Abstract
Emergency (stress) granulopoiesis is an episodic process for the production of granulocytes in response to infectious challenge. We previously determined that Fanconi C, a component of the Fanconi DNA-repair pathway, is necessary for successful emergency granulopoiesis. Fanconi anemia results from mutation of any gene in this pathway and is characterized by bone marrow failure (BMF) in childhood and clonal progression in adolescence. Although murine Fanconi anemia models exhibit relatively normal steady-state hematopoiesis, FANCC−/− mice are unable to mount an emergency granulopoiesis response. Instead, these mice develop BMF and die during repeated unsuccessful emergency granulopoiesis attempts. In FANCC−/− mice, BMF is associated with extensive apoptosis of hematopoietic stem and progenitor cells through an undefined mechanism. In this study, we find that TP53 haploinsufficiency completely rescues emergency granulopoiesis in FANCC−/− mice and protects them from BMF during repeated emergency granulopoiesis episodes. Instead, such recurrent challenges accelerated clonal progression in FANCC−/−TP53+/− mice. In FANCC−/− mice, BMF during multiple emergency granulopoiesis attempts was associated with increased ataxia telangiectasia and Rad3-related protein (Atr) and p53 activation with each attempt. In contrast, we found progressive attenuation of expression and activity of Atr, and consequent p53 activation and apoptosis, in the bone marrow of FANCC−/−TP53+/− mice during this process. Therefore, activation of Atr—with consequent Fanconi-mediated DNA repair or p53-dependent apoptosis—is an essential component of emergency granulopoiesis and it protects the bone marrow from genotoxic stress during this process.
Introduction
Emergency granulopoiesis is an episodic process for the production of granulocytes in response to infectious challenge, a key component of the innate immune response (1–3). In contrast, steady-state granulopoiesis is an ongoing process to replace granulocytes lost to normal programmed cell death. Emergency granulopoiesis involves cell cycle shortening in hematopoietic stem cells (HSC) and progenitor cells, commitment of HSCs to myelopoiesis, and accelerated differentiation from myeloid progenitors to phagocytes compared with the steady-state (4). S phase shortening facilitates myeloid expansion but provides less time for replication fork rescue, increasing the risk for adverse events due to unrepaired collapsed or stalled replication forks.
Studies in murine models demonstrated that emergency granulopoiesis requires IL-1β and is enhanced by an IL-1β–induced increase in G-CSF (2, 3). Although aspects of the innate immune response are enhanced by TNF-α or IFN-γ, these inflammatory mediators are not essential for emergency granulopoiesis (5). Studies in murine models also determined that Stat3 and C/ebpβ are required to initiate and maintain emergency granulopoiesis (6, 7). During this process, IFN consensus sequence binding protein (Icsbp)/IFN regulatory factor 8 increase the expression of Fanconi DNA-repair proteins and phagocyte effectors (8–11). Icsbp also is required to terminate emergency granulopoiesis by increasing Fas sensitivity and decreasing β-catenin activity in myeloid progenitors (12). In contrast, steady-state granulopoiesis is enhanced by GM-CSF or G-CSF (but not IL-1β) and requires the transcription factors Pu.1, C/ebpα, and Stat5 (1, 3, 13–16).
Emergency granulopoiesis is studied in mice by i.p. injection of an OVA/alum mixture (referred to as Alum), Listeria monocytogenes, or Candida albicans. These approaches induce the same cytokine profile and bone marrow response, but Alum does not cause death or chronic infection, permitting study of multiple emergency granulopoiesis episodes (2, 8, 12, 17). In wild-type (WT) mice, stimulation of emergency granulopoiesis induces maximal peripheral granulocytosis and myeloid progenitor expansion at 2 wk, with a return to the steady-state by 4 wk (2, 8, 12, 17). Alum injection every 4 wk induces repeated emergency granulopoiesis episodes without morbidity or mortality in WT mice (8).
We previously used the Alum injection model to study the Fanconi DNA-repair pathway during emergency granulopoiesis. In addition to their classical role in DNA cross-link repair, Fanconi proteins rescue collapsed or stalled replication forks (18, 19). In response to DNA damage, Fanconi D2 and I are ubiquitinated by the activation complex (Fanconi A, B, C, E, F, G, L, and M), resulting in recruitment of an effector complex (Fanconi D1, J, O, and P and Rad51) to sites of DNA damage (20–23). Fanconi D2 is recruited to sites of replication during the S phase, even without DNA damage, further supporting a role in cell cycle–related repair. Stimulation of myeloid progenitor cells with G-CSF or IL-1β increased Fanconi C and J and Rad51 in chromatin-enriched fractions, indicating Fanconi anemia (FA) pathway activation by these cytokines (8).
Inherited mutation of any Fanconi DNA-repair gene results in FA (20–23). FA has a spectrum of clinical manifestations, including skeletal abnormalities, progressive cytopenias, and development of hematologic or epithelial malignancies (21). Anemia is the first cytopenia to develop in FA, perhaps because steady-state turnover is greatest in the erythroid lineage. Granulocytopenia often develops during childhood, and many FA patients develop bone marrow failure (BMF). Clonal progression to acute myeloid leukemia (AML) occurs in some FA patients surviving BMF in childhood and is heralded by myeloid-lineage dysplasia (24, 25).
Steady-state hematopoiesis is unremarkable in mice with engineered Fanconi gene disruption, but we found that repeated episodes of emergency granulopoiesis induced BMF in Fanconi C–knockout (FANCC−/−) mice (8). These mice failed to develop granulocytosis upon Alum injection, and most expired with profound cytopenias by the third emergency granulopoiesis attempt (8). BMF in these mice was associated with apoptosis of HSCs, myeloid progenitors, and differentiating granulocytes (8). FANCC−/− mice surviving three or more emergency granulopoiesis attempts developed AML, similar to the course of human FA (8). Treatment with an IL-1R antagonist blocked emergency granulopoiesis in WT mice and prevented BMF in FANCC−/− mice (8). However, the mechanisms for apoptosis during emergency granulopoiesis in FANCC−/− mice were undefined.
During the S phase of the cell cycle, ataxia telangiectasia and Rad3-related protein (Atr) is phosphorylated/activated by ssDNA/dsDNA hybrids, as occur with stalled or collapsed replication forks. Atr activation results in an S phase pause for DNA repair and facilitates Fanconi pathway activation (26–28). If repair is unsuccessful, Atr activates Chk1 and p53 to induce apoptosis of cells with DNA damage (29, 30). We hypothesized that accumulation of unrepaired DNA in FA patients during episodes of emergency granulopoiesis results in Atr activation and consequent p53-dependent apoptosis of bone marrow stem and progenitor cells, leading to BMF. If this is correct, blocking p53 would rescue emergency granulopoiesis in FA. This might improve morbidity in FA by increasing the competence of the innate immune response and preventing BMF.
Materials and Methods
Murine studies
Animal studies were performed according to a protocol approved by the Animal Care and Use Committees of Northwestern University and Jesse Brown VA Medical Center.
Mice.
FANCC+/− mice were a gift from Dr. D.W. Clapp (Indiana University, Indianapolis, IN) (31). TP53+/− mice were a gift from Dr. A. Minella (Blood Research Institute of Wisconsin, Milwaukee, WI) (32).
Emergency granulopoiesis.
Mice were injected i.p. with Alum (0.5 ml) or saline control every 4 wk (15 mice per group). Alum was prepared as described (2, 8).
Analysis of peripheral blood.
Blood was obtained from the tail vein of each mouse every 2 wk, and complete blood counts with leukocyte differential were determined using an automated cell counter. Myeloid blast counts were verified by hand-counting of May-Grünwald-Giemsa–stained peripheral blood smears (blinded for automated differential results; 300 cells per slide). Mice with leukocytosis (>100,000 cells per milliliter) or a hemoglobin (Hgb) concentration < 6.0 were sacrificed. Images of peripheral blood were captured by light microscopy (100× magnification).
Murine bone marrow analysis
Analysis of bone marrow histology.
Sternal bone marrow was decalcified and stained using H&E, according to standard techniques, by the Pathology Core of the Robert H. Lurie Cancer Center. Light microscopy was performed, and digital images were captured (40× magnification).
Bone marrow immunohistochemistry.
