We read with interest the paper of Suofu et al. (1), demonstrating that neuronal mitochondria produce melatonin, which upon binding to its melatonin type 1 (MT1) receptor on the mitochondrial membrane (MM) inhibits cytochrome c release, caspase activation, and apoptosis. We commend the authors on their thorough investigation but wish to point out some major questions: (i) Is the presence of the MT1 receptor in mitochondria limited to the neuronal cells or may it also apply to other cells, for example, endothelial cells that are components of blood vessels and are critical for delivery of oxygen and nutrients to all tissues, vessels’ regeneration, and angiogenesis? (ii) Is the MT2 receptor expressed on mitochondria and what is its role?
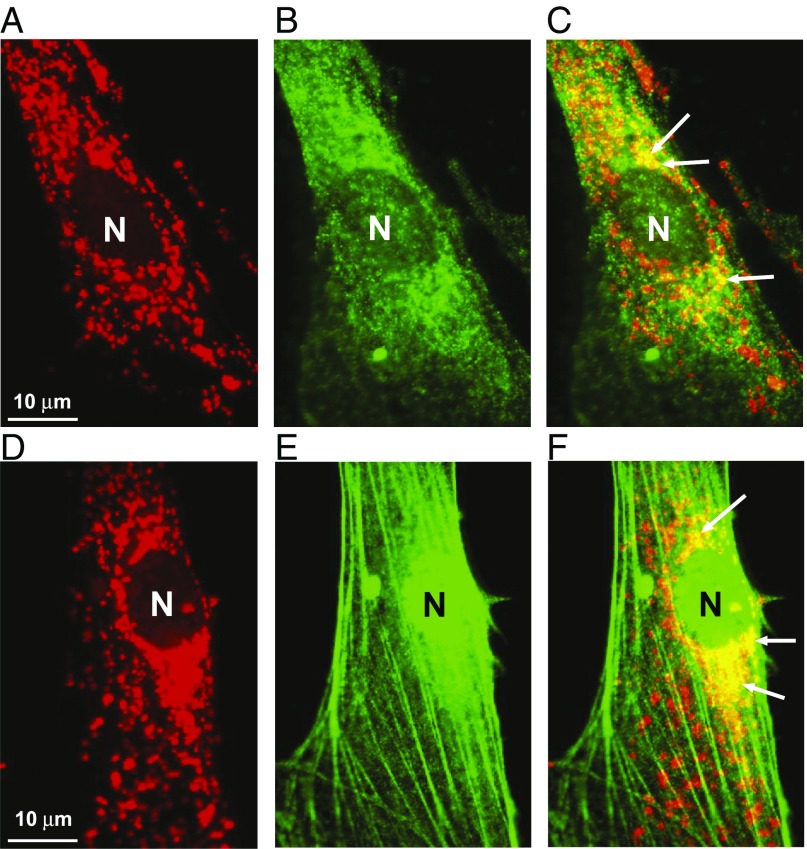
Melatonin is abundantly expressed beyond the neural system; for example, in gastrointestinal tissues, including the stomach, where it has been shown to protect gastric mucosa against stress, ischemia, and nonsteroidal antiinflammatory drug-induced injury, and to accelerate ulcer healing (2–6). We recently demonstrated that both MT1 and MT2 are expressed on the MM of gastric endothelial cells (GECs); MT1 was expressed in MM while MT2 was expressed in the nucleus and MM (Fig. 1). However, in contrast to the study of Suofu et al. (1), we found that treatment of GECs with exogenous melatonin (10 µM) increased by 1.7-fold (P < 0.001) MM potential, which drives ATP synthesis (7). Moreover, treatment of GECs with exogenous melatonin increased the MM expression of MT1 and MT2 by 2.5- and 1.6-fold, respectively, and increased in vitro angiogenesis (new blood vessel formation) by 1.4-fold (P < 0.001). It should be noted that the angiogenic response to melatonin was independent of either cell proliferation or apoptosis, leading us to speculate that, at least in GECs, melatonin signaling via mitochondria extends beyond cellular protection. Although, we have not yet determined the relative contributions of MT1 vs. MT2 in the angiogenic response of GECs to melatonin signaling, our finding that exogenous melatonin increased the MM expression of both MT2 and MT1 is at least indicative that both receptors are involved. Suofu et al. (1) did not assess the relative MM expression levels of MT1 vs. MT2, but rather used the MT2 selective inhibitor, 4P-PDOT, as evidence that melatonin signaling in neuronal mitochondria is mediated exclusively through MM-expressed MT1. Nevertheless, luzindole, which did prevent melatonin from blocking Ca2+-mediated cytochrome c release, has a much greater affinity for MT2 vs. MT1. Moreover, the possibility that MT2 plays a “decoy” role, akin to Flt1 in VEGF signaling, cannot be excluded. In such a scenario, MT2 may play a more subtle role in regulating (e.g., dampening) melatonin signaling. On the other hand, the role of MT2 in mitochondrial signaling by melatonin may be restricted to certain cell types (e.g., endothelial cells but not to brain neurons). The work of Suofo et al. (1) demonstrates novel melatonin signaling in neuronal mitochondria. Our work uncovered that this mechanism operates in endothelial cells that are major components of all blood vessels. We further suggest that melatonin signaling in mitochondria may also be applicable to other physiological processes, such as angiogenesis, gastroprotection, cardioprotection, and aging (3–6, 8, 9).
