Abstract
E7777, a recombinant cytotoxic fusion protein comprising diphtheria toxin fragments A and B and human interleukin‐2, shares an amino acid sequence with denileukin diftitox but has improved purity and an increased percentage of active protein monomer species. A phase I study was carried out to evaluate the tolerability, safety, pharmacokinetics, and antitumor activity of E7777 in Japanese patients with relapsed/refractory peripheral and cutaneous T‐cell lymphoma. E7777 (6, 12, and expanded 9 μg/kg/day) was given to 13 patients by i.v. infusion on five consecutive days per 21‐day cycle. Dose‐limiting toxicities, including increased alanine aminotransferase, hyponatremia (n = 2), hypokalemia, lymphopenia, fatigue, hypoalbuminemia, rash, and increased lipase (n = 1), were observed in all three patients in the 12 μg/kg/day cohort, whereas two of six patients in the 9 μg/kg/day cohort showed decreased appetite or fatigue. The maximum tolerated and recommended dose of E7777 was 9 μg/kg/day for five consecutive days per 21‐day cycle. The objective response rate was 38% (5/13) and did not appear to depend on tumor expression of CD25. E7777 was well tolerated, assuming careful management of adverse events during treatment, and preliminary but clinically meaningful antitumor activity was observed. Subsequent studies of E7777 for T‐cell lymphomas are warranted. This study was registered with www.ClinicalTrials.gov (NCT1401530).
Keywords: cutaneous T‐cell lymphoma, E7777, Japanese patients, peripheral T‐cell lymphoma, phase I study
Abbreviations
- AE
adverse event
- AITL
angioimmunoblastic T‐cell lymphoma
- ALCL
anaplastic large cell lymphoma
- ALK
anaplastic lymphoma kinase
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- CCR4
CC chemokine receptor 4
- CTCL
cutaneous T‐cell lymphoma
- DD
denileukin diftitox
- DLT
dose‐limiting toxicity
- IL‐2
interleukin‐2
- IL‐2R
interleukin‐2 receptor
- ISC
independent safety committee
- MF
mycosis fungoides
- MTD
maximum tolerated dose
- NHL
non‐Hodgkin's lymphoma
- NOS
not otherwise specified
- ORR
objective response rate
- PK
pharmacokinetics
- PTCL
peripheral T‐cell lymphoma
- RD
recommended dose
- Treg
regulatory T‐cell
- ULN
upper limit of normal
1. INTRODUCTION
Peripheral T‐cell lymphoma and CTCL are classified as mature T‐cell neoplasms and as rare and heterogeneous forms of NHL. Of these, PTCL is the most common histological subtype of the T‐cell lymphomas that are usually characterized by an aggressive clinical presentation, frequent relapse, and eventual development of refractory disease.1 The standard first‐line treatment for PTCL is multi‐agent chemotherapy, such as cyclophosphamide, doxorubicin, vincristine, and prednisone therapy;2 however, the outcomes have been disappointing, with reported long‐term survival rates of only 20%‐30%.3 Moreover, median overall survival and median progression‐free survival in patients with PTCL after relapse or progression were reported to be 5.5 and 3.1 months, respectively.4 Cutaneous T‐cell lymphoma is a generally indolent and heterogeneous NHL, and advanced cases generally have a poor prognosis.5
Currently, several agents for relapsed or refractory T‐cell NHLs have been approved by the US FDA: pralatrexate, romidepsin, and belinostat for PTCL, brentuximab vedotin for ALCL, vorinostat and romidepsin for CTCL. However, the ORRs of these agents have remained at approximately 30%, except the highest ORR of 86% of brentuximab vedotin for ALCL.6, 7, 8, 9, 10, 11 In Japan, a phase II study of mogamulizumab yielded an ORR of 35% among patients with relapsed CCR4‐positive PTCL and CTCL12 and has been approved for CCR4‐positive T‐cell NHL. In contrast, a European phase II study of mogamulizumab yielded an ORR of only 11% for relapsed or refractory CCR4‐positive PTCL patients.13 Accordingly, the lack of a clear optimal treatment regimen for T‐cell NHL has encouraged the search for new therapies.
One potential therapeutic agent is E7777, a recombinant cytotoxic fusion protein comprising the enzymatically active portions of the diphtheria toxin fragments A and B, and the receptor‐binding domain of human IL‐2. E7777 shares an amino acid sequence with DD, and is manufactured using similar but modified processes. Despite these similarities, E7777 has improved purity and an increased percentage of active protein monomer species and reduced level of misfolded and/or aggregated protein impurities. Both E7777 and DD directly bind to the IL‐2R expressed on malignant cells and are subsequently taken up by receptor‐mediated endocytosis, where the diphtheria toxin domain is cleaved and translocated to the cytoplasm to catalyze the covalent linkage of ADP‐ribose to elongation factor‐2, thus inhibiting protein synthesis and causing cell death.14
Two phase III studies of DD have been reported in patients with CD25‐positive (CD25 on ≥20% of tumor cells) CTCL who had previously received other therapeutic interventions. In these studies, patients were assigned to receive DD at 9 or 18 μg/kg/day on five consecutive days per 21‐day cycle, and the resulting ORRs were 30% and 44%, respectively. Although the two doses did not differ significantly regarding efficacy and safety, the DD 18 μg/kg/day cohorts showed superior outcomes.15, 16 A companion study further examined the efficacy and safety of DD 18 μg/kg/day in patients with low CD25‐expressing (CD25 on <20% of tumor cells) CTCL. The study showed an ORR of 31%, which suggested that low CD25 expression does not preclude a meaningful clinical response to DD.17
Yet another phase II study evaluated DD 18 μg/kg/day for the treatment of other relapsed/refractory T‐cell NHLs, and yielded ORRs of 62% for CD25‐positive tumors (CD25 on ≥10% of tumor cells) and 45% for CD25‐negative tumors (CD25 on <10% of tumor cells).18 As noted above, E7777 and DD share the same construction; however, in the former, the purity is improved and the percentage of active protein monomer species is higher. Furthermore, the specific bioactivity of E7777 is 1.5‐2 times higher than that of DD. Accordingly, in this report we describe the results of a phase I study of E7777 in Japanese patients with PTCL and CTCL.
