Abstract
In the hepatocellular carcinoma (HCC) microenvironment, chemokine receptors play a critical role in tumorigenesis and metastasis. Our previous studies have found that osteopontin (OPN) is a promoter for HCC metastasis. However, the role of chemokine receptors in OPN‐induced HCC metastasis remains unclear. In this study, we demonstrate that OPN is dramatically elevated in HCC tissues with metastasis and that high expression of OPN correlates with poorer overall survival and higher recurrence rate. OPN upregulates chemokine receptor expression, migration, invasion and pulmonary metastasis in HCC. We find that C‐C chemokine receptor type 1 (CCR1) and C‐X‐C chemokine receptor type 6 (CXCR6) are the most upregulated chemokine receptors induced by OPN. CCR1 knockdown results in reduction of migration, invasion and pulmonary metastasis induced by OPN in vitro and in vivo, whereas CXCR6 knockdown does not reverse OPN‐promoted migration and invasion. Moreover, OPN upregulates the expression of CCR1 through activating phosphoinositide 3‐kinase (PI3K)/AKT and hypoxia‐inducible factor 1α (HIF‐1α) in HCC cells. Furthermore, blockade of OPN‐CCR1 axis with CCR1 antagonist significantly restrains the promoting effects of OPN on HCC progression and metastasis. In human HCC tissues, OPN expression shows significantly positive correlation with CCR1 expression, and the patients with high levels of both OPN and CCR1 have the most dismal prognosis. Collectively, our results indicate that the OPN‐CCR1 axis in HCC is important for accelerating tumor metastasis and that CCR1 is a potential therapeutic target for controlling metastasis in HCC patients with high OPN.
Keywords: BX471, CCR1, hepatocellular carcinoma, metastasis, osteopontin
1. INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most prevalent cancer worldwide, and its incidence is predicted to increase in most countries.1, 2 In China, HCC is the fourth and sixth most common cause of death from cancer in men and women, respectively.3 The current best strategy for a potential cure is surgical resection or liver transplantation.4 Although major advances have been achieved in the clinical diagnosis and treatment of HCC over the past two decades, the outcome for patients is still dismal.2 It is mainly due to the high probabilities of intrahepatic metastases and postoperative relapse.2 Therefore, elucidating the mechanism of metastatic relapse of HCC is of great importance.
Osteopontin (OPN) is a secretary phosphorylated glycoprotein that is highly expressed in various human cancers.5 In our previous studies, we have demonstrated that OPN is one of the leading genes that promote HCC metastasis,6, 7, 8, 9 and elevated OPN levels in both HCC tissues and plasma are closely related to poor prognosis and postoperative tumor recurrence of HCC patients.10, 11 Thus, understanding how OPN is involved in maintaining the metastatic phenotype of HCC cells may help to identify novel ways to combat HCC metastasis.
Chemokines and their receptors have been shown to play crucial roles not only in physiological cell migration but also in pathological processes, such as invasion and metastasis of cancer.12 A growing body of research highlights their importance in determining the metastatic destination of cancers, including HCC.13, 14, 15 Moreover, chemokine receptor antagonists have already been applied in clinical trials of inflammatory diseases and cancers.16
Among them, C‐C chemokine receptor type 1 (CCR1) and its 3 ligands, chemokine (C‐C motif) ligand 3, 5 and 7 (CCL3, CCL5 and CCL7), have been characterized by the progression and metastasis of various of cancers.17, 18 An increasing amount of preclinical evidence suggests that the inhibitory compounds of CCL3, CCL5, CCL7/CCR1 axis can be used for treatment of HCC and other tumors.19, 20, 21 CCR1 is upregulated in human HCC tissues and animal HCC models.18, 22, 23 Meanwhile, its ligands CCL3, CCL5 and CCL7 can promote HCC cell growth, migration and invasion.18, 24, 25, 26 Moreover, the contribution of CCL3‐CCR1 axis to HCC progression and metastasis is further confirmed in the diethylnitrosamine (DEN)‐induced HCC model.18, 22 In the present study, we aimed to explore the possible roles and functional mechanisms of CCR1 activation involved in OPN facilitating HCC metastasis.
2. METHODS
2.1. Cell lines and cell culture
Immortalized liver cell line (LO2) and human HCC cells (Hep3B, HepG2 and SMMC7721) were purchased from the Shanghai cell bank, Chinese Academy of Sciences (Shanghai, China). Additional human HCC cell lines (HCC97H and HCC‐LM3) were obtained from the Liver Cancer Institute at Fudan University (Shanghai, China). Cells were cultured at 37°C with 5% CO2 in DMEM supplemented with 10% FBS, 100 mg/mL penicillin and 100 mg/mL streptomycin.
2.2. Immunohistochemical staining of human hepatocellular carcinoma tissue samples
Human HCC tissue specimens were obtained following the guidelines approved by the Ethics Committee of the Liver Cancer Institute, Fudan University, and written informed consent was obtained from patients in all cases. Immunohistochemical (IHC) staining was performed as described previously.6 Briefly, tissue specimens were incubated using antibodies against OPN (1:200 dilution, Abcam, Cambridge, MA, USA), CCR1 (1:100 dilution, Novus, St. Louis, MO, USA) and a biotin‐conjugated secondary antibody and then incubated with an avidin‐biotin‐peroxidase complex.
2.3. Plasmid and cell transfections
Expression vectors for human OPN and CCR1 were constructed. Human OPN or CCR1 was cloned into pWPI.1 lentiviral vectors. In addition, OPN shRNA, CCR1 shRNA, CXCR6 shRNA, and HIF‐1α shRNA and non‐target shRNA control (pLKO.1 TRC, Mission RNAi) were obtained commercially (Sigma‐Aldrich, St. Louis, MO, USA). The CCR1 promoter was generated from human genomic DNA by PCR, and was cloned into the MLUI and Hind III sites of the pGL3‐Basic Vector. All constructs were confirmed by enzyme digestion and DNA sequencing. Detailed primers are presented in Table S2.
pWPI.1‐OPN was transfected into lower metastatic HCC cells (Hep3B, HepG2 and SMMC7721). pWPI.1 lentiviral vectors were used as controls. pLKO.1‐CCR1 shRNA and CXCR6 shRNA were transfected into high OPN‐expression HCC cells (Hep3B, HepG2, and SMMC7721‐OPN). pLKO.1 lentiviral vectors were used as controls. All these constructs and oligonucleotides were transfected into HCC cells using Lipofectamine 2000 according to the product manual (Invitrogen, CA, USA). Stably transfected clones were validated by quantitative PCR (qPCR) and western blot analysis.
2.4. Statistical analysis
All the analyses were performed using SPSS (Version 16; SPSS, Chicago, IL, USA). In IHC analysis, the Fisher exact test and Spearman rank correlation coefficient were used. Student's t‐test was used to compare experimental data. The cumulative recurrence and survival rates were determined by using the Kaplan‐Meier survival analyses and the log‐rank test. Univariate and multivariate Cox proportional hazards models were performed to determine the independent factors that influence survival and recurrence. P < .05 was considered statistically significant. Additional methods are detailed in Document S1.
