Abstract
Growing evidence suggests that protocadherins (PCDH) play crucial roles in pathogenesis and progression of cancers, including gastric cancer (GC). Protocadherin‐8 (PCDH8) was previously reported to be involved in metastasis of GC, but functional studies yielded inconsistent results and the molecular mechanism remained unknown. The present study aimed to explore the clinical relevance, function and molecular mechanism of PCDH8 in GC. Data from the GEPIA and Kaplan–Meier plotter databases showed that high expression of PCDH8 was significantly correlated with poorer prognosis in GC. Ectopic expression of PCDH8 in GC cells promoted invasion and migration in vitro and metastasis in vivo, and knockdown of PCDH8 inhibited invasion and migration in vitro. RNA sequencing followed by gene set enrichment analysis found a remarkable enrichment in the extracellular matrix receptor interaction pathway, with the expression of laminin subunit γ2 (LAMC2) being significantly increased in the PCDH8‐overexpressing group. High expression of LAMC2 was significantly correlated to poor prognosis in GC in GEPIA database. Upregulation of LAMC2 following PCDH8 overexpression was further confirmed by immunohistochemistry in liver metastatic lesions of nude mice. To our knowledge, this is the first report of the metastasis‐enhancing property and molecular mechanism through upregulation of LAMC2 of PCDH8 in cancer. High expression of PCDH8 could be used as a biomarker for poor prognosis in clinical practice.
Keywords: gastric cancer, invasion, laminin subunit γ2, metastasis, protocadherin‐8
1. INTRODUCTION
Growing evidence suggests that protocadherins (PCDH), which belong to the cadherin superfamily, are widely involved in the pathogenesis and progression of multiple cancers.1 Research into the functions of PCDH has found that members of this family have diverse biological roles, from tumor suppressors to oncogenes.1 For example, PCDH10 was found to suppress tumor growth and induce apoptosis in endometrioid endometrial carcinoma by negatively regulating the Wnt/β‐catenin signaling pathway,2 while PCDH11Y and PCDH7 were found to have oncogenic properties of enhancing growth, cell migration and drug resistance.1 However, for most of the PCDH, the functions and the underlying mechanisms remain largely unexplored.
Protocadherin‐8 (PCDH8) is another member of the protocadherin family. Methylation of PCDH8 was found to be related to poor prognosis or unfavorable clinicopathological features in various cancers, including gastric cancer (GC).3, 4, 5, 6, 7 Exogenous expression of PCDH8 inhibited proliferation and migration in breast cancer cells and nasopharyngeal cancer cells.3, 6 Re‐expression of PCDH8 in GC cells using demethylation reagent inhibited migration and induced apoptosis in vitro.7 Given these studies, PCDH8 was proposed to be a tumor suppressor. However, a study found that PCDH8 was reversely regulated by microRNA‐429, and microRNA‐429 inhibitor increased the motility of human endometrial cancer cells through epithelial–mesenchymal transition (EMT).8 This study provided circumstantial evidence that PCDH8 might have metastasis‐enhancing properties. Therefore, the role of PCDH8 in cancer metastasis has been controversial, with limited results of in vitro experiments and no in vivo studies to our knowledge, and the molecular mechanisms remaining unknown.
Gastric cancer is one of the most common malignancies and causes of cancer‐related death worldwide.9 Prognosis for GC patients remains poor. One reason for the poor outcome of GC is that many patients are diagnosed at an advanced or late stage, with lymphatic or distant metastasis, and many patients experience regional recurrence or distal metastasis after radical resection and systemic therapy. Understanding the mechanisms of invasion and metastasis of GC is very important for discovering new molecules involved in GC progression and developing new biomarkers and therapeutic strategies.
Considering the controversial role of PCDH8 in cancer metastasis and its unexplored molecular mechanisms, the present study aimed to investigate the clinical relevance and impact on cell motility of PCDH8 in GC. To our knowledge, this is the first report of the molecular mechanisms of PCDH8 in cancer. We found that high expression of PCDH8 was significantly correlated with poor prognosis in GC. Ectopic expression of PCDH8 promoted the invasion and migration abilities of GC cells, both in vitro and in vivo, and knockdown of PCDH8 inhibited invasion and migration in vitro. PCDH8 might function through interaction with extracellular matrix (ECM) receptors.
2. MATERIALS AND METHODS
2.1. Patients and specimens
All primary gastric adenocarcinoma specimens were obtained by surgical resection between 2007 and 2010 at Fudan University Shanghai Cancer Center (FUSCC), Shanghai, China. Samples were acquired after informed consent was given, and under the protocol approved by the Clinical Research Ethics Committee of FUSCC. All procedures were in accordance with the 1964 Declaration of Helsinki and its later amendments. Paraffin‐embedded tumor tissues were collected from 144 consecutive patients with gastric adenocarcinoma, and intratumoral area were selected to construct tissue microarrays in collaboration with Shanghai Biochip (Shanghai, China). The clinical data collection and postoperative follow‐up procedures followed uniform guidelines of FUSCC.
