Abstract
Cancer immunotherapy with human γδ T cells expressing Vγ2Vδ2 T cell receptor (also termed Vγ9Vδ2) has shown promise because of their ability to recognize and kill most types of tumors in a major histocombatibility complex (MHC) ‐unrestricted fashion that is independent of the number of tumor mutations. In clinical trials, adoptive transfer of Vγ2Vδ2 T cells has been shown to be safe and does not require preconditioning. In this report, we describe a method for preparing highly enriched human Vγ2Vδ2 T cells using the bisphosphonate prodrug, tetrakis‐pivaloyloxymethyl 2‐(thiazole‐2‐ylamino)ethylidene‐1,1‐bisphosphonate (PTA). PTA stimulated the expansion of Vγ2Vδ2 cells to purities up to 99%. These levels were consistently higher than those observed after expansion with zoledronic acid, the most commonly used stimulator for clinical trials. Cell numbers also averaged more than those obtained with zoledronic acid and the expanded Vγ2Vδ2 cells exhibited high cytotoxicity against tumor cells. The high purity of Vγ2Vδ2 cells expanded by PTA increased engraftment success in immunodeficient NOG mice. Even low levels of contaminating αβ T cells resulted in some mice with circulating human αβ T cells rather than Vγ2Vδ2 cells. Vγ2Vδ2 cells from engrafted NOG mice upregulated CD25 and secreted tumor necrosis factor‐α and interferon‐γ in response to PTA‐treated tumor cells. Thus, PTA expands Vγ2Vδ2 T cells to higher purity than zoledronic acid. The high purities allow the successful engraftment of immunodeficient mice without further purification and may speed up the development of allogeneic Vγ2Vδ2 T cell therapies derived from HLA‐matched normal donors for patients with poor autologous Vγ2Vδ2 T cell responses.
Keywords: adoptive cancer immunotherapy, bisphosphonate, farnesyl diphosphate synthase, Vγ2Vδ2 T cells, zoledronic acid
1. INTRODUCTION
Recent advances in cancer immunotherapy have revolutionized treatment for a number of cancers. By targeting checkpoint receptors, durable remissions have been achieved in patients with advanced metastatic melanoma, non‐small cell lung cancer (NSCLC), bladder cancer and kidney cancer that would have had little chance of survival with conventional chemotherapies or targeted therapies. Similarly, chimeric antigen receptor‐T cells (CAR‐T) bearing receptors specific for CD19 have successfully treated patients with relapsing B‐cell acute lymphoblastic leukemia and diffuse large B‐cell lymphoma. However, both these treatments have limitations. Checkpoint blockade targeting PD‐1 or its ligand, PD‐L1, provides clinical benefits for a minority of the patients; approximately 30% for melanoma and kidney cancer and approximately 20% for lung cancer.1, 2 Moreover, the effectiveness of checkpoint blockade correlates with the numbers of nonsynonymous mutations present in the tumors from patients with NSCLC3 and melanoma.4 Thus, patients with cancers that have low numbers of mutations, such as many of the pediatric cancers and glioblastomas,5 would be predicted to respond infrequently as has been observed.6 CAR‐T therapy is limited because there are few clearly defined tumor‐specific antigens on solid tumors.7 Therefore, additional types of immunotherapy are needed to realize the full potential of cancer immunotherapy.
Adoptive immunotherapy with Vγ2Vδ2 T cells (also termed Vγ9Vδ2) is a potential therapy for a variety of cancers and is independent of the mutational status of the tumor. Stimulation of Vγ2Vδ2 T cells is not dependent on peptides presented by MHC proteins and is, therefore, major histocombatibility complex (MHC) ‐unrestricted.8 Instead, Vγ2Vδ2 T cells respond to the presence of small isoprenoid metabolites, such as self isopentenyl pyrophosphate (IPP)9 or foreign microbial (E)‐4‐hydroxy‐3‐methylbut‐2‐enyl pyrophosphate (HMBPP),10, 11 in a process requiring the butyrophilin 3A1 (BTN3A1) protein, an immunoglobulin superfamily protein present on all normal and tumor cells.12, 13, 14 The isoprenoid metabolites bind to the B30.2 intracellular domains of BTN3A1,15, 16 which alters the cell through an unknown process to allow the Vγ2Vδ2 TCR17 to recognize this intracellular binding. TCR recognition leads to the activation of Vγ2Vδ2 T cells for cytotoxicity and cytokine secretion.
In 9 clinical trials involving a total of 213 patients, adoptive transfer of Vγ2Vδ2 T cells18, 19, 20, 21, 22, 23, 24, 25, 26 has proven to be safe,20, 21 does not require pretreatment with cytotoxic agents, and has resulted in a durable remission in a patient with metastatic clear cell renal cancer,27 complete and partial responses in patients with breast and cervical cancer,20 and stable disease in 50% of patients with advanced NSCLC.28 However, most patients progressed, underscoring the need for improvements in the efficacy of this therapy.
The most successful adoptive transfer γδ trials gave the nitrogen‐containing bisphosphonate zoledronic acid (Zol) prior to the transfer of the cells.20, 21 Bisphosphonates indirectly stimulate Vγ2Vδ2 T cells by inhibiting farnesyl diphosphate synthase (FDPS), resulting in the subsequent intracellular accumulation of its substrate, isopentenyl pyrophosphate (IPP), which is bound by BTN3A1.29, 30
Bisphosphonates enter cells through fluid‐phase endocytosis and uptake can be enhanced by Ca2+, suggesting that the negatively charged P‐C‐P structure limits entry.31 To improve cellular uptake and activity, we recently synthesized a bisphosphonate prodrug, tetrakis‐pivaloyloxymethyl 2‐(thiazole‐2‐ylamino)ethylidene‐1,1‐bisphosphonate (PTA), where these negative charges are masked with pivoxil esters.32, 33 PTA is highly hydrophobic, allowing its efficient entry into cells where intracellular esterases convert it to its active acid form that blocks FDPS. PTA is a highly potent inhibitor of tumor cell proliferation that is 796‐fold more potent against hematopoietic tumors and 27‐fold more potent against solid tumors than Zol (the most potent bisphosphonate in current use).32 Similarly, PTA efficiently stimulates Vγ2Vδ2 T cells to secrete TNF‐α with 75 different tumor cell lines, being 903‐fold more potent on average than Zol.33
In this study, we examine the effect of the PTA bisphosphonate prodrug on the expansion of peripheral blood Vγ2Vδ2 T cells ex vivo from patients with prostate cancer and breast cancer and analyze the engraftment success and effector functions of the expanded Vγ2Vδ2 T cells after adoptive transfer to immunodeficient NOG mice.
2. MATERIALS AND METHODS
2.1. Reagents
Tetrakis‐pivaloyloxymethyl 2‐(thiazole‐2‐ylamino)ethylidene‐1,1‐bisphosphonate (PTA) and 2‐(thiazole‐2‐ylamino)ethylidene‐1,1‐bisphosphonate (TA) were synthesized as described.32 Zoledronic acid (Zol) was purchased from Novartis AG (Basel, Switzerland). (E)‐4‐hydroxy‐3‐methylbut‐2‐enyl pyrophosphate (HMBPP) was synthesized as described.34
2.2. Solubilization of PTA
PTA (10 μmoles) was dissolved in 10 mL of either DMSO or ethanol containing 10 mM of trimethyl β‐cyclodextrin (TMβCD). TMβCD, a cyclic compound composed of 7 trimehtyl‐D‐glucopyranoside units linked α−1−>4, was synthesized as described.35
2.3. Expansion of Vγ2Vδ2 T cells
Blood was obtained from healthy adult donors and prostate and breast cancer patients following approval by the institutional review board of Kyoto University and the Tokyo Women's Medical University and written informed consent being provided by patients. Patients' characteristics are summarized for prostate cancer patients in Table S1 and for breast cancer patients in Table S2. Peripheral blood mononuclear cells (PBMC) were purified by Ficoll‐Paque PLUS (GE Healthcare Bio‐Sciences AB, Uppsala, Sweden) gradient centrifugation. The cells were washed 2 times with PBS, then resuspended in modified Yssel's medium supplemented with 10% human AB serum (Cosmobio, Koto‐ku, Tokyo, Japan) or enriched RPMI 1640 medium.36 They were cultured at 2.5 × 106 cells/1.5 mL/well in medium with either 1 μM PTA, 5 μM TA or 5 μM ZOL in the presence of 100 U/mL interleukin‐2 (IL‐2) (Shionogi Pharmaceutical, Chuo‐ku, Osaka, Japan) in 4 wells of a 24‐well plate (Corning Incorporated, Corning, NY). The culture medium was replaced every day from day 2 to day 9 with fresh medium containing IL‐2. On day 10 for Yssel's media or on day 14 for RPMI 1640 media, the cells were harvested. Purity of the Vγ2Vδ2 T cells were assessed by flow cytometry and the cells were either directly transferred or frozen in liquid nitrogen for later use.