3. RESULTS
3.1. Osteopontin is positively associated with hepatocellular carcinoma metastasis in hepatocellular carcinoma patients
To evaluate the clinical outcomes of metastasis in HCC patients, we analyzed the survival of 96 HCC patients who received surgery (Table S1). The results showed that HCC patients within metastasis group had a significantly worse prognosis than the other patients (Figure 1A). As OPN is one of the leading genes that promote HCC metastasis, we examined OPN mRNA level in normal livers, tumors without metastasis and tumors with metastasis (n = 10 in each group; Figure 1B). OPN levels were positively associated with HCC metastasis in HCC patients (Figure 1B). Moreover, we found significantly strong staining for OPN in the tumors with metastasis (Figure 1C,D). Furthermore, upregulation of OPN was significantly associated with poorer overall survival and higher recurrence rates in HCC patients (P < .001, respectively; Figure 1E,F). Collectively, these findings suggested that OPN was positively associated with metastasis and indicated poor prognosis in HCC patients.
Figure 1.
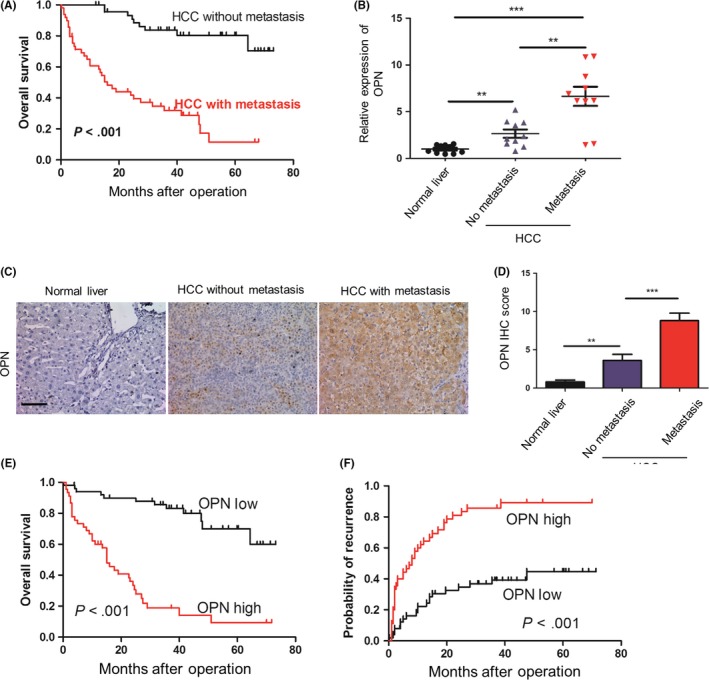
Osteopontin (OPN) is positively associated with metastasis in hepatocellular carcinoma patients. A, Cumulative overall survival of hepatocellular carcinoma patients with metastasis compared with those without metastasis over time, estimated using the Kaplan‐Meier method and compared by the log‐rank test. B, Relative mRNA levels of OPN measured by quantitative PCR analysis and normalized to β‐actin, in normal livers, and in hepatocellular carcinoma tumors without and with metastasis (n = 10, respectively). Data are expressed as means ± SEM and compared using the unpaired t‐test (**P < .01 and ***P < .001). C, Representative immunohistochemical (IHC) staining images showing the accumulation of OPN in normal liver, tumors without metastasis and tumors with metastasis. Scale bar, 100 μm. D, Statistical analysis of the pathologic scores of OPN in the various groups. Data are expressed as means ± SEM and compared using the unpaired t‐test (**P < .01 and ***P < .001). E,F, Cumulative overall survival (E) and recurrence survival (F) of hepatocellular carcinoma patients with high or low OPN expression levels estimated using the Kaplan‐Meier method and compared by the log‐rank test. HCC, hepatocellular carcinoma
3.2. Osteopontin upregulates C‐C chemokine receptor type 1 and C‐X‐C chemokine receptor type 6 expression in hepatocellular carcinoma cells
To evaluate whether chemokine receptors are involved in this promoting effect of OPN on HCC metastasis, we first used a qPCR array to quantify the expression of a panel of chemokine receptors in HCC cell lines stably overexpressing OPN (SMMC7721‐OPN, HepG2‐OPN and Hep3B‐OPN) and found that many chemokine receptors were upregulated (Figure 2A). Among them, we focused on CCR1 and CXCR6 for further study because they were stably upregulated in all 3 OPN‐overexpressed HCC cell lines (Figure 2A,B), which were further confirmed in protein levels by western blot (Figure 2C).
Figure 2.
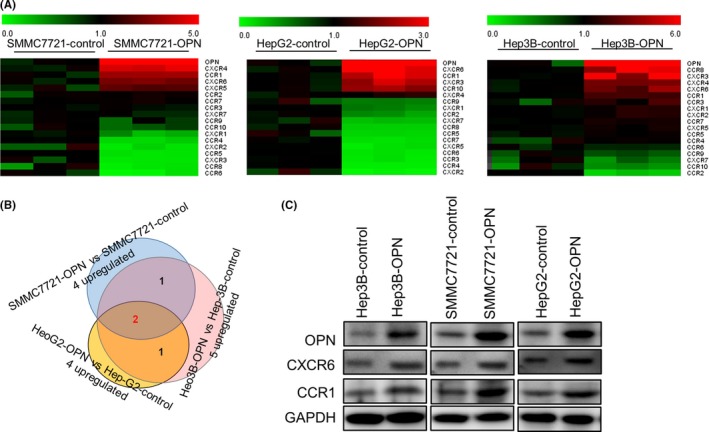
Osteopontin (OPN) upregulates CCR1 and CXCR6 expression in hepatocellular carcinoma cells. A, Heat map of a partial list of mRNA with chemokine receptors expression patterns that correlated with OPN overexpression in hepatocellular carcinoma (HCC) cells. SMMC7721, HepG2 and Hep3B cells were stably transfected with lentiviral (LV)‐OPN, and a quantitative PCR array analysis was carried out to determine the expression profile based a panel of chemokine receptors. B, HCC cells transfected with LV‐OPN vs those transfected with LV‐control. C, HCC cells were stably transfected with LV‐OPN or control. CCR1 and CXCR6 expression was detected by western blot
3.3. C‐C chemokine receptor type 1, but not C‐X‐C chemokine receptor type 6, knockdown repressed osteopontin‐induced migration and invasion in hepatocellular carcinoma cells
Because CCR1 and CXCR6 have been reported to play important roles in HCC metastasis,22, 27 we further investigated whether CCR1 and CXCR6 were involved in the promoting effects of OPN on HCC metastasis. We stably downregulated CCR1 or CXCR6 in OPN‐overexpressing HCC cells (Figure 3A), and found that downregulation of CCR1 could significantly decrease the migration and invasion abilities of OPN‐overexpression HCC cells (Figure 3B,C and Figure S1A‐C), but knockdown of CXCR6 did not show significant effect on the ability of migration and invasion in HCC cells. Moreover, to exclude the possibility of off target effects, we reintroduced CCR1 with engineered cDNA that was not sensitive to shRNA (shRES) into the shCCR1 cells and found that re‐expression of CCR1 could rescue the decreased effect on both migration and invasion (Figure 3B,C).
Figure 3.
