Significance
Prostate cancer is one of most common cancers in men worldwide, and osteoblastic bone metastasis is frequently observed in prostate cancer patients. However, the mechanisms responsible for the predominantly osteoblastic phenotype have not been fully elucidated. Cancer-secreted microRNAs (miRNAs) were recently shown to be significant in the modification of the tumor microenvironment. Here, hsa-miR-940, which was highly secreted by prostate cancer cells, promoted osteogenic differentiation of human mesenchymal stem cells in vitro, and induced extensive osteoblastic lesions in the bone metastatic microenvironment in vivo. Our study provides a demonstration that osteoblastic bone metastasis can be induced by miRNAs secreted by cancer cells in the bone microenvironment.
Keywords: cancer-secreted microRNA, osteoblastic bone metastasis, exosome, prostate cancer, bone microenvironment
Abstract
Bone metastatic lesions are classified as osteoblastic or osteolytic lesions. Prostate and breast cancer patients frequently exhibit osteoblastic-type and osteolytic-type bone metastasis, respectively. In metastatic lesions, tumor cells interact with many different cell types, including osteoblasts, osteoclasts, and mesenchymal stem cells, resulting in an osteoblastic or osteolytic phenotype. However, the mechanisms responsible for the modification of bone remodeling have not been fully elucidated. MicroRNAs (miRNAs) are transferred between cells via exosomes and serve as intercellular communication tools, and numerous studies have demonstrated that cancer-secreted miRNAs are capable of modifying the tumor microenvironment. Thus, cancer-secreted miRNAs can induce an osteoblastic or osteolytic phenotype in the bone metastatic microenvironment. In this study, we performed a comprehensive expression analysis of exosomal miRNAs secreted by several human cancer cell lines and identified eight types of human miRNAs that were highly expressed in exosomes from osteoblastic phenotype-inducing prostate cancer cell lines. One of these miRNAs, hsa-miR-940, significantly promoted the osteogenic differentiation of human mesenchymal stem cells in vitro by targeting ARHGAP1 and FAM134A. Interestingly, although MDA-MB-231 breast cancer cells are commonly known as an osteolytic phenotype-inducing cancer cell line, the implantation of miR-940–overexpressing MDA-MB-231 cells induced extensive osteoblastic lesions in the resulting tumors by facilitating the osteogenic differentiation of host mesenchymal cells. Our results suggest that the phenotypes of bone metastases can be induced by miRNAs secreted by cancer cells in the bone microenvironment.
Bone is a preferred site of metastasis for many types of cancer, including prostate and breast cancer. Based on their radiographic appearance, bone metastatic lesions are classified as osteoblastic or osteolytic lesions. These lesions are believed to be induced by an imbalance between bone formation and bone resorption in the bone microenvironment (1, 2).
In bone metastatic lesions, tumor cells interact with many types of cells, including osteoblasts, osteoclasts, and mesenchymal stem cells. Prostate cancer cells frequently induce osteoblastic-type bone metastasis. Previous studies have demonstrated that prostate cancer cells supply several osteoblast-stimulating factors, such as bone morphogenetic protein (BMP), VEGF, PDGF, endothelin-1 (ET-1), Wnt ligands, or urokinase plasminogen activator, to osteoblasts or osteoblast precursor cells to promote bone formation in the bone microenvironment (3–5). In contrast, cancer cells originating from the breast, lung, or kidney cancer activate osteoclasts, leading to osteolytic-type bone metastasis. According to previous reports, the increased osteoclast activity is mediated by several osteoclastogenic factors, such as RANKL, IL-6, IL-8, or IL-11, secreted by metastasized cancer cells (6, 7). However, the detailed mechanisms responsible for the osteoblastic or osteolytic phenotype remain to be fully elucidated.
MicroRNAs (miRNAs) are small noncoding RNA molecules that bind to the 3′UTRs of target messenger RNAs and induce gene silencing by suppressing protein production or facilitating mRNA degradation (8). miRNAs play a critical role in various developmental processes and physiological and pathological conditions (9, 10). Our previous reports have demonstrated that mmu-miR-206 and mmu-miR-145 play important roles in osteoblast differentiation in vivo (11, 12). To date, numerous studies have shown that multiple miRNAs regulate the differentiation of osteoblasts and osteoclasts. In addition, a recent study showed that miRNAs transfer between cells via exosomes to serve as intercellular communication tools (13, 14). In the tumor microenvironment, various exosomal miRNAs are secreted by cancer cells. Thus, we hypothesized that in bone metastatic lesions, cancer-secreted miRNAs are incorporated into the surrounding stromal cells, resulting in the modification of bone remodeling.
Results
Identifying Exosomal miRNAs Markedly Secreted by Osteoblastic Phenotype-Inducing Cancer Cell Lines.
