Mouse embryonic stem cells (ESC) were successfully isolated and characterized in the early 1980s (1, 2), followed by the establishment of ESC from primates more than a decade later (3). However, despite continuous efforts since then, the establishment of ESC lines from domesticated species has remained elusive. Over the years, there have been numerous, sometimes contradictory, reports of success from economically important ungulates, such as cattle and pigs, but the general consensus is that no stable, well-characterized ESC line from these species presently exists (4). This situation may have now changed. Bogliotti et al. (5) employed fibroblast growth factor 2 (FGF2) and a canonical WNT signaling pathway inhibitor in their culture condition, and derived stable pluripotent cell lines from bovine blastocysts. Their work is now published in PNAS (5).
One major obstacle that was thought to hamper the establishment of ESC lines from ungulates was an uncertainty about the precise mechanisms of how signaling pathways controlled the pluripotent state and early embryo development, and whether these pathways were conserved in domesticated ungulates (6, 7). On the assumption that similar regulatory pathways existed, it was assumed that direct application of the protocols established successfully in mouse and human studies would permit establishment of stable ESC lines from ungulates. However, these approaches were generally unsuccessful. Still, in 2015, Wu et al. (8) reported the derivation of a novel type of epiblast stem cells (EpiSC), termed region-selective epiblast stem cells (rsEpiSC) due to their ability to engraft to the posterior part of the gastrula-stage mouse epiblast. The combination of FGF2 and a WNT signaling inhibitor, IWR1, in a simple serum-free medium allows the clonal expansion of both human ESC and mouse EpiSC, as well as EpiSC derivation from mouse embryos (8). Bogliotti et al. (5) used the same human rsEpiSC culture condition, the custom TESR1 medium containing FGF2 and a WNT signaling inhibitor IWR1 (CTFR), and derived bovine ESC (bESC) from blastocysts, termed CTFR-bESC. The derivation of CTFR-bESC can be achieved by whole-blastocyst plating, mechanical isolation of the inner cell mass by microdissection, and inner cell mass isolation by immunosurgery. The efficiency of the process was high and at least comparable to that reported for derivation of mouse ESC. By week three, colonies with homogenous morphology started to appear, and retained a stable morphology for more than 50 passages. Cell karyotype, population doubling time, and transcriptome profile also remained stable over extended culture. These bovine cells expressed typical pluripotent markers, including SOX2 and POU5F1, and were negative for the trophectoderm marker, CDX2, and the primitive endoderm marker, GATA6. They were able to form well-differentiated teratomas containing tissue representing all three germ layers when introduced into immunodeficient mice. Finally, these bESC could be used as nuclear donors to produce cloned embryos from which the “secondary” bESC could be derived. Analysis of their transcriptional and epigenetic profiles indicate that the CTFR-bESC were more similar to human ESC than to mouse ESC, and possessed features more characteristic of a primed rather than a naïve pluripotent state. Unlike human ESC, however, the bESC form colonies lacking clear boundaries, and can tolerate dissociation into single cells when passaged by trypsin.
Perhaps what the Bogliotti et al. (5) paper reinforces is the concept that multiple kinds of pluripotent state exist in laboratory settings (9). Until a few years ago, only two types of pluripotency were generally recognized: the naïve state, as exemplified by ESC derived from the inner cell mass of mouse blastocysts, and primed, as the human ESC or mouse EpiSC established from postimplantation epiblast cells (10). The ground-state naïve pluripotency found in rodent ESC appeared to provide many desirable features, such as unbiased differentiation ability and efficient single-cell clonogenicity, and encouraged a hunt for homologous types of cells of human origin. Indeed, numerous naïve-like human lines have been recently reported, although whether authentic human naïve pluripotency has been attained is still being debated (11). What is clear from the Bogliotti et al. (5) study is that the bESC described are more of the epiblast than naïve type, but still do not entirely mimic either type. It will be important, for example, to apply the gold-standard criterion for pluripotency: namely, to demonstrate convincingly whether or not the bESC can contribute to blastocyst chimeras and ultimately to the gonads of chimeric calves.