Sternal sections were analyzed by immunohistochemistry for phospho-p53, total p53, phospho-Atr, or total Atr. Following deparaffinization and rehydration, Ag retrieval was performed by boiling in sodium citrate buffer (pH 6) for 12 min in a pressure cooker. Endogenous peroxidase activity was blocked by 3% H2O2, and slides were washed in PBS with 0.1% Tween. Slides were blocked with 5% goat serum in PBS. Total or phospho-Ser6 p53 Ab (1:50 dilution; LSBio) or total or phospho-Thr1989 Atr Ab (1:100 dilution; GeneTex) was applied (4°C overnight). Washed slides were incubated for 60 min with biotin-conjugated secondary Ab, washed again, and incubated at 25°C with streptavidin HRP. Ab was visualized following incubation with 3,3′-diaminobenzidine (DAB) and hematoxylin counterstain. Sections were visualized with a Zeiss Axioskop microscope/Nuance Camera in the Northwestern University imaging center.
Three mice in each group were sampled, and 10 high power fields were at 40× magnification counted for each sample. Results are presented as an average of the 10 fields for each of the three individual mice (normalized to 1.0 for saline-injected WT mice).
Apoptosis assays and flow cytometry
TUNEL assay.
To detect apoptosis in situ, assays were performed using a TACS 2 TdT DAB (diaminobenzidine) Kit (Trevigen, Gaithersburg, MD), according to the manufacturer’s instructions. Sternal bone marrow was fixed in formalin for 24 h and decalcified, and 4-μm sections were cut. Paraffin was removed, and DAB-stained slides were counterstained with eosin. Three mice in each group were sampled, and 10 40× fields were counted for each sample. Results are presented as an average of the 10 fields for each of three individual mice in each group (normalized to 100 for saline-injected WT mice).
Flow cytometry.
Bone marrow was obtained from mice 2 wk after injection with Alum or saline. Cells were washed with PBS, counted, and labeled with anti-mouse FITC-conjugated Abs to Sca1 (Ly-6A/E), CD34, or Gr1 (Ly-6G) (eBioscience, San Diego, CA). Bone marrow cells were depleted of CD34 prior to Gr1 assessment and of lineage prior to Sca1 or CD34 assessment using MACS separation kits (Miltenyi Biotec, Auburn, CA). In control experiments, we noted >85% depletion of the target populations using this technique.
Apoptosis was assessed by flow cytometry using an Annexin V: PE Apoptosis Detection Kit I (BD Pharmingen, San Diego, CA), according to the manufacturer’s instructions. For assessment of Gr1+ cells, bone marrow was depleted of CD34; for CD34+ cells, bone marrow was depleted of lineage and Sca1; and for Sca1+ cells, bone marrow was depleted of lineage and CD34. In preliminary studies, we found that ∼50% of Lin−CD34+ cells were ckit+ but <10% were Sca1+ in WT or FANCC−/− mice. Similarly, ∼20% of Lin−Sca1+ cells were ckit+, but very few were CD34+.
Chromosomal breaks and radials
Murine bone marrow was harvested, and RBCs were lysed in ACK Lysing Buffer. Cells were cultured in DMEM supplemented with 10% FBS, 1% penicillin/streptomycin, GM-CSF (10 ng/ml), IL-3 (10 ng/ml), and GM-CSF (20 ng/ml) for 24 h. Mitomycin C (40 ng/ml) was added for 48 h. Chromosome spreads were made by incubating bone marrow cells with 50 ng/ml Colcemid (25 min at 37°C), followed by 0.075 M KCl for 8 min. Cells were transferred to freshly prepared methanol/acetic acid (3:1) fixative, followed by air drying. Slides were aged for 12 h at 60°C, followed by Giemsa staining. Chromosome number and structure were examined and recorded with a Zeiss Axioskop microscope/Nuance Camera (100×) in the Northwestern University imaging center. A total of 25 metaphases (1000 chromosomes) was scored for breaks and radials in three samples for each condition.
Statistical analysis
Statistical significance was determined by an unpaired two-tailed Student t test, ANOVA (for more than two samples), or log-rank analysis (survival curves) using SigmaPlot and SigmaStat software. The p values < 0.04 were considered statistically significant. Error bars represent ± SE.
Results
TP53 haploinsufficiency rescues emergency granulopoiesis in FANCC−/− mice
FANCC−/− mice are unable to mount an emergency granulopoiesis response, and most of these animals exhibit profound pancytopenia and die after a third attempt (8). In this study, we investigated the role of p53 in the apoptosis of HSCs and progenitor cells in FANCC−/− mice during this process. This was based on the hypothesis that unrepaired replication forks in FA during emergency granulopoiesis result in activation of Atr and, consequently, p53, leading to apoptosis and BMF. For these studies, we compared FANCC−/−, FANCC−/−TP53+/−, TP53+/−, and WT mice (33, 34). TP53+/− mice develop lymphoma with aging, but abnormalities in the innate immune response are not described (32). Mice were injected with Alum every 4 wk to induce emergency granulopoiesis or with saline as a control for steady-state granulopoiesis, and peripheral blood counts were analyzed every 2 wk (8, 35). Some mice in each cohort were sacrificed 2 wk after the first, second, or third injection for additional studies.
In WT or TP53+/− mice, we found a significant increase in circulating granulocytes 2 wk after Alum injection (p < 0.0001, n = 10) (Fig. 1A). Granulocytosis was not significantly different between the two groups (p > 0.2, n = 10) or with subsequent cycles within each group (p > 0.3, n = 10). The number of circulating granulocytes returned to baseline levels by 4 wk after Alum injection in both groups. FANCC−/− mice did not exhibit granulocytosis in response to Alum, but they did develop granulocytopenia with repeated injections (p = 0.01, n = 10 for baseline granulocytes versus cycle three) (Fig. 1A) (8).
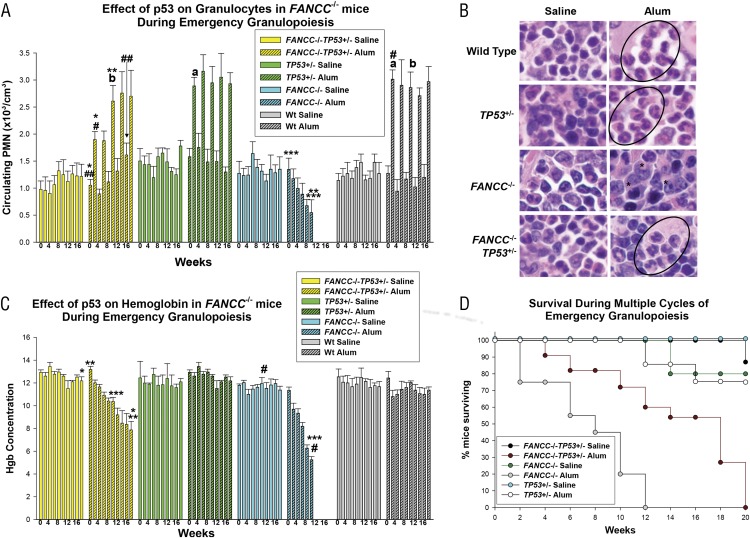
FIGURE 1.
TP53 haploinsufficiency rescued emergency granulopoiesis in FANCC−/− mice. FANCC−/−, FANCC−/−TP53+/−, TP53+/−, and WT mice were injected on day 0, and every 4 wk thereafter, with Alum (to induce emergency granulopoiesis) or saline (as a steady-state control). Peripheral blood counts were performed every 2 wk. (A) Granulocytosis during emergency granulopoiesis in FANCC−/−TP53+/− mice was equivalent to WT mice by the third Alum injection. Statistically significant differences in peripheral blood granulocytes are indicated by *, **, ***, #, and ## (p < 0.001 for comparisons between granulocyte counts under various conditions) (n = 10). a,bNonstatistically significant differences. (B) Granulocytes increased in FANCC−/−TP53+/− bone marrow, but not in FANCC−/− bone marrow, 2 wk after Alum injection. Sternal bone marrow was stained with Wright–Giemsa (original magnification ×40). Granulocytes are circled, and abnormal myeloid cells are indicated by an asterisk (*). (C) FANCC−/−TP53+/− mice developed anemia more slowly than did FANCC−/− mice during multiple episodes of emergency granulopoiesis. Statistically significant differences in Hgb concentration are indicated by *, **, ***, and # (p < 0.001 for comparison between hemoglobin values under various conditions) (n = 10). (D) FANCC−/−TP53+/− mice survived significantly more episodes of emergency granulopoiesis than did FANCC−/− mice. Kaplan–Meyer survival plots reveal a significant difference between Alum-injected FANCC−/−TP53+/− mice and FANCC−/− mice (p < 0.01 for comparison in survival in different genotypes under various conditions, n = 10). Error bars represent the SEM in all graphs.
