Abstract
Uterine cervical adenocarcinoma is rare, but its prevalence has increased. To improve outcomes and ensure the suitability of recent immunotherapies, the aim of this study was to evaluate the clinicopathological impact of the tumor immune microenvironment of uterine cervical adenocarcinoma. We investigated 148 adenocarcinoma cases, including 21 cases of adenocarcinoma in situ (AIS) and 127 cases of invasive adenocarcinoma, using immunohistochemistry to detect tumor‐infiltrating immune cells and the expression of programmed cell death 1 ligand‐1 (PD‐L1) and p16 on tumor cells. We then carried out correlation and survival analyses. The density of immune cells and expression levels were compared between the tumor cell nest and stroma and between AIS and invasive adenocarcinoma using digital image analysis. A higher density of tumor‐infiltrating CD204+ M2 macrophages was significantly associated with shorter disease‐free survival, although no other tumor‐infiltrating immune cells were prognostic, including CD4+, CD8+, FOXP3+, and PD‐1+ lymphocytes and CD68+ macrophages. The density of stroma‐infiltrating lymphocytes and macrophages was significantly higher in invasive adenocarcinoma than in AIS. The density of tumor‐infiltrating lymphocytes in p16‐expressing human papillomavirus (HPV)‐positive tumors was significantly higher than that in HPV‐negative tumors. The HPV status was not associated with patient outcome. Expression of PD‐L1 on tumor cells was found only in invasive adenocarcinoma cases (17.3%). A higher density of stroma‐infiltrating lymphocytes and macrophages was found in PD‐L1‐positive tumors than in negative tumors. Patients with PD‐L1‐positive tumors tended to experience longer survival. It is suggested that tumor‐infiltrating CD204+ M2 macrophages may predict poor prognoses in patients with cervical adenocarcinoma.
Keywords: cervical adenocarcinoma, human papillomavirus infection, programmed cell death 1 ligand‐1, tumor‐associated macrophage, tumor‐infiltrating lymphocyte
1. INTRODUCTION
Uterine cervical adenocarcinoma has become an important women's health issue because of its high prevalence and poor prognosis. Adenocarcinoma accounts for approximately 10%‐25% of uterine cervical cancer cases;1, 2 the remaining cases are due to squamous cell carcinoma (SCC). During the past two decades, the incidence of cervical adenocarcinoma has more than doubled, affecting mostly younger women.1 Approximately 90% of cervical adenocarcinomas develop in relation to human papillomavirus (HPV) infection; however, some histological subtypes of cervical adenocarcinoma that are unassociated with HPV infection had worse prognosis than HPV‐positive adenocarcinomas.3 Although patients with adenocarcinoma are diagnosed at an early stage (stage I) and receive appropriate standard therapy, nearly 20% of patients die from the disease.3 Therefore, more effective and optimized treatments have been demanded to improve the prognosis of cervical adenocarcinoma.
Recently, the development of new treatment options and cancer immunotherapies that restimulate the host immune response has resulted in sustained tumor elimination. Treatment with nivolumab or pembrolizumab, antibodies against programmed cell death‐1 (PD‐1), has dramatically improved the overall survival of patients with malignant tumors with poor prognoses.4 However, immune checkpoint inhibitors are not always effective, and indeed, only patients with limited types of cancer or under limited conditions have benefitted. To identify potential predictive markers for these new immune therapies, numerous researchers have investigated the tumor microenvironment and clarified the mechanisms of immune editing and tumor immunogenicity, and the composition of the tumor infiltrating cells, such as lymphocytes and macrophages.5 For example, programmed cell death 1 ligand‐1 (PD‐L1) expression on tumor cells has been reported to be associated with shorter survival in many cancer types and is one of the predictive markers for immunotherapy.6 Furthermore, macrophage polarization has been considered a potential target of immunotherapy because it can promote tumor progression and therapeutic resistance.7 The accumulation of evidence from many studies suggests that host antitumor immune reactions and tumor immune microenvironments differ according to the tumor histological type and tissue in which the cancer develops.8, 9, 10
Regarding uterine cervical cancer, PD‐L1 expression and a high ratio of FOXP3+/CD3+ tumor‐infiltrating lymphocytes (TILs)11 were associated with poor prognosis. However, most of these cases were SCCs, and only a small number of adenocarcinomas were included in these studies. Therefore, we performed an evaluation of the tumor immune microenvironment in 148 uterine cervical adenocarcinomas and explored the association with prognosis and HPV infection status to elucidate the unique tumor microenvironment and potential immuno‐therapeutic targets of uterine cervical adenocarcinoma.