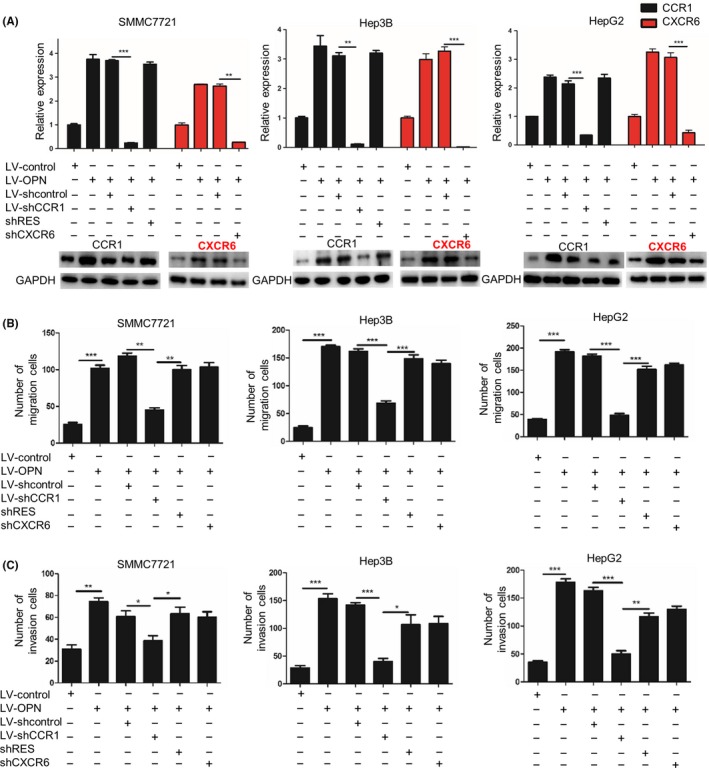
CCR1, but not CXCR6, knockdown repressed osteopontin (OPN)‐induced migration and invasion in hepatocellular carcinoma cells. SMMC7721, Hep3B and HepG2 cells were infected with lentiviral (LV) shCCR1, shCXCR6 or shRES to inhibit the expression of CCR1 and CXCR6 or rescue the expression of CCR1. shcontrol was used as a control. A, CCR1 and CXCR6 mRNA expression and protein expression levels were examined by quantitative PCR and western blot, respectively. B,C, Migration (B) and invasion (C) abilities of the indicated hepatocellular carcinoma (HCC) cells were determined by transwell assay. Data are expressed as means ± SEM of 3 independent experiments and compared using Student's t‐test (*P < .05, **P < .01 and ***P < .001). OPN, osteopontin
3.4. C‐C chemokine receptor type 1 is essential for osteopontin‐induced hepatocellular carcinoma metastasis
Next, we investigated the importance of CCR1 in OPN‐induced HCC metastasis. The in vivo orthotopic assay showed that the overexpression of OPN increased the tumor volumes, the incidence of lung metastasis and the number of metastatic lung nodules, while decreasing the overall survival time of mice in the SMMC7721‐control group. In contrast, downregulation of CCR1 significantly decreased the tumor volumes, the incidence of lung metastasis and the number of metastatic lung nodules in the orthotopic implantation models of OPN‐upregulated HCC cells (SMMC7721‐OPN), and also significantly prolonged the overall survival time of mice (Figure 4A‐E and Figure S2B).
Figure 4.
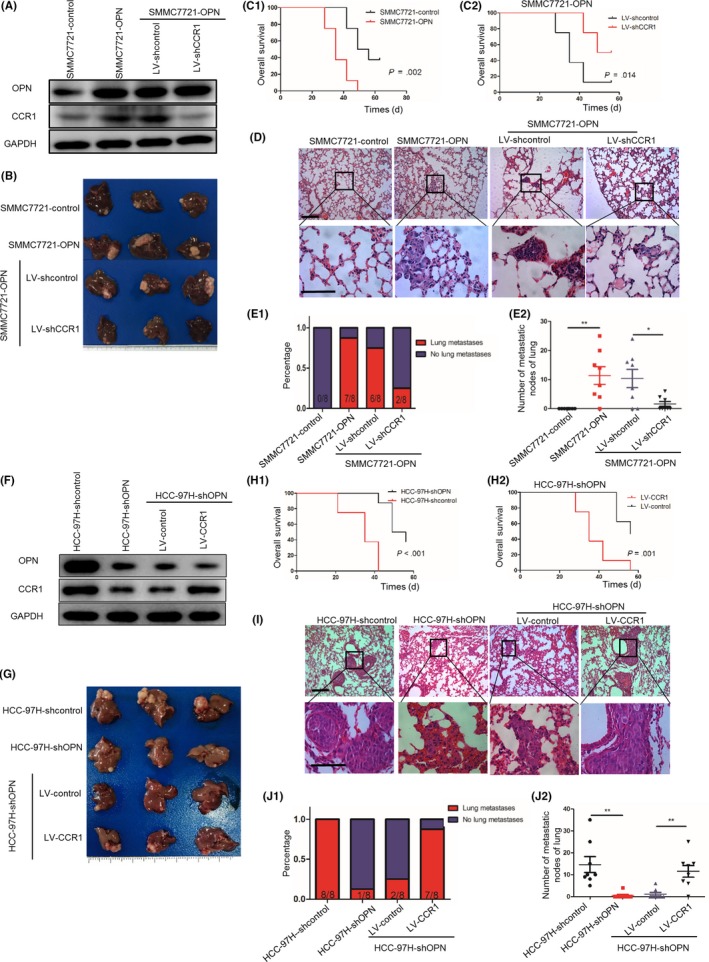
CCR1 is essential for osteopontin (OPN)‐induced hepatocellular carcinoma (HCC) metastasis. A‐E, Downregulation of CCR1 decreased OPN‐mediated HCC metastasis. A, After SMMC7721‐OPN cells were infected with lentivirus (LV)‐shCCR1 or LV‐shcontrol, OPN and CCR1 expression was detected by western blot. B, Subcutaneous tumors with different cells were transplanted into the livers of nude mice. Representative images of the different groups are shown at 6 wk following orthotropic implantation. C1‐C2, Overall survival of xenograft mice models of different groups using the Kaplan‐Meier method and compared by the log‐rank test. D, Representative HE staining images of lung tissues of different groups. Scale bar, 100 μm. E1, Incidence of lung metastases in the different groups. E2, Number of lung metastatic foci in each group. Error bars indicate means ±SEM. *P < .05 and **P < .01. F‐J, Upregulation of CCR1 rescued the decreased HCC metastasis induced by OPN knockdown. F, After HCC‐97H‐shOPN cells were infected with LV‐CCR1 or LV‐control, OPN and CCR1 expression was detected by western blot. G, Subcutaneous tumors with different cells were transplanted into the livers of nude mice. Representative images of the different groups are shown at 6 wk following orthotropic implantation. H1‐H2, Overall survival of xenograft mice models of different groups using the Kaplan‐Meier method and compared using the log‐rank test. I, Representative HE staining images of lung tissues of different groups. Scale bar, 100 μm. J1, The incidence of lung metastases in the different groups. J2, Number of lung metastatic foci in each group. Error bars indicate means ± SEM. *P < .05 and **P < .01
Next, the lentivirus LV‐shOPN was used to downregulate the OPN expression of HCC‐97H cells. Knockdown of OPN decreased the migration and invasion of HCC‐97H cells with OPN highly expressed, and upregulation of CCR1 rescued the decreased migration and invasion abilities induced by OPN knockdown (Figure S2A). The in vivo orthotopic assay showed that the knockdown of OPN decreased the tumor volumes, the incidence of lung metastasis and the number of metastatic lung nodules, and prolonged the overall survival time of mice in the HCC‐97H‐control group. In contrast, the upregulation of CCR1 rescued the decreased tumor volumes, the incidence of lung metastasis and the number of metastatic lung nodules, and prolonged the overall survival time of mice in the HCC‐97H‐shOPN group (Figure 4F‐J and Figure S2C). The data above indicated that CCR1 was essential for OPN‐induced HCC metastasis.