In contrast, in FANCC−/−TP53+/− mice, circulating granulocytes increased significantly 2 wk after each Alum injection (p < 0.001, n = 10) (Fig. 1A). Alum-induced granulocytosis in FANCC−/−TP53+/− mice was significantly less than in WT mice for the first two episodes (p < 0.001, n = 10), but it was comparable in FANCC−/−TP53+/−, TP53+/−, and WT mice after the third Alum injection (p > 0.4, n = 10). Although circulating granulocytes returned to baseline by 4 wk after the first Alum injection in FANCC−/−TP53+/− mice, baseline circulating granulocytes increased significantly between the subsequent injections (p < 0.001, n = 10 comparing baseline before the first and fifth Alum injections). Saline injections did not significantly alter granulocyte counts in any group.
Sternal bone marrow was examined 2 wk after the first injection of Alum or saline. We identified a relative increase in mature-appearing granulocytes in WT, TP53+/−, and FANCC−/−TP53+/− murine bone marrow post-Alum injection compared with saline control (Fig. 1B). In contrast, bone marrow from FANCC−/− mice had many irregular apoptotic-appearing cells 2 wk after Alum injection but no increase in differentiating granulocytes (Fig. 1B) (8).
FANCC−/− mice developed progressive anemia with multiple episodes of Alum injection, consistent with our prior findings (p < 0.01, n = 10 for Hgb concentration in the first versus third cycle) (Fig. 1C) (8). Hgb concentration also decreased over five cycles of Alum injection in FANCC−/−TP53+/− mice (p < 0.001, n = 10), but the decrease was significantly less than in FANCC−/− mice (p < 0.01, n = 10, comparison at the third cycle) (Fig. 1C). Hgb levels were stable during multiple episodes of emergency granulopoiesis in WT and TP53+/− mice and were not significantly different between the two groups (Fig. 1C). Saline injection did not alter Hgb concentration in any genotype.
Different responses to emergency granulopoiesis stimuli in FANCC−/− and FANCC−/−TP53+/− mice were also reflected in survival rates. Fifty percent of Alum-injected FANCC−/−TP53+/− mice survived to 18 wk (p = 0.01, n = 10), and some survived for up to five episodes (Fig. 1D). In contrast, 50% of FANCC−/− mice expired within a week of the second Alum injection, and none of these animals survived a third emergency granulopoiesis attempt (Fig. 1D). All Alum-injected WT mice survived the duration of the experiment as did 80% of Alum-injected TP53+/− mice (Fig. 1D). All saline-injected mice survived the experiment.
These results indicated that p53 haploinsufficiency rescued the emergency granulopoiesis response in FANCC−/− mice and delayed or prevented BMF. This suggested the possibility that p53 mediates the abnormal innate immune response in FA and contributes to BMF. We designed experiments to specifically investigate this hypothesis.
TP53 haploinsufficiency in FANCC−/− mice prevents apoptosis of bone marrow progenitors during emergency granulopoiesis
We first assessed the effect of p53 by examining bone marrow apoptosis during emergency granulopoiesis. For these experiments, we performed TUNEL assays on sternal bone marrow from FANCC−/−, FANCC−/−TP53+/−, TP53+/−, and WT mice 2 wk after injection with Alum or saline control over several treatment cycles (Fig. 2A) (8). Apoptotic cells were quantified for presentation in graphic format (Fig. 2B). We found that TUNEL+ cells increased significantly in FANCC−/− bone marrow 2 wk after the first Alum injection (p < 0.0001, with versus without Alum, n = 3) and increased further with each subsequent episode (p < 0.01 comparing the first and third injections, n = 3) (Fig. 2B).
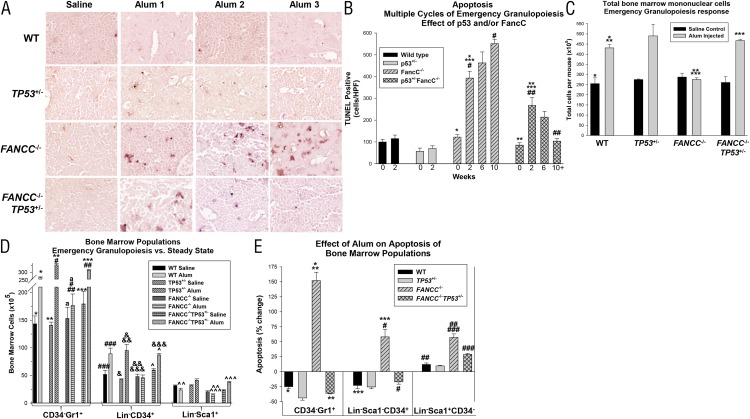
FIGURE 2.
TP53 haploinsufficiency inhibited apoptosis in the bone marrow of FANCC−/− mice exposed to multiple episodes of emergency granulopoiesis. FANCC−/−, FANCC−/−TP53+/−, TP53+/−, and WT mice were injected on day 0 and every 4 wk thereafter with Alum or saline control. (A) Apoptosis progressively increased in FANCC−/− bone marrow during multiple episodes of emergency granulopoiesis but decreased in FANCC−/−TP53+/− mice. TUNEL assays were performed 2 wk post-Alum injection. TUNEL staining as described in Materials and Methods. Original magnification ×40. (B) Quantification of TUNEL assays revealed a significant decrease in total apoptotic cells during multiple episodes of emergency granulopoiesis in FANCC−/−TP53+/− mice compared with FANCC−/− mice. Apoptosis was not significantly altered by Alum injection in WT or TP53+/− mice (p > 0.1, n = 3 for comparison in apoptotic cells under various conditions for each cycle; only the first cycle is shown). Statistically significant differences are indicated by *, **, ***, #, and ## (p < 0.01, n = 3). (C) Total bone marrow mononuclear cells increased 2 wk after Alum injection in WT, TP53+/−, and FANCC−/−TP53+/− mice but not in FANCC−/− mice. Statistically significant differences are indicated by *, **, and *** (p < 0.01 for comparison between cell numbers under various conditions) (n = 3). (D) Expansion of CD34−Gr1+ and Lin−CD34+ cells in FANCC−/−TP53+/− bone marrow was greater than in FANCC−/− bone marrow 2 wk after Alum injection. Statistically significant differences are indicated by *, **, ***, #, ##, ###, &, &&, &&&, ^, ^^, and ^^^ (p < 0.001 for comparison between cell populations under various conditions, n = 3). aDifference in cell populations that is not statistically significant (p > 0.05). (E) The increase in apoptotic CD34−Gr1+ and Lin−CD34+ cells in FANCC−/− bone marrow 2 wk after Alum injection was reversed by TP53 haploinsufficiency. Statistically significant differences are indicated by *, **,***, #, ##, and ### (p < 0.001 for comparison in change in apoptosis under various conditions, n = 3). Error bars represent the SEM.
The number of TUNEL+ cells also increased significantly in FANCC−/−TP53+/− mice 2 wk after the first Alum injection (p < 0.001, n = 3), but it was less than in FANCC−/− bone marrow (p < 0.001, n = 3) (Fig. 2B). In contrast to FANCC−/− mice, there were decreasing numbers of apoptotic cells in FANCC−/−TP53+/− bone marrow with successive episodes of Alum injection (Fig. 2A). This difference was statistically significant (p < 0.0001, comparing the first and third injections, n = 3) (Fig. 2B). Apoptosis in the bone marrow of WT mice was not significantly altered by the first Alum injection (Fig. 2B) or by subsequent injections (data not shown). There were slightly fewer TUNEL+ cells in the bone marrow of TP53+/− mice in comparison with WT mice, and this was not altered by multiple cycles of Alum injection (Fig. 2B).
We also investigated the impact of p53 haploinsufficiency on the apoptosis of specific cell populations in FANCC−/− murine bone marrow during Alum-induced emergency granulopoiesis. In initial studies, we found a significant (p < 0.001, n = 3) and equivalent (p = 0.4, n = 3) increase in total bone marrow mononuclear cells 2 wk after Alum injection in WT, TP53+/−, and FANCC−/−TP53+/− mice (Fig. 2C). In contrast, Alum injection did not alter the number of total bone marrow mononuclear cells in FANCC−/− mice (p = 0.1, n = 3) (Fig. 2C).
