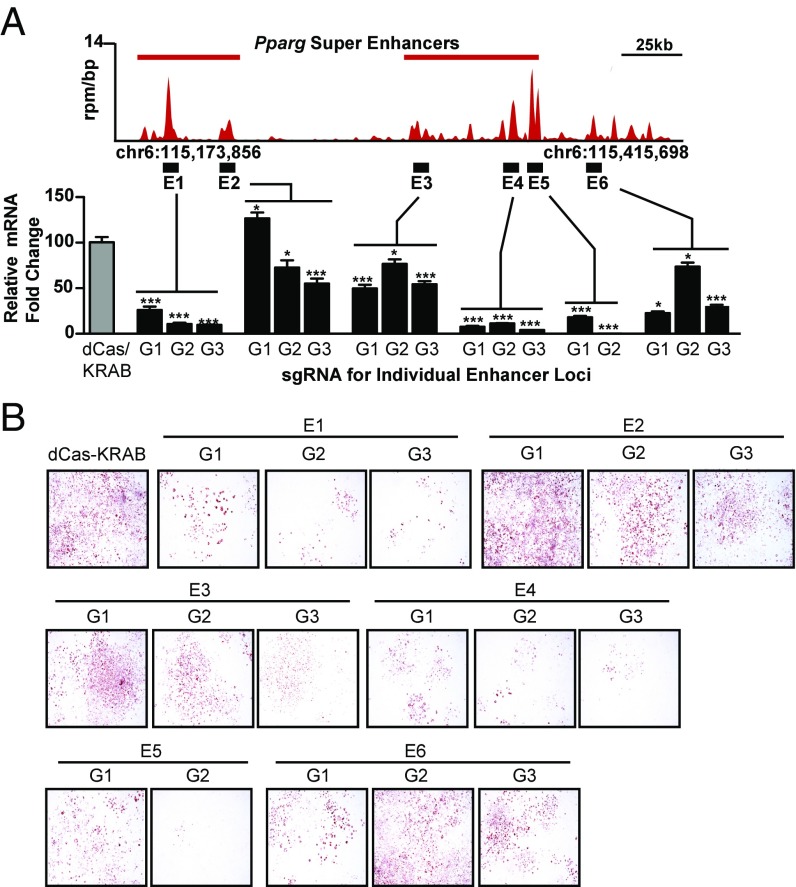
BRD4 binding sites regulate Pparg expression and adipogenesis. (A) Gene track of ChIP-Seq signal of BRD4 on D2 of L1 differentiation at the upstream and intragenic Pparg super-enhancer regions (Upper) and bar plot of Pparg mRNA expression (D4 of L1 differentiation) in cells stably expressing dCas9-KRAB alone or with gRNAs targeting individual enhancer constituent sites (E1–E6). (Lower) Three unique gRNAs per enhancer site were used in separate cell lines. (B) Oil red O staining of cells (D7) treated as in A. The statistical significance of the difference in expression between individual gRNA and dCas9-KRAB was determined using two-tailed t test. (Magnification: 4×.) *P < 0.05; ***P < 0.001.
BRD4 Super-Enhancer Binding Sites Regulate Pparg Gene Expression and Adipogenesis.
Recruitment of BRD4 to super-enhancers at the Pparg locus suggests that these cis-regulatory elements may directly control Pparg gene expression. To test this hypothesis, we employed clustered, regularly interspaced, palindromic repeats interference (CRISPRi) to silence individual cis-regulatory elements occupied by BRD4 (30). L1 cells were selected for stable expression of dCas9-KRAB along with individual guide RNAs (gRNAs) complementary to DNA at BRD4-enriched loci within the Pparg super-enhancers (Figs. 3H and 4). Targeting these sites by multiple, individual gRNAs repressed Pparg gene induction on D4 of L1 differentiation, compared with cells expressing dCas9-KRAB with no gRNA (Fig. 4). Notably, silencing of the distal 5′ site (E1) and intragenic sites (E4, E5) resulted in the most significant reduction in gene expression, with more modest effects at the other regions tested. CRISPRi of these Pparg enhancers also attenuated adipogenesis, as measured by oil red O staining (Fig. 4B). These data establish an important functional role for these de novo formed, BRD4-enriched cis-regulatory elements in adipogenesis in the endogenous chromatin context of the cell.
