Significance
Immunopathology after Chlamydia trachomatis infection is the major cause of human suffering associated with this pathogen, yet the immune responses that drive pathology are not well defined. We demonstrate that a mucosal influx of neutrophils and CXCR3-driven CD4+ and CD8+ T cells is required for C. trachomatis pathology and does not contribute to bacterial clearance. Our study decouples immune-mediated pathology from immune-mediated protection for C. trachomatis, which will have important translational implications for the development of vaccines and CXCR3-mediated treatments.
Keywords: Chlamydia, infection, pathology, neutrophils, CXCR3
Abstract
Infection with Chlamydia trachomatis drives severe mucosal immunopathology; however, the immune responses that are required for mediating pathology vs. protection are not well understood. Here, we employed a mouse model to identify immune responses required for C. trachomatis-induced upper genital tract pathology and to determine whether these responses are also required for bacterial clearance. In mice as in humans, immunopathology was characterized by extravasation of leukocytes into the upper genital tract that occluded luminal spaces in the uterus and ovaries. Flow cytometry identified these cells as neutrophils at early time points and CD4+ and CD8+ T cells at later time points. To determine what draws these cells to C. trachomatis-infected tissue, we measured the expression of 700 inflammation-related genes in the upper genital tract and found an up-regulation of many chemokines, including a node of interaction between CXCL9/10/11 and their common receptor CXCR3. Either depleting neutrophils or reducing T-cell numbers by CXCR3 blockade was sufficient to significantly ameliorate immunopathology but had no effect on bacterial burden, demonstrating that these responses are necessary for mucosal pathology but dispensable for C. trachomatis clearance. Therapies that specifically target these host responses may therefore prove useful in ameliorating C. trachomatis-induced pathology without exacerbating infection or transmission.
The obligate intracellular pathogen Chlamydia trachomatis (Ct) is the most common reportable infection in the United States with an estimated incidence of three million cases per year (1). Ct pathogenesis is serovar specific: serovars A–C cause ocular infection and serovars D–L cause urogenital tract infection (2). Although acute infection is asymptomatic, mucosal immunopathology can develop after infection, causing severe disease outcomes: blindness caused by ocular infection and pelvic inflammatory disease (PID), ectopic pregnancy, and infertility caused by female upper genital tract infection (3).
Although it is the mucosal immunopathology secondary to Ct infection that is the major cause of human suffering associated with this pathogen, it is not well understood which host responses cause pathology (3). In the few studies of histopathology of human tissue, Ct-induced PID has been associated with neutrophil recruitment to the uterine epithelium and lumen, as well as lymphocyte infiltration into the subepithelial stroma (4). However, these observations in human tissues are not able to determine which host responses are necessary and/or sufficient to cause disease. Similarly, while Ct infection in cell culture has been shown to induce proinflammatory cytokines (5), such data do not address whether these responses cause pathology in vivo.
We sought to identify the host immune responses that are required to cause pathology and determine whether these are separable from the host immune responses necessary for bacterial clearance. This required experimentation using a Ct infection model that reproduces human disease pathology. Although pathology can be modeled in mice using a related species with 80% sequence identity, Chlamydia muridarum, the extent to which the molecular pathogenesis of C. muridarum represents that of Ct is unknown (6). Ct serovar L2 (Ct L2) is capable of infecting the mouse upper genital tract when inoculated across the cervix into the uterus (7, 8) but it does not induce robust immunopathology. This is consistent with the human disease phenotype caused by Ct L2, which disseminates to the lymph nodes causing lymphogranuloma venereum (LGV) and is not a major cause of mucosal immunopathology in the female upper genital tract (uterus and ovaries).
Here, we examine female upper genital tract infection of mice with Ct serovar D (Ct D), one of the serovars responsible for reproductive damage in women. We show that Ct D induces upper genital tract immunopathology in mice following transcervical inoculation and describe the immune responses that cause mucosal pathology. We find that an influx of neutrophils and CXCR3-driven CD4+ and CD8+ T cells is required for Ct pathology and is distinct from protective antigen-specific responses, demonstrating that the host responses that drive pathology can be decoupled from those that drive protection.
Results
C. trachomatis Serovar D Infection of the Murine Female Upper Genital Tract Induces Significant Immunopathology.
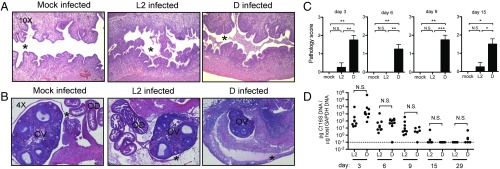
We first tested whether the Ct serovar-specific differences in mucosal immunopathology observed in human infection could be reproduced in mice. For each of the two groups of Ct serovars that infect via the genital tract in humans (D–K or L1–L3), we selected for our experiments a serovar with high incidence of human infection. Ct D was chosen to represent group D–K, which is associated with robust and long-lasting immunopathology in women. Ct L2 was chosen to represent group L1–L3, which is not associated with significant mucosal immunopathology. Our goal in comparing infection between these two Ct serovars, rather than comparing infection vs. no infection, was to hold constant those immune responses involved in bacterial clearance and therefore reveal those immune responses that specifically drive pathology. We predicted that, as in humans, both Ct serovars would be capable of infecting the upper genital tracts of mice but only infection with Ct D and not Ct L2 would result in robust mucosal immunopathology. Six- to 8-wk-old female C57BL/6 mice were infected transcervically with 5 × 106 inclusion forming units (IFUs) of either Ct D or Ct L2 or were mock infected with buffer only. Over a 42-d time course of infection, pathology was assessed by blinded scoring of H&E-stained tissue sections using a scale of 0 (none), 1 (mild/rare, less than 1/3 of tissue affected), 2 (moderate/multifocal, between 1/3 and 2/3 of tissue affected), and 3 (severe/coalescing, greater than 2/3 of tissue affected) (Fig. 1 A–C) (9). Bacterial burden was measured by qPCR (Fig. 1D). Both Ct D and Ct L2 robustly and equally infected the upper genital tracts of C57BL/6 mice, with no significant differences in bacterial burden observed between the serovars at any time point over the course of infection (Fig. 1D). These data demonstrate that any difference observed in pathology between these two serovars was not simply the result of a difference in pathogen load.
