Significance
Hybrid sterility, a major reproductive barrier between species, hinders the transfer of desirable traits from one species to another. We report a forward genetic approach for creating a “neutral” allele of the S1 locus, a major interspecific hybrid sterility locus in rice. This neutral allele does not induce hybrid sterility in combination with alleles from either Asian or African rice species. The allele carries a deletion in the peptidase-coding gene, SSP, in the S1 locus. This work provides mechanistic and evolutionary insights into hybrid sterility and demonstrates the feasibility of the approach that allows broader access to desirable traits in distantly related species during crop breeding.
Keywords: rice, reproductive barrier, hybrid sterility, transmission ratio distortion, mutagenesis
Abstract
Understanding the genetic basis of reproductive barriers between species has been a central issue in evolutionary biology. The S1 locus in rice causes hybrid sterility and is a major reproductive barrier between two rice species, Oryza sativa and Oryza glaberrima. The O. glaberrima-derived allele (denoted S1g) on the S1 locus causes preferential abortion of gametes with its allelic alternative (denoted S1s) in S1g/S1s heterozygotes. Here, we used mutagenesis and screening of fertile hybrid plants to isolate a mutant with an allele, S1mut, which does not confer sterility in the S1mut/S1g and S1mut/S1s hybrids. We found that the causal mutation of the S1mut allele was a deletion in the peptidase-coding gene (denoted “SSP”) in the S1 locus of O. glaberrima. No orthologous genes of SSP were found in the O. sativa genome. Transformation experiments indicated that the introduction of SSP in carriers of the S1s allele did not induce sterility. In S1mut/S1s heterozygotes, the insertion of SSP led to sterility, suggesting that SSP complemented the loss of the functional phenotype of the mutant and that multiple factors are involved in the phenomenon. The polymorphisms caused by the lineage-specific acquisition or loss of the SSP gene were implicated in the generation of hybrid sterility. Our results demonstrated that artificial disruption of a single gene for the reproductive barrier creates a “neutral” allele, which facilitates interspecific hybridization for breeding programs.
Reproductive barriers prevent gene flow and maintain genetic discontinuities between species. One of the central issues in evolutionary biology is how genic incompatibility between species arose and became fixed without causing deleterious interaction within a species. The Bateson–Dobzhansky–Muller (BDM) model indicates that incompatibilities are generally caused by an interaction between genes that have functionally diverged in each population (1). BDM-type genic incompatibilities are caused by several types of genetic differences between species, including nucleotide substitutions, the presence of transposable elements or noncoding repeats, gene transposition, and reciprocal gene loss (2). In addition to these differences, recent genome sequence analyses in animal and plant species revealed widespread gene-presence/absence polymorphisms represented by coding sequences that are present in some genomes but completely absent in others (3, 4). The species-specific genes, also known as “orphan genes,” are shown to contribute to species-specific adaptations (5). However, it has not been known whether gene-presence/absence polymorphisms are involved in the reproductive barrier between species.
There are two cultivated rice species, Asian (Oryza sativa) and African (Oryza glaberrima) (6). Although O. glaberrima is rapidly being replaced by O. sativa cultivars that produce higher yields, O. glaberrima has tolerance to various abiotic and biotic stresses (7, 8) and is therefore a valuable source of useful genes (9). Moreover, hybrids between O. glaberrima and O. sativa are very vigorous (Fig. 1A and Fig. S1) (10), suggesting the potential usefulness of interspecific hybrids for increasing grain production. However, strong hybrid sterility between O. glaberrima and O. sativa (Fig. 1 B and C) has prevented the use of desirable traits of O. glaberrima (9, 11–14). Therefore, elucidating the genetic basis of interspecific hybrid sterility between the two rice species is important not only to understand the evolutionary biology of speciation but also to improve crops for future food production.
Fig. 1.
Effects of lines carrying S1s, S1g, and the Acc108S1M on hybrid sterility. (A) Hybrid vigor observed in F1 plants derived from a cross between O. sativa (cultivar, NERICA10) and O. glaberrima (cultivar, CG14). (Scale bar: 10 cm.) (B) Panicles of O. sativa (cultivar, T65), O. glaberrima (cultivar, CG14), and an interspecific hybrid. (Scale bar: 2 cm.) (C) Mean ± SD seed fertility of O. sativa (cultivar, T65), O. glaberrima (cultivar, CG14), and an interspecific hybrid. Means labeled with different letters differed significantly in Tukey’s honestly significant difference (HSD) test (n = 3 plants). (D) Plant type of Acc108, Acc108S1, and their F1 progeny. (Scale bars: 10 cm.) (E) Light micrographs of developing female gametophytes of Acc108 and F1 derived from a cross between Acc108 and Acc108S1 stained with Delafield’s hematoxylin (Muto Pure Chemicals). (Scale bars: 50 μm.) (F) I2-KI–stained pollen of Acc108 and of F1 plants derived from a cross between Acc108 and Acc108S1. TY and SP indicate typical and spherical-type aborted pollen grains, respectively. (Scale bar: 50 μm.) (G) Plant types of Acc108S1M, Acc108S1M × Acc108, and Acc108S1M × Acc108S1. (Scale bars: 10 cm.) (H) Panicles of Acc108 × Acc108S1, Acc108S1M, Acc108S1M × Acc108, and Acc108S1M × Acc108S1 plants. (Scale bars: 4 cm.) (I) Mean ± SD seed fertility (Upper) and frequency of pollen types (Lower) of Acc108 and Acc108S1 plants and their F1 progeny. Means labeled with different letters differed significantly in Tukey’s HSD test (n = 3 plants). (J) Interfertility relationships among Acc108, Acc108S1, and Acc108S1M. F1 plants derived from a cross between Acc108 and Acc108S1 are seed and pollen sterile, while F1 plants derived from a cross between Acc108S1M and either Acc108 or Acc108S1 are seed and pollen fertile.