2. MATERIALS AND METHODS
2.1. Study design and treatment
This multicenter, open‐label, phase I study of E7777 in Japanese patients with relapsed or refractory PTCL and CTCL aimed to evaluate the tolerability, safety, PK, immunogenicity and antitumor activity of this agent and to estimate the RD for subsequent studies. E7777 was given by i.v. infusion over a 60‐min period on five consecutive days per 21‐day cycle, with up to eight cycles. The planned dose levels were 6, 12, 15, and 18 μg/kg/day, with evaluation of intermediate dose levels if necessary. All patients received required premedication comprising acetaminophen, antihistamine drugs, anti‐emetic drugs, systemic steroids, and i.v. hydration.
The DLT of E7777 was evaluated during the first cycle, and the MTD was defined as the highest dose at which fewer than two of six patients experienced a DLT. If two patients experienced DLT at a particular dose level, an ISC was consulted to determine whether that dose could be considered the MTD. One dose reduction in the following cycle was allowed if a DLT or similar toxicity event occurred. The RD was comprehensively determined using the MTD and safety data. Furthermore, tumor CD25 expression was explored in this study.
2.2. Patient eligibility
The key inclusion criteria included: (i) age of 20‐79 years; (ii) histological or cytological diagnosis of PTCL or CTCL; (iii) relapsed or refractory disease after systemic therapy, including psoralen‐UV A radiation and retinoid; (iv) adequate renal (serum creatinine ≤1.5× ULN or calculated creatinine clearance ≥50 mL/min), hepatic (total bilirubin ≤1.5× ULN, serum albumin ≥3.0 g/dL, and AST or ALT ≤3.0× ULN) and hematologic (absolute neutrophil count ≥1.0×103/μL, platelet count ≥5.0 × 104/μL, and hemoglobin ≥8.0 g/dL) functions; (v) ECOG performance status of 0 or 1; and (vi) a life expectancy ≥3 months. The key exclusion criteria included: (i) central nervous system invasion with clinical symptoms or requiring treatment; (ii) serious or severe active infection requiring treatment; (iii) prior radiotherapy and chemotherapy within 4 weeks; (iv) prior antibody therapy within 3 months; (v) history of hypersensitivity to therapeutic protein; (vi) pleural effusions; (vii) relapse within 6 months of autologous hematopoietic stem cell transplantation; (viii) history of allogeneic hematopoietic stem cell transplantation; and (ix) pregnancy or breastfeeding. This study was undertaken in compliance with the Good Clinical Practice guidelines, the Declaration of Helsinki, and local laws. The study protocol was approved by the institutional review board of each institution, and all patients provided written informed consent prior to any study procedure.
2.3. Dose‐limiting toxicity definition
The following toxicities were regarded as DLTs if they occurred in cycle 1 and were potentially related to the study treatment: (i) grade 4 neutropenia and leukopenia lasting ≥7 days; (ii) grade 4 thrombocytopenia and grade 3 thrombocytopenia requiring platelet transfusion; (iii) other grade 4 hematological toxicities; (iv) grade 3 or 4 non‐hematological toxicities except for grade 3 nausea, vomiting, or diarrhea controlled by anti‐emetic or antidiarrheal drugs; (v) grade 3 or 4 infusion‐related reactions except the case recovering to ≤grade 2 within 24 h; and (vi) capillary leak syndrome requiring inpatient hospitalization for medical treatment other than fluid replacement. According to the ISC recommendation, the DLT definition was modified after investigating a 12‐μg/kg/day cohort so as to exclude grade 4 lymphopenia, grade 3 hepatic enzyme abnormalities that recovered to grade ≤2, and any other asymptomatic grade 3 clinical abnormal laboratory results that recovered within 7 days.
2.4. Safety
Safety assessments were undertaken throughout the study to address AEs, vital signs, body weight, 12‐lead electrocardiogram data, concomitant medication use, clinical laboratory values (hematology, blood biochemistry, and urinalysis), and ophthalmological findings. Adverse event severity was classified using the Common Terminology Criteria for Adverse Events, version 4.0. Ophthalmological examinations were carried out at screening, on day 15 of an odd‐numbered cycle, and if clinically indicated.
