Significance
Activation of T cells via their antigen receptor (TCR) and the formation of a multimolecular signaling complex in or near the plasma membrane is a key initial event in the generation of an immune response. Here we find that p38, a serine/threonine kinase activated by TCR signaling, in turn phosphorylates its upstream tyrosine kinase ZAP-70. This results in a decrease in the size and longevity of the TCR signaling complex, limiting T cell effector responses. Therefore, cross-talk between an upstream and downstream kinase in the signaling complex itself generates a negative feedback loop that may limit excessive T cell responses.
Keywords: T cell antigen receptor, signal transduction, MAP kinase, immune synapse
Abstract
ZAP-70 is a tyrosine kinase that is essential for initiation of T cell antigen receptor (TCR) signaling. We have found that T cell p38 MAP kinase (MAPK), which is directly phosphorylated and activated by ZAP-70 downstream of the TCR, in turn phosphorylates Thr-293 in the interdomain B region of ZAP-70. Mutant T cells expressing ZAP-70 with an alanine substitution at this residue (ZAP-70T293A) had enhanced TCR proximal signaling and increased effector responses. Lack of ZAP-70T293 phosphorylation increased association of ZAP-70 with the TCR and prolonged the existence of TCR signaling microclusters. These results identify a tight negative feedback loop in which ZAP-70–activated p38 reciprocally phosphorylates ZAP-70 and destabilizes the signaling complex.
The adaptive immune response is orchestrated by T cells that proliferate, differentiate, and carry out effector functions upon activation. Physiologic T cell activation is induced by occupancy of the T cell receptor (TCR) with a peptide/MHC complex presented by antigen-presenting cells. Engaged TCRs form a proximal signaling complex (1) in which the coreceptors CD4 and CD8, whose intracellular domains associate with the Src family kinase Lck, are brought into proximity with the TCR, resulting in Lck-mediated phosphorylation of immunoreceptor tyrosine-based activation motifs (ITAMs) in the TCR-ζ and CD3 chains. These are docking sites for the SH2 domains of the tyrosine kinase ZAP-70 (TCR-ζ chain-associated 70-kDa tyrosine phosphoprotein). Once recruited to TCR-ζ, ZAP-70 assumes an “unlocked” conformation that enables phosphorylation by Lck, and subsequent autophosphorylation fully activates ZAP-70 (2, 3). Fully activated ZAP-70 phosphorylates the adaptors linker of activated T cells (LAT) and SH2 domain-containing leukocyte protein of 76 kDa (SLP-76), providing essential phosphotyrosine moieties that enable these molecules to work as scaffolds for the assembly of the full signalosome (4). The importance of ZAP-70 in TCR signaling is demonstrated by the fact that genetic abnormalities in ZAP-70 expression are associated with severe combined immunodeficiency (SCID) (5, 6), and thymocytes from ZAP-70 knockout mice fail to undergo positive selection and develop into mature T cells (7).
Given its importance at the apex of TCR signaling, the manner in which ZAP-70 is activated has received much attention. The two tandem N-terminal SH2 domains that bind phosphorylated ITAMs are separated by a region named interdomain A, and the second SH2 domain is separated from the kinase domain by interdomain B (6). ZAP-70 activity is primarily regulated by tyrosine phosphorylation, in both a positive and negative fashion (6). Among the 31 Tyr residues of ZAP-70, 11 have been identified as phosphorylation sites by mass spectroscopy, but the majority have not been studied (8, 9). Interdomain B contains three Tyr (residues 292, 315, and 319) that are phosphorylated by Lck following TCR stimulation (6). Phosphorylation of Tyr-292 is a negative regulatory event due to binding of the ubiquitin protein ligase c-Cbl, although the mechanism by which this binding inhibits TCR signaling is not fully understood (4). On the other hand, phosphorylation of Tyr-315 and Tyr-319 have a positive effect on kinase activity by stabilizing ZAP-70 in an active conformation and recruiting other molecules to the signalosome, in this case ZAP-70 functioning as a scaffold (6). Tyr-492 and Tyr-493 in the kinase domain are phosphorylated by either Lck or ZAP-70 itself, and also have important effects on function. A ZAP-70 Y492F mutant exhibited increased kinase activity, while activity of the Y493F mutant was impaired, indicating a negative regulatory role for phospho- (p)Tyr-492 and a positive regulatory one for pTyr-493 (9). A similar approach demonstrated that phosphorylation of Tyr-493 is essential for full activation of some downstream signaling molecules (e.g., SLP-76, LAT, and p38) but not others (PLC-γ1 and ERK) (10).
The mitogen-activated protein kinase (MAPK) p38, which in most cells is activated by a kinase cascade that results in phosphorylation on Tyr-180 and Tyr-182 in the activation loop, is activated by an alternative pathway in TCR-signaled T cells (11). In this case, activated ZAP-70 phosphorylates p38 on Tyr-323, which leads to p38 autophosphorylation on Thr-180. Dual (Thr180/Tyr182)- and mono (Thr180)-phosphorylated p38 differ in their substrate specificities, and thus biological outcomes (12, 13). In particular, ZAP-70–activated monophosphorylated p38 is crucial for TCR-induced up-regulation of the transcription factors NFATc1 and IRF4, which have major roles in proliferation and IL-17 production (13). In this report, we find that ZAP-70 is a substrate for alternatively activated but not classically activated p38, and that its phosphorylation on Thr-293 negatively regulates signaling by shortening the time of association of ZAP-70 with TCR-ζ in the signaling complex.
Results
ZAP-70–Activated Recombinant p38 Phosphorylates ZAP-70 on Thr-293.
In most cells, the MAPK p38 is activated by a kinase cascade that results in dual phosphorylation of the activation loop on residues Thr-180 and Tyr-182 (11). Activation of T cells via the TCR, however, uses an alternative pathway in which the tyrosine kinase ZAP-70 phosphorylates p38 on Tyr-323, resulting in p38 autophosphorylation on Thr-180 (14). During the course of studies using [γ-32P]ATP labeling of recombinant proteins in vitro, we observed that when ZAP-70 and p38 were coincubated, there was, in addition to the expected labeled band at 38 kDa, a radiolabeled band with a molecular mass of ∼70 kDa (Fig. S1A). To characterize this protein, a nonradioactive kinase reaction was performed with recombinant active ZAP-70 and p38, the material resolved by SDS/PAGE, and the 70-kDa band excised, cleaved with trypsin, and subjected to tandem mass spectrometry analysis (Fig. S1B). The peptide corresponding to ZAP-70 residues 283–298 was observed with a phosphorylation on Thr-293 (m/z = 629.32, z = 3+).
