Every year around Christmas we receive a visitor from the Nordic countries. A hawk keeps returning to a particular branch of a single tree throughout the entire winter, knowing that he will obtain daily treats with little effort. With the advent of spring, he happily returns to his breeding grounds, only to reappear the following winter. This raptor has associated that particular tree branch with easy food and distinguishes it from millions of virtually identical tree branches between Scandinavia and Flanders. Linking sensory stimuli with value is essential to survival, not only for this particular bird but for any organism, both invertebrates and vertebrates. This type of information needs to be encoded, stored, and quickly retrieved after the first stimulus–value associations have been made, and to remain available even after extensive periods of time in the absence of contingent stimulus–reward associations. While short-term memory allows recall of recently acquired information for up to a minute, its capacity is very limited. Long-term memory, on the other hand, has a much greater storage capacity, lasting up to a lifetime. The initial phase of stimulus–value associations depends on dopaminergic-dependent reward-prediction error signals broadcasted by the ventral midbrain, which can be either positive or negative. These signals are ideally suited to promote or suppress behavior associated with the reward (1, 2). The storage in short-term memory is thought to depend on the hippocampus and/or amygdala in the medial temporal lobe, in concert with several divisions of the frontal cortex (3). Over time, however, the memory becomes consolidated and is represented in a distributed cortical network independent of the hippocampus. Major, largely unanswered, questions relate to how and where item value is stored in long-term memory. In PNAS, Ghazizadeh et al. (4) address the “where” question by charting the brain network in nonhuman primates involved in the long-term storage of values associated with many visual objects.
In the first phase of their experiments, they trained monkeys using a conditioning task whereby subjects were required to make a saccadic eye movement to the only object present on a screen. The stimuli were selected from a huge set of fractals, half of which were consistently paired with high rewards and half with low rewards, thus creating “good” and “bad” object categories for the monkey.
After 10 d of training, the second phase of the experiment began. Whole-brain activity was measured using functional magnetic resonance imaging (fMRI) while the monkeys stared at a central fixation point. Meanwhile, good and bad fractals were randomly presented in blocks, either on the left-hand or right-hand side of the screen. In contrast to the preceding training phase of the experiment, rewards were noncontingent relative to the visual stimuli and given only to encourage fixation behavior. Hence, measured differences in fMRI activity should be related to value memory signals that were encoded during the training phase of the experiment.
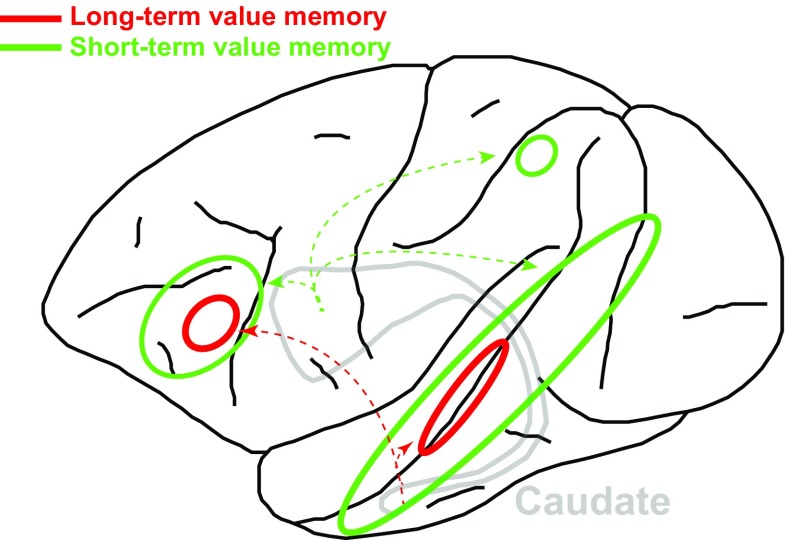
The fMRI signals acquired immediately after the training phase revealed mainly higher activities for good objects than for bad objects (green outlines in Fig. 1), especially in the ventral visual stream in and around the superior temporal sulcus. Similar value memory signals were observed in the ventrolateral prefrontal and orbitofrontal cortex; a small region in the parietal cortex; and also, to a lesser extent, in early visual areas (V1–V4).
Fig. 1.
Schematic of main cortical areas in the monkey (lateral view of the left hemisphere, frontal cortex to the left) showing high-capacity short-term (green) and long-term (red) value memory signals. Arrows indicate projections from the head and tail of the caudate nucleus, respectively.
Remarkably, when the same animals were again tested 6–13 mo after training, without having been exposed to the stimuli during this period, a restricted set of the above-mentioned regions still retained long-term value memory signals (red outlines in Fig. 1). Extrastriate regions characterized by such long-term value memory signals included areas in and near the fundus of the superior temporal sulcus. In the ventral frontal cortex, these months-old value memory signals were largely restricted to area 45B. In contrast, the previously good-preferring voxels in the early visual, parietal, and orbitofrontal cortices had lost their differential activations (good > bad) during the months following training. Thus, value signals were restricted to a subset of the cortex that had initially shown memory signals. Value-coding areas showed strong laterality effects immediately after training, reflecting the representation of the unilaterally presented stimuli in the contralateral hemisphere. This lateralization was reduced in the months after training, which may indicate that the cortex generalized across value memory signals independent of the original stimulus appearance.