2. MATERIALS AND METHODS
2.1. Study cohort
A total of 148 patients with uterine cervical adenocarcinoma surgically resected between 2001 and 2014 at the National Cancer Center Hospital (Tokyo, Japan) were enrolled in this study. Patients who received chemotherapy or radiotherapy prior to surgery were excluded. Clinical information was obtained retrospectively from electronic medical records. The median follow‐up time of the censored cases was 5.7 years (range, 5.0‐12.9 years) from the date of surgery. Patient demographics are shown in Table S1. This study was approved by the Institutional Review Board of the National Cancer Center (Tokyo, Japan). Informed consent was obtained from all participants involved in the study, and all clinical investigations were carried out in accordance with the principles of the Declaration of Helsinki.
2.2. Pathological examination
All specimens were examined pathologically and classified according to the WHO classification of tumors2 and the UICC TNM classification12 by more than 2 pathologists. We also classified the invasion pattern of adenocarcinomas into three groups using the Silva system:13 pattern A, tumors composed of well‐demarcated glands without desmoplastic reaction and lymphovascular invasion; pattern B, tumors with early destructive stromal invasion arising from otherwise well‐demarcated glands; pattern C, tumors showing extensive destructive stromal invasion.
2.3. Immunohistochemistry and quantitative evaluation of tumor‐infiltrating immune cells, PD‐L1+ cells, and p16+ cells
Immunohistochemistry was undertaken on formalin‐fixed, paraffin‐embedded tissue sections as described previously.14 We used 4‐μm‐thick serial sections of representative blocks with antibodies (Table S2). Immunohistochemistry without the primary antibody was carried out as a negative control.
After immunohistochemistry, microscopic images were scanned and digitized using a NanoZoomer Digital Pathology system (Hamamatsu Photonics, Hamamatsu, Japan), and the density of the immune‐labeled cells was analyzed using the image analysis software Tissue Studio (Definiens, Munich, Germany) (Figure S1).14 Numbers of TILs and tumor‐associated macrophages (TAMs) were counted separately within tumor cell nests and the tumor stroma. The density was calculated as the number of stained cells divided by the interested area. The PD‐L1 expression on tumor cells was assessed manually and more than 5% of stained cells were positive.15 The HPV infection status was surrogated by expression of p16INK4a,16, 17 and evaluated based on histological subtypes. Human papillomavirus infection is evident by detection of HPV genes by PCR or in situ hybridization, although a systematic review of correlation between HPV infection and p16 overexpression indicated that p16 staining is better to predict the presence of HPV at the cut‐off value of 70%.18 Therefore, a case was considered HPV‐positive when there were ≥70% tumor cells with p16 diffuse and strongly positive staining of nuclei and cytoplasm.16, 17, 18 For survival and correlation analyses, patients were divided into two groups using the median as a cut‐off.
2.4. Statistical analysis
The non‐parametric Wilcoxon signed‐rank test was used to compare the densities of TILs and TAMs between adenocarcinoma in situ (AIS) and invasive tumors, and between HPV‐positive and ‐negative tumors. Differences between PD‐L1‐positive and ‐negative tumors were evaluated with the χ2‐test or Fisher's exact test. Among patients with invasive adenocarcinoma who were followed up for more than 5 years (n = 93), the disease‐free survival (DFS) rates were analyzed with the Kaplan–Meier method using the log–rank test. The multivariate Cox proportional hazard regression model was used with the following covariates and stratified by adjuvant radiation therapy, TNM stage, lymph node metastasis, and age. Spearman's rank correlation analysis was applied to test for associations among the different types of infiltrating immune cells. A P‐value of < .05 was considered statistically significant. Statistical calculations were undertaken using SAS version 9.3 (SAS, Cary, NC, USA).
3. RESULTS
3.1. Destructive stromal invasion in relation to the density and type of TILs
Few TILs and TAMs were observed in non‐cancerous cervical tissues without apparent inflammatory changes. In contrast, many cancer cases containing both AIS and invasive adenocarcinoma showed some host immune reaction representing TILs and TAMs (Table S3).