2.4. Statistical analysis
Statistical analyses were performed in GraphPad Prism v7.0A using the non‐parametric 2‐tailed Wilcoxon signed rank test for paired samples. P‐values are as given with values <.05 considered significant.
2.5. Cell aggregation assay
Cell aggregation during the culture was recorded under a microscope (Eclipse TS100, Nikon, Minato‐ku, Japan) equipped with a digital camera (CoolPIX L20, Nikon).
2.6. Flow cytometric analysis
Peripheral blood mononuclear cells before and after expansion were plated out at 2 × 105 cells/50 μL in a 96‐well plate (Corning Incorporated, Corning, NY). The cells were then treated with 3 μL of FITC‐conjugated anti‐TCR Vδ2 mAb (clone IMMU 389, Beckman Coulter, Flullerton, CA, USA) and phycoerythrin (PE)‐conjugated anti‐CD3 mAb (clone SK7, BD Biosciences, San Diego, CA, USA) on ice for 30 min. After being washed 3 times with PBS, the cells were resuspended in 200 μL of 1% paraformaldehyde in PBS and analyzed using a FACSCalibur or an LSR II flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA).
2.7. TNF‐α production by peripheral blood mononuclear cells in response to bisphosphonates
Peripheral blood mononuclear cells derived from a healthy adult donor were cultured for 24 h at 2.5 × 106 cells/1.5 mL in modified Yssel's medium with 1 μM PTA, 5 μM TA or 5 μM Zol in the presence of 100 U/mL IL‐2 in a 24‐well plate. The culture medium was removed and the content of TNF‐α was determined using the standard ELISA according to the manufacturer's protocol (Peprotech, Rocky Hill, NJ, USA).
2.8. Cytotoxicity assay
Peripheral blood mononuclear cells from 2 healthy adult volunteers were stimulated with 1 μM PTA and IL‐2. On day 11, the proportion of Vγ2Vδ2 T cells among CD3 T cells was confirmed to be greater than 99%. Vγ2Vδ2 T cell‐mediated cellular cytotoxicty against tumor cells was determined using a non‐radioactive cellular cytotoxicity assay according to the manufacturer's protocol (Techno Suzuta, Heiwa‐machi, Nagasaki, Japan). In brief, RPMI8226 (a plasmacytoma cell line that directly stimulates Vγ2Vδ2 T cells), K562 (an erythroleukemic cell line that is sensitive to killing by natural killer (NK) cells and to NK‐like killing by T cells) and PTA‐pretreated U937 (a monocyte‐like histiocytic lymphoma cell line) were incubated with the europium‐chelate‐forming compound, bis(butyryloxymethyl) 4′‐hydroxymethyl‐2,2′:6′,2″‐terpyridine‐6,6″‐dicarboxylate, for 15 min at 37°C.37 After being washed with RPMI 1640 medium, the tumor cells were incubated with PTA‐expanded Vγ2Vδ2 T cells at effector to target ratios of 0, 0.625, 1.25, 2.5, 6, 10, 20 and 40. The cells were centrifuged and after 40 min, the culture supernatants harvested and mixed with a europium solution to form a europium‐chelate complex with released labeling compound. Time‐resolved europium fluorescence was measured on a PHERAStar FS multiplate reader (BMG LABTECH GmbH, Allmendgruen, Ortenberg, Germany) or a Berthold multiplate reader (Berthold Technologies GmbH, KG, Bad Wildbad, Germany).
2.9. Adoptive transfer of Vγ2Vδ2 T cells into immunodeficient NOG mice
Peripheral blood mononuclear cells derived from healthy donors or prostate cancer patients were stimulated with 1 μM of PTA and IL‐2 as detailed above and harvested on day 10 for direct transfer (for healthy donors) or frozen for later use (for cancer patients). Vγ2Vδ2 T cells constituted >98% of lymphocytes. Vγ2Vδ2 T cells from healthy donors were transferred directly, whereas Vγ2Vδ2 T cells from cancer patients were thawed, washed and then transferred. For both types of donors, 5 × 107 Vγ2Vδ2 T cells were i.p. injected into immunodeficient NOD.Cg‐Prkdc scid I12rg tm1Sug/Jic (NOG) mice (Central Institute for Experimental Animals, Kawasaki, Kanagawa, Japan) that were maintained under specific‐pathogen‐free conditions. Fourteen or fifteen days later, peripheral blood was obtained and the red blood cells (RBC) were lysed with 1 mL of ACK lysis buffer (8.024 g of NH4Cl, 1.001 g of KHCO3 and 3.722 mg of EDTA/Na2/2H2O in 1000 mL of Milli‐Q water). After washing with PBS/2% FCS, the resulting cells were stained with FITC‐conjugated anti‐Vδ2 TCR and PE‐conjugated anti‐human CD3 mAb. Animal use was approved by the institutional review board of Kyoto University Medical School. All experiments were performed in accordance with the relevant guidelines and regulations of Kyoto University Medical School.
2.10. TNF‐α production by adoptively transferred Vγ2Vδ2 T cells in vitro
NOG mice were i.p. injected with 5 × 107 Vγ2Vδ2 T cells from a breast cancer patient (BC21). Two weeks later peripheral blood was taken from the mice, RBC were lysed with ACK buffer, and the resulting peripheral blood cells were stimulated with 1 μM of HMBPP at a cell concentration of 1 × 105 cells/100 μL in vitro. After 24 h, the culture supernatants were harvested and TNF‐α levels were determined in triplicate by ELISA (Peprotech, Rocky Hill, NJ, USA).
2.11. CD25 expression on adoptively transferred Vγ2Vδ2 T cells stimulated in vivo
NOG mice were i.p. injected with 1 × 106 EJ‐1 cells. Four weeks later the mice were i.p. injected with 1 × 107 Vγ2Vδ2 T cells and 1 μg of PTA. Vγ2Vδ2 T cells were harvested 24 h after the injection and stained with PE‐conjugated anti‐human CD25 mAb (clone BC96, Biolegend, San Diego, CA, USA).
2.12. Determination of IFN‐γ mRNA in adoptively transferred Vγ2Vδ2 T cells stimulated in vivo
NOG mice were i.p. injected with 1 × 106 EJ‐1 cells. Four weeks later, the mice were i.p. injected with 1 × 107 Vγ2Vδ2 T cells with or without 1 μg of PTA. Peripheral blood was taken from the mice 4 h and 24 h after the challenge, and human IFN‐γ mRNA levels measured in the harvested cells by quantitative PCR. Total RNA was purified using an RNeasy Mini Kit (Qiagen, Valencia, CA, USA) and complementary DNA was synthesized from 1 μg of total RNA using SuperScript III reverse transcriptase kit and random hexamers (Invitrogen, Carlsbad, CA, USA). Then, RT‐PCR was performed using a 0.2 μM concentration of both forward and reverse primers and iTaq SYBR Green Supermix with ROX (Bio Rad, Hercules, CA, USA). The quantity of each cDNA was normalized by GAPDH. The following PCR primers were used for amplification: IFN‐γ, 5′‐ TGACCAGAGCATCCAAAAGA‐3′ and 5′‐CTCTTCGACCTCGAAACAGC‐3′; GAPDH, 5′‐ CGACCACTTTGTCAAGCTCA ‐3′ and 5′‐AGGGGAGATTCAGTGTGGTG ‐3′.