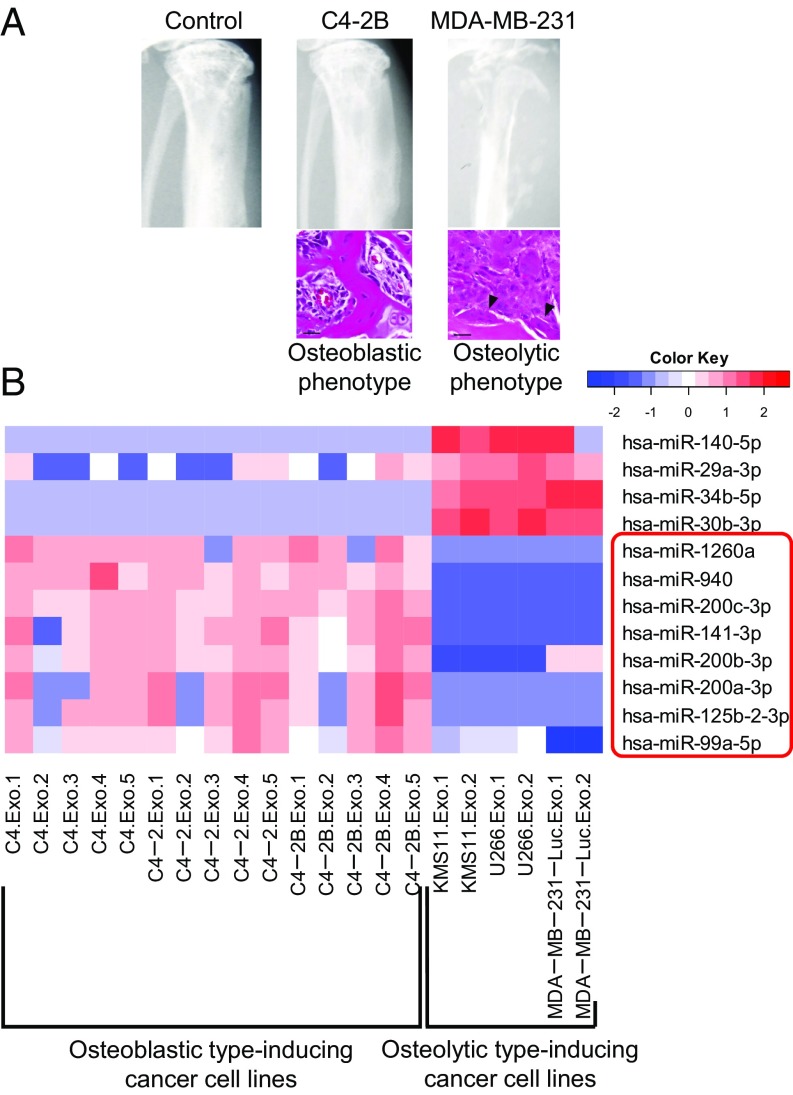
Based on previous studies, we divided human cancer cell lines into two groups: osteoblastic phenotype- and osteolytic phenotype-inducing cell lines. The osteoblastic phenotype-inducing cancer cell lines included human prostate cancer cell lines (e.g., C4, C4-2, and C4-2B) and represent the osteoblastic phenotype when the cells are implanted into the tibia of immunodeficient mice (Fig. 1A) (15). In contrast, human breast cancer cell lines (e.g., MDA-MB-231-Luc) and human myeloma cell lines (e.g., KMS11 and U266) are known to induce the osteolytic phenotype in bone metastatic lesions (Fig. 1A) (6, 16).
Fig. 1.
Identifying exosomal microRNAs markedly secreted by osteoblastic phenotype-inducing cancer cell lines. (A) Representative X-ray images and H&E stains of tibiae at 2 wk after the intratibial injection of 1 × 106 tumor cells into NOD/SCID mice. Several osteoclasts (arrowheads) were observed in the osteolytic lesions. (Scale bars, 20 μm.) (B) The expression profile of miRNAs in exosomes secreted by cancer cell lines. Exosomes from noncancerous cells (HEK293) were used as a normalization control. Red color denotes higher expression, and blue color denotes lower expression relative to the control.
We first examined the expression profile of miRNAs in exosomes secreted by the above osteoblastic or osteolytic phenotype-inducing cell lines. The cancer-secreted exosomes were isolated by ultracentrifugation of the culture supernatant (Fig. S1A), and total RNAs, including miRNAs, were extracted from the isolated exosomes and subjected to miRNA microarray. In advance of the analysis, we confirmed that the exosomal RNAs had an abundance of small RNAs compared with cellular RNAs (Fig. S1B). The comprehensive microarray analysis showed that eight human miRNAs, hsa-miR-99a-5p, 125b-2-3p, 141-3p, 200a-3p, 200b-3p, 200c-3p, 940, and 1260a, were highly expressed in the exosomes secreted by osteoblastic phenotype-inducing prostate cancer cell lines compared with the exosomes from osteolytic phenotype-inducing cell lines (Fig. 1B).
hsa-miR-940 Promotes the Osteogenic Differentiation of Human Mesenchymal Stem Cells.
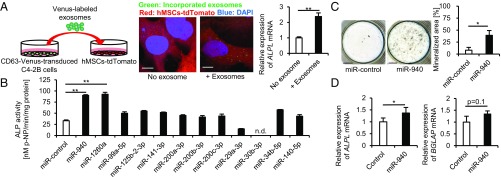
To visualize the transfer of miRNA-containing exosomes from cancer cells to mesenchymal cells, we constructed a retroviral vector containing CD63, which is a well-known exosome marker (13), fused with improved yellow fluorescent protein (Venus). We then established a CD63-Venus stably expressing cancer cell line, C4-2B-CD63-Venus, using a retroviral infection system. Venus-labeled exosomes were isolated from the supernatant of the cells by ultracentrifugation and added to tdTomato-expressing immortalized human mesenchymal stem cell lines (hMSCs) (Fig. 2A, Left). After a 24-h culture, we detected Venus-labeled exosomes incorporated into hMSCs (Fig. 2A, Center). We also confirmed that the expression of ALPL was significantly up-regulated in hMSCs cultured with the exosomes for 48 h (Fig. 2A, Right). These findings suggest that miRNA-containing exosomes derived from osteoblastic phenotype-inducing cell lines have the potential to promote the osteogenic differentiation of hMSCs.
Fig. 2.
hsa-miR-940 promotes the osteogenic differentiation of human mesenchymal stem cells. (A) Confocal microscopy images showing that Venus-labeled C4-2B exosomes were incorporated into hMSCs (Center). qPCR analysis showing the increased expression of ALPL in hMSCs cultured with C4-2B exosomes (Right). (Scale bars, 10 μm.) (B and C) The effects of transient overexpression of cancer-secreted miRNAs on the osteogenic differentiation of hMSCs. ALP activity assay (B) and von Kossa staining (C) showed that miR-940 overexpression induced the osteogenic differentiation. n.d., not detected. (Scale bars, 4 mm.) (D) qPCR analysis showing higher expression of ALPL in miR-940–overexpressing hMSCs after 6-d osteogenic induction. All of the data are the means ± SEMs (n = 3). n.s., not significant, *P < 0.05, **P < 0.01 by one-way ANOVA with Tukey’s HSD test (B) or Student’s t test (C and D).
