Significance
Locus coeruleus (LC) integrity in cognitively normal older adults is a potentially important preclinical marker in dementia. Our study establishes a link between variability in LC integrity and cognitive decline related to noradrenergic modulation in old age. We find that in older adults, reduced LC integrity explains lower memory performance. This effect was more pronounced for memory related to negative events, and accompanied by increased pupil diameter size in response to negative events. The study provides a strong motivation for future research investigating the role of LC integrity in healthy, as well as in pathological, aging.
Keywords: locus coeruleus, aging, noradrenaline, episodic memory, pupillometry
Abstract
The locus coeruleus (LC) is the principal origin of noradrenaline in the brain. LC integrity varies considerably across healthy older individuals, and is suggested to contribute to altered cognitive functions in aging. Here we test this hypothesis using an incidental memory task that is known to be susceptible to noradrenergic modulation. We used MRI neuromelanin (NM) imaging to assess LC structural integrity and pupillometry as a putative index of LC activation in both younger and older adults. We show that older adults with reduced structural LC integrity show poorer subsequent memory. This effect is more pronounced for emotionally negative events, in accord with a greater role for noradrenergic modulation in encoding salient or aversive events. In addition, we found that salient stimuli led to greater pupil diameters, consistent with increased LC activation during the encoding of such events. Our study presents novel evidence that a decrement in noradrenergic modulation impacts on specific components of cognition in healthy older adults. The findings provide a strong motivation for further investigation of the effects of altered LC integrity in pathological aging.
Postmortem histology of the human brain shows that cell density in the locus coeruleus (LC) can vary substantially between nondemented older individuals (1). Earlier postmortem studies even reported a 20 to 40% total cell loss by the age of 60 compared with younger adults (2, 3). Furthermore, reduced LC integrity, and the related decline in noradrenergic modulation, compared with younger adults in their 20s, is suggested to contribute to altered cognitive functions during healthy aging (4, 5). Although a prevalent hypothesis, there is little direct evidence to support this notion in humans.
Recent studies suggest that in vivo measures of LC integrity, using MRI, have the potential to serve as a biomarker for presymptomatic stages of Alzheimer’s disease (1). Cell loss in the LC is already observable in early stages of tau pathology before cognitive symptoms reach clinically relevant stages (1, 6). An important question to ask is how variability in LC integrity relates to interindividual variability in cognition in otherwise cognitively normal older adults.
An indirect structural MRI measure of LC integrity can be attained by exploiting the magnetic properties of neuromelanin (NM), a byproduct of noradrenaline synthesis (7–9). NM accumulates in noradrenergic LC cells as we get older (9–11). Postmortem studies suggest that the lifespan pattern of this accumulation is nonlinear (12, 13). However, the NM content per cell appears to remain stable between 50 and 80 y of age (12) and only starts to decline by the ninth decade (12). This suggests that between 50 and 80 y of age, the overall NM signal in MRI [T1-weighted (T1w) scans] may covary with the number of LC neurons (12). Consequently, a positive relationship between NM signal intensity and cognitive function in this age range would support a hypothesis that LC integrity explains interindividual differences in cognition. In contrast to older adults, the NM signal in younger adults likely reflects a moderate accumulation of NM in some of the LC cells, with no significant implications for function.
To assess the cognitive impact of altered noradrenergic modulation, we focused on the episodic memory encoding of negative emotional events. In emotional memory encoding, phasic burst firing in the LC is thought to modulate long-lasting synaptic plasticity in the medial temporal lobe, resulting in improved memory (14, 15). Compatible with this memory-promoting effect of LC activation, memory enhancement for emotional items can be attenuated by the beta-adrenergic antagonist propanolol (16, 17). The proposed role for the LC in memory encoding suggests that an altered LC integrity might contribute to altered memory performance in healthy older adults. Indeed, a recent study in rats showed that, in older animals, a deficit in negative emotional memory formation is accompanied by reduced levels of extracellular noradrenaline, and that this effect can be reversed by administering noradrenaline or by blockade of noradrenaline reuptake (18). The examination of this hypothesis in healthy older adults, however, is still in its infancy (19).
Here we tested this hypothesis by examining the relationship between neuromelanin signal-based imaging of the LC, pupil responses, and memory performance in healthy younger and older adults. Participants made choice judgments to novel indoor and outdoor scene stimuli and received negative or positive feedback, where outcome contingencies underwent unpredictable reversals (Fig. 1). We investigated the effect of negative feedback (negative emotional events) and reversals (unexpected events) on pupil responses and incidental memory for scene stimuli. We included reversals as an experimental manipulation, since a growing literature suggests that phasic LC responses support shifts in behavioral goals or attentional sets (20, 21). In vivo recordings in monkeys showed that increased firing of noradrenergic neurons in the LC is reflected in larger pupil diameters (22, 23). We thus expected a greater pupil diameter size in response to negative feedback, as well as to feedback indicating a reversal, in conjunction with a modulation of memory for associated novel images. Our core hypothesis was that interindividual differences in LC integrity are predictive of interindividual differences in incidental memory for scenes in older adults.
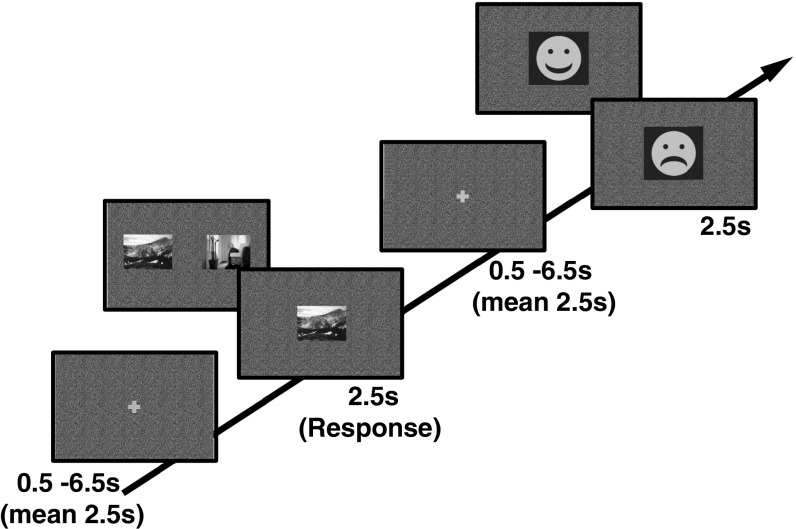
Fig. 1.
