Abstract
Exosomes derived from cells have been found to mediate signal transduction between cells and to act as efficient carriers to deliver drugs and small RNA. Hepatocyte growth factor (HGF) is known to promote the growth of both cancer cells and vascular cells, and the HGF‐cMET pathway is a potential clinical target. Here, we characterized the inhibitory effect of HGF siRNA on tumor growth and angiogenesis in gastric cancer. In addition, we showed that HGF siRNA packed in exosomes can be transported into cancer cells, where it dramatically downregulates HGF expression. A cell co‐culture model was used to show that exosomes loaded with HGF siRNA suppress proliferation and migration of both cancer cells and vascular cells. Moreover, exosomes were able to transfer HGF siRNA in vivo, decreasing the growth rates of tumors and blood vessels. The results of our study demonstrate that exosomes have potential for use in targeted cancer therapy by delivering siRNA.
Keywords: angiogenesis, exosomes, hepatocyte growth factor, siRNA, tumor therapy
1. INTRODUCTION
Exosomes are small vesicles proactively secreted by several cell types, and they mediate signal transduction between cells.1, 2 Generally, extracellular vesicles (EV) comprise 2 vesicle types, exosomes (30‐200 nm) and shedding vesicles (SV, 200‐1000 nm).3, 4, 5 Previous studies have shown that exosomes can deliver proteins, microRNA, mRNA and DNA to neighboring or distant cells, playing key roles in modulating the regulatory pathways of target cells.6, 7, 8 Recently, exosomes have been hypothesized to be potential carriers of drugs, including both chemical drugs and microRNA, but more effort is needed to explore the role of exosomes in the transport of siRNA.9, 10
RNA interference has become a promising tool for clinical therapy, guiding sequence‐specific endonucleolytic cleavage of mRNA and resulting in specific gene silencing.11 However, there are major limitations of the use of siRNA as a therapeutic tool, including its degradation by extracellular nucleases and poor cellular uptake. Although a number of vehicles, such as cationic liposomes, viral vectors and nanoparticles, have been used to transport siRNA, there are disadvantages of each method.12, 13 Therefore, it remains a challenge to deliver interfering RNA to tumor cells in vivo.
Hepatocyte growth factor (HGF) was first discovered in mouse livers and is a multifunctional cytokine that has important roles in cell proliferation, survival, motility and morphogenesis.14, 15 HGF has been found to be upregulated in various tumor tissues, and its upregulation is closely linked with tumor development. cMET, the receptor of HGF, is widely expressed in various types of epithelial, endothelial and hematopoietic progenitor cells.16, 17, 18 The HGF‐cMET axis is involved in several biological processes, such as embryogenesis, organogenesis, adult tissue regeneration and carcinogenesis, including both solid and hematological malignancies.19 Thus, the HGF‐cMET pathway has been considered as a novel target for cancer therapy.20, 21
The present study is designed to investigate whether cell‐derived exosomes can be used as a delivery vehicle for siRNA and to evaluate the inhibitory effects of exosome‐delivered HGF siRNA on tumor growth and angiogenesis. Human Embryonic Kidney 293T cells (HEK293T) transfected with HGF siRNA were selected as exosome donors. We demonstrated that exosomes packaged with HGF siRNA fused with SGC‐7901 cells and significantly decreased HGF and vascular endothelial growth factor (VEGF) expression. Human Umbilical Vein Endothelial Cells (HUVEC) were co‐cultured with SGC‐7901 cells to simulate the tumor microenvironment, and results clearly showed that exosomes delivered HGF siRNA, suppressing the cell proliferation and vascular ring formation of HUVEC. Data from tumor‐implanted mice demonstrated that exosomes containing HGF siRNA, administered by tail vein injection, effectively suppressed tumor growth and angiogenesis. Therefore, we conclude that cell‐derived exosomes are effective carriers of siRNA and have potential value for future clinical application.
2. MATERIALS AND METHODS
2.1. Human tissue
Human gastric cancer tissues and paired adjacent non‐cancerous tissues were acquired from patients undergoing surgical procedures at the Tianjin Medical University Cancer Institute and Hospital (Tianjin, China). Both tumor tissues and non‐cancerous tissues were confirmed histologically. The pathological type of each cancer was determined to be glandular carcinoma. Written consent (for both study participation and publication of identifying information/images) was provided by all patients, and the Ethics Committee of the Tianjin Medical University Cancer Institute and Hospital approved all aspects of this study. Tissue fragments were immediately frozen in liquid nitrogen at the time of surgery and stored at −80°C. All experiments were performed in accordance with relevant guidelines and regulations.
2.2. Animals
Male nude mice (BALB/c‐nu, 5‐6 weeks) were housed in a pathogen‐free animal facility with access to water and food ad libitum. All experimental procedures were performed in accordance with protocols approved by the Institutional Animal Care and Research Advisory Committee of the Tianjin Medical University Cancer Institute and Hospital.
2.3. Cell culture
Human gastric cell line SGC‐7901 and human embryo kidney epithelial cell line (HEK293T) were cultured in DMEM (Gibco, New York, NY, USA) supplemented with 10% FBS (Gibco); HUVEC were cultured in MCDB131 (Gibco) supplemented with 10% FBS, 10% MVGS, 1% Glutamine and 1% HEPES. All cells were maintained in a humidified incubator at 37°C with 5% CO2.
2.4. Cell transfection
Cells were seeded into a 10 cm or 6‐well plate, and transfection was conducted after 24 hours. Transfection with siRNA was performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocols. For each well, equal doses (100 pmol) of HGF siRNA (sc‐29002, Santa Cruz, California, CA, USA) or scrambled negative control RNA were used. After 4‐6 hours, the medium was changed to exclude remaining liposome. Cells were harvested 24 hours after transfection for real‐time PCR analysis or 48 hours after transfection for western blotting.
2.5. Isolation exosomes from medium
Exosomes were isolated from cell culture medium by differential centrifugation, as previously published.22, 23 After removing cells and other debris by centrifugation at 300 and 3000 g, the supernatants were centrifuged at 110 000 g for 70 minutes (all steps were performed at 4°C). Exosomes were collected from the resulting pellets and resuspended in PBS.