In Oryza, several causal genes for hybrid sterility have been cloned (15–19). The presence of “neutral” alleles that do not cause sterility in the heterozygous state at intraspecific hybrid sterility loci in O. sativa has been reported (Table S1). Although neutral alleles have facilitated a breakthrough in hybrid rice production in Asia (18, 20), this type of naturally occurring allele has never been found in interspecific cross-combinations in plants (Table S1). Recently, reverse-genetic approaches through CRISPR/Cas9-mediated gene knockout successfully created neutral alleles of intra- and interspecific hybrid sterility genes in rice (21–23). However, such reverse-genetic approaches need detailed information about the causative genes before application; therefore, these approaches are not practical for disrupting reproductive barriers for which the causative genes have not been characterized.
To date, more than 10 loci for hybrid sterility between O. sativa and O. glaberrima have been reported (24–26). Among them, the HYBRID STERILITY 1 (S1) locus on chromosome 6 has frequently been detected, suggesting that the S1 locus is the major cause of the sterility barrier (11, 13, 14). The hybrid between O. sativa and near-isogenic lines (NILs) of O. sativa containing a segment of chromosome 6 from O. glaberrima showed partial sterility in pollen and seeds (11). This phenomenon was explained by Sano in 1990 as the interaction between two genetically defined alleles (12), the S1g allele [formerly the S1 allele of Sano (12), renamed here to avoid potential confusion with the name of the locus] identified in the chromosomal segment derived from O. glaberrima and its alternative allele, the S1s allele [formerly the S1a allele of Sano (12)], in O. sativa at the S1 locus. The S1g allele acts as a “gamete eliminator,” and both male and female gametes possessing the S1s allele are aborted only in the heterozygote (S1g/S1s). As a result, the S1g allele is transmitted more frequently than the S1s allele through both male and female gametes, causing a sex-independent transmission ratio distortion (siTRD) in later generations (27). Previous genetic-mapping studies suggest that multiple components affecting hybrid sterility are located in a region on chromosome 6 from O. glaberrima [in this article the region is denoted as the “glaberrima sterility complex” (GSC), equivalent to the “S1 regions” of Garavito et al. (13)], and a factor causing hybrid sterility is located in the 27.8-kb interval (13) (the S1 locus) between two DNA markers, RM19357 and RMC6_22028, in the GSC (for more detail, see SI Introduction and Fig. S2). A recent attempt at a CRISPR/Cas9-mediated knockout of OgTPR1, a gene coding a protein containing trypsin-like peptidase and ribosome biogenesis regulatory domains located in the S1 locus, resulted in an increase in the level of pollen and seed fertility in the heterozygote (S1g/S1s) (22). Although this study showed that OgTPR1 is necessary for the S1 locus-mediated siTRD and suggested that a nucleotide substitution causes a functional change between OgTPR1 and its allelic counterpart OsTP1, whether OgTPR1 is sufficient for inducing this phenomenon has not been examined. Thus, the overall mechanism of the S1 locus-mediated siTRD system has remained unknown. Interestingly, previous studies showed that there are large insertions in the S1 locus in the O. glaberrima genome (the S1 locus is 27.8 kb in O. sativa, while it is 50.3 kb in O. glaberrima). In these insertions, five genes that are not present in O. sativa genome are predicted (13). These results raised the possibility of the involvement of species-specific genes in the S1 locus-mediated siTRD.
Here, we describe a forward genetic approach to screen a mutant that does not induce sterility in a hybrid with either the S1g or S1s allele. Using this mutant, we found that a gene coding for a serine/trypsin-like peptidase domain-containing protein, which is not OgTPR1, is also an essential gene for inducing hybrid sterility in heterozygotes (S1g/S1s). The causative gene is located on the O. glaberrima-specific insertion, and no orthologous genes were found in the O. sativa genome. We demonstrate that the presence of the causal gene alone is insufficient for inducing S1 locus-mediated siTRD, but its presence with another factor(s) is sufficient. This study provides evidence that the gene-presence/absence polymorphism is involved in the reproductive barrier between species. This study also demonstrated that a neutral allele for a hybrid sterility locus can be created through a forward genetic approach, which potentially enables crop breeding using distantly related species between which even uncharacterized hybrid sterility exists.
Results
Histological Analysis and Genetic Mapping of the S1 Locus.
Rice strains Acc108 (S1s carrier) and the NIL Acc108S1 (S1g carrier) had a high level of pollen and seed fertility, whereas seed and pollen of their F1 hybrids were sterile (Fig. 1 D and F). In female gametogenesis, abnormal embryo sacs were observed in the F1 hybrid (Fig. 1E and Fig. S1). During male gametogenesis, a typical (TY) type of abortion was frequently noted in pollen grains in the F1 hybrid (Fig. 1F and Fig. S1; for abortion types of pollen grains, see SI Materials and Methods). These results indicate that seed and pollen sterility in the F1 hybrids is caused by developmental failure during male and female gametogenesis (for details, see SI Results).