To compare the immune microenvironments between non‐invasive and invasive cancers appropriately, we evaluated tumor‐infiltrating immune cells both within the tumor nest and in the adjacent tumor stroma separately (Figure 1, Table S3). Significantly higher numbers of CD4+, CD8+, FOXP3+, and PD‐1+ TILs were observed in the stroma of invasive adenocarcinomas than in those of AIS (Table S3). The differences in TIL numbers within the tumor nest were not statistically significant. Similar findings were found for CD68+ and CD204+ macrophages, although few macrophages infiltrated the tumor nest. Among cases of invasive adenocarcinoma, those with cancer stroma pattern A of the Silva classification13 had significantly lower densities of CD3+ TILs and CD68+ TAMs than those with pattern C (Table S4). These results suggest that cancer cell invasion into the stroma with destruction enhances the local infiltration of immune cells.
Figure 1.
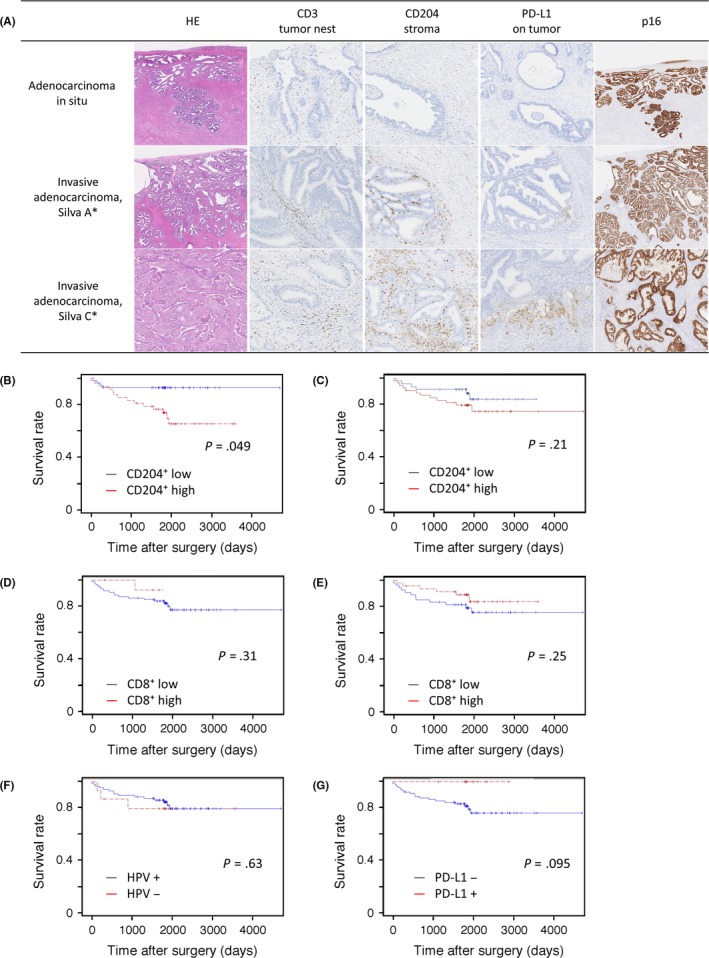
(A) Representative images of CD3+, CD204+, and programmed cell death 1 ligand‐1 (PD‐L1) expression and p16 immunohistochemistry according to tumor histology based on the Silva classification of invasion patterns. Histology (H&E staining) is categorized into adenocarcinoma in situ and Silva patterns A (well‐demarcated glands without desmoplastic reaction or lymphovascular invasion) and C (extensive destructive stromal invasion). Corresponding immunohistochemistry images are shown. (B‐G) Kaplan–Meier survival curves for 5‐year disease‐free survival in patients with cervical adenocarcinoma. Patients were dichotomized by median density of tumor‐infiltrating lymphocytes (TILs) or tumor‐associated macrophages (TAMs). Stromal infiltration of CD204+ TAMs (B) and CD8+ TILs (D), infiltration of the tumor nest by CD204+ TAMs (C) and CD8+ TILs (E), human papillomavirus (HPV) status (F), and expression of PD‐L1 on tumor cells (G). Data were statistically analyzed by log–rank test
3.2. Disease‐free survival analysis
Kaplan–Meier analysis revealed that only the density of stromal CD204+ TAMs was significantly associated with survival rates (P = .049; Figure 1). Patients with high densities of CD8+ TILs in both the tumor nest and stroma tended to have favorable outcomes (P = .25 and P = .31, respectively), although this was not statistically significant. Tumor‐infiltrating CD4+, FOXP3+, PD‐1+, and CD68+ cells, and the ratios of tumor‐infiltrating CD4+/CD8+, FOXP3+/CD8+, PD‐1+/CD8+, or CD8+/CD204+ cells, were evaluated but were not associated with patient outcome (Figure 1, Figure S2, and data not shown). According to the multivariate Cox proportional hazard model, a weak negative association between a high density of CD204+ TAMs and survival was observed (hazard ratio, 1.88; 95% confidence interval, 0.61‐5.85; data not shown).