To examine the effect of the cancer-secreted miRNAs that were identified in Fig. 1B on osteogenic differentiation of hMSCs, each miRNA mimic was subsequently transfected into hMSCs, and the cells were cultured in osteogenic induction medium. After a 14-d osteogenic induction, an alkaline phosphatase (ALP) assay revealed that the overexpression of miR-940 or miR-1260a significantly promoted the osteogenic differentiation of hMSCs as shown by the increase in the ALP activity (Fig. 2B). After a 28-d induction, von Kossa staining was performed to investigate the effect of the miRNAs on the matrix mineralization of hMSCs, and miR-940 overexpression induced extensive mineralized nodules (Fig. 2C). We also established stable miR-940–overexpressing hMSCs using a lentiviral infection system, and the osteogenic potential of the cells was examined. After a 7-d osteogenic induction, miR-940–overexpressing hMSCs showed significantly increased ALP activity, as well as a higher expression of osteogenic markers than the empty vector-infected hMSCs (Fig. 2D and Fig. S2A). The mineralized areas of the cultured cells were also significantly increased in miR-940–overexpressing hMSCs after a 28-d osteogenic induction (Fig. S2B). In contrast, miR-940 overexpression in human osteoclast precursor cells, which were isolated from CD14+ peripheral blood mononuclear cells, did not affect osteoclastogenesis. The number of tartrate-resistant acid phosphatase (TRAP)-positive multinucleated osteoclasts was not altered by miR-940 overexpression (Fig. S2C). The gene expression of an osteoclast marker, CTSK, was also not changed (Fig. S2D).
ARHGAP1 and FAM134A Are Targets of hsa-miR-940 to Promote Osteogenic Differentiation.
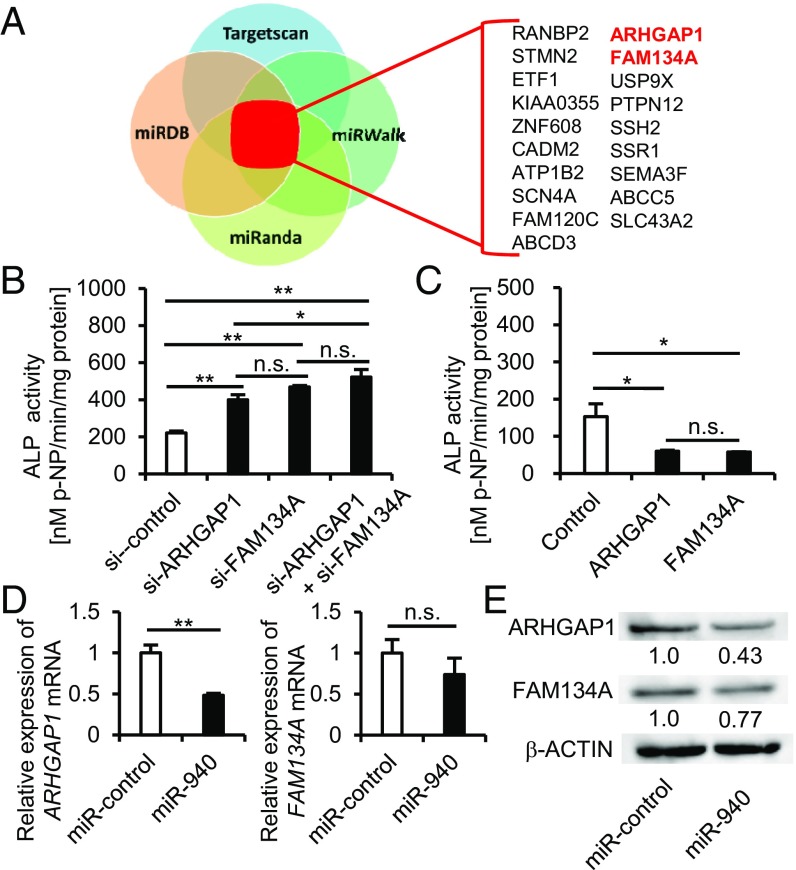
To identify the target genes of hsa-miR-940 to regulate osteogenic differentiation, we performed in silico analysis using four target prediction databases: Target Scan (17), miRDB (18), miRanda (19), and miRWalk (20). According to the analysis, 19 candidate genes were identified as targets of miR-940 (Fig. 3A). To determine whether miR-940 regulates osteogenic differentiation through targeting these genes, hMSCs were transfected with siRNA of each candidate gene and cultured in osteogenic induction medium. After a 14-d osteogenic induction, the down-regulation of ARHGAP1 and FAM134A significantly increased the ALP activity of hMSCs (Fig. 3B). In contrast, hMSCs with stable overexpression of ARHGAP1 or FAM134A also showed a significant decrease in the ALP activity of the cells (Fig. 3C). Moreover, we also confirmed that miR-940 overexpression in hMSCs decreased ARHGAP1 and FAM134A protein levels, as well as their mRNA expression levels (Fig. 3 D and E), indicating that ARHGAP1 and FAM134A were targets of miR-940. According to the in silico analysis, ARHGAP1 and FAM134A have the binding sites for miR-940 in each 3′UTR region (Fig. S3A). To confirm that miR-940 binds to the lesions, we performed a luciferase activity assay using a reporter plasmid in which the above putative miR-940 binding sites were cloned into the 3′UTR of the luciferase gene. In accordance with the in silico prediction, transient overexpression of miR-940 significantly decreased the luciferase activity of both binding sites of ARHGAP1 and FAM134A (Fig. S3B, Left), whereas each mutation in these binding sites abrogated the response to miR-940 (Fig. S3B, Right). miR-940 also significantly decreased the luciferase activity of a construct harboring the entire length of the ARHGAP1 or FAM134A 3′UTR (Fig. S3C, Left), and mutations in the binding sites of the 3′UTR diminished the response to miR-940 (Fig. S3C, Right). These findings suggest that miR-940 promotes osteogenic differentiation of hMSCs by targeting ARHGAP1 and FAM134A.