Reversal learning task. Subjects saw a mixture of forced and free choice trials involving pictures that were associated with rewards. On forced (single-picture) trials, they only saw one picture in the middle of the screen, and had to choose it by pressing either the left or right button. They were then rewarded according to the current contingency for the relevant picture. On free choice (two-picture) trials, they saw two pictures, one indoor and one outdoor, and pressed the button according to the side of the picture they preferred. Presentation of indoor or outdoor pictures was randomized for the left and right sides of the display for free choice trials. In both trial types, a smiling face indicated a reward and a sad face indicated a loss.
Results
Memory Performance.
The reversal learning task (Fig. 1) required learning from forced and free choice trials whether indoor or outdoor scenes are rewarded. The rewarded stimulus category alternated approximately every 20 trials throughout the task without warning. Rewards were administered deterministically for every correct choice of indoor or outdoor stimulus (that is, choosing the indoor stimulus when indoor stimuli are rewarded resulted in a gain, and choosing the outdoor stimulus when indoor stimuli are rewarded resulted in a loss). Half of the trials were forced trials (cf. Fig. 1), with only one stimulus (indoor or outdoor) which could be selected with either a left or right button press. As this picture could be the currently rewarded or unrewarded type of scene, forced trials allowed us to increase the number of losses that subjects experienced and to reduce a correlation between informativeness and valence. Reversal trials were defined as trials which contained the first feedback on a new reversal condition (e.g., feedback for the first indoor trial following an outdoor condition). On average across subjects, 13.8 choice trials resulted in a loss and 120.04 choice trials resulted in a gain, whereas 66.26 forced trials resulted in a loss and 60.64 forced trials resulted in a gain.
Early and late recognition tests required identifying old scene stimuli presented in the reversal learning task among new foil scene stimuli. Early recognition was tested 1 h after scanning, and late recognition was tested between 4 and 6 h after scanning. Old pictures from the reversal task were split across these two recognition tests. Performance on the reversal learning task was overall high and did not differ between age groups [mean accuracy younger adults, 89%; mean accuracy older adults, 90%; t(1,48) = −0.38, P = 0.70; note that reversal and single-picture trials were excluded from accuracy assessment].
Both age groups showed incidental memory formation for the scene stimuli used in the reversal learning task [mean hit-FA (false alarm) across both tests and age groups 19%, mean hits 50%, mean FA 32%, main effect hit > false alarms F(1,48) = 289.84, P < 0.05, residual Intraclass Correlation Coefficient (rICC) = 0.89]. However, we observed neither age-related differences in memory [F(1,48) = 0.26, P = 0.61] nor the expected decline in memory performance between early and late recognition tests. Instead, memory performance was increased on delayed tests in both age groups [F(1,48) = 20.79, P < 0.05, rICC = 0.55]. This effect was not due to increased hit rates on delayed tests [F(1,48) = 0.96, P = 0.33] but was instead driven by participants making more false alarms on the early recognition test [F(1,48) = 29.18, P < 0.05, rICC = 0.61]. It is conceivable that weaker memory traces, which were still present on the immediate memory test, caused more interference with the new foil scene stimuli and resulted in more false positives on immediate recognition (24). Alternatively, some form of reorganization may have occurred during the intervening time, restricting overgeneralization to new stimuli after 4 to 6 h. In what follows, we consequently report memory performance aggregated across the two recognition tests and in all analyses control for an influence of interindividual differences in false alarms on the first, compared with the second, recognition test by including this difference as a regressor of no interest.
Noradrenaline is thought to support increased memory for more noticeable or alerting events, often entailed by negative emotional stimuli (15, 25, 26). In our task, these noticeable events were negative outcomes in the reversal learning task. Negative outcomes occurred on average in 33% of trials. There was no age difference in overall frequency of negative feedback [t(1,48) = −1.18, P = 0.24], partly because 74% of these events occurred unavoidably on single-picture trials which old and young experienced equally frequently. We investigated whether incidental memory in the vicinity of such negative events is increased. Specifically, we examined incidental memory for stimuli immediately before losses or gains, in other words scenes that were followed by a gain or loss within the same trial.
In both age groups, we observed increased memory in particular for scene stimuli that occurred on trials with loss feedback compared with gain feedback [F(1,48) = 12.86, P < 0.05, rICC = 0.50] (Fig. 2A), an effect that did not differ between early and delayed recognition tests [F(1,48) = 2.06, P = 0.16; repeated measures ANOVA age group x immediate versus delayed tests x gain versus loss effect]. Note that the effect of better memory for stimuli before losses was also present for trials with single pictures alone [F(1,48) = 8.83, P < 0.05, rICC = 0.43, repeated measures ANOVA age group x gain versus loss effect for forced trials only]. This suggests that the memory effect was not driven by the fact that losses occurred relatively more frequently on trials with single-scene stimuli compared with trials with two scene stimuli. Note, however, that a separate assessment of memory performance for losses obtained after errors on free choice trials as well as losses on reversal trials was not possible due to insufficient trial numbers (23 and 14 trials on average, respectively). Similarly, no effect of reversal trials on subsequent memory was observed (SI Results), which may have been due to an insufficient number of trials (40 reversals altogether). Finally, analyses using multiple regression confirmed that the increased memory for scene stimuli occurred before losses on the same trial, controlling for different trial types (i.e., free choice and reversal trials; Fig. 2F). Regression analyses were performed for all trials and on forced trials alone (Fig. 2 and Fig. S1). This stronger effect of incidental memory for stimuli occurring before losses is in line with evidence showing an effect of incidental memory for stimuli which have been in the focus of attention before emotionally arousing events (29).
