Abstract
In oncology, actionable mutations (alterations) in cancer‐associated genes are critical in terms of the selection of therapeutic approaches. Next‐generation sequencing of tumor sample DNA (ie, clinical sequencing) can guide clinical management by providing diagnostic or prognostic data, and facilitating the identification of potential treatment regimens, such as molecular‐targeted and immune checkpoint blockade therapies. In the USA, a variety of tumor‐profiling multiplex gene panels have been developed and implemented for this purpose. In Japan, several academic institutions have now carried out detailed investigations of the feasibility and value of clinical sequencing, and cancer societies have issued consensus clinical practice guidance for next‐generation sequencing‐based gene panel tests. These efforts will facilitate the implementation of cancer genome medicine in Japan.
Keywords: actionable mutation, cancer genome medicine, clinical sequencing, implementation, insurance reimbursement
1. INTRODUCTION
An “actionable gene mutation (alteration)” in a tumor sample is defined as a DNA change with an expected (or predicted) impact on response to treatment.1 Drivers of tumorigenesis, and thus promising targets for therapeutic intervention, include recurrent hotspot mutations, fusions, and high‐level (eg, >6‐copy) focal amplifications. Screening for actionable mutations facilitates the identification of patients who will benefit from targeted therapy.
Cancer genome research has revealed a number of actionable gene mutations. In this context, the most extensively investigated cancer type is lung adenocarcinoma (LADC), which represents the most common histological lung cancer subtype. LADC can be classified according to the presence of mutually exclusive oncogene alterations, all of which drive tumorigenesis: EGFR (hotspot mutations) KRAS (hotspot mutations), and ALK (fusion). Research by the group of the present author and others added the RET and ROS1 gene fusions and the BRAF gene mutation to the list of actionable alterations, as it showed that alteration‐positive cases benefit from treatment with tyrosine kinase inhibitors (Figure 1).2, 3, 4, 5 These alterations are present in 1%‐2% of LADC cases. Genetic screening to diagnose actionable mutations is thus a critical step in terms of treatment selection in cancer patients. In February 2013, research, government, and pharmaceutical agencies in Japan initiated a nationwide genome screening program (LC‐SCRUM‐Japan) as a clinical research project to detect multiple oncogene alterations, including RET and ROS1 fusions and BRAF mutations, in lung cancer patients.6 As of December 2017, more than 5000 patients from 251 institutions had been enrolled. Patients who were positive for those oncogene alterations have been receiving (or received) targeted therapies using investigational drugs in clinical trials according to their gene alterations.3, 6, 7
Figure 1.
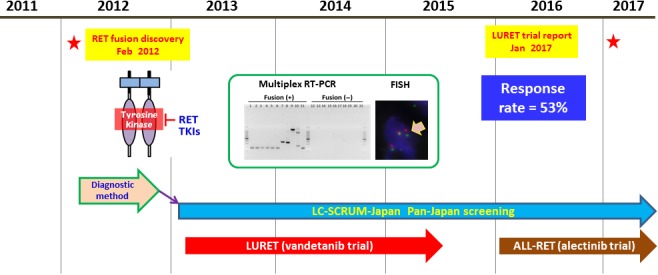
Discovery of RET fusion and its translation to clinical oncology. RET fusion was discovered in 2012.4 Nationwide screening for RET and ROS1 fusions within the context of LC‐SCRUM‐Japan commenced in 2