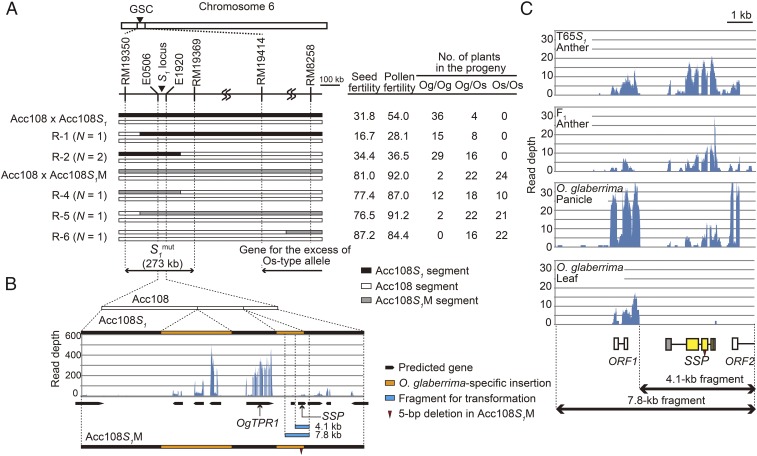
By using the segregating population derived from the cross between Acc108 and Acc108S1, the gene causing hybrid sterility and siTRD [excess of the O. glaberrima-type (Og-type) allele] was mapped onto the 273-kb interval between two markers (RM19350 and RM19369) where the S1 locus was located (Fig. 2A and SI Results).
Fig. 2.
Map-based cloning of the S1mut allele in the Acc108S1M mutant. (A, Left) Substitution mapping of S1mut. S1mut was mapped onto the region between markers RM19350 and RM19369, where the S1 locus was mapped. The genotypes of plants used for the mapping are shown graphically. Black, white, and gray boxes indicate chromosomal segments from O. glaberrima, O. sativa, and the Acc108S1M mutant, respectively. (Right) The table shows the means for seed and pollen fertility in each genotype and the number of O. glaberrima-type homozygotes (Og/Og) and heterozygotes (Og/Os) and O. sativa-type homozygotes (Os/Os) in progenies. (B) Genomic structures of the S1 locus in Acc108 and the Acc108S1 and Acc108S1M mutants. Sequence analysis revealed that Acc108S1 and Acc108S1M possess insertions specific to O. glaberrima in this region (orange boxes). Ten genes were predicted in the S1 locus in Acc108S1 based on the RNA-seq analysis using young anthers of T65S1. The number of mapped reads in each nucleotide is shown. The Acc108S1M had a 5-bp deletion in the O. glaberrima-specific insertion in the S1 locus (red downward-pointing triangle). The positions of the SSP and OgTPR1 are shown by arrows. Two WT genomic fragments (4.1 and 7.8 kb; pale blue boxes) were used for transgenic experiments. (C) RNA-seq analysis to determine the gene structure surrounding the 5-bp deletion in the Acc108S1M mutant. Short reads obtained from RNA samples of young anthers in T65S1 and T65wx × T65S1 were mapped to the 7.8-kb genomic sequence surrounding the 5-bp deletion. In addition, RNA-seq data of young panicle and leaf samples from O. glaberrima were obtained from the public database and used (SI Materials and Methods). The number of mapped reads in each nucleotide is shown. Three predicted genes in the region are depicted by a box with the gene name below.
Mutant Acc108S1M Has a Deletion in the S1 Locus-Specific Peptidase Domain-Containing Protein Gene.
The mutant Acc108S1M, which does not induce sterility in the hybrid with either the S1g or the S1s carrier, was obtained through mutagenesis using heavy-ion beam irradiation applied to the seeds derived from the cross between Acc108 and Acc108S1 (for details, see SI Results). Acc108S1M had a high level of pollen and seed fertility in hybrids with both Acc108 and Acc108S1 (Fig. 1 G–J), whereas quantitative values for other morphological traits were similar to those of Acc108S1 (Fig. S1B).
Genetic experiments suggest that the mutant phenotype is controlled by a single locus (SI Results). The crossing experiments and genetic mapping revealed that the mutation was located in the 273-kb region on the chromosome of O. glaberrima that contains the S1 locus (Fig. 2A and SI Results). Therefore, we speculated that the mutant Acc108S1M has a mutated allele, designated “S1mut,” at the S1 locus and that the S1mut allele acts as the neutral allele in the S1 locus-mediated siTRD system. In addition to the S1mut allele, the gene responsible for the excess of the Os-type allele was also mapped in a region different from that of the S1 locus (Fig. 2A and SI Results). This gene was not investigated further in this study.
Comparison of the 273-kb sequence in the Acc108S1M and Acc108S1 revealed that the Acc108S1M has a 5-bp deletion in the S1 locus (Fig. 2B), with no other mutations observed in the 273-kb region. The gene structure determined by RNA-sequencing (RNA-seq) analysis in the S1 locus indicated that the deletion had occurred in the gene coding for a serine/trypsin-like peptidase domain-containing protein (Fig. 2B). The gene was not found in the corresponding chromosomal region of O. sativa. In addition, no homologous genes with >50% amino acid sequence identity were detected in the O. sativa genome in a BLASTP search (Fig. S3). A serine proteinase-specific catalytic triad was present in the protein sequence of this gene (Fig. S3). Therefore, we tentatively designated this gene as the S1 locus-specific peptidase domain-containing protein (SSP). The SSP of Acc108S1 (denoted “SSP-gla”) encodes 246 amino acids, whereas that of the Acc108S1M (denoted “SSP-mut”) has a 5-bp deletion in the second exon, causing a frame-shift mutation and thereby generating a premature stop codon in a peptidase domain (Fig. S3). These results suggested that the 5-bp deletion in the SSP gene is the cause of the S1mut allele in Acc108S1M.