Fig. 1.
Infection with C. trachomatis serovar D induces immunopathology in the upper genital tract of female C57BL/6 mice. (A and B) Representative H&E-stained sections of uteri (A) and ovaries (B) from mock-infected, Ct L2-infected, and Ct D-infected mice at day 6 postinfection. Moderate-to-severe inflammation is observed only in the Ct D-infected mice, characterized by an influx of cells into the uterine lumen (* in A) and thickening and separation of the ovarian membrane from the ovary due to cellular infiltration (* in B). OD, oviduct; OV, ovary. (C) Upper genital tract pathology scores (0, none; 1, mild; 2, moderate; and 3, severe) for mock-infected, Ct L2-infected, and Ct D-infected mice. Day 3: mock vs. D, **P = 0.0020; L2 vs. D, **P = 0.0054. Day 6: mock vs. D, **P = 0.0083; L2 vs. D, **P = 0.0025. Day 9: mock vs. L2, **P = 0.0095; L2 vs. D, ***P = 0.0004. Day 15: mock vs. D, *P = 0.0257; L2 vs. D, *P = 0.0170. (D) Time course of bacterial burden in the upper genital tract of Ct L2- vs. Ct D-infected mice shows no significant differences between serovars. N.S., not significant. Day 3: P = 0.39. Day 6: P = 0.59. Day 9: P = 0.31. Day 15: P = 0.35. Day 29: P = 0.31.
By contrast, significant differences were observed in the ability of Ct D and Ct L2 to induce mucosal immunopathology, with only Ct D infection resulting in immediate and lasting disease in the female upper genital tract (Fig. 1 A–C). Mock-infected animals displayed no pathology, having uterine lumens (asterisk, Fig. 1A) that were clear of any infiltrating cells. These data show that the transcervical inoculation procedure alone was not sufficient to induce pathology. Infection with Ct L2 (Fig. 1A, Center) resulted in mild and transient inflammatory infiltration while infection with Ct D (Fig. 1A, Right) caused significant inflammatory cellular infiltration of the uterine lumen (asterisk, Fig. 1A) that persisted for weeks (Fig. 1C). Indeed, pathology scores for Ct D-infected animals remained significantly elevated at day 15 postinfection, a time point when acute inflammation would be typically resolved and when bacterial burdens hovered around the limit of detection (Fig. 1D). Similar trends in pathology were observed in ovarian tissue (Fig. 1B). Only following Ct D infection (Fig. 1B, Right) and not mock or Ct L2 infection (Fig. 1B, Left or Fig. 1B, Center) was moderate-to-severe immunopathology observed, characterized by a thickening of the ovarian membrane (asterisk, Fig. 1B) and its separation from the ovary (OV, Fig. 1B) as a result of substantial inflammatory cellular infiltration and edema. Qualitative assessments of gross pathology also intermittently identified hydrosalpinx, a blockage of the oviducts that results in ovarian edema, in Ct D-infected but not Ct L2-infected animals at later time points (Fig. S1A). Since pathology was observed only in a small subset of animals at these late times postinfection (days 29–42), pathology scores hovered around 0 and were no longer significantly different between groups. Whether inconsistent data at later time points reflect stochastic differences between individual animals or meaningful biological variability reminiscent of the minority of human infections that progress to chronic disease remains to be determined in future studies. Taken together, these data demonstrate that the human pathogen Ct can be used to induce robust upper genital tract immunopathology in a mouse model. Moreover, this model reproduces serovar-specific differences in human disease phenotypes, with Ct D but not Ct L2 resulting in significant and lasting immunopathology.
Immune Cell Infiltrates in C. trachomatis Serovar D-Infected Upper Genital Tracts Are Mainly Composed of Neutrophils and Monocytes.