3.5. Osteopontin upregulates C‐C chemokine receptor type 1 expression via phosphoinositide 3‐kinase/AKT/hypoxia‐inducible factor 1α pathway
We have determined that OPN overexpression could upregulate CCR1 expression in HCC cells. To elucidate the molecular mechanisms, HCC cells were transfected with a reporter plasmid containing the promoter of the human CCR1 gene, and we found that OPN upregulation significantly enhanced the promoter activity of CCR1 gene (Figure 5A).
Figure 5.
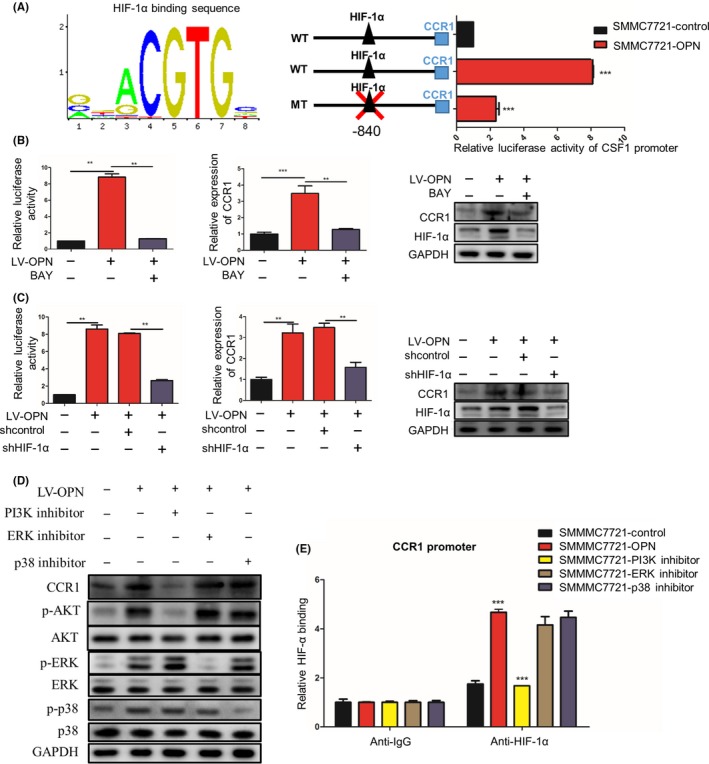
Osteopontin (OPN) upregulates CCR1 expression via PI3K/Akt/HIF‐1α pathway. A, SMMC7721 cells with or without lentivirus (LV)‐OPN were transiently transfected with a wild‐type CCR1 promoter‐dependent luciferase construct or a mutant construct wherein HIF‐1α binding sites were mutated (WT: GGCACGTGCAG; MT: GGCATAGACAG). Luciferase activity was detected 24 h after LV‐OPN transfection. B, SMMC7721‐OPN cells were treated by HIF‐1α inhibitor BAY 87‐2243. The promoter activity and expression of CCR1 were measured by luciferase reporter assay, quantitative PCR (qPCR) and western blot, respectively. C, SMMC7721‐OPN cells were transfected with HIF‐1α shRNA or control shRNA. The promoter activity and expression of CCR1 were measured by luciferase reporter assay, qPCR and western blot, respectively. D, SMMC7721‐OPN were treated by PI3K, ERK or p38 inhibitors. The expression of CCR1, phosphorylated and total AKT, ERK and p38 were analyzed by western blot. E, ChIP‐qPCR of HIF‐1α on the CCR1 promoter in the presence of LV‐OPN and PI3K inhibitor. Data are expressed as means ± SEM and compared using Student's t‐test (*P < .05, **P < .01 and ***P < .001)
Interestingly, we also found that CCR1 was highly expressed at the invasive front and significantly associated with hypoxic regions (detected by HIF‐1α staining). Considering that hypoxia is a common feature that contributes to invasion and metastasis of cancers, including HCC,28, 29, 30 we next analyzed the expression levels of HIF‐1α and CCR1 in HCC tissues and found that the CCR1 levels were closely correlated to HIF‐1α levels (Figure S3A,B). In addition, in in vivo orthotopic models of OPN‐upregulated SMMC7721 cells, HIF‐1α was significantly higher in the xenograft tumors than in the controls (Figure S3C,D). Based on bioinformatics analysis using the Jaspar database, a putative HIF‐1α‐binding site was found at the promoter region of CCR1 gene (Figure 5A). Furthermore, site‐directed mutagenesis of HIF‐1α‐binding sites in CCR1 promoter significantly decreased the CCR1 promoter‐driven luciferase activity in HCC cells (Figure 5A). A luciferase reporter assay showed that the HIF‐1α inhibitor (BAY 87‐2243) or HIF‐1α knockdown (sh‐HIF‐1α) significantly blocked the OPN‐mediated activation of CCR1 promoter activity (Figure 5B,C). These studies suggest that the HIF‐1α binding sites are required for the activation of the CCR1 promoter activity by OPN, and OPN upregulates the expression of CCR1 through HIF‐1α.
To further elucidate the signaling pathways responsible for OPN‐induced CCR1 expression in HCC, we treated HCC cells with several pathways inhibitors, which were reported to be activated by OPN.31, 32, 33, 34, 35 The results revealed that only blocking the PI3K/AKT pathways by using LY294002 could significantly inhibit the OPN‐induced CCR1 expression, while U0126 or SB203580 did not have similar effects (Figure 5D). In addition, ChIP‐qPCR assay showed reduced binding of HIF‐1α to the CCR1 promoter only in the presence of PI3K inhibitor, but not ERK or p38 inhibitors (Figure 5E). Taken together, these studies suggested that OPN upregulated CCR1 expression through the PI3K/AKT/HIF‐1α signaling pathway (Figure 5A‐E).
3.6. C‐C chemokine receptor type 1 is positively associated with hepatocellular carcinoma metastasis in hepatocellular carcinoma patients
To further investigate the role of CCR1 in HCC metastasis, we detected CCR1 expression in HCC cell lines with different metastatic potentials, and found that the CCR1 levels in high‐metastatic HCC cell lines (HCC‐97H and HCC‐LM3) were much higher than those with low metastatic potential (HepG2, Hep3B and SMMC7721) (Figure 6A,B).36 Moreover, we measured mRNA levels of CCR1 by qPCR and IHC in normal livers, tumors without metastasis and tumors with metastasis, and found that CCR1 expression was significantly increased in the group of HCC with metastasis (Figure 6C‐E). Furthermore, upregulation of CCR1 was significantly associated with poorer overall survival of the patients and higher recurrence rates (P < .001, respectively; Figure 6F,G). Collectively, these findings suggested that CCR1 was positively associated with metastasis and indicated poor prognosis in HCC patients.
Figure 6.