3.3. Human papillomavirus infection status correlated positively with TIL density in cervical adenocarcinoma
Patients' HPV infection status was evaluated by p16 immunohistochemistry among those with invasive adenocarcinoma. There were no p16‐stained adenocarcinoma cells in tumors categorized as p16‐negative tumors (21/22), whereas all cases of p16‐positive tumors showed cancer cells with strong and diffuse positive staining for p16 (Figure 1). Most p16‐negative cases were mucinous adenocarcinoma (gastric type), serous carcinoma, or clear cell carcinoma, which are known to have little association with HPV infection. As p16‐negative and ‐positive tumors corresponded well with WHO histological classification, we determined that p16 status could be used as a surrogate marker of HPV infection status.
Cases of HPV‐positive tumors were found more frequently among tumors <40 mm in diameter (P = .019; Table 1). The HPV‐positive tumors had a statistically higher density of TILs, except for CD8+ and PD‐1+ TILs, in the tumor nest compared with HPV‐negative tumors. No association was observed between HPV status and the density of TAMs. Patients with HPV‐positive tumors did not have a more favorable prognosis than those with HPV‐negative tumors in our cohort (P = .63; Figure 1). Disease recurrence was observed in 3/5 (16.7%) and 12/71 (14.5%) patients with HPV‐positive and ‐negative tumors, respectively.
Table 1.
Correlation of human papillomavirus (HPV) status with clinicopathological variables in invasive adenocarcinoma cases (n = 127)
| Clinicopathological factor | HPV‐negative | HPV‐positive | P‐value |
|---|---|---|---|
| LVIa | |||
| Negative | 7 (13.5) | 45 (86.5) | .470 |
| Positive | 14 (18.9) | 60 (81.1) | |
| Nodal metastasisa | |||
| Negative | 17 (17.2) | 82 (82.8) | 1.000 |
| Positive | 4 (14.8) | 23 (85.2) | |
| Depth of invasiona | |||
| <5 mm | 8 (12.2) | 61 (87.7) | .340 |
| ≥5 mm | 16 (20.0) | 64 (80.0) | |
| Length of tumora | |||
| <40 mm | 11 (12.1) | 80 (87.9) | .019 |
| ≥40 mm | 11 (30.6) | 25 (69.4) | |
| TILsb | |||
| CD4+ T cells | |||
| Tumor nest | 5.1 (0.0‐154.7) | 19.4 (0.0‐862.1) | <.0010 |
| Stroma | 49.4 (1.0‐684.7) | 113.2 (0.0‐1052.3) | .0049 |
| CD8+ T cells | |||
| Tumor nest | 14.6 (0.0‐155.5) | 13.4 (0.0‐412.2) | .9300 |
| Stroma | 32.3 (6.6‐624.6) | 85.7 (3.8‐587.4) | <.0010 |
| FOXP3+ Tregs | |||
| Tumor nest | 2.7 (0.0‐44.5) | 6.0 (0.0‐487.7) | .0440 |
| Stroma | 13.4 (1.8‐232.5) | 23.2 (0.2‐487.7) | .0013 |
| PD‐1+ T cells | |||
| Tumor nest | 10.6 (0.0‐378.0) | 19.2 (0.0‐407.7) | .0680 |
| Stroma | 13.5 (0.0‐600.6) | 48.2 (1.1‐552.9) | <.0010 |
| TAMsb | |||
| CD68+ MΦ (Stroma) | 222.5 (0.0‐453.0) | 202.0 (0.0‐771.3) | .8700 |
| CD204+ MΦ (Stroma) | 191.1 (0.0‐421.3) | 190.6 (0.0‐684.4) | .6500 |
Data are shown as number of cases (%) or median cell number/mm2 (minimum‐maximum).