The SSP gene was located ∼7 kb from the OgTPR1 gene, a gene recently reported to be involved in S1 locus-mediated siTRD (22). Although both these genes encode a protein containing trypsin-like peptidase domains, there is less than 12.5% identity between the domains encoded by these genes (Fig. S3). In O. sativa, 17 genes have been reported to encode trypsin-like peptidase domain-containing protein. A phylogenetic analysis indicated that trypsin-like peptidase domains encoded by SSP and OgTPR1 belong to different clades, which is consistent with the low level of sequence similarity between them (Fig. S3). The expression profile of genes encoding trypsin-like peptidase suggested that they are not preferentially expressed in any specific tissues or stages (Fig. S3). The gene ontology analysis showed that some of these genes (Fig. S3) are related to protein folding, proteolysis, intracellular signal transductions, and apoptotic processes.
The SSP and OgTPR1 Genes Are Coexpressed in Developing Gametophytes.
RT-PCR analysis showed that SSP is expressed in the plumule, young root, anther, and ovule in O. glaberrima, while OgTPR1 is expressed in various tissues including the anther and ovule (Fig. S4A). SSP and OgTPR1 were also expressed in young panicles in Acc108S1, Acc108 × Acc108S1 hybrids, and Acc108S1M, suggesting the coexpression of these two genes in developing gametophytes (Fig. S4B).
To investigate the spatiotemporal expression of SSP and OgTPR1 in detail, we performed single-target (Fig. S4 C–L) and double-target in situ hybridizations (Fig. 3). In the functional sporophyte stage of female gametogenesis of Acc108S1, SSP transcripts were detected in the tissues surrounding the embryo sac, including the nucellus and the inner and outer integuments (Fig. 3C and Fig. S4C). In the early to middle embryo sac stages, a weak signal for the SSP transcript was also detected in the nucellus (Fig. 3H and Fig. S4 D and E). The same expression pattern of the SSP gene was observed during female gametogenesis in Acc108 × Acc108S1 hybrids (Fig. S4 F–H). The OgTPR1 transcripts were also detected in tissues where the SSP transcripts were detected during female gametogenesis (Fig. 3 D and I and Fig. S4 K and L). Furthermore, the coexpression of the SSP and OgTPR1 genes in the nucellus and the inner and outer integuments was confirmed by colocalization of the signals in the double-target in situ hybridization analysis (Fig. 3 E and J).
Fig. 3.
Double-target in situ hybridization of SSP and OgTPR1 in developing ovules in Acc108S1. A biotin-labeled probe of the SSP transcript and a digoxygenin-labeled probe of the OgTPR1 transcript were used. (A–E) Longitudinal sections of ovules at the functional sporophyte stage. (F–J) Longitudinal sections of ovules at the early-middle stage of embryo sac development. Bright-field light micrograph (A and F), DAPI staining (B and G), and expression pattern of SSP (C and H) and OgTPR1 (D and I) are shown. A merged view of C and D is shown in E; a merged view of H and I is shown in J. Em, embryo sac; In, inner integuments; Nu, nucellus; Oi, outer integuments. (Scale bars: 50 μm.)
We could not detect any specific signals in the developing anthers of Acc108S1 using the SSP antisense probe. To confirm the expression of SSP and OgTPR1 during male gametogenesis, we carried out quantitative RT-PCR analysis and confirmed the expression of SSP and OgTPR1 in young anthers in homozygotes for the S1g allele in the genetic background of a japonica rice strain, T65 (T65S1) (Fig. S4M). The expression of the SSP and OgTPR1 genes in the young anthers and young panicles was also confirmed by RNA-seq analysis of T65S1 and RNA-seq data of O. glaberrima, in the public database, respectively (Fig. 2 B and C).
Introduction of the SSP Gene Complements the Hybrid Sterility Phenotype.
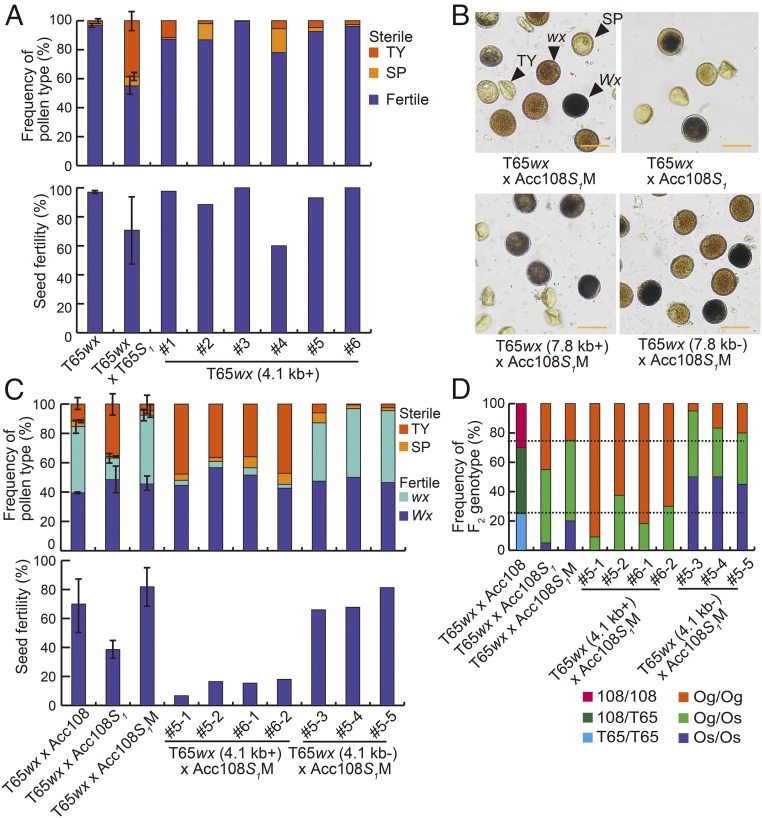
If the SSP gene is a necessary and sufficient factor for inducing hybrid sterility, transformation of an S1s carrier with the intact SSP gene (SSP-gla) might induce hybrid sterility. To examine this hypothesis, we used two types of WT genomic fragments (4.1 and 7.8 kb) (Fig. 2) containing SSP-gla to transform rice strain T65wx (S1s carrier). Intriguingly, pollen and seed fertility of the resultant transgenic lines was not significantly lower than that of nontransgenic controls (T65wx) (Fig. 4A and Fig. S5B). This result suggested that SSP-gla is not sufficient to induce sterility and that another factor(s) including OgTPR1 collaboratively acts to induce sterility.