Fig. 1.
Localization of melatonin receptors MT1 and MT2 in MM of gastric endothelial cells (GECs). (A) Staining of mitochondria using MitoTracker, a fluorescent dye that stains mitochondria (red) in a manner dependent on mitochondrial membrane potential. (B) Immunofluorescence staining for MT1 in GECs (green). (C) Overlay of MitoTracker and MT1 immunostaining images showing localization of MT1 in MM as yellow/orange staining (arrows). (D) Staining of mitochondria using MitoTracker (red). (E) Immunofluorescence staining for MT2 in GECs (green); please note strong MT2 expression in the nucleus (N). (F) Overlay of MitoTracker and MT2 immunostaining images showing localization of MT2 in MM as yellow/orange staining (arrows).
Acknowledgments
This work was supported by Merit Review Award I01 BX000626-05A2 from the US Department of Veterans Affairs Biomedical Laboratory Research and Development Service (to A.S.T.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Suofu Y, et al. Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc Natl Acad Sci USA. 2017;114:E7997–E8006. doi: 10.1073/pnas.1705768114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bubenik GA. Thirty four years since the discovery of gastrointestinal melatonin. J Physiol Pharmacol. 2008;59:33–51. [PubMed] [Google Scholar]
- 3.Karasek M. Melatonin, human aging, and age-related diseases. Exp Gerontol. 2004;39:1723–1729. doi: 10.1016/j.exger.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 4.Ma Z, et al. Melatonin and mitochondrial function during ischemia/reperfusion injury. Cell Mol Life Sci. 2017;74:3989–3998. doi: 10.1007/s00018-017-2618-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brzozowska I, et al. Role of prostaglandins, nitric oxide, sensory nerves and gastrin in acceleration of ulcer healing by melatonin and its precursor, L-tryptophan. J Pineal Res. 2002;32:149–162. doi: 10.1034/j.1600-079x.2002.1o811.x. [DOI] [PubMed] [Google Scholar]
- 6.Brzozowska I, Strzalka M, Drozdowicz D, Konturek SJ, Brzozowski T. Mechanisms of esophageal protection, gastroprotection and ulcer healing by melatonin. Implications for the therapeutic use of melatonin in gastroesophageal reflux disease (GERD) and peptic ulcer disease. Curr Pharm Des. 2014;20:4807–4815. doi: 10.2174/1381612819666131119110258. [DOI] [PubMed] [Google Scholar]
- 7.Klusch N, Murphy BJ, Mills DJ, Yildiz Ö, Kühlbrandt W. Structural basis of proton translocation and force generation in mitochondrial ATP synthase. eLife. 2017;6:6. doi: 10.7554/eLife.33274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tengattini S, et al. Cardiovascular diseases: Protective effects of melatonin. J Pineal Res. 2008;44:16–25. doi: 10.1111/j.1600-079X.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- 9.Lochner A, Huisamen B, Nduhirabandi F. Cardioprotective effect of melatonin against ischaemia/reperfusion damage. Front Biosci (Elite Ed) 2013;5:305–315. doi: 10.2741/e617. [DOI] [PubMed] [Google Scholar]