Fig. 3.
ARHGAP1 and FAM134A are targets of hsa-miR-940 to promote osteogenic differentiation. (A) A diagram illustrating in silico analysis. (B and C) The effects of ARHGAP1 or FAM134A knockdown (B) or overexpression (C) on the osteogenic differentiation of hMSCs. (D and E) The down-regulation of mRNA levels (D) and protein levels (E) of target genes by the transient overexpression of miR-940. All of the data are the means ± SEMs (n = 3). n.s., not significant, *P < 0.05, **P < 0.01 by one-way ANOVA with Tukey’s HSD test (B and C) and Student’s t test (D).
Cancer-Secreted hsa-miR-940 Is Transferred via Exosomes to Mesenchymal Stem Cells and Promotes Osteogenesis.
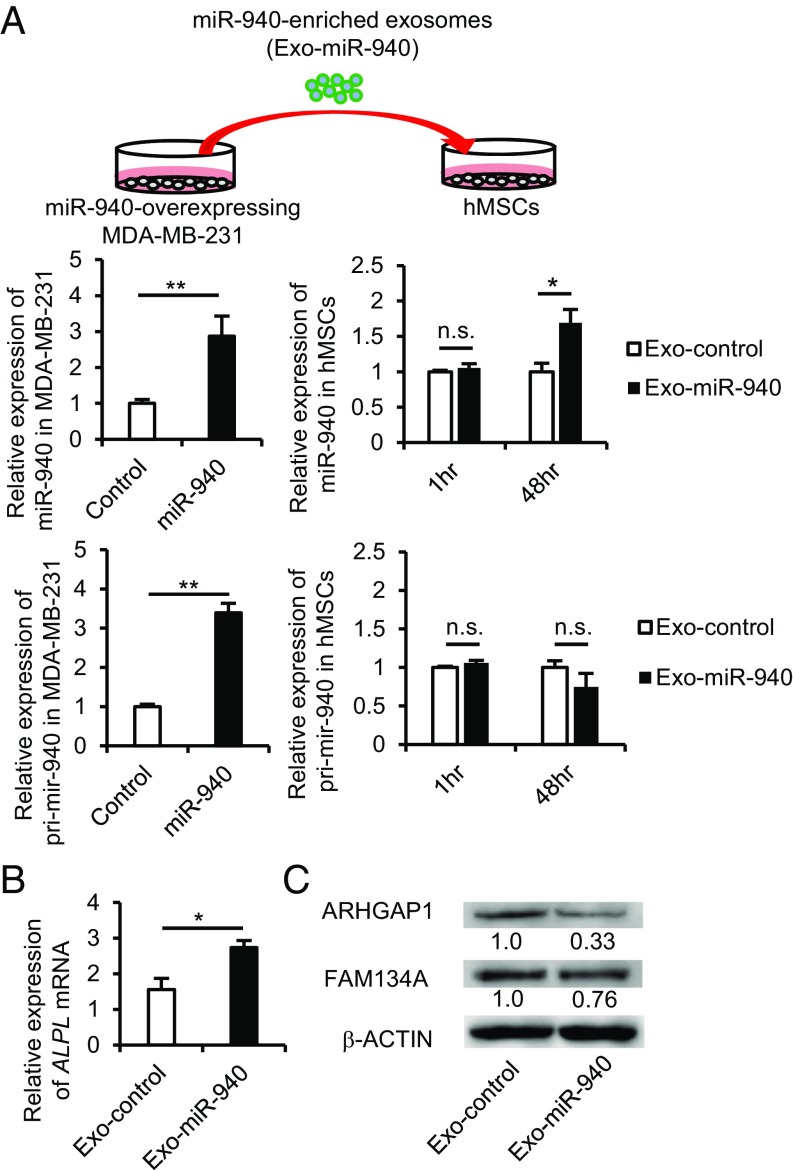
To investigate whether miR-940 overexpression in the osteolytic phenotype-inducing cancer cells would induce the characteristics of osteoblastic phenotype-inducing cancer cells, we established hsa-miR-940–overexpressing MDA-MB-231 cells using a lentiviral infection system (Fig. 4A, Upper). Empty vector-infected MDA-MB-231 cells were also established as a control. As MDA-MB-231 cells were transduced with the genomic fragment of pri-mir-940, the overexpressing cells showed higher expression of mature miR-940 as well as pri-mir-940 compared with the control cells (Fig. 4A, Left). Exosomes from the miR-940–overexpressing MDA-MB-231 cells (Exo-miR-940) or exosomes from the empty vector-infected cells (Exo-control) were added to the culture medium of hMSCs. Exo-miR-940–incorporated hMSCs showed a higher expression of mature miR-940 than Exo-control-incorporated cells. In contrast, the expression level of pri-mir-940 was not altered (Fig. 4A, Right), suggesting that the increased expression of miR-940 in the Exo-miR-940–incorporated hMSCs was due to the transfer of mature miR-940 via exosomes. Indeed, to investigate whether miR-940 is enriched in the exosomes from miR-940–overexpressing cells, MDA-MB-231-CD63-Venus cells were transfected with miR-940 mimic, and Venus+ exosomes were sorted from the culture supernatant by flow cytometry using magnetic beads (Fig. S4A). qPCR analysis showed that miR-940 was significantly expressed in the exosomes from miR-940–overexpressing cancer cells (Fig. S4B). We also examined the expression of ALPL and targets of miR-940 in the Exo-miR-940–incorporated hMSCs. qPCR analysis showed that the expression of ALPL was significantly up-regulated in hMSCs cultured with Exo-miR-940 (Fig. 4B). Western blot analysis revealed that the protein levels of ARHGAP1 and FAM134A were down-regulated in the cells (Fig. 4C), suggesting that the exosomes from miR-940–overexpressing cells were incorporated into the hMSCs and promoted the osteogenic differentiation of the cells by regulating the targets of miR-940.