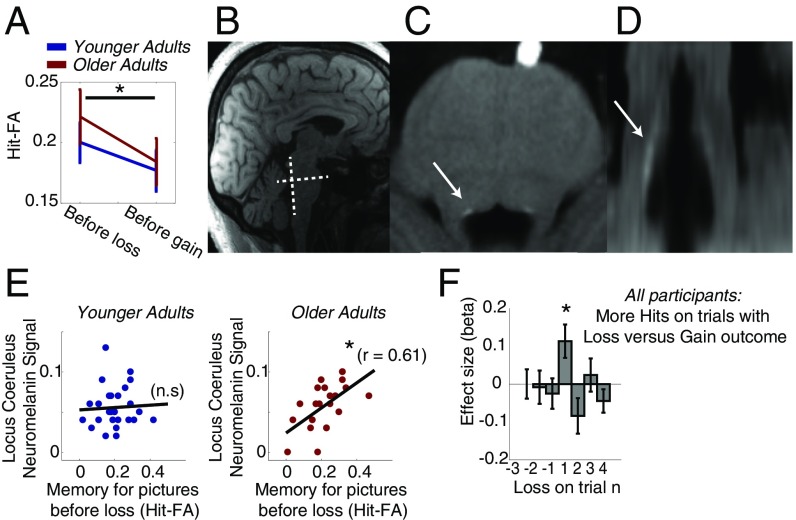
Fig. 2.
Emotional memory effects and their correlation with NM signal intensity in the LC. (A) Both age groups show better incidental emotional memory for scene stimuli that appeared before losses in the reversal learning task. (B) Illustration of the slice orientation perpendicular to the dorsal pons for LC NM imaging. (C and D) Example of an NM-sensitive image of one older adult in axial and coronal views, respectively, of the slice selection illustrated in B. The LC is evident as a hyperintense area at the dorsal border of the pons, just above the fourth ventricle (arrows). Note: Anisotropic voxel sizes (0.4 × 0.4 × 3.0 mm) result in lower resolution in the coronal view in D. (Voxel resolution is upsampled to isotropic 0.4 mm for the purpose of display.) (E) Better memory for scene stimuli before losses was observed in individuals with larger NM signals in the LC. However, this was only the case in the group of older adults. This suggests a positive link between LC integrity and emotional memory capacity in older adults. (F) Effect sizes of predicting memory success based on receiving a gain or a loss feedback on current, preceding, and following trials. Only losses on the current trial (n = 1) were predictive of memory success. Significant effects were determined via permutation tests. Error bars show SEM. n.s, not significant. *P < 0.05.
Neuromelanin-Sensitive Scans.
LC integrity was assessed as signal intensity in T1-weighted imaging, using a ratio score of mean T1w signal in the LC and mean T1w signal (Fig. 2 and Fig. S2) in a control area in the pons as previously described (10, 27). We observed no age differences in mean NM signal intensity [t(1,48) = 0.39, P = 0.71] or LC volume [t(1,48) = −1.07, P = 0.30]. However, as outlined above, the number of LC cells with NM deposits is likely to differ between age groups, making interpretation of age group differences in NM signal in terms of LC integrity difficult. Still, in older age, the majority of LC cells can be assumed to contain NM and, on this basis, we expect higher NM values in older adults to be a marker of greater LC integrity.
Indeed, within the age group of older adults, we observed that participants with higher NM-related signal intensity in the LC showed better memory overall [older adults: r = 0.44, P < 0.05; younger adults: r = −0.01, P = 0.95, reliable age difference based on permutation tests (2.5 percentile in older adults = 0.33), memory measured as mean of hit-FA across both recognition tests]. Moreover, in line with evidence that noradrenaline is especially relevant for the encoding of negative events, the link to LC integrity in older adults was particularly pronounced for stimuli before salient negative events [Fig. 2E; older adults: r = 0.61, P < 0.05; younger adults: r = 0.06, P = 0.78; older adults for stimuli before gains: r = 0.22, P = 0.34, reliable condition and age difference based on permutation tests (2.5 percentile in older adults for losses = 0.50)]. This was the case for memory before losses on the first memory test (r = 0.46, P < 0.05) as well as the second memory test (r = 0.52, P < 0.05) in older adults. No reliable correlations with NM signal intensity and first or second test memory performance were observed in younger adults or in older adults for stimuli before gains. The observed link between memory for stimuli before salient negative events and NM signal intensity within older adults survived controlling for multiple comparisons (eight correlations across age groups, feedback valence, and scene position). It was also robust to controlling for interindividual differences in age, reversal task performance, and difference in false alarms between first and second recognition tests (partial correlation, r = 0.47, P < 0.05). This suggests that the observed correlation is not due to a mediating effect of confounding interindividual variance within the group of older adults or interindividual differences in the overall frequency of negative feedback in the incidental encoding task.
Finally, negative feedback on forced trials might be considered to provide a less confounded assessment of the impact of salient or negative feedback since the frequency of gains and losses was more balanced on these trials and was less dependent on the correctness of performance. We therefore also examined the relationship between NM signal intensity and memory performance on just forced trials. These analyses confirmed the pattern of results observed on all trials (SI Results).
Taken together, we observed that NM signal intensity as a signature of LC integrity explains interindividual differences in memory performance in older adults. Moreover, this link is more pronounced for emotionally negative salient events, in keeping with an assumed greater importance of the LC for incidental encoding of memories related to negative events.
Changes in Pupil Diameter.