Fig. 4.
Transgenic experiments. (A) Mean ± SD frequency of pollen types and seed fertility of T65wx, T65S1, and six independent transgenic plants (T65wx, 4.1 kb+). TY and SP indicate typical- and spherical-type aborted pollen grains, respectively. n = 3 plants for T65wx and T65wx × T65S1; n = 1 plant for transgenic plants. (B) Pollen grains of T65wx × Acc108S1M, T65wx × Acc108, T65wx (7.8 kb+) × Acc108S1M, and T65wx (7.8 kb−) × Acc108S1M plants. Wx and wx indicate Wx- and wx-type fertile pollen grains with a functional and nonfunctional allele, respectively. (Scale bar: 50 μm.) (C) Mean ± SD frequency of pollen types and seed fertility of T65wx × Acc108, T65wx × Acc108S1, T65wx × Acc108S1M, and transgenic plants. For transgenic plants, two genotypes, T65wx (4.1 kb+) × Acc108S1M and T65wx (4.1 kb−) × Acc108S1M, were used to assess pollen and seed fertility. A plus sign (+) indicates a transgenic positive; a minus sign (−) indicates a null segregant. n = 3 plants for T65wx × Acc108, T65wx × Acc108S1, and T65wx × Acc108S1M; n = 1 plant for transgenic plants. (D) Mean ± SD frequency of F2 genotypes obtained from the self-pollination of plants of each genotype. T65wx × Acc108, T65wx × Acc108S1, T65wx × Acc108S1M, T65wx (4.1 kb+) × Acc108S1M, and T65wx (4.1 kb−) × Acc108S1M were used. 108/108, Acc108-type homozygotes; 108/T65, heterozygotes for Acc108 and T65wx types; T65/T65, T65wx-type homozygotes; Og/Og, O. glaberrima-type homozygotes; Og/Os, heterozygotes for O. glaberrima and O. sativa types; Os/Os, O. sativa-type homozygotes. Dotted lines mark 25% and 75%.
We then conducted a complementation test by crossing these transgenic lines with the Acc108S1M (S1mut carrier). If the inability to induce sterility in the Acc108S1M is caused by the 5-bp deletion in the SSP gene, transformation with the WT genomic fragment containing the SSP-gla gene will complement the loss-of-function phenotype of the Acc108S1M (Fig. S5A). As a consequence, the F1 hybrids between the Acc108S1M and the transgenic lines will show hybrid sterility and siTRD identical to that mediated by the S1 locus, although the SSP-gla transgene is present at a different place in the genome. All hybrids harboring the S1mut allele and transformed with WT genomic fragments indeed showed a high proportion of TY-type aborted pollen, as observed in T65wx × T65S1 (S1g/S1s) (Fig. 4 B and C and Fig. S5C). In addition, a marked reduction of the proportion of visibly distinguishable waxy (wx)-type pollen was observed in these hybrids (Fig. 4 B and C and Fig. S5C; for analysis of pollen abortion on the basis of the wx phenotype, see SI Materials and Methods). Since the wx allele is linked in a coupling phase with the S1s allele (27), this result indicated the presence of male transmission ratio distortion (mTRD) at the S1 locus in these hybrids. Seed fertility of these hybrids was also lower than that of the hybrids between T65wx and Acc108 or Acc108S1M (Fig. 4C and Fig. S5C). Furthermore, a marked excess of the Og-type allele was observed in the progenies of the F1 hybrids between Acc108S1M and transgenic lines (Fig. 4D and Fig. S5D), indicating the occurrence of siTRD at the S1 locus. Based on these results, we concluded that the 5-bp deletion in the SSP gene caused the functional change from the S1g allele to the S1mut allele at the S1 locus and that SSP-gla acts with other factor(s) at the S1 locus to induce sterility.
SSP Is Not Present in the Asian Rice Gene Pool.