Fig. 4.
Cancer-secreted hsa-miR-940 is transferred via exosomes to mesenchymal stem cells and promotes osteogenesis. (A) A diagram illustrating an in vitro experiment (Upper). qPCR analysis showed that hMSCs cultured with Exo-miR-940 for 48 h exhibited higher expression of mature miR-940 but not pri-mir-940 (Lower). (B and C) The increased expression level of ALPL (B) and down-regulation of the ARHGAP1 and FAM134A protein levels (C) in hMSCs cultured with Exo-miR-940. Exo-control, exosomes from empty vector-infected MDA-MB-231 cells. Exo-miR-940, exosomes from miR-940–overexpressing MDA-MB-231 cells. All of the data are the means ± SEMs (n = 3–4). n.s., not significant, *P < 0.05, **P < 0.01 by Student’s t test.
Cancer-Secreted hsa-miR-940 Induced Osteoblastic Lesions in the Bone Microenvironment in Vivo.
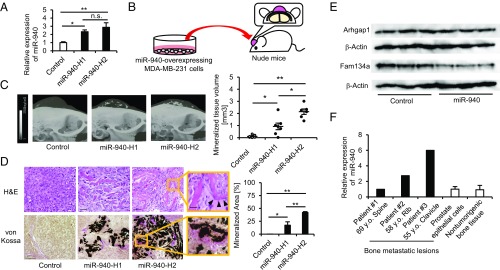
To investigate whether miR-940 overexpression can induce an osteoblastic phenotype in bone metastatic lesions in vivo, we established two clones of miR-940–overexpressing MDA-MB-231-CD63-Venus cells, miR-940-H1 and miR-940-H2, which exhibited different levels of miR-940 overexpression (Fig. 5A). These clonal cells were implanted on the calvarial bones of immunodeficient mice (Fig. 5B). This mouse model is useful to assess the interaction between implanted cancer cells and host cells in the bone microenvironment (21). Interestingly, miR-940–overexpressing MDA-MB-231 cells formed tumors with extensive mineralized tissues inside (Fig. 5C). H&E or von Kossa staining confirmed that the mineralized tissues were composed of bone matrix and surrounded by osteoblast-like cells or osteoids (Fig. 5D). The volume of osteoblastic lesions correlated with the overexpression levels of miR-940 (Fig. 5 C and D). miR-940–overexpressing MDA-MB-231 cells were also injected into the tibiae of immunodeficient mice. The miR-940-–overexpressing cells also induced osteoblastic lesions at the tibial sites (Fig. S5).
Fig. 5.
Cancer cell-derived hsa-miR-940 induces osteoblastic lesions in the bone microenvironment in vivo. (A) qPCR analysis showing the expression level of miR-940 in the established miR-940-overexpressing MDA-MB-231 cell clones. (B) A diagram illustrating the design of in vivo experiments. The miR-940–overexpressing tumor cells were implanted on the calvarial bones of nude mice. (C) Representative micro-CT images reconstructed to 3D images (Left). The volume of mineralized tissues correlated with the overexpression levels of miR-940 (Right). (D) Representative H&E staining (Upper) and von Kossa staining (Lower) of the osteoblastic lesions, which comprised mineralized bone matrix surrounded by osteoblast-like cells (black arrowheads) or osteoids (Right). [Scale bars, 50 μm (Upper), 100 μm (Lower), and 20 μm (enlargements).] (E) Down-regulation of protein levels of target genes of miR-940 in host cells that incorporated cancer cell-derived exosomes. (F) qPCR analysis showing the expression level of miR-940 in bone metastatic lesions of prostate cancer patients. All of the data are the means ± SEMs (n = 3–7). *P < 0.05, **P < 0.01 by one-way ANOVA with Tukey’s HSD test (A and D) or the Kruskal–Wallis test with the Mann–Whitney U test (C).
Furthermore, to ensure that the osteoblastic lesions were induced by the transfer of miR-940–containing exosomes in vivo, CD63 fused with red fluorescent protein (CD63-tdTomato) was stably transduced into miR-940–overexpressing MDA-MB-231 cells, and the cells were implanted on the calvarial bones of GFP-transgenic nude mice with ubiquitous GFP expression (Fig. S6A). Confocal microscopy analysis of the developed tumors showed that tdTomato-labeled exosomes secreted by the cancer cells were incorporated into GFP-expressing host cells (Fig. S6B, arrowheads). In addition, we dissociated the developed tumors and isolated GFP+ host cells using flow cytometry. We extracted proteins from the sorted cells and performed Western blot analyses. The results showed that the protein levels of the miR-940 target genes, Arhgap1 and Fam134a, were suppressed in the GFP+ host cells that incorporated cancer cell-derived exosomes (Fig. 5E). We also performed immunofluorescent staining of the osteoblastic lesions using primary antibodies against ALP. Confocal microscopy analysis showed that GFP-expressing host cells around the osteoblastic lesions expressed ALP, suggesting that the osteogenic potential of host cells was up-regulated by cancer cell-derived exosomes (Fig. S6C). We also investigated the expression level of miR-940 in bone metastatic lesions of prostate cancer patients. Three samples were analyzed for qPCR analysis, and the results showed that two samples had higher expression of miR-940 compared with human nontumorigenic prostate epithelial cells (RWPE-1 cells) or bone tissues (Fig. 5F).
