Abstract
Although humoral responses against CTL epitope peptides from lymphocyte‐specific protein tyrosine kinase (Lck) antigen have been observed in the majority of healthy donors and cancer patients, the biological activity of the antibody has not been determined. We investigated the biological activity of mAb against CTL epitope peptide of Lck antigen at positions 486‐494 (anti‐Lck‐486 mAb). This mAb induced dendritic cell maturation from murine bone marrow cells by the immune complex form in vitro, and inhibited tumor growth in association with a suppression of tumor‐infiltrating T cells, including T regulatory cells in a murine model using female BALB/cCrlCrlj mice (H‐2Kd). More potent tumor inhibition was observed when this mAb was given prior to peptide vaccination. These results may help to unveil the biological activity of anti‐Lck peptide antibodies against CTL epitope peptides.
Keywords: anti‐Lck‐486 peptide antibody, cancer vaccine, dendritic cells, lymphocyte‐specific protein tyrosine kinase, T‐cell epitope peptide
Abbreviations
- APC
Allophycocyanin
- DC
dendritic cell
- ELISPOT
enzyme‐linked immunospot
- Lck
lymphocyte‐specific protein tyrosine kinase
- PPV
personalized peptide vaccination
- TIL
tumor‐infiltrating lymphocyte
- Treg
regulatory T cell
- VC
vaccine
1. INTRODUCTION
We previously reported that the IgG against CTL epitope peptides from Lck antigen have been observed in the majority of healthy donors and cancer patients in correlation with the overall survival of cancer patients.1 We also reported that an increase in the IgG responses to the vaccinated peptides were favorable for the overall survival of cancer patients under PPV, in which four of 31 warehouse peptides were selected by pre‐existing peptide‐specific IgG levels.2, 3 Among the warehouse peptides for PPV, peptides derived from Lck have been used most frequently for PPV for advanced cancer patients, with favorable clinical outcomes.2, 3 Lymphocyte‐specific protein tyrosine kinase is a member of the Src family of non‐receptor protein tyrosine kinases expressed in both activated T lymphocytes and metastatic cancer cells with oncogenic properties in human cancers.4, 5 Lymphocyte‐specific protein tyrosine kinase is pivotal for Tregs and program death‐1‐positive T‐cell activities.6, 7 The Lck gene was reported to encode CTL epitope peptides that were cytotoxic to tumor cells from metastatic cancer patients.8
Immunoglobulin G reactive to Lck peptide at positions 486‐494 (Lck‐486) was found to be expressed in some metastatic tumor cells and T cells at the tumor site, but not expressed in tumor cells from non‐metastatic primary tumor cells.1, 2, 3 In the present study, we investigated the biological activity of a mAb reacting to the Lck peptide at positions 486‐494 (anti‐Lck‐486 mAb), and showed it had antitumor activity.
2. MATERIALS AND METHODS
2.1. Antibody production and purification
The hybridoma clone producing IgG2b specific to the Lck‐486 peptide (TFDYLRSVL), which is shared by both humans and mice, was carried out by Cell Engineering Corp. (Osaka, Japan). The hybridoma producing IgG2b reactive to the SART3 peptide at positions 109‐117 (SART3‐109) (VYDYNCHVDL, which is different from the mouse SART3 sequence at position 117 (D to E in the mouse SART3 gene as reported),9 was also provided by the study. Both peptides were able to induce peptide‐specific CTLs in HLA‐A24+ cancer patients.3, 10 We measured the levels of IgG produced by these hybridoma clones reactive to Lck‐486 or to SART3‐109 by carrying out a multiplex bead suspension array using the Luminex system (Luminex, Austin, TX, USA), as described.11
The specificity of the anti‐Lck‐486 and that of the anti‐SART3‐109 mAb was confirmed by competition assay (Figure S1). For the competition assay, 10 000‐fold diluted antibody was incubated with 100 μL peptide‐coupled color‐coded beads and 5 μL each of the corresponding peptides (10 μg/mL) for 1.5 hours at 30°C. The binding of anti‐peptide IgG was detected by the same method as that described above.1 For purification, the cloned cells were cultured with hybridoma serum‐free medium (Life Technologies, Carlsbad, CA, USA). The supernatant was collected, and IgG was purified with a Protein‐A column (GE Healthcare, Uppsala, Sweden) according to the manufacturer's instructions.