2.5. Pharmacokinetics and immunogenicity
Blood samples were taken for PK analysis on days 1 and 5 of cycle 1 and day 1 of cycles 3, 5, and 7. Samples were collected before E7777 treatment, 30 min after the start of treatment, and 30, 60, 90, and 120 min after completing treatment. The lower limit of quantitation was 30 ng/mL. The following PK parameters were calculated by a non‐compartmental approach, using WinNonlin software version 6.2 (Pharsight, Sunnyvale, CA, USA): area under the curve extrapolated to infinity (AUC(0‐inf)), terminal half‐life (t1/2), total clearance (CL), and volume of distribution at steady state (V ss). The maximum observed serum concentration (C max) and time to C max (t max) were directly derived from these data. For immunogenicity assessments, blood samples were collected before treatment on day 1 of each cycle, and used to evaluate the presence of anti‐E7777/anti‐IL‐2 antibodies.
2.6. Antitumor activity
Antitumor activity was assessed according to the International Working Group (IWG‐2007) criteria except PET assessment for PTCL19 and modified Severity Weighted Assessment Tool criteria for CTCL.10 Assessments were carried out within 28 days prior to the start of treatment, 6 weeks after the first dose, and every 6 weeks thereafter, or sooner if there was clinical suspicion of disease progression.
2.7. CD25 expression
CD25 expression on tumor cells was determined at The Tohkai Cytopathology Institute by immunohistochemical staining with an anti‐CD25 mAb (4C9; NCL‐CD25‐305, Leica Biosystems).
3. RESULTS
3.1. Patient characteristics
Thirteen patients were enrolled in this study and received E7777 dose levels of 6 (n = 3), 9 (n = 7), or 12 μg/kg/day (n = 3). Of these patients, 10 had been diagnosed with PTCL (PTCL‐NOS, n = 4; AITL, n = 3; ALCL‐ALK positive, n = 1; ALCL‐ALK negative, n = 1; enteropathy‐associated T‐cell lymphoma, n = 1) and 3 with CTCL (MF, n = 3). The median age was 64 years (range, 23‐75 years), and the median number of prior chemotherapy regimens was 1 (range, 0‐8). Psoralen‐UV A radiation, interferons, and retinoids were excluded as prior chemotherapy. The patient characteristics are shown in Table 1.
Table 1.
Patient characteristics
| Characteristics | 6 μg/kg/day | 9 μg/kg/day | 12 μg/kg/day | Total |
|---|---|---|---|---|
| (n = 3) | (n = 7) | (n = 3) | (N = 13) | |
| Median age, years (range) | 70 (64‐75) | 50 (23‐75) | 66 (63‐71) | 58 (23‐75) |
| Sex, n (%) | ||||
| Male | 2 | 4 | 2 | 8 (62) |
| ECOG PS, n (%) | ||||
| 0 | 3 | 6 | 2 | 11 (85) |
| 1 | 0 | 1 | 1 | 2 (15) |
| Histopathologic subtype, n (%) | ||||
| PTCL‐NOS | 1 | 3 | 0 | 4 (31) |
| AITL | 2 | 0 | 1 | 3 (23) |
| ALCL‐ALK+ | 0 | 1 | 0 | 1 (8) |
| ALCL‐ALK‐ | 0 | 1 | 0 | 1 (8) |
| EATL | 0 | 0 | 1 | 1 (8) |
| MF | 0 | 2 | 1 | 3 (23) |
| Number of prior chemotherapy, n (%) | ||||
| 0 | 0 | 1a | 0 | 1 (8) |
| 1 | 1 | 3 | 2 | 6 (46) |
| 2 | 1 | 0 | 1 | 2 (15) |
| ≥3 | 1 | 3 | 0 | 4 (31) |
| Median (range) | 2 (1‐3) | 1 (0‐8) | 1 (1‐2) | 1 (0‐8) |
| Other therapy, n (%)b | 0 | 2 | 1 | 3 (23) |
| Radiation, n (%)c | 0 | 2 | 1 | 3 (23) |
| Auto‐HSCT, n (%) | 0 | 0 | 1 | 1 (8) |
ECOG PS, Eastern Cooperative Oncology Group performance status; PTCL‐NOS, peripheral T‐cell lymphoma‐not otherwise specified; AITL, angioimmunoblastic T‐cell lymphoma; ALCL‐ALK, anaplastic large cell lymphoma‐anaplastic lymphoma kinase; EATL, enteropathy‐associated T‐cell lymphoma; MF, mycosis fungoides; Auto‐HSCT, autologous haematopoietic stem cell transplantation.
The chemotherapy‐naïve patient had prior therapy of interferon and PUVA.
Psoralen‐ultraviolet A radiation (PUVA) and interferon were counted as other therapies.
Radiation administered to MF patients.
3.2. Treatment
Overall, E7777 was given for a median of three cycles (range, 1‐8), with six patients receiving ≥4 cycles and one patient receiving all 8 cycles. Four patients (31%) discontinued treatment because of disease progression, and three (23%) and five patients (38%) withdrew because of AEs or personal decisions/other reasons, respectively.