This study was approved by the Clinical Research Ethics Committee of FUSCC. All samples of patients obtained were acquired from the tissue bank of FUSCC under the protocol approved by the Clinical Research Ethics Committee of FUSCC. Consent for the use of surgical samples was given from patients before surgery and all procedures were in accordance with the 1964 Declaration of Helsinki and its later amendments. All animal procedures in this study complied with protocols approved by the Shanghai Medical Experimental Animal Care Commission. All applicable international, national and institutional guidelines for the care and use of animals were followed.
2.2. Immunohistochemistry and scoring system
A GA tissue microarray was subjected to immunohistochemistry assays using a MaxVision HRP‐Polymer Detection System (Maixin, Fuzhou, China), as described previously.10 Primary antibody against PCDH8 (Santa Cruz Biotechnology, Paso Robles, CA, USA) and laminin subunit γ2 (LAMC2) (Abcam, Cambridge, MA, USA) were used according to the manufacturers’ guidelines. Slides were independently evaluated by 2 investigators who were blinded to the patients’ clinical information. The staining intensity of PCDH8 membrane immunostaining was evaluated (score 0 = none; 1 = weak; 2 = moderate; 3 = strong). Samples that scored ≥2 were regarded as high expression.
2.3. Cell lines
Gastric cancer cell lines MKN‐28 and MGC‐803, and immortalized human gastric epithelial cell line GES‐1, were obtained from 3D Biopharm Biotech (Shanghai, China). SGC‐7901 cell line was obtained from the Cell Bank of Type Culture Collection of Chinese Academy of Sciences (Shanghai, China). SNU‐216 cell line was a gift from the Medical College of Xiamen University (Fuzhou, China). Cells were cultured in Roswell Park Memorial Institute 1640 medium containing 10% FBS (Gibco, Carlsbad, CA, USA) and 1% penicillin‐streptomycin (Invitrogen, Carlsbad, CA, USA) at 37°C in a humidified atmosphere with 5% carbon dioxide.
2.4. Lentivirus production and transduction
The PCDH8‐overexpressing and empty vector plasmids were purchased from Hanyin Biotechnology (Shanghai, China), and were transfected into human embryonic kidney 293T cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). Viruses were harvested 48 h after transfection. MKN‐28 and MGC‐803 were infected with filtered lentivirus in the presence of 6 μg/mL of polybrene (Sigma‐Aldrich, St Louis, MO, USA) for 12 hour and subjected to selection with 2 μg/mL puromycin 72 hour after transfection.
2.5. siRNA knockdown
siRNA for PCDH8 and negative control (NC) were purchased from GenePharma (Shanghai, China) and transfected into SNU‐216 cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. The final concentration of siRNA was 100 nmol/L.
2.6. Western blot and antibodies
Standard western blot procedures were performed as described previously.10 The primary antibodies against GAPDH (Cell Signaling Technology, Cambridge, MA, USA) and PCDH8 (Santa Cruz Biotechnology, CA, USA) were used according to the manufacturers’ guidelines. HRP‐conjugated secondary antibody (Cell Signaling Technology, Cambridge, MA, USA) was used according to the manufacturer's guidelines. Detection was performed using enhanced chemiluminescence reagent (Pierce, Rockford, IL, USA).
2.7. In vitro invasion and migration assays
In vitro invasion and migration assays were performed in chambers with 8‐μm pores (BD Biosciences, San Jose, CA, USA) with or without Matrigel (BD Biosciences); 4 × 104 MGC‐803, 5 × 104 MKN‐28 and 5 × 104 SNU‐216 cells were used for the invasion assay and 3 × 104 MGC‐803, 4 × 104 MKN‐28 and 4 × 104 SNU‐216 cells were used for the migration assay. Cells were diluted in 300 μL of serum‐free medium and seeded into upper inserts, with 600 μL of 20% serum medium in the lower chamber. After incubating for 36 and 30 hours for invasion and migration assays, respectively, cells were fixed with 500 μL 4% paraformaldehyde. Then, each well was washed 3 times with PBS, and stained with 0.6 mL of 0.1% crystal violet solution. Cells on the upper chamber were gently removed using cotton swabs. The number of cells was counted at 5 fields per membrane at 200× magnification from each group of 3 independent experiments using an IX71 inverted microscope (Olympus, Tokyo, Japan).
2.8. In vivo studies
Male BALB/c (nu/nu) nude mice aged 6 weeks were purchased from Shanghai Slac Laboratory Animal (Shanghai, China) and raised under specific pathogen‐free conditions. MKN‐28 cells (4 × 106 per mouse) stably transfected with empty vector or PCDH8 lentivirus were injected into the tail veins. All mice were killed 6 weeks later by cervical dislocation. Lungs and livers were dissected, fixed in 10% formalin, and embedded in paraffin. After being processed for HE staining, presence of metastases and number of metastatic sites were observed under the microscope. All animal work was conducted according to relevant national and international guidelines and NIH guidelines for the ethical use of animals.