2.2. Mice, cell line, and reagents
Female BALB/cCrlCrlj mice (H‐2Kd) were obtained from Charles River Japan (Yokohama, Japan). The mice were maintained under specific pathogen‐free conditions and provided for the study at 6‐10 weeks of age. The murine rectal cancer cell line, Colon26 (H‐2Kd), was used in this study to determine the biological activity of HLA‐A24‐restricted CTL epitope peptides in a mouse model as described.10 This is because H‐2Kd molecules hold the binding motif to CTL epitope short peptides capable of binding to human HLA‐A24 molecules.
The expression of Lck antigen in Colon26 tumor cells was confirmed by real‐time PCR before the cells' use (data not shown). The synthetic peptides used for the study were the Lck‐486 peptide or SART3‐109. In addition, we provided the SART2 peptide at positions 161‐169 (AYDFLYNYL) (SART2‐161 peptide), known as the other HLA‐A24 restricted CTL epitope peptide.12 The sequence of SART2‐161 peptide is shared by humans and mice.
2.3. Generation of myeloid DCs
Marrow from the bones (femurs and tibias) was flushed and collected into RPMI medium. Ammonium chloride lysis buffer was added to lyse red blood cells, and then the bone marrow cells were washed and suspended in RPMI medium. These cells were incubated in the presence of 500 U/mL granulocyte/macrophage colony‐stimulating factor and 1000 U/mL interleukin‐4 for 48 hours for induction of immature DCs based on the methods reported.13 After 48 hours of incubation, non‐adherent cells were removed, followed by replacement of half the medium, and cultured for an additional 48 hours.
Immature DCs were then cultured with: anti‐Lck‐486 mAb alone, anti‐SART3‐109 mAb alone, or an isotype control antibody (IgG2b < clone: MG2b‐57>) alone; anti‐Lck‐486 mAb plus Lck‐486 peptide, anti‐Lck‐486 mAb plus SART2‐161 peptide, isotype control plus Lck‐486 peptide, or anti‐SART3‐109 mAb plus SART3‐109 peptide; and Lck‐486 peptide alone or SART3‐109 peptide alone for another 48 hours for the subsequent experiments. For these experiments, to make the immune complex form, 2 μg Lck‐486 or SART3‐109 peptide was cultured with 20 μg anti‐Lck‐486 or anti‐SART3‐109 mAb, respectively, for 60 minutes at 37°C prior to the addition to the culture of DCs based on methods reported with slight modification.14
As negative controls, 2 μg SART2‐161 peptide was cultured with 20 μg anti‐Lck‐486 mAb, or 2 μg SART3‐109 peptide was cultured with 20 μg anti‐SART3‐109 mAb for 60 minutes at 37°C.
2.4. Animal study
All animal experiments were approved by the Animal Experiments Committee of Kurume University (Kurume, Japan). One million Colon26 cells in 100 μL PBS was injected s.c. into the right flank of female BALB/cCrlCrlj mice. The tumor size was measured every 3 days with a caliper, and tumor volume was calculated as the product of 1/2(length × width2). Tumor sizes are presented as the means of each group (4‐6 mice per group). Twenty‐four mice were divided into six groups for the first study, in which mice were killed at day 19, followed by measurement of the tumor size and harvesting of TILs after the tumor was minced. Tumors were minced with a tumor dissociation kit (Miltenyi Biotec Japan, Tokyo, Japan). The cells were then stained with antibodies for subsequent experiments.
For the second series of experiments investigating the antitumor effect of anti‐Lck‐486 mAb injection prior to the peptide vaccine, the treatment was started on day 3; in this experiment, the mice were killed on day 21.
2.5. Vaccination
The Lck‐486 and SART2‐161 peptides were dissolved in Meylon 7% injection (Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan), then diluted with Otsuka Distilled Water (Tokyo, Japan). The vaccine was formulated with incomplete Freund's adjuvant (Montanide ISA‐51VG; Seppic, Paris, France) and peptide solution at a 1:1 ratio.