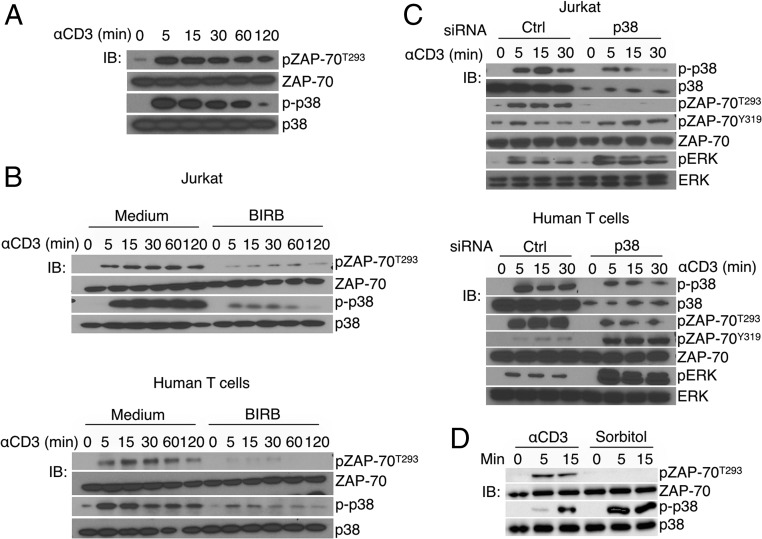
To determine whether ZAP-70 is phosphorylated on Thr-293 in a physiologic setting, we generated a rabbit polyclonal antibody specific for pZAP-70T293 (anti–pZAP-70T293). The purified polyclonal antibodies were highly specific, as evaluated by comparing their binding to the phosphorylated versus nonphosphorylated immunizing peptide (Fig. S2). Anti-CD3 stimulation of Jurkat cells or primary human T cells activated p38 and induced the appearance of a 70-kDa band detected by the anti–pZAP-70T293 (Fig. 1A). The highly selective p38 inhibitor BIRB796 markedly reduced both activation-induced p38 autophosphorylation and phosphorylation of ZAP-70T293, indicating that p38 mediated ZAP-70T293 phosphorylation in cells (Fig. 1B). To confirm this, we used a genetic approach in which p38 was knocked down by RNAi. siRNA duplexes specific for the human p38 α/β isoforms were introduced into Jurkat or primary human T cells. As observed with the p38 small molecule inhibitor, in the p38 knockdown cells there was reduced phosphorylation of ZAP-70T293, confirming a role for p38 (Fig. 1C). Importantly, although activation of p38 by osmotic shock (i.e., via the classic MAPK cascade) was even better than activation induced by TCR signaling, it did not result in phosphorylation of ZAP-70T293 (Fig. 1D).
Fig. 1.
p38 phosphorylates ZAP-70T293 in vitro and in T cells activated via the TCR. (A) Jurkat cells were activated with cross-linked anti-CD3 and cell lysates immunoblotted (IB) with antibodies against the indicated proteins. The antibody used to detect p-p38 recognizes both Thr-180/Tyr-182 dual phosphorylation and Thr-180 monophosphorylation (12). (B) Jurkat cells and primary human T cells were activated in the absence or presence of the p38 inhibitor BIRB796 and cell lysates immunoblotted. (C) Validated RNAi to p38α/β were electroporated in Jurkat and primary human T cells, which after 48 were activated with anti-CD3 and analyzed by immunoblotting for the indicated proteins. (D) Jurkat T cells were stimulated with cross-linked anti-CD3 or sorbitol, lysed, and immunoblotted for the indicated proteins. The data are representative of three (A, B, and D) and two (C) independent experiments.
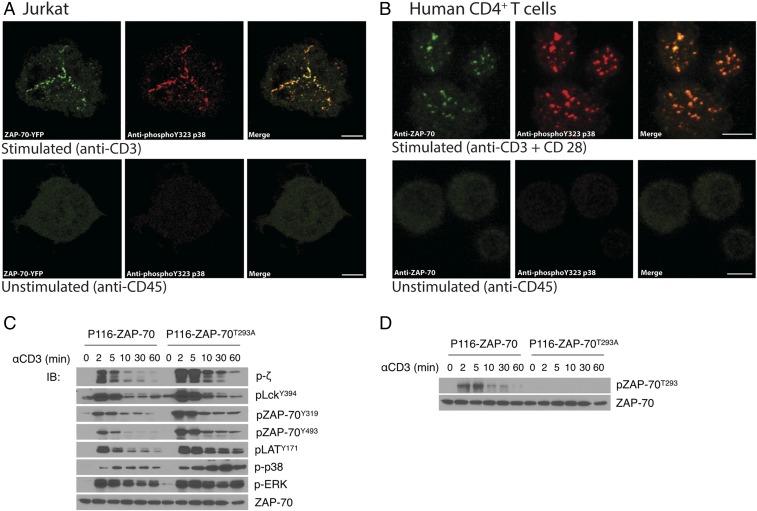
ZAP-70 was first identified as the kinase most proximal to alternative p38 activation because TCR-mediated stimulation of Jurkat cells lacking ZAP-70 did not result in phosphorylation of p38Y323 (14). This would imply that p38 is recruited to the plasma membrane-associated TCR signaling complex, which is where activated ZAP-70 resides. To test this, Jurkat T cells expressing a ZAP-70–YFP fusion protein or primary human T cells were stimulated on anti-CD45 (control)- or anti-CD3 (activating)-coated slides and TCR microclusters at the T cell:slide interface imaged by fluorescence confocal microscopy. Whereas no microclusters, as defined by accumulation of ZAP-70–YFP, were detected in nonactivated Jurkat T cells, they were abundant at 3 and 6 min after activation (Fig. 2A). Notably, antibodies specific for phospho-p38Y323 also detected this species only in activated cells, where it colocalized with ZAP-70. Similar results were obtained with primary human T cells using antibodies against ZAP-70 and phospho-p38Y323 (Fig. 2B). Taken together, these results demonstrate that p38 is activated by ZAP-70 in the TCR signaling complex, and in turn phosphorylates ZAP-70 on Thr-293.