2.9. RNA extraction
RNA extraction was performed for RNA sequencing and, for quantitative real‐time PCR, cells were cultured in 6‐well plates and harvested at a concentration of 70%‐80%. RNA was extracted using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA).
2.10. RNA sequencing analysis
To enrich polyA+ RNA, 1 μg of RNA was treated with VAHTS mRNA Capture Beads (Vazyme, Nanjing, China). Subsequently, RNA‐sequencing libraries were constructed using a VAHTS mRNA‐Seq v2 Library Preparation Kit for Illumina (Vazyme, Nanjing, China) following the manufacturer's instructions. Briefly, approximately 100 ng of polyA+ RNA samples were fragmented and used for first‐strand and second‐strand cDNA synthesis with random hexamer primers. The cDNA fragments were treated with a DNA End Repair Kit (Vazyme, Nanjing, China), modified with a DNA Polymerase I Klenow Fragment Kit (Vazyme, Nanjing, China) and ligated to adapters. Purified dsDNA was subjected to 12 cycles of PCR amplification to construct libraries. The libraries were sequenced by the Illumina sequencing platform on a 150‐bp paired‐end run. Data for sequencing reads were aligned using the spliced read aligner HISAT2 (Center for Computational Biology, Johns Hopkins University, Baltimore, MD, USA) using Ensembl human genome assembly (Genome Reference Consortium GRCh38) as a reference. Gene expression levels were calculated by fragments per kilobase of transcript per million mapped reads. Gene set enrichment analysis was used for the pathway analysis.
2.11. Quantitative real‐time PCR
Total RNA was reversely transcribed to synthesize cDNA using PrimeScript RT‐PCR Kit (TaKaRa, Shiga, Japan) according to the manufacturer's instructions. Expression levels of mRNA were quantified using the SYBR Premix Ex Taq Kit (TaKaRa, Shiga, Japan) on a 7900 Real‐Time PCR System (Applied Biosystems, Foster City, CA, USA) at the following thermal cycling settings: 1 initial cycle at 95°C for 30 seconds followed by 40 cycles of 5 seconds at 95°C and 30 seconds at 60°C. The paired primers for LAMC2 were forward primer GTCACTGGAGAACGCTGTGA and reverse primer ACATCTTGATGGCGCTGTGA. Beta‐actin was used as an internal control and paired primers were forward primer CTCCATCCTGGCCTCGCTGT and reverse primer GCTGTCACCTCCACCGTTCC, as previously described.11
2.12. Statistical analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences version 19.0 (SPSS, Chicago, IL, USA) or GraphPad Prism version 5.0 (GraphPad Software, La Jolla, CA, USA). Quantitative variables were analyzed by Student's t‐test or 1‐way ANOVA with Bonferroni's test. Survival analyses were performed using the Kaplan–Meier method and the log‐rank test. Relations between clinicopathological characteristics and PCDH8 expressions were analyzed with Pearson's χ2‐test. Two‐tailed P < .05 was considered statistically significant.
3. RESULTS
3.1. High expression of protocadherin‐8 was associated with poor prognosis in gastric cancer patients
To characterize the association between PCDH8 expression and prognosis in GC, the GEPIA database12 and the Kaplan–Meier plotter database13 were used. In the GEPIA cohort, high expression of PCDH8 was significantly associated with shorter disease‐free survival (DFS) and shorter overall survival (OS) (P = .0054 and P = .035, respectively; Figure 1A,B). Exploration in the Kaplan–Meier plotter cohort showed similar results, that high expression of PCDH8 was significantly associated with shorter relapse‐free survival (RFS) and shorter OS (P < .001 and P < .001, Figure 1C,D).
Figure 1.