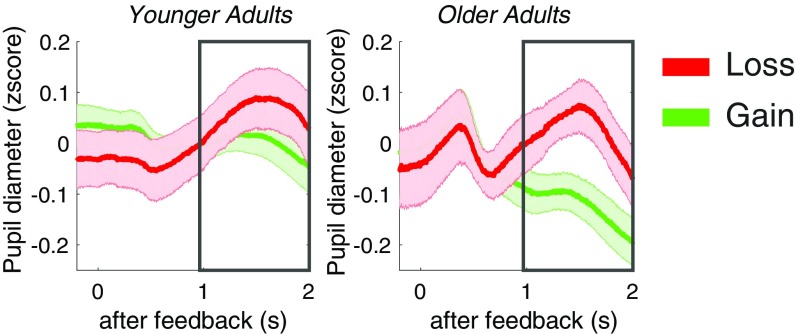
Increased firing of LC noradrenergic neurons is reflected in larger pupil diameters (22, 23). Given that we would expect a stronger LC response to negative events (18, 21), we expected to see increased pupil diameter during loss feedback. Indeed, pupil diameters were larger during loss compared with gain feedback [Fig. 3; main effect loss > gain in two-way MANOVA loss versus gain and younger versus older adults, F(1,40) = 4.35, P < 0.05, rICC = 0.31; no age interaction or main effect of age]. However, we only observed a trend for a relationship between pupil diameters and NM signal intensity, with pupil diameters to losses being larger in those older adults with stronger NM signal intensity (r = 0.44, P = 0.08; see also SI Results and Fig. S3). Also, interindividual differences in pupil diameter to losses did not mediate the relationship between memory and NM signal intensity in older adults (SI Results). It should be noted that only a reduced sample (26 younger adults and 17 older adults) could be used to assess pupil-related effects due to noisy pupil recordings in the scanner (of these, one younger and one older adult did not have sufficient trials to examine reversal effects, and one older adult did not have enough trials to examine loss effects). It is likely that we lack power to detect reliable effects related to interindividual differences in pupil diameter.
Fig. 3.
Pupil diameters to loss and gain feedback. Increased pupil diameters were observed during loss feedback.
Discussion
We show that NM signal intensity in the LC explains variance in incidental memory for novel items in older adults. Moreover, this link is more pronounced for novel items occurring before emotionally negative compared with positive events. This specific relationship is in line with animal studies showing that the LC supports encoding of memories related to negative or salient events (15, 18, 28), as well as evidence for an effect of incidental memory for stimuli that have been in the focus of attention before emotionally negative events (29). The present study provides an important missing link that ties together evidence on variability in LC integrity in older adults (1, 11, 27) with evidence from animal work showing that memory encoding in aging is affected by altered levels of noradrenaline during arousing negative events (18).
What is the source of the linked variability between LC NM signal and memory performance in old age? In principle, three sources for variability in the LC NM signal in older adults are conceivable: (i) Variability could be reflective of interindividual variability that is age-independent but not accessible in younger adults given the lack of LC NM intensity. (ii) Variability could be due to age-related loss of LC neurons during healthy aging. Indeed, earlier postmortem studies suggested a linear decline in LC neuron numbers with aging (2, 3, 12). However, recent postmortem studies assessing tau pathology, a marker of pathological aging, in addition to neuronal number, suggest that the observed age-related decline in LC neuron numbers might in part reflect a decline related to early stages of preclinical tau pathology (1). (iii) Reduced NM signal in cognitively normal older adults could, in part, encompass subjects at a preclinical stage of Alzheimer’s disease impacting on LC integrity. This in turn might affect subtle aspects of cognitive function even though this is not yet expressed at clinically relevant levels (6). Indeed, postmortem data show that LC cell numbers are reduced in the early phases of Alzheimer’s disease and decline further as the disease progresses (1, 30). In vivo imaging studies using radioactive ligands that bind to tau protein show that cognitive impairment is preclinical and subtle in Braak stages I and II (31). The observed link between variability in LC NM signal and variability in memory performance in older adults might thus express variability in LC integrity that occurs in healthy aging, or a variability in LC integrity indicative of preclinical dementia. Note that our study did not include an extensive cognitive or neurological assessment which would allow us to examine this possibility in greater depth. To resolve this question, it is necessary to combine molecular imaging and histological validation to further assess the link between LC NM signal intensity, LC neural cell counts, and LC tau pathology. Our findings of a linked variability between the LC NM signal and cognition in vivo provide a strong motivation to conduct such studies.
We did not observe differences between age groups in LC volume or LC NM signal intensity as evident in a ratio score of T1-weighted signal intensity. Nevertheless, our finding that NM signal explains variance in memory performance in older, but not in younger, adults is unsurprising. Indeed, postmortem studies indicate that a link between NM signal intensity and cell density in the LC can be expected to be tighter in old age than in young adults because NM is more widely distributed among LC cells beyond the fifth decade (11, 12). Indeed, our study provides confirmation that the LC NM signal in old age may explain differences in memory performance. Furthermore, an accelerated decline in LC neuron numbers with Alzheimer’s and Parkinson’s diseases is well-documented (32). Reduced NM signal intensity has been reported in mild cognitive impairment, early Alzheimer’s, as well as Parkinson patients compared with healthy age-matched controls (10, 33). On this basis, neuromelanin imaging could provide an interesting investigative tool for populations where an accelerated decline in LC neuron numbers can be expected.
A large body of evidence supports the notion that noradrenergic modulation promotes the encoding of memories related to negative events (15, 34). We therefore hypothesized that variability in LC NM signal would relate to those aspects of memory formation that are known to depend on noradrenergic neuromodulation. This hypothesis was confirmed by our finding of improved memory for scenes related to negative events. Noradrenergic modulation of memory encoding likely takes place via projections of the LC to the amygdala as well as the hippocampus (34). Stimulation of the LC in rats induces long-term potentiation in the hippocampus (14), whereby increased levels of hippocampal noradrenaline facilitate the synaptic delivery of AMPA receptors necessary for long-term potentiation (35). Interestingly, case studies on lesions in humans suggest a double dissociation, with hippocampus lesions affecting declarative recall of fear conditioning and amygdala lesions affecting only the arousal response (36). It is conceivable that reciprocal connections of the LC and amygdala support an arousal response, while LC projections to the hippocampus might be relevant for the improved encoding of memories related to the arousing event (37, 38).