2.6. Antibodies and cell surface marker analysis
For the staining surface marker of DCs and TILs, APC anti‐mouse CD4, FITC‐CD11c, APC‐CD40, PerCP/Cy5.5‐CD80, and PE/Cy7‐CD86, and the Treg flow kit were purchased from BioLegend Japan (Tokyo). Fluorescein isothiocyanate‐labeled mouse CD8 was purchased from MBL (Nagoya, Japan). Cultured DCs were harvested, centrifuged, and suspended in 2% FBS/PBS. Their surfaces were then stained with FITC‐CD11c, APC‐CD40, PerCP/Cy5.5‐CD80, and PE/Cy7‐CD86 for 30 minutes at 4°C. Tumor‐infiltrating lymphocytes were incubated with the APC‐CD4, FITC‐CD8, or CD4/CD25/Foxp3 cocktail according to the manufacturer's instructions, followed by an analysis with a FACSVerse flow cytometer (BD Biosciences, Mountain View, CA, USA) and FlowJo software (version 7.6.5).
2.7. Cytotoxic T‐lymphocyte assay
Mice were inoculated s.c. with 100 μg Lck486 peptide plus incomplete Freund's adjuvant to the flank once a week for 6 weeks, as reported previously.10 Spleens were harvested and homogenized into single cell suspensions using two pairs of slide glasses, and a 100‐μm cell strainer (Greiner Bio‐One) was used to remove debris. Cells were centrifuged and ammonium chloride lysis buffer was added to lyse red blood cells. The cells were washed and resuspended in RPMI medium for counting. Cells were resuspended at 2 × 107/mL in RPMI complete media and were incubated with or without 10 μg/mL Lck486 peptide for 4 days at 37°C. After incubation, the cells were harvested and tested for their ability to produce γ‐interferon in response to Lck486 peptide, or without peptide as a negative control. Antigen‐specific γ‐interferon secretion after 18 hours of incubation was determined by ELISPOT assay in accordance with the manufacturer's instructions. All assays were carried out in triplicate, at minimum, and spots were counted by an ELISPOT reader (CTL‐ImmunoSpot S5 series, Cellular Technology Ltd., Cleveland, OH, USA). For in vitro stimulation of spleen cells, the same method of preparation was used.
2.8. Statistical analysis
Two‐sided P‐values <.05 were considered significant. All statistical analyses were undertaken using JMP Pro 11.0 software (SAS Institute, Cary, NC, USA).
3. RESULTS AND DISCUSSION
3.1. Cytotoxic T lymphocytes specific for Lck468 in this mouse system
To facilitate better understanding of the T cell‐recognition of Lck486 peptide, we tested whether Lck486 peptide‐specific CTLs were detectable in a murine system measured by ELISPOT assay. We found that Lck486 peptide‐specific CTLs were detected by ELISPOT assay in spleen cells when mice were vaccinated with Lck468 peptide. The number of spots for the immunized group and the non‐immunized control group were 13 ± 4.5 and 3 ± 1.9 (P = .006), respectively. In addition, it was detectable when spleen cells were stimulate by addition of Lck468 peptide in vitro. The number of spots for the peptide‐stimulated group and the control group were 175 ± 36.6 and 48 ± 21.5 (P = .001), respectively.
3.2. Induction of maturation of DCs by anti‐Lck486 mAb in vitro
We investigated whether anti‐Lck486 mAb induced the maturation of DCs from bone marrow cells. Representative results are shown in Figure 1. In CD11c+ cells, the expressions of CD86 and CD80 (which bind to CD28 molecules for T‐cell activation and thus are markers for matured DC capable of presenting antigenic peptides to T cells)15, 16 were significantly increased in culture with only this immune complex form (Figure 1, column 2) compared to that with both the isotype control mAb and the Lck‐486 peptide (column 4) (P < .05). The expression of CD40, the other marker of mature DCs, on CD11c+ cells also tended to increase. None of the other peptides or antibodies tested induced the maturation. The failure of maturation by anti‐SART3‐109 mAb plus the corresponding peptide could be explained by the fact that the amino acid sequence of SART3‐109 used for the study was different to that of the corresponding murine peptide.
Figure 1.