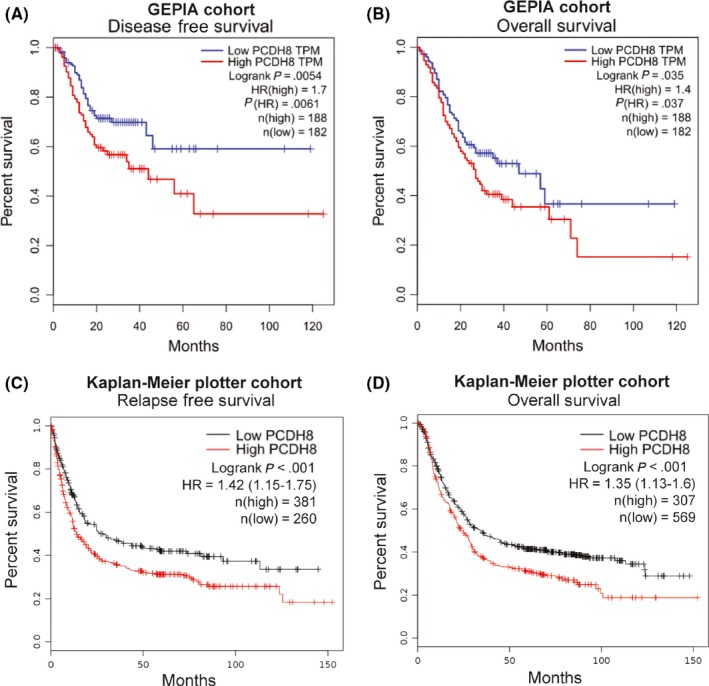
High expression of protocadherin‐8 (PCDH8) was correlated with poorer prognosis in gastric cancer. A,B, High expression of PCDH8 was significantly correlated with shorter disease free survival (A) and shorter overall survival (B) in the GEPIA cohort. C,D, High expression of PCDH8 was significantly correlated with shorter relapse free survival (C) and overall survival (D) in the Kaplan–Meier plotter cohort. P‐values by log‐rank test are displayed
In addition, we further explored the clinical relevance of PCDH8 using tissue microarray containing samples from 144 GC patients in FUSCC (Figure S1A). PCDH8 expression was high in 27.1% (39/144) of patients (Figure S1B). Correlations between clinicopathological characteristics of GC patients with different PCDH8 expressions are presented in Table 1. Although no statistically significant link was observed, we did find that high PCDH8 expression tended to be more common in elderly (>50 years old) GC patients (P = .078), and was related to lower 3‐year progression free survival (PFS) rate and 3‐year OS rate (P = .147 and P = .101), respectively (Table 1). There was no significant association between PCDH8 expression and PFS and OS (Figure S1C). However, patients with high PCDH8 expression had a tendency towards shorter PFS and OS than patients with low PCDH8 expression, which is consistent with the above results.
Table 1.
Clinicopathological characteristics in gastric cancer patients with different PCDH8 expressions
| Features | PCDH8 expression in gastric cancer tissue | ||||
|---|---|---|---|---|---|
| Low | High | ||||
| Number of patients | % | Number of patients | % | P‐value | |
| Total | 105 | 72.9 | 39 | 27.1 | |
| Age | |||||
| ≤50 | 21 | 87.5 | 3 | 12.5 | .078 |
| >50 | 84 | 70.0 | 36 | 30.0 | |
| Sex | |||||
| Female | 24 | 72.7 | 9 | 27.3 | .978 |
| Male | 81 | 73.0 | 30 | 27.0 | |
| Differential status | |||||
| Undifferentiated/poorly | 87 | 71.3 | 35 | 28.7 | .307 |
| Moderate/well | 18 | 81.8 | 4 | 18.2 | |
| T | |||||
| T1/2 | 16 | 76.2 | 5 | 23.8 | .715 |
| T3/4 | 89 | 72.4 | 34 | 27.6 | |
| N | |||||
| N0 | 28 | 77.8 | 8 | 22.2 | .449 |
| N1/2/3 | 77 | 71.3 | 31 | 28.7 | |
| Vascular invasion | |||||
| Negative | 44 | 74.6 | 15 | 25.4 | .709 |
| Positive | 61 | 71.8 | 24 | 28.2 | |
| Nerve invasion | |||||
| Negative | 45 | 76.3 | 14 | 23.7 | .450 |
| Positive | 60 | 70.6 | 25 | 29.4 | |
| Stage | |||||
| I/II | 35 | 79.5 | 9 | 20.5 | .235 |
| III/IV | 70 | 70.0 | 30 | 30.0 | |
| 3‐year progression free survival | 60 | 57.1 | 17 | 43.6 | .147 |
| 3‐year overall survival | 67 | 63.8 | 19 | 48.7 | .101 |
PCDH8, protocadherin‐8.
3.2. Stable expression of protocadherin‐8 promoted invasion and migration and knockdown of protocadherin‐8 inhibited invasion and migration in vitro
Endogenous expression of PCDH8 in immortalized normal cell line (GES‐1) and several GC cell lines was not abundant, except for SNU‐216. Expression of PCDH8 in most GC cell lines was slightly higher than that in GES‐1 (Figure 2A).
Figure 2.