2.6. Transmission electron microscopy
For conventional transmission electron microscopy (TEM), exosome pellets were placed in a droplet of 2.5% glutaraldehyde in PBS buffer at pH 7.2 and fixed overnight at 4°C. The samples were rinsed in PBS (3 times, 10 minutes each) and post‐fixed in 1% osmium tetroxide for 60 minutes at room temperature. The samples were then embedded in 10% gelatin, fixed in glutaraldehyde at 4°C and cut into several blocks (smaller than 1 mm3). The samples were dehydrated for 10 minutes per step in increasing concentrations of alcohol (30%, 50%, 70%, 90%, 95% and 100% × 3). Next, pure alcohol was replaced with propylene oxide, and the specimens were infiltrated with increasing concentrations (25%, 50%, 75% and 100%) of Quetol‐812 epoxy resin mixed with propylene oxide for a minimum of 3 hours per step. The samples were embedded in pure, fresh Quetol‐812 epoxy resin and polymerized at 35°C for 12 hours, 45°C for 12 hours, and 60°C for 24 hours. Ultrathin sections (100 nm) were cut using a Leica UC6 ultramicrotome and post‐stained, first with uranyl acetate for 10 minutes and then with lead citrate for 5 minutes at room temperature, prior to observation using an FEI Tecnai T20 transmission electron microscope (Hillsboro, TX, USA) operated at 120 kV.
2.7. In vitro model of tumor microenvironment
Cell co‐culture was performed in Boyden chambers (6.5 mm; Costar, Washington, DC, USA) with 0.4‐μm polyester membranes. For each assay, 1 × 105 SGC‐7901 cells were seeded in the Boyden chamber, and 1 × 105 HUVEC cells were seeded in a 24‐well plate. Both types of cells were co‐cultured for 24 hours in DMEM medium supplemented with 10% FBS. SGC‐7901 cells were treated with exosomes or transfection prior to co‐culture.
2.8. Vascular ring formation of HUVEC cells
In vitro ring formation assays were performed with endothelial cells as previously described.24, 25 Briefly, 100 μL of Matrigel (BD Bioscience, Bedford, MA, USA) was added to each well of a 24‐well plate and allowed to polymerize at 37°C for 30 minutes. HUVEC cells were first co‐cultured with pre‐treated SGC‐7901 cells. Next, the HUVEC cells were re‐suspended in FBS‐free DMEM medium and seeded into each well at a concentration of 1 × 105 cells/well. After 6 hours, the cells were examined under a light microscope to assess the formation of capillary‐like structures. The branch points of the formed tubes, which represent the degree of angiogenesis in vitro, were scanned and quantified in at least 5 low‐power fields (200×).
2.9. ELISA analysis
The release of VEGF from SGC7901 cells was examined using an ELISA kit (Thermo, EHVEGFACL, Carlsbad, CA, USA) according to the manufacturer's protocols. Briefly, 100 μL of each standard and sample was added into appropriate wells and agitated gently at room temperature.
2.10. Exosome incubation with cells
Empty exosomes, or exosomes loaded with HGF siRNA or scramble RNA (100 μg exosomes per 106 cells), were incubated with SGC‐7901 cells for 24 hours. The recipient cells were then collected for subsequent analysis.
2.11. RNA isolation and quantitative RT‐PCR
Total RNA was isolated from cultured cells and tissues using TRIzol Reagent (Invitrogen) according to the manufacturer's protocol. Subsequently, 2 μL of total RNA was reverse‐transcribed to cDNA (16°C for 15 minutes, 42°C for 60 minutes and 85°C for 5 minutes). Gene‐specific PCR products were measured using qRT‐PCR with a SYBR Green PCR Kit (Takara, Dalian, China) on a CFX96 Real‐time RT‐PCR System. The relative expression levels of target genes were normalized to the control using the method. To calculate the expression levels of target siRNA, a series of siRNA oligonucleotides at known concentrations in water were reverse‐transcribed and amplified to generate a standard curve. Then, siRNA was quantified based on the standard curve.26
2.12. Cell proliferation assay
SGC‐7901 and HUVEC cells were incubated with 50 μm EdU (RiboBio, Guangzhou, China) for 12 hours, and fixed with 4% paraformaldehyde for 30 minutes at 25°C. Next, the cells were washed in PBS (2 × 5 minutes, room temperature [RT]) and then permeabilized using PBS containing 0.3% Triton X‐100 for 10 minutes. After extensive washing in PBS, the cells were incubated in Apollo staining solution (RiboBio) for 20 minutes, washed with NaCl/Pi (3 × 10 minutes, RT), and then incubated in Hochest (1:2500; Roche Diagnostics, Mannheim, Germany) for 10 minutes at RT.
2.13. Cell migration assay
The migratory capacity of the cells was tested using a Transwell Boyden chamber (6.5‐mm, Costar) with polycarbonate membranes (8‐μm pore size) on the bottom of the upper compartment. In total, 1 × 105 cells were suspended in serum‐free DMEM and 0.5 mL DMEM with 10% FBS was added to the lower compartment, and the Transwell‐containing plates were incubated for 6 hours. At the end of the incubation, the cells that had migrated through the filter membranes were fixed with 90% ethanol for 15 minutes at room temperature and stained with 0.1% crystal violet solution. Images of migrated cells were captured with a photomicroscope; cell migration was quantified by blind counting with 5 fields per chamber.
2.14. Western blotting analysis
The expression of HGF was assessed by western blotting, and protein levels were normalized to glyceraldehyde 3‐phosphate dehydrogenase (GAPDH). Immunoblots were blocked with PBS containing 5% fat‐free dried milk at room temperature for 1 hour and incubated at 4°C overnight with anti‐HGF (1:1000; Santa Cruz) and anti‐GAPDH (1:2000; Santa Cruz) antibodies.
2.15. Immunohistochemistry
Tumors were fixed in 4% paraformaldehyde, embedded in paraffin, sectioned and then stained with anti‐CD31 (Abcam, Cambridge, MA, USA) and anti‐HGF antibodies (Santa Cruz). The fluorescence intensity was quantified from at least 5 sections.
2.16. Establishment of tumor xenografts in nude mice
SGC‐7901 cells were injected subcutaneously into nude mice (1 × 107 cells per mouse). Twenty micrograms of cell‐derived exosomes per mouse were injected through tail veins every 2 days. Mice were killed on the 23th day and the weights and diameters of their tumors were recorded.
2.17. Statistical analyses
All data are representative of 5 or 6 independent experiments. Data are expressed as the means ± SE of at least 5 separate experiments. Differences with P < .05 were considered statistically significant using Student's t‐test. In this study, *, ** and *** indicate P < .05, P < .01 and P < .001, respectively.