2.13. IFN‐γ production by adoptively transferred Vγ2Vδ2 T cells stimulated in vivo
NOG mice were i.p. injected with 1 × 106 EJ‐1 cells. Four weeks later the mice were further i.p. injected with 1 × 107 Vγ2Vδ2 T cells with or without PTA. Peripheral blood was taken from the mice 4 h and 24 h after the injection, serum was prepared, and serum IFN‐γ levels were measured by ELISA (Peprotech, Rocky Hill, NJ).
3. RESULTS
3.1. Delivery of PTA in an inclusion complex with trimethyl β‐cyclodextrin
We recently described a new thiazole bisphosphonate prodrug, PTA, that is highly active relative to its acid form, TA, and to Zol (the most potent bisphosphonate in clinical use) (structures shown in Figure 1A).32, 33 PTA is highly hydrophobic and is insoluble in water or ethanol. Although PTA can be dissolved in DMSO for research use, few drugs solubilized in DMSO have been approved for use in patients. Therefore, we tested a number of nonionic detergents for their ability to solubilize PTA in ethanol. A trimethyl derivative of β‐cyclodextrin (TMβCD) had the best ability to solubilize PTA in ethanol (Figure S1). Cyclodextrin compounds can form inclusion complexes with drugs where hydrophobic drugs bind to their central hydrophobic cavity and where the polar surface of cyclodextrin makes the complex soluble in ethanol.38, 39 PTA could be dissolved up to a concentration of 1 mM in ethanol containing 10 mM of TMβCD. Importantly, PTA solubilized in TMβCD/ethanol exhibited identical bioactivity to PTA solubilized in DMSO. For example, PTA in TMβCD/ethanol inhibited the proliferation of the EJ‐1 bladder carcinoma and the U937 histocytoma cell lines identically to PTA in DMSO (Figure S1C,D). Thus, TMβCD/ethanol can be used as the solvent for PTA, as was done in this study.
Figure 1.

Structure and immunological activity of bisphosphonates. A, Structure of the bisphosphonate prodrug, PTA, its active acid form, TA, and zoledronic acid (Zol). B, Effect of bisphosphonates on the aggregation of Vγ2Vδ2 T cells in peripheral blood mononuclear cells (PBMC). PBMC from a healthy donor where Vδ2 T cells constituted 20.2% of total T cells, were exposed to varying concentrations of the bisphosphonates for 2 days, and cell aggregation was monitored under a microscope. C, TNF‐α production by PBMC in response to bisphosphonates. After incubation of PBMC with bisphosphonates for 2 days, supernatants were harvested and TNF‐α levels measured by ELISA
3.2. Stimulation of Vγ2Vδ2 T cells by PTA
PTA potently inhibits the growth of tumor cell lines in vitro32 and selectively activates Vγ2Vδ2 T cells in PBMC to proliferate, secrete cytokines and kill tumor cells.33 Consistent with these results, when we compared the activity of Zol to the PTA prodrug and its TA acid form, PTA was strongly active. Zol and TA stimulated Vγ2Vδ2 T cell‐dependent cell aggregation at concentrations of approximately 10 μM, whereas PTA caused aggregation at approximately 0.1 μM (Figure 1B). Similarly, PTA stimulated TNF‐α production by Vγ2Vδ2 T cells at an EC50% of 3 nM, whereas TA and Zol stimulated at an EC50% of 3000 nM (Figure 1C). Thus, PTA was 1000‐fold more potent than TA and Zol in stimulating Vγ2Vδ2 T cells in PBMC.
3.3. Ex vivo expansion by PTA of Vγ2Vδ2 T cells for adoptive immunotherapy
To determine whether PTA can be used to expand Vγ2Vδ2 T cells for adoptive immunotherapy, PMBC from cancer patients and from healthy donors were stimulated with PTA and IL‐2 and the resulting Vγ2Vδ2 T cells were assessed for purity and biological activity after 10 days. Similar to what we have previously shown for Zol,36 PTA stimulated maximum expansion within a relatively narrow dose range when PBMC were continuously exposed to PTA as it was slowly diluted (Figure 2). Thus, Vγ2Vδ2 T cell expansion was maximal at a PTA concentration of 1 μM, while a PTA concentration of 2 μM was highly toxic (Figure 2A). PTA stimulation resulted in highly enriched populations of Vγ2Vδ2 T cells that approached 100% of CD3 T cells, whereas Zol stimulation was consistently less (Figure 2A,B). To determine the effect of PTA on Vγ2Vδ2 T cells from cancer patients, PBMC from 8 cancer patients with prostate or breast cancer were stimulated with either PTA or Zol (Figure 3). PTA stimulation resulted in significantly higher enrichment of the Vγ2Vδ2 T cell population as compared with Zol stimulation (95.6 ± 5.7% of total cells vs 90.3 ± 10.0%, P = .0078, Figure 3A). All donors exhibited higher enrichment with PTA compared to Zol. In addition, the number of Vγ2Vδ2 T cells was 20% higher on average for stimulation with PTA as compared with Zol (6.3 × 108 ± 5.5 × 108 cells with PTA vs 5.7 × 108 ± 4.3 × 108 cells with Zol, P = .38, Figure 3B), although this was observed for only 6 out of 8 patients and was not statistically significant. The increase in Vγ2Vδ2 cell numbers varied widely ranging from 959 to 28 722‐fold (6344‐fold for PTA vs 4302‐fold for Zol, P = .31) and was not significantly different (Figure 3C). Examples of Vγ2Vδ2 T cell expansion in response to PTA are shown for prostate cancer patients (Figure 4) and healthy donors (Figure S2). Thus, ex vivo PTA stimulation of Vγ2Vδ2 T cells in PBMC from cancer patients results in Vγ2Vδ2 T cell populations that were of significantly higher purity and slightly higher number than when Zol was used for stimulation.
Figure 2.

Expansion of Vγ2Vδ2 T cells by PTA or Zol. PBMC derived from a (A) healthy adult donor or a (B) breast cancer patient (BC37B) were stimulated either by PTA or Zol with interleukin‐2 (IL‐2) in either enriched RPMI 1640 (panel A) or Yssel's media (panel B) for 10 days and then analyzed by flow cytometry
Figure 3.