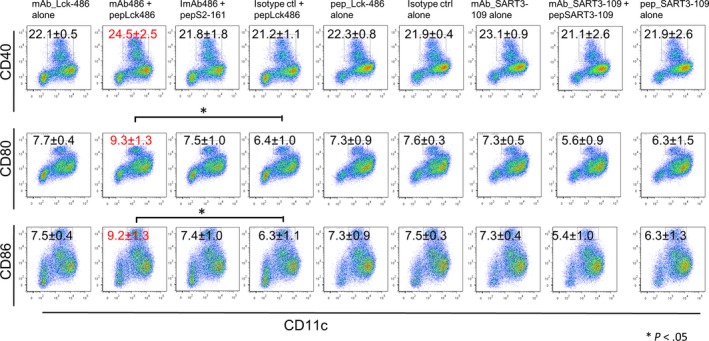
Dendritic cell maturation with antibodies with/without peptides. Immature dendritic cells from bone marrow cells were cultured with nine different culture conditions: column 1, anti‐lymphocyte‐specific protein tyrosine kinase (Lck)‐486 mAb alone; column 2, anti‐Lck‐486 mAb plus Lck‐486 peptide; column 3, anti‐Lck‐486 mAb plus SART2‐161 peptide; column 4, isotype control plus Lck‐486 peptide; column 5, Lck‐486 peptide alone; column 6, isotype control (ctrl) antibody alone; column 7, anti‐SART3‐109 mAb alone; column 8, anti‐SART3‐109 mAb plus SART3‐109 peptide; and column 9, SART3‐109 peptide alone
Collectively, these results suggest that immature DCs differentiated into mature DCs through the capture of the immune complex of the Lck‐486 peptide and anti‐Lck‐486 mAb by the Fc receptor on the surface, followed by the presentation of the Lck‐486 peptide to H‐2k molecules.
3.3. Inhibition of tumor growth by anti‐Lck486 mAb in vivo
To investigate anti‐Lck‐486 mAb‐mediated inhibition of tumor growth in vivo, we injected 106 Colon26 cells (which serve as murine tumor models of human HLA‐A24+ cancer)10 s.c. into the right flank of Balb/c mice (H‐2k). Treatment was started on day 7 when the tumors reached approximately 25 mm in size. Representative results are shown in Figure 2(A). The tumor size of each group on day 19 was as follows: Group 1 (vaccination of Lck‐486 peptide), 403 ± 108 mm2; Group 2 (vaccination of SART2‐161 peptide), 615 ± 235 mm2; Group 3 (injection of Lck‐486 mAb), 255 ± 103 mm2; Group 4 (isotype mAb), 1637 ± 378 mm2; Group 5 (LCK‐486 peptide plus Lck‐486 mAb), 329 ± 46 mm2; and Group 6 (SART2‐161 peptide plus Lck486 mAb), 1361 ± 328 mm2 (Figure 2A). The tumor growth in mice that received Lck‐486 peptide alone, Lck‐486 mAb alone, or both together was significantly suppressed compared to the mice that received the isotype control mAb.
Figure 2.