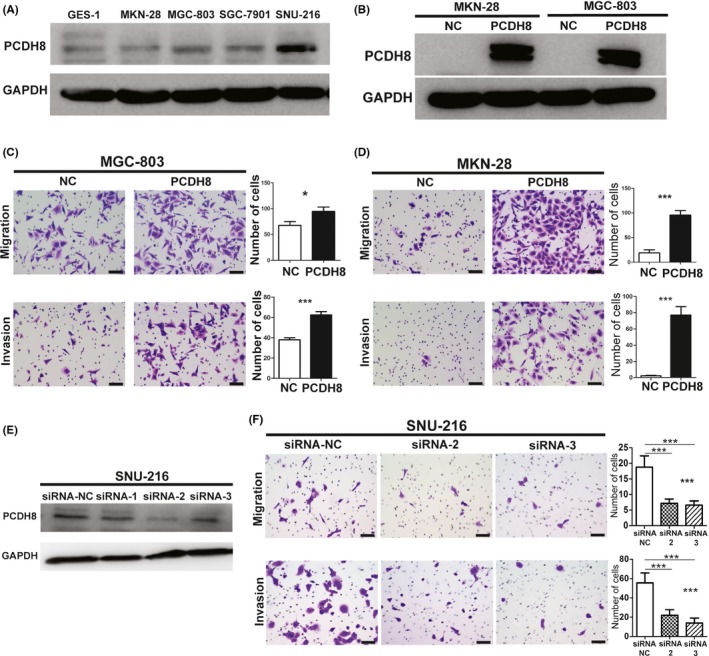
Overexpression of protocadherin‐8 (PCDH8) promoted invasion and migration and knockdown of PCDH8‐inhibited invasion and migration in gastric cancer cells in vitro. A, Expression of PCDH8 in GES‐1 and several gastric cancer cell lines. B, Stable overexpression of PCDH8 in MKN‐28 and MGC‐803 cells was confirmed by western blotting. C, Overexpression of PCDH8 promoted invasion and migration in vitro in MGC‐803 cells. Scale bar, 50 μm. Values are mean ± SD of 3 replicates. D, Overexpression of PCDH8 promoted invasion and migration in vitro in MKN‐28 cells. Scale bar, 50 μm. Values are mean ± SD of 3 replicates. E, Knockdown of PCDH8 in SNU‐216 cells was confirmed by western blotting. F, Knockdown of PCDH8 inhibited invasion and migration in vitro in SNU‐216 cells. Scale bar, 50 μm. Values are mean ± SD of 3 replicates. *P < .05; **P < 0.01; ***P < .001
Two GC cell lines, MGC‐803 and MKN‐28, were stably transfected with PCDH8‐overexpressing lentivirus. Successful stable overexpression of PCDH8 was confirmed by western blotting (Figure 2B). The results of a transwell invasion and migration assay showed that overexpression of PCDH8 significantly promoted invasion and migration in MGC‐803 and MKN‐28 cells (Figure 2C,D).
A knockdown experiment of PCDH8 by 3 siRNAs was performed in SNU‐216 cells, and cells with successful knockdown of PCDH8 by 2 of the 3 siRNAs were subjected to further transwell invasion and migration assay (Figure 2E). The results showed that knockdown of PCDH8 significantly inhibited invasion and migration in SNU‐216 cells (Figure 2F).
3.3. Stable expression of protocadherin‐8 promoted metastasis in vivo
An in vivo murine experimental metastasis assay was performed by transplanting MKN‐28 cells from the negative control (NC) group or the PCDH8‐overexpressing group (PCDH8) into the caudal vein of nude mice. Expression of PCDH8 was detected by immunohistochemistry in liver metastatic lesions (Figure S1D). Histologic analysis of the lungs showed no obvious lung metastasis nodule in the NC group (0/5), while 3 of 5 nude mice developed microscopically visible lung metastasis nodules in the PCDH8‐overexpressing group (Figure 3A). Histologic analysis of the livers showed that the number and size of liver metastasis nodules in the PCDH8‐overexpressing group significantly increased when compared with the NC group (Figure 3B).
Figure 3.
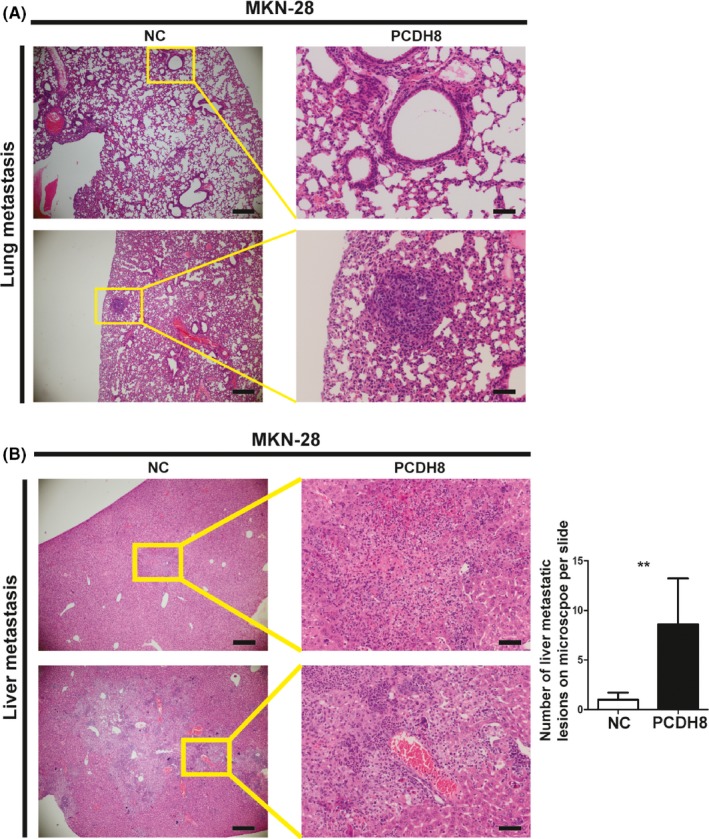
Protocadherin‐8 (PCDH8) promoted metastasis in vivo of gastric cancer. A, PCDH8 promoted metastasis to lung in vivo in MKN‐28 cells. Left panel, scale bar, 500 μm; right panel, scale bar, 100 μm. B, PCDH8 promoted metastasis to liver in vivo in MKN‐28 cells. Left panel, scale bar, 500 μm; right panel, scale bar, 100 μm. Bar graph shows the number of metastatic lesions in the microscope per slide. Values are mean ± SD of 3 replicates. *P < .05; **P < .01; ***P < .001
3.4. Identification of extracellular matrix interaction as a possible functioning mechanism of protocadherin‐8 in gastric cancer
Previous studies found that PCDH8 phosphorylated p38 MAPK in neurons to regulate synaptic morphology. However, in both MGC‐803 and MKN‐28 cells, overexpression of PCDH8 did not cause changes in phosphorylation of p38 MAPK (Figure S1E).