Transcription of Pparg and Cebpa Is BET Bromodomain-Dependent.
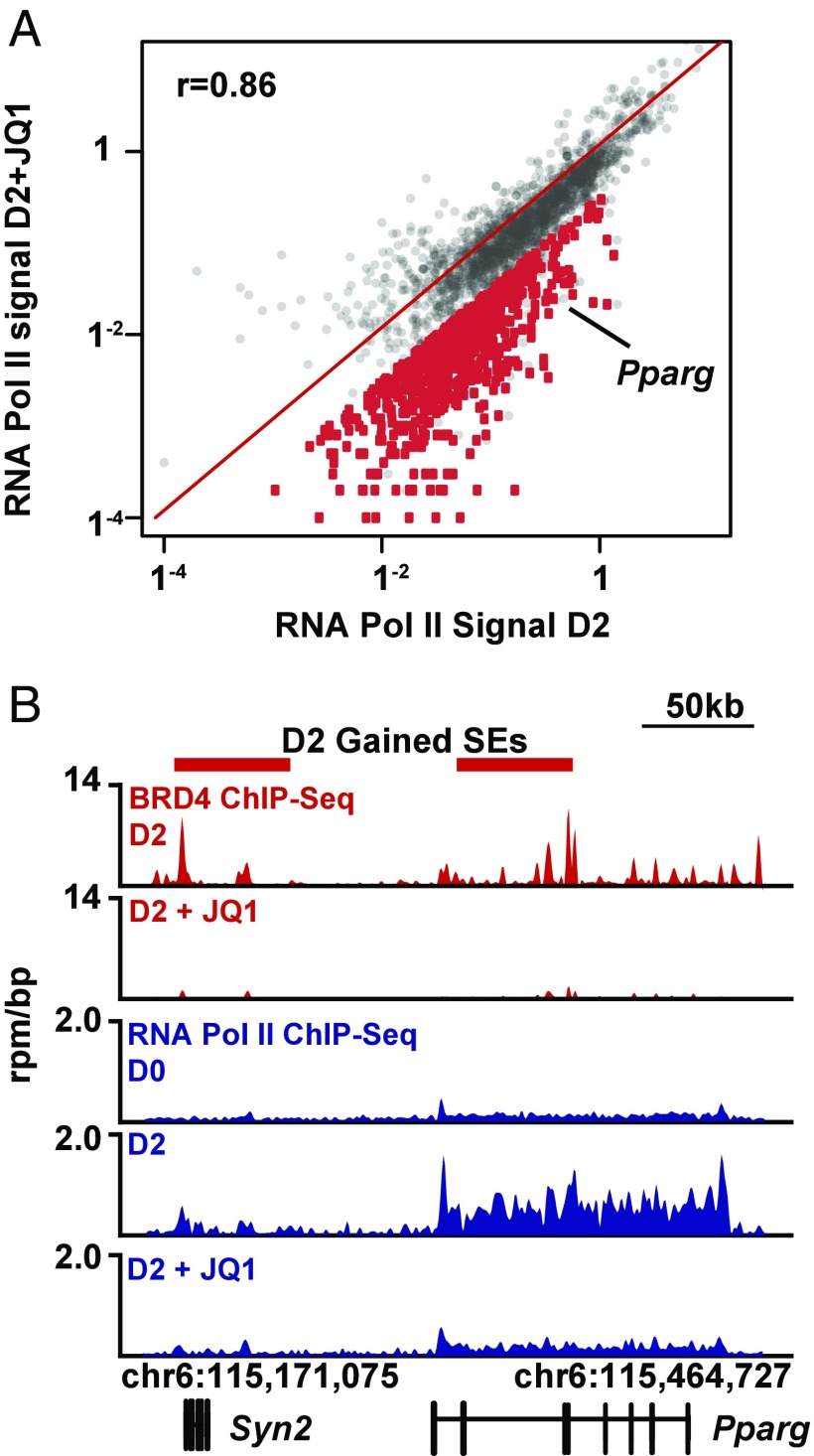
The dynamic redistribution of BRD4 to de novo super-enhancers predicts that depletion of BRD4 from chromatin would reduce transcription at super enhancer-associated genes. To analyze the transcriptional consequences of disruption of BET super-enhancers, we performed ChIP-seq analysis of RNA Pol II occupancy in L1 preadipocytes (D0) as well as differentiating L1s (D2) in the presence or absence of the selective BET bromodomain inhibitor (JQ1, 500 nM). Comparison of RNA Pol II signal from actively transcribed genes in vehicle versus JQ1-treated L1s revealed overall statistical correlation between these two groups (Fig. 5A). However, at a subset of genes, RNA Pol II signal in JQ1-treated cells was lower than vehicle (Fig. 5A). Within this cluster, reductions in RNA Pol II signal at the Pparg gene locus were noted in JQ1-treated L1 cells. A comparison of RNA Pol II signal at the Pparg and Cebpa loci on D0 versus D2 revealed a robust increase during differentiation, coincident with acquisition of BRD4 at super-enhancers (Figs. 3H and 5B and Fig. S4A). BET bromodomain inhibition with JQ1 depleted BRD4 from chromatin, and this resulted in reduced transcription of Pparg and Cebpa. This effect on Pparg and Cebpa expression also blocked transcription of known downstream target genes such as Fabp4 (Fig. S4B). Finally, we considered the possibility that TF genes induced at earlier time points during L1 adipogenesis (Fig. 2E) are less sensitive to BET inhibition because they are not controlled by super-enhancers. Analysis of a previously published dataset of genome-wide binding of MED1—a constituent of super-enhancers (8)—at 4 h after the start of L1 differentiation revealed no super-enhancers assigned to Krox20, Cebpd, and Cebpb genes (Fig. S4C) (7). Collectively, these data reveal that BET inhibition blocks transcription of super enhancer-associated genes including Pparg and Cebpa.
Fig. 5.
Transcription of adipogenic master regulatory factors is BET bromodomain-dependent. (A) Scatter plot of RNA Pol II ChIP-Seq signal at gene bodies of all actively transcribed genes in L1 cells on D2 of differentiation versus D2 + JQ1 (500 nM). (B) Gene tracks of ChIP-Seq signal (rpm/bp) for BRD4 (D2 versus D2 + JQ1 of differentiation) and RNA Pol II on D0 (Top), D2 (Middle), and D2 + JQ1 (500 nM, Bottom) of differentiation at the Pparg (B) locus in L1 cells.