Values in bold indicate significance.
LVI, lymphovascular invasion; MΦ, macrophage; PD‐1, programmed cell death‐1; TAM, tumor‐associated macrophage; TIL, tumor‐infiltrating lymphocyte; Treg, regulatory T cell.
Comparisons were carried out using aFisher's exact test or bWilcoxon's signed‐rank test.
3.4. Programmed cell death 1 ligand‐1 expression on cancer cells found in invasive adenocarcinoma cases only
None of the AIS cases expressed PD‐L1 (0/21); however, 19/127 (15.0%) of invasive adenocarcinoma cases showed PD‐L1 expression, which was markedly observed in the invasive front of invasive adenocarcinoma (Figure 1). In terms of clinicopathological factors, tumors <40 mm in diameter tended to be more frequent among PD‐L1‐positive tumors (P = .067; Table 2). The PD‐L1‐positive tumors also had a statistically higher density of stromal CD8+, FOXP3+, and PD‐1+ TILs and CD204+ TAMs, as well as a higher ratio of CD8+/CD204+ compared to PD‐L1‐negative tumors among invasive adenocarcinoma cases. None of the patients with PD‐L1‐positive tumors (0/12) relapsed, whereas 18/89 (20.2%) cases with PD‐L1‐negative tumors relapsed (P = .12). Patients with PD‐L1‐positive tumors tended to have a favorable DFS (P = .095; Figure 1).
Table 2.
Correlation of PD‐L1 expression on tumor cells with clinicopathological variables in invasive adenocarcinoma cases (n = 127)
| Clinicopathological factors | PD‐L1 negative | PD‐L1 positive | P‐value |
|---|---|---|---|
| LVIa | |||
| Negative | 46 (85.2%) | 8 (14.8%) | .99 |
| Positive | 63 (85.1%) | 11 (14.9%) | |
| Nodal metastasisa | |||
| Negative | 87 (86.1%) | 14 (13.9%) | .55 |
| Positive | 22 (81.5%) | 5 (18.5) | |
| Depth of invasiona | |||
| <5 mm | 43 (87.8%) | 6 (12.2%) | .53 |
| ≥5 mm | 67 (83.6%) | 13 (16.3%) | |
| Length of tumora | |||
| <40.0 mm | 76 (81.2%) | 17 (18.3%) | .067 |
| ≥40.0 mm | 34 (94.4%) | 2 (5.56%) | |
| TILsb (Area) | |||
| CD4+ T cells (Stroma) | 9.9 (0.0‐68) | 13.7 (4.2‐39.0) | .13 |
| CD8+ T cells (Stroma) | 6.7 (0.6‐62) | 11.9 (5.6‐58.2) | .0034 |
| FOXP3+ Tregs (Stroma) | 2.3 (0.0‐35.6) | 6.2 (0.3‐48.8) | .0010 |
| PD‐1+ T cells (Stroma) | 4.4 (0.0‐60) | 9.5 (3.8‐55) | .004 |
| TAMsb (Area) | |||
| CD68+ MΦ (Stroma) | 21.2 (3.7‐121) | 27.3 (6.9‐61.4) | .076 |
| CD204+ MΦ (Stroma) | 19.8 (4.0‐68.4) | 26.8 (11.8‐63.0) | .0046 |
| Ratio of TILs and TAMsb (Area) | |||
| CD4+/CD8+ (Stroma) | 1.46 (0.00‐8.86) | 0.79 (0.172‐1.466) | .028 |
| FOXP3+/CD8+ (Stroma) | 0.36 (0.0025‐9.39) | 0.33 (0.11‐5.56) | .99 |
| PD‐1+/CD8+ (Stroma) | 143.9 (34.9‐453.0) | 179.2 (20.2‐3173.6) | .14 |
| CD8+/CD204+ (Stroma) | 0.33 (0.038‐4.88) | 0.58 (0.21‐2.43) | .018 |
Data are shown as number of cases (%) or median cell number/mm2 (minimum‐maximum). Values in bold font indicate significance.
LVI, lymph‐vascular invasion; TILs, tumor‐infiltrating lymphocytes; TAMs, tumor‐associated macrophages; Tregs, regulatory T cells; MΦ, macrophages.
Comparisons were conducted using aFisher's exact test and bWilcoxon signed‐rank test.