3.3. Dose escalation and DLTs
Dose‐limiting toxicities were evaluated during the first cycle, and at that time, the initial cohort (n = 3) received 6 μg/kg/day E7777. As none of the patients experienced DLTs, the dose level was then escalated to 12 μg/kg/day. However, DLTs were observed in all three patients at this dose level: the first patient showed ALT increased, hyponatremia, hypokalemia, and lymphopenia; the second showed increased ALT, fatigue, hypoalbuminemia, hyponatremia, and rash; and the third showed increased lipase. This dose level was considered to exceed the MTD, and the 9 μg/kg/day dose level was expanded accordingly. According to the ISC recommendation, the DLT definition was modified to exclude grade 4 lymphopenia, grade 3 hepatic enzyme abnormalities that recovered to grade ≤2 within one cycle, and any other asymptomatic grade 3 clinical abnormal laboratory results that recovered within 7 days. In the 9 μg/kg/day cohort, two of six patients evaluable for DLTs experienced decreased appetite or fatigue, and recovered from these events within 2‐3 weeks after treatment interruption. One patient did not experience DLT, but was regarded as DLT non‐evaluable because of the dose interruption by grade 3 AST/ALT increased on day 5 of cycle 1. According to the protocol, the ISC evaluated the toxicity profiles of all patients and recommended an MTD and RD of 9 μg/kg/day for five consecutive days per 21‐day cycle.
3.4. Adverse events
Table 2 presents an overall summary of treatment‐emergent AEs. The most common respective hematologic toxicities of all grades (≥30%) and ≥grade 3 were lymphopenia (77% and 62%, respectively), thrombocytopenia (62% and 15%), leukocytosis (54% and 0%), anemia (39% and 15%), and neutropenia (31% and 23%). Grade 4 hematologic toxicities included lymphopenia (23%), thrombocytopenia (8%), and neutropenia (8%). The most common respective non‐hematologic toxicities in all grades (≥60%) and ≥grade 3 were increased ALT (92% and 69%, respectively), increased AST (85% and 46%), decreased appetite (85% and 15%), fatigue (85% and 15%), hypoalbuminemia (77% and 15%), and nausea (69% and 0%). Grade 3 capillary leak syndrome was observed in one patient from the 9 μg/kg/day cohort who recovered within 10 days. Three patients in the 9 μg/kg/day cohort reported serious AEs associated with study drug use; one patient experienced increased ALT/AST and fatigue, the second reported a decreased appetite and hypoxia, and the third developed delirium. Additionally, three patients reported AEs leading to study discontinuation: hypersensitivity in one patient from the 6 μg/kg/day cohort, respiratory failure (not related to the study drug) in one patient from the 12 μg/kg/day cohort, and delirium in one patient from the 9 μg/kg/day cohort. No treatment‐related deaths occurred during the study.
Table 2.
Treatment‐emergent adverse events in ≥30% of total patients
| Dose (μg/kg/day) | 6 μg/kg/day (n = 6) | 9 μg/kg/day (n = 7) | 12 μg/kg/day (n = 3) | Total (N = 13) | ||||
|---|---|---|---|---|---|---|---|---|
| Grade | All Grades | ≥Grade 3 | All Grades | ≥Grade 3 | All Grades | ≥Grade 3 | All Grades | ≥Grade 3 |
| Haematologic toxicity | ||||||||
| Lymphopenia | 3 | 3 | 4 | 2 | 3 | 3 | 10 (77) | 8 (62) |
| Thrombocytopenia | 2 | 0 | 4 | 1 | 2 | 1 | 8 (62) | 2 (15) |
| Leukocytosis | 3 | 0 | 3 | 0 | 1 | 0 | 7 (54) | 0 |
| Anaemia | 2 | 0 | 1 | 0 | 2 | 2 | 5 (39) | 2 (15) |
| Neutropenia | 1 | 0 | 1 | 1 | 2 | 2 | 4 (31) | 3 (23) |
| Non‐haematologic toxicity | ||||||||
| ALT increased | 3 | 1 | 6 | 6 | 3 | 2 | 12 (92) | 9 (69) |
| AST increased | 2 | 0 | 6 | 4 | 3 | 2 | 11 (85) | 6 (46) |
| Decreased appetite | 3 | 0 | 5 | 1 | 3 | 1 | 11 (85) | 2 (15) |
| Fatigue | 3 | 0 | 5 | 1 | 3 | 1 | 11 (85) | 2 (15) |
| Hypoalbuminaemia | 3 | 0 | 5 | 0 | 2 | 2 | 10 (77) | 2 (15) |
| Nausea | 2 | 0 | 4 | 0 | 3 | 0 | 9 (69) | 0 |
| Constipation | 2 | 0 | 3 | 0 | 2 | 0 | 7 (54) | 0 |
| Hypertriglyceridaemia | 3 | 0 | 4 | 2 | 0 | 0 | 7 (54) | 2 (15) |
| Pyrexia | 0 | 0 | 4 | 0 | 2 | 1 | 6 (46) | 1 (8) |
| Lipase increased | 2 | 0 | 1 | 1 | 2 | 1 | 5 (39) | 2 (15) |
| Peripheral oedema | 2 | 0 | 2 | 0 | 1 | 0 | 5 (39) | 0 |
| ALP increased | 1 | 0 | 2 | 0 | 1 | 0 | 4 (31) | 0 |
| Hypokalaemia | 1 | 0 | 0 | 0 | 3 | 2 | 4 (31) | 2 (15) |
| Malaise | 0 | 0 | 4 | 0 | 0 | 0 | 4 (31) | 0 |
| Rash | 1 | 0 | 1 | 0 | 2 | 1 | 4 (31) | 1 (8) |
| Weight increased | 1 | 0 | 1 | 0 | 2 | 1 | 4 (31) | 1 (8) |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase
3.5. Pharmacokinetics and immunogenicity
The serum E7777 concentration‐time profiles after the start of treatment on day 1, cycle 1 are shown in Figure 1. Once reaching Cmax, E7777 was eliminated rapidly from the serum. The mean t1/2 value, CL, and Vss after treatment ranges on day 1, cycle 1 were 75.5‐92.5 min, 0.398‐0.430 mL/min/kg, and 47.9‐58.4 mL/kg, respectively.