To elucidate the underlying mechanism of PCDH8, RNA‐sequencing was performed and transcriptome differences between the NC group and the PCDH8‐overexpression group in MGC‐803 and MKN‐28 cells were compared. We identified a significant enrichment in the ECM receptor interaction pathway (P < .01) in the PCDH8‐overexpression group compared to the NC group (Figure 4A). Among the genes from the ECM receptor interaction pathway that showed core enrichments (Figure 4B), we noticed the presence of LAMC2, which has been previously reported to promote invasion and metastasis in cancer.14, 15, 16, 17, 18 Further validation by quantitative real‐time PCR showed that LAMC2 was significantly upregulated in the PCDH8‐overexpression group (P < .05) (Figure 4C). Exploration of correlations between LAMC2 expression and survival in GC patients in the GEPIA database12 showed that high expression of LAMC2 was significantly associated with shorter DFS (P = .014) and tended to be associated with shorter OS (P = .44; Figure 4D). Detection of protein expression by immunohistochemistry in liver metastatic lesions of nude mice showed that LAMC2 was upregulated in the PCDH8‐overexpression group compared to the NC group (Figure 4E).
Figure 4.
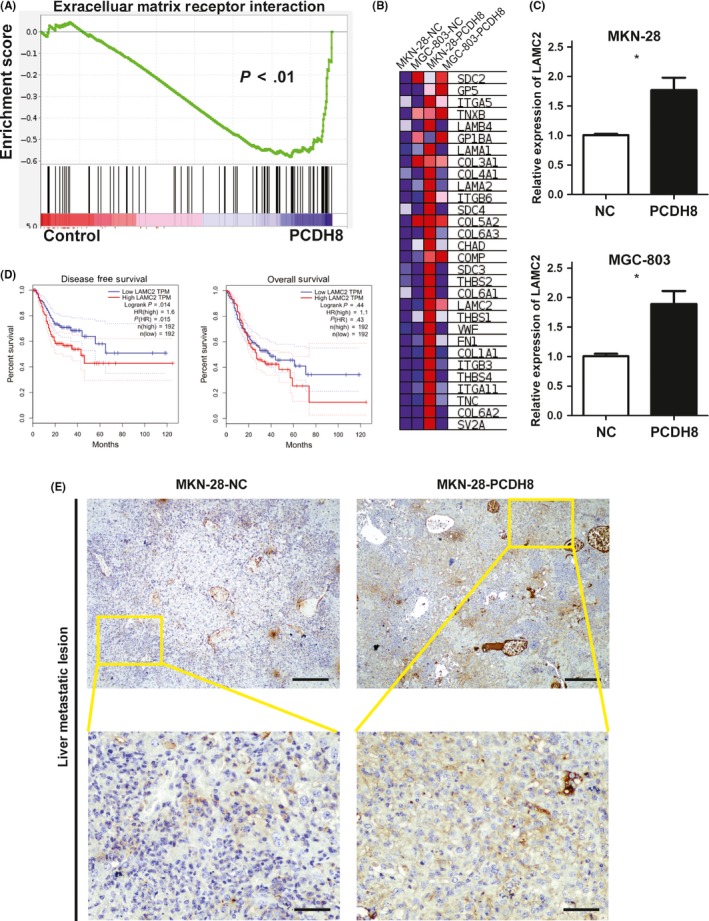
Identification of extracellular matrix receptor interaction pathway as a possible mechanism of protocadherin‐8 (PCDH8) in gastric cancer. A, Enrichment plot of extracellular matrix receptor interaction pathway regulated by PCDH8 overexpression (P < .01) by gene set enrichment analysis. B, Heat map of genes in extracellular matrix receptor interaction pathway that showed core enrichment with PCDH8 overexpression. C, Relative expression of LAMC2 was significantly upregulated with PCDH8 overexpression in MKN28 and MGC803 cells. D, High expression of LAMC2 was significantly correlated with a shorter disease free survival (left) and tended to correlated with shorter overall survival (right) in the GEPIA cohort. P‐values by log‐rank test are displayed. E, Protein expression of LAMC2 was upregulated when PCDH8 was overexpressed in liver metastatic lesions of nude mice. Upper panel, scale bar, 200 μm; lower panel, scale bar, 50 μm. Values are mean ± SD of 3 replicates. *P < .05; **P < .01; ***P < .001
4. DISCUSSION
Recurrence and metastasis are crucial factors of cancer‐related death. Understanding the underlying molecular mechanisms in the process of recurrence and metastasis is very important.