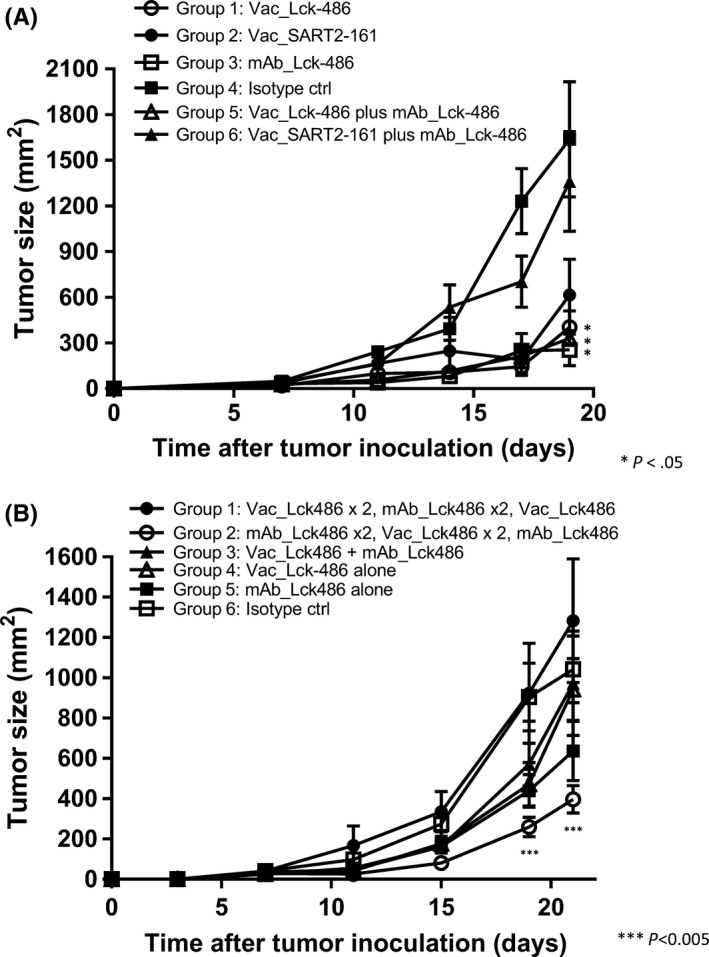
Tumor regression with anti‐lymphocyte‐specific protein tyrosine kinase (Lck)‐486 mAb in vivo. A, One million Colon26 cells were injected s.c. into the right flank of female BALB/cCrlCrlj mice. Treatment was started on day 7 when the tumors reached a size of approximately 25 mm. Twenty‐four mice were divided into six groups (n = 4): Lck‐486 (50 mg/mouse; ○) or SART2‐161 (●) peptide was injected s.c. to the inguinal region on days 7, 11, 14, and 17; purified anti‐Lck‐486 mAb (13.7 mg/mouse; □) or an isotype control (ctrl) IgG2b (clone: MG2b‐57; ■) was given ip, on days 7, 11, 14, and 17; or mice received an ip, injection of anti‐Lck‐486 mAb with an s.c. vaccination of Lck‐486 (▵) or SART2‐161 (▲) peptide. Arrow indicates the day of treatment. *P < .05. B, One million Colon26 cells were s.c. injected into the right flank of 48 mice. Treatment was started on day 3 after the tumor inoculation, and mice were divided into six groups (n = 8) as follows: one group each received an ip, injection of anti‐Lck‐486 mAb alone (■), isotype control antibody alone (□), an s.c. injection of Lck‐486 peptide alone (▵), or Lck‐486 peptide and ip, injection of anti‐Lck‐486 mAb (▲), respectively, on days 3, 7, 11, 15, and 19. The other group received an ip, injection of anti‐Lck‐486 mAb alone on days 3 and 7, and then an s.c. injection of Lck‐486 peptide alone on days 11 and 15 followed by an ip, injection of anti‐Lck‐486 mAb alone on day 19 (○). The remaining group received an s.c. injection of the Lck‐486 peptide alone on days 3 and 7, an ip, injection of the anti‐Lck‐486 mAb alone on days 11 and 15, and an s.c. injection of Lck‐486 peptide alone on day 19 (●). ***P < .005
3.4. Effect of anti‐Lck486 mAb injection prior to peptide vaccination
We then hypothesized that more potent antitumor activity could be obtained by an injection of the antibody prior to the vaccination, given the induction of matured DCs at regional lymph nodes through the activation of CD80/CD86 and the CD28 pathway,16 which in turn results in the activation of CTLs rather than Tregs. We also speculated that the antitumor effect by the peptide vaccination could be decreased by a subsequent injection of the antibody, given the induction of tolerogenic DCs, which would in turn result in the activation of Tregs rather than CTLs.
We investigated whether the injection of anti‐Lck‐486 mAb prior to the peptide vaccination could increase antitumor activity. Our findings indicated that tumor growth was significantly suppressed in mice that were given anti‐Lck‐486 mAb alone, Lck‐486 peptide alone, or both together (Table 1, Figure 2B), consistent with the results shown in Figure 2(A). Notably, the strongest tumor growth suppression among all groups throughout the experiment was observed in mice that received the anti‐Lck‐486 mAb prior to the Lck‐486 peptide vaccination, with a significant level of suppression compared to that in mice that received the Lck‐486 peptide alone on days 19 and 21 (P < .05). In contrast, tumor growth suppression was completely cancelled by vaccination with the Lck‐486 peptide prior to the injection of anti‐Lck‐486 mAb throughout the experiment. These results could partly support the hypotheses described above, but this issue needs to be further tested by examinations of DC and T‐cell subsets at the lymph nodes.