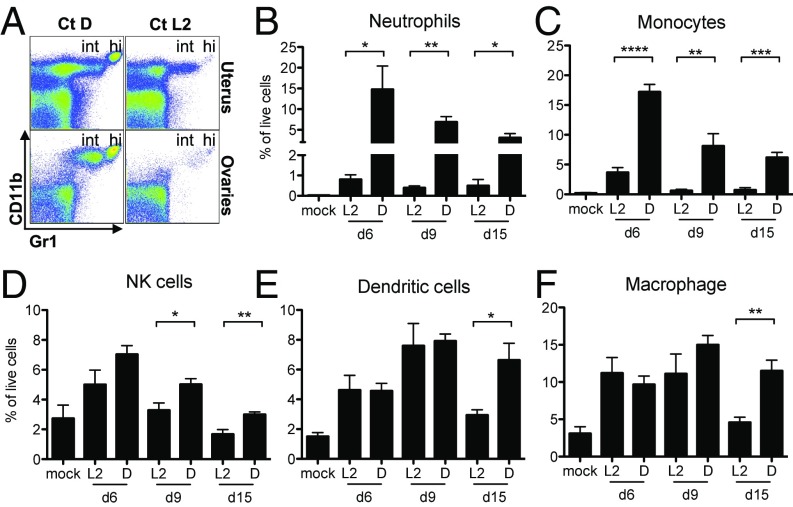
We next sought to differentiate the immune response(s) specific to Ct D infection that drives pathology from the immune response(s) common to both Ct D and Ct L2 infection that results in the same bacterial clearance. Flow cytometry was used to characterize the innate (Fig. 2) immune cells present in the upper genital tract over a time course of infection with Ct L2 or Ct D. Representative plots of Ct D-infected (Fig. 2A, Left) vs. Ct L2-infected (Fig. 2A, Right) uterus (Top) and ovaries (Bottom) illustrate that the innate cellular infiltrates specific to the Ct D-infected tissues were composed of large populations of Gr1hiCD11b+ neutrophils and Gr1intCD11b+ monocytes. Because we consistently observed the same relative phenotypes in the uterus and ovaries within each animal (compare Top vs. Bottom in Fig. 2A; Fig. S2), for all subsequent experiments, we processed the uteri and ovaries together and refer to the site as the upper genital tract.
Fig. 2.
Innate immune cellular infiltrates in C. trachomatis serovar D-infected upper genital tracts persist for weeks and are composed mainly of neutrophils and monocytes. (A) Representative flow cytometry plots of Ct D-infected (Left) vs. Ct L2-infected (Right) uterus (Top) and ovary (Bottom) from day 3 postinfection showing large populations of Gr1hiCD11b+ neutrophils (hi) and Gr1intCD11b+ monocytes (int) only in Ct D-infected tissues. (B–F) Time course quantification by flow cytometry of each of the indicated cell types in the upper genital tracts of mock-infected, Ct L2-infected, or Ct D-infected mice, where significant infiltration of neutrophils and monocytes is seen only after Ct D infection. (B) Day 6: *P = 0.0264. Day 9: **P = 0.0012. Day 15: *P = 0.0350. (C) Day 6: ****P < 0.0001. Day 9: **P = 0.0066. Day 15: ***P = 0.0004. (D) Day 9: *P = 0.0236. Day 15: **P = 0.0057. (E) Day 15: *P = 0.0141. (F) Day 15: **P = 0.0024.
Neutrophils and monocytes were found with significantly greater frequency in Ct D-infected compared with Ct L2-or mock-infected upper genital tracts at every time postinfection (Fig. 2 B and C). The influx of neutrophils was the most dramatic difference observed between the two serovars, with 10–20 times greater frequency of these cells found in Ct D-infected compared with Ct L2-infected upper genital tracts (Fig. 2B). In contrast to neutrophils and monocytes, there were no significant differences between the two serovars in the frequency of natural killer cells, dendritic cells, or macrophages seen at early times postinfection (Fig. 2 D–F). Only at day 15 postinfection, minor serovar-specific differences could be observed (Fig. 2 D–F). Overall, however, these three innate populations diminished over time as would be expected during the resolution of transient inflammation. Consequently, we hypothesized that it is the large, immediate, and lasting mucosal influx of neutrophils and monocytes following Ct D infection that causes tissue pathology.
Neutrophils Are Required for Immunopathology and Dispensable for Pathogen Clearance.
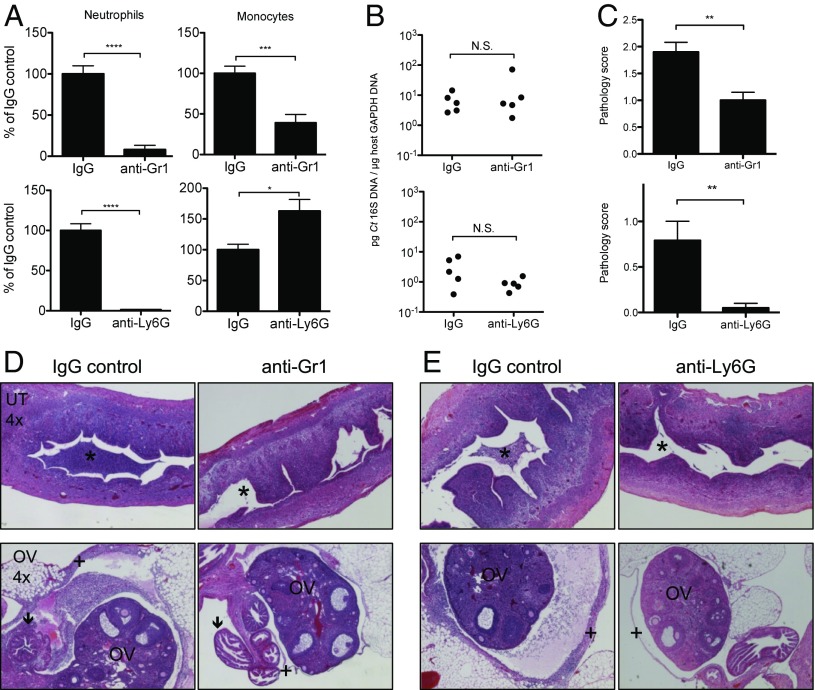
To test whether the observed Gr1+CD11b+ cell influx was required for Ct-induced immunopathology, we employed two independent antibody depletion protocols to assess the relative roles of neutrophils and monocytes. Gr1hiCD11b+ neutrophils were specifically depleted using anti-Ly6G monoclonal antibody compared with its isotype control (Fig. 3A, Bottom). This treatment did not reduce the number of Gr1intCD11b+ monocytes; rather a slight increase was observed (Fig. 3A, Bottom Right). By contrast, depletion using anti-Gr1 monoclonal antibody compared with its isotype control resulted in the significant reduction of both neutrophils and monocytes (Fig. 3A, Top). Neither depletion protocol significantly affected the burden of Ct in the upper genital tract (Fig. 3B), demonstrating that neither of these innate cell types are required to control pathogen load, a finding consistent with the obligate intracellular lifestyle of this organism.