Ghazizadeh et al. (4) also analyzed correlations in fMRI signals across cortical regions while the monkeys simply stared at the fixation dot, without additional visual stimulation. Such resting-state fMRI analyses provide a measure of functional connectivity across regions. Interestingly, they observed particularly strong functional connectivity, during rest, between the ventrolateral prefrontal cortex and posterior inferotemporal regions showing long-term value memory signals, which are also known to be anatomically connected (5). Moreover, the strength of the functional connectivity to this frontotemporal network predicted the persistence of long-term value coding for other cortical areas. It is unlikely that the value memory signals are driven (only) by increased attention toward previously highly rewarded objects (6), as typical selective-attention areas in the parietal and frontal cortex (7, 8) lack long-term value memory signals.
Value-based long-term memory signals were also observed subcortically in the caudal-ventral putamen and claustrum, the tail of the caudate, and the dorsal and lateral nuclei of the amygdala. These subcortical structures also showed significant resting-state functional connectivity with the frontotemporal network, expressing long-term value memory signals. The apparent lack of hippocampal involvement in value memory coding may be related to the role of this medial temporal role structure in explicit (declarative) rather than implicit (nondeclarative) memory (9). Indeed, no conscious process was required to explicitly retrieve the object information in the monkey’s task.
Finally, behavioral preferences reflected long-term differences in fMRI activity between good and bad objects. In a free-viewing task, monkeys showed a clear preference for good versus bad objects, which can be regarded as an index of memory strength. One monkey actually showed an even stronger bias for good objects months after the training phase than he had in the days immediately afterwards. Thus, despite the shrinkage of the cortical territory showing value memory signals over time, the behavioral preference for good objects increased. This may indicate that the consolidation process, possibly requiring additional resources, was still ongoing in days immediately after training. Alternatively, fewer or more efficient neurons may be required to sustain a long-term behavioral preference.
The combined functional and behavioral results point to a high-capacity, long-term value memory system that is housed in a frontotemporal cortical network. This network connects with ventral parts of the amygdala, putamen, caudate, and claustrum. Signatures of reward processing or learned associations between objects and reward are ubiquitous in the brain and have previously been shown in the orbitofrontal (10, 11), prefrontal (12), temporal (13, 14), parietal (15), and even early visual (16, 17) cortex. Most of these previous studies, however, focused on reward-related signals that are evident either during or immediately after training, as required for immediate decision-making and flexible cognitive processes. The present study stands out, as it has found very-long-term value effects for an extensive set of stimuli far beyond the context of the conditioning phase of the experiment. The engagement of prefrontal areas in long-term memory processes has
The combination of both short- and long-term value memories in the prefrontal cortex may be key to making correct decisions based on the combination of remote and recent memories.
been hinted at before (18). The prefrontal cortex, however, is more typically associated with short-term memory processes ideally suited for executive control, whereby current information needs to be kept online for short periods of time (19). How can that be reconciled with the present findings, whereby the (lateral) prefrontal cortex is involved in both short-term and long-term value memory processes? The answer may lie in the prefrontal cortex’s connectivity with the ventral basal ganglia and posterior inferotemporal areas. Kim and Hikosaka (20) have recently shown that short-term and long-term value memory for visual stimuli is coded in different compartments of the basal ganglia. Specifically, the tail of the caudate nucleus and the caudolateral sectors of the substantia nigra encode long-term memories. Exactly these nuclei are connected with the prefrontal regions showing long-term value memory signals (red arrows in Fig. 1). The combination of both short- and long-term value memories in the prefrontal cortex may be key to making correct decisions based on the combination of remote and recent memories.
Although the high-capacity network for long-term reward value largely overlaps with the short-term memory network, future research is required to examine convergence at the single-cell level, as well as the functional, anatomical, and biochemical changes underlying long-term memory storage at the neuronal or microcircuit level in primates. The present type of imaging experiments will be crucial for guiding such research (21). Insofar as Ghazizadeh et al. (4) have tapped into mainly implicit memory processes, lingering questions remain regarding the degree of overlap with other long-term memory systems, such as declarative memory. Finally, the present data call for causal experiments to evaluate the contributions of the individual nodes in the long-term value-coding network (21).
Long-term value memory is essential for survival, as in the case of our yearly returning hawk. However, it is also highly relevant in the diseased brain, such as in addiction, where old, very-high-value memories can trigger relapse during periods of drug abstinence. Understanding the macro- (22) and microcircuitry and mechanisms underlying long-term memory in such cases may be key in our fight against these devastating disorders for the patients, their families, and society at large.