3. RESULTS
3.1. Hepatocyte growth factor is upregulated in gastric cancer
The expression of HGF has been reported to be upregulated in various cancers and, thus, HGF is a potential serum marker for tumor diagnosis.27, 28, 29 Here, we first checked HGF levels in both the tissues and sera of gastric cancer patients. The HGF protein was clearly increased in cancer tissues compared with adjacent tissues (Figure 1A,B). We also quantified the levels of HGF mRNA by quantitative RT‐PCR (RT‐qPCR), and the results showed that HGF mRNA is upregulated in cancer tissues (Figure 1C). As expected, serum HGF was much higher in gastric cancer patients than in healthy subjects (Figure 1D). These data suggest that HGF acts as an oncogene in gastric cancer.
Figure 1.
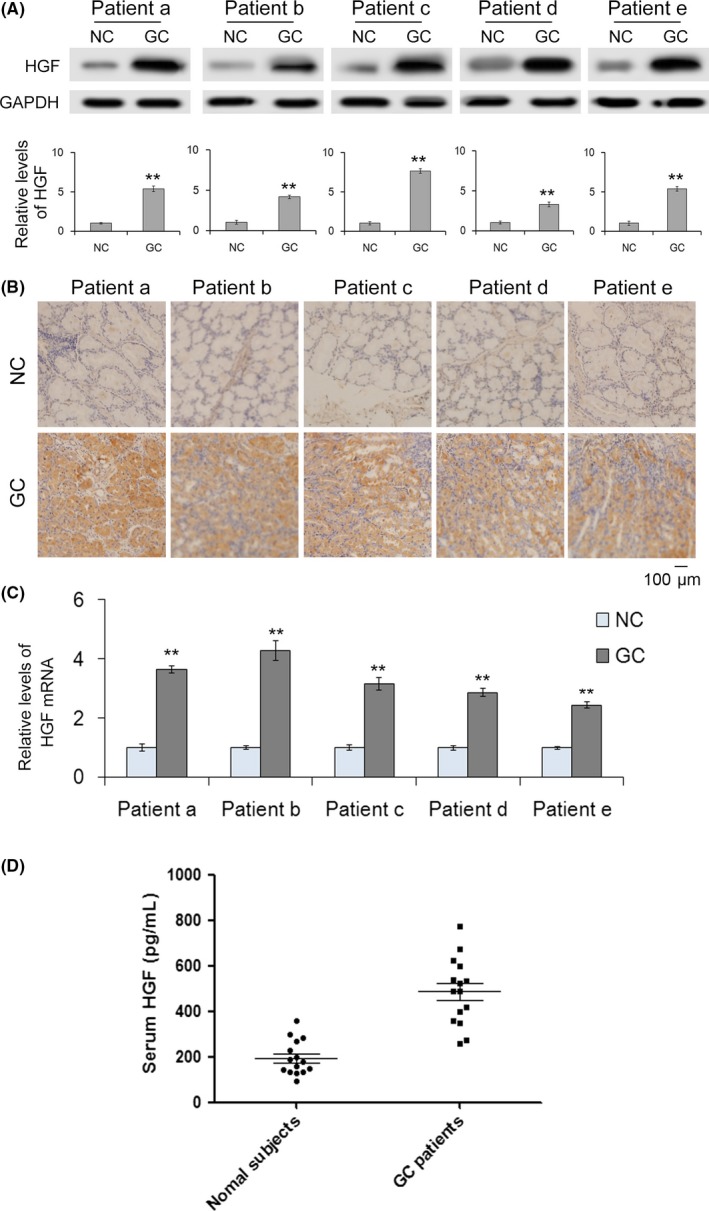
Hepatocyte growth factor (HGF) is upregulated in gastric cancer. A, Western blot analysis of HGF expression and quantitative analysis of HGF in gastric cancer (GC) tissues and paired adjacent noncancerous tissues (n = 5). B, Immunohistochemistry of paraffin‐embedded human gastric cancer tissues and adjacent noncancerous tissues (n = 5). C, Quantitative RT‐PCR analysis of HGF mRNA levels in GC tissues and paired adjacent noncancerous tissues (n = 5). D, Relative levels of HGF in GC serum and normal serum (n = 15). NC indicates noncancerous tissues paired with GC. ** indicates P < .01
3.2. Selection of hepatocyte growth factor siRNA for gastric cancer cells
To verify that HGF regulates cell proliferation and migration in gastric cancer cells and to select a sensitive siRNA against HGF (si.HGF), an siRNA strategy was used to knock down HGF in SGC‐7901 cells. The relative abundance of HGF protein was significantly reduced in SGC‐7901 cells transduced with 3 siRNA, as evidenced by western blotting, and siRNA‐1 had the most pronounced effects (Figure 2A). The levels of HGF mRNA also showed the same trend as protein content (Figure 2B). Meanwhile, knockdown of HGF resulted in a decrease in VEGF expression (Figure 2C). These results showed that si.HGF‐1 was the most sensitive si.HGF. Subsequently, the biological effects of silencing HGF in SGC‐7901 cells were investigated. Proliferation, transwell and scratch‐wound healing assays all indicated that SGC‐7901 cells transfected with si.HGF‐1, compared with control cells, had significantly lower rates of proliferation and decreased migration (Figure 2D–F).
Figure 2.