Fig. 2.
Phosphorylation of ZAP-70T293 enhances TCR signaling. (A) Jurkat cells stably expressing YFP-tagged ZAP-70 or (B) purified primary human CD4+ T cells were plated on coverslips coated with anti-CD45 (nonactivating) or anti-CD3 (activating) for 3 min, fixed, and immunostained. ZAP-YFP is shown in green and anti-pY323 p38 is shown in red for Jurkat–ZAP-70–YFP cells; ZAP-70 is shown in green and anti-pY323 p38 is shown in red for human T cells. (C and D) ZAP-70–deficient P116 cells expressing WT ZAP-70 or ZAP-70T293A were stimulated with cross-linked anti-CD3 for the indicated times and cell lysates immunoblotted for the indicated proteins. The data are representative of three (A, C, and D) and two (B) independent experiments.
Phosphorylation of ZAP-70T293 Inhibits TCR Proximal Signaling and Effector Functions.
To assess possible functional implications of phosphorylation of ZAP-70T293, WT ZAP-70 or ZAP-70T293A were stably expressed in otherwise ZAP-70–deficient P116 cells. These cells were stimulated with anti-CD3 and proximal signaling was assessed by immunoblotting. As shown in Fig. 2C, whereas both cells expressed comparable amounts of ZAP-70, robust phosphorylation of TCR-ζ was detected as early as 2 min. Phosphorylation of ZAP-70 itself was much more robust when ZAP-70 residue 293 could not be phosphorylated. Notably, we observed a modest enhancement in the phosphorylation of Lck on Y394 (active) and enhanced phosphorylation of ZAP-70 on Y319 in interdomain B, which is mediated by autophosphorylation and transphosphorylation by Lck (4). Phosphorylation of ZAP-70Y493 in the kinase domain, which is thought to be mediated by Lck as well as being an autophosphorylation site (4, 8, 15, 16), was elevated in the mutant. Consistent with studies showing that phosphorylation of both the residues is required for full function (4), phosphorylation of the ZAP-70 substrates LAT and p38 were enhanced, as was further downstream events such as ERK phosphorylation (Fig. 2C). Knockdown of p38, which reduced ZAP-70T293 phosphorylation, resulted in a similar increase in downstream signaling in both Jurkat and primary human T cells (Fig. 1C). Anti–pZAP-70T293 did not detect a signal in cells expressing ZAP-70T293A (Fig. 2D). Therefore, phosphorylation of ZAP-70T293 is a negative regulator of TCR signaling along with the previously reported residue Y292.
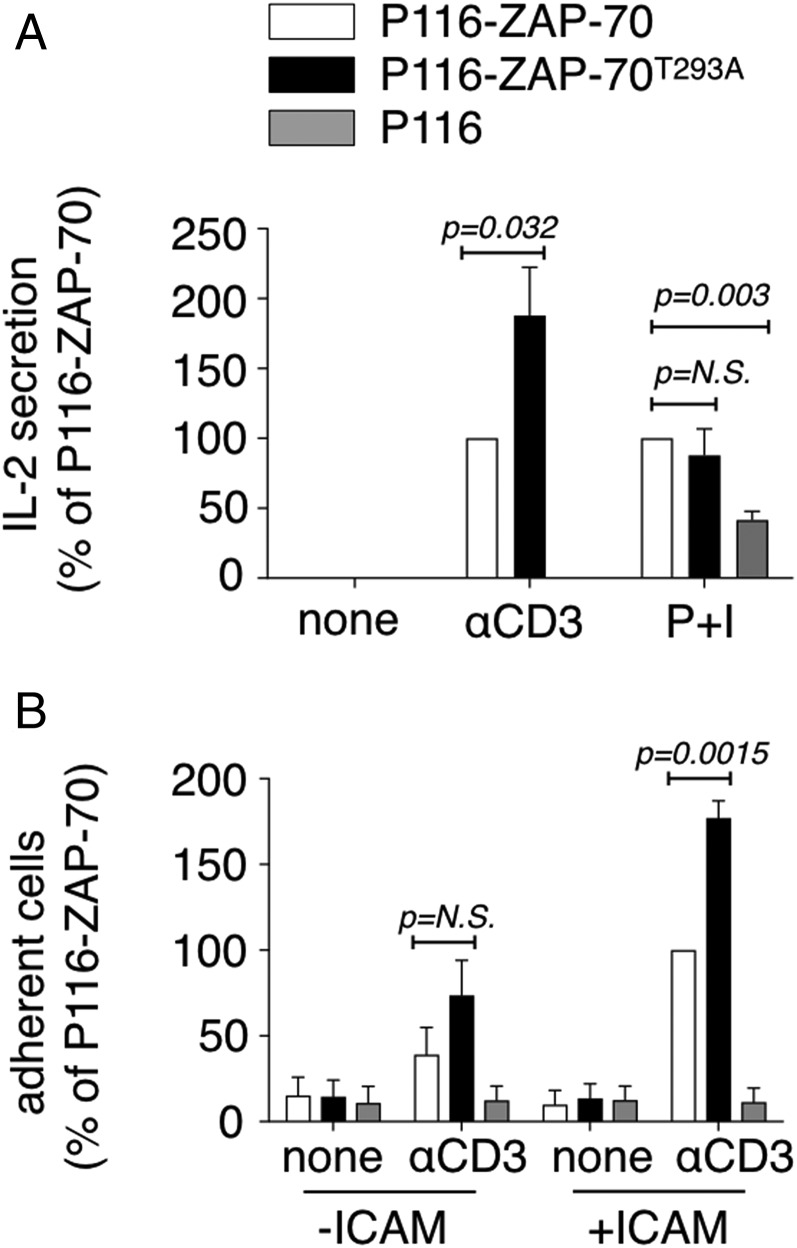
The effect of phosphorylation of ZAP-70T293 on important functional effector responses was determined in two ways. P116 cells and those stably expressing WT ZAP-70 or ZAP-70T293A were activated via the TCR, and IL-2 secretion was measured. Engagement of the TCR induced very little IL-2 in P116 cells (Fig. 3A). Notably, there was a substantially greater amount of IL-2 produced by cells expressing ZAP-70T293A compared with WT ZAP-70. This was specific for TCR-signaled IL-2 production, because the cells in which the TCR was bypassed by the combination of phorbol myristate acetate (PMA) and ionomycin made equal amounts.