Table 1.
Injection schedule of anti‐lymphocyte‐specific protein tyrosine kinase (Lck)‐486 mAb, Lck‐486 peptide, and isotype conrol (crl) to evaluate tumor regression in mice in vivo
| Day 0 | Day 3 | Day 7 | Day 11 | Day 15 | Day 19 | Day 21 | |
|---|---|---|---|---|---|---|---|
| Group 1 | One million Colon 26 cells | Vac_Lck486 | Vac_Lck486 | mAb_Lck486 | mAb_Lck486 | Vac_Lck486 | End point |
| Group 2 | One million Colon 26 cells | mAb_Lck486 | mAb_Lck486 | Vac_Lck486 | Vac_Lck486 | mAb_Lck486 | End point |
| Group 3 | One million Colon 26 cells | Vac_Lck486 + mAb_Lck486 | Vac_Lck486 + mAb_Lck486 | Vac_Lck486 + mAb_Lck486 | Vac_Lck486 + mAb_Lck486 | Vac_Lck486 + mAb_Lck486 | End point |
| Group 4 | One million Colon 26 cells | Vac_Lck486 | Vac_Lck486 | Vac_Lck486 | Vac_Lck486 | Vac_Lck486 | End point |
| Group 5 | One million Colon 26 cells | mAb_Lck486 | mAb_Lck486 | mAb_Lck486 | mAb_Lck486 | mAb_Lck486 | End point |
| Group 6 | One million Colon 26 cells | Isotype ctrl | Isotype ctrl | Isotype ctrl | Isotype ctrl | Isotype ctrl | End point |
3.5. T‐lymphocyte subsets at tumor sites
We next investigated the T‐lymphocyte subsets at the tumor sites of these mice at day 19 to better understand the mechanisms involved in the anti‐Lck‐486 mAb‐mediated inhibition of tumor growth. Decreases of all CD4+ T‐cell and CD8+ T‐cell subsets (Figure 3A), and the CD4+CD25+Foxp3+ Treg subset (Figure 3B) were observed in mice that received anti‐Lck486 mAb alone compared to the level in mice given an isotype control mAb. Notably, significantly decreased levels of CD8+ T cells and CD4+CD25+Foxp3+ Tregs were observed in the mice that received both the Lck‐486 peptide and anti‐Lck‐486 mAb compared to those given the SART2‐161 peptide plus anti‐Lck‐486 mAb (P < .05) (considered the negative control group). We think that the decrease of CD8+ TIL in mice receiving Lck‐486 peptide might be partly responsible for tumor reduction although the mechanisms are presently unclear. We also think that Tregs in tumor sites were suppressed in mice receiving Lck‐486 peptide and Lck‐486 mAb primarily because of the antibody‐mediated suppression of the Lck‐486 peptide‐induced Treg activation in a peptide‐specific manner. These issue needs to be further studied in the near future by a relatively large‐scale study.
Figure 3.