A sympathetic modulation of memories is likely to depend on inputs via paragigantocellularis neurons situated in the lateral part of the medulla into dorsolateral aspects of the LC, which in turn project to the hippocampus, amygdala, and septum (37, 39). However, the LC also receives afferent projections from the prefrontal cortex as well as amygdala (34, 40) which are suggested to mediate increased LC responses to stimuli considered relevant in a particular task context (21, 22). It is conceivable that sympathetic regulation underlying memory encoding in our task might also involve a top-down–mediated saliency of negative feedback stimuli (29). The noradrenergic system is known to also react to events that are salient because they are unexpected, given current outcome expectations (20, 41, 42). As our task included deterministic feedback, feedback on reversal trials and perhaps errors on free choice trials can be considered to represent unexpected outcomes. Indeed, as for negative feedback, we observed an increased pupil diameter on reversal trials (Fig. S2). However, their overall number was too small to investigate whether the expectedness of loss (or gain) outcomes affects incidental memory encoding differentially.
Although we observed the expected increase in memory for stimuli associated with negative emotional events in our incidental memory task, we did not find that this effect was reduced in older adults. It is possible that this reflects floor effects in our comparatively difficult memory task. As we aimed for sufficient power to compare effects of comparatively rare loss events, this entailed our recognition tests required assessing a large number of comparatively similar grayscale stimuli (Fig. 1). Indeed, the fact that we observed increased false alarm rates on early recognition tests suggests that participants were relying on weaker memory representations. This was especially so in the first recognition test and therefore resulted in more interference early after encoding (24).
Also, while variability in LC signal showed the expected relationship to memory performance in older adults, it is evident that other cognitive factors must also be considered to appreciate the full profile of memory performance in old age. It is, for instance, conceivable that a stronger top-down focus on salient events [e.g., top-down modulation of LC activity (21, 43)] in a memory episode in older adults might compensate for weaker memory representations and LC decline. We observed increased pupil diameters to loss and reversal stimuli in older adults, but not in younger adults, which might point to a stronger top-down focus on salient events in older adults. For the present study, however, the notion of a stronger top-down focus in older adults has to remain a tentative explanation, and other factors such as lack of recall tests (which have been reported to show increased effects of emotional encoding) have to be considered as well when trying to understand an absence of age group differences in emotional memory in our study.
In vivo recordings in monkeys as well as functional MRI studies in humans suggest a link between pupil diameter and functional LC activation (23, 44). Our study found mixed evidence for a link between pupil diameter and LC integrity. In line with reports of stronger LC responses to aversive events (18, 21) as well as the observed better memory for stimuli before losses, we found increased pupil diameter to loss compared with gain feedback. On the other hand, we did not observe a reliable link between interindividual differences in the size of the pupil diameter to losses and memory strength or LC integrity. However, we could only use data from a reduced sample to assess interindividual differences in pupil diameters. The smaller power in the resulting measurements might allow only for a reliable detection of large effect sizes related to interindividual differences in pupil diameter.
Nonetheless, given that the present study can only report correlational effects of LC NM signal intensity and memory performance, it remains an open question as to whether the correlation observed in our study is indicative of a causal relationship between functional LC activity and memory encoding or whether memory encoding and LC signal intensity are both caused by a common third factor [e.g., altered sleep quality or tonically altered levels of sympathetic arousal (45, 46)].
Finally, it should be noted that our study, as well as animal studies exploring the LC response to negative events (15, 18), cannot disambiguate whether negative events in our task were more salient because they were aversive in nature or because they attracted extra top-down saliency, which is known to engage the noradrenergic system (21). Indeed, incidental learning has also been found to be related to salient positive feedback (47). There are at least two potential factors contributing to top-down saliency of negative events in the context of our task. First, loss feedback could be more salient given that it was overall less common than gain feedback and therefore less expected. However, in vivo recordings show that this is not a sufficient condition for eliciting LC responses; nontarget stimuli in oddball tasks do not lead to phasic LC firing even if they are as rare as target stimuli. This suggests that a focus of attention based on a task set can overrule unexpectedness effects (48). Second, several studies have shown that focusing on loss feedback can be an adaptive strategy in reinforcement learning contexts (49, 50). It is thus conceivable that negative feedback was more salient, as it was a more relevant benchmark for the aim of achieving an overall correct performance (49).
In summary, our study was able to confirm the venerable but as of yet untested hypothesis that a lower LC integrity is related to lower memory performance in aging. By establishing this link, our study provides strong motivation to further explore the role of LC integrity in preclinical cognitive decline in pathological aging.
Methods
Participants.
Twenty-eight healthy younger adults (age range 20 to 31 y) and 22 healthy older adults (age range 65 to 84 y) were recruited via existing participant databases as well as through advertisements. All participants enrolled in the study are included in the reported sample. In recruitment, assessment of study eligibility was standardized in a telephone questionnaire and carried out by research assistants during recruitment and once more by radiographers in person before experimental testing. Volunteers unsuitable for scanning (e.g., metallic implants, claustrophobia) as well as volunteers with a history of neurological or psychiatric conditions were excluded. All subjects were right-handed (Oldfield questionnaire lateralization quotient >80) (51). A shortened version of Raven’s progressive matrices (52) was used to examine whether our sample is consistent with well-established markers of adult age differences in fluid intelligence (53). Performance was evaluated as the number of correctly solved matrices of the 18 given matrices within 20 min. Only 19 younger adults obtained a fluid intelligence measure due to changes in the test setup. However, as expected, younger adults performed better than older adults [t(1,39) = 3.59, P < 0.05], suggesting that our sample is consistent with known age differences in general cognitive abilities. The study was approved by the local ethics committee (University College London ethics reference no. 5506/001), and written consent was obtained from each participant before participation. Participants were paid 50 pounds for participation, including a bonus payment of 6 pounds that depended on task performance (all participants showed a high performance level and obtained the bonus payment).
Study Overview.
Participants completed three test sessions. The first session included functional midbrain MRI during a reversal task and pupillometric recordings as well as (quantitative) structural midbrain imaging (with eyes closed). For the purpose of the present study, only data acquired from structural MRI recordings, behavioral performance, as well as concurrent pupillometry data during the reversal task are reported. The second and third test sessions included early and delayed memory tests as well as covariate assessments, all of which were performed on the same day.
Task Design and Procedure.