Fig. 3.
Phosphorylation of ZAP-70T293 inhibits effector functions. (A) ZAP-70–deficient P116 cells or p116 cells expressing WT ZAP-70 or ZAP-70T293A were stimulated with plate-bound anti-CD3 or PMA and ionomycin (P+I) for 24 h and IL-2 was measured in the supernatant. The data represent the average of three independent experiments and were normalized to the amount of IL-2 produced by P116 cells expressing WT ZAP-70 in each experiment. N.S., not significant. (B) The same cells used in A were activated with cross-linked anti-CD3 and seeded on ICAM1-coated plates for 30 min. Adherent cells were recovered and counted. The data represent the average of five independent experiments and were normalized to binding of P116 cells expressing WT ZAP-70 to iCAM-1 in each experiment.
TCR engagement results in conformation changes in the integrin LFA-1 that results in binding to ICAM-1 on the surface of endothelial cells and dendritic cells, events that are important for diapedesis and prolonged and effective antigen presentation (17). It is possible to assess this in vitro by quantitating the number of T cells that adhere to ICAM-1–coated plates (10). In the absence of either activation or ICAM-1, few if any cells bound (Fig. 3B). Whereas activated P116 cells did not bind, P116 cells expressing WT ZAP-70 adhered to the ICAM-1–coated wells. As observed for IL-2 production, P116 cells expressing ZAP-70T293A bound substantially better. These results demonstrate that phosphorylation of ZAP-70T293 negatively regulates signals and functional responses downstream of the TCR.
Phosphorylation of ZAP-70T293 Acts Independently of ZAP-70Y292 Phosphorylation.
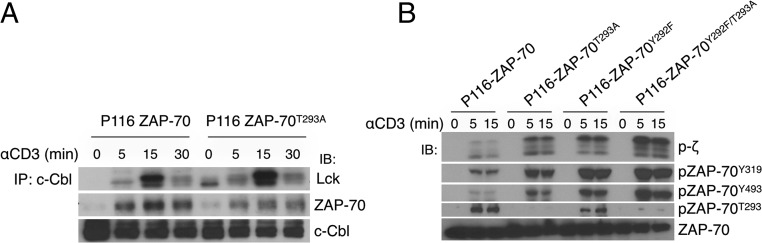
The ubiquitin protein ligase c-Cbl binds ZAP-70 phosphorylated on Tyr-292 and attenuates TCR signaling by as yet undetermined mechanisms (18). The ZAP-70 amino acid substitutions D290A or Y292F reduce c-Cbl binding to ZAP-70 (18), and c-Cbl–deficient thymocytes have marked hyperphosphorylation of cellular substrates following TCR-mediated stimulation (19). To determine whether phosphorylation of Thr-293, which is adjacent to the c-Cbl binding site, contributes to ZAP-70 interactions with c-Cbl, we activated P116 cells stably expressing WT ZAP-70 or ZAP-70T293A and looked for ZAP-70 in anti–c-Cbl immunoprecipitates. As shown in Fig. 4A, the association of ZAP-70T293A with c-Cbl was modestly diminished compared with ZAP-70, suggesting that pZAP-70T293 contributes somewhat to c-Cbl recruitment. To ask whether this was responsible for the effects or pZAP-70T293 on TCR cell signaling, we analyzed TCR signaling in P116 cells stably expressing wild-type ZAP-70, ZAP-70Y292F, ZAP-70T293A, or ZAP-70Y292F/T293A (double mutant). As shown in Fig. 4B, despite expressing similar amounts of ZAP-70, cells expressing either single mutation had enhanced signaling compared with the wild type. Notably, the double mutant had a further substantial enhancement in phosphorylation of proximal signaling molecules. This argues that the consequences of ZAP-70T293 phosphorylation are independent of phosphorylation of ZAP-70Y292.
Fig. 4.
Phosphorylation of ZAP-70T293 acts independently of ZAP-70Y292 phosphorylation. (A) ZAP-70–deficient P116 cells expressing WT ZAP-70 or ZAP-70T293A were activated with cross-linked anti-CD3, and cell lysates prepared at the indicated times were immunoprecipitated (IP) with anti–c-Cbl. The immunoprecipitates were resolved by SDS/PAGE and immunoblotted (IB) with antibodies against the indicated proteins. (B) The indicated stable P116 transfectants were stimulated and analyzed as in A. The data are representative of three (A) and two (B) independent experiments.
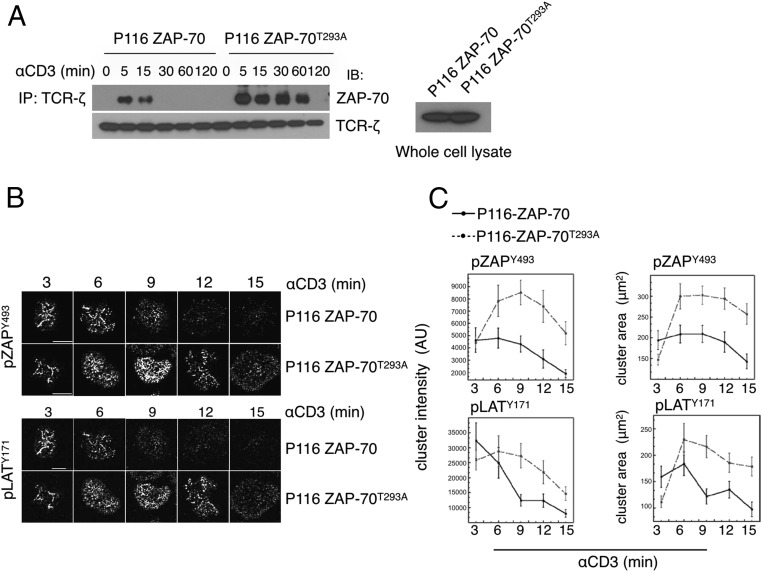
Phosphorylation of ZAP-70T293 Reduces ZAP-70 Recruitment to Signaling Microclusters.