Fig. 3.
Depletion of neutrophils ameliorates genital tract immunopathology following C. trachomatis infection, but does not affect bacterial burden. Ct D-infected mice were depleted of neutrophils using either anti-Gr1 or anti-Ly6G monoclonal antibodies and their matching isotype controls (IgG) and assessed at day 5 postinfection. Depletion was detected and quantified by flow cytometry using a detection epitope other than the one used for antibody-mediated depletion. (A) Quantification of neutrophil depletion by anti-Gr1 (Top) and anti-Ly6G (Bottom). Monocytes are also partially depleted by anti-Gr1 (Top). Neutrophils: IgG vs. anti-Gr1, ****P < 0.0001; IgG vs. anti-Ly6G, ****P < 0.0001. Monocytes: IgG vs. anti-Gr1, ***P = 0.0003; IgG vs. anti-Ly6G, *P = 0.0163. (B) Bacterial burden in the upper genital tracts of mice treated with anti-Gr1 (Top, P = 0.42) or anti-Ly6G (Bottom, P = 0.10). N.S., not significant. (C) Upper genital tract pathology scores for anti–Gr1- (Top, **P = 0.0012) or anti–Ly6G-treated (Bottom, **P = 0.0012) Ct D-infected mice. (D and E) Representative H&E-stained sections of Ct D-infected uteri (Top) and ovaries (Bottom) treated with anti-Gr1 (D) or anti-Ly6G (E). *, uterine lumen; OV, ovary; arrow, oviduct; +, ovarian membrane, where pathology is ameliorated by both anti-Gr1 and anti-Ly6G.
Both neutrophil depletion protocols were found to significantly ameliorate immunopathology in the upper genital tract (Fig. 3 C–E). Compared with isotype controls, anti-Ly6G and anti-Gr1 treatment resulted in a marked clearance of the uterine lumen (asterisk, Fig. 3 D and E, Top) and oviducts (arrow, Fig. 3D, Bottom). Ovarian pathology was also reversed by neutrophil depletion as evidenced by the loss of membrane thickening and separation from the ovary (plus sign, Fig. 3 D and E, Bottom) and by a significant reduction in the occurrence of gross hydrosalpinx (Fig. S1B). Indeed, neutrophil-depleted Ct-infected ovaries resembled mock-infected controls (see Fig. 1B, Left, for comparison). Similar reductions in upper genital tract immunopathology were observed after either depletion protocol (Fig. 3 C–E), suggesting that neutrophils and not monocytes (depleted only by anti-Gr1 treatment and not by anti-Ly6G treatment) are the dominant innate cell type contributing to pathology. In summary, these data demonstrate that neutrophils are required for Ct-induced immunopathology in the upper genital tract. Importantly, if neutrophils were also involved in protection against Ct, then we would expect to see increases in bacterial burden following neutrophil depletion. Instead, neutrophil depletion had no affect on bacterial burden, suggesting that neutrophils drive immune-mediated pathology that can be separated from immune-mediated protection.
Immunopathology at Later Time Points Coincides with Significant Influxes of CD4+ and CD8+ T Cells That Do Not Contribute to Protection.
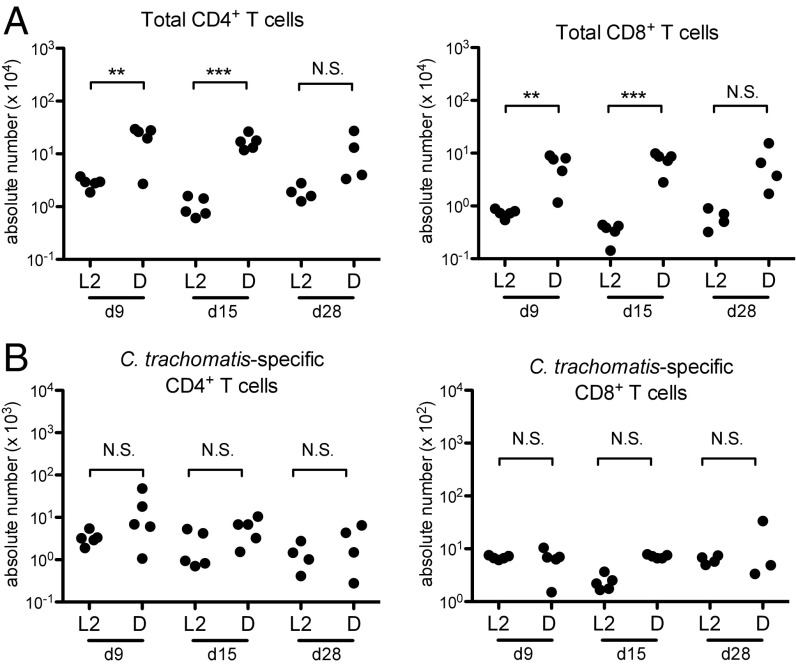
In addition to examining the role of innate cellular infiltrates in immunopathology, we also investigated whether adaptive immune cell populations were differentially recruited following upper genital tract infection with the pathology-inducing Ct D compared with Ct L2 (Fig. 4). Very few B cells were detected in the upper genital tract under any experimental conditions and no significant differences were observed between any groups. By contrast, we did observe large, significant increases in the numbers of total CD4+ and CD8+ T cells in Ct D-infected upper genital tracts compared with Ct L2-infected upper genital tracts (Fig. 4A). Elevated total T cell numbers in the genital tract persisted at least 1 mo after Ct D infection, more than 2 wk after clearance of bacteria from the tissue.