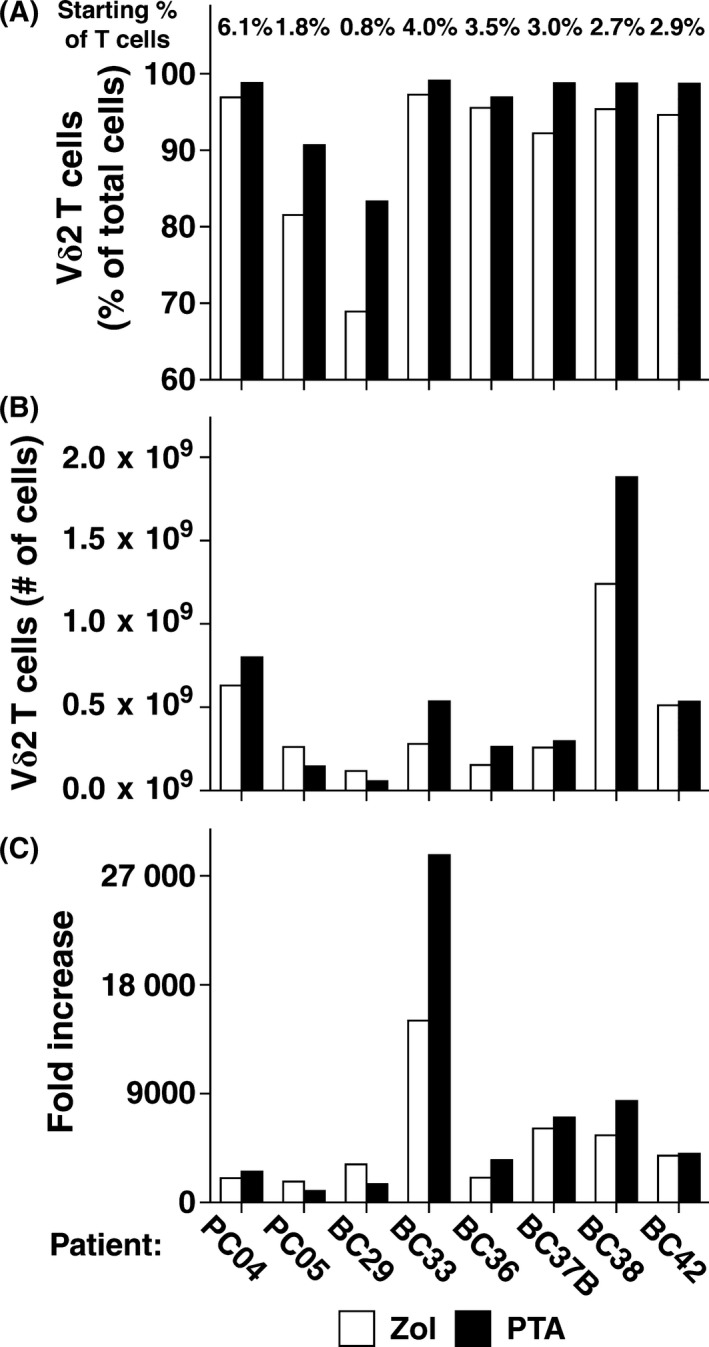
Expansion of Vγ2Vδ2 T cells by PTA increases their purity and number compared with expansion by Zol. Vγ2Vδ2 T cells were expanded from peripheral blood mononuclear cells (PBMC) derived from patients with prostate or breast cancer by either PTA or Zol. PBMC were cultured with PTA or Zol in the presence of interleukin‐2 (IL‐2) for 10 days and then analyzed before and after by flow cytometry. A, Purity of Vγ2Vδ2 T cells expanded by PTA or Zol. Vγ2Vδ2 T cells averaged 5.3% higher purity (ranging from 1.4 to 14.4%) (95.6 ± 5.7% of total cells for PTA vs 90.3 ± 10.0% for Zol, P = .0078). The starting percentage of Vγ2Vδ2 T cells of total T cells is listed for each patient at the top of the panel. The mean ± SD was 3.1 ± 1.6% for the patients versus 2.9 ± 3.9% for normal adults as determined in an earlier study.49 B, Number of Vγ2Vδ2 T cells expanded by PTA or Zol. Cell numbers averaged 30% higher with PTA compared to Zol. Vγ2Vδ2 T cells expanded on average to 5.6 × 108 ± 5.8 × 108 cells with PTA versus 4.3 × 108 ± 3.7 × 108 cells with Zol (P = .20, not significant). (c) Fold increase of Vγ2Vδ2 T cells expanded by PTA or Zol. PTA expansion compared to Zol ranged from 0.5 to 1.9‐fold different (7085‐fold for PTA vs 4936‐fold for Zol, P = .25, not significant). BC, breast cancer; PC, prostate cancer
Figure 4.
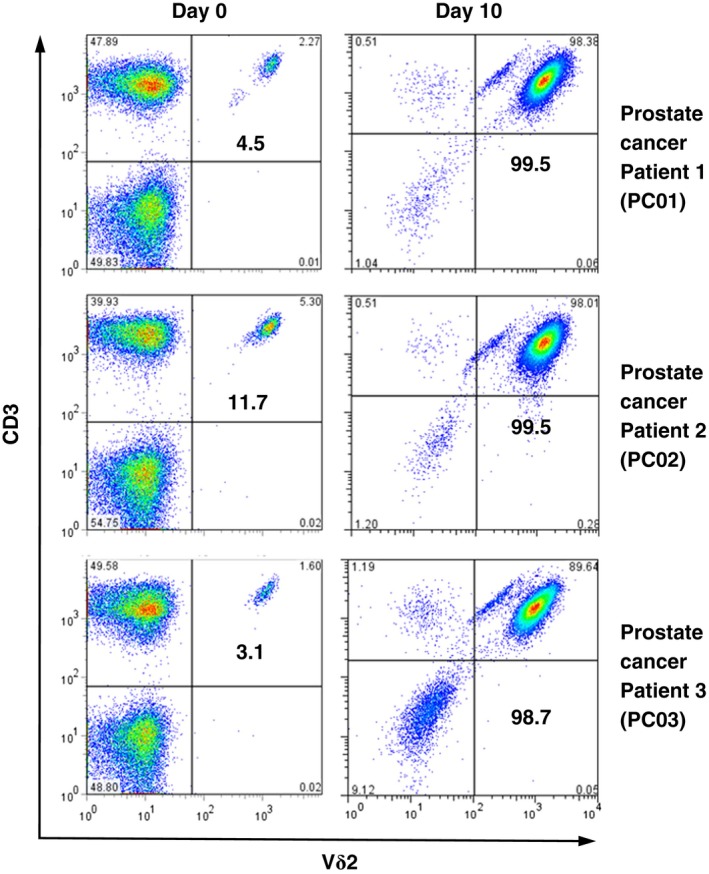
Expansion of Vγ2Vδ2 T cells from prostate cancer patients by PTA leads to high purity. Peripheral blood mononuclear cells (PBMC) isolated from prostate cancer patients were analyzed by flow cytometry before (left panels) and after (right panels) stimulation with PTA and interleukin‐2 (IL‐2) for 10 days. Values shown are % of CD3 T cells. The absolute number of Vδ2 T cells increased from 2.0 × 105 to 2.4 × 108 for PC01, 5.7 × 105 to 6.4 × 108 for PC02, and 1.7 × 105 to 7.6 × 108 for PC03, with the expansion rate being 1175‐fold for PC01, 1118‐fold for PC02, and 4398‐fold for PC03
3.4. Cytotoxic activity of Vγ2Vδ2 T cells expanded by PTA
Adult Vγ2Vδ2 T cells are highly cytotoxic40 and exhibit cytotoxicity for most tumor cell lines.8, 33 Vγ2Vδ2 T cells expanded with PTA were, therefore, assessed for their cytotoxic activity against an NK‐sensitive cell line, a stimulatory plasmacytoma and a tumor cell line treated with PTA. PTA‐expanded Vγ2Vδ2 T cells exhibited strong NK‐like cytotoxic activity efficiently lysing the NK target cell line, K562, which lacks MHC class I (Figure 5A). PTA‐expanded Vγ2Vδ2 T cells also lysed the RPMI 8226 plasmacytoma cell line that is directly stimulatory for Vγ2Vδ2 T cells through a TCR‐dependent mechanism (Figure 5A).17, 41 Finally, PTA‐expanded Vγ2Vδ2 T cells efficiently lysed U937 tumor cells that had been treated with PTA (Figure 5B). Therefore, expansion of Vγ2Vδ2 T cells by PTA preserves their cytotoxic activity.
Figure 5.