Previous studies have already found that many signals and molecules are implicated in the process of GC recurrence and metastasis, the most notable being members of the cadherin family.1 The cadherin family includes well‐known molecules such as CDH1, the loss of which underlies the pathogenesis of hereditary diffuse GC.1, 19 Members of the cadherin superfamily, including protocadherins and cadherin‐related proteins, have also been widely implicated in the recurrence and metastasis of cancer.1 Members of the protocadherin family demonstrated various functions from tumor suppression to tumor promotion.1 PCDH8 was found to be epigenetically silenced in various cancers.3, 4, 5, 6, 7 Zhang and colleagues restored PCDH8 expression by treating cells with 5‐aza‐2′‐deoxycytidine, a reagent that causes demethylation, and found proliferation and migration of cells to be suppressed.7 However, we should not ignore the fact that demethylation by 5‐aza‐2′‐deoxycytidine was not gene specific such that not only PCDH8 but also many other genes were demethylated. Therefore, the influence on GC cancer cells may be caused by the joint force of multiple genes rather than the function of PCDH8 alone. In addition, a recent study found that microRNA‐429 reversely regulated PCDH8, and its inhibition leads to upregulation of PCDH8 expression and an increase in the motility of cancer cells, suggesting a pro‐metastasis role of PCDH8.8
In this study, using the GC data from the GEPIA and Kaplan–Meier plotter databases, we found that high expression of PCDH8 in cancer tissues was significantly correlated with poor prognosis. In our FUSCC cohort, although no significant correlation was found, there was a consistent tendency towards high PCDH8 expression being correlated with shorter PFS and OS and lower 3‐year PFS rate and 3‐year OS rate. The negative association we found may be caused by the relatively small sample size and the short follow‐up of our cohort. Functional studies have found that overexpression of PCDH8 remarkably promoted invasion and migration in vitro as well as metastasis in vivo, and knockdown of PCDH8 inhibited invasion and migration in vitro. Overexpression of PCDH8 induced a significant enrichment in the ECM receptor interaction pathway, with expression of LAMC2 being significantly upregulated.
Extracellular matrix, a vital component of the tumor microenvironment, has an important influence on tumor formation and invasion.20 Aberrant expression of the composition of ECM could lead to tumor formation and spread.20 LAMC2 encodes the laminin γ chain of laminin‐332, an adhesive component of epithelial basement membranes.14 Previous studies have found that LAMC2 is significantly upregulated and promotes the ability of invasion and metastasis in several carcinoma cell lines, probably through induction of the EMT or epidermal growth factor receptor pathways.15, 16, 17, 18 In addition, high expression of LAMC2 in tumor tissues is significantly correlated with poorer prognosis in lung adenocarcinoma.16 Regarding GC, LAMC2 was found to be overexpressed at the invading fronts,21 and its expression was considerably higher in cancerous tissue than in corresponding normal tissues.22 Our study found that high expression of LAMC2 was significantly associated with shorter DFS in GC in GEPIA cohort, which was consistent with results in lung adenocarcinoma. The downstream mechanism of LAMC2‐induced motility of GC cells remains unknown, but research has found that LAMC2 was an important mediator in Wnt5a‐induced invasion of GC cells.23 This study found that expression of LAMC2 was significantly upregulated when PCDH8 was overexpressed, suggesting that PCDH8 might function through LAMC2 to promote invasion and migration of GC.
In conclusion, for the first time, to our knowledge, our study found that PCDH8 promoted invasion and metastasis of GC by enriching the pathway of interaction with ECM receptors, probably through upregulation of LAMC2. High expression of PCDH8 was significantly correlated with poor prognosis, and could potentially be used as a biomarker in clinical practice.
Supporting information
ACKNOWLEDGMENTS
We thank Dr Menghong Sun (chief of the Tissue Bank at Fudan University Shanghai Cancer Center) for sample preservation and acquisition, and Dr Shenglin Huang (professor at Fudan University Shagnhai Cancer Center and Institutes of Biomedical Sciences, Shanghai Medical School, Fudan University) for advice in the analysis of RNA‐sequencing data. We also thank Mary Smith (PhD) for language editing.
Lin Y, Ge X, Zhang X, et al. Protocadherin‐8 promotes invasion and metastasis via laminin subunit γ2 in gastric cancer. Cancer Sci. 2018;109:732–740. https://doi.org/10.1111/cas.13502
Funding information
National Science and Technology Major Projects of China (Grant No.: 2012ZX09303‐018‐002).
Ying Lin, Xiaoxiao Ge and Xiaofei Zhang contributed equally to this work.