Discussion
Prostate cancer is one of the most common cancers in men worldwide, and osteoblastic-type bone metastasis is observed in up to 70% of patients with prostate cancer. Bone metastasis can cause severe pain, pathological fractures, and spinal cord compression. An improved understanding of the mechanisms underlying bone metastasis can facilitate the development of new therapeutic options and improve patient survival.
The bone microenvironment comprises osteoblasts, osteoclasts, and many other cell types, including MSCs. Therefore, it is believed that the cross-talk between metastasized cancer cells and the surrounding bone cells is critical for the formation of the osteoblastic or osteolytic phenotype. Previous studies have demonstrated that metastasized prostate cancer cells secrete several osteotropic factors, such as BMPs, VEGF, PDGF, and ET-1, and promote osteoblastic-type bone metastasis (3–5). Identifying such factors is important because the signaling pathways mediated by the molecules may be potential targets to prevent or treat osteoblastic-type bone metastasis.
miRNAs also serve as important intercellular communication tools, as they are transferred between cells via exosomes and influence the phenotypes of their recipient cells (14). Numerous studies have shown that cancer-secreted miRNAs can modify the tumor microenvironment to facilitate tumor development, progression, and metastasis (22, 23). These findings raise the possibility that bone remodeling in the bone metastatic lesions can be regulated by miRNAs secreted by cancer cells. However, the role of cancer-secreted miRNAs in the bone metastatic phenotype is not yet understood.
In our study, miRNA microarray analysis identified eight human miRNAs that were highly expressed in the exosomes isolated from the prostate cancer cells. In vitro analysis showed that hsa-miR-940 significantly promoted the osteogenic differentiation of hMSCs (Fig. 2 B and C). To date, several studies have shown that miR-940 plays diverse roles in cell proliferation, migration, metastasis, and apoptosis in various types of cancers (24, 25). However, the role of miR-940 in bone metabolism has not previously been reported. Therefore, our study reveals the osteogenic effect of cancer-secreted miR-940. To investigate whether miR-940 is generally expressed in prostate cancer cell lines that induce osteoblastic bone metastasis, we also examined the expression of miR-940 in other osteoblastic phenotype-inducing cancer cell lines, VCaP and MDA-PCa-2b cells. However, the expression level of miR-940 in these cells was comparable to that observed in the osteolytic phenotype-inducing prostate cancer cell lines, DU-145 and PC-3 cells (Fig. S7). These findings suggest that miR-940 is not generally involved in the induction of osteoblastic bone metastasis of prostate cancer. VCaP or MDA-PCa-2b cells might induce osteoblastic bone metastasis via different mechanisms, such as prostatic acid phosphatase, BMP-2, TGF-β, bFGF, or other soluble osteoblast-stimulating factors (26, 27).
We identified ARHGAP1 and FAM134A as targets of hsa-miR-940 in the regulation of osteogenic differentiation. ARHGAP1 is a factor comprising GTPase-activating proteins, which enhance intrinsic GTPase activity, leading to G protein inactivation. Previous studies have reported that ARHGAP1 regulated the epithelial-to-mesenchymal transition by inhibiting RhoA/ROCK signaling (28). RhoA/ROCK signaling is known to be involved in regulating the proliferation, differentiation, and apoptosis of various cell types. Previous studies have also reported that the RhoA/ROCK pathway stimulated osteogenic differentiation in mesenchymal stem cells and that inhibition of the pathway reduced hMSC osteogenesis (29). In our study, ARHGAP1 overexpression decreased the ALP activity levels of hMSCs (Fig. 3C). This inhibitory effect may be attributable to RhoA/ROCK pathway suppression. FAM134A is broadly expressed in many types of adult and fetal tissues, as well as cancer tissues. A previous study showed that FAM134A played important roles in promoting tumor metastasis by enhancing cancer cell invasion, mobility, and adherence capabilities (30). However, the physiological function of FAM134A in osteogenesis remains unclear.
MDA-MB-231 cells are commonly known as an osteolytic phenotype-inducing cancer cell line (Fig. 1A). We established miR-940–overexpressing MDA-MB-231 cells, which had a miR-940 expression level comparable to C4 lineage cell lines (Fig. 4A and Fig. S7), and implanted the cells on the calvaria of mice. Interestingly, miR-940–overexpressing MDA-MB-231 cells induced extensive osteoblastic lesions in the resulting tumors (Fig. 5 C and D), indicating that miR-940 is a crucial osteogenic factor in vivo. Moreover, we demonstrated that the osteoblastic lesions were induced by the transfer of exosomes from cancer cells to host stromal cells (Fig. S6B) and that the osteogenic activity of host cells was up-regulated through suppressing the protein levels of Arhgap1 and Fam134a (Fig. 5E and Fig. S6C). miR-940–overexpressing MDA-MB-231 cells were also implanted into the subcutaneous tissues of mice. However, osteoblastic lesions were not observed in the developed tumors (Fig. S8). This finding indicated that the osteoblastic lesions induced by miR-940 overexpression could be microenvironment-dependent and that additional factors in the bone microenvironment may mediate the formation of the osteoblastic lesions.
Finally, in preliminary analyses, we also showed that miR-940 enhanced the resistance of cancer cells against chemotherapy agents. Clinically, advanced prostate or breast cancers become hormone-independent and rapidly develop resistance to chemotherapy, leading to aggressive bone metastasis. Recent numerous studies have shown the significance of miRNAs in cancer therapeutic response or drug resistance. Several miRNAs were also identified as predictive biomarkers of drug resistance (31, 32). Our high-throughput inhibitor screening revealed that miR-940 increased resistance to several chemotherapy agents, such as 5-FU, methotrexate, and vinblastine (Fig. S9).