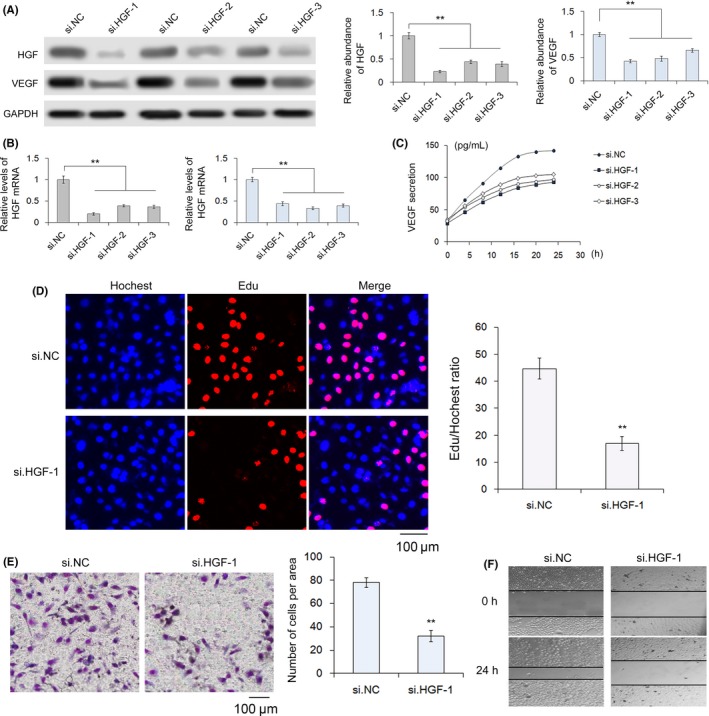
Selection of hepatocyte growth factor (HGF) siRNA and effects of siHGF‐1 on the proliferation and migration of SGC‐7901 cells. A–C, Silencing of HGF expression by siRNA. SGC‐7901 cells were transfected with HGF siRNA, and the protein levels of HGF and VEGF were measured along with the mRNA levels of HGF (n = 3). D, Silencing of HGF clearly decreases the proliferation of SGC‐7901 cells (n = 3). E, Quantitative analysis of transwell assays demonstrate that knockdown of HGF inhibits cell migration (n = 3). F, Validation of HGF‐mediated cell migration by scratch‐wound healing assays. si.NC indicates negative control siRNA (n = 3). ** indicates P < .01. GAPDH, glyceraldehyde 3‐phosphate dehydrogenase; VEGF, vascular endothelial growth factor
3.3. Packaging of hepatocyte growth factor siRNA in HEK293T‐derived exosomes
We next sought to separate exosomes from other extracellular vesicles by differential centrifugation. We defined exosomes as approximately 30‐100 nm vesicles as determined with TEM (Figure 3A,B). The enrichment of exosome markers TSG101, CD9 and CD63 confirmed that we had successfully isolated exosomes (Figure 3C). For exosome loading, HGF siRNA were transfected into HEK293T cells and then exosomes were isolated. As shown in Figure 3D, HEK293T‐derived exosomes selectively packaged HGF siRNA. The concentration of HGF siRNA in these exosomes was calculated through qRT‐PCR and the results clearly showed that HGF siRNA was effectively packaged into exosomes (Figure 3E). The encapsulation efficiency was also calculated to prove the high efficiency of HGF siRNA packaged into exosomes (Figure 3F). In conclusion, we verified that exosomes are potential carriers of siRNA.
Figure 3.
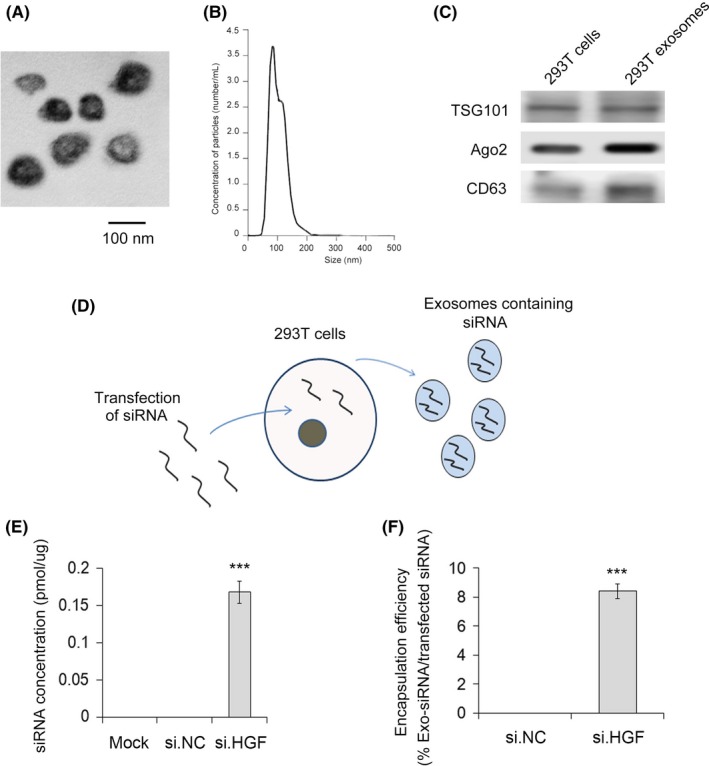
HEK293T‐derived exosomes are packaged with hepatocyte growth factor (HGF) siRNA. A, Image of exosomes under transmission electron microscopy. B, Particle concentration and size distribution of exosomes as evaluated by nanoparticle tracking analysis (n = 3). C, Exosomes were enriched, and exosomal markers TSG101, CD9 and CD63 were detected by western blot (n = 3). D, Sketch map of HEK293T‐derived exosomes selectively packaged with HGF siRNA. E, Concentration of HGF siRNA packaged in exosomes (n = 3). F, Encapsulation efficiency of HGF siRNA packaged into exosomes. *** indicates P < .001
3.4. siRNA against hepatocyte growth factor exosomes suppress hepatocyte growth factor/VEGF expression in SGC‐7901 cells
To determine whether exosomes packaged with si.HGF‐1 regulate the expression of HGF and VEGF, SGC‐7901 cells were incubated with empty exosomes, exosomes loaded with si.HGF‐1 (20 or 40 μg), exosomes loaded with negative control siRNA (si.NC) or free si.HGF‐1. Exosomes were labeled with the fluorescent dye PKH26, and then PKH26‐labeled exosomes were incubated with recipient cells. As shown in Figure 4C, we found that the recipient cells displayed red fluorescence, which confirmed that the exosomes had fused with recipient cells. Figure 4A,B,D show that exosomes packaged with si.HGF‐1 significantly decreased the protein and mRNA expression levels of HGF and VEGF, and this relationship was dose‐dependent. Next, the release of VEGF from SGC‐7901 cells was analyzed using an ELISA kit. We observed similar changes in HGF along with a range of inhibitory effects across siRNA treatments (Figure 4E). Therefore, exosomes can serve as carries to deliver HGF siRNA to inhibit HGF and VEGF expression.
Figure 4.