Acknowledgments
This work was supported by the Research Foundation Flanders Grants G0D5817N, G090714N, and G000712; KU Leuven Grant C14/17/109; and the European Union’s Horizon 2020 Framework Programme for Research and Innovation under Grant Agreement 720270 (Human Brain Project Specific Grant Agreement1).
Footnotes
The author declares no conflict of interest.
See companion article on page E2135.
References
- 1.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 2.Arsenault JT, Rima S, Stemmann H, Vanduffel W. Role of the primate ventral tegmental area in reinforcement and motivation. Curr Biol. 2014;24:1347–1353. doi: 10.1016/j.cub.2014.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDonald RJ, White NM. A triple dissociation of memory systems: Hippocampus, amygdala, and dorsal striatum. Behav Neurosci. 1993;107:3–22. doi: 10.1037//0735-7044.107.1.3. [DOI] [PubMed] [Google Scholar]
- 4.Ghazizadeh A, Griggs W, Leopold DA, Hikosaka O. Temporal–prefrontal cortical network for discrimination of valuable objects in long-term memory. Proc Natl Acad Sci USA. 2018;115:E2135–E2144. doi: 10.1073/pnas.1707695115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerbella M, Borra E, Tonelli S, Rozzi S, Luppino G. Connectional heterogeneity of the ventral part of the macaque area 46. Cereb Cortex. 2013;23:967–987. doi: 10.1093/cercor/bhs096. [DOI] [PubMed] [Google Scholar]
- 6.Maunsell JH. Neuronal representations of cognitive state: Reward or attention? Trends Cogn Sci. 2004;8:261–265. doi: 10.1016/j.tics.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Schall JD. On the role of frontal eye field in guiding attention and saccades. Vision Res. 2004;44:1453–1467. doi: 10.1016/j.visres.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 8.Colby CL, Goldberg ME. Space and attention in parietal cortex. Annu Rev Neurosci. 1999;22:319–349. doi: 10.1146/annurev.neuro.22.1.319. [DOI] [PubMed] [Google Scholar]
- 9.Eichenbaum H. A cortical-hippocampal system for declarative memory. Nat Rev Neurosci. 2000;1:41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- 10.Noonan MP, Kolling N, Walton ME, Rushworth MF. Re-evaluating the role of the orbitofrontal cortex in reward and reinforcement. Eur J Neurosci. 2012;35:997–1010. doi: 10.1111/j.1460-9568.2012.08023.x. [DOI] [PubMed] [Google Scholar]
- 11.Rolls ET. The orbitofrontal cortex and reward. Cereb Cortex. 2000;10:284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- 12.Roesch MR, Olson CR. Impact of expected reward on neuronal activity in prefrontal cortex, frontal and supplementary eye fields and premotor cortex. J Neurophysiol. 2003;90:1766–1789. doi: 10.1152/jn.00019.2003. [DOI] [PubMed] [Google Scholar]
- 13.Mogami T, Tanaka K. Reward association affects neuronal responses to visual stimuli in macaque te and perirhinal cortices. J Neurosci. 2006;26:6761–6770. doi: 10.1523/JNEUROSCI.4924-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koyano KW, et al. Laminar module cascade from layer 5 to 6 implementing cue-to-target conversion for object memory retrieval in the primate temporal cortex. Neuron. 2016;92:518–529. doi: 10.1016/j.neuron.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 15.Kubanek J, Snyder LH. Reward size informs repeat-switch decisions and strongly modulates the activity of neurons in parietal cortex. Cereb Cortex. 2017;27:447–459. doi: 10.1093/cercor/bhv230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frankó E, Seitz AR, Vogels R. Dissociable neural effects of long-term stimulus-reward pairing in macaque visual cortex. J Cogn Neurosci. 2010;22:1425–1439. doi: 10.1162/jocn.2009.21288. [DOI] [PubMed] [Google Scholar]
- 17.Arsenault JT, Nelissen K, Jarraya B, Vanduffel W. Dopaminergic reward signals selectively decrease fMRI activity in primate visual cortex. Neuron. 2013;77:1174–1186. doi: 10.1016/j.neuron.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blumenfeld RS, Ranganath C. Dorsolateral prefrontal cortex promotes long-term memory formation through its role in working memory organization. J Neurosci. 2006;26:916–925. doi: 10.1523/JNEUROSCI.2353-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 20.Kim HF, Hikosaka O. Distinct basal ganglia circuits controlling behaviors guided by flexible and stable values. Neuron. 2013;79:1001–1010. doi: 10.1016/j.neuron.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vanduffel W, Zhu Q, Orban GA. Monkey cortex through fMRI glasses. Neuron. 2014;83:533–550. doi: 10.1016/j.neuron.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nelissen K, et al. Neural correlates of the formation and retention of cocaine-induced stimulus-reward associations. Biol Psychiatry. 2012;72:422–428. doi: 10.1016/j.biopsych.2012.02.021. [DOI] [PubMed] [Google Scholar]