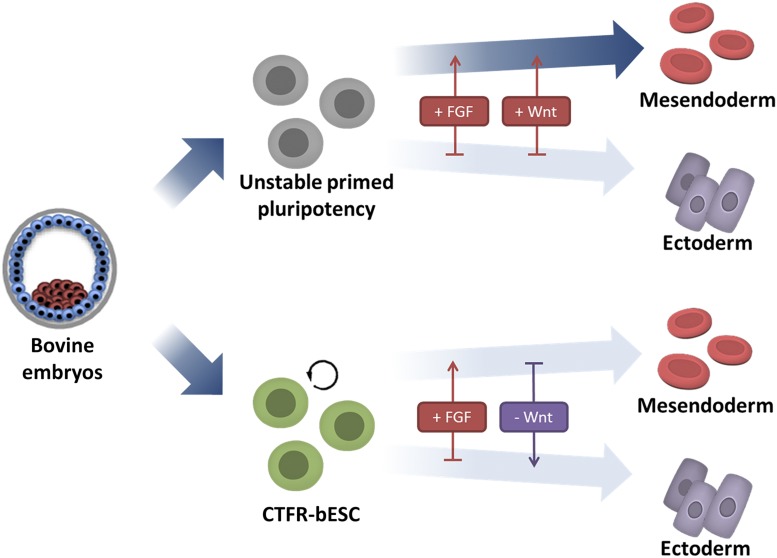
It is intriguing that a combination of FGF2 and WNT signaling inhibition is critical for capturing bovine pluripotency. The WNT signaling pathway is highly conserved and known to play crucial roles in virtually every aspect of embryonic development (12). It promotes self-renewal of mouse naïve-type ESC and prevents their conversion to EpiSC (13). A high level of WNT signaling, on the other hand, triggers differentiation of both mouse and human EpiSC toward mesoendoderm lineages (14, 15), while FGF restricts ectoderm formation. Therefore, blockage of endogenous WNT and the presence of FGF2 in the culture medium can be used to balance two opposing lineage forces and thereby contribute to a more stable state of pluripotency (8, 16). The same concept may also apply in attaining bovine pluripotency (Fig. 1). For example, without the WTN inhibitor presence, potential bovine ESC likely have a tendency to destabilize and shift toward mesoderm lineage. With human ESC, the presence of WNT inhibitor is not necessary to maintain pluripotency, but does reduce heterogeneity in the cell population as a whole (8). In the bovine, however, inhibition of WNT signaling appears to have become a necessity if pluripotency is to be attained.
Fig. 1.
Balancing the stem-cell seesaw, a theory to explain how FGF signaling and WNT inhibition recapitulate pluripotency in cattle. Under standard primed culture condition, the potential bESC differentiate into mesendoderm cells in response to FGF and endogenous WNT. Bogliotti et al. (5) demonstrate the primed-state bESC can be stabilized by FGF2 supplementation and inhibition of WNT. The balance of lineage specification can result in a stabilized pluripotency state in cattle. Modified with permission from ref. 16.
Even though the Bogliotti et al. (5) paper uses a previously established protocol, and provides only limited information toward our understanding of bovine pluripotency and the pathways necessary to maintain that state, the outcomes of the Bogliotti et al. study are likely to be of significant, long-term value to basic animal sciences, agriculture, and biomedicine. First, it seems likely that the protocol described might be applied to other species and not just to cattle to produce pluripotent stem cells that are free of the transgenes normally used to create induced pluripotent cells. Second, these cells possess several desirable features. They can be efficiently established, proliferate quickly, and are stable in long-term culture. They also can be clonally propagated from single cells, thereby permitting multiple rounds of genetic modification and selection, and can serve as nuclear donors for cloning purposes. One potential use of these cells will be for the in vitro differentiation of gametes, as has been achieved in mice (17). As pointed out by Bogliotti et al. (5), if such production of gametes can be achieved, in vitro breeding schemes involving multiple rounds of genomic selection, in vitro gamete production, in vitro fertilization, and bESC derivation, could be used to create genetically superior cattle over a significantly shorter generational interval than could be achieved by standard breeding procedures. Another long-term possibility is that ESC from large farm animal species will be important in creating large animal models for testing the safety and efficacy of stem cell therapies for regenerative medicine, generating organs for transplantation and even producing cultured meat, topics that have been reviewed in more detail elsewhere (18).