Recruitment of ZAP-70 to TCR-ζ ITAMs is a critical early step in the signaling cascade. To ask whether phosphorylation of ZAP-70T293 affects this interaction, the association of the TCR with ZAP-70 was determined by quantitating the amount of ZAP-70 coimmunoprecipitated with TCR-ζ. WT ZAP-70 was found in anti–TCR-ζ immunoprecipitates 5 and 15 min after stimulation but not thereafter (Fig. 5A). In contrast, ZAP-70T293A association was far more robust and endured for at least 1 h. The composition and longevity of TCR microclusters in activated T cells was assessed by fluorescence microscopy of cells expressing WT or ZAP-70T293A. pZAP-70 was found in microclusters 3 min after activation and maintained for 9–12 min, afterward decreasing in area and intensity, which are indicative of protein levels (Fig. 5 B and C). In contrast, ZAP-70T293A–containing microclusters continued to increase in size and intensity until 6–9 min and were maintained above baseline even at 15 min. The cluster intensity of the ZAP-70 target pLATY171 peaked at 3 min and the area at 6 min in ZAP-70–expressing cells, whereas both were maintained at a high level even 15 min after activation in cells expressing ZAP-70T293A. Thus, preventing phosphorylation of ZAP-70T293 stabilized the TCR signaling machinery and prolonged signaling.
Fig. 5.
Phosphorylation of ZAP-70T293 diminishes recruitment of ZAP-70 to the TCR and destabilizes the signaling complex. (A) ZAP-70–deficient P116 cells expressing WT ZAP-70 or ZAP-70T293A were activated with cross-linked anti-CD3 and cell lysates prepared at the indicated times were immunoprecipitated with anti–TCR-ζ. The immunoprecipitates were resolved by SDS/PAGE and immunoblotted with antibodies against the indicated proteins. The data are representative of three independent experiments. (B, Upper) Representative images illustrating the time course of phosphorylated ZAP-70 clustering in activated Jurkat cells. The images shown here were taken from different staining experiments. (B, Lower) Representative images illustrating the time course of phosphorylated LAT clustering in activated Jurkat cells. The images shown in the Upper and Lower panels were taken from different staining experiments. (C) Quantification of the area of pZAP-70 and pLAT clusters. Error bars show SEM. For WT ZAP-70 cells, 3 min n = 51, 6 min n = 55, 9 min n = 52, 12 min n = 55, and 15 min n = 60. For ZAP-70T293A cells, 3 min n = 55, 6 min n = 50, 9 min n = 52, 12 min n = 53, and 15 min n = 52, taken from three experiments. AU, arbitrary units.
Discussion
T cells have a number of mechanisms to limit the intensity and duration of signals generated by TCR engagement, one being changes in phosphorylation of downstream kinases (20). The present study describes a mechanism for negative regulation of TCR signaling based on cross-talk between ZAP-70 and p38. In this tight regulatory loop, TCR-activated ZAP-70 phosphorylates and activates p38, which in turn phosphorylates the ZAP-70 inhibitory residue T293. Activation of p38 MAPK through the classic (stress-induced) or alternative (TCR-induced) pathway leads to dual or monophosphorylation of the p38 activation loop, respectively, which results in different substrate fine specificities (12). As a result, the biological outcomes of having mono- or dual-phospho p38 differ, and indeed can have diametrically opposed effects on T cell functions (13). In general, although monophosphorylated p38 shares most substrates with dual-phospho p38 (e.g., STAT4, MK2, and MEF2A), it does not phosphorylate them as well as the dual-phospho form (12, 21, 22). ZAP-70 is the first reported exception, being preferentially phosphorylated on Thr-293 by monophosphorylated p38. One possible explanation for this may be enzyme-substrate proximity. That is, TCR-induced and ZAP-70–mediated phosphorylation of p38 has been shown to require the scaffolding activity of Discs Large Homolog 1 (Dlgh1), which colocalizes with the TCR at the immunological synapse and is thought to bridge Lck, ZAP-70, and p38 (23). In this case, the juxtaposition of activated ZAP-70 with its substrate would increase the likelihood of “backtalk” from activated p38, which would not be the case for MAPK-activated p38. Alternatively, ZAP-70T293 may be a preferred substrate for alternatively activated p38. Although ZAP-70T293 is followed by a proline, typical of p38 target sites, kinase interaction motifs in the target protein that interact with docking sites on p38 also contribute to substrate specificity (24). It is possible that differences in the conformation of the docking sites differ between mono- and dual-phospho p38, resulting in a preference for the former in binding and phosphorylating ZAP-70.
The role of c-Cbl in the negative regulation of TCR signaling is well documented, although the precise mechanism of action remains elusive, possibly involving ubiquitination and degradation of TCR-ζ, internalization of the liganded TCR, or other as yet unidentified mechanisms (4). We found that recruitment of c-Cbl to ZAP-70 occurs but is modestly decreased in the absence of Thr-293 phosphorylation. The reduced binding of c-Cbl to ZAP-70T293A could be because phosphorylation of ZAP-70Y292 may depend upon pZAP-70T293, even though phosphorylation of T293 occurred in the absence of pY292. In this case, reduced c-Cbl binding would be due to reduced availability of its docking site. It is also conceivable that the c-Cbl docking site must contain both phosphorylated residues. The possibility that c-Cbl docking sites other than pZAP-70Y292 exist is supported by the finding that after TCR cross-linking, ZAP-70Y292F–expressing T cells retained the ability to increase phosphorylation of c-Cbl (25). It is possible that phosphorylation of ZAP-70Y292 is facilitated by phosphorylation of the adjacent T293. Unfortunately, the commercially available antibodies recognizing pZAP-70Y292 that we tested do not recognize their epitope if ZAP-70T293 is either phosphorylated or replaced with an alanine, making it difficult to distinguish between these possibilities. However, we were able to ask whether the effects of pZAP-70T293 on signaling are mediated by affecting phosphorylation of Y292 and found that signaling in cells expressing the ZAP-70Y292F/T293A double mutant was greater than with either mutation alone. It is therefore likely that the effect of pZAP-70T293 on TCR-mediated signaling is independent of pZAP-70Y292, and is unlikely to be the result of the modest reduction in c-Cbl binding.