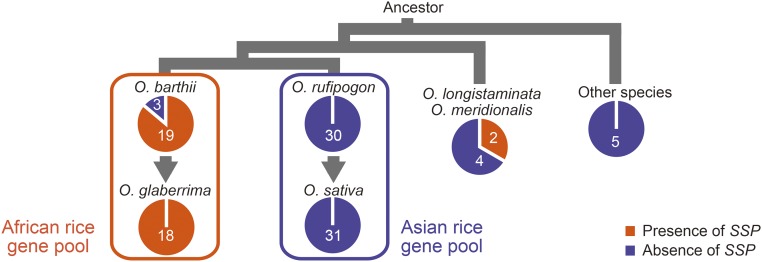
A BLASTP search showed that there is no homologous gene for SSP in the genus Oryza other than in Oryza barthii, a wild ancestral species of O. glaberrima (Fig. S3), suggesting that the SSP gene is present only in limited species in this genus. To comprehensively analyze the distribution of the SSP gene, we examined 112 rice accessions in nine species of the genus Oryza (Fig. 5 and Table S2). The SSP gene was detected in O. glaberrima, O. barthii, and Oryza meridionalis but not in the Asian rice gene pool and other distantly related Oryza species.
Fig. 5.
Distribution of the SSP in Oryza species. The proportions of the number of strains with and without the SSP gene and the number of strains are shown in pie charts. The charts are arranged on phylogenetic relationships of Oryza species (for details, see SI Materials and Methods). The process of domestication is shown by arrows. Oryza longistaminata and O. meridionalis have the same AA genome as species in the Asian and African rice gene pools. Oryza officinalis, Oryza minuta, and Oryza ridleyi were used as outgroups.
Discussion
The present study showed that the neutral allele S1mut at the S1 locus was obtained through the forward genetic approach via mutagenesis using heavy-ion beam irradiation. Such a neutral allele will greatly promote the transfer of elite genes from different species in rice. The cause of the S1mut was the 5-bp deletion in the SSP gene (SSP-mut). These findings showed that the intact SSP gene (SSP-gla) is essential for the S1 locus-mediated siTRD system. However, the introduction of the SSP-gla gene into the S1s carrier did not induce hybrid sterility or siTRD. These results suggested that the ability to induce sterility and siTRD is due to the SSP-gla gene and other factor(s): the S1 locus consists of multiple genes, and the epistatic interaction between them induces hybrid sterility and the S1 locus-mediated siTRD. Recently, Xie et al. (22) suggested the involvement of another gene, OgTPR1, in the S1 locus-mediated siTRD. However, it was still unclear whether the presence of OgTPR1 is sufficient for the S1 locus-mediated siTRD. Our results showed that mutant Acc108S1M, which does not have any mutations in OgTPR1, produces fertile hybrids when crossed with Acc108 (Fig. 1 G–I). These results indicated that the intact OgTPR1 alone does not induce the siTRD mediated by the S1 locus.
In well-characterized examples of hybrid sterility involving Segregation distorter (SD) in Drosophila, the t-haplotype in mouse, and the Sa locus in O. sativa, selective dysfunction of gametes is induced by the interaction between two factors, a “distorter” and a “responder” (15, 28, 29). In this distorter–responder system, a distorter and a responder are linked in a repulsion phase, and the distorter acts to raise its transmission by inducing dysfunction of gametes carrying the responder. On the other hand, Yang et al. (18) proposed a “killer–protector” system at the S5 locus encoded by three tightly linked genes (ORF3–ORF5) in hybrid sterility. In this killer–protector system, a killer and a protector are linked in a coupling phase, and the killer selectively kills the gametes that do not have the protector, thus raising its transmission. In this model, ORF5, which encodes an aspartic protease, acts like a killer when partnered with ORF4, whereas the functional ORF3 (protector) allele prevents dysfunction of gametes that have this allele. Thus, all these systems of hybrid sterility involve two or more factors: a distorter–responder or a killer–protector to express hybrid sterility.
Are these systems involved in the hybrid sterility mediated by the S1 locus? The fact that Acc108S1M and the CRISPR/Cas9-mediated mutant of OgTRP1 produced fertile hybrids in crosses between both S1g and S1s carriers indicates that neither of these mutants has such a killer (distorter) function. Because Acc108S1M and the CRISPR/Cas9-mediated mutant of OgTPR1 possess intact OgTPR1 and SSP-gla, respectively, these results suggested that both SSP-gla and OgTPR1 are necessary but not sufficient for the killer function. This idea is strongly supported by the lack of sterility induction when the SSP-gla gene is introduced into the S1s carrier. Although the SSP-gla transgene did not singly induce sterility, it complemented the phenotype of the Acc108S1M and induced the S1 locus-mediated siTRD; the observed frequency of sterility suggests that the SSP gene acts in a sporophytic manner, which is also consistent with its expression in the nucellus (the sporophytic tissue surrounding the developing embryo sac). Considering the results collectively, we propose a model in which at least three genes are involved in this phenomenon (Fig. S6 A and B): The SSP-gla gene, which acts in a sporophytic manner, and the OgTPR1 gene cooperate as a killer, and another gene(s) in the S1 locus for factor(s) corresponding to the responder or protector controls the selective dysfunction of gametes in hybrids. Interestingly, both the SSP-gla and the OgTPR1 genes encode peptidase domain-containing proteins, suggesting that genes related to protein degradation are involved in the killer function, not only in the case of the S5 locus in intraspecific hybrid sterility but also in interspecific hybrid sterility.
Our expression analysis showed the coexpression of SSP and OgTPR1 in developing gametophytes, whereas their expression patterns in other tissues are not identical. These results suggest that SSP and OgTPR1 genes may collaboratively act in specific tissues and developmental stages including developing gametophytes. Although there is no experimental support, Xie et al. (22) suggested that OgTPR1 was involved either in the activation of certain precursor molecules via the peptidase domains or in the ribosome assembly through the RRS domain. It can be hypothesized that the SSP gene may also be involved in the activation of the precursor together with the OgTPR1 or in the activation of the OgTPR1 protein by the peptidase domain. Identification of all the other causative factor(s) in the S1 locus might provide mechanistic insights regarding the protein interactions involved in the S1 locus-mediated hybrid sterility.