Cytotoxic activity of PTA‐expanded Vγ2Vδ2 T cells against tumor cells. A, Cytotoxicity of PTA‐expanded Vγ2Vδ2 T cells against K562 and RPMI 8226 tumor cells. K562 erythroleukemia cells (right panel) and RPMI 8226 plasmacytoma cells (left panel) were labeled with a europium‐chelate‐forming procompound, washed, and then incubated with PTA‐expanded Vγ2Vδ2 T cells at effector to target ratios of 0, 0.625, 1.25, 2.5, 5, 10, 20 and 40 for 40 min at 37°C in a 5% CO 2 incubator. The culture supernatants were combined with an europium solution and the levels of the europium–chelate complex determined by measuring time‐resolved fluorescence. Specific lysis (%) was calculated as [(experimental release − spontaneous release)/(maximum release − spontaneous release)]× 100. All assays were performed in triplicate. B, Cytotoxicity of PTA‐expanded Vγ2Vδ2 T cells against PTA‐pulsed U937 histiocytic lymphoma cells. U937 cells were either not treated (open circles) or treated with 500 nM PTA (solid circles) at 37°C with 5% CO 2 for 2 h followed by labeling and testing for specific lysis as detailed in (A)
3.5. Purity of Vγ2Vδ2 T cells helps to determine their engraftment success after adoptive transfer in NOG mice
To assess their potential use for cancer immunotherapy, Vγ2Vδ2 T cells expanded by PTA were adoptively transferred into immunodeficient NOG mice. Vγ2Vδ2 T cells expanded from prostate cancer patient 2 (PC02) and 4 (PC04) using PTA with IL‐2 (shown in Figure 4) were i.p. injected into immunodeficient NOG mice and continued to circulate 15 days later. Mice receiving cells from prostate cancer patient 2 (PC02) averaged 2.5% Vγ2Vδ2 T cells, whereas mice receiving cells from prostate cancer patient 4 (PC04) averaged 6.4% Vγ2Vδ2 T cells of total cells (Figure 6). Similarly, NOG mice receiving Vγ2Vδ2 T cells derived from a normal donor averaged 18.8% in Experiment 1 and 12.6% in Experiment 2 (Figure S3), whereas breast cancer patients averaged 8.8% for BC36 and 4.8% for BC35 (Figure S4). The higher proportions of Vγ2Vδ2 T cells observed with the normal donor were likely due to the transfer of freshly expanded Vγ2Vδ2 T cells instead of the previously frozen cells used from prostate and breast cancer patients.
Figure 6.

Adoptively transferred human Vγ2Vδ2 T cells from prostate cancer patients persist in the blood of NOG mice. Vγ2Vδ2 T cells (>98%) were expanded from prostate cancer patient 2 (PC02) (left panels) and patient 4 (PC04) (right panels) and frozen for later use. 5 × 107 thawed Vγ2Vδ2 T cells were then adoptively transferred into each NOG mice. Fifteen days after transfer, peripheral blood was obtained. After RBC lysis, the resulting cells were stained and then analyzed by flow cytometry for expression of Vδ2 TCR and human CD3. Values shown are % of total cells. Mean ± SD PC02 2.5% ± 0.9%, n = 3 PC04 6.4% ± 2.4%, n = 3
Transfer of highly enriched Vγ2Vδ2 T cells (>98% of total cells) reduced the proportion of NOG mice with elevated levels of αβ T cells (Figure 7A,B). For example, transfer of Vγ2Vδ2 T cells expanded using Zol from patient BC35 which were 90% pure resulted in only 1 out of 9 NOG mice having Vγ2Vδ2 T cells that constituted greater than 80% of circulating T cells while transfer of Vγ2Vδ2 T cells which were 97% pure resulted in only 1 out of 5 NOG mice (Figure S4). In contrast, transfer of Vγ2Vδ2 T cells with purities >98% resulted in 29 out of 32 NOG mice with Vγ2Vδ2 T cells constituting greater than 80% of circulating T cells (Figure 7A, lower panel). Thus, the high purity of Vγ2Vδ2 T cells expanded using PTA helped to ensure successful engraftment of NOG mice without αβ T cell outgrowth.
Figure 7.
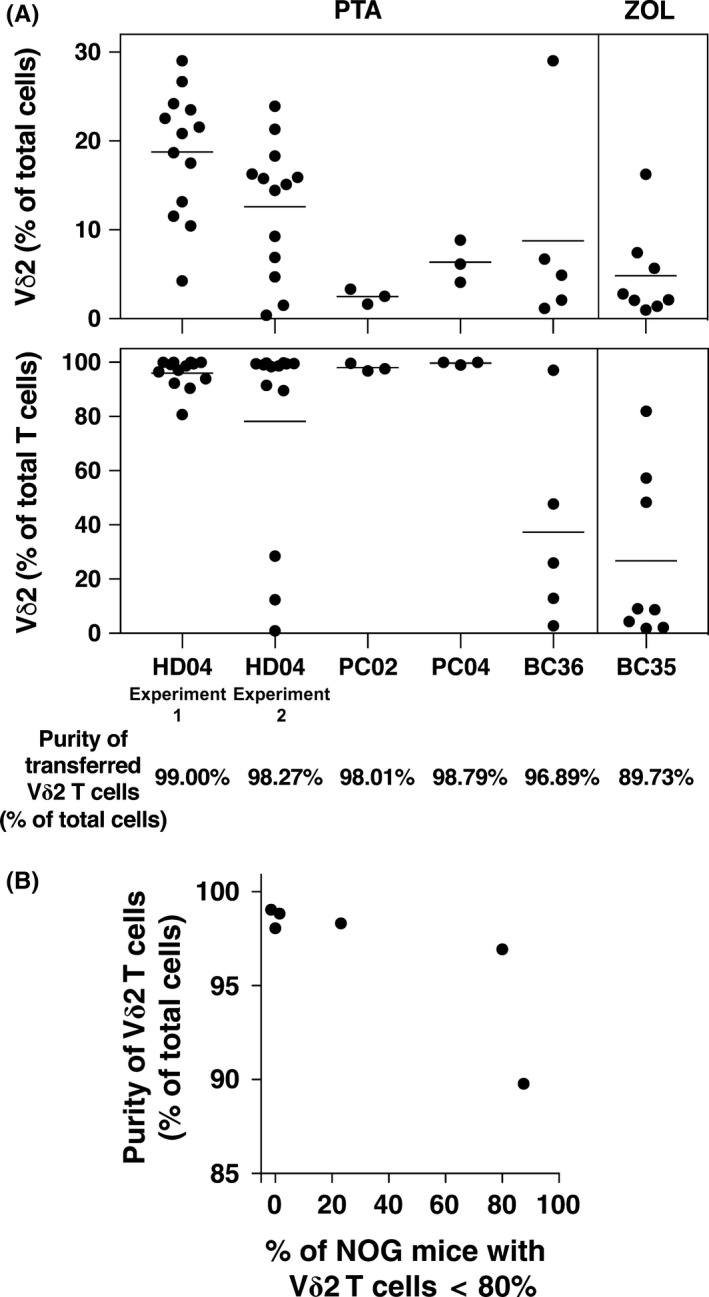
Purity of Vγ2Vδ2 T cells helps to determine their engraftment success after adoptive transfer in NOG mice. A, Summary of the results of NOG mouse engraftment with expanded Vγ2Vδ2 T cells. Top panel: Levels of Vγ2Vδ2 T cells as a percentage of total cells for each transfer experiment. Bottom panel: Levels of Vγ2Vδ2 T cells as a percentage of T cells for each transfer experiment. Each point represents 1 NOG mouse. Vγ2Vδ2 T cells were expanded using PTA except for BC35 that was expanded using Zol. Thawed Vγ2Vδ2 T cells were used for cells derived from cancer patients whereas freshly expanded Vγ2Vδ2 T cells were used for the healthy donor. The purity of the starting populations as a percent of total cells is listed at the bottom. B, Purity of the starting population of Vγ2Vδ2 T cells helps to determine engraftment success. The purity of the transferred Vγ2Vδ2 T cells is correlated with the percentage of NOG mice with predominant Vγ2Vδ2 T cell populations (>80%) at 2 weeks. Each data point represents 1 transfer experiment
3.6. Adoptively transferred Vγ2Vδ2 T cells from NOG mice are fully functional
To assess the functional capabilities of Vγ2Vδ2 T cells expanded by PTA in vivo, we tested various immunological functions of the cells after their adoptive transfer to immunodeficient NOG mice. First, recovered Vγ2Vδ2 T cells were tested for their ability to secrete TNF‐α. Despite 2 weeks in NOG mice without exogenous human IL‐2 or IL‐15, recovered Vγ2Vδ2 T cells secreted TNF‐α upon in vitro stimulation with the bacterial metabolite, HMBPP (Figure 8A). Note that murine cells and other human T cells do not respond to HMBPP or its analogs, as we have shown in earlier studies.11, 42
Figure 8.