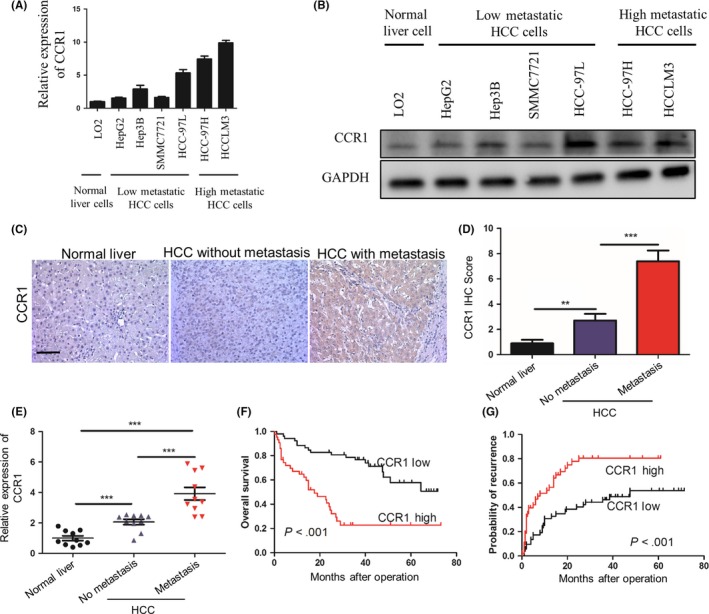
CCR1 is positively associated with metastasis in hepatocellular carcinoma patients. A,B, quantitative PCR (qPCR) (A) and western blot analysis (B) of CCR1 expression in differential hepatocellular carcinoma (HCC) cell lines. C, Representative immunohistochemical (IHC) staining images showing the accumulation of CCR1 in normal liver, tumors without metastasis and tumors with metastasis. Scale bar, 100 μm. D, Statistical analysis of the pathologic scores of CCR1 in the various groups. Data are expressed as means ± SEM and compared using the unpaired t‐test (**P < .01 and ***P < .001). E, Relative mRNA levels of CCR1 measured by qPCR analysis and normalized to β‐actin, in normal livers, and in hepatocellular carcinoma tumors without and with metastasis (n = 10, respectively). Data are expressed as means ± SEM and compared using the unpaired t‐test (**P < .01 and ***P < .001). F,G, The cumulative overall survival (F) and recurrence survival (G) of hepatocellular carcinoma patients with high or low CCR1 expression levels estimated using the Kaplan‐Meier method and compared by the log‐rank test
3.7. C‐C chemokine receptor type 1 antagonist has more significant effects on hepatocellular carcinoma progression when osteopontin is upregulated
To further validate the essential roles of CCR1 in OPN‐enhanced HCC progression, we evaluated the effects of BX471, a selective non‐peptide CCR1 antagonist, on cell proliferation by the CCK‐8 cell viability assay.18, 22, 23 We found that BX471 could significantly inhibit SMMC7721 cell proliferation in a dose‐dependent manner, and the IC50 of OPN‐upregulated cells (SMMC7721‐OPN, 44.09 ± 1.10 μmol L−1) was much lower than that of the controls (71.59 ± 1.07 μmol L−1, P < .05); that is, SMMC7721‐OPN cells (with higher CCR1 levels) is much more sensitive to BX471 than the SMMC7721‐control cells (with a lower level of CCR1) (Figure 7A). In addition, more significant inhibitory effects of BX471 were found on the clone formation, migration and invasion of OPN‐overexpressed cells compared with the control cells (Figure 7B,C and Figure S4A,B). These data indicated that the effects of BX471 on HCC cells were significantly correlated with the basal OPN and CCR1 levels in HCC cells.
Figure 7.
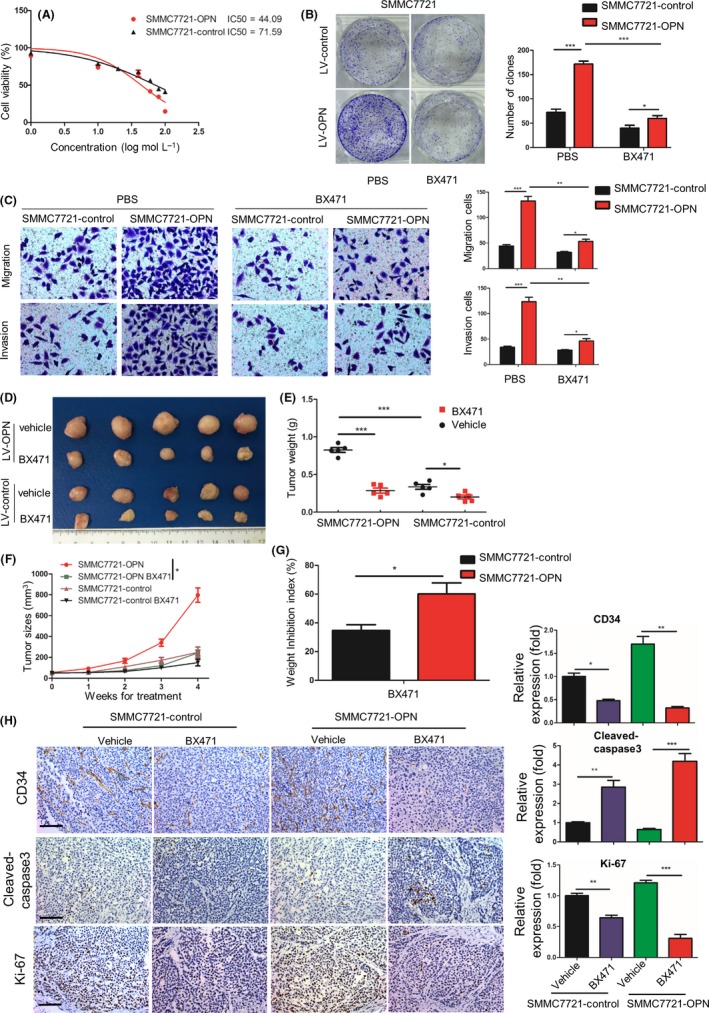
CCR1 antagonist has more significant effects on hepatocellular carcinoma (HCC) progression when osteopontin (OPN) is upregulated. A, Proliferation curves of human HCC cells after exposure to the various concentrations of BX471. B, Comparison of clone formation ability among HCC cells with treatment of BX471 (20 μmol L−1). C, OPN‐induced HCC cells migration is attenuated upon BX471. Migration and invasion of SMMC7721 cells stably expressing LV‐control or LV‐OPN in the presence or absence of BX471. D‐F, Tumor growth in SMMC7721 cells stably expressing lentivirus (LV)‐control or LV‐OPN treated with BX471 (20 mg/kg) or vehicle for 4 wk. Representative images of dissected tumors at the end of the experiment (D), tumor weight (E) and tumor growth curves (F) of mice were analyzed. G, Relative inhibition rate of subcutaneous tumors of SMMC7721 cells stably expressing LV‐control or LV‐OPN treated with BX471 (20 mg/kg). H, Left, representative immunohistochemical (IHC) images of CD34, cleaved‐caspase3 and Ki‐67 on subcutaneous tumors after the indicated treatment. Scale bar, 100 μm. Right, quantification of CD34, cleaved‐caspase3 and Ki‐67 expression according to IHC scores. Data are shown as means ± SEM and compared using Student's t‐test (*P < .05, **P < .01, ***P < .001)
To further evaluate the effects of BX471 in in vivo tumor growth of HCC, we established subcutaneous implantation models of mice with SMMC7721 cells stably expressing LV‐OPN or LV‐control, and found that BX471 treatment could more remarkably inhibit the in vivo tumor growth of SMMC7721‐OPN xenograft compared with controls (Figure 7D‐F). The inhibitory rates of these 2 groups were 60.1% and 34.6%, respectively (Figure 7G, P < .05). Furthermore, IHC demonstrated an increased apoptosis (cleaved caspase‐3 staining), reduced cell proliferation (Ki‐67 staining) and less angiogenesis (CD34 staining) in SMMC7721‐OPN xenograft tumor tissues after BX471 treatment compared with the control group (Figure 7H). In addition, no significant changes in average mice body weight or toxicity in the liver and kidney were observed in the treatment of BX471 (Figure S5A,B). Collectively, these results suggested that blockade of CCR1 with CCR1 antagonist could significantly inhibit HCC growth and metastasis in both in vitro and in vivo experiments, and CCR1 antagonist had more significant effects on HCC progression when OPN was upregulated.