We next investigated the abundance of various bone marrow populations in these mice 2 wk after injection of Alum or saline control. To determine the effect of Alum on granulocytes, we analyzed CD34-depleted bone marrow cells for the abundance of the Gr1+ population by flow cytometry. After Alum injection, we found that CD34−Gr1+ cells increased significantly in bone marrow from WT, TP53+/−, and FANCC−/−TP53+/− mice (p < 0.001, n = 3) but not FANCC−/− mice (p = 0.5, n = 3) (Fig. 2D, Supplemental Fig. 1A).
We found that Alum injection resulted in significant (p < 0.01, n = 3) and equivalent (p = 0.8, n = 3) expansion of the Lin−CD34+ population in WT, TP53+/−, and FANCC−/−TP53+/− lineage-depleted murine bone marrow using flow cytometry (Fig. 2D, Supplemental Fig. 1B). However, this population was unchanged in FANCC−/− mice (p = 0.7, n = 3) (Fig. 2D). Alum injection did not significantly alter the number of Lin−Sca1+ cells in WT or FANCC−/− murine bone marrow, although there was a trend toward a decrease. However, Lin−Sca1+ cells were significantly increased in the bone marrow of FANCC−/−TP53+/− and TP53+/− mice 2 wk after Alum injection (p = 0.01, n = 3) (Fig. 2D, Supplemental Fig. 1C).
To investigate the contribution of apoptosis to these observations, we used flow cytometry to analyze bone marrow populations from FANCC−/−, TP53+/−FANCC−/−, TP53+/−, and WT mice for annexin V staining 2 wk after injection with Alum or saline. We found that the number of apoptotic Gr1+ cells in the CD34-depleted bone marrow population from WT, TP53+/−, and FANCC−/−TP53+/− mice decreased significantly post-Alum injection (p < 0.001, n = 3) (Fig. 2E, Supplemental Fig. 2A). In contrast, there was a significant increase in apoptotic CD34−Gr1+ cells post-Alum injection in FANCC−/− mice compared with WT, TP53+/−, and FANCC−/−TP53+/− mice (p < 0.001, n = 3).
We next investigated the effect of Alum on apoptosis in the CD34+ bone marrow population. We found that the number of apoptotic CD34+ cells in the lineage and Sca1–depleted population of bone marrow from WT, TP53+/−, and FANCC−/−TP53+/− mice decreased after Alum injection but increased in FANCC−/− mice (Fig. 2E, Supplemental Fig. 2B). We also tested the effect of Alum on apoptosis in the Sca1+ population. Alum injection increased apoptosis in Sca1+ cells in the lineage and CD34–depleted bone marrow population from each of the genotypes, but this was significantly greater in FANCC−/− and FANCC−/−TP53+/− mice compared with WT and TP53+/− mice (p < 0.001, n = 3) (Fig. 2E, Supplemental Fig. 2C). Data in Fig. 2E are presented as the percentage change in apoptosis with versus without Alum injection to capture population dynamics.
Cell cycle checkpoint activation is progressively impaired during multiple episodes of emergency granulopoiesis in FANCC−/−TP53+/− mice
The above studies implicated p53 in excess apoptosis of myeloid progenitors and developing granulocytes in FANCC−/− murine bone marrow during emergency granulopoiesis. We were interested in determining whether protection from apoptosis during multiple episodes of emergency granulopoiesis in FANCC−/−TP53+/− mice was associated with decreased p53 activation or whether another mechanism was responsible. We investigated this by analyzing bone marrow from WT, TP53+/−, FANCC−/−, and FANCC−/−TP53+/− mice for phospho-p53 (activated) and total p53 protein. First, sternal bone marrow was analyzed by immunohistochemistry for p53 phosphorylation 2 wk after the injection of Alum or saline control. Ab specific to a p53 serine residue that is phosphorylated in response to DNA damage was used in these studies (36, 37). Results were compared over several episodes, performed every 4 wk.
We found increased phospho-p53 in FANCC−/− murine bone marrow 2 wk after Alum injection compared with saline control (Fig. 3A). Results of immunohistochemistry were quantified and are presented graphically (Fig. 3B). p53 activation increased with each subsequent Alum injection cycle in FANCC−/− mice (p < 0.01, n = 3), correlating with increasing apoptosis and development of anemia and neutropenia. We found similar phospho-p53 levels 2 wk after the first Alum injection in FANCC−/−TP53+/− mice (p = 0.8 versus FANCC−/− mice, n = 3), despite the loss of a TP53 allele (Fig. 3B). In contrast to FANCC−/− mice, p53 activation decreased significantly in FANCC−/−TP53+/− mice during subsequent Alum injections (p < 0.01, n = 3). Therefore, activation of p53 was increasingly inefficient with repeated emergency granulopoiesis episodes in FANCC−/−TP53+/− mice, but it was increasingly efficient in FANCC−/− mice.
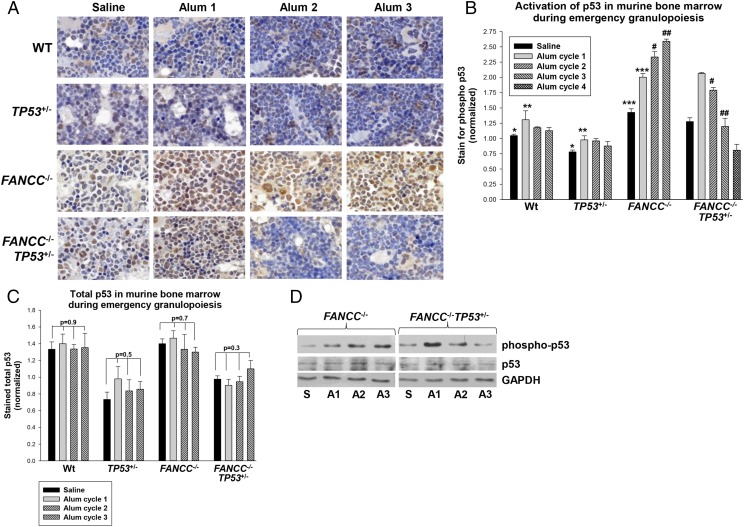
FIGURE 3.
Activation of p53 increased with repeated episodes of emergency granulopoiesis in FANCC−/− mice but decreased in FANCC−/−TP53+/− mice. FANCC−/−, FANCC−/−TP53+/−, TP53+/−, and WT mice were injected on day 0 and every 4 wk thereafter with Alum or saline control. Studies were performed 2 wk after injection during several episodes. (A) Phospho-p53 increased in the bone marrow of FANCC−/− mice with each episode of emergency granulopoiesis but decreased subsequent to the first episode in FANCC−/−TP53+/− mice. Sternal bone marrow was analyzed by immunohistochemistry for phospho-p53 (yellow; original magnification ×40). (B) Quantification of these results revealed significant differences in p53 phosphorylation after the second episode of emergency granulopoiesis in FANCC−/− versus FANCC−/−TP53+/− murine bone marrow. Activation of p53 was not significantly altered by Alum injection in WT or TP53+/− mice (p > 0.05 for comparison in phospho-p53 under various conditions, n = 3). Statistically significant differences are indicated by *, **, ***, #, and ## (p < 0.001 for comparison in phospho-p53, n = 3). (C) Total p53 protein was not altered during multiple episodes of emergency granulopoiesis in any of the genotypes. Immunohistochemistry for total p53 was performed on these samples. Nonstatistically significant p values are shown for comparison of total p53 under various conditions (n = 3). (D) Phospho-p53 progressively increased in bone marrow cell lysates from FANCC−/− mice during multiple episodes of emergency granulopoiesis but decreased after the first episode in FANCC−/−TP53+/− mice. Western blots of bone marrow lysate proteins from these mice were serially probed with Abs to p53, phospho-p53, and Gapdh (loading control). Error bars represent the SEM for all bar graphs. A1, first Alum injection; A2, second Alum injection; A3, third Alum injection; S, saline injection.