REFERENCES
- 1. van Roy F. Beyond E‐cadherin: roles of other cadherin superfamily members in cancer. Nat Rev Cancer. 2014;14:121‐134. [DOI] [PubMed] [Google Scholar]
- 2. Zhao Y, Yang Y, Trovik J, et al. A novel wnt regulatory axis in endometrioid endometrial cancer. Cancer Res. 2014;74:5103‐5117. [DOI] [PubMed] [Google Scholar]
- 3. He D, Zeng Q, Ren G, et al. Protocadherin8 is a functional tumor suppressor frequently inactivated by promoter methylation in nasopharyngeal carcinoma. Eur J Cancer Prev. 2012;21:569‐575. [DOI] [PubMed] [Google Scholar]
- 4. Lin YL, Wang YL, Fu XL, Ma JG. Aberrant methylation of PCDH8 is a potential prognostic biomarker for patients with clear cell renal cell carcinoma. Med Sci Monit. 2014;20:2380‐2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lin YL, Wang YL, Ma JG, Li WP. Clinical significance of protocadherin 8 (PCDH8) promoter methylation in non‐muscle invasive bladder cancer. J Exp Clin Cancer Res. 2014;33:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yu JS, Koujak S, Nagase S, et al. PCDH8, the human homolog of PAPC, is a candidate tumor suppressor of breast cancer. Oncogene. 2008;27:4657‐4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang D, Zhao W, Liao X, Bi T, Li H, Che X. Frequent silencing of protocadherin 8 by promoter methylation, a candidate tumor suppressor for human gastric cancer. Oncol Rep. 2012;28:1785‐1791. [DOI] [PubMed] [Google Scholar]
- 8. Li Z, Gou J, Jia J, Zhao X. MicroRNA‐429 functions as a regulator of epithelial‐mesenchymal transition by targeting Pcdh8 during murine embryo implantation. Hum Reprod. 2015;30:507‐518. [DOI] [PubMed] [Google Scholar]
- 9. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87‐108. [DOI] [PubMed] [Google Scholar]
- 10. Wu Z, Zhang Z, Ge X, et al. Identification of short‐form RON as a novel intrinsic resistance mechanism for anti‐MET therapy in MET‐positive gastric cancer. Oncotarget. 2015;6:40519‐40534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu X, Ge X, Zhang Z, et al. MicroRNA‐940 promotes tumor cell invasion and metastasis by downregulating ZNF24 in gastric cancer. Oncotarget. 2015;6:25418‐25428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017; 3 July;45(web server issue):W98‐W102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Szasz AM, Lanczky A, Nagy A, et al. Cross‐validation of survival associated biomarkers in gastric cancer using transcriptomic data of 1,065 patients. Oncotarget. 2016;7:49322‐49333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Garg M, Braunstein G, Koeffler HP. LAMC2 as a therapeutic target for cancers. Expert Opin Ther Targets. 2014;18:979‐982. [DOI] [PubMed] [Google Scholar]
- 15. Garg M, Kanojia D, Okamoto R, et al. Laminin‐5gamma‐2 (LAMC2) is highly expressed in anaplastic thyroid carcinoma and is associated with tumor progression, migration, and invasion by modulating signaling of EGFR. J Clin Endocrinol Metab. 2014;99:E62‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moon YW, Rao G, Kim JJ, et al. LAMC2 enhances the metastatic potential of lung adenocarcinoma. Cell Death Diff. 2015;22:1341‐1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tsubota Y, Ogawa T, Oyanagi J, Nagashima Y, Miyazaki K. Expression of laminin gamma2 chain monomer enhances invasive growth of human carcinoma cells in vivo. Int J Cancer. 2010;127:2031‐2041. [DOI] [PubMed] [Google Scholar]
- 18. Zboralski D, Warscheid B, Klein‐Scory S, et al. Uncoupled responses of Smad4‐deficient cancer cells to TNFalpha result in secretion of monomeric laminin‐gamma2. Mol Cancer. 2010;9:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lin Y, Wu Z, Guo W, Li J. Gene mutations in gastric cancer: a review of recent next‐generation sequencing studies. Tumour Biol. 2015;36:7385‐7394. [DOI] [PubMed] [Google Scholar]
- 20. Martin M, Wei H, Lu T. Targeting microenvironment in cancer therapeutics. Oncotarget. 2016;7:52575‐52583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Koshikawa N, Moriyama K, Takamura H, et al. Overexpression of laminin gamma2 chain monomer in invading gastric carcinoma cells. Cancer Res. 1999;59:5596‐5601. [PubMed] [Google Scholar]
- 22. Kwon OH, Park JL, Kim M, et al. Aberrant up‐regulation of LAMB3 and LAMC2 by promoter demethylation in gastric cancer. Biochem Biophys Res Comm. 2011;406:539‐545. [DOI] [PubMed] [Google Scholar]
- 23. Yamamoto H, Kitadai Y, Yamamoto H, et al. Laminin gamma2 mediates Wnt5a‐induced invasion of gastric cancer cells. Gastroenterol. 2009;137:242‐252,252 e1‐6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