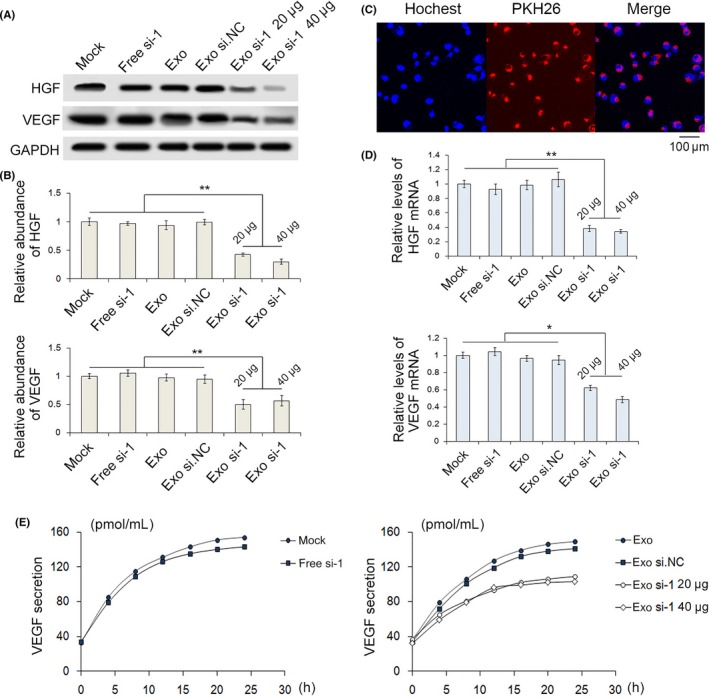
HEK293T‐derived exosomes packaged with siRNA against HGF (si.HGF) decrease HGF and VEGF expression in SGC‐7901 cells. A,B,D, Exosomes loaded with si.HGF‐1 decreased HGF and VEGF expression in SGC‐7901 cells, as shown by western blot (n = 3). C, PKH26‐labeled exosomes successfully fused with recipient cells (n = 3). E, Release of VEGF from SGC‐7901 cells was analyzed using an ELISA kit (n = 3). * indicates P < .05, ** indicates P < .01. GAPDH, glyceraldehyde 3‐phosphate dehydrogenase; HGF, hepatocyte growth factor; VEGF, vascular endothelial growth factor
3.5. Exosomes delivering siRNA against hepatocyte growth factor regulate the biological behavior of SGC‐7901 cells
We next examined the biological effects of exosomes packed with si.HGF‐1 on SGC‐7901 cells. First, the proliferation of SGC‐7901 cells treated with si.HGF‐1 exosomes was analyzed in vitro by EdU proliferation assays (Figure 5A,B). As expected, the proliferation rate of SGC‐7901 cells treated with exosomes packed with si.HGF‐1 was significantly decreased compared with that of control cells. To determine whether exosomes packed with si.HGF‐1 affected cell migration, transwell (Figure 5C,D) and scratch‐wound healing assays (Figure 5E) were performed. Both assays demonstrated that SGC‐7901 cells transfected with exosomes packed with si.HGF‐1 showed a lower migration rate than did control cells. These results demonstrate that exosomes packed with si.HGF‐1 restrain proliferation and migration, thus potentially playing a vital role in future studies.
Figure 5.
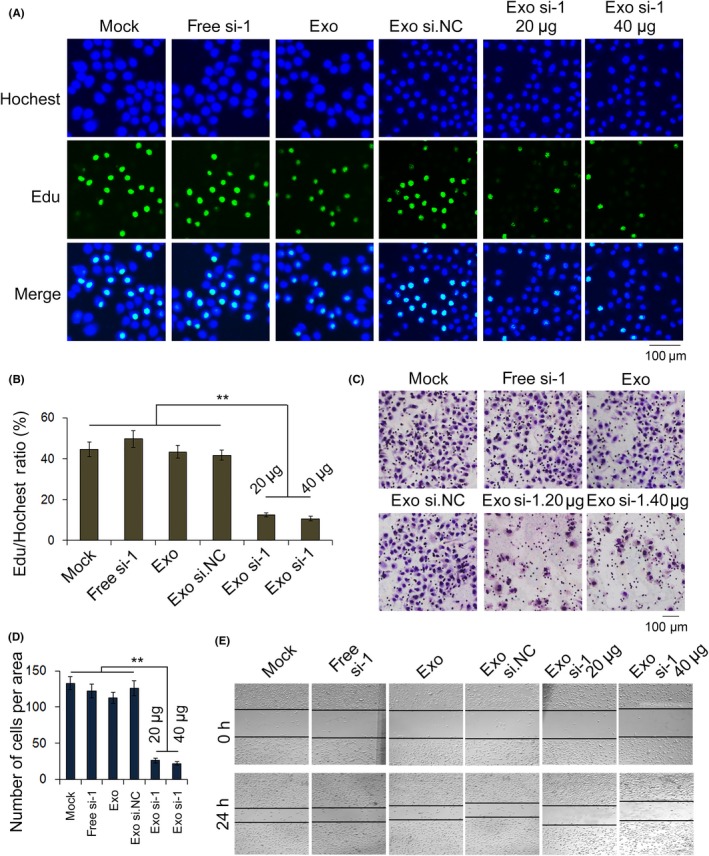
Exosomes deliver si.HGF‐1 to regulate the proliferation and migration of SGC‐7901 cells. A, EdU assays demonstrate that exosomes delivered si.HGF‐1 and suppressed the proliferation of SGC‐7901 cells (n = 3). B, Quantification of (A) (n = 3). C, Transwell assays demonstrate that exosomes carrying si.HGF‐1 suppress the migration of gastric cancer cells (n = 3). D, Quantitative analysis of (C) (n = 3). E, Validation that exosomes packed with si.HGF‐1 inhibit cell migration by scratch‐wound healing assays (n = 3). ** indicates P < .01. HGF, hepatocyte growth factor; si.HGF, siRNA against HGF. VEGF, vascular endothelial growth factor
3.6. Exosomes delivering siRNA against hepatocyte growth factor suppress the angiogenesis of SGC‐7901 cells
Angiogenesis is one of the most important conditions in the progression of tumor development. We created a cell co‐culture model to simulate the tumor microenvironment using SGC‐7901 cells and HUVEC cells. In this model, SGC‐7901 cells were treated with exosomes or transfected prior to co‐culture. To assess the angiogenesis of HUVEC cells, we designed a vascular ring formation assay (Figure 6A,B). In this assay, we observed sparse vascular rings formed by SGC‐7901 cells treated with exosomes packed with si.HGF‐1. Moreover, EdU and transwell assays indicated that SGC‐7901 cells transfected with exosomes containing HGF siRNA had a significantly lower rate of proliferation and reduced migration compared with control cells.
Figure 6.