We initially hypothesized that this might be due to loss of the other TP53 allele in FANCC−/−TP53+/− mice during the genotoxic stress of multiple emergency granulopoiesis episodes. We concluded that this was likely, because TP53 loss of heterozygosity was advantageous for cell survival in FANCC−/− murine bone marrow during this process. To analyze this, we performed immunohistochemistry on murine sternal bone marrow for total p53 abundance. We found significantly less total p53 protein in TP53+/− and FANCC−/−TP53+/− murine bone marrow compared with WT bone marrow in the steady-state (p = 0.004, n = 3) (Fig. 3C). However, total p53 was not significantly altered during multiple episodes of emergency granulopoiesis in any of the four genotypes (p > 0.3, n = 3) (Fig. 3C). Immunohistochemistry results were verified by Western blots of bone marrow cell lysates for total or phospho-p53 (Fig. 3D). Samples for these studies were obtained 2 wk after the first, second, or third injection of Alum, as above. We found that phospho-p53, but not total p53, increased with repeated episodes of Alum injection in FANCC−/− mice but decreased with repeated Alum injections in FANCC−/−TP53+/− mice.
We performed additional studies to investigate the mechanisms increasing p53 activation during multiple episodes of emergency granulopoiesis in FANCC−/− mice but not in FANCC−/−TP53+/− mice. The presence of unrepaired DNA during the S phase of the cell cycle activates (phosphorylates) Atr. Atr activation by DNA damage induces phosphorylation of p53 at multiple residues, directly or indirectly (26–30).
We first investigated activation of Atr during multiple episodes of emergency granulopoiesis in FANCC−/− and FANCC−/−TP53+/− mice. Comparisons were made to WT and TP53+/− mice. Mice were injected with Alum or saline control, and sternal bone marrow was analyzed for phospho-Atr 2 wk later by immunohistochemistry (Fig. 4A). The immunohistochemistry results were quantified and are presented graphically (Fig. 4B). This procedure was repeated during several episodes of Alum injection, 4 wk apart.
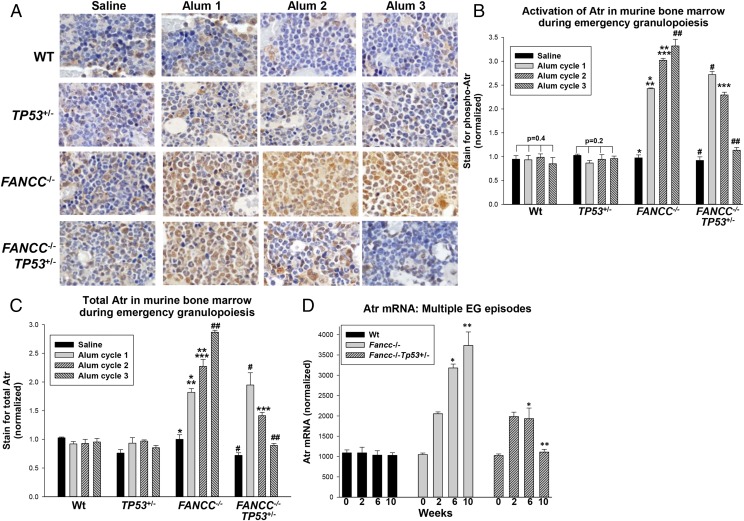
FIGURE 4.
Atr activation and expression increased with repeated episodes of emergency granulopoiesis in FANCC−/− mice but decreased in FANCC−/−TP53+/− mice. FANCC−/−, FANCC−/−TP53+/−, TP53+/−, and WT mice were injected on day 0 and every 4 wk thereafter with Alum or saline control. Studies were performed 2 wk after injection during several episodes. (A) Phospho-Atr increased in the bone marrow of FANCC−/− mice with each episode of emergency granulopoiesis but decreased subsequent to the first in FANCC−/−TP53+/− mice. Sternal bone marrow was analyzed by immunohistochemistry for phospho-Atr (yellow, original magnification ×40). (B) Quantification of these results revealed significant differences in Atr phosphorylation after the second episode of emergency granulopoiesis in FANCC−/− versus FANCC−/−TP53+/− murine bone marrow. Activation of Atr was not significantly different with Alum injection in WT or TP53+/− bone marrow (p > 0.1 for comparison of phospho-Atr under various conditions, n = 3). Statistically significant differences are indicated by *, **, ***, #, and ## (p < 0.001, n = 3). (C) Total Atr protein increased in the bone marrow of FANCC−/− mice during multiple episodes of emergency granulopoiesis but decreased during this process in FANCC−/−TP53+/− mice. Total Atr protein did not increase in response to Alum injection in WT or TP53+/− murine bone marrow (p > 0.2, n = 3). Statistically significant differences are indicated by *, **, ***, #, and ## (p < 0.01 for quantification of total Atr under various conditions, n = 3). (D) Atr mRNA increased in the bone marrow of FANCC−/− mice during multiple episodes of emergency granulopoiesis but decreased after the first episode in FANCC−/−TP53+/− mice. Statistically significant differences are indicated by * and ** (p < 0.001 for comparison of Atr mRNA under various conditions, n = 3). Error bars represent the SEM for all bar graphs.
Similar to phospho-p53, we found a statistically significant and equivalent increase in phospho-Atr in FANCC−/− and TP53+/−FANCC−/− mice 2 wk after the first Alum injection (p < 0.001, n = 3) (Fig. 4B). In FANCC−/− mice, phospho-Atr also increased significantly with each subsequent Alum injection (p < 0.01, n = 3), consistent with Atr-induced activation of p53 and apoptosis in the bone marrow of these mice (Fig. 4B). However, in FANCC−/−TP53+/− mice, phospho-Atr decreased significantly with subsequent Alum injections (p < 0.001, n = 3) (Fig. 4B), suggesting that impaired cell cycle checkpoint control (i.e., decreased Atr activation) was a mechanism for decreased apoptosis during repeated episodes of emergency granulopoiesis. There was no significant change in phospho-Atr 2 wk after Alum in WT or TP53+/− mice during multiple Alum injections (p > 0.5, n = 3) (Fig. 4B).
To investigate the mechanism of impaired Atr activation in TP53+/−FANCC−/− mice during multiple episodes of emergency granulopoiesis, we quantified total Atr protein. Because transcription of the ATR gene increases during the S phase (28), we were interested in determining whether expression of total Atr was progressively impaired in TP53+/−FANCC−/− mice. For these studies, immunohistochemistry was performed on bone marrow from WT, TP53+/−, FANCC−/−, and TP53+/−FANCC−/− mice 2 wk postinjection of Alum or saline, as above. Staining for total Atr protein was quantified and is presented graphically (Fig. 4C). We found that total Atr paralleled phospho-Atr during multiple episodes of Alum-induced emergency granulopoiesis in each of the genotypes. These results suggested that inhibition of p53-induced apoptosis in FANCC−/−TP53+/− mice during multiple episodes of emergency granulopoiesis was related to progressively impaired Atr expression, an event that did not occur in FANCC−/− bone marrow in the absence of p53 loss of heterozygosity.
We performed experiments to quantify Atr by another approach and identify the mechanism for increased expression during emergency granulopoiesis in FANCC−/− mice. For these studies, we obtained bone marrow 2 wk after injection of Alum or saline, as above, and quantified Atr mRNA by real-time PCR. We found that Atr mRNA expression paralleled protein expression (Fig. 4D).