3.8. Combination of osteopontin and C‐C chemokine receptor type 1 has a better prediction performance for hepatocellular carcinoma prognosis
We further evaluated the association between OPN and CCR1 in HCC tissues. Immunohistochemistry (IHC) showed that OPN expression was positively correlated with CCR1 expression (Figure 8A,B). Based on the expression levels of OPN and CCR1, the HCC patients enrolled were divided into 3 groups. Kaplan‐Meier analysis showed that patients with both high OPN and high CCR1 expression had the highest tumor recurrence rates and the shortest overall survival (Figure 8C,D). Univariate analysis showed that OPN, CCR1 and serum GGT levels, tumor size, numbers and capsule were significantly associated with OS and DFS of HCC patients. Multivariate analysis showed that OPN, CCR1 expression, tumor size and combination of OPN and CCR1 were independent prognostic indicators for survival and recurrence (Table 1). Moreover, combination of OPN and CCR1 thus served as a powerful prognostic factor for HCC patients. To determine whether the OPN/CCR1 signaling shows significance during in vivo hepatocarcinogenesis, we started with the analysis of tumors in a model of DEN‐induced experimental liver tumorigenesis in mice. IHC revealed that the increased expression of OPN correlated with enhanced CCR1 staining (Figure 8E). Taken together, these studies suggested that the OPN/CCR1 signaling pathway promoted HCC metastasis and indicated poor prognosis.
Figure 8.
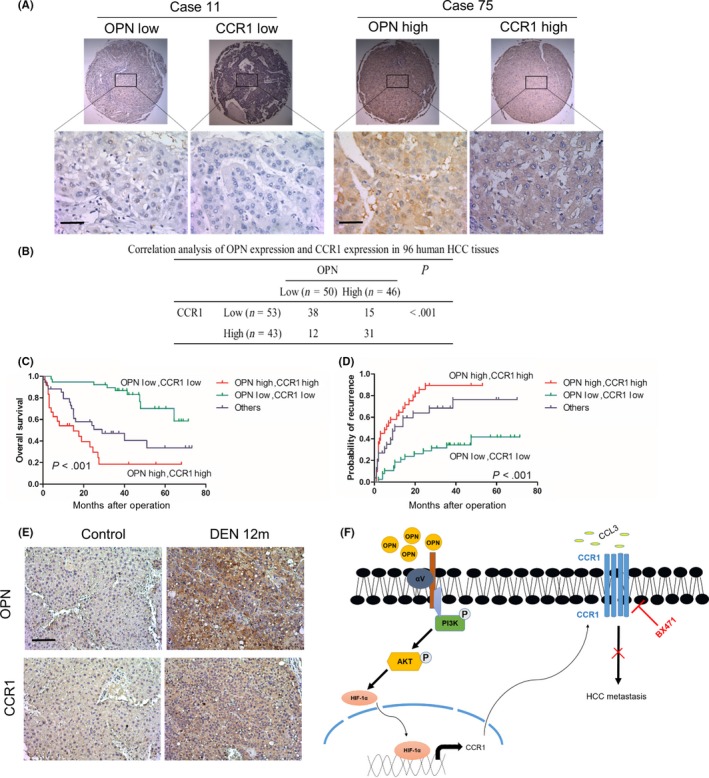
Combination of osteopontin (OPN) and CCR1 has a better prediction performance for hepatocellular carcinoma (HCC) prognosis. A, Representative immunohistochemical (IHC) images showing the expression of OPN and CCR1 in HCC tissues. Scale bar, 50 μm. B, Association between the expression of OPN and CCR1 in HCC tissues. Significance was determined using the χ2‐test. C,D, HCC tissues with both high OPN and CCR1 level had poorer overall survival (C) and higher probability of recurrence (D) among the 3 subgroups. Significance was determined using the log‐rank test. E, Representative IHC images of serial sections of liver from mice showing increased expression of OPN and CCR1 after diethylnitrosamine (DEN) compared with PBS treatment (12 mo). Scale bar, 100 μm. F, Working model for the role of OPN‐CCR1 axis in HCC metastasis. OPN promotes HCC migration, invasion and metastasis by upregulating CCR1 through PI3K/AKT/HIF‐1α signaling pathway, which is inhibited by CCR1 antagonist, BX471
Table 1.
Univariate and multivariate analysis of factors associated with overall survival and disease‐free survival in hepatocellular carcinoma patients
| Variables | Overall survival | Disease‐free survival | ||||
|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||
| P | HR (95% CI) | P | P | HR (95% CI) | P | |
| Sex (female vs male) | .311 | NA | .784 | NA | ||
| Age, (y) (≤50 vs >50) | .371 | NA | .256 | NA | ||
| HBsAg (negative vs positive) | .588 | NA | .468 | NA | ||
| HCV Ab (negative vs positive) | .600 | NA | .515 | NA | ||
| ALT,U/L (>75 vs ≤75) | .211 | NA | .491 | NA | ||
| GGT,U/L (>54 vs ≤54) | .006** | .181 | .089 | NA | ||
| AFP, ng/mL (>20 vs ≤20) | .08 | NA | .066 | NA | ||
| Tumor size, cm (>5 vs ≤5) | <.001** | 2.639 (1.266‐5.502) | .010** | <.001** | 1.859 (1.061‐3.255) | .030** |
| Tumor number (multiple vs single) | <.001** | .543 | .536 | NA | ||
| Tumor differentiation (III + IV vs I + II) | .243 | NA | .726 | NA | ||
| OPN (high vs low) | <.001** | 2.896 (1.329‐6.315) | .007** | <.001** | 3.233 (1.796‐5.821) | <.001** |
| CCR1 (high vs low) | <.001** | 2.015 (1.037‐3.914) | .039** | <.001** | .143 | |
| Combination OPN and CCR1 | ||||||
| Double high vs double low | <.001** | 5.585 (2.480‐12.577) | <.001** | <.001** | 4.146 (2.093‐8.212) | <.001** |
| Others vs double low | .016** | .062 | .001** | 2.750 (1.348‐5.611) | .005** | |
| Double high vs others | .012** | 2.414 (1.189‐4.905) | .015** | .131 | .182 | |
AFP, α‐fetoprotein; ALT, alanine transaminase; anti‐HCV Ab, anti‐hepatitis C virus antibody; CI, confidence interval; HBsAg, hepatitis B surface antigen; GGT, gamma‐glutamyl transpeptidase; HR, hazard ratio; NA, not adopt.
Univariate analysis, Cox proportional hazards regression model. **P < .05.
4. DISCUSSION
Accumulating evidence has demonstrated that OPN contributes to metastasis and poor prognosis of cancers, including HCC.6, 7, 8, 9, 10, 11, 37, 38 Thus, understanding how OPN are involved in maintaining the aggressive phenotype of HCC cells may help to identify novel potential targets for HCC.35, 39, 40 Indeed, dissecting the downstream signals of the OPN signaling pathway in HCC may help in the design of more specific targeted therapies and treatment selection to which a potential positive response is predicted. In the present study, we found that OPN upregulated CCR1 expression through the PI3K/AKT/HIF‐1α signaling pathway. Downregulation of CCR1 decreased OPN‐enhanced HCC invasion and metastasis, and upregulation of CCR1 rescued the decreased HCC invasion and metastasis induced by OPN knockdown. Furthermore, CCR1 expression was positively correlated with OPN expression, and both high OPN and high CCR1 expression had the highest tumor recurrence rates and shortest overall survival time in human HCC patients. Taken together, these studies indicated that upregulated CCR1 expression induced by OPN played an important role in HCC metastasis and poor prognosis in human HCC with high OPN.