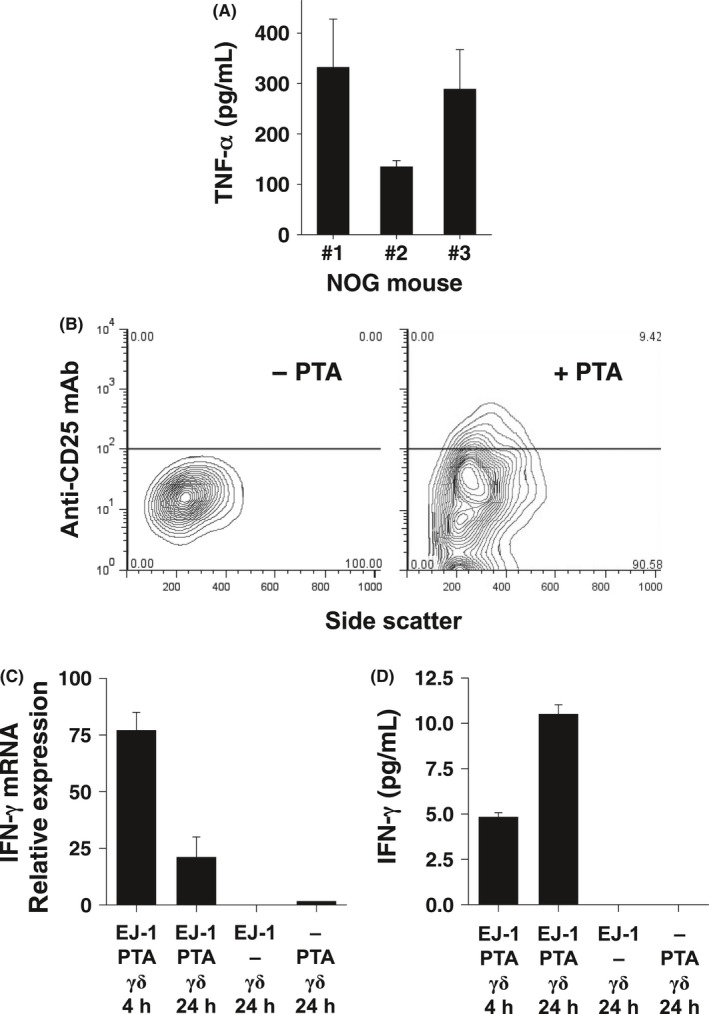
Functional analyses of Vγ2Vδ2 T cells adoptively transferred into NOG mice. A, TNF‐α production by adoptively transferred Vγ2Vδ2 T cells from NOG mice. Three NOG mice were each i.p. injected with 2 × 107 Vγ2Vδ2 T cells derived from a healthy donor. Two weeks later, peripheral blood cells were collected and Vγ2Vδ2 T cells stimulated by HMBPP in vitro. After 24 h of incubation, culture supernatants were collected and TNF‐α levels assessed in triplicate. B, Expression of CD25 on Vγ2Vδ2 T cells adoptively transferred into NOG mice bearing EJ‐1 bladder cancer tumors treated with PTA. NOG mice were i.p. injected with EJ‐1 tumor cells. Four weeks later, the mice were i.p. injected with 1 × 107 Vγ2Vδ2 T cells with or without PTA. Vγ2Vδ2 T cells were harvested 24 h later and CD25 expression assessed by flow cytometry. C, IFN‐γ mRNA expression by Vγ2Vδ2 T cells adoptively transferred into NOG mice bearing EJ‐1 tumors treated with PTA. NOG mice were i.p. injected with EJ‐1 tumor cells. Four weeks later, the mice were i.p. injected with 1 × 107 Vγ2Vδ2 T cells with or without PTA or PTA alone. Peripheral blood samples were obtained 4 h and 24 h after the challenge and IFN‐γ mRNA levels determined by quantitative PCR. D, IFN‐γ production by Vγ2Vδ2 T cells adoptively transferred into NOG mice bearing EJ‐1 tumors treated with PTA. NOG mice were treated as described in (C). Blood samples were collected 4 h and 24 h after PTA injection, serum prepared and serum IFN‐γ levels measured by ELISA
Second, we examined the ability of transferred Vγ2Vδ2 T cells to respond to bisphosphonate stimulation in vivo. For these experiments, NOG mice bearing human EJ‐1 bladder carcinoma cells were i.p. injected with Vγ2Vδ2 T cells with or without PTA and Vγ2Vδ2 T cells recovered from the mice 4 h and 24 h later. After 24 h, Vγ2Vδ2 T cells upregulated expression of the CD25 IL‐2α receptor when stimulated with PTA (right panel, Figure 8B) but not if PTA was omitted (left panel, Figure 8B). Moreover, IFN‐γ mRNA expression in Vγ2Vδ2 T cells was upregulated, reaching maximum levels at 4 h and declining thereafter (Figure 8C). Serum human IFN‐γ protein levels increased later such that levels were higher at 24 h compared with 4 h (Figure 8D), consistent with the requirement for translation of IFN‐γ to produce the cytokine rather than its release from preformed stores as has been demonstrated for in vivo responses to lymphocytic choriomeningitis virus.43 IFN‐γ production was dependent on Vγ2Vδ2 T cells given that NOG mice bearing EJ‐1 cancer cells with PTA but no Vγ2Vδ2 T cells or receiving only PTA alone failed to upregulate IFN‐γ expression at either the mRNA or protein levels. These findings clearly demonstrate that adoptively transferred Vγ2Vδ2 T cells that have been expanded by PTA stimulation can be specifically activated in vivo with bisphosphonate stimulation to upregulate CD25 for proliferation and IFN‐γ for its myriad effector functions.
4. DISCUSSION
In the present study, we show that PTA, a novel bisphosphonate prodrug, expands peripheral blood Vγ2Vδ2 T cells up to several thousand‐fold in 10 days, with very high purity in both cancer patients and healthy adult donors. PTA is, thus, ideal for the preparation of large numbers of highly homogeneous Vγ2Vδ2 T cells for use in adoptive immunotherapy for cancer. These expanded Vγ2Vδ2 T cells exhibited full immunological functions when tested in preclinical immunodeficient mouse models and their high purity helped to limit the outgrowth of human αβ T cells in these mice.
The pivaloyloxymethyl groups of PTA mask the hydrophilic phosphonate moieties, allowing cell entry but also making PTA hydrophobic such that it dissolves in DMSO but not in ethanol. Because of the possible toxicity of DMSO and its chemical properties (high freezing point and garlic taste), few intravenous drugs are solubilized in DMSO for patient use. However, because none of the conventional detergents used in clinical practice allowed PTA to dissolve in ethanol, we tested TMβCD. TMβCD is highly soluble in both water and ethanol and its parent compound, βCD, has been used for drug delivery. The less hydrophilic interiors of these compounds allow them to form complexes with hydrophobic drugs. Accordingly, TMβCD solubilizes PTA in ethanol. This TMβCD/ethanol solubilization method could be applicable to other hydrophobic therapeutics, such as alkoxymethyl derivatives of anionic compounds. PTA dissolved in TMβCD/ethanol exhibited identical biological activity to PTA dissolved in DMSO, suggesting that PTA preparation in TMβCD/ethanol could be used for both preclinical and clinical studies.
In previous clinical studies, it was not clear whether IL‐2 infusion was essential for adoptive immunotherapy with γδ T cells because some studies gave IL‐2 in vivo. This study clearly shows that Vγ2Vδ2 T cells continued to circulate in the peripheral blood even 2 weeks after i.p. injection into NOG mice in the absence of exogenous IL‐2. In addition, the transferred Vγ2Vδ2 T cells were functionally active, as evidenced by the expression of TNF‐α, CD25 and IFN‐γ in response to PTA‐sensitized tumor cells or HMBPP stimulation. Although IL‐2 is absolutely required for the maintenance of Vγ2Vδ2 T cells in vitro, these findings suggest that IL‐2 is not absolutely necessary in adoptive immunotherapy with Vγ2Vδ2 T cells. Thus, IL‐2 infusion might be omitted or reduced in patients undergoing adoptive cancer immunotherapy with Vγ2Vδ2 T cells.