Discussion
Adipocyte differentiation requires the coordinated activity of the master TFs, C/EBPα, and PPARγ. Disruption of their expression or function in humans profoundly alters metabolism, in part, through effects on adipocyte differentiation (31). In this study, we explored the role of chromatin-dependent signal transduction in this process. We demonstrate that the BET bromodomain-containing protein, BRD4, transduces the adipogenic program through formation of de novo super-enhancers that drive transcription of Pparg and Cebpa and promote adipocyte differentiation.
Super or stretch enhancers are clusters of enhancers where TFs and coactivator proteins are concentrated and drive transcription of genes controlling cell state (8, 9). In this study, we identified that BRD4 redistributes to de novo super-enhancers in proximity to genes encoding TFs, including Cebpa and Pparg, in response to adipocyte differentiation cues. These data build on prior reports demonstrating that TF hotspots colocalize with super-enhancers containing MED1 in differentiating L1 cells (7). Functional interactions between Mediator and BRD4 may partially explain how BRD4 redistributes to these regions (32). In addition, the colocalization of BRD4 with multiple TF-binding motifs, as observed in our study, suggests that site-specific BRD4 redistribution may occur via interactions between BRD4 and specific DNA-binding TFs. In the context of inflammation, biochemical studies have demonstrated that BRD4 can bind acetylated p65-RelA, implicating this interaction as a possible mechanism for genome-wide redistribution of BRD4 to inflammatory super-enhancers during innate immune activation (19, 33). Furthermore, a functional relationship between C/EBPβ and BRD4 has been described in NIH 3T3 fibroblasts (14). Overall, these data are consistent with a model in which lineage-specific TFs activated during adipogenesis direct state-specific flux of BRD4 and other coregulators to de novo super-enhancers that control cell state.