4. DISCUSSION
Uterine cervical adenocarcinoma is rare but has an increasing prevalence and a poor prognosis. To improve the outcome and ensure the suitability of recent immunotherapies, we characterized the tumor immune microenvironment of cervical adenocarcinoma using the largest cohort so far. We revealed that a higher density of CD204+ M2 TAMs was significantly associated with shorter DFS (P = .049); no other TILs or TAMs tested were significantly associated with survival. It is interesting that patients with PD‐L1‐positive tumors tended to be associated with longer DFS, as the densities of TILs and TAMs in PD‐L1‐positive tumors were higher than those in PD‐L1‐negative tumors. Furthermore, the density of TILs but not TAMs in HPV‐positive tumors was significantly higher than that in HPV‐negative tumors, and no prognostic impact of HPV status was observed.
We still have very limited information regarding the immune microenvironment of cervical adenocarcinoma.11, 19, 20, 21 No reports on tumor‐infiltrating CD8+ T cells, CD4+ T cells, or TAMs in cervical adenocarcinoma have yet been published. We showed that CD204+ TAMs were associated with poorer prognosis, as in most solid tumors.10, 22 Tumor‐associated macrophages promote tumor progression or certain forms of malignant phenotypes, such as tumoral invasion and metastasis at the invasive edge.7 However, neither tumor‐infiltrating CD8+, CD4+, FOXP3+, PD‐1+, or CD68+ cells, nor the ratios of tumor‐infiltrating CD4+/CD8+, FOXP3+/CD8+, PD‐1+/CD8+, or CD8+/CD204+ cells significantly impacted survival in our cohort. Punt et al11 examined 67 patients with cervical adenocarcinoma and showed that higher levels of both tumor‐infiltrating CD3+ and FOXP3+ cells were associated with longer survival and that lower levels of tumor‐infiltrating interleukin‐17+ cells also correlated with malignant phenotypes. These findings were not confirmed in our study. A possible reason for this is that the two studies used different methods to measure TILs. A higher ratio of tumor‐infiltrating CD4+/CD8+ T cells and a higher density of tumor‐infiltrating FOXP3+ T cells have been reported to be associated with poor prognosis in cervical SCC.11, 20, 23 It has been suggested that the tumor immune microenvironments in uterine cervical cancer differ between adenocarcinoma and SCC.
Here, we showed that patients with tumors expressing PD‐L1 tended to have favorable prognoses (Figure 1). Because of the low incidence of disease relapse, however, a statistically significant difference was not detected. Programmed cell death 1 ligand‐1 has generally been reported to be a marker of attenuated antitumor immunity and poor prognosis.6 Such paradoxical findings have been observed in other tumors, including breast cancer, lung cancer, Merkel cell carcinoma,24, 25 and cervical SCC,20 although these discordant results have not been explained clearly.
Expression of PD‐L1 was less frequently observed in adenocarcinomas than in SCC (14% vs 54%),19 and it was also observed in 95% of cervical SCCs, including non‐invasive lesions, in another published study.26 Heeren et al19 reported that there were two patterns of PD‐L1 expression on tumor cells in cervical SCC: marginal PD‐L1 tumor expression and diffusely positive PD‐L1 expression. Interestingly, patients with marginal PD‐L1‐positive SCC (7.2%) showed significantly longer DFS than both patients with PD‐L1‐negative SCC (46.4%) and patients with diffuse PD‐L1‐positive SCC (46.4%). In marginal PD‐L1 expression, in which expression on tumor cells is localized at the invasive margin, PD‐L1 expression might be induced by cytokines, including interferon‐γ, tumor necrosis factor‐α, and interleukin‐1β, produced in the local area. In such circumstances, the antitumor immune reaction responsible for secreting cytokines also provides cytotoxic T‐cell infiltration, resulting in a type 1 immune response. In contrast, the diffusely positive PD‐L1 expression pattern results from constitutive expression of PD‐L1 by intrinsic tumor mechanisms due to gene abnormalities. In our study, expression of PD‐L1 was characteristically found in the invasive front (Figure 1) and was closely correlated with higher densities of tumor‐infiltrating CD8+, FOXP3+, PD‐1+, and CD204+ cells and higher ratios of CD4+/CD8+ and CD8+/CD204+ compared to those in PD‐L1‐negative tumors (Table 2). Although the number of PD‐1+ T cells and FOXP3+ regulatory T cell infiltration increased, these findings, together with CD8+ T‐predominant TIL profiles and better trends in patient outcomes, suggest that the expression of PD‐L1 on tumor cells was induced by a relatively immune‐responsive microenvironment in our cases. These findings regarding the frequency of PD‐L1 expression on tumor cells, the pattern of PD‐L1 expression, and their impact on survival raise the possibility that PD‐1/PD‐L1 axis inhibitors may be more suitable for SCC than for adenocarcinoma in the uterine cervix.