Figure 1.
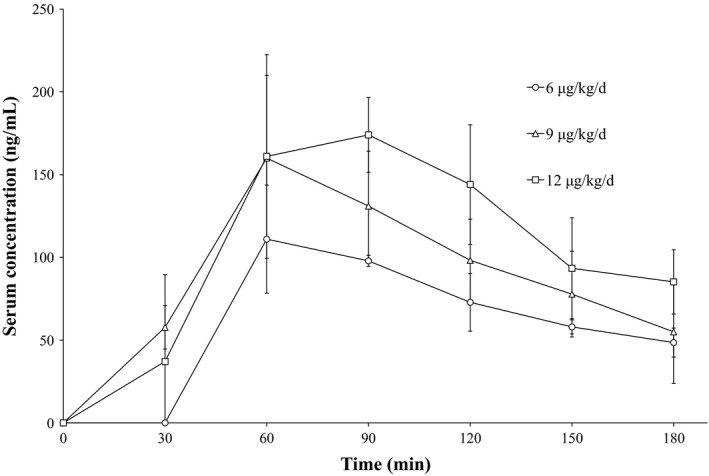
Mean serum concentrations of E7777 on day 1, cycle 1 of a phase I study to evaluate the tolerability, safety, pharmacokinetics, and antitumor activity of E7777 in Japanese patients with relapsed/refractory peripheral and cutaneous T‐cell lymphoma. Patients were allocated to three treatment regimens: 6 μg/kg/day cohort (n = 3), 9 μg/kg/day cohort (n = 5), and 12 μg/kg/day cohort (n = 2). Error bars indicate SD
Table 3 presents the PK parameters after treatment, by dose level. Similar to other large molecules, the distribution volume of E7777 was small, and nearly equal to the plasma volume at 3000 mL/70 kg (43 mL/kg). The total clearance rate, however, was much lower than the hepatic blood flow rate at 1450 mL/min/70 kg (21 mL/min/kg).20 In other words, the PK characteristics of E7777 indicate a small distribution volume and low clearance.
Table 3.
Pharmacokinetic parameters after drug administration on cycle 1, day 1
| Parameter | Dose (μg/kg/day) | ||
|---|---|---|---|
| 6 μg/kg/day (actual dose: 6.84 μg/kg/day)a (n = 3) | 9 μg/kg/daya (n = 5) | 12 μg/kg (actual dose: 13.68 μg/kg/day)a (n = 2) | |
| Cmax (ng/mL) | 120 ± 16.5 | 164 ± 46.1 | 158, 204 |
| tmax (min) | 64 (64‐86) | 64 (60‐98) | 64, 85 |
| AUC(0‐t) (ng•min/mL) | 11000 ± 1530 | 16400 ± 4200 | 14000, 23200 |
| AUC(0‐inf) (ng•min/mL) | 17300 ± 1380 | 23100 ± 6990b | 35600c |
| t 1/2 (min) | 92.5 ± 14.8 | 75.5 ± 21.0b | 87.4c |
| CL (mL/min/kg) | 0.398 ± 0.0241 | 0.430 ± 0.169b | 0.393c |
| V ss (mL/kg) | 58.4 ± 3.24 | 47.9 ± 12.5b | 54.1c |
| MRT (min) | 147 ± 1.53 | 117 ± 30.6b | 138c |
C max, maximum observed concentration; t max, time at which the highest drug concentration occurred; AUC(0‐t), area under the concentration‐time curve from zero time to the time of the last quantifiable concentration; AUC(0‐inf), area under the concentration‐time curve from zero time, extrapolated to infinite time; t 1/2, terminal elimination phase half‐life; CL, total clearance; V ss, volume of distribution at steady state; MRT, mean residence time
Data are shown as means ± standard deviations except t max, which is shown as a median (minimum‐maximum). Individual values are shown if n ≤ 2.
A change in the protein concentration assay led to recalculations of the actual doses in the first 2 cohorts to 6.84 μg and 13.68 μg, and the 9 μg doses were calculated using the new assay.
n = 4.
n = 1.
Regarding immunogenicity, anti‐E7777 and anti‐IL‐2 antibodies were detected in nine (69%) and eight (62%) of 13 patients, respectively. In this study, the serum E7777 concentration decreased as the anti‐E7777 antibody titer increased (data not shown). However, this decrease in E7777 concentration was not observed in patients with a low anti‐E7777 antibody titer. All patients with tumor response showed positive results for antibodies against both E7777 and IL‐2.
3.6. Antitumor activity and CD25 expression
Preliminary antitumor activity was observed in five of 13 patients (ORR, 38%; 95% confidence interval, 14‐68), including four of 10 patients with PTCL and one of three patients with CTCL. All responses were partial. The dose levels were as follows: two patients with AITL at 6 μg/kg/day, one patient with MF at 12 μg/kg/day followed by 6 μg/kg/day from cycle 2, and two patients with PTCL‐NOS at 9 μg/kg/day. Nine of 13 patients were evaluable for tumor CD25 expression, resulting in a range of <1% to >90%. The best overall response and CD25 expression status of all patients are listed in Table 4.