Participants completed an extensive practice session before the task which included familiarization with the use of the button box. The reversal learning task (Fig. 1) required learning whether indoor or outdoor scenes are rewarded. The rewarded stimulus category alternated approximately every 20 trials throughout the task without warning. Rewards were administered deterministically for every correct choice of indoor or outdoor stimuli. Rewards (2 points) and losses (1 point) were indicated by smiling and frowning faces, respectively, after a choice was made. Rewards were larger than losses, as participants are generally loss-averse in tasks involving gains as well as losses (54). Missed responses were counted as a loss of 2 points. Participants were encouraged to collect as many points as possible. Reaching 20 points per scanner run was required to receive a bonus payment of 6 pounds. Participants were informed about the obtained points after each scanner run.
We aimed to examine subsequent memory effects of the indoor and outdoor stimuli related to positive and negative reinforcement. The cover task (reversal learning of indoor or outdoor choices) was therefore held comparatively simple to avoid age differences in attentional focus during incidental encoding. However, in simple reversal learning tasks, the valence of the feedback is often highly correlated with the expectedness or informativeness of the feedback. This is because losses can in general be avoided and occur only on the few reversal trials or error trials. To increase the number of losses and reduce the correlation of informativeness and valence, we therefore had equal numbers of forced and free choice trials. On a forced trial, only one picture was presented on the screen (Fig. 1). This single picture could be the currently rewarded or the currently unrewarded type of scene (50% of the single-picture trials, respectively). To balance gains and losses on reversals, reversal trials occurred more frequently on single-picture trials (66% of reversals were single-picture trials, which yielded 41% gains on reversals in younger adults, 44% gains in older adults, and no age differences in gain frequency on reversals [t(1,48) = −1.21, P = 0.23]). None of the participants reported to have noticed that reversals were more frequent on forced trials.
On free choice trials, participants had to press a button on an MRI-compatible button box to choose the scene stimuli that they expected to be rewarded via left or right button presses. Presentation sides of indoor and outdoor scenes were randomized across left and right sides of the screen. Participants used the outer buttons on a box with four buttons. On forced trials, participants were asked to press left or right buttons to respond to the single picture, and then experienced the gain or loss accordingly. Participants had to respond even for the scene type that was associated with a loss, as otherwise they would lose 2 points for missing the trial. On these trials, left or right button presses did not carry any significance. Participants were asked to balance responses between left and right buttons to keep conditions comparable. Our analyses showed that participants showed an overall tendency to respond with the right button on free choice trials (67% right responses in younger adults; 57% right responses in older adults). The observed link between emotional memory and NM signal intensity was robust to controlling for this tendency to respond right (younger adults: r = 0.05, P = 0.80; older adults: r = 0.56, P < 0.05). In total, losses occurred on 33% of the trials (no age differences in overall loss frequency [t(1,48) = −1.18, P = 0.24]). Seventy-four percent of these losses were obtained on single-picture trials.
Behavioral Tests After Scanning.
Following scanning, participants performed two unannounced memory tests, the first one immediately after functional and structural scans (early memory test, about 1 h after the incidental encoding task) and the second one between 4 and 6 h after the incidental encoding task (delayed memory test), each taking ∼35 min. In the memory test, participants were asked to classify stimuli as old or new and, if they chose old, they were asked to indicate whether they “remembered” (explicit information on episodic memory trace accessible) or “knew” (feeling of familiarity) the stimuli. On average, each recognition test included 50 new and 150 old stimuli (half indoor and half outdoor scenes, respectively). Then, participants were asked to indicate on a 6-point scale how certain they were in their respective responses (i.e., “new,” “remember,” “know”). Only stimuli that were chosen by participants in the reversal learning task (either on forced trials or free choice trials) were included in the recognition tests. Before the recognition test, participants were familiarized with the distinction between remembering and knowing stimuli. There was no response deadline on the recognition test, but participants were instructed to go with their first impression and not to overthink responses. Stimuli were presented on a laptop and responses were recorded via a keyboard.
Structural MRI Acquisition.
Scans were acquired on a 3T Tim Trio System (Siemens Healthcare) with a standard 32-channel radiofrequency (RF) coil used for signal reception and the body coil for transmission. Neuromelanin-sensitive scans were acquired with a 3D T1-weighted multiecho FLASH acquisition as part of a modified multiparameter mapping protocol (55). The voxel size (0.4 × 0.4 × 3 mm) was adjusted to optimize capture of the LC, and transverse planes were oriented perpendicular to the dorsal pons (cf. Fig. 2). Protocol parameters were as follows: Repetition time (TR), 27 ms; flip angle, 21°; field of view (FOV), 206 mm (anterior–posterior, generalized autocalibrating partially parallel acquisitions factor 2 with 40 integrated reference lines) × 180 mm (right–left) × 60 mm (head–foot, excluding 20% oversampling), slab-selective RF excitation, readout bandwidth (BW) 444 Hz per pixel. Echoes were acquired with alternating readout polarity at six equidistant echo times between 3.35 and 16.95 ms. To improve signal-to-noise ratio (SNR), this acquisition was repeated six times.
In addition, an isotropic T1-weighted FLASH acquisition was acquired to improve the assessment of inferior and superior boundaries of the LC. Scans were averaged across two repetitions to increase SNR [protocol parameters: voxel sizes (0.75 isotropic), TR, 20 ms; flip angle, 23°; echo time, 5.56 ms; FOV 240 × 240 × 64 mm3; slab-selective RF excitation, readout BW 130 Hz per pixel, partial Fourier factor 7/8 in each phase-encoded direction].
Supplementary Material
Acknowledgments
This work was supported by the German Academic Exchange Service (ID 91515956), the Human Brain Project (SP3 WP 3.3.1), and SFB (Sonderforschungsbereich) 779, Project A7.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1712268115/-/DCSupplemental.