In conclusion, we demonstrated that prostate cancer-secreted hsa-miR-940 promoted the osteogenic differentiation of hMSCs in vitro and induced extensive osteoblastic lesions in the bone metastatic microenvironment in vivo. This study provides a demonstration that a cancer-secreted miRNA induced osteoblastic-type bone metastasis, serving as an osteotropic factor in the bone microenvironment.
Materials and Methods
Cell Culture and Osteogenic Differentiation.
The human prostatic carcinoma cell lines C4, C4-2, and C4-2B, the human breast adenocarcinoma cell line MDA-MB-231-Luc, and the immortalized human mesenchymal stem cell line UCB408E6E7TERT-33 were cultured as previously reported (15, 16, 33). The osteogenic differentiation potential of hMSCs was examined using the ALP assay and von Kossa staining, as previously described (12). Further details are provided in the SI Materials and Methods.
Exosome Isolation and miRNA Microarray Analysis.
Exosomes were isolated from the culture medium by ultracentrifugation as previously reported (34). Microarray analysis was performed using an Agilent Human miRNA Microarray Kit (V3). The data were subsequently normalized and analyzed using GeneSpring GX software (Agilent Technologies). Additional details are provided in the SI Materials and Methods.
qPCR and Western Blot Analysis.
Relative miRNA expression levels were determined by qPCR using miScript SYBR Green PCR Kit (Qiagen). Western blot analysis was performed as previously described (12). Additional details are provided in the SI Materials and Methods.
Cancer Cell Implantation and Microcomputed Tomography Analysis.
MDA-MB-231 cells were implanted on the calvaria of BALB/cAJcl-nu/nu mice, as previously reported (21). Microcomputed tomography (Micro-CT) analysis was performed using R_mCT2 (Rigaku) and TRI/FCS-BON (Ratoc System Engineering). Additional details are provided in the SI Materials and Methods. All animal experiments were performed with approval from the Animal Study Committee of Tokyo Medical and Dental University and conformed to the relevant guidelines and legislations.
Human Bone Metastasis Specimens.
The Cancer Institute of the Japanese Foundation for Cancer Research stocks frozen samples of bone metastatic lesions that were previously harvested from prostate cancer patients. Informed consent for their use in medical research was obtained under an Institutional Review Board-approved protocol. The ethics committee of the Cancer Institute approved the use of such samples in this study. The expression of miR-940 in human samples was examined by qPCR.
Statistical Analyses.
All of the data are presented as the means ± SEMs. The values were considered significant at P < 0.05. The results are representative of more than three individual experiments. Additional details are provided in the SI Materials and Methods.
Supplementary Material
Acknowledgments
The methods for human osteoclast isolation and differentiation were instructed by Dr. Toru Yago (Tokyo Women’s Medical University). The antialkaline phosphatase primary antibody was generously provided by Dr. Kimimitsu Oda (Niigata University). This work was supported by Grants-in-Aid for Scientific Research (KAKENHI, 24791567, 26893068, and 16H06276). This work was also supported by the Core Research for Evolutional Science and Technology (JP17gm0610008) and the Japan Agency for Medical Research and Development (JP17gk0210008).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1717363115/-/DCSupplemental.
References
- 1.Kingsley LA, Fournier PG, Chirgwin JM, Guise TA. Molecular biology of bone metastasis. Mol Cancer Ther. 2007;6:2609–2617. doi: 10.1158/1535-7163.MCT-07-0234. [DOI] [PubMed] [Google Scholar]
- 2.Papachristou DJ, Basdra EK, Papavassiliou AG. Bone metastases: Molecular mechanisms and novel therapeutic interventions. Med Res Rev. 2012;32:611–636. doi: 10.1002/med.20224. [DOI] [PubMed] [Google Scholar]
- 3.Dai J, et al. Vascular endothelial growth factor contributes to the prostate cancer-induced osteoblast differentiation mediated by bone morphogenetic protein. Cancer Res. 2004;64:994–999. doi: 10.1158/0008-5472.can-03-1382. [DOI] [PubMed] [Google Scholar]
- 4.Dolloff NG, et al. Bone-metastatic potential of human prostate cancer cells correlates with Akt/PKB activation by alpha platelet-derived growth factor receptor. Oncogene. 2005;24:6848–6854. doi: 10.1038/sj.onc.1208815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin JJ, et al. A causal role for endothelin-1 in the pathogenesis of osteoblastic bone metastases. Proc Natl Acad Sci USA. 2003;100:10954–10959. doi: 10.1073/pnas.1830978100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farrugia AN, et al. Receptor activator of nuclear factor-kappaB ligand expression by human myeloma cells mediates osteoclast formation in vitro and correlates with bone destruction in vivo. Cancer Res. 2003;63:5438–5445. [PubMed] [Google Scholar]
- 7.Boucharaba A, et al. Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer. J Clin Invest. 2004;114:1714–1725. doi: 10.1172/JCI22123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 9.Hobert O. Gene regulation by transcription factors and microRNAs. Science. 2008;319:1785–1786. doi: 10.1126/science.1151651. [DOI] [PubMed] [Google Scholar]