We find that selective depletion of BRD4 from chromatin impairs adipocyte differentiation. These results occurred in three models of adipogenesis (3T3L1, 10T1/2, and SVF) in response to a panel of structurally dissimilar BET bromodomain inhibitors or siRNA-mediated knockdown of Brd4. Notably, two independent studies have recently emerged that used BET bromodomain inhibition or conditional Brd4 deletion to demonstrate a role for BETs in adipogenesis (34, 35). The results presented here align with these reports while also providing additional significant insights, including the use of CRISPR interference to demonstrate directly that silencing of individual, BRD4-enriched endogenous enhancer elements at the Pparg locus resulted in disrupted Pparg expression and adipogenesis. Whether BRD4 enrichment at specific cis-regulatory elements is sufficient to drive adipogenesis and which elements are required for this will be of interest to investigate in future studies. Overall, when considered collectively, these data demonstrate a requirement for BETs and BRD4 in adipogenesis through collaboration with lineage determining TFs and may in part explain why systemic Brd4 haploinsufficient mice have reduced adipose tissue formation (36). The effects of BET bromodomain inhibition diminished over time, revealing the presence of precise temporal windows during which enhancer activity is required to regulate distinct stages of adipocyte differentiation. The muted effect of BET bromodomain inhibition at later time points may reflect compensation by other coactivators such as MED1 at established super-enhancers, functional cooperativity between multiple TFs bound at these regions or bromodomain-independent recruitment of BETs to these enhancers (13, 37). Our findings in adipogenesis extend prior work describing the role of BRD4-enriched super-enhancers in cell state. In embryonic stem cells (ESCs) and in certain cancers, super-enhancers regulate expression of pluripotency factors (8, 17) or oncogenes, respectively (16, 22). In these contexts, BET bromodomain inhibition triggers differentiation, growth arrest, and/or cell death (22). The differentiation block by JQ1 in L1 and 10T1/2 cells stands in sharp contrast to the prodifferentiation effects of BET inhibition in ESCs and NUT midline carcinoma (17, 22). These differences highlight how BETs transduce signals controlling the establishment of specific, TF-driven cell states by coactivating context-specific enhancer activity to RNA polymerase II.
BET bromodomain inhibitors are a new class of drugs in cancer therapy now under investigation in human clinical trials. The data presented herein have implications for this therapeutic approach. Disruption of BET-occupied enhancers may have adverse consequences in noncancer cellular compartments including adipocytes, where stimulus-coupled transcription is an essential mechanism controlling metabolic homeostasis. Furthermore, analysis of BET-dependent super-enhancer wiring may provide insight into potential on-target toxicities of pharmacologic BET bromodomain inhibitors that target cell-state defining genes. Considered more broadly, understanding the precise mechanisms underlying context-specific, BET bromodomain-dependent coactivation may deepen our understanding of the gene regulatory circuits governing tissue-specific responses in development and diseases outside of cancer.
Experimental Procedures
Animal experiments were approved by Institutional Animal Care and Use Committee (IACUC) at Harvard Medical School. Adipocyte differentiation was performed in media supplemented with fetal bovine serum (10%), insulin, dexamethasone, and IBMX. For 10T1/2 cells, 1 nM T3 and 0.125 mM indomethacin was also included. Summary data are presented as mean ± SEM, and Student’s t test was used to compare groups. For kinetic gene expression, ANOVA was conducted. Methods for ChIP-Seq, coimmunoprecipitation, CRISPRi, RNA preparation, and cell staining are provided in SI Experimental Procedures.
Supplementary Material
Acknowledgments
We thank the Gersbach and Boehm/Hahn/Root laboratories for Addgene plasmids and M. Steinhauser and Y. Zhang in the Division of Genetics, Brigham and Women’s Hospital, for comments during manuscript preparation. Research was supported by Grant NIH-K08 HL105678 (to J.D.B.); the US Department of Defense (C.Y.L.); National Heart, Lung, and Blood Institute (NHLBI) Grant P01 HL048743 (to J.P.); NIH/NHLBI Grants R56-HL125894 and R01-HL133665 (to J.P.); NIH/National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK107239 (to J.P.); the Burroughs-Wellcome Fund (J.E.B.); the Damon-Runyon Cancer Research Foundation (J.E.B.); and the Next Generation Award (to J.E.B.).