References
- 1.Theofilas P, et al. Locus coeruleus volume and cell population changes during Alzheimer’s disease progression: A stereological study in human postmortem brains with potential implication for early-stage biomarker discovery. Alzheimers Dement. 2017;13:236–246. doi: 10.1016/j.jalz.2016.06.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.German DC, et al. The human locus coeruleus: Computer reconstruction of cellular distribution. J Neurosci. 1988;8:1776–1788. doi: 10.1523/JNEUROSCI.08-05-01776.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mann DM. The locus coeruleus and its possible role in ageing and degenerative disease of the human central nervous system. Mech Ageing Dev. 1983;23:73–94. doi: 10.1016/0047-6374(83)90100-8. [DOI] [PubMed] [Google Scholar]
- 4.Arnsten AF, Goldman-Rakic PS. Catecholamines and cognitive decline in aged nonhuman primates. Ann N Y Acad Sci. 1985;444:218–234. doi: 10.1111/j.1749-6632.1985.tb37592.x. [DOI] [PubMed] [Google Scholar]
- 5.Arnsten AFT, Goldman-Rakic PS. Alpha 2-adrenergic mechanisms in prefrontal cortex associated with cognitive decline in aged nonhuman primates. Science. 1985;230:1273–1276. doi: 10.1126/science.2999977. [DOI] [PubMed] [Google Scholar]
- 6.Mather M, Harley CW. The locus coeruleus: Essential for maintaining cognitive function and the aging brain. Trends Cogn Sci. 2016;20:214–226. doi: 10.1016/j.tics.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sasaki M, Shibata E, Kudo K, Tohyama K. Neuromelanin-sensitive MRI. Clin Neuroradiol. 2008;18:147–153. [Google Scholar]
- 8.Trujillo P, et al. Contrast mechanisms associated with neuromelanin-MRI. Magn Reson Med. 2017;78:1790–1800. doi: 10.1002/mrm.26584. [DOI] [PubMed] [Google Scholar]
- 9.Zecca L, et al. The role of iron and copper molecules in the neuronal vulnerability of locus coeruleus and substantia nigra during aging. Proc Natl Acad Sci USA. 2004;101:9843–9848. doi: 10.1073/pnas.0403495101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sasaki M, et al. Neuromelanin magnetic resonance imaging of locus ceruleus and substantia nigra in Parkinson’s disease. Neuroreport. 2006;17:1215–1218. doi: 10.1097/01.wnr.0000227984.84927.a7. [DOI] [PubMed] [Google Scholar]
- 11.Shibata E, et al. Age-related changes in locus ceruleus on neuromelanin magnetic resonance imaging at 3 tesla. Magn Reson Med Sci. 2006;5:197–200. doi: 10.2463/mrms.5.197. [DOI] [PubMed] [Google Scholar]
- 12.Manaye KF, McIntire DD, Mann DM, German DC. Locus coeruleus cell loss in the aging human brain: A non-random process. J Comp Neurol. 1995;358:79–87. doi: 10.1002/cne.903580105. [DOI] [PubMed] [Google Scholar]
- 13.Mann DM, Yates PO. Lipoprotein pigments—Their relationship to ageing in the human nervous system. II. The melanin content of pigmented nerve cells. Brain. 1974;97:489–498. doi: 10.1093/brain/97.1.489. [DOI] [PubMed] [Google Scholar]
- 14.Klukowski G, Harley CW. Locus coeruleus activation induces perforant path-evoked population spike potentiation in the dentate gyrus of awake rat. Exp Brain Res. 1994;102:165–170. doi: 10.1007/BF00232449. [DOI] [PubMed] [Google Scholar]
- 15.Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci. 2009;10:211–223. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- 16.Kroes MCW, Strange BA, Dolan RJ. Beta-adrenergic blockade during memory retrieval in humans evokes a sustained reduction of declarative emotional memory enhancement. J Neurosci. 2010;30:3959–3963. doi: 10.1523/JNEUROSCI.5469-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strange BA, Dolan RJ. Beta-adrenergic modulation of emotional memory-evoked human amygdala and hippocampal responses. Proc Natl Acad Sci USA. 2004;101:11454–11458. doi: 10.1073/pnas.0404282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo Y, et al. Reversal of aging-related emotional memory deficits by norepinephrine via regulating the stability of surface AMPA receptors. Aging Cell. 2015;14:170–179. doi: 10.1111/acel.12282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mammarella N, Di Domenico A, Palumbo R, Fairfield B. Noradrenergic modulation of emotional memory in aging. Ageing Res Rev. 2016;27:61–66. doi: 10.1016/j.arr.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Dayan P, Yu AJ. Phasic norepinephrine: A neural interrupt signal for unexpected events. Network. 2006;17:335–350. doi: 10.1080/09548980601004024. [DOI] [PubMed] [Google Scholar]
- 21.Sara SJ, Bouret S. Orienting and reorienting: The locus coeruleus mediates cognition through arousal. Neuron. 2012;76:130–141. doi: 10.1016/j.neuron.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 22.Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- 23.Joshi S, Li Y, Kalwani RM, Gold JI. Relationships between pupil diameter and neuronal activity in the locus coeruleus, colliculi, and cingulate cortex. Neuron. 2016;89:221–234. doi: 10.1016/j.neuron.2015.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brainerd CJ, Reyna VF. Fuzzy-trace theory and false memory. Curr Dir Psychol Sci. 2002;11:164–169. [Google Scholar]
- 25.Bradley MM, Greenwald MK, Petry MC, Lang PJ. Remembering pictures: Pleasure and arousal in memory. J Exp Psychol Learn Mem Cogn. 1992;18:379–390. doi: 10.1037//0278-7393.18.2.379. [DOI] [PubMed] [Google Scholar]
- 26.Wittmann BC, Dolan RJ, Düzel E. Behavioral specifications of reward-associated long-term memory enhancement in humans. Learn Mem. 2011;18:296–300. doi: 10.1101/lm.1996811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clewett DV, et al. Neuromelanin marks the spot: Identifying a locus coeruleus biomarker of cognitive reserve in healthy aging. Neurobiol Aging. 2016;37:117–126. doi: 10.1016/j.neurobiolaging.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chamberlain SR, Robbins TW. Noradrenergic modulation of cognition: Therapeutic implications. J Psychopharmacol. 2013;27:694–718. doi: 10.1177/0269881113480988. [DOI] [PubMed] [Google Scholar]