Human papillomavirus infection was not a prognostic factor in cervical adenocarcinoma in our cohort, although the density of TILs, especially stromal TILs, was significantly higher in HPV‐positive cancers than in HPV‐negative cancers. These findings suggest that HPV‐positive tumors are more immunogenic but that lymphocytic cytotoxicity is probably severely disabled. Virus‐related tumors other than cervical cancer, such as head and neck cancer and Merkel cell carcinoma,24 often show relatively higher immunogenicities and host immune reactions, resulting in more favorable outcomes.
Does the state of TILs or TAMs change from non‐invasive cancer to invasive cancer? The infiltration of stromal CD4+, CD8+, PD‐1+, and FOXP3+ TILs and CD204+ TAMs was more severe in invasive adenocarcinoma than in AIS. In contrast to this stromal infiltration of immune cells, intraepithelial infiltration of immune cells was comparable between the two types, or higher in invasive cancer. This suggests that the densities of TILs and TAMs in the stroma are associated with tumor invasion as well as the degree of stromal destruction. However, it was difficult to conclude how the host immune reaction changed between AIS and invasive adenocarcinoma, as the infiltration of both immune‐reactive and ‐tolerant T cells increases during the progression of carcinogenesis.
There are some limitations to our study. We evaluated each subset of T cells by a single immunohistochemical staining, which could not determine detailed subsets of immune cells. Because of the low incidence of disease recurrence, we did not have enough statistical power to detect associations with prognosis using multivariate analysis, especially for the density of CD204+ macrophages, even though our study contains the largest number of cervical adenocarcinomas yet reported. Further multicenter studies are required to verify our findings.
In conclusion, tumor‐infiltrating CD204+ M2 macrophages may be a predictor of poor prognosis in patients with cervical adenocarcinoma.
CONFLICT OF INTEREST
The authors have no conflict of interest.
Supporting information
ACKNOWLEDGMENTS
This research was supported by a Grant‐in‐Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to N.H.), and the National Cancer Center Research and Development Fund (to N.H.). The authors thank Ms. Sachiko Miura, Ms. Toshiko Sakaguchi, and Ms. Chizu Kina for excellent technical support.
Kawachi A, Yoshida H, Kitano S, Ino Y, Kato T, Hiraoka N. Tumor‐associated CD204+ M2 macrophages are unfavorable prognostic indicators in uterine cervical adenocarcinoma. Cancer Sci. 2018;109:863–870. https://doi.org/10.1111/cas.13476
Funding Information
Ministry of Education, Culture, Sports, Science and Technology of Japan; National Cancer Center.
REFERENCES
- 1. Vinh‐Hung V, Bourgain C, Vlastos G, et al. Prognostic value of histopathology and trends in cervical cancer: a SEER population study. BMC Cancer. 2007;7:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wilbur DC, Colgan TJ, Ferenczy AS, et al. Glandular tumorus and precursors In: Kurman RJ, Carcangiu ML, Herrington CS, Young RH, eds. World Health Organization Classification of Tumours Pathology & Genetics Tumours of Female Reproductive Organs, 4th edn Lyon: IARCPress; 2014:183‐189. [Google Scholar]
- 3. Galic V, Herzog TJ, Lewin SN, et al. Prognostic significance of adenocarcinoma histology in women with cervical cancer. Gynecol Oncol. 2012;125:287‐291. [DOI] [PubMed] [Google Scholar]
- 4. Borghaei H, Paz‐Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non‐Small‐Cell Lung Cancer. N Engl J Med. 2015;373:1627‐1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beatty GL, Gladney WL. Immune escape mechanisms as a guide for cancer immunotherapy. Clin Cancer Res. 2015;21:687‐692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu P, Wu D, Li L, Chai Y, Huang J. PD‐L1 and Survival in Solid Tumors: a Meta‐Analysis. PLoS ONE. 2015;10:e0131403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11:750‐761. [DOI] [PubMed] [Google Scholar]
- 8. Fridman WH, Pages F, Sautes‐Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298‐306. [DOI] [PubMed] [Google Scholar]