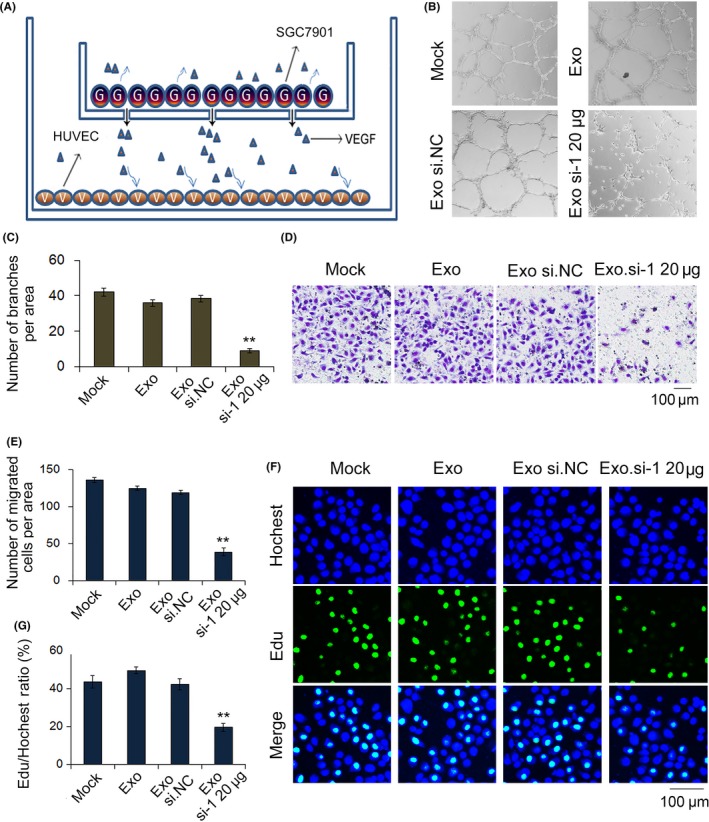
Exosomes deliver si.HGF‐1 to suppress angiogenesis in SGC‐7901 cells and HUVEC. A, Sketch map of cell co‐culture tumor microenvironment with SGC‐7901 cells and HUVEC. B,C, Vascular ring formation assay of HUVEC (n = 3). D, Transwell assays demonstrate that exosomes delivered si.HGF‐1 suppresses the migration of SGC‐7901 cells and HUVEC (n = 3). E, Quantitative analysis of (D) (n = 3). F, EdU assays demonstrate that exosomes carrying si.HGF‐1 suppressed the proliferation of SGC‐7901 cells and HUVEC (n = 3). G, Quantitative analysis of (F) (n = 3). ** indicates P < .01. HGF, hepatocyte growth factor; si.HGF, siRNA against HGF. VEGF, vascular endothelial growth factor
3.7. In vivo effects of siRNA against hepatocyte growth factor exosomes in tumor xenograft mice
Finally, we assessed the effects of HGF siRNA delivered by exosomes on tumor growth in a mouse model. SGC‐7901 cells were injected subcutaneously into the armpits of nude mice. Twenty micrograms of cell‐derived exosomes per mouse were administered through intravenous tail injection every 2 days. As is shown in Figure 7A,B, exosomes packed with si.HGF‐1 significantly decreased tumor size and weight (Figure 7C,D); the levels of HGF and VEGF were then detected in the excised tumors. Our results indicated that exosomes packed with si.HGF‐1, compared with control exosomes, decreased the protein levels of HGF and VEGF (Figure 7E–G), as well as their mRNA levels. To further confirm that exosomes packed with si.HGF‐1 regulate the expression of VEGF, which usually suppresses angiogenesis, tumor angiogenesis was evaluated by immunohistochemistry using CD31 as a vascular marker. The results showed that the relative vessel density of tumors treated with si.HGF‐1 exosomes was lower than with other treatments (Figure 7H).
Figure 7.
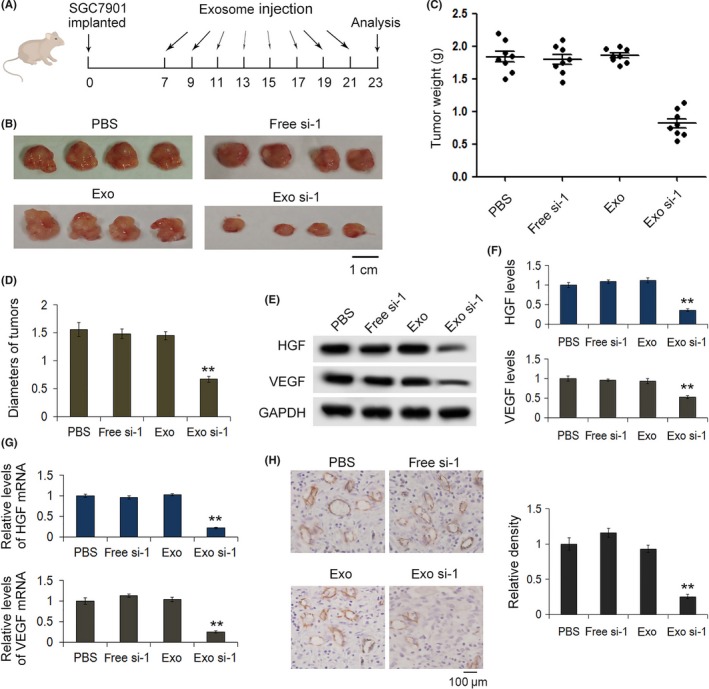
Exosomes deliver HGF siRNA in vivo. A, Sketch map of mouse implanted tumor model. SGC‐7901 cells were implanted into BALB/c mice and then exosomes packaged with siRNA against HGF (si.HGF‐1) were administered to mice via intravenous tail injection every 2 days. B–D, Representative image of tumors excised from nude mice (n = 8). The weights and sizes of tumors were clearly reduced by exosomes packaged with si.HGF‐1. E, HGF and vascular endothelial growth factor (VEGF) expression in implanted tumors (n = 8). F, Quantitative analysis of (E) (n = 8). G, Quantitative RT‐PCR analysis of HGF and VEGF mRNA levels in implanted tumors (n = 8). H, Immunohistochemical analysis of paraffin‐embedded tumor tissues from nude mice using CD31 antibodies, and quantitative analysis of CD31 intensity in tumor sections (n = 8). ** indicates P < .01. GAPDH, glyceraldehyde 3‐phosphate dehydrogenase; HGF, hepatocyte growth factor; VEGF, vascular endothelial growth factor
These in vivo results further demonstrate that exosomes can act as carriers of si.HGF‐1 to decrease HGF and VEGF expression in gastric cancer cells.