Fig. 4.
At later time points, cellular infiltrates in C. trachomatis serovar D-infected upper genital tracts include a significant increase in total T cells, but not C. trachomatis-specific T cells. (A and B) Time course quantification by flow cytometry of each of the indicated cell types in the upper genital tracts of Ct L2-infected or Ct D-infected mice, showing significant increases in total CD4+ and CD8+ T cell populations only in Ct D-infected mice (A) but no serovar-dependent significant differences in C. trachomatis-specific T cell populations (B). (A) CD4+ T cells: day 9, **P = 0.0058; day 15, ***P = 0.0002; day 28, P = 0.12. CD8+ T cells: day 9, **P = 0.0058; day 15, ***P = 0.0004; day 28, P = 0.08. (B) CD4+ T cells: day 9, P = 0.17; day 15, P = 0.10; day 28, P = 0.29. CD8+ T cells: day 9, P = 0.78; day 15, P = 0.50; day 28, P = 0.39. N.S., not significant.
We compared this response of the total T cells to that of Ct-specific T cells in the upper genital tract during infection. Ct-specific CD8+ T cells were enumerated in the upper genital tract with a MHC class I tetramer. The available MHC class II tetramer for Ct-specific CD4+ T cells is not sensitive enough to detect cells in peripheral tissue. Therefore, to track Ct-specific CD4+ T cells, we transferred TCR transgenic Ct-specific CD4+ T cells i.v. and then measured their recruitment to the upper genital tract following infection. We found no significant differences in the amount of Ct-specific CD4+ or CD8+ T cells in the upper genital tract between the Ct L2- and Ct D-infected mice (Fig. 4B). These data are consistent with previous reports that antigen-specific CD4+ T cells are necessary and sufficient for clearance of Ct burden in the upper genital tract, which we show in Fig. 1D is the same for Ct L2 and Ct D (7, 8). The additional influx of nonprotective CD4+ and CD8+ T cells that occurs only after Ct D infection suggests a role for these cells in driving pathology. We predict that this population is composed of bystander T cells that are recruited in an antigen-independent manner in response to inflammation. Our data support a model wherein bystander T cells are recruited in large numbers to Ct-infected tissues where they are unable to contribute to pathogen clearance and instead contribute to immunopathology.
Comparison of Early Gene Expression Profiles in C. trachomatis-Infected Upper Genital Tracts.
We then considered whether antigen-independent recruitment mechanisms like cytokine and chemokine expression levels might be responsible for driving the differential cellular response to Ct D and Ct L2 infection in the upper genital tract. It has previously been reported that infection of HeLa cells with a genital Ct serovar (serovar E) vs. Ct L2 results in differential cytokine expression in vitro (5). We took an unbiased in vivo approach to survey the expression of over 700 murine inflammation- and immunity-related genes using a commercially available NanoString panel (Fig. S3). mRNA was extracted from mock-infected, Ct L2-infected, and Ct D-infected murine upper genital tracts 2 d after infection, before cellular influxes were observed and when there was no difference in bacterial burden (as in all other time points) (Fig. S3B). We found that not only did host gene expression differ in Ct-infected vs. mock-infected upper genital tracts in a serovar-independent manner (Fig. S3A, Right cluster), it also varied between serovars, with specific genes being expressed in a Ct D-specific manner (Left cluster) or Ct L2-specific manner (Center cluster). Serovar-dependent changes in gene expression were further stratified by magnitude and significance (Fig. S3C) and overlaid onto all Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways containing these genes to infer biological function (Fig. S3D). We observed a node of interaction in the KEGG chemokine and cytokine pathway (Fig. S3D, asterisk) where the expression of CXCL9/10/11 message was similarly up-regulated only in Ct D-infected upper genital tracts. The expression of these three chemokines remained elevated in Ct D- vs. Ct L2-infected mice at days 6 and 9 postinfection (Fig. S4). While each of these chemokines has been independently implicated in a variety of nonredundant biological functions, the coordinate expression of all three, signaling through their common receptor CXCR3, has been particularly associated with chronic inflammatory diseases such as inflammatory bowel disease and rheumatoid arthritis (10). Moreover, since CXCL10 was one of the genes most disproportionately up-regulated in Ct D- vs. Ct L2-infected upper genital tracts (Fig. S3C), we next sought to test whether CXCL9/10/11 signaling through CXCR3 is required for the induction of immunopathology by Ct D.
Blockade of CXCR3 Significantly Reduces Immunopathology Following C. trachomatis Infection.