Table 4.
Anti‐tumour activity and CD25 expression
| Patient number | Dose (μg/kg/day) | Subtype | No. of prior chemo‐therapies | Treatment cycles | Best overall response | CD25 expression (IHC) |
|---|---|---|---|---|---|---|
| 1 | 6 | PTCL‐NOS | 3 | 1 | PD | <1% |
| 2 | 6 | AITL | 2 | 5 | PR | <1% |
| 3 | 6 | AITL | 1 | 4 | PR | 10‐20% |
| 4 | 12a | MF | 1 | 4 | PR | ‐a |
| 5 | 12a | EATL | 2 | 1 | PD | ‐a |
| 6 | 12a | AITL | 1 | 2 | SD | ‐a |
| 7 | 9 | ALCL‐ALK‐ | 1 | 3 | SD | 1‐10% |
| 8 | 9 | PTCL‐NOS | 1 | 2 | PR | 1‐10% |
| 9 | 9 | PTCL‐NOS | 1 | 8 | PR | 1‐10% |
| 10 | 9 | PTCL‐NOS | 5 | 1 | PD | <1% |
| 11 | 9 | ALCL‐ALK+ | 7 | 1 | NE | >90% |
| 12 | 9 | MF | 8 | 5 | SD | ‐a |
| 13 | 9 | MF | 0 | 6 | SD | 30‐40% |
PTCL‐NOS, peripheral T‐cell lymphoma‐not otherwise specified; AITL, angioimmunoblastic T‐cell lymphoma; ALCL‐ALK, anaplastic large cell lymphoma‐anaplastic lymphoma kinase; EATL, enteropathy‐associated T‐cell lymphoma; MF, mycosis fungoides; PR, partial response; SD, stable disease; PD, progressive disease; IHC, immunohistochemistry
Dose was decreased to 6 μg/kg/day from the second cycle.
Archival samples were not available for measurement.
4. DISCUSSION
In this multicenter, open‐label phase I study of E7777 in Japanese patients with relapsed or refractory PTCL and CTCL, we determined an MTD and RD of 9 μg/kg/day on five consecutive days per 21‐day cycle. Although this dosage is only half of the previously selected higher RD for DD (18 μg/kg/day),15, 16 we consider this result reasonable, as E7777 has a higher level of active monomer purity and reduced level of misfolded and/or aggregated protein impurities, compared with DD. However, DLTs such as decreased appetite and fatigue were observed in two of six evaluable patients from 9 μg/kg/day cohort and other common AEs were also observed, suggesting that these events should be carefully monitored and managed during treatment. Previous studies reported capillary leak syndrome (the occurrence of two or more of the following: hypoalbuminemia, edema, and hypotension) rates of 10‐25%.15, 16 In contrast, we observed grade 3 capillary leak syndrome in only one patient (8%); however, the high rates of hypoalbuminemia (77%) and peripheral edema (39%) suggest the risk of capillary leak syndrome development. These findings suggest the need for appropriate hydration at the time of drug infusion and careful AE management.
In the previous DD study, 60% of patients developed an infusion reaction, characterized by immediate events such as back pain, chest pain/tightness, hypotension, pruritus, vasodilation, and dyspnea, within 24 h of study drug infusion.15 However, the frequency of infusion reaction in the present study was low, likely due to the required use of dexamethasone (4‐8 mg, i.v.) as premedication, which was based on several previous reports of DD optimal use.21, 22 Furthermore, the lower levels of impurities such as misfolded and/or aggregated proteins might also have contributed to this discrepancy. Another report described a reduced frequency of AEs in subsequent cycles of DD treatment,16 and a similar tendency was observed in the present study. However, we should carefully consider the influence of confounding from early discontinued patients and evaluate the similarities of these AE profiles in further studies.
The PK profiles of E7777 (9 μg/kg/day) in this study, and of DD (9 μg/kg/day) in previous reports15 were similar in cycle 1 and subsequent cycles, including the tendency toward a decreased drug concentration after cycle 1. The decreased concentration of E7777 in later cycles was attributed to the presence of anti‐E7777 antibodies, and might also account for the reduced frequency of AEs in subsequent cycles. Interestingly, all patients who showed tumor responses were positive for antibodies against both E7777 and IL‐2, thus warranting a further investigation of the relationship between tumor response and immunogenicity.
Tumor response was observed in five of 13 subjects (38%), as described in the previous section. We note that the high‐affinity IL‐2 receptor is a heterotrimer comprising the IL‐2 receptor‐α (CD25) and ‐β (CD122) chains and the common γ (CD132) chain. CD122 and CD132 form a heterodimer that binds to IL‐2 with intermediated affinity, whereas a CD25 monomer is known as a low‐affinity IL‐2 receptor. Although E7777 binds to all three forms of the IL‐2 receptor, but only in cells that commonly express high or intermediate affinity receptors, such as mature T‐cells.23 E7777 can internalize this drug by receptor‐mediated endocytosis through both high affinity (CD25‐positive) and intermediate (CD25‐negative) IL‐2 receptors.24 Because E7777 exerted antitumor activity even in low CD25‐expressing patients in this study (Table 4), its efficacy does not appear to depend on tumor CD25 expression; therefore, expression of the intermediate affinity IL‐2 receptor might be sufficient to induce a tumor response. Further investigation of the relationship between tumor response and tumor CD25 expression will be carried out in a larger cohort of patients treated with E7777 at the RD of 9 μg/kg/day.