4. DISCUSSION
siRNA are produced from cytoplasmic dsRNA, which are cut into 21‐23 base‐pair fragments by an endonuclease called DICER.30 Following their formation, siRNA are incorporated into the RNA‐induced silencing complex and bind to target mRNA, leading to target mRNA degradation by cellular nucleases, and silencing of the target gene's expression. Because the expression and functionality of siRNA are significantly reduced in several diseases, siRNA have become attractive targets for the development of innovative therapeutics. Since 2010, a number of preclinical and clinical studies have been carried out to exploit the potential of siRNA for treating various genetic disorders, viral diseases, cancer and other non‐cancerous diseases.31 The preference for siRNA is related to their ease of synthesis, and the fact that they act in the cytoplasm rather than the nucleus, which facilitates their delivery.32 Even if few molecules of siRNA are delivered intact into the cytosol, they are likely sufficient to trigger a sustained gene silencing effect. Therefore, to enhance the clinical use of siRNA, finding an appropriate carrier to deliver siRNA to target cells is urgent.
Exosomes are naturally secreted, nano‐sized, lipid bilayer membrane‐enclosed vesicles that can cross biological membranes/barriers and deliver their payloads to recipient cells with virus‐like efficiency. Moreover, exosomes are less immunogenic, non‐cytotoxic and non‐mutagenic than currently existing viral or liposome‐based gene delivery vehicles. These characteristics suggest that exosomes can be developed as an ideal vehicle for therapeutic delivery.33 Over the past 5 years, exosomes have been seriously considered as drug delivery systems.34, 35, 36 Furthermore, exosomes can promote receptor‐ligand interactions, based on the affinity of signaling molecules on the surface of exosomes for targets on the receiving cells. Overall, compared to other candidate bio‐carriers, exosomes have been demonstrated to be the most suitable for therapeutic delivery.
We confirmed the high expression of HGF/c‐Met in gastric cancer tissues, which indicates that the inhibition of HGF/c‐Met may have a significant role in treating gastric cancer.37, 38 HGF also plays a prominent role in inducing the expression of VEGF.39 VEGF is one of the key factors for the migration of endothelial cells and the formation of new vessels during angiogenesis.40 Previous studies have described that the HGF/c–Met signaling pathway targets MAPK, PIK3 and Stat3 to upregulate VEGF expression.41, 42 Thus, the HGF/c–Met signaling pathway acts on VEGF to promote angiogenesis in cancer cells.
RNAi has become a promising tool for targeted therapy, but the technical obstacles for in vivo transport of siRNA have not yet been overcome. Our results show that cell‐derived exosomes are potential siRNA carriers which may provide novel treatment strategies for cancer.
CONFLICTS OF INTEREST
The authors have no conflicts of interest.
Zhang H, Wang Y, Bai M, et al. Exosomes serve as nanoparticles to suppress tumor growth and angiogenesis in gastric cancer by delivering hepatocyte growth factor siRNA. Cancer Sci. 2018;109:629–641. https://doi.org/10.1111/cas.13488
Funding information
National Natural Science Foundation of China (Nos. 81772629, 81602158, 81372394, 81602156 and 81702437); Tianjin Health and Family Planning Commission Foundation of Science and Technology (15KG142); CSCO‐Merck Serono Oncology Research Fund (Y‐MX2015‐092).
Haiyang Zhang, Yi Wang, Ming Bai, Junyi Wang and Kegan Zhu are contributed equally to this work.
Contributor Information
Guoguang Ying, Email: yingguoguang163@163.com.
Yi Ba, Email: bayi@tjmuch.com.
REFERENCES
- 1. Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569‐579. [DOI] [PubMed] [Google Scholar]
- 2. Fevrier B, Vilette D, Laude H, Raposo G. Exosomes: a bubble ride for prions? Traffic. 2005;6:10‐17. [DOI] [PubMed] [Google Scholar]
- 3. Cocucci E, Meldolesi J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015;25:364‐372. [DOI] [PubMed] [Google Scholar]
- 4. Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha‐granules. Blood. 1999;94:3791‐3799. [PubMed] [Google Scholar]
- 5. Johnstone RM. Revisiting the road to the discovery of exosomes. Blood Cells Mol Dis. 2005;34:214‐219. [DOI] [PubMed] [Google Scholar]
- 6. Gibbings DJ, Ciaudo C, Erhardt M, Voinnet O. Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat Cell Biol. 2009;11:1143‐1149. [DOI] [PubMed] [Google Scholar]
- 7. Cho JA, Yeo DJ, Son HY, et al. Exosomes: a new delivery system for tumor antigens in cancer immunotherapy. Int J Cancer. 2005;114:613‐622. [DOI] [PubMed] [Google Scholar]
- 8. Buschow SI, Liefhebber JM, Wubbolts R, Stoorvogel W. Exosomes contain ubiquitinated proteins. Blood Cells Mol Dis. 2005;35:398‐403. [DOI] [PubMed] [Google Scholar]
- 9. Zhang H, Bai M, Deng T, et al. Cell‐derived microvesicles mediate the delivery of miR‐29a/c to suppress angiogenesis in gastric carcinoma. Cancer Lett. 2016;375:331‐339. [DOI] [PubMed] [Google Scholar]
- 10. Jang SC, Kim OY, Yoon CM, et al. Bioinspired exosome‐mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano. 2013;7:7698‐7710. [DOI] [PubMed] [Google Scholar]
- 11. Guo J, Fisher KA, Darcy R, Cryan JF, O'Driscoll C. Therapeutic targeting in the silent era: advances in non‐viral siRNA delivery. Mol BioSyst. 2010;6:1143‐1161. [DOI] [PubMed] [Google Scholar]
- 12. Ozpolat B, Sood AK, Lopez‐Berestein G. Nanomedicine based approaches for the delivery of siRNA in cancer. J Intern Med. 2010;267:44‐53. [DOI] [PubMed] [Google Scholar]
- 13. Min JJ, Nguyen VH, Gambhir SS. Molecular imaging of biological gene delivery vehicles for targeted cancer therapy: beyond viral vectors. Nucl Med Mol Imaging. 2010;44:15‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koh SA, Kim MK, Lee KH, Kim SW, Kim JR. RhoGDI2 is associated with HGF‐mediated tumor invasion through VEGF in stomach cancer. Clin Exp Metastasis. 2014;31:805‐815. [DOI] [PubMed] [Google Scholar]