Footnotes
The author declares no conflict of interest.
See companion article on page 2090.
References
- 1.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 2.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomson JA, et al. Isolation of a primate embryonic stem cell line. Proc Natl Acad Sci USA. 1995;92:7844–7848. doi: 10.1073/pnas.92.17.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ezashi T, Yuan Y, Roberts RM. Pluripotent stem cells from domesticated mammals. Annu Rev Anim Biosci. 2016;4:223–253. doi: 10.1146/annurev-animal-021815-111202. [DOI] [PubMed] [Google Scholar]
- 5.Bogliotti YS, et al. Efficient derivation of stable primed pluripotent embryonic stem cells from bovine blastocysts. Proc Natl Acad Sci USA. 2018;115:2090–2095. doi: 10.1073/pnas.1716161115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malaver-Ortega LF, Sumer H, Liu J, Verma PJ. The state of the art for pluripotent stem cells derivation in domestic ungulates. Theriogenology. 2012;78:1749–1762. doi: 10.1016/j.theriogenology.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 7.Brevini TA, Pennarossa G, Gandolfi F. No shortcuts to pig embryonic stem cells. Theriogenology. 2010;74:544–550. doi: 10.1016/j.theriogenology.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 8.Wu J, et al. An alternative pluripotent state confers interspecies chimaeric competency. Nature. 2015;521:316–321. doi: 10.1038/nature14413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu J, Izpisua Belmonte JC. Dynamic pluripotent stem cell states and their applications. Cell Stem Cell. 2015;17:509–525. doi: 10.1016/j.stem.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Nichols J, Smith A. Naive and primed pluripotent states. Cell Stem Cell. 2009;4:487–492. doi: 10.1016/j.stem.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 11.Ying QL, Smith A. The art of capturing pluripotency: Creating the right culture. Stem Cell Reports. 2017;8:1457–1464. doi: 10.1016/j.stemcr.2017.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 13.ten Berge D, et al. Embryonic stem cells require Wnt proteins to prevent differentiation to epiblast stem cells. Nat Cell Biol. 2011;13:1070–1075. doi: 10.1038/ncb2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davidson KC, et al. Wnt/β-catenin signaling promotes differentiation, not self-renewal, of human embryonic stem cells and is repressed by Oct4. Proc Natl Acad Sci USA. 2012;109:4485–4490. doi: 10.1073/pnas.1118777109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurek D, et al. Endogenous WNT signals mediate BMP-induced and spontaneous differentiation of epiblast stem cells and human embryonic stem cells. Stem Cell Reports. 2015;4:114–128. doi: 10.1016/j.stemcr.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loh KM, Lim B. Stem cells: Equilibrium established. Nature. 2015;521:299–300. doi: 10.1038/521299a. [DOI] [PubMed] [Google Scholar]
- 17.Hikabe O, et al. Reconstitution in vitro of the entire cycle of the mouse female germ line. Nature. 2016;539:299–303. doi: 10.1038/nature20104. [DOI] [PubMed] [Google Scholar]
- 18.Roberts RM, Yuan Y, Genovese N, Ezashi T. Livestock models for exploiting the promise of pluripotent stem cells. ILAR J. 2015;56:74–82. doi: 10.1093/ilar/ilv005. [DOI] [PMC free article] [PubMed] [Google Scholar]