We first assessed the role of the chemokine CXCL10 in inducing immunopathology after Ct infection since it was one of our top Ct D-specific hits in the NanoString panel and has been found by others to be associated with Chlamydia infection (11). CXCL10−/− mice were compared with wild-type mice in their ability to recruit neutrophils and T cells to Ct D-infected upper genital tracts (Fig. S5A) and to induce immunopathology (Fig. S5 B and C). Bacterial burden was also compared in these mice (Fig. S5D). Contrary to our prediction of a specific role for CXCL10 in driving immunopathology, we observed no significant differences in any of these metrics between wild-type and CXCL10−/− mice. These data show that CXCL10 deficiency is not sufficient to reduce immunopathology, suggesting a functional redundancy of CXCL10 with other chemokines such as CXCL9 and CXCL11 as is seen in other systems of chronic inflammation.
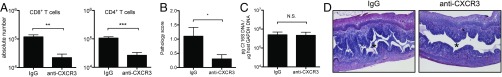
Therefore, we endeavored to test the role of the entire CXCL9/10/11–CXCR3 node of interaction through blockade of their common receptor using an anti-CXCR3 monoclonal antibody (Fig. 5). Compared with isotype controls, anti–CXCR3-treated mice displayed significant improvements in upper genital tract immunopathology (Fig. 5 B and D). CXCR3 blockade also resulted in a significant reduction in total CD4+ and CD8+ T cell numbers in the upper genital tract (Fig. 5A and Fig. S6). The amelioration of pathology was observed independently of bacterial burden, which was unaffected by anti-CXCR3 treatment (Fig. 5C). These data are consistent with the observation that CXCR3 blockade was not sufficient to significantly reduce protective Ct-specific CD4+ T-cell numbers below the threshold required to affect bacterial load (Fig. S6C) (7). Indeed, CXCR3 blockade more significantly inhibited the recruitment of total CD4+ and CD8+ T cells than Ct-specific CD4+ and CD8+ T cells to the infected upper genital tract (Fig. S6). Moreover, neutrophil recruitment was also not affected by anti-CXCR3 treatment (Figs. S6E and S7). These data suggest that a CXCR3-dependent influx of nonprotective T cells contributes to Ct-mediated disease in the upper genital tract and is not involved in pathogen clearance.
Fig. 5.
Antibody blockade of CXCR3, the common receptor for chemokines CXCL9-11, reduces the influx of CD4+ and CD8+ T cells to C. trachomatis-infected upper genital tracts and ameliorates pathology, but does not affect bacterial burden. Ct D-infected mice were treated with anti-CXCR3 monoclonal antibody or matching isotype control (IgG) and assessed at day 8 postinfection. (A) Quantification by flow cytometry of each indicated cell type in the upper genital tract. CD8+ T cells, **P = 0.0027. CD4+ T cells, ***P = 0.0005. (B) Upper genital tract pathology scores, *P = 0.0344. (C) Bacterial burden in the upper genital tract, P = 0.92. N.S., not significant. (D) Representative H&E-stained sections of Ct D-infected uteri treated with anti-CXCR3 vs. isotype control. *, uterine lumen, where cellular infiltration is ameliorated by anti-CXCR3 treatment.
The Development of Protective and Pathology-Driving T-Cell Responses Is Independent of Neutrophils.
Finally, we sought to determine whether the neutrophil influx that precedes the infiltration of T cells is required for the development of protective or pathology-inducing adaptive responses. To test the relationship between these innate and adaptive cell types, we repeated and extended the duration of neutrophil depletion to assess its effect on T cells. We found that neutrophil depletion had no effect on the number of total CD4+ or CD8+ T nor Ct-specific CD4+ or CD8+ T cells in the Ct-infected upper genital tract (Fig. S8). These data demonstrate that neutrophils themselves are not responsible for the downstream influx of nonprotective T cells and suggest that each cell type independently responds to Ct infection in the upper genital tract.
Discussion
The data presented here support the following model for how infection with Ct causes immunopathology in the upper genital tract (Fig. S9). We have shown that the human pathogen Ct causes robust mucosal infection and immunopathology in wild-type mice when a genital-specific serovar (serovar D) is inoculated into the anatomical site of disease (female upper genital tract) (Fig. S9A). As in humans, murine upper genital tract immunopathology following Ct infection is characterized by an immediate and lasting inflammatory response that persists beyond the duration of infection. Immunopathology corresponds to an up-regulation of chemokines CXCL9/10/11 and requires their common receptor CXCR3. Extravasation of leukocytes into the reproductive tissues occludes luminal spaces in the uterus, oviduct, and ovaries. These cellular infiltrates are composed of neutrophils at early time points (Fig. S9B) and the addition of CD4+ and CD8+ T cells at later time points (Fig. S9C). Depletion of these cells significantly ameliorates immunopathology but has no effect on bacterial burden, demonstrating that they are required for mucosal disease but do not contribute to clearance of Ct. Rather, we predict that the invasion of the upper genital tract by nonprotective immune cells results in a positive feedback loop of tissue damage, chemokine release, and further cellular infiltration that is unable to clear Ct and instead perpetuates immunopathology (Fig. S9C).
Neutrophil recruitment to the upper genital tract has been previously associated with Ct disease sequelae in women (4) and our study demonstrates their causative role. Tissue damage due to dysregulated neutrophil recruitment and degranulation has been documented in many other settings such as rheumatoid arthritis and systemic lupus erythematosus (12). We found that neutrophil depletion during Ct infection abrogated mucosal immunopathology and reduced the occurrence of gross pathology (hydrosalpinx), suggesting that neutrophils could be a fruitful therapeutic target for ameliorating disease in humans. The fact that neutrophils are not required to control bacterial replication is consistent with the obligate intracellular niche of Ct and other bacterial pathogens. This suggests that therapies to reduce tissue-trophic neutrophils would not exacerbate infection or transmission in humans. We did not observe any significant differences in known neutrophil chemoattractants in the NanoString dataset, nor did CXCR3 blockade affect neutrophil recruitment, and so further study is needed to identify factors that draw neutrophils into the infected upper genital tract.