Phosphorylation of Thr-293 reduces recruitment of ZAP-70 to the TCR, as evidenced by prolonged interactions of ZAP-70T293A with TCR-ζ in both coimmunoprecipitation and confocal studies. A recent paper reported that in the initial phase after stimulation ZAP-70 undergoes cycles of recruitment and release into the plasma membrane from phosphorylated TCR ITAMs (26). A subsequent decrease in cycling was accompanied by stabilization of the ZAP-70–TCR interaction. It is an interesting speculation that phosphorylation of ZAP-70T293 interferes with this second phase, and that in its absence there is an increase in stable complex formation that prolongs signaling. We note that signaling in cells expressing ZAP-70T293A was increased at early times as well, with heightened phosphorylation of Lck and its most proximal substrate, TCR-ζ. Our data support the notion that phosphorylation of Thr-293 may prevent ZAP-70 recruitment to phosphorylated ITAMs after release.
The findings in this study reveal a negative feedback loop at the very earliest levels of T cell activation. The T cell alternative p38 pathway seems to have evolved at least in part so that activation via the TCR induces NFAT-mediated gene transcription and cytokine production, which is prevented by activation of p38 via the classical MAPK cascade (13). The role of alternatively activated p38 in the attenuation of proximal TCR signaling may be another example, in this case with the acquisition rather than loss of substrate specificity.
Materials and Methods
Cells.
E6.1 Jurkat and the ZAP-70–deficient Jurkat derivative, P116, cells were maintained in RPMI 1640 (Gibco) medium supplemented with 10% FBS, 2 mM glutamine, 50 μM β-mercaptoethanol, and 100 μM gentamicin (complete medium). Stably transfected cell lines were cultured in complete medium plus 1.3 mg/mL geneticin sulfate G418 (KSE Scientific). Human CD4+ T cells were isolated from buffy coats of healthy volunteers (NIH Blood Bank) using the human CD4 cell recovery column kit (Cedarlane), according to the manufacturer’s instructions.
Reagents and Antibodies.
The p38 inhibitors, SB203580 and BIRB796, were obtained from Cell Signaling and Axon Biomed, respectively. The MEK1/2 inhibitor PD0325901 and p38 siRNA (catalog nos. sc-39116 and sc-29433) were from Santa Cruz. GST-human ZAP-70 was purchased from R&D Systems. SDS sample buffer was purchased from Quality Biologicals, and Restore PLUS Western blot stripping buffer was from Thermo Scientific. Agarose-conjugated anti–TCR-ζ was from Santa Cruz. The indicated antibodies to the following proteins were used for immunoblotting: phospho-LckY394 (catalog no. 101728), Lck (catalog no. 433), and anti–TCR-ζ (catalog no. 1239) from Santa Cruz,; pZAP-70Y319 (catalog no. 2701), pZAP-70Y493 (catalog no. 2704), ZAP-70 (catalog no. 2705), pLATY171 (catalog no. 3581), LAT (catalog no. 9166), SLP-76 (catalog no. 4958), phospho-PLCγ1 (catalog no. 2821), PLCγ1 (catalog no. 2822), phospho-ERK1/2 (catalog no. 9012), ERK1/2 (catalog no. 9101), phospho-p38 (catalog no. 9211, which recognizes both Thr-180/Tyr-182 dual- and Thr-180 monophosphorylation) (12), and p38 (catalog no. 9212) from Cell Signaling; anti–pSLP-76 (catalog no. 75829) from Abcam; anti–c-Cbl from Santa Cruz (catalog no. 1651); and β-actin from Sigma (catalog no. 5316).
Generation of Anti-pZAPT293 Antibodies.
Threonine-phosphorylated [Ac-NSDGY-pT-PEPAC] and its nonphosphorylated [Ac-NSDGY-T-PEPAC] peptide analogs corresponding to ZAP-70 (residues 288–297)-Cys were synthesized by the solid-phase peptide synthesis utilizing 9-fluorenylmethoxycarbonyl (Fmoc)/tert-butyl chemistry. The purity of the peptides was >95%. Purified peptides were characterized by matrix-associated laser desorption ionization time-of-flight mass spectrometry (MALDI microMX; Waters). For KLH conjugation, 6 mg of purified peptide was dissolved in 120 µL 1× PBS (50 mg peptide/mL), immediately mixed with 350 µL IMA-KLH (10 mg/mL in H2O) (Thermo Fisher Scientific), and stirred at room temperature for 2 h. The sample was dialyzed against 1× PBS overnight. Antisera were raised by immunizing rabbits with the KLH-coupled peptide in Complete Freund’s Adjuvant (Pocono Rabbit Farm & Laboratory). Phospho-specific antibodies were isolated by affinity purification.
Cell Activation.
Cells were incubated with soluble monoclonal anti-CD3 (OKT3; eBioscience) at a concentration of 10 μg/mL on ice for 10 min. OKT3 was cross-linked with anti-mouse IgG (Jackson Immunoresearch) for the indicated times. Activation was stopped by placing the cells on ice and diluting them with chilled PBS at 1:10 ratio before processing. In some experiments, cells were stimulated with PMA (1 ng/mL) and ionomycin (500 ng/mL). Cell lysates were prepared in lysis buffer supplemented with protease and phosphatase inhibitors. Lysates were centrifuged at 14,000 × g for 20 min and boiled in SDS sample buffer followed by SDS/PAGE and immunoblot analysis (Trans-Blot Turbo; Bio-Rad).
Retroviral Transduction.