Clonal progression develops more rapidly in FANCC−/−TP53+/− mice undergoing multiple episodes of emergency granulopoiesis than in FANCC−/− mice
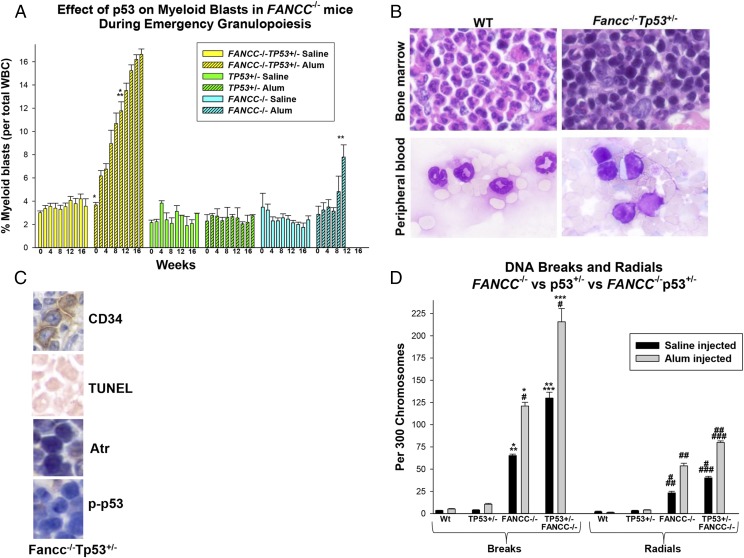
Although TP53 haploinsufficiency rescued emergency granulopoiesis in FANCC−/− mice and protected them from BMF, the mortality for FANCC−/−TP53+/− mice was significantly greater than for WT or TP53+/− mice during sequential episodes (Fig. 1D). Mortality in FANCC−/−TP53+/− mice correlated with progressive anemia and baseline granulocytosis (Fig. 1A, 1C). Further examination of blood counts in these mice identified an accumulation of myeloid blasts in the circulation during repeated episodes of Alum injection in FANCC−/− and FANCC−/−TP53+/− mice (Fig. 5A), an effect not seen in TP53+/− or WT mice. However, circulating myeloid blasts increased significantly more rapidly in FANCC−/−TP53+/− mice compared with FANCC−/− mice (p < 0.001, 2 wk after the second Alum injection, n = 5 ). Myeloid blasts did not increase in these mice during the steady-state over the same time period.
FIGURE 5.
TP53 haploinsufficiency increased the rate of progression to AML in FANCC−/− mice during multiple episodes of emergency granulopoiesis. FANCC−/−, FANCC−/−TP53+/−, TP53+/−, and WT mice were injected on day 0 and every 4 wk thereafter with Alum or saline control. Peripheral blood counts were performed every 2 wk, and mice were sacrificed for analysis 2 wk after the fourth injection. (A) Circulating myeloid blasts were increased significantly earlier in FANCC−/−TP53+/− mice compared with FANCC−/− mice. Statistically significant differences are indicated by * and ** (p < 0.01 for comparison of percent blasts under various conditions, n = 10). (B) Myeloid blasts accumulated in the bone marrow of FANCC−/−TP53+/− mice during multiple episodes of emergency granulopoiesis. Wright–Giemsa stain of bone marrow and peripheral blood at week 2 of the fourth episode of emergency granulopoiesis in WT or FANCC−/−TP53+/− mice (original magnification ×40 [upper panels], original magnification ×100 [lower panels]). (C) CD34+ bone marrow blasts did not have increased apoptosis, Atr expression, or p53 activity during emergency granulopoiesis. Bone marrow from FANCC−/−TP53+/− mice described above was analyzed by immunohistochemistry for CD34, Atr, and phospho-p53. Cells were analyzed for apoptosis using a TUNEL assay. Original magnification ×40. (D) More DNA breaks and radials accumulated in the bone marrow of FANCC−/−TP53+/− mice 2 wk after Alum injection compared with FANCC−/−, TP53+/−, and WT mice. Statistically significant differences are indicated by *, **, ***, #, ##, and ### (p < 0.001 for comparison of DNA breaks or radials under various conditions, n = 6). Error bars represent the SEM for all bar graphs.
We examined sternal bone marrow from these mice 2 wk after Alum injection (Fig. 5B). FANCC−/−TP53+/− mice with peripheral evidence of AML were studied to confirm the diagnosis; we found extensive infiltration of myeloid blasts in the bone marrow of these mice. This was not observed in WT or TP53+/− mice but was similar to findings in rare FANCC−/− mice surviving multiple emergency granulopoiesis episodes (8). We also determined that CD34+ leukemic blasts in the bone marrow of these mice did not undergo apoptosis in response to Alum injection, nor was Atr expression or p53 activation increased (Fig. 5C).
We were interested in the mechanism for simultaneous protection from BMF and acceleration of leukemogenesis during multiple episodes of emergency granulopoiesis in FANCC−/−TP53+/− versus FANCC−/− mice. We hypothesized that this was explained by accumulation of mutations in HSCs and progenitor cells in FANCC−/−TP53+/− mice due to decreased apoptosis of cells with DNA damage. To investigate this, we quantified DNA breaks and radials in the bone marrow of FANCC−/−, FANCC−/−TP53+/−, TP53+/−, and WT control mice subsequent to Alum or saline injection. Because we performed this analysis using standard chromosome spreads, only DNA breaks or radials in viable cells were detected.
We found significantly more DNA breaks and radials in bone marrow from FANCC−/−TP53+/− mice compared with FANCC−/− mice, even without Alum injection (p < 0.001, n = 3) (Fig. 5D). The number of breaks and radials increased significantly in FANCC−/− and FANCC−/−TP53+/− mice after Alum injection (p < 0.0001, n = 3). The percentage increase in DNA breaks or radials with Alum was approximately equivalent in the two genotypes (p > 0.4, n = 3) (Fig. 5D); however, the absolute number of breaks and radials in FANCC−/−TP53+/− murine bone marrow was significantly greater than in FANCC−/− mice (p < 0.0001, n = 3). This indicated that p53 haploinsufficiency increased the absolute number of cells with DNA damage that persisted in the bone marrow of FANCC−/− mice after an emergency granulopoiesis challenge. Significantly fewer breaks and radials were present in the bone marrow of WT and TP53+/− mice in comparison with FANCC−/− mice, with or without TP53 haploinsufficiency, under all conditions (p < 0.0001, n = 3) (Figs. 5D, 6).
FIGURE 6.
Schematic representation of emergency granulopoiesis in FANCC−/− versus FANCC−/−TP53+/− mice. Events in normal bone marrow are highlighted in green, those in FANCC−/−TP53+/− bone marrow are highlighted in blue, and those in FANCC−/− bone marrow are highlighted in purple.
Discussion
Repeated unsuccessful episodes of emergency granulopoiesis resulted in BMF in FANCC−/− mice (8). These results suggest the importance of the Fanconi DNA-repair pathway in maintaining genomic integrity during this process, but they did not identify relevant mechanisms. Induction of emergency granulopoiesis in mice by Alum injection is IL-1β dependent and associated with increased G-CSF expression relative to the steady-state (2, 8, 35). In prior studies, we found no difference in serum levels of IL-1β or G-CSF between WT or FANCC−/− mice during Alum-induced emergency granulopoiesis (8). These results indicated that the aberrant response in FANCC−/− mice was not driven by abnormal cytokine levels. G-CSF stimulates expansion and phenotypic differentiation of myeloid progenitor cells. Because the Fanconi DNA-repair pathway is involved in replication fork rescue during the S phase, and the cell cycle is shortened during emergency granulopoiesis (4), we hypothesized that impaired function of the Fanconi DNA-repair pathway might be especially critical for this aspect of the innate immune response.
The ATR gene is transcriptionally activated during the S phase of the cell cycle (28). Atr is activated (phosphorylated) by signaling events triggered by ssDNA/dsDNA hybrids, as found at the site of a stalled or collapsed replication fork (29). Activated Atr induces S phase arrest for replication fork repair and directly activates some Fanconi proteins (27). If repair is not successful, Atr activates a signaling cascade, resulting in p53-mediated apoptosis (28, 30). In this study, we hypothesized that the latter was a mechanism for BMF during failed emergency granulopoiesis in FANCC−/− mice.
The data reported in this article support this hypothesis. We found that introduction of TP53 haploinsufficiency into FANCC−/− mice rescued Alum-induced emergency granulopoiesis. In addition, anemia developed more slowly in FANCC−/−TP53+/− mice compared with FANCC−/− mice and was associated with prolonged survival. TP53+/− mice exhibited an emergency granulopoiesis response that was not significantly different from WT mice. The slightly increased mortality in Alum-injected TP53+/− mice compared with saline-injected mice had variable causes in the few involved mice. We also performed experiments testing the emergency granulopoiesis response in FANCC−/−TP53−/− mice (data not shown). These animals are very small and sickly and were completely intolerant of Alum injection; they were not investigated further.
We found significantly less bone marrow apoptosis during emergency granulopoiesis in FANCC−/−TP53+/− mice in comparison with FANCC−/− mice. Subsequent episodes of emergency granulopoiesis further increased apoptosis and p53 activation in FANCC−/− murine bone marrow, but these parameters improved with subsequent episodes in FANCC−/−TP53+/− mice. Decreased p53 activation during emergency granulopoiesis in FANCC−/−TP53+/− mice was not associated with further decreases in p53 protein, and phosphorylation was not associated with stabilization and an increased abundance of p53 protein in FANCC−/− murine bone marrow. Because recent data suggest that p53 stability does not require phosphorylation under some conditions, p53 may not be further stabilized by phosphorylation in bone marrow progenitors (38). Alternatively, cells with very abundant p53 may undergo apoptosis and be underrepresented in the population.