Our study also suggests that T cells contribute to upper genital tract pathology after Ct infection. By comparing two infections (Ct L2 vs. Ct D) that differ in pathology but not bacterial burden, we found that the pathology-inducing Ct D infection recruits significantly more CD4+ and CD8+ T cells to the site of infection that do not affect bacterial clearance. We predict that this nonprotective T cell influx is composed of bystander T cells that activate in response to inflammation without antigen-specific T-cell receptor engagement (13). Our results showing that CXCR3 blockade more significantly inhibits the recruitment of bystander vs. Ct-specific T cells to the Ct-infected upper genital tract raise the question of whether nonprotective T cells rely solely on CXCR3-mediated recruitment to the genital tract while protective, antigen-specific T cells have supplemental mechanisms that drive their trafficking. Indeed, we have previously shown that antigen-specific T cells use CXCR3, CCR5, and integrin α4β1 to modulate their trafficking to the genital mucosa following Ct infection (14). This may result from the fact that antigen-specific priming by dendritic cells is known to imprint distinct receptor profiles on activated T cells, a signal that bystander T cells may not receive. Taken together, we predict that robust activation of antigen-specific T cells induces alternative homing molecules that allow their efficient trafficking to the genital mucosa where they restrict bacterial levels even in the presence of anti-CXCR3 blockade. Future studies that aim to identify alternative homing molecules specific to bystander T cells may reveal important druggable targets that could relieve immunopathology.
Nonspecific activation of bystander CD4+ and CD8+ T cells has been linked to pathology in other settings of chronic inflammation (15) and here we show their requirement for Ct-induced disease since chemokine-mediated blockade of bystander T-cell recruitment to the upper genital tract was sufficient to reduce pathology. In considering whether this immunopathology-driving T-cell population may have undetected antigen specificity, our data do not rule out the possibility that they include unidentified subdominant T-cell epitopes specific to Ct or even pathology-specific epitopes that are distinct from the known protective Ct T-cell epitopes. Therefore, it will be an exciting area of future research to determine whether there is indeed specificity to these pathology-driving populations and if so to undergo rigorous epitope mapping studies to identify and track pathology-specific T cells.
We hypothesize that the pathology-inducing recruitment of neutrophils and T cells to the upper genital tract is driven by damage to the epithelium, initially caused by Ct replication-induced lysis of epithelial cells. Eliminating either neutrophils (via anti-Ly6G treatment) or bystander T cells (via anti-CXCR3 treatment) was not sufficient to inhibit the development of the other cellular response, supporting the idea that both cell types are independently responding to the underlying and ongoing epithelial damage being driven by the intracellular bacterial infection. The observed up-regulation of CXCL9/10/11 in the uterus at a time before any significant leukocyte infiltration also suggests that epithelial cells are directly responsible for secreting these chemokines. Previous studies have reported an increase in CXCL10 expression following Chlamydia infection (11) but its direct role in vivo has never been tested. We demonstrate that deletion of CXCL10 in mice is not sufficient to affect infection or pathology, highlighting the functional redundancy of this chemokine and revealing the relative importance of the other CXCR3 ligands (10). Indeed, the CXCL9/10/11–CXCR3 axis has been documented in other examples of chronic inflammation, including inflammatory bowel disease and rheumatoid arthritis, and in the T cell-mediated pathology observed in hepatitis, malaria, and atherosclerosis (10). Our data add a bacterial infection to the growing list of immunopathologies mediated by CXCL9/10/11–CXCR3. Treatments being developed to target these chemokines in other chronic inflammatory conditions have shown efficacy in phase II clinical trials (10) and might also prove useful in ameliorating Ct-induced disease in humans.
In ongoing studies, we are investigating how the observed serovar-specific differences in immunopathology can be linked to variation in bacterial virulence factors between Ct L2 and Ct D. The strains used in this study share 99% sequence identity and differ mostly at a genetic region known as the plasticity zone (6). It has long been appreciated that the plasticity zone of Ct serovars A–K contains ORFs with homology to the Clostridium difficile toxin TcdA/B (6, 16). TcdA/B is an essential virulence factor that induces the inflammatory colitis characteristic of C. difficile infection due to actin rearrangement and cell rounding that disrupts the epithelial barrier and causes proinflammatory enterocyte death (17). During Ct infection of HeLa cells, Ct D but not Ct L2 induces actin rearrangement and cytotoxicity, a phenotype that can be reproduced with ectopic expression of the putative Ct toxin ORF (16). The presence of the toxin homolog in Ct serovars A–K and in C. muridarum, all of which cause mucosal pathology, but not in the Ct LGV serovars, makes it an attractive target for investigation as a pathology-inducing virulence factor. Testing this hypothesis and others will benefit from the mouse model of Ct-induced immunopathology described here. Such studies will be key to the development of treatments for Ct-infected women at risk for reproductive disease and the development of Ct vaccines that can prevent infection without inducing immunopathology.
Materials and Methods
See Supporting Information for full description of methods.
Transcervical Infection.
All mouse procedures were performed in accordance with protocols approved by Harvard’s Institutional Animal Care and Use Committee. Transcervical infections were performed as has been previously described (7, 8). At time points described in the text, the upper genital tract was harvested and processed for flow cytometry or submitted for pathology processing by the Rodent Histopathology Core Facility at Harvard Medical School. Images of H&E-stained tissue sections were acquired on a Nikon Eclipse Ti-E inverted microscope using a DS-QiMc camera and the manufacturer’s acquisition software.