Footnotes
Conflict of interest statement: J.D.B., J.P., and J.E.B. have a patent filed on Compositions and Methods for Modulating Metabolism (application no. PCT/US2011/036647) in which BET bromodomain inhibition is used to modulate metabolic diseases including obesity and fatty liver. J.E.B. is now an executive and shareholder of Novartis AG.
This article is a PNAS Direct Submission.
Data deposition: ChIP-Seq data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo (accession no.GSE109940).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1711155115/-/DCSupplemental.
References
- 1.Long HK, Prescott SL, Wysocka J. Ever-changing landscapes: Transcriptional enhancers in development and evolution. Cell. 2016;167:1170–1187. doi: 10.1016/j.cell.2016.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarjeant K, Stephens JM. Adipogenesis. Cold Spring Harb Perspect Biol. 2012;4:a008417. doi: 10.1101/cshperspect.a008417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 4.Lefterova MI, Haakonsson AK, Lazar MA, Mandrup S. PPARγ and the global map of adipogenesis and beyond. Trends Endocrinol Metab. 2014;25:293–302. doi: 10.1016/j.tem.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mikkelsen TS, et al. Comparative epigenomic analysis of murine and human adipogenesis. Cell. 2010;143:156–169. doi: 10.1016/j.cell.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siersbæk R, et al. Extensive chromatin remodelling and establishment of transcription factor ‘hotspots’ during early adipogenesis. EMBO J. 2011;30:1459–1472. doi: 10.1038/emboj.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siersbæk R, et al. Transcription factor cooperativity in early adipogenic hotspots and super-enhancers. Cell Rep. 2014;7:1443–1455. doi: 10.1016/j.celrep.2014.04.042. [DOI] [PubMed] [Google Scholar]
- 8.Whyte WA, et al. Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell. 2013;153:307–319. doi: 10.1016/j.cell.2013.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parker SC, et al. NISC Comparative Sequencing Program National Institutes of Health Intramural Sequencing Center Comparative Sequencing Program Authors NISC Comparative Sequencing Program Authors Chromatin stretch enhancer states drive cell-specific gene regulation and harbor human disease risk variants. Proc Natl Acad Sci USA. 2013;110:17921–17926. doi: 10.1073/pnas.1317023110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lovén J, et al. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell. 2013;153:320–334. doi: 10.1016/j.cell.2013.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saint-André V, et al. Models of human core transcriptional regulatory circuitries. Genome Res. 2016;26:385–396. doi: 10.1101/gr.197590.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LeRoy G, Rickards B, Flint SJ. The double bromodomain proteins Brd2 and Brd3 couple histone acetylation to transcription. Mol Cell. 2008;30:51–60. doi: 10.1016/j.molcel.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu SY, Lee AY, Lai HT, Zhang H, Chiang CM. Phospho switch triggers Brd4 chromatin binding and activator recruitment for gene-specific targeting. Mol Cell. 2013;49:843–857. doi: 10.1016/j.molcel.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roe JS, Mercan F, Rivera K, Pappin DJ, Vakoc CR. BET bromodomain inhibition suppresses the function of hematopoietic transcription factors in acute myeloid leukemia. Mol Cell. 2015;58:1028–1039. doi: 10.1016/j.molcel.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Z, et al. Recruitment of P-TEFb for stimulation of transcriptional elongation by the bromodomain protein Brd4. Mol Cell. 2005;19:535–545. doi: 10.1016/j.molcel.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 16.Chapuy B, et al. Discovery and characterization of super-enhancer-associated dependencies in diffuse large B cell lymphoma. Cancer Cell. 2013;24:777–790. doi: 10.1016/j.ccr.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Micco R, et al. Control of embryonic stem cell identity by BRD4-dependent transcriptional elongation of super-enhancer-associated pluripotency genes. Cell Rep. 2014;9:234–247. doi: 10.1016/j.celrep.2014.