Retroviruses encoding WT ZAP-70, ZAP-70T293A, ZAP-70Y292F, and ZAP-70T293A/Y292F were generated by transient transfection of the retroviral vector pMSCV (10 μg) together with 2 μg of gag-pol and 2 μg of VSV-g into HEK293T cells. The supernatant containing retrovirus was collected, passed through a 0.45-μm filter, and aliquots were stored at −80 °C. For infection, 106 ZAP-70–deficient P116 cells were plated in six-well plates, the virus-containing supernatant was added, and the plate was spun at 400 × g at 30 °C for 2 h. Cells were selected in puromycin (0.5 μg/mL) and maintained in culture.
Confocal Microscopy and Image Analysis.
Jurkat ZAP-70–YFP cells, p116 ZAP-70–deficient cells stably expressing wild-type ZAP-70 or mutant ZAP-70T293A, or purified primary human CD4+ T cell were allowed to spread on coverslips as described (27). Briefly, poly-l-lysine–covered four-chambered glass coverslips (LabTek II; Nunc/Nalgene) were coated with 10 μg/mL of the anti-CD3 antibody Hit3A (BD Biosciences). The chambers were loaded with 300 μL of complete medium without phenol red supplemented with 25 mM Hepes, pH 7.0, and warmed. Cells were resuspended in the same buffer, plated into the bottom of the chamber, and incubated at 37 °C. After the indicated times, the cells were fixed in 2.4% (Jurkat cells) or 1.2% (human T cells) paraformaldehyde for 30 min. Cells were permeabilized with Triton X-100, incubated with blocking buffer for 30 min, and incubated with primary antibodies for 60 min (anti-pZAP Tyr493/Syk526 from Cell Signaling was used at 1:50 dilution; anti-pLAT pY171 from BD Pharmingen at 5 μg/mL), followed by washes and a 60-min incubation with either a mouse isotype control or anti-rabbit Alexa-conjugated secondary antibodies (secondary Alexa-568 conjugated antibodies were diluted 1:1,000, Alexa-647–conjugated antibodies were diluted 1:500). Images were collected with a Leica SP8 laser scanning confocal microscope using a 63× 1.4 N.A. objective (Leica Microsystems). Z stacks (2–3 μm), with a spacing of 0.3 μm were taken of the area contacting the coverslip. Leica AF software was used to produce images. Single confocal slices were exported and Adobe Photoshop and Illustrator (Adobe Systems) were used to prepare composite figures. Scale bars were removed from the original images and replaced with a more visible version in the final composite image. To quantify cluster area and summed intensities, surface renderings were made of pZAP and pLAT clusters in the confocal images using Imaris (Bitplane-Oxford Instruments). The total fluorescence intensity of each channel and the total area of the clusters were obtained from the defined surfaces.
In Vitro Kinase Assay and Mass Spectrometry.
A total of 3 μg of recombinant GST–ZAP-70 and 1 μg of bacterially expressed p38 or MKK6 were added to kinase buffer (25 mM Hepes, 25 mM MgCl2, 2 mM DTT, 50 μM ATP) supplemented with 1× protease and phosphatase inhibitor to a final volume of 15 μL and incubated at 30 °C for 1 h. Laemmli SDS buffer at 2× concentration was added to terminate the assay and samples were electrophoresed and bands visualized by SilverQuest silver staining kit (Thermo). The ZAP-70 band was excised and in-gel trypsin digested overnight at 37 °C; the resultant peptides were extracted and lyophilized to dry. Phosphopeptides were enriched using TiO2 magnetic Sepharose (GE Healthcare) following manufacturer’s protocols. The flow through and eluted peptides were desalted by C18 ZipTip (Millipore) before mass spectrometry analysis. Dried peptides were resuspended in water containing 2% acetonitrile, 0.5% acetic acid and injected onto a 0.2 × 50 mm Magic C18AQ reverse phase column (Michrom Bioresources, Inc.) using the Paradigm MS4 HPLC (Michrom Bioresources, Inc.). Peptides were gradient eluted at a flow rate of 2 μL/min followed by online analysis by tandem mass spectrometry using an LTQ ion trap mass spectrometer (Thermo Scientific) equipped with an ADVANCE CaptiveSpray ion source (Michrom Bioresources, Inc.). Peptides were detected in positive ion mode using a data-dependent method in which the nine most abundant ions detected in an initial survey scan were selected for MS/MS analysis. The raw data were analyzed using Bioworks (Thermo).
Immunoblotting and Immunoprecipitation.
Cells were normalized to cell number and lysed in lysis buffer (150 mM NaCl, 20 mM Tris pH 7.5, 1% Triton X-100, supplemented with 1× protease inhibitor and 1× phosphatase inhibitor; Roche). The lysates were either resolved by SDS/PAGE and immunoblotted with the indicated antibodies, or immunoprecipitated using agarose-conjugated anti–TCR-ζ or anti–c-Cbl antibody for 4–6 h, washed in lysis buffer, eluted by boiling in SDS sample buffer, and resolved by SDS/PAGE.
ELISA.
The 105 cells were stimulated in 24-well plates coated with anti-CD3 and after 24 h, supernatants were collected. IL-2 was quantitated with a human IL-2 ELISA Ready-SET-Go! kit, following the manufacturer’s instructions. For validation of the rabbit anti–pZAP-70T293 antibodies, 96-well plates were coated with the unphosphorylated or phosphorylated immunizing peptide and incubated with serial dilutions of the affinity-purified antibody.
Adhesion Assay.
LFA-1–mediated cell adhesion to ICAM-1–coated plates was performed as described (10). The 106 cells per condition were activated for 30 min and seeded into 24-well plates that had been coated with 3 μg/mL of ICAM-Fc (R&D Systems). After 30 min, the wells were washed gently at least three times with PBS at room temperature. Trypsin-EDTA was added to each well and cells were detached by gentle pipetting and counted by light microscopy.
Statistical Analysis.
Statistical analysis was performed with GraphPad Prism 6 software. Error bars indicate SEMs unless otherwise specified.
Supplementary Material
Acknowledgments
We thank Bei Dong for expert technical assistance and David Venson [National Cancer Institute (NCI)] for help with statistical analyses. This work was supported by the Intramural Research Program of the Center for Cancer Research, NCI, NIH. M.M.G. was supported by the German Research Foundation (DFG, GA 1818/2-1).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1713301115/-/DCSupplemental.