- 15. Yang X, Zhang XF, Lu X, et al. MicroRNA‐26a suppresses angiogenesis in human hepatocellular carcinoma by targeting hepatocyte growth factor‐cMet pathway. Hepatology. 2014;59:1874‐1885. [DOI] [PubMed] [Google Scholar]
- 16. Fajardo‐Puerta AB, Mato Prado M, Frampton AE, Jiao LR. Gene of the month: HGF. J Clin Pathol. 2016;69:575‐579. [DOI] [PubMed] [Google Scholar]
- 17. Husmann K, Ducommun P, Sabile AA, Pedersen EM, Born W, Fuchs B. Signal transduction and downregulation of C‐MET in HGF stimulated low and highly metastatic human osteosarcoma cells. Biochem Biophys Res Commun. 2015;464:1222‐1227. [DOI] [PubMed] [Google Scholar]
- 18. Cao HH, Cheng CY, Su T, et al. Quercetin inhibits HGF/c‐Met signaling and HGF‐stimulated melanoma cell migration and invasion. Mol Cancer. 2015;14:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li M, Xin X, Wu T, Hua T, Wang H. HGF and c‐Met in pathogenesis of endometrial carcinoma. Front Biosci (Landmark Ed). 2015;20:635‐643. [DOI] [PubMed] [Google Scholar]
- 20. Ciamporcero E, Miles KM, Adelaiye R, et al. Combination strategy targeting VEGF and HGF/c‐met in human renal cell carcinoma models. Mol Cancer Ther. 2015;14:101‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hu HJ, Lin XL, Liu MH, Fan XJ, Zou WW. Curcumin mediates reversion of HGF‐induced epithelial‐mesenchymal transition via inhibition of c‐Met expression in DU145 cells. Oncol Lett. 2016;11:1499‐1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome‐mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654‐659. [DOI] [PubMed] [Google Scholar]
- 23. Zhang Y, Liu D, Chen X, et al. Secreted monocytic miR‐150 enhances targeted endothelial cell migration. Mol Cell. 2010;39:133‐144. [DOI] [PubMed] [Google Scholar]
- 24. Akhtar N, Dickerson EB, Auerbach R. The sponge/Matrigel angiogenesis assay. Angiogenesis. 2002;5:75‐80. [DOI] [PubMed] [Google Scholar]
- 25. Malinda KM. In vivo matrigel migration and angiogenesis assay. Methods Mol Biol. 2009;467:287‐294. [DOI] [PubMed] [Google Scholar]
- 26. Liu Y, Li D, Liu Z, et al. Targeted exosome‐mediated delivery of opioid receptor Mu siRNA for the treatment of morphine relapse. Sci Rep. 2015;5:17543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ma R, Xu H, Wu J, et al. Identification of serum proteins and multivariate models for diagnosis and therapeutic monitoring of lung cancer. Oncotarget. 2017;8:18901‐18913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Graziano F, Catalano V, Lorenzini P, et al. Clinical impact of the HGF/MET pathway activation in patients with advanced gastric cancer treated with palliative chemotherapy. Pharmacogenomics J. 2014;14:418‐423. [DOI] [PubMed] [Google Scholar]
- 29. Day RM, Cioce V, Breckenridge D, Castagnino P, Bottaro DP. Differential signaling by alternative HGF isoforms through c‐Met: activation of both MAP kinase and PI 3‐kinase pathways is insufficient for mitogenesis. Oncogene. 1999;18:3399‐3406. [DOI] [PubMed] [Google Scholar]
- 30. Hannon GJ. RNA interference. Nature. 2002;418:244‐251. [DOI] [PubMed] [Google Scholar]
- 31. Zatsepin TS, Kotelevtsev YV, Koteliansky V. Lipid nanoparticles for targeted siRNA delivery – going from bench to bedside. Int J Nanomedicine. 2016;11:3077‐3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wittrup A, Lieberman J. Knocking down disease: a progress report on siRNA therapeutics. Nat Rev Genet. 2015;16:543‐552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. O'Loughlin AJ, Woffindale CA, Wood MJ. Exosomes and the emerging field of exosome‐based gene therapy. Curr Gene Ther. 2012;12:262‐274. [DOI] [PubMed] [Google Scholar]
- 34. Kong W, He L, Coppola M, et al. MicroRNA‐155 regulates cell survival, growth, and chemosensitivity by targeting FOXO3a in breast cancer. J Biol Chem. 2010;285:17869‐17879. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35. van Dommelen SM, Vader P, Lakhal S, et al. Microvesicles and exosomes: opportunities for cell‐derived membrane vesicles in drug delivery. J Control Release. 2012;161:635‐644. [DOI] [PubMed] [Google Scholar]
- 36. Kooijmans SA, Vader P, van Dommelen SM, van Solinge WW, Schiffelers RM. Exosome mimetics: a novel class of drug delivery systems. Int J Nanomedicine. 2012;7:1525‐1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kang YK, Muro K, Ryu MH, et al. A phase II trial of a selective c‐Met inhibitor tivantinib (ARQ 197) monotherapy as a second‐ or third‐line therapy in the patients with metastatic gastric cancer. Invest New Drugs. 2014;32:355‐361. [DOI] [PubMed] [Google Scholar]
- 38. Li K, Li J. Current molecular targeted therapy in advanced gastric cancer: a comprehensive review of therapeutic mechanism, clinical trials, and practical application. Gastroenterol Res Pract. 2016;2016:4105615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mohri Y, Miki C, Tanaka K, et al. Clinical correlations and prognostic relevance of tissue angiogenic factors in patients with gastric cancer. Clin Oncol (R Coll Radiol). 2012;24:610‐615. [DOI] [PubMed] [Google Scholar]
- 40. Guo D, Jia Q, Song HY, Warren RS, Donner DB. Vascular endothelial cell growth factor promotes tyrosine phosphorylation of mediators of signal transduction that contain SH2 domains. Association with endothelial cell proliferation. J Biol Chem. 1995;270:6729‐6733. [DOI] [PubMed] [Google Scholar]
- 41. Kaga T, Kawano H, Sakaguchi M, Nakazawa T, Taniyama Y, Morishita R. Hepatocyte growth factor stimulated angiogenesis without inflammation: differential actions between hepatocyte growth factor, vascular endothelial growth factor and basic fibroblast growth factor. Vascul Pharmacol. 2012;57:3‐9. [DOI] [PubMed] [Google Scholar]
- 42. Villaume K, Blanc M, Gouysse G, et al. VEGF secretion by neuroendocrine tumor cells is inhibited by octreotide and by inhibitors of the PI3K/AKT/mTOR pathway. Neuroendocrinology. 2010;91:268‐278. [DOI] [PubMed] [Google Scholar]