- 29.Sakaki M, Fryer K, Mather M. Emotion strengthens high-priority memory traces but weakens low-priority memory traces. Psychol Sci. 2014;25:387–395. doi: 10.1177/0956797613504784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braak H, Del Tredici K. The preclinical phase of the pathological process underlying sporadic Alzheimer’s disease. Brain. 2015;138:2814–2833. doi: 10.1093/brain/awv236. [DOI] [PubMed] [Google Scholar]
- 31.Schöll M, et al. PET imaging of tau deposition in the aging human brain. Neuron. 2016;89:971–982. doi: 10.1016/j.neuron.2016.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zarow C, Lyness SA, Mortimer JA, Chui HC. Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Arch Neurol. 2003;60:337–341. doi: 10.1001/archneur.60.3.337. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi J, et al. Detection of changes in the locus coeruleus in patients with mild cognitive impairment and Alzheimer’s disease: High-resolution fast spin-echo T1-weighted imaging. Geriatr Gerontol Int. 2015;15:334–340. doi: 10.1111/ggi.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: Modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- 35.Hu H, et al. Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell. 2007;131:160–173. doi: 10.1016/j.cell.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 36.Bechara A, et al. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995;269:1115–1118. doi: 10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- 37.Van Bockstaele EJ, Colago EE, Aicher S. Light and electron microscopic evidence for topographic and monosynaptic projections from neurons in the ventral medulla to noradrenergic dendrites in the rat locus coeruleus. Brain Res. 1998;784:123–138. doi: 10.1016/s0006-8993(97)01250-x. [DOI] [PubMed] [Google Scholar]
- 38.Van Bockstaele EJ, Chan J, Pickel VM. Input from central nucleus of the amygdala efferents to pericoerulear dendrites, some of which contain tyrosine hydroxylase immunoreactivity. J Neurosci Res. 1996;45:289–302. doi: 10.1002/(SICI)1097-4547(19960801)45:3<289::AID-JNR11>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 39.Loughlin SE, Foote SL, Bloom FE. Efferent projections of nucleus locus coeruleus: Topographic organization of cells of origin demonstrated by three-dimensional reconstruction. Neuroscience. 1986;18:291–306. doi: 10.1016/0306-4522(86)90155-7. [DOI] [PubMed] [Google Scholar]
- 40.Arnsten AF, Goldman-Rakic PS. Selective prefrontal cortical projections to the region of the locus coeruleus and raphe nuclei in the rhesus monkey. Brain Res. 1984;306:9–18. doi: 10.1016/0006-8993(84)90351-2. [DOI] [PubMed] [Google Scholar]
- 41.Bouret S, Sara SJ. Reward expectation, orientation of attention and locus coeruleus-medial frontal cortex interplay during learning. Eur J Neurosci. 2004;20:791–802. doi: 10.1111/j.1460-9568.2004.03526.x. [DOI] [PubMed] [Google Scholar]
- 42.Nassar MR, et al. Rational regulation of learning dynamics by pupil-linked arousal systems. Nat Neurosci. 2012;15:1040–1046. doi: 10.1038/nn.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mather M, Clewett D, Sakaki M, Harley CW. Norepinephrine ignites local hot spots of neuronal excitation: How arousal amplifies selectivity in perception and memory. Behav Brain Sci. 2015;1:1–100. doi: 10.1017/S0140525X15000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murphy PR, Robertson IH, Balsters JH, O’Connell RG. Pupillometry and P3 index the locus coeruleus-noradrenergic arousal function in humans. Psychophysiology. 2011;48:1532–1543. doi: 10.1111/j.1469-8986.2011.01226.x. [DOI] [PubMed] [Google Scholar]
- 45.Mattis J, Sehgal A. Circadian rhythms, sleep, and disorders of aging. Trends Endocrinol Metab. 2016;27:192–203. doi: 10.1016/j.tem.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mather M, et al. Higher locus coeruleus MRI contrast is associated with lower parasympathetic influence over heart rate variability. Neuroimage. 2017;150:329–335. doi: 10.1016/j.neuroimage.2017.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mather M, Schoeke A. Positive outcomes enhance incidental learning for both younger and older adults. Front Neurosci. 2011;5:129. doi: 10.3389/fnins.2011.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aston-Jones G, Rajkowski J, Kubiak P, Alexinsky T. Locus coeruleus neurons in monkey are selectively activated by attended cues in a vigilance task. J Neurosci. 1994;14:4467–4480. doi: 10.1523/JNEUROSCI.14-07-04467.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hämmerer D, Eppinger B. Dopaminergic and prefrontal contributions to reward-based learning and outcome monitoring during child development and aging. Dev Psychol. 2012;48:862–874. doi: 10.1037/a0027342. [DOI] [PubMed] [Google Scholar]
- 50.Hämmerer D, Li S-C, Müller V, Lindenberger U. Life span differences in electrophysiological correlates of monitoring gains and losses during probabilistic reinforcement learning. J Cogn Neurosci. 2011;23:579–592. doi: 10.1162/jocn.2010.21475. [DOI] [PubMed] [Google Scholar]
- 51.Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 52.Raven JC, Court JH. Raven’s Progressive Matrices and Vocabulary Scales. Oxford Psychologists Press; Oxford: 1998. [Google Scholar]
- 53.Li S-C, et al. Transformations in the couplings among intellectual abilities and constituent cognitive processes across the life span. Psychol Sci. 2004;15:155–163. doi: 10.1111/j.0956-7976.2004.01503003.x. [DOI] [PubMed] [Google Scholar]
- 54.McGraw AP, Larsen JT, Kahneman D, Schkade D. Comparing gains and losses. Psychol Sci. 2010;21:1438–1445. doi: 10.1177/0956797610381504. [DOI] [PubMed] [Google Scholar]
- 55.Weiskopf N, et al. Quantitative multi-parameter mapping of R1, PD(*), MT, and R2(*) at 3T: A multi-center validation. Front Neurosci. 2013;7:95. doi: 10.3389/fnins.2013.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.