If hybrid sterility induced by the S1 locus fits the killer–receptor or killer–protector models, its evolutionary process may be explained by a model similar to the BDM model. Assuming that the killer and receptor arose separately and were fixed in two divergent lineages or that the killer and protector were fixed in one of two divergent lineages, hybrid sterility could evolve without having a deleterious effect in each lineage. One of significant features of our results is that the SSP gene is not found in one of the two diverged lineages (i.e., the Asian rice gene pool). Thus, the present results broaden our understanding of the BDM-type hybrid incompatibility: Hybrid incompatibility can occur as a consequence of a lineage-specific gene acquisition or loss that generated the gene-presence/absence polymorphism between the two lineages (Fig. S6 C and D). Interestingly, in the genus Oryza, we did not find any genes homologous to SSP except for three species. If the SSP gene exists only in limited species in the genus Oryza, our results lead to a hypothesis that the species-specific gene is involved in a reproductive barrier between species.
In plants, neutral alleles have frequently been reported for intra- but not for interspecific hybrid sterility loci (Table S1); thus, further knowledge regarding the neutral allele in the latter locus is required. In the present study, we used the S1 locus as a model system and created an artificial neutral allele by disrupting one of the causative genes of hybrid sterility through forward genetic screening of fertile hybrids. In the case of the S1 locus, the causal gene, SSP, was not found and annotated in the reference genome of rice (cv. Nipponbare). In general, there are numerous hybrid sterility loci that have not been annotated and characterized in crop gene pools. In such a case, a forward genetic screening is more practical than approaches for which gene identification and characterization are prerequisite (e.g., genome editing) for creating neutral alleles. Thus, the present study demonstrated the feasibility of an approach that allows broader access to desirable traits in distantly related species during crop breeding.
Materials and Methods
Pehkuh (an indica type of O. sativa, denoted “Acc108”), Taichung 65 (a japonica type of O. sativa, denoted “T65”) and three NILs (Acc108S1, T65wx, and T65S1) were used. T65wx contains the wx allele at the Wx locus. Acc108S1 and T65S1 have the chromosomal segment proximate to the S1 region, introduced from a strain of O. glaberrima (W025 from Guinea). Acc108, T65, and T65wx contain the S1s allele, whereas Acc108S1 and T65S1 contain the S1g allele at the S1 locus.
Heavy-ion beam irradiation was carried out at the RIKEN RI-beam factory, Wako, Japan. Mutant screening was carried out in the experimental field at the Japan International Research Center for Agricultural Sciences, Tsukuba, Japan, and at the experimental farm at Kyoto University, Kyoto, Japan. Detailed procedures of cytological observation, gene mapping, sequencing analysis, expression analysis, in situ hybridizations, transformation analysis, and gene distribution analysis are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Y. Sano for invaluable advice, encouragement, and the gift of rice materials; Drs. E. Coen, Y. Kishima, I. Takamure, T. Tagami, K. Matsubara, N. Uwatoko, R. Ishikawa, T. Ishii, D. Fujita, M. Obara, S. Teramoto, T. Yokoo, and T. Tanisaka for their suggestions and encouragement; Drs. D. Bradley and C. Whitewoods for comments on the manuscript; Mr. I. Masuda for technical assistance; and Dr. T. Nakagawa for providing the pGWB1 vector. Some of the wild rice accessions were provided by the National Institute of Genetics supported by the National Bioresource Project of Agency for Medical Research and Development, Japan. Computations were partially performed on the National Institute of Genetics supercomputer at the Research Organization of Information and Systems, National Institute of Genetics. This work was partly supported by Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research JP25892014, JP26850003, and JP16KT0034 (to Y. Koide), Takano Life Science Research Foundation (Y. Koide), The Hakubi Project coordinated by the Hakubi Center for Advanced Research, Kyoto University (Y. Koide), the Program for Fostering Researchers for the Next Generation conducted by Consortium Office for Fostering of Researchers in Future Generations, Hokkaido University (Y. Koide), and Japan International Research Center for Agricultural Sciences research project “Rice Innovation for Environmentally Sustainable Production Systems” from 2011–2015.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Sequencing data have been deposited in the DNA Data Bank of Japan (DDBJ) (accession nos. DRA006416 to DRA006419).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1711656115/-/DCSupplemental.