Antibody Treatments.
To deplete neutrophils, mice were treated i.p. with 200 μg of either anti-Gr1 (RB6-8C5) vs. its IgG2b isotype control (LTF-2) or anti-Ly6G (IA8) vs. its IgG2a isotype control (2A3). To block CXCR3, mice were treated transcervically (t.c.) with 200 μg of anti-CXCR3 (CXCR3-173) or its IgG2a isotype control (C1.18.4). All antibody treatments began 1 d before infection and continued every other day for the experiment’s duration. All antibodies were purchased from Bio-X-Cell.
Statistical Analysis.
Two-to-three independent experiments were performed for each analysis, with at least five mice per group per treatment. Statistical analysis was performed using Prism (GraphPad) and a P value of <0.05 was considered significant. All bar graphs are shown as mean ± SEM, where ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05, and N.S. indicates not significant by unpaired Student’s t test.
Supplementary Material
Acknowledgments
This work was supported by NIH Grants AI39558 and AI062827, and Grant AI113187 from the the Epidemiology and Prevention Interdisciplinary Center for Sexually Transmitted Infections. R.S.L. was supported by NIH Grant 5F32AI108144.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1711356115/-/DCSupplemental.
References
- 1.Wiesenfeld HC. Screening for Chlamydia trachomatis infections in women. N Engl J Med. 2017;376:765–773. doi: 10.1056/NEJMcp1412935. [DOI] [PubMed] [Google Scholar]
- 2.Choroszy-Król IC, Frej-Mądrzak M, Jama-Kmiecik A, Bober T, Jolanta Sarowska J. Characteristics of the Chlamydia trachomatis species–Immunopathology and infections. Adv Clin Exp Med. 2012;21:799–808. [PubMed] [Google Scholar]
- 3.Darville T, Hiltke TJ. Pathogenesis of genital tract disease due to Chlamydia trachomatis. J Infect Dis. 2010;201(Suppl 2):S114–S125. doi: 10.1086/652397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiviat NB, et al. Histopathology of endocervical infection caused by Chlamydia trachomatis, herpes simplex virus, Trichomonas vaginalis, and Neisseria gonorrhoeae. Hum Pathol. 1990;21:831–837. doi: 10.1016/0046-8177(90)90052-7. [DOI] [PubMed] [Google Scholar]
- 5.Dessus-Babus S, Darville TL, Cuozzo FP, Ferguson K, Wyrick PB. Differences in innate immune responses (in vitro) to HeLa cells infected with nondisseminating serovar E and disseminating serovar L2 of Chlamydia trachomatis. Infect Immun. 2002;70:3234–3248. doi: 10.1128/IAI.70.6.3234-3248.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Read TD, et al. Genome sequences of Chlamydia trachomatis MoPn and Chlamydia pneumoniae AR39. Nucleic Acids Res. 2000;28:1397–1406. doi: 10.1093/nar/28.6.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gondek DC, Olive AJ, Stary G, Starnbach MN. CD4+ T cells are necessary and sufficient to confer protection against Chlamydia trachomatis infection in the murine upper genital tract. J Immunol. 2012;189:2441–2449. doi: 10.4049/jimmunol.1103032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stary G, et al. VACCINES. A mucosal vaccine against Chlamydia trachomatis generates two waves of protective memory T cells. Science. 2015;348:aaa8205. doi: 10.1126/science.aaa8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibson-Corley KN, Olivier AK, Meyerholz DK. Principles for valid histopathologic scoring in research. Vet Pathol. 2013;50:1007–1015. doi: 10.1177/0300985813485099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Raemdonck K, Van den Steen PE, Liekens S, Van Damme J, Struyf S. CXCR3 ligands in disease and therapy. Cytokine Growth Factor Rev. 2015;26:311–327. doi: 10.1016/j.cytogfr.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Porcella SF, et al. Transcriptional profiling of human epithelial cells infected with plasmid-bearing and plasmid-deficient Chlamydia trachomatis. Infect Immun. 2015;83:534–543. doi: 10.1128/IAI.02764-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bardoel BW, Kenny EF, Sollberger G, Zychlinsky A. The balancing act of neutrophils. Cell Host Microbe. 2014;15:526–536. doi: 10.1016/j.chom.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Thompson LJ, et al. Conditioning of naive CD4(+) T cells for enhanced peripheral Foxp3 induction by nonspecific bystander inflammation. Nat Immunol. 2016;17:297–303. doi: 10.1038/ni.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davila SJ, Olive AJ, Starnbach MN. Integrin α4β1 is necessary for CD4+ T cell-mediated protection against genital Chlamydia trachomatis infection. J Immunol. 2014;192:4284–4293. doi: 10.4049/jimmunol.1303238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGavern DB. The role of bystander T cells in CNS pathology and pathogen clearance. Crit Rev Immunol. 2005;25:289–303. doi: 10.1615/critrevimmunol.v25.i4.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thalmann J, et al. Actin re-organization induced by Chlamydia trachomatis serovar D–Evidence for a critical role of the effector protein CT166 targeting Rac. PLoS One. 2010;5:e9887. doi: 10.1371/journal.pone.0009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li S, et al. Critical roles of Clostridium difficile toxin B enzymatic activities in pathogenesis. Infect Immun. 2015;83:502–513. doi: 10.1128/IAI.02316-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.