The mechanisms mediating the pro‐metastatic effects of CCR1 can be multifarious. A recent report has described that CCR1 promotes invasion of prostate cancer cells through activation of ERK and Rac signaling and increasing secretion of MMP2/9.41 In addition, CCR1 promotes colon cancer liver metastasis through accumulation of myeloid cells.42 Inhibition of CCR1 either by small interfering RNA or antagonist dramatically decreases both in vitro invasion and in vivo metastasis of cancer cells.17, 22, 43 These studies indicate that CCR1 is a critical regulator of cancer invasion and metastasis. In the present study, we provided evidence to support that OPN‐CCR1 axis plays a critical role in promoting HCC metastasis. Moreover, we also demonstrated that OPN upregulates CCR1 expression through the PI3K/AKT/HIF‐1α signaling pathway and blocking CCR1 significantly decreased HCC invasion and metastasis induced by OPN. These suggest that OPN promotes HCC progression and metastasis at least in part by activating CCR1 expression. This is further confirmed in the DEN‐induced liver carcinogenesis models of C57BL/6 mice. All these factors support the existence of OPN‐CCR1 pathways in HCC, which may contribute to tumor progression and metastases.
Osteopontin has been confirmed to play critical roles in HCC metastasis in the present as well as previous studies, and could be an ideal target to combat HCC metastasis.9, 40, 44 However, targeting OPN in cancer with multiple inhibitors proved to be ineffective and frequently elicited unwanted adverse effects.44, 45 In the present study, we have demonstrated that OPN promotes HCC progression and metastasis at least in part by activating CCR1 expression, and blocking CCR1 significantly decreases HCC invasion and metastasis induced by OPN. Chemokines and their receptors are highly promising therapeutic targets for cancers, many of them have been applied in preclinical researches and clinical trials.16, 46, 47, 48 Therefore, targeting CCR1 can be an alternative way to control the progression and metastasis of HCC with OPN overexpression. Another important finding of the present study is that blockade of CCR1 with CCR1 antagonist could significantly inhibit HCC growth and metastasis in both in vitro and in vivo experiments. Given that CCR1 antagonist has more significant effects on HCC progression when OPN is upregulated, OPN can be used as biomarkers to predict the beneficial response to CCR1 blockade.
In conclusion, we have uncovered a new molecular mechanism of OPN in HCC metastasis (Figure 8F). OPN can activate CCR1 expression through the PI3K/AKT/HIF‐1α signaling pathway to promote HCC progression and metastasis. CCR1 blockade is an alternative way to control the progression and metastasis of HCC with OPN overexpression.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
Supporting information
Zhu Y, Gao X‐M, Yang J, et al. C‐C chemokine receptor type 1 mediates osteopontin‐promoted metastasis in hepatocellular carcinoma. Cancer Sci. 2018;109:710–723. https://doi.org/10.1111/cas.13487
Funding information
“973” State Key Basic Research Program of China (2013CB910500 and 2014CB542101); China National Natural Science Foundation (81372647 and 81672820); National Key Research and Development Program of China (2017YFC1308604).
Ying Zhu, Xiao‐Mei Gao, Jing Yang, Da Xu and Yu Zhang authors are co‐first authors.
Contributor Information
Qiong‐Zhu Dong, Email: qiongzhudong@163.com.
Lun‐Xiu Qin, Email: qinlx@fudan.edu.cn.
REFERENCES
- 1. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87‐108. [DOI] [PubMed] [Google Scholar]
- 2. Siegel R, Naishadham D, Jemal A. Cancer statistics for hispanics/latinos, 2012. CA Cancer J Clin. 2012;62:283‐298. [DOI] [PubMed] [Google Scholar]
- 3. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115‐132. [DOI] [PubMed] [Google Scholar]
- 4. Kassahun WT, Fangmann J, Harms J, et al. Liver resection and transplantation in the management of hepatocellular carcinoma: a review. Exp Clin Transplant. 2006;4:549‐558. [PubMed] [Google Scholar]
- 5. El‐Tanani MK. Role of osteopontin in cellular signaling and metastatic phenotype. Front Biosci. 2008;13:4276‐4284. [DOI] [PubMed] [Google Scholar]
- 6. Dong Q, Zhu X, Dai C, et al. Osteopontin promotes epithelial‐mesenchymal transition of hepatocellular carcinoma through regulating vimentin. Oncotarget. 2016;7:12997‐13012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xue YH, Zhang XF, Dong QZ, et al. Thrombin is a therapeutic target for metastatic osteopontin‐positive hepatocellular carcinoma. Hepatology. 2010;52:2012‐2022. [DOI] [PubMed] [Google Scholar]
- 8. Sun BS, Dong QZ, Ye QH, et al. Lentiviral‐mediated miRNA against osteopontin suppresses tumor growth and metastasis of human hepatocellular carcinoma. Hepatology. 2008;48:1834‐1842. [DOI] [PubMed] [Google Scholar]
- 9. Ye QH, Qin LX, Forgues M, et al. Predicting hepatitis B virus‐positive metastatic hepatocellular carcinomas using gene expression profiling and supervised machine learning. Nat Med. 2003;9:416‐423. [DOI] [PubMed] [Google Scholar]
- 10. Sun J, Xu HM, Zhou HJ, et al. The prognostic significance of preoperative plasma levels of osteopontin in patients with TNM stage‐I of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2010;136:1‐7. [DOI] [PubMed] [Google Scholar]
- 11. Zhang H, Ye QH, Ren N, et al. The prognostic significance of preoperative plasma levels of osteopontin in patients with hepatocellular carcinoma. J Cancer Res Clin Oncol. 2006;132:709‐717. [DOI] [PubMed] [Google Scholar]
- 12. Sheu BC, Chang WC, Cheng CY, et al. Cytokine regulation networks in the cancer microenvironment. Front Biosci. 2008;13:6255‐6268. [DOI] [PubMed] [Google Scholar]
- 13. Lazennec G, Richmond A. Chemokines and chemokine receptors: new insights into cancer‐related inflammation. Trends Mol Med. 2010;16:133‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kruizinga RC, Bestebroer J, Berghuis P, et al. Role of chemokines and their receptors in cancer. Curr Pharm Des. 2009;15:3396‐3416. [DOI] [PubMed] [Google Scholar]
- 15. Fingleton B. Molecular targets in metastasis: lessons from genomic approaches. Cancer Genomics Proteomics. 2007;4:211‐221. [PubMed] [Google Scholar]
- 16. Horuk R. Chemokine receptor antagonists: overcoming developmental hurdles. Nat Rev Drug Discov. 2009;8:23‐33. [DOI] [PubMed] [Google Scholar]
- 17. Karash AR, Gilchrist A. Therapeutic potential of CCR1 antagonists for multiple myeloma. Future Med Chem. 2011;3:1889‐1908. [DOI] [PubMed] [Google Scholar]