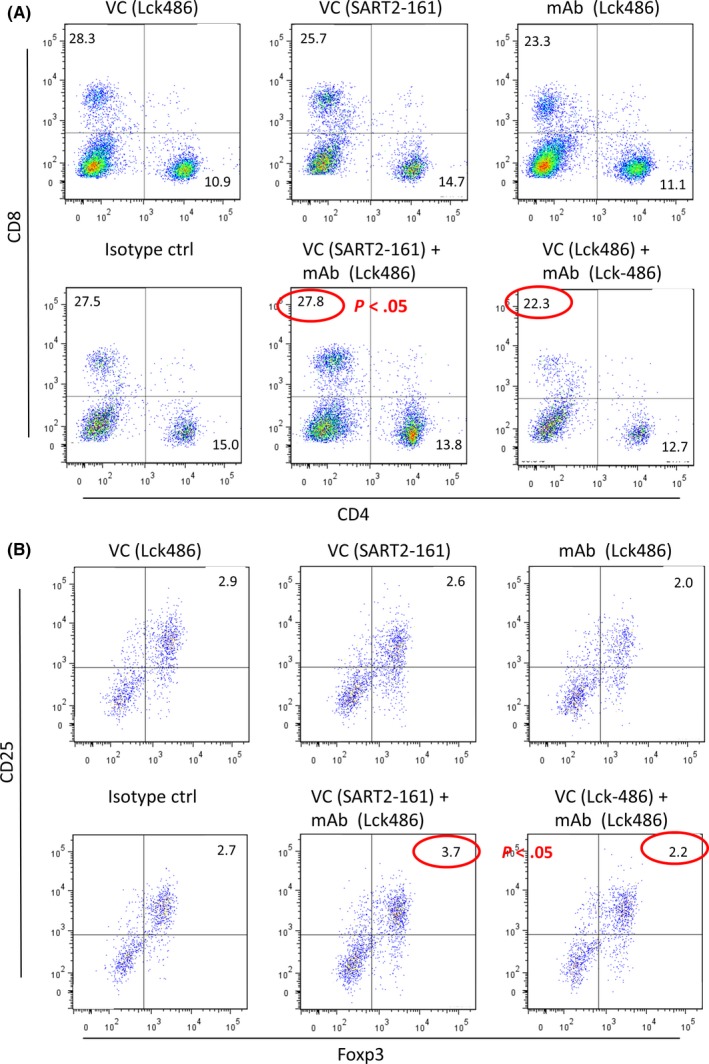
Phenotypic analysis of tumor‐infiltrating lymphocytes. Tumor infiltrating cells were incubated with APC‐CD4, FITC‐CD8 or CD4/CD25/Foxp3 cocktail according to the manufacturer's instructions, followed by analysis with a FACSVerse flow cytometer (BD Biosciences) and FlowJo software (version 7.6.5). A, Results of CD4+ T cell and CD8+ T‐cell subsets. B, Results of CD4+ CD25+Foxp3+ regulatory T‐cell subsets. ctrl, control; VC, vaccine
Since 1989, numerous studies have been undertaken on the potential of Lck antigen as a druggable molecule for cancer therapy, but, to the best of our knowledge, the efforts to achieve this have not been successful.17, 18 This failure might be due in part to the unique properties of Lck, which is involved in both immune activation and oncogenesis.5, 6, 7, 8 Targeted molecules against the Lck antigen might inhibit both antitumor immunity and tumor cell growth. Our present findings also indicate that anti‐Lck‐486mAb suppressed both CD8+ T cells and CD4+CD25+Foxp3+ Tregs at tumor sites, suggesting that this mAb suppresses both CTL‐mediated antitumor immunity and Treg‐mediated CTL suppression. These bi‐potential properties of anti‐Lck‐486 mAb might be responsible for the finding that the co‐administration of both molecules showed no synergistic effect compared to that by either the antibody or peptide alone.
The presence of humoral responses to certain CTL epitope peptides has been reported over the past decade, and vaccination of these peptides for cancer patients often induced increased peptide‐specific IgG responses.1, 2, 3 However, to our knowledge, there are no published reports on the biological activity of antibodies against CTL epitope peptides. The precise understanding of this activity might be important to the development of clinically effective peptide‐based cancer vaccines. Therefore, the results shown herein could open a new avenue for basic and clinical research on the biological activity of anti‐peptide antibodies against CTL epitope peptides.
CONFLICT OF INTEREST
Kyogo Itoh received research funding from Taiho Pharmaceutical Co. Ltd. The other authors have no conflict of interest.
Supporting information
ACKNOWLEDGMENTS
We thank Ms. Sayaka Nagata for technical assistance. This study was supported in part by a grant from the Developing Innovation Systems Program for Fostering Regional Innovation (Global Type) and the Japan Society for the Promotion of Science (KAKENHI grant nos. 24791452 and 26861105) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to S.M.).