- 9. Hiraoka N, Ino Y, Yamazaki‐Itoh R. Tertiary Lymphoid Organs in Cancer Tissues. Front Immunol. 2016;7:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ino Y, Yamazaki‐Itoh R, Shimada K, et al. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer. 2013;108:914‐923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Punt S, van Vliet ME, Spaans VM, et al. FoxP3(+) and IL‐17(+) cells are correlated with improved prognosis in cervical adenocarcinoma. Cancer Immunol Immunother. 2015;64:745‐753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours, 7th edn Hoboken, NJ: Wiley‐Blackwell; 2011. [Google Scholar]
- 13. Roma AA, Diaz De Vivar A, Park KJ, et al. Invasive endocervical adenocarcinoma: a new pattern‐based classification system with important clinical significance. Am J Surg Pathol. 2015;39:667‐672. [DOI] [PubMed] [Google Scholar]
- 14. Oguro S, Ino Y, Shimada K, et al. Clinical significance of tumor‐infiltrating immune cells focusing on BTLA and Cbl‐b in patients with gallbladder cancer. Cancer Sci. 2015;106:1750‐1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang X, Teng F, Kong L, Yu J. PD‐L1 expression in human cancers and its association with clinical outcomes. Onco Targets Ther. 2016;9:5023‐5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Houghton O, Jamison J, Wilson R, Carson J, McCluggage WG. p16 Immunoreactivity in unusual types of cervical adenocarcinoma does not reflect human papillomavirus infection. Histopathology. 2010;57:342‐350. [DOI] [PubMed] [Google Scholar]
- 17. Negri G, Egarter‐Vigl E, Kasal A, Romano F, Haitel A, Mian C. p16INK4a is a useful marker for the diagnosis of adenocarcinoma of the cervix uteri and its precursors: an immunohistochemical study with immunocytochemical correlations. Am J Surg Pathol. 2003;27:187‐193. [DOI] [PubMed] [Google Scholar]
- 18. Gronhoj Larsen C, Gyldenlove M, Jensen DH, et al. Correlation between human papillomavirus and p16 overexpression in oropharyngeal tumours: a systematic review. Br J Cancer. 2014;110:1587‐1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heeren AM, Punt S, Bleeker MC, et al. Prognostic effect of different PD‐L1 expression patterns in squamous cell carcinoma and adenocarcinoma of the cervix. Mod Pathol. 2016;29:753‐763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Karim R, Jordanova ES, Piersma SJ, et al. Tumor‐expressed B7‐H1 and B7‐DC in relation to PD‐1 + T‐cell infiltration and survival of patients with cervical carcinoma. Clin Cancer Res. 2009;15:6341‐6347. [DOI] [PubMed] [Google Scholar]
- 21. Spaans VM, Peters AA, Fleuren GJ, Jordanova ES. HLA‐E expression in cervical adenocarcinomas: association with improved long‐term survival. J Transl Med. 2012;10:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hatogai K, Kitano S, Fujii S, et al. Comprehensive immunohistochemical analysis of tumor microenvironment immune status in esophageal squamous cell carcinoma. Oncotarget. 2016;7:47252‐47264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shah W, Yan X, Jing L, Zhou Y, Chen H, Wang Y. A reversed CD4/CD8 ratio of tumor‐infiltrating lymphocytes and a high percentage of CD4(+)FOXP3(+) regulatory T cells are significantly associated with clinical outcome in squamous cell carcinoma of the cervix. Cell Mol Immunol. 2011;8:59‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lipson EJ, Vincent JG, Loyo M, et al. PD‐L1 expression in the Merkel cell carcinoma microenvironment: association with inflammation, Merkel cell polyomavirus and overall survival. Cancer Immunol Res. 2013;1:54‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schalper KA, Velcheti V, Carvajal D, et al. In situ tumor PD‐L1 mRNA expression is associated with increased TILs and better outcome in breast carcinomas. Clin Cancer Res. 2014;20:2773‐2782. [DOI] [PubMed] [Google Scholar]
- 26. Mezache L, Paniccia B, Nyinawabera A, Nuovo GJ. Enhanced expression of PD L1 in cervical intraepithelial neoplasia and cervical cancers. Mod Pathol. 2015;28:1594‐1602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