Previously, DD was reported to suppress Tregs and alter immune functions.25, 26 Naturally occurring Tregs strongly express CD25 and the master transcription factor Forkhead box P3, and have been implicated in the lack of an effective antitumor immune response. CD25‐positive Tregs have been shown to infiltrate CTCLs, and CTCL cells have been reported to show a Treg‐like phenotype, which includes CD25 and CTLA‐4 expression.27, 28 Therefore, the mechanism of action by which E7777 depletes Tregs might have also contributed to the observed tumor response.
In conclusion, this was the first phase I study of E7777. Regarding the safety profile, E7777 was well tolerated at the RD determined herein, assuming careful AE management during treatment, and preliminary but clinically meaningful antitumor activity was observed. Subsequent studies of the efficacy and safety of E7777 in patients with PTCL and CTCL are warranted, including investigations of the relationships between tumor response and factors such as the E7777 PK profile/immunogenicity, tumor CD25 expression, and Treg depletion.
CONFLICT OF INTEREST
K.A. has received honoraria and research funding from Eisai Co., Ltd. M.O. has received research funding from Eisai Co., Ltd., Kyowa Hakko Kirin Co., Ltd., Zenyaku Kogyo Co., Ltd., Chugai Pharmaceutical Co., Ltd., Pfizer Japan Inc., Janssen Pharmaceutical K.K., GlaxoSmithKline K.K., MSD K.K., AstraZeneca K.K., Takeda Pharmaceutical Company Limited, Symbio Pharmaceuticals Limited, Solasia Pharma K.K., Mundipharma K.K., Celgene K.K., and Sumitomo Dainippon Pharma Co., Ltd. T.U. has received research funding from Eisai Co., Ltd. K.T. has received research funding from Eisai Co., Ltd., Mundipharma K.K., Celgene K.K., Solasia Pharma K.K., Kyowa Hakko Kirin Co., Ltd., and HUYA Bioscience International. D.M. has received honoraria from Eisai Co., Ltd and Takeda Pharmaceutical Co., Ltd. M.N. and T.N. are employees of Eisai Co., Ltd. K.O. has no conflict of interest.
ACKNOWLEDGMENTS
We thank all of the patients who participated in this study and their families as well as investigators, physicians, nurses, and clinical research coordinators who helped this study. We would also like to thank Dr. Kiyohiko Hatake (The Cancer Institute Hospital) who was the medical advisor of the study, Dr. Kazunori Ohnishi (Hamamatsu University School of Medicine), Dr. Noriko Usui (The Jikei University Daisan Hospital), and Dr. Tomohiro Kinoshita (Aichi Cancer Center Hospital) as members of the Independent Safety Committee. We also acknowledge Dr. Kenzo Muramoto (Eisai Co., Ltd.) for his help in preparing this manuscript. This study was funded and supported by Eisai Co., Ltd.
Ohmachi K, Ando K, Ogura M, et al. E7777 in Japanese patients with relapsed/refractory peripheral and cutaneous T‐cell lymphoma: A phase I study. Cancer Sci. 2018;109:794–802. https://doi.org/10.1111/cas.13513
Funding Information
Eisai Co., Ltd.
REFERENCES
- 1. Phan A, Veldman R, Lechowicz MJ. T‐cell lymphoma epidemiology: the known and unknown. Curr Hematol Malig Rep. 2016;6:492‐503. [DOI] [PubMed] [Google Scholar]
- 2. Fisher RI, Gaynor ER, Dahlberg S, et al. Comparison of standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non‐Hodgkin's lymphoma. N Engl J Med. 1993;328:1002‐1006. [DOI] [PubMed] [Google Scholar]
- 3. Hamadani M, Kar SM, Usmani SZ, Sabani BN, Ayala E, Kharfan‐Dabaja MA. Management of relapses after hematopoietic cell transplantation in T‐cell non‐Hodgkin lymphomas. Semin Hematol. 2014;51:73‐86. [DOI] [PubMed] [Google Scholar]
- 4. Mak V, Hamm J, Chhanabhai M, et al. Survival of patients with peripheral T‐cell lymphoma after first relapse or progression: spectrum of disease and rare long‐term survivors. J Clin Oncol. 2013;31:1970‐1976. [DOI] [PubMed] [Google Scholar]
- 5. Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010;28:4730‐4739. [DOI] [PubMed] [Google Scholar]
- 6. O'Connor OA, Pro B, Pinter‐Brown L, et al. Pralatrexate in patients with relapsed or refractory peripheral T‐cell lymphoma: results from the pivotal PROPEL study. J Clin Oncol. 2011;29:1182‐1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coiffer B, Pro B, Prince HM, et al. Results from a pivotal, open‐label, phase II study of romidepsin in relapsed or refractory peripheral T‐cell lymphoma after prior systemic therapy. J Clin Oncol. 2012;30:631‐636. [DOI] [PubMed] [Google Scholar]