- 10.Kloosterman WP, Plasterk RH. The diverse functions of microRNAs in animal development and disease. Dev Cell. 2006;11:441–450. doi: 10.1016/j.devcel.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Inose H, et al. A microRNA regulatory mechanism of osteoblast differentiation. Proc Natl Acad Sci USA. 2009;106:20794–20799. doi: 10.1073/pnas.0909311106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukuda T, et al. MicroRNA-145 regulates osteoblastic differentiation by targeting the transcription factor Cbfb. FEBS Lett. 2015;589:3302–3308. doi: 10.1016/j.febslet.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 13.Suetsugu A, et al. Imaging exosome transfer from breast cancer cells to stroma at metastatic sites in orthotopic nude-mouse models. Adv Drug Deliv Rev. 2013;65:383–390. doi: 10.1016/j.addr.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Kosaka N, Yoshioka Y, Fujita Y, Ochiya T. Versatile roles of extracellular vesicles in cancer. J Clin Invest. 2016;126:1163–1172. doi: 10.1172/JCI81130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thalmann GN, et al. Androgen-independent cancer progression and bone metastasis in the LNCaP model of human prostate cancer. Cancer Res. 1994;54:2577–2581. [PubMed] [Google Scholar]
- 16.Yoneda T, Williams PJ, Hiraga T, Niewolna M, Nishimura R. A bone-seeking clone exhibits different biological properties from the MDA-MB-231 parental human breast cancer cells and a brain-seeking clone in vivo and in vitro. J Bone Miner Res. 2001;16:1486–1495. doi: 10.1359/jbmr.2001.16.8.1486. [DOI] [PubMed] [Google Scholar]
- 17.Agarwal V, Bell GW, Nam JW, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. eLife. 2015;4:e05005. doi: 10.7554/eLife.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong N, Wang X. miRDB: An online resource for microRNA target prediction and functional annotations. Nucleic Acids Res. 2015;43:D146–D152. doi: 10.1093/nar/gku1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Betel D, Koppal A, Agius P, Sander C, Leslie C. Comprehensive modeling of microRNA targets predicts functional non-conserved and non-canonical sites. Genome Biol. 2010;11:R90. doi: 10.1186/gb-2010-11-8-r90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dweep H, Gretz N. miRWalk2.0: A comprehensive atlas of microRNA-target interactions. Nat Methods. 2015;12:697. doi: 10.1038/nmeth.3485. [DOI] [PubMed] [Google Scholar]
- 21.Futakuchi M, et al. Transforming growth factor-beta signaling at the tumor-bone interface promotes mammary tumor growth and osteoclast activation. Cancer Sci. 2009;100:71–81. doi: 10.1111/j.1349-7006.2008.01012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou W, et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 2014;25:501–515. doi: 10.1016/j.ccr.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melo SA, et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell. 2014;26:707–721. doi: 10.1016/j.ccell.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan B, Liang Y, Wang D, Luo F. MiR-940 inhibits hepatocellular carcinoma growth and correlates with prognosis of hepatocellular carcinoma patients. Cancer Sci. 2015;106:819–824. doi: 10.1111/cas.12688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rashed MH, et al. Exosomal miR-940 maintains SRC-mediated oncogenic activity in cancer cells: A possible role for exosomal disposal of tumor suppressor miRNAs. Oncotarget. 2017;8:20145–20164. doi: 10.18632/oncotarget.15525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirschenbaum A, Liu XH, Yao S, Leiter A, Levine AC. Prostatic acid phosphatase is expressed in human prostate cancer bone metastases and promotes osteoblast differentiation. Ann N Y Acad Sci. 2011;1237:64–70. doi: 10.1111/j.1749-6632.2011.06198.x. [DOI] [PubMed] [Google Scholar]
- 27.Yang J, et al. Prostate cancer cells induce osteoblast differentiation through a Cbfa1-dependent pathway. Cancer Res. 2001;61:5652–5659. [PubMed] [Google Scholar]
- 28.Clay MR, Halloran MC. Rho activation is apically restricted by Arhgap1 in neural crest cells and drives epithelial-to-mesenchymal transition. Development. 2013;140:3198–3209. doi: 10.1242/dev.095448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J, Liu G, Meng Y, Lin H, Lu Y. MAG-2 promotes invasion, mobility and adherence capability of lung cancer cells by MMP-2, CD44 and intracellular calcium in vitro. Oncol Rep. 2009;21:697–706. [PubMed] [Google Scholar]
- 31.Li F, Mahato RI. MicroRNAs and drug resistance in prostate cancers. Mol Pharm. 2014;11:2539–2552. doi: 10.1021/mp500099g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurozumi S, et al. Recent trends in microRNA research into breast cancer with particular focus on the associations between microRNAs and intrinsic subtypes. J Hum Genet. 2017;62:15–24. doi: 10.1038/jhg.2016.89. [DOI] [PubMed] [Google Scholar]
- 33.Terai M, et al. Immortalization of human fetal cells: The life span of umbilical cord blood-derived cells can be prolonged without manipulating p16INK4a/RB braking pathway. Mol Biol Cell. 2005;16:1491–1499. doi: 10.1091/mbc.E04-07-0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshioka Y, et al. Ultra-sensitive liquid biopsy of circulating extracellular vesicles using ExoScreen. Nat Commun. 2014;5:3591. doi: 10.1038/ncomms4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yago T, Nanke Y, Kawamoto M, Yamanaka H, Kotake S. Tacrolimus potently inhibits human osteoclastogenesis induced by IL-17 from human monocytes alone and suppresses human Th17 differentiation. Cytokine. 2012;59:252–257. doi: 10.1016/j.cyto.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 37.R Core Team 2016. R: A Language and Environment for Statistical Computing, v3.3.1 (R Foundation for Statistical Computing, Vienna)
- 38.Fukuda T, et al. Sema3A regulates bone-mass accrual through sensory innervations. Nature. 2013;497:490–493. doi: 10.1038/nature12115. [DOI] [PubMed] [Google Scholar]
- 39.Sato S, et al. Central control of bone remodeling by neuromedin U. Nat Med. 2007;13:1234–1240. doi: 10.1038/nm1640. [DOI] [PubMed] [Google Scholar]
- 40.Oda K, et al. A general method for rapid purification of soluble versions of glycosylphosphatidylinositol-anchored proteins expressed in insect cells: An application for human tissue-nonspecific alkaline phosphatase. J Biochem. 1999;126:694–699. doi: 10.1093/oxfordjournals.jbchem.a022505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.