Using PTA to expand Vγ2Vδ2 T cells for adoptive immunotherapy has potential advantages over Zol, the most commonly used stimulator for clinical trials. PTA stimulation consistently resulted in highly enriched populations of Vγ2Vδ2 T cells that were of significantly higher purity than those expanded by Zol. In many cases, Vγ2Vδ2 T cells were >99% of total T cells and it may be possible to further increase cell yields by using pulse PTA stimulation, as we have shown for pulse stimulation with Zol.36 The ability to generate highly enriched Vγ2Vδ2 T cells would be useful for preclinical studies in immunodeficient mice. Our findings show that relatively low levels of αβ T cell contamination can lead to the outgrowth of αβ T cells in the mice. This contamination could lead to xenogeneic graft‐versus‐host‐disease that is observed when human PBMC are xenotransplanted into NSG mice and that leads to their deaths with a median survival of 40 days.44, 45 The contamination could also compromise tumor immunity by Vγ2Vδ2 T cells through competition with αβ T cells. Our findings suggest that Vγ2Vδ2 T cells should generally be purified prior to use in long‐term tumor studies in immunodeficient NSG or NOG mice. Using PTA‐expanded Vγ2Vδ2 T cells as a starting population would ensure that purification would give highly enriched Vγ2Vδ2 T cells with few αβ T cells.
Another potential use of such highly enriched populations would be allogenic adoptive transfer of Vγ2Vδ2 T cells for tumor immunotherapy. Thus far, all clinical trials have used syngeneic Vγ2Vδ2 T cells. However, because Vγ2Vδ2 T cells are not alloreactive and because the BTN3A1 is not polymorphic,46 Vγ2Vδ2 T cells can be stimulated by allogeneic tumor cells treated with PTA or a prenyl pyrophosphate. Because they are not alloreactive, transfer of Vγ2Vδ2 T cells will not cause graft‐versus‐host disease. Note that in natural infections, very high numbers of Vγ2Vδ2 T cells can be observed (up to 50% of circulating T cells) without autoimmunity or other toxicity (reviewed in Morita et al.8).
The ability to generate highly enriched Vγ2Vδ2 T cells preparations without contaminating αβ T cells would allow their use as “off‐the‐shelf” reagents for cancer immunotherapy. This would result in major cost savings and greatly increase the feasibility of such treatments because normal donors that expand particularly well could be selected and these individuals can be repeatedly leukophoresed to obtain starting PBMC. To avoid host‐versus‐graft disease, the donors could be HLA typed and donors selected based on their degree of HLA match to the recipient. If host‐versus‐graft responses develop, the donor for the Vγ2Vδ2 T cells could be switched. If necessary, αβ T cells could be depleted using anti‐αβ TCR magnetic beads as this process would be very efficient because of their low abundance. Such an approach is being investigated commercially for CAR‐NKT cells. Moreover, infusion of HLA‐matched allogeneic virus‐specific cytotoxic lymphocytes derived by in vitro stimulation is already being used to treat patients undergoing bone marrow transplantation who have developed severe viral infections with Epstein–Barr virus or cytomegalovirus.47 In one study, the transferred cells could be detected in the blood for a median period of 10 weeks and there was minimal toxicity.48 The ability to generate large numbers of highly enriched Vγ2Vδ2 T cells that can be banked and used as “off‐the‐shelf” reagents could greatly facilitate the development of adoptive Vγ2Vδ2 T cell therapies.
CONFLICT OF INTEREST
Y.T. is a co‐inventor of novel terpyridine‐derivative proligands for measuring cytotoxicity: PCT/JP2015/059838. C.T.M. is a co‐inventor of US Patent 8,012,466 on the development of live bacterial vaccines for activating γδ T cells and has no other financial or non‐financial conflict of interest. The other authors declare no financial or non‐financial conflicts of interest.
Supporting information
Tanaka Y, Murata‐Hirai K, Iwasaki M, et al. Expansion of human γδ T cells for adoptive immunotherapy using a bisphosphonate prodrug. Cancer Sci. 2018;109:587–599. https://doi.org/10.1111/cas.13491
Funding information
This study was supported by grants from the Ministry of Education, Science, Culture, Sports, and Technology of Japan. To Y. Tanaka: Grants‐in‐Aid for Scientific Research, 16K08844, and Platform Project for Supporting Drug Discovery and Life Science Research, 17933802, from Ministry of Education, Science, Culture, Sports, and Technology of Japan and Astellas Pharma. To Y. Tanaka: “Special Coordination Funds for Promoting Science and Technologies” program through the “Formation of Center for Innovation by Fusion of Advanced Technologies”, 11800121, from Kyoto University and Japan Agency for Medical Research and Development. To Y. Tanaka: Grants‐in‐Aid for Translational Research, A48 and A90), from the Department of Veterans Affairs. To C. T. Morita: Veterans Health Administration, 1 I01 BX000972‐01A1, and from the National Cancer Institute. To C. T. Morita: CA097274 (University of Iowa/Mayo Clinic Lymphoma Specialized Program of Research Excellence) and P30CA086862 (Core Support). C. T Morita is the Kelting Family Scholar in Rheumatology.
Toi and Morita authors contributed equally to this work.
REFERENCES
- 1. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti‐PD‐1 antibody in cancer. N Engl J Med. 2012;366:2443‐2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti‐PD‐L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455‐2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rizvi NA, Hellmann MD, Snyder A, et al. Mutational landscape determines sensitivity to PD‐1 blockade in non‐small cell lung cancer. Science. 2015;348:124‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goodman AM, Kato S, Bazhenova L, et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther. 2017;16:2598‐2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alexandrov LB, Nik‐Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Khagi Y, Goodman AM, Daniels GA, et al. Hypermutated circulating tumor DNA: correlation with response to checkpoint inhibitor‐based immunotherapy. Clin Cancer Res. 2017;23:5729‐5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Klebanoff CA, Rosenberg SA, Restifo NP. Prospects for gene‐engineered T cell immunotherapy for solid cancers. Nat Med. 2016;22:26‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morita CT, Jin C, Sarikonda G, Wang H. Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vγ2Vδ2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol Rev. 2007;215:59‐76. [DOI] [PubMed] [Google Scholar]
- 9. Tanaka Y, Morita CT, Tanaka Y, Nieves E, Brenner MB, Bloom BR. Natural and synthetic non‐peptide antigens recognized by human γδ T cells. Nature. 1995;375:155‐158. [DOI] [PubMed] [Google Scholar]
- 10. Hintz M, Reichenberg A, Altincicek B, et al. Identification of (E)‐4‐hydroxy‐3‐methyl‐but‐2‐enyl pyrophosphate as a major activator for human γδ T cells in Escherichia coli . FEBS Lett. 2001;509:317‐322. [DOI] [PubMed] [Google Scholar]
- 11. Puan K‐J, Jin C, Wang H, et al. Preferential recognition of a microbial metabolite by human Vγ2Vδ2 T cells. Int Immunol. 2007;19:657‐673. [DOI] [PubMed] [Google Scholar]