References
- 1.Coyne JA, Orr HA. Speciation. Sinauer Associates; Sunderland, MA: 2004. [Google Scholar]
- 2.Maheshwari S, Barbash DA. The genetics of hybrid incompatibilities. Annu Rev Genet. 2011;45:331–355. doi: 10.1146/annurev-genet-110410-132514. [DOI] [PubMed] [Google Scholar]
- 3.Ding J, et al. Highly asymmetric rice genomes. BMC Genomics. 2007;8:154. doi: 10.1186/1471-2164-8-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marroni F, Pinosio S, Morgante M. Structural variation and genome complexity: Is dispensable really dispensable? Curr Opin Plant Biol. 2014;18:31–36. doi: 10.1016/j.pbi.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Wissler L, Gadau J, Simola DF, Helmkampf M, Bornberg-Bauer E. Mechanisms and dynamics of orphan gene emergence in insect genomes. Genome Biol Evol. 2013;5:439–455. doi: 10.1093/gbe/evt009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oka HI. Origin of Cultivated Rice. Elsevier; Amsterdam: 1988. [Google Scholar]
- 7.Jones MP, Semon M, Aluko K. Diversity and potential of Oryza glaberrima Steud in upland rice breeding. Breed Sci. 1997;47:395–398. [Google Scholar]
- 8.Li XM, et al. Natural alleles of a proteasome α2 subunit gene contribute to thermotolerance and adaptation of African rice. Nat Genet. 2015;47:827–833. doi: 10.1038/ng.3305. [DOI] [PubMed] [Google Scholar]
- 9.Jones MP, Dingkuhn M, Aluko GK, Semon M. Interspecific Oryza sativa L. X O. glaberrima Steud. progenies in upland rice improvement. Euphytica. 1997;92:237–246. [Google Scholar]
- 10.Ghesquière A, Séquier J, Second G, Lorieux M. First steps towards a rational use of African rice, Oryza glaberrima, in rice breeding through a ‘contig line’ concept. Euphytica. 1997;96:31–39. [Google Scholar]
- 11.Sano Y, Chu YE, Oka HI. Genetic studies of speciation in cultivated rice, 1. Genic analysis for the F1 sterility between O. sativa L. and O. glaberrima Steud. Jpn J Genet. 1979;54:121–132. [Google Scholar]
- 12.Sano Y. The genic nature of gamete eliminator in rice. Genetics. 1990;125:183–191. doi: 10.1093/genetics/125.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garavito A, et al. A genetic model for the female sterility barrier between Asian and African cultivated rice species. Genetics. 2010;185:1425–1440. doi: 10.1534/genetics.110.116772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou J, et al. Genetic dissection of a chromosomal region conferring hybrid sterility using multi-donors from Oryza glaberrima. Euphytica. 2010;175:395–407. [Google Scholar]
- 15.Long Y, et al. Hybrid male sterility in rice controlled by interaction between divergent alleles of two adjacent genes. Proc Natl Acad Sci USA. 2008;105:18871–18876. doi: 10.1073/pnas.0810108105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mizuta Y, Harushima Y, Kurata N. Rice pollen hybrid incompatibility caused by reciprocal gene loss of duplicated genes. Proc Natl Acad Sci USA. 2010;107:20417–20422. doi: 10.1073/pnas.1003124107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamagata Y, et al. Mitochondrial gene in the nuclear genome induces reproductive barrier in rice. Proc Natl Acad Sci USA. 2010;107:1494–1499. doi: 10.1073/pnas.0908283107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang J, et al. A killer-protector system regulates both hybrid sterility and segregation distortion in rice. Science. 2012;337:1336–1340. doi: 10.1126/science.1223702. [DOI] [PubMed] [Google Scholar]
- 19.Kubo T, Takashi T, Ashikari M, Yoshimura A, Kurata N. Two tightly linked genes at the hsa1 locus cause both F1 and F2 hybrid sterility in rice. Mol Plant. 2016;9:221–232. doi: 10.1016/j.molp.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 20.Wang GW, He YQ, Xu CG, Zhang Q. Identification and confirmation of three neutral alleles conferring wide compatibility in inter-subspecific hybrids of rice (Oryza sativa L.) using near-isogenic lines. Theor Appl Genet. 2005;111:702–710. doi: 10.1007/s00122-005-2055-z. [DOI] [PubMed] [Google Scholar]
- 21.Shen R, et al. Genomic structural variation-mediated allelic suppression causes hybrid male sterility in rice. Nat Commun. 2017;8:1310. doi: 10.1038/s41467-017-01400-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie Y, et al. Interspecific hybrid sterility in rice is mediated by OgTPR1 at the S1 locus encoding a peptidase-like protein. Mol Plant. 2017;10:1137–1140. doi: 10.1016/j.molp.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Xie Y, et al. Suppression or knockout of SaF/SaM overcomes the Sa-mediated hybrid male sterility in rice. J Integr Plant Biol. 2017;59:669–679. doi: 10.1111/jipb.12564. [DOI] [PubMed] [Google Scholar]
- 24.Doi K, Yasui H, Yoshimura A. Genetic variation in rice. Curr Opin Plant Biol. 2008;11:144–148. doi: 10.1016/j.pbi.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Li J, et al. Identification of four genes for stable hybrid sterility and an epistatic QTL from a cross between Oryza sativa and Oryza glaberrima. Euphytica. 2008;164:699–708. [Google Scholar]
- 26.Shen Y, et al. Fine mapping of S37, a locus responsible for pollen and embryo sac sterility in hybrids between Oryza sativa L. and O. glaberrima Steud. Plant Cell Rep. 2015;34:1885–1897. doi: 10.1007/s00299-015-1835-4. [DOI] [PubMed] [Google Scholar]
- 27.Koide Y, et al. Sex-independent transmission ratio distortion system responsible for reproductive barriers between Asian and African rice species. New Phytol. 2008;179:888–900. doi: 10.1111/j.1469-8137.2008.02490.x. [DOI] [PubMed] [Google Scholar]
- 28.Kusano A, Staber C, Chan HY, Ganetzky B. Closing the (Ran)GAP on segregation distortion in Drosophila. BioEssays. 2003;25:108–115. doi: 10.1002/bies.10222. [DOI] [PubMed] [Google Scholar]
- 29.Bauer H, Willert J, Koschorz B, Herrmann BG. The t complex-encoded GTPase-activating protein Tagap1 acts as a transmission ratio distorter in mice. Nat Genet. 2005;37:969–973. doi: 10.1038/ng1617. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.