- 8. O'Connor OA, Horwitz S, Masszi T, et al. Belinostat in patients with relapsed or refractory peripheral T‐cell lymphoma: results of the pivotal phase II BELIEF (CLN‐19) study. J Clin Oncol. 2015;33:2492‐2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pro B, Advani R, Brice P, et al. Brentuximab vedotin (SGN‐35) in patients with relapsed or refractory systemic anaplastic large‐cell lymphoma: results of a phase II study. J Clin Oncol. 2012;30:2190‐2196. [DOI] [PubMed] [Google Scholar]
- 10. Olsen EA, Kim YH, Kuzel TM, et al. Phase IIb multicentre trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T‐cell lymphoma. J Clin Oncol. 2007;25:3109‐3115. [DOI] [PubMed] [Google Scholar]
- 11. Whittaker SJ, Demierre MF, Kim EJ, et al. Final results from a multicentre, international, pivotal study of romidepsin in refractory cutaneous T‐cell lymphoma. J Clin Oncol. 2010;28:4485‐4491. [DOI] [PubMed] [Google Scholar]
- 12. Ogura M, Ishida T, Hatake K, et al. Multicenter phase II study of mogamulizumab (KW‐0761), a defucosylated anti‐cc chemokine receptor 4 antibody, in patients with relapsed peripheral T‐cell lymphoma and cutaneous T‐cell lymphoma. J Clin Oncol. 2014;32:1157‐1163. [DOI] [PubMed] [Google Scholar]
- 13. Zinzani PL, Karlin L, Radford J, et al. European phase II study of mogamulizumab, an anti‐CCR4 monoclonal antibody, in relapsed/refractory peripheral T‐cell lymphoma. Haematologica. 2016;101:e407‐e410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. VanderSpek JC, Mindell JA, Finkelstein A, Murphy JR. Structure/function analysis of the transmembrane domain of DAB389–interleukin‐2, an interleukin‐2 receptor‐targeted fusion toxin. The amphipathic helical region of the transmembrane domain is essential for the efficient delivery of the catalytic domain to cytosol of target cells. J Biol Chem. 1993;268:12077‐12082. [PubMed] [Google Scholar]
- 15. Olsen E, Duvic M, Frankel A, et al. Pivotal phase III trial of two dose levels of denileukin diftitox for the treatment of cutaneous T‐cell lymphoma. J Clin Oncol. 2001;19:376‐388. [DOI] [PubMed] [Google Scholar]
- 16. Prince HM, Duvic M, Martin A, et al. Phase III placebo‐controlled trial of denileukin diftitox for patients with cutaneous T‐cell lymphoma. J Clin Oncol. 2010;28:1870‐1877. [DOI] [PubMed] [Google Scholar]
- 17. Prince HM, Martin AG, Olsen EA, Fivenson DP, Duvic M. Denileukin diftitox for the treatment of CD25 low‐expression mycosis fungoides and Sézary syndrome. Leuk Lymphoma. 2013;54:69‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dang NH, Pro B, Hagemeister FB, et al. Phase II trial of denileukin diftitox for relapsed/refractory T‐cell non‐Hodgkin lymphoma. Br J Haematol. 2007;136:439‐447. [DOI] [PubMed] [Google Scholar]
- 19. Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579‐586. [DOI] [PubMed] [Google Scholar]
- 20. Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharm Res. 1993;10:1093‐1095. [DOI] [PubMed] [Google Scholar]
- 21. Foss FM, Bacha P, Osann KE, Demierre MF, Bell T, Kuzel T. Biological correlates of acute hypersensitivity events with DAB(389)IL‐2(denileukin diftitox, ONTAK) in cutaneous T‐cell lymphoma: decreased frequency and severity with steroid premedication. Clin lymphoma. 2001;1:298‐302. [DOI] [PubMed] [Google Scholar]
- 22. Duvic M. Optimizing denileukin diftitox (Ontak) therapy. Haematol rep. 2006;2:57‐60. [DOI] [PubMed] [Google Scholar]
- 23. Létourneau S, Krieg C, Pantaleo G, Boyman O. Il‐2‐and CD25‐dependent immunoregulatory mechanisms in the homeostasis of T‐cell subsets. J Allergy and Clin Immunol. 2009;123:758‐762. [DOI] [PubMed] [Google Scholar]
- 24. Morris SC, Gause WC, Finkelman FD. IL‐4 supression of in vivo T cell activation and antibody production. J Imunology. 2000;165:1734‐1740. [DOI] [PubMed] [Google Scholar]
- 25. Mahnke K, Schönfeld K, Fondel S, et al. Depletion of CD4 + CD25 + human regulatory T cells in vivo: linetics of Treg depletion and alterations in immune functions in vivo and in vitro. Int J Cancer. 2007;120:2723‐2733. [DOI] [PubMed] [Google Scholar]
- 26. Litzinger MT, Fernando R, Curiel TJ, Grosenbach DW, Schlom J, Palena C. IL‐2 immunotoxin denileukin diftitox reduces regulatory T cells and enhabces vaccine‐mediated T‐cell immunity. Blood. 2007;110:3192‐3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Knol AC, Quéreux G, Brocard A, et al. Absence of modulation of CD4 + CD25 + T cells in CTCL patients treated with bexarotene. Exp Dermatol. 2010;19:e95‐e102. [DOI] [PubMed] [Google Scholar]
- 28. Berger CL, Tigelaar R, Cohen J, et al. Cutaneous T‐cell lymphoma: malignant proliferation of T‐regulatory cells. Blood. 2005;105:1640‐1647. [DOI] [PubMed] [Google Scholar]