Matsueda S, Itoh K, Shichijo S. Antitumor activity of antibody against cytotoxic T lymphocyte epitope peptide of lymphocyte‐specific protein tyrosine kinase. Cancer Sci. 2018;109:611–617. https://doi.org/10.1111/cas.13522
REFERENCES
- 1. Matsueda S, Shichijo S, Nagata S, et al. Identification of novel Lck‐derived T helper epitope long peptides applicable for HLA‐A2 (+) cancer patients as cancer vaccine. Dev Comp Immunol. 2013;41:68‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Noguchi M, Matsumoto K, Uemura H, et al. An open‐label, randomized phase II trial of personalized peptide vaccination in patients with bladder cancer that progressed after platinum‐based chemotherapy. Clin Cancer Res. 2016;22:54‐60. [DOI] [PubMed] [Google Scholar]
- 3. Sasada T, Yamada A, Noguchi M, Itoh K. Personalized peptide vaccine for treatment of advanced cancer. Curr Med Chem. 2014;21:2332‐2345. [DOI] [PubMed] [Google Scholar]
- 4. Cooper JC, Shi M, Chueh FY, Venkitachalam S, Yu CL. Enforced SOCS1 and SOCS3 expression attenuates Lck‐mediated cellular transformation. Int J Oncol. 2010;36:1201‐1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Balbin OA, Prensner JR, Sahu A, et al. Nat Commun. 2013;4:2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Taskén K. Waking up regulatory T cells. Blood. 2009;114:1136‐1137. [DOI] [PubMed] [Google Scholar]
- 7. Bardhan K, Patsoukis N, Weaver J, Freeman G, Li L, Boussiotiset AV. PD‐1 inhibits the TCR signaling cascade by sequestering SHP‐2 phosphatase, preventing its translocation to lipid rafts and facilitating Csk‐mediated inhibitory phosphorylation of Lck. J Immunol. 2016;196(1 Suppl):128.15 http://www.jimmunol.org/content/196/1_Supplement/128.15. [Google Scholar]
- 8. Harashima N, Tanaka K, Sasatomi T, et al. Recognition of the Lck tyrosine kinase as a tumor antigen by cytotoxic T lymphocytes of cancer patients with distant metastases. Eur J Immunol. 2001;31:323‐332. [DOI] [PubMed] [Google Scholar]
- 9. Harada K, Yamada A, Mine T, Kawagoe N, Takasu H, Itoh K. Mouse homologue of the human SART3 gene encoding tumor‐rejection antigen. Jpn J Cancer Res. 2000;91:239‐247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ono T, Harada M, Yamada A, et al. Antitumor effects of systemic and local immunization with a CTL‐directed peptide in combination with a local injection of OK‐432. Clin Cancer Res. 2006;12:1325‐1332. [DOI] [PubMed] [Google Scholar]
- 11. Komatsu N, Shichijo S, Nakagawa M, Itoh K. New multiplexed flow cytometric assay to measure anti‐peptide antibody: a novel tool for monitoring immune responses to peptides used for immunization. Scand J Clin Lab Invest. 2004;64:535‐546. [DOI] [PubMed] [Google Scholar]
- 12. Nakao M, Shichijo S, Imaizumi T, et al. Identification of a gene coding for a new squamous cell carcinoma antigen recognized by the CTL. J Immunol. 2000;164:2565‐2574. [DOI] [PubMed] [Google Scholar]
- 13. Sim WJ, Malinarich F, Fairhurst AM, Connolly JE. Generation of immature, mature and tolerogenic dendritic cells with differing metabolic phenotypes. J Vis Exp. 2016;112:e54128 https://doi.org/10.3791/54128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jefferis R. Aggregation, immune complexes and immunogenicity. MAbs. 2011;3:503‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419‐426. [DOI] [PubMed] [Google Scholar]
- 16. Shin T, Kennedy G, Gorski K, et al. Cooperative B7‐1/2 (CD80/CD86) and B7‐DC costimulation of CD4 + T cells independent of the PD‐1 receptor. J Exp Med. 2003;198:31‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harr MW, Caimi PF, McColl KS, et al. Inhibition of Lck enhances glucocorticoid sensitivity and apoptosis in lymphoid cell lines and in chronic lymphocytic leukemia. Cell Death Differ. 2010;17:1381‐1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kükenshöner T, Schmit NE, Bouda E, et al. Selective targeting of SH2 domain‐phosphotyrosine interactions of src family tyrosine kinases with monobodies. J Mol Biol. 2017;429:1364‐1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