We investigated the role of Atr activation in p53 activity during repeated episodes of emergency granulopoiesis. In FANCC−/− murine bone marrow, increasing p53 activation during repeated episodes of emergency granulopoiesis was associated with activation of Atr and increasing levels of Atr protein and mRNA. In contrast, Atr protein and mRNA declined with subsequent episodes of emergency granulopoiesis in FANCC−/−TP53+/− mice.
We found that apoptosis resistance in FANCC−/−TP53+/− mice permitted accumulation of DNA damage in bone marrow cells during repeated episodes of emergency granulopoiesis relative to FANCC−/− mice. Mutation of cell cycle checkpoint control genes, such as those regulating Atr, would further impair p53 activation and the DNA damage response. Such mutations might permit expansion of myeloid progenitors with DNA damage, rescuing emergency granulopoiesis but accelerating leukemogenesis. Increased granulocyte density in the bone marrow provides negative feedback on granulopoiesis (17). Mechanisms for this may include inhibition of proliferative signals and resetting the cell cycle checkpoint to the steady-state. Therefore, successful emergency granulopoiesis in FANCC−/−TP53+/− mice might decrease Atr expression, with further loss of the p53 response to unrepaired DNA (Fig. 6). A Ter119+ subset of Lin−CD34+ cells can undergo erythroid differentiation (39). Inhibition of Atr/p53-induced apoptosis of these cells may also ameliorate anemia in FANCC−/−TP53+/− mice compared with FANCC−/− mice during recurrent emergency granulopoiesis episodes. This is a topic of interest in the laboratory.
A human FA kindred was described with attenuated BMF, accelerated clonal progression, and undefined abnormalities in cell cycle checkpoint events, suggesting the clinical relevance of our studies (40). Our findings also have translational implications for the possible use of p53 inhibitors in FA. Our studies suggest that the innate immune response might be improved by therapeutic p53 inhibition, but acceleration of clonal progression may be an unintended consequence of this approach. In contrast, prevention of unsuccessful episodes of emergency granulopoiesis, by inhibiting IL-1R or Irak4, might be useful approaches.
These issues may be of interest for understanding the role of emergency granulopoiesis in other myeloid leukemias. For example, progression to AML in G-CSF–treated patients with severe congenital neutropenia is heralded by a switch from the steady-state to emergency granulopoiesis signaling (41). Exaggerated granulocytosis is observed during infection in chronic myeloid leukemia and chronic myelomonocytic leukemia, but the role of emergency granulopoiesis in these diseases is unknown. This may be a useful area of investigation, because chronic myeloid leukemia and chronic myelomonocytic leukemia are characterized by decreased activity of Icsbp, a key activator of FANCC and FANCF transcription (42–44).
Studies by other investigators identified increased susceptibility to DNA cross-link damage in FA bone marrow progenitors treated with inflammatory mediators, including TNF-α and IFN-γ (31, 45). Mechanisms were hypothesized to include increased generation of reactive oxygen species or activation of SAPK or MAPK (46). These mechanisms may also contribute to sustained unrepaired DNA damage during emergency granulopoiesis in FA. Although mature granulocytes were not produced in FANCC−/− mice during episodes of emergency granulopoiesis, they were produced in FANCC−/−TP53+/− mice. These issues are obvious areas of interest and additional investigation in the laboratory.
Supplementary Material
Acknowledgments
We thank Dr. Alex Minella (Blood Research Institute of Wisconsin, Milwaukee WI) for providing TP53+/− mice and helpful discussions, Dr. D. Wade Clapp (Indiana University, Indianapolis, IN) for FANCC+/− mice and insight into FA murine models, and Dr. Katrine Carlson-Leuer (Lurie Children’s Hospital, Chicago, IL) for assistance and advice on murine chromatin spread techniques.
This work was supported by National Institutes of Health Grants R01 CA174205 and R01 DK098812, Veterans Administration Merit Review Grant BX002067, and the Fanconi Anemia Research Fund (all to E.A.E.).
The online version of this article contains supplemental material.
- Alum
- OVA/alum mixture
- AML
- acute myeloid leukemia
- Atr
- ataxia telangiectasia and Rad3-related protein
- BMF
- bone marrow failure
- DAB
- 3,3′-diaminobenzidine
- FA
- Fanconi anemia
- Hgb
- hemoglobin
- HSC
- hematopoietic stem cell
- Icsbp
- IFN consensus sequence binding protein
- WT
- wild-type.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Lord B. I., Molineux G., Pojda Z., Souza L. M., Mermod J. J., Dexter T. M. 1991. Myeloid cell kinetics in mice treated with recombinant interleukin-3, granulocyte colony-stimulating factor (CSF), or granulocyte-macrophage CSF in vivo. Blood 77: 2154–2159. [PubMed] [Google Scholar]
- 2.Ueda Y., Cain D. W., Kuraoka M., Kondo M., Kelsoe G. 2009. IL-1R type I-dependent hemopoietic stem cell proliferation is necessary for inflammatory granulopoiesis and reactive neutrophilia. J. Immunol. 182: 6477–6484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panopoulos A. D., Watowich S. S. 2008. Granulocyte colony-stimulating factor: molecular mechanisms of action during steady state and ‘emergency’ hematopoiesis. Cytokine 42: 277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lord B. I., Bronchud M. H., Owens S., Chang J., Howell A., Souza L., Dexter T. M. 1989. The kinetics of human granulopoiesis following treatment with granulocyte colony-stimulating factor in vivo. Proc. Natl. Acad. Sci. USA 86: 9499–9503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scumpia P. O., Kelly-Scumpia K. M., Delano M. J., Weinstein J. S., Cuenca A. G., Al-Quran S., Bovio I., Akira S., Kumagai Y., Moldawer L. L. 2010. Cutting edge: bacterial infection induces hematopoietic stem and progenitor cell expansion in the absence of TLR signaling. J. Immunol. 184: 2247–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirai H., Zhang P., Dayaram T., Hetherington C. J., Mizuno S., Imanishi J., Akashi K., Tenen D. G. 2006. C/EBPbeta is required for ‘emergency’ granulopoiesis. Nat. Immunol. 7: 732–739. [DOI] [PubMed] [Google Scholar]
- 7.Panopoulos A. D., Zhang L., Snow J. W., Jones D. M., Smith A. M., El Kasmi K. C., Liu F., Goldsmith M. A., Link D. C., Murray P. J., Watowich S. S. 2006. STAT3 governs distinct pathways in emergency granulopoiesis and mature neutrophils. Blood 108: 3682–3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu L., Huang W., Hjort E., Eklund E. A. 2013. Increased Fanconi C expression contributes to the emergency granulopoiesis response. J. Clin. Invest. 123: 3952–3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saberwal G., Horvath E., Hu L., Zhu C., Hjort E., Eklund E. A. 2009. The interferon consensus sequence binding protein (ICSBP/IRF8) activates transcription of the FANCF gene during myeloid differentiation. J. Biol. Chem. 284: 33242–33254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eklund E. A., Jalava A., Kakar R. 1998. PU.1, interferon regulatory factor 1, and interferon consensus sequence-binding protein cooperate to increase gp91(phox) expression. J. Biol. Chem. 273: 13957–13965. [DOI] [PubMed] [Google Scholar]
- 11.Eklund E. A., Kakar R. 1999. Recruitment of CBP by PU.1, IRF1 and ICSBP is necessary for gp91phox and p67phox expression. J. Immunol. 163: 6095–6105. [PubMed] [Google Scholar]
- 12.Hu L., Huang W., Hjort E. E., Bei L., Platanias L. C., Eklund E. A. 2016. The interferon consensus sequence binding protein is required for termination of emergency granulopoiesis. J. Biol. Chem. 291: 4107–4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson K. L., Smith K. A., Conners K., McKercher S. R., Maki R. A., Torbett B. E. 1998. Myeloid development is selectively disrupted in PU.1 null mice. Blood 91: 3702–3710. [PubMed] [Google Scholar]