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anand P, et al. BET bromodomains mediate transcriptional pause release in heart failure. Cell. 2013;154:569–582. doi: 10.1016/j.cell.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown JD, et al. NF-κB directs dynamic super enhancer formation in inflammation and atherogenesis. Mol Cell. 2014;56:219–231. doi: 10.1016/j.molcel.2014.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Picaud S, et al. PFI-1, a highly selective protein interaction inhibitor, targeting BET bromodomains. Cancer Res. 2013;73:3336–3346. doi: 10.1158/0008-5472.CAN-12-3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Picaud S, et al. RVX-208, an inhibitor of BET transcriptional regulators with selectivity for the second bromodomain. Proc Natl Acad Sci USA. 2013;110:19754–19759. doi: 10.1073/pnas.1310658110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Filippakopoulos P, et al. Selective inhibition of BET bromodomains. Nature. 2010;468:1067–1073. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mirguet O, et al. From ApoA1 upregulation to BET family bromodomain inhibition: Discovery of I-BET151. Bioorg Med Chem Lett. 2012;22:2963–2967. doi: 10.1016/j.bmcl.2012.01.125. [DOI] [PubMed] [Google Scholar]
- 24.Nicodeme E, et al. Suppression of inflammation by a synthetic histone mimic. Nature. 2010;468:1119–1123. doi: 10.1038/nature09589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Filippakopoulos P, Knapp S. Targeting bromodomains: Epigenetic readers of lysine acetylation. Nat Rev Drug Discov. 2014;13:337–356. doi: 10.1038/nrd4286. [DOI] [PubMed] [Google Scholar]
- 26.Kim SM, et al. Loss of white adipose hyperplastic potential is associated with enhanced susceptibility to insulin resistance. Cell Metab. 2014;20:1049–1058. doi: 10.1016/j.cmet.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reznikoff CA, Brankow DW, Heidelberger C. Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res. 1973;33:3231–3238. [PubMed] [Google Scholar]
- 28.Gu D, et al. The effect of pleiotrophin signaling on adipogenesis. FEBS Lett. 2007;581:382–388. doi: 10.1016/j.febslet.2006.12.043. [DOI] [PubMed] [Google Scholar]
- 29.Sue N, et al. Targeted disruption of the basic Krüppel-like factor gene (Klf3) reveals a role in adipogenesis. Mol Cell Biol. 2008;28:3967–3978. doi: 10.1128/MCB.01942-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thakore PI, et al. Highly specific epigenome editing by CRISPR-Cas9 repressors for silencing of distal regulatory elements. Nat Methods. 2015;12:1143–1149. doi: 10.1038/nmeth.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hegele RA, Cao H, Frankowski C, Mathews ST, Leff T. PPARG F388L, a transactivation-deficient mutant, in familial partial lipodystrophy. Diabetes. 2002;51:3586–3590. doi: 10.2337/diabetes.51.12.3586. [DOI] [PubMed] [Google Scholar]
- 32.Wu SY, Chiang CM. The double bromodomain-containing chromatin adaptor Brd4 and transcriptional regulation. J Biol Chem. 2007;282:13141–13145. doi: 10.1074/jbc.R700001200. [DOI] [PubMed] [Google Scholar]
- 33.Huang B, Yang XD, Zhou MM, Ozato K, Chen LF. Brd4 coactivates transcriptional activation of NF-kappaB via specific binding to acetylated RelA. Mol Cell Biol. 2009;29:1375–1387. doi: 10.1128/MCB.01365-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee JE, et al. Brd4 binds to active enhancers to control cell identity gene induction in adipogenesis and myogenesis. Nat Commun. 2017;8:2217. doi: 10.1038/s41467-017-02403-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goupille O, et al. Inhibition of the acetyl lysine-binding pocket of bromodomain and extraterminal domain proteins interferes with adipogenesis. Biochem Biophys Res Commun. 2016;472:624–630. doi: 10.1016/j.bbrc.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 36.Houzelstein D, et al. Growth and early postimplantation defects in mice deficient for the bromodomain-containing protein Brd4. Mol Cell Biol. 2002;22:3794–3802. doi: 10.1128/MCB.22.11.3794-3802.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shu S, et al. Response and resistance to BET bromodomain inhibitors in triple-negative breast cancer. Nature. 2016;529:413–417. doi: 10.1038/nature16508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.