- 12. Harly C, Guillaume Y, Nedellec S, et al. Key implication of CD277/butyrophilin‐3 (BTN3A) in cellular stress sensing by a major human γδ T‐cell subset. Blood. 2012;120:2269‐2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang H, Henry O, Distefano MD, et al. Butyrophilin 3A1 plays an essential role in prenyl pyrophosphate stimulation of human Vγ2Vδ2 T cells. J Immunol. 2013;191:1029‐1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Palakodeti A, Sandstrom A, Sundaresan L, et al. The molecular basis for modulation of human Vγ9Vδ2 T cell responses by CD277/butyrophilin‐3 (BTN3A)‐specific antibodies. J Biol Chem. 2012;287:32780‐32790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sandstrom A, Peigné C‐M, Léger A, et al. The intracellular B30.2 domain of butyrophilin 3A1 binds phosphoantigens to mediate activation of human Vγ9Vδ2 T cells. Immunity. 2014;40:490‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang H, Morita CT. Sensor function for butyrophilin 3A1 in prenyl pyrophosphate stimulation of human Vγ2Vδ2 T Cells. J Immunol. 2015;195:4583‐4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bukowski JF, Morita CT, Tanaka Y, Bloom BR, Brenner MB, Band H. Vγ2Vδ2 TCR‐dependent recognition of non‐peptide antigens and Daudi cells analyzed by TCR gene transfer. J Immunol. 1995;154:998‐1006. [PubMed] [Google Scholar]
- 18. Bennouna J, Bompas E, Neidhardt EM, et al. Phase‐I study of Innacell γδ, an autologous cell‐therapy product highly enriched in γ9δ2 T lymphocytes, in combination with IL‐2, in patients with metastatic renal cell carcinoma. Cancer Immunol Immunother. 2008;57:1599‐1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abe Y, Muto M, Nieda M, et al. Clinical and immunological evaluation of zoledronate‐activated Vγ9γδ T‐cell‐based immunotherapy for patients with multiple myeloma. Exp Hematol. 2009;37:956‐968. [DOI] [PubMed] [Google Scholar]
- 20. Nicol AJ, Tokuyama H, Mattarollo SR, et al. Clinical evaluation of autologous gamma delta T cell‐based immunotherapy for metastatic solid tumours. Br J Cancer. 2011;105:778‐786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kobayashi H, Tanaka Y, Yagi J, Minato N, Tanabe K. Phase I/II study of adoptive transfer of γδ T cells in combination with zoledronic acid and IL‐2 to patients with advanced renal cell carcinoma. Cancer Immunol Immunother. 2011;60:1075‐1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sakamoto M, Nakajima J, Murakawa T, et al. Adoptive immunotherapy for advanced non‐small cell lung cancer using zoledronate‐expanded γδ T cells: a phase I clinical study. J Immunother. 2011;34:202‐211. [DOI] [PubMed] [Google Scholar]
- 23. Izumi T, Kondo M, Takahashi T, et al. Ex vivo characterization of γδ T‐cell repertoire in patients after adoptive transfer of Vγ9Vδ2 T cells expressing the interleukin‐2 receptor β‐chain and the common γ‐chain. Cytotherapy. 2013;15:481‐491. [DOI] [PubMed] [Google Scholar]
- 24. Wada I, Matsushita H, Noji S, et al. Intraperitoneal injection of in vitro expanded Vγ9Vδ2 T cells together with zoledronate for the treatment of malignant ascites due to gastric cancer. Cancer Med. 2014;3:362‐375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Okawaki M, Hironaka K, Yamanura M, Yamaguchi Y. Adoptive immunotherapy using autologous lymphocytes activated ex vivo with antigen stimulation for patients with incurable cancer. Kawasaki Med J. 2014;40:33‐39. [Google Scholar]
- 26. Noguchi A, Kaneko T, Kamigaki T, et al. Zoledronate‐activated Vγ9γδ T cell‐based immunotherapy is feasible and restores the impairment of γδ T cells in patients with solid tumors. Cytotherapy. 2011;13:92‐97. [DOI] [PubMed] [Google Scholar]
- 27. Kobayashi H, Tanaka Y, Shimmura H, Minato N, Tanabe K. Complete remission of lung metastasis following adoptive immunotherapy using activated autologous γδ T‐cells in a patient with renal cell carcinoma. Anticancer Res. 2010;30:575‐579. [PubMed] [Google Scholar]
- 28. Kakimi K, Matsushita H, Murakawa T, Nakajima J. γδ T cell therapy for the treatment of non‐small cell lung cancer. Transl Lung Cancer Res. 2014;3:23‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gober H‐J, Kistowska M, Angman L, Jenö P, Mori L, De Libero G. Human T cell receptor γδ cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;197:163‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang H, Sarikonda G, Puan K‐J, et al. Indirect stimulation of human Vγ2Vδ2 T cells through alterations in isoprenoid metabolism. J Immunol. 2011;187:5099‐5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thompson K, Rogers MJ, Coxon FP, Crockett JC. Cytosolic entry of bisphosphonate drugs requires acidification of vesicles after fluid‐phase endocytosis. Mol Pharmacol. 2006;69:1624‐1632. [DOI] [PubMed] [Google Scholar]
- 32. Matsumoto K, Hayashi K, Murata‐Hirai K, et al. Targeting cancer cells with a bisphosphonate prodrug. ChemMedChem. 2016;11:2656‐2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tanaka Y, Iwasaki M, Murata‐Hirai K, et al. Anti‐tumor activity and immunotherapeutic potential of a bisphosphonate prodrug. Sci Rep. 2017;7:5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Iwasaki M, Tanaka Y, Kobayashi H, et al. Expression and function of PD‐1 in human γδ T cells that recognize phosphoantigens. Eur J Immunol. 2011;41:345‐355. [DOI] [PubMed] [Google Scholar]
- 35. Hakomori S. A rapid permethylation of glycolipid, and polysaccharide catalyzed by methylsulfinyl carbanion in dimethyl sulfoxide. J Biochem. 1964;55:205‐208. [PubMed] [Google Scholar]
- 36. Nada MH, Wang H, Workalemahu G, Tanaka Y, Morita CT. Enhancing adoptive cancer immunotherapy with Vγ2Vδ2 T cells through pulse zoledronate stimulation. J Immunother Cancer. 2017;5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tanaka Y, Sakai Y, Mizuta S, et al. Live cell labeling with terpyridine derivative proligands to measure cytotoxicity mediated by immune cells. ChemMedChem. 2017;12:2006‐2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Savjani KT, Gajjar AK, Savjani JK. Drug solubility: importance and enhancement techniques. ISRN Pharm. 2012;2012:195727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kalepu S, Nekkanti V. Insoluble drug delivery strategies: review of recent advances and business prospects. Acta Pharm Sin B. 2015;5:442‐453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Morita CT, Verma S, Aparicio P, Martinez AC, Spits H, Brenner MB. Functionally distinct subsets of human γ/δ T cells. Eur J Immunol. 1991;21:2999‐3007. [DOI] [PubMed] [Google Scholar]
- 41. Selin LK, Stewart S, Shen C, Mao HQ, Wilkins JA. Reactivity of γδ T cells induced by the tumour cell line RPMI 8226: functional heterogeneity of clonal populations and role of GroEL heat shock proteins. Scand J Immunol. 1992;36:107‐117. [DOI] [PubMed] [Google Scholar]
- 42. Tanaka Y, Sano S, Nieves E, et al. Nonpeptide ligands for human γδ T cells. Proc Natl Acad Sci USA. 1994;91:8175‐8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Araki K, Morita M, Bederman AG, et al. Translation is actively regulated during the differentiation of CD8+ effector T cells. Nat Immunol. 2017;18:1046‐1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ali N, Flutter B, Sanchez Rodriguez R, et al. Xenogeneic graft‐versus‐host‐disease in NOD‐scid IL‐2Rγnull mice display a T‐effector memory phenotype. PLoS One. 2012;7:e44219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ito R, Katano I, Kawai K, et al. Highly sensitive model for xenogenic GVHD using severe immunodeficient NOG mice. Transplantation. 2009;87:1654‐1658. [DOI] [PubMed] [Google Scholar]
- 46. Kabelitz D, Bender A, Schondelmaier S, da Silva Lobo ML, Janssen O. Human cytotoxic lymphocytes. V. Frequency and specificity of γδ+ cytotoxic lymphocyte precursors activated by allogeneic or autologous stimulator cells. J Immunol. 1990;145:2827‐2832. [PubMed] [Google Scholar]
- 47. Bollard CM, Heslop HE. T cells for viral infections after allogeneic hematopoietic stem cell transplant. Blood. 2016;127:3331‐3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Heslop HE, Ng CY, Li C, et al. Long‐term restoration of immunity against Epstein‐Barr virus infection by adoptive transfer of gene‐modified virus‐specific T lymphocytes. Nat Med. 1996;2:551‐555. [DOI] [PubMed] [Google Scholar]
- 49. Wang H, Lee HK, Bukowski JF, et al. Conservation of nonpeptide antigen recognition by rhesus monkey Vγ2Vδ2 T cells. J Immunol. 2003;170:3696‐3706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