- 14.Zhang D. E., Zhang P., Wang N. D., Hetherington C. J., Darlington G. J., Tenen D. G. 1997. Absence of granulocyte colony-stimulating factor signaling and neutrophil development in CCAAT enhancer binding protein alpha-deficient mice. Proc. Natl. Acad. Sci. USA 94: 569–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takeda K., Kaisho T., Yoshida N., Takeda J., Kishimoto T., Akira S. 1998. Stat3 activation is responsible for IL-6-dependent T cell proliferation through preventing apoptosis: generation and characterization of T cell-specific Stat3-deficient mice. J. Immunol. 161: 4652–4660. [PubMed] [Google Scholar]
- 16.Holtschke T., Löhler J., Kanno Y., Fehr T., Giese N., Rosenbauer F., Lou J., Knobeloch K. P., Gabriele L., Waring J. F., et al. 1996. Immunodeficiency and chronic myelogenous leukemia-like syndrome in mice with a targeted mutation of the ICSBP gene. Cell 87: 307–317. [DOI] [PubMed] [Google Scholar]
- 17.Cain D. W., Snowden P. B., Sempowski G. D., Kelsoe G. 2011. Inflammation triggers emergency granulopoiesis through a density-dependent feedback mechanism. PLoS One 6: e19957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schlacher K., Wu H., Jasin M. 2012. A distinct replication fork protection pathway connects Fanconi anemia tumor suppressors to RAD51-BRCA1/2. Cancer Cell 22: 106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L. C., Stone S., Hoatlin M. E., Gautier J. 2008. Fanconi anemia proteins stabilize replication forks. DNA Repair (Amst.) 7: 1973–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moldovan G. L., D’Andrea A. D. 2009. How the fanconi anemia pathway guards the genome. Annu. Rev. Genet. 43: 223–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kee Y., D’Andrea A. D. 2012. Molecular pathogenesis and clinical management of Fanconi anemia. J. Clin. Invest. 122: 3799–3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson L. H., Hinz J. M. 2009. Cellular and molecular consequences of defective Fanconi anemia proteins in replication-coupled DNA repair: mechanistic insights. Mutat. Res. 668: 54–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X., Heyer W. D. 2008. Homologous recombination in DNA repair and DNA damage tolerance. Cell Res. 18: 99–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Auerbach A. D., Allen R. G. 1991. Leukemia and preleukemia in Fanconi anemia patients. A review of the literature and report of the International Fanconi Anemia Registry. Cancer Genet. Cytogenet. 51: 1–12. [DOI] [PubMed] [Google Scholar]
- 25.Cioc A. M., Wagner J. E., MacMillan M. L., DeFor T., Hirsch B. 2010. Diagnosis of myelodysplastic syndrome among a cohort of 119 patients with fanconi anemia: morphologic and cytogenetic characteristics. Am. J. Clin. Pathol. 133: 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stiff T., Walker S. A., Cerosaletti K., Goodarzi A. A., Petermann E., Concannon P., O’Driscoll M., Jeggo P. A. 2006. ATR-dependent phosphorylation and activation of ATM in response to UV treatment or replication fork stalling. EMBO J. 25: 5775–5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shigechi T., Tomida J., Sato K., Kobayashi M., Eykelenboom J. K., Pessina F., Zhang Y., Uchida E., Ishiai M., Lowndes N. F., et al. 2012. ATR-ATRIP kinase complex triggers activation of the Fanconi anemia DNA repair pathway. Cancer Res. 72: 1149–1156. [DOI] [PubMed] [Google Scholar]
- 28.Shiotani B., Zou L. 2009. ATR signaling at a glance. J. Cell Sci. 122: 301–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiotani B., Nguyen H. D., Håkansson P., Maréchal A., Tse A., Tahara H., Zou L. 2013. Two distinct modes of ATR activation orchestrated by Rad17 and Nbs1. Cell Reports 3: 1651–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koundrioukoff S., Carignon S., Técher H., Letessier A., Brison O., Debatisse M. 2013. Stepwise activation of the ATR signaling pathway upon increasing replication stress impacts fragile site integrity. PLoS Genet. 9: e1003643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitney M. A., Royle G., Low M. J., Kelly M. A., Axthelm M. K., Reifsteck C., Olson S., Braun R. E., Heinrich M. C., Rathbun R. K., et al. 1996. Germ cell defects and hematopoietic hypersensitivity to gamma-interferon in mice with a targeted disruption of the Fanconi anemia C gene. Blood 88: 49–58. [PubMed] [Google Scholar]
- 32.Donehower L. A., Harvey M., Slagle B. L., McArthur M. J., Montgomery C. A., Jr., Butel J. S., Bradley A. 1992. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 356: 215–221. [DOI] [PubMed] [Google Scholar]
- 33.Chen M., Tomkins D. J., Auerbach W., McKerlie C., Youssoufian H., Liu L., Gan O., Carreau M., Auerbach A., Groves T., et al. 1996. Inactivation of Fac in mice produces inducible chromosomal instability and reduced fertility reminiscent of Fanconi anaemia. Nat. Genet. 12: 448–451. [DOI] [PubMed] [Google Scholar]
- 34.Freie B., Li X., Ciccone S. L., Nawa K., Cooper S., Vogelweid C., Schantz L., Haneline L. S., Orazi A., Broxmeyer H. E., et al. 2003. Fanconi anemia type C and p53 cooperate in apoptosis and tumorigenesis. Blood 102: 4146–4152. [DOI] [PubMed] [Google Scholar]
- 35.Kool M., Pétrilli V., De Smedt T., Rolaz A., Hammad H., van Nimwegen M., Bergen I. M., Castillo R., Lambrecht B. N., Tschopp J. 2008. Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J. Immunol. 181: 3755–3759. [DOI] [PubMed] [Google Scholar]
- 36.Higashimoto Y., Saito S., Tong X. H., Hong A., Sakaguchi K., Appella E., Anderson C. W. 2000. Human p53 is phosphorylated on serines 6 and 9 in response to DNA damage-inducing agents. J. Biol. Chem. 275: 23199–23203. [DOI] [PubMed] [Google Scholar]
- 37.Appella E., Anderson C. W. 2001. Post-translational modifications and activation of p53 by genotoxic stresses. Eur. J. Biochem. 268: 2764–2772. [DOI] [PubMed] [Google Scholar]
- 38.Ashcroft M., Kubbutat M. H., Vousden K. H. 1999. Regulation of p53 function and stability by phosphorylation. Mol. Cell. Biol. 19: 1751–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akashi K., Traver D., Miyamoto T., Weissman I. L. 2000. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 404: 193–197. [DOI] [PubMed] [Google Scholar]
- 40.Ceccaldi R., Briot D., Larghero J., Vasquez N., Dubois d’Enghien C., Chamousset D., Noguera M. E., Waisfisz Q., Hermine O., Pondarre C., et al. 2011. Spontaneous abrogation of the G2DNA damage checkpoint has clinical benefits but promotes leukemogenesis in Fanconi anemia patients. J. Clin. Invest. 121: 184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skokowa J., Welte K. 2009. Dysregulation of myeloid-specific transcription factors in congenital neutropenia. Ann. N. Y. Acad. Sci. 1176: 94–100. [DOI] [PubMed] [Google Scholar]
- 42.Huang W., Luan C. H., Hjort E. E., Bei L., Mishra R., Sakamoto K. M., Platanias L. C., Eklund E. A. 2016. The role of Fas-associated phosphatase 1 in leukemia stem cell persistence during tyrosine kinase inhibitor treatment of chronic myeloid leukemia. Leukemia 30: 1502–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang L. W., Hu L., Bei E., Hjort, Eklund E. A. 2012. The leukemia-associated fusion-protein Tel-PdgfRB inhibits transcriptional repression of the PTPN13 gene by the interferon consensus sequence binding protein. J. Biol. Chem. 287: 8110–8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Oncomine Research Edition. Available at: https://www.oncomine.org/resource/main.html. Accessed: September 15, 2017.
- 45.Schultz J. C., Shahidi N. T. 1993. Tumor necrosis factor-alpha overproduction in Fanconi’s anemia. Am. J. Hematol. 42: 196–201. [DOI] [PubMed] [Google Scholar]
- 46.Garbati M. R., Hays L. E., Keeble W., Yates J. E., Rathbun R. K., Bagby G. C. 2013. FANCA and FANCC modulate TLR and p38 MAPK-dependent expression of IL-1β in macrophages. Blood 122: 3197–3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.