- 18. Yang X, Lu P, Fujii C, et al. Essential contribution of a chemokine, CCL3, and its receptor, CCR1, to hepatocellular carcinoma progression. Int J Cancer. 2006;118:1869‐1876. [DOI] [PubMed] [Google Scholar]
- 19. Forssmann U, Magert HJ, Adermann K, et al. Hemofiltrate CC chemokines with unique biochemical properties: HCC‐1/CCL14a and HCC‐2/CCL15. J Leukoc Biol. 2001;70:357‐366. [PubMed] [Google Scholar]
- 20. Karash A, Mazzoni MR, Gilchrist A. Pharmacological intervention at CCR1 and CCR5 as an approach for cancer: help or hindrance. Curr Top Med Chem. 2014;14:1553‐1573. [DOI] [PubMed] [Google Scholar]
- 21. Vallet S, Anderson KC. CCR1 as a target for multiple myeloma. Expert Opin Ther Targets. 2011;15:1037‐1047. [DOI] [PubMed] [Google Scholar]
- 22. Wu X, Fan J, Wang X, et al. Downregulation of CCR1 inhibits human hepatocellular carcinoma cell invasion. Biochem Biophys Res Commun. 2007;355:866‐871. [DOI] [PubMed] [Google Scholar]
- 23. Lu P, Nakamoto Y, Nemoto‐Sasaki Y, et al. Potential interaction between CCR1 and its ligand, CCL3, induced by endogenously produced interleukin‐1 in human hepatomas. Am J Pathol. 2003;162:1249‐1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chew V, Chen J, Lee D, et al. Chemokine‐driven lymphocyte infiltration: an early intratumoural event determining long‐term survival in resectable hepatocellular carcinoma. Gut. 2012;61:427‐438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koh MY, Gagea M, Sargis T, et al. A new HIF‐1alpha/RANTES‐driven pathway to hepatocellular carcinoma mediated by germline haploinsufficiency of SART1/HAF in mice. Hepatology. 2016;63:1576‐1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu J, Chen S, Wang W, et al. Cancer‐associated fibroblasts promote hepatocellular carcinoma metastasis through chemokine‐activated hedgehog and TGF‐beta pathways. Cancer Lett. 2016;379:49‐59. [DOI] [PubMed] [Google Scholar]
- 27. Gao Q, Zhao YJ, Wang XY, et al. CXCR6 upregulation contributes to a proinflammatory tumor microenvironment that drives metastasis and poor patient outcomes in hepatocellular carcinoma. Cancer Res. 2012;72:3546‐3556. [DOI] [PubMed] [Google Scholar]
- 28. Lanzen J, Braun RD, Klitzman B, et al. Direct demonstration of instabilities in oxygen concentrations within the extravascular compartment of an experimental tumor. Cancer Res. 2006;66:2219‐2223. [DOI] [PubMed] [Google Scholar]
- 29. Rofstad EK, Galappathi K, Mathiesen B, et al. Fluctuating and diffusion‐limited hypoxia in hypoxia‐induced metastasis. Clin Cancer Res. 2007;13:1971‐1978. [DOI] [PubMed] [Google Scholar]
- 30. Wong CC, Kai AK, Ng IO. The impact of hypoxia in hepatocellular carcinoma metastasis. Front Med. 2014;8:33‐41. [DOI] [PubMed] [Google Scholar]
- 31. Wai PY, Kuo PC. Osteopontin: regulation in tumor metastasis. Cancer Metastasis Rev. 2008;27:103‐118. [DOI] [PubMed] [Google Scholar]
- 32. Gimba ER, Tilli TM. Human osteopontin splicing isoforms: known roles, potential clinical applications and activated signaling pathways. Cancer Lett. 2013;331:11‐17. [DOI] [PubMed] [Google Scholar]
- 33. Qin L. Osteopontin is a promoter for hepatocellular carcinoma metastasis: a summary of 10 years of studies. Front Med. 2014;8:24‐32. [DOI] [PubMed] [Google Scholar]
- 34. Wen Y, Jeong S, Xia Q, et al. Role of osteopontin in liver diseases. Int J Biol Sci. 2016;12:1121‐1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nagoshi S. Osteopontin: versatile modulator of liver diseases. Hepatol Res. 2014;44:22‐30. [DOI] [PubMed] [Google Scholar]
- 36. Li Y, Tian B, Yang J, et al. Stepwise metastatic human hepatocellular carcinoma cell model system with multiple metastatic potentials established through consecutive in vivo selection and studies on metastatic characteristics. J Cancer Res Clin Oncol. 2004;130:460‐468. [DOI] [PubMed] [Google Scholar]
- 37. Shang S, Plymoth A, Ge S, et al. Identification of osteopontin as a novel marker for early hepatocellular carcinoma. Hepatology. 2012;55:483‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhou C, Zhou HJ, Zhang XF, et al. Postoperative serum osteopontin level is a novel monitor for treatment response and tumor recurrence after resection of hepatitis B‐related hepatocellular carcinoma. Ann Surg Oncol. 2013;20:929‐937. [DOI] [PubMed] [Google Scholar]
- 39. Wang KX, Denhardt DT. Osteopontin: role in immune regulation and stress responses. Cytokine Growth Factor Rev. 2008;19:333‐345. [DOI] [PubMed] [Google Scholar]
- 40. Zhao J, Dong L, Lu B, et al. Down‐regulation of osteopontin suppresses growth and metastasis of hepatocellular carcinoma via induction of apoptosis. Gastroenterology. 2008;135:956‐968. [DOI] [PubMed] [Google Scholar]
- 41. Kato T, Fujita Y, Nakane K, et al. CCR1/CCL5 interaction promotes invasion of taxane‐resistant PC3 prostate cancer cells by increasing secretion of MMPs 2/9 and by activating ERK and Rac signaling. Cytokine. 2013;64:251‐257. [DOI] [PubMed] [Google Scholar]
- 42. Hirai H, Fujishita T, Kurimoto K, et al. CCR1‐mediated accumulation of myeloid cells in the liver microenvironment promoting mouse colon cancer metastasis. Clin Exp Metastasis. 2014;31:977‐989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kitamura T, Fujishita T, Loetscher P, et al. Inactivation of chemokine (C‐C motif) receptor 1 (CCR1) suppresses colon cancer liver metastasis by blocking accumulation of immature myeloid cells in a mouse model. Proc Natl Acad Sci U S A. 2010;107:13063‐13068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bandopadhyay M, Bulbule A, Butti R, et al. Osteopontin as a therapeutic target for cancer. Expert Opin Ther Targets. 2014;18:883‐895. [DOI] [PubMed] [Google Scholar]
- 45. Johnston NI, Gunasekharan VK, Ravindranath A, et al. Osteopontin as a target for cancer therapy. Front Biosci. 2008;13:4361‐4372. [DOI] [PubMed] [Google Scholar]
- 46. Weitzenfeld P, Ben‐Baruch A. The chemokine system, and its CCR5 and CXCR4 receptors, as potential targets for personalized therapy in cancer. Cancer Lett. 2014;352:36‐53. [DOI] [PubMed] [Google Scholar]
- 47. Solari R, Pease JE, Begg M. Chemokine receptors as therapeutic targets: why aren't there more drugs? Eur J Pharmacol. 2015;746:363‐367. [DOI] [PubMed] [Google Scholar]
- 48. Klarenbeek A, Maussang D, Blanchetot C, et al. Targeting chemokines and chemokine receptors with antibodies. Drug Discov Today Technol. 2012;9:e227‐e314. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