References
- 1.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acuto O, Di Bartolo V, Michel F. Tailoring T-cell receptor signals by proximal negative feedback mechanisms. Nat Rev Immunol. 2008;8:699–712. doi: 10.1038/nri2397. [DOI] [PubMed] [Google Scholar]
- 3.Yan Q, et al. Structural basis for activation of ZAP-70 by phosphorylation of the SH2-kinase linker. Mol Cell Biol. 2013;33:2188–2201. doi: 10.1128/MCB.01637-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang H, et al. ZAP-70: An essential kinase in T-cell signaling. Cold Spring Harb Perspect Biol. 2010;2:a002279. doi: 10.1101/cshperspect.a002279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hivroz C, Fischer A. Immunodeficiency diseases. Multiple roles for ZAP-70. Curr Biol. 1994;4:731–733. doi: 10.1016/s0960-9822(00)00162-7. [DOI] [PubMed] [Google Scholar]
- 6.Au-Yeung BB, et al. The structure, regulation, and function of ZAP-70. Immunol Rev. 2009;228:41–57. doi: 10.1111/j.1600-065X.2008.00753.x. [DOI] [PubMed] [Google Scholar]
- 7.Negishi I, et al. Essential role for ZAP-70 in both positive and negative selection of thymocytes. Nature. 1995;376:435–438. doi: 10.1038/376435a0. [DOI] [PubMed] [Google Scholar]
- 8.Watts JD, et al. Identification by electrospray ionization mass spectrometry of the sites of tyrosine phosphorylation induced in activated Jurkat T cells on the protein tyrosine kinase ZAP-70. J Biol Chem. 1994;269:29520–29529. [PubMed] [Google Scholar]
- 9.Szabo M, et al. Fine-tuning of proximal TCR signaling by ZAP-70 tyrosine residues in Jurkat cells. Int Immunol. 2012;24:79–87. doi: 10.1093/intimm/dxr105. [DOI] [PubMed] [Google Scholar]
- 10.Dutta D, et al. Recruitment of calcineurin to the TCR positively regulates T cell activation. Nat Immunol. 2017;18:196–204. doi: 10.1038/ni.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashwell JD. The many paths to p38 mitogen-activated protein kinase activation in the immune system. Nat Rev Immunol. 2006;6:532–540. doi: 10.1038/nri1865. [DOI] [PubMed] [Google Scholar]
- 12.Mittelstadt PR, Yamaguchi H, Appella E, Ashwell JD. T cell receptor-mediated activation of p38α by mono-phosphorylation of the activation loop results in altered substrate specificity. J Biol Chem. 2009;284:15469–15474. doi: 10.1074/jbc.M901004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alam MS, et al. Counter-regulation of T cell effector function by differentially activated p38. J Exp Med. 2014;211:1257–1270. doi: 10.1084/jem.20131917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salvador JM, et al. Alternative p38 activation pathway mediated by T cell receptor-proximal tyrosine kinases. Nat Immunol. 2005;6:390–395. doi: 10.1038/ni1177. [DOI] [PubMed] [Google Scholar]
- 15.Chan AC, et al. Activation of ZAP-70 kinase activity by phosphorylation of tyrosine 493 is required for lymphocyte antigen receptor function. EMBO J. 1995;14:2499–2508. doi: 10.1002/j.1460-2075.1995.tb07247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kong G, et al. Distinct tyrosine phosphorylation sites in ZAP-70 mediate activation and negative regulation of antigen receptor function. Mol Cell Biol. 1996;16:5026–5035. doi: 10.1128/mcb.16.9.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muller WA. Mechanisms of leukocyte transendothelial migration. Annu Rev Pathol. 2011;6:323–344. doi: 10.1146/annurev-pathol-011110-130224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lupher ML, Jr, Songyang Z, Shoelson SE, Cantley LC, Band H. The Cbl phosphotyrosine-binding domain selects a D(N/D)XpY motif and binds to the Tyr292 negative regulatory phosphorylation site of ZAP-70. J Biol Chem. 1997;272:33140–33144. doi: 10.1074/jbc.272.52.33140. [DOI] [PubMed] [Google Scholar]
- 19.Thien CB, Langdon WY. c-Cbl and Cbl-b ubiquitin ligases: Substrate diversity and the negative regulation of signalling responses. Biochem J. 2005;391:153–166. doi: 10.1042/BJ20050892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Navarro MN, Cantrell DA. Serine-threonine kinases in TCR signaling. Nat Immunol. 2014;15:808–814. doi: 10.1038/ni.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang YY, Mei ZQ, Wu JW, Wang ZX. Enzymatic activity and substrate specificity of mitogen-activated protein kinase p38α in different phosphorylation states. J Biol Chem. 2008;283:26591–26601. doi: 10.1074/jbc.M801703200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Askari N, Beenstock J, Livnah O, Engelberg D. p38alpha is active in vitro and in vivo when monophosphorylated at threonine 180. Biochemistry. 2009;48:2497–2504. doi: 10.1021/bi900024v. [DOI] [PubMed] [Google Scholar]
- 23.Round JL, et al. Dlgh1 coordinates actin polymerization, synaptic T cell receptor and lipid raft aggregation, and effector function in T cells. J Exp Med. 2005;201:419–430. doi: 10.1084/jem.20041428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peti W, Page R. Molecular basis of MAP kinase regulation. Protein Sci. 2013;22:1698–1710. doi: 10.1002/pro.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magnan A, et al. T cell development and T cell responses in mice with mutations affecting tyrosines 292 or 315 of the ZAP-70 protein tyrosine kinase. J Exp Med. 2001;194:491–505. doi: 10.1084/jem.194.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katz ZB, Novotná L, Blount A, Lillemeier BF. A cycle of Zap70 kinase activation and release from the TCR amplifies and disperses antigenic stimuli. Nat Immunol. 2017;18:86–95. doi: 10.1038/ni.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bunnell SC, Barr VA, Fuller CL, Samelson LE. High-resolution multicolor imaging of dynamic signaling complexes in T cells stimulated by planar substrates. Sci STKE. 2003;2003:PL8. doi: 10.1126/stke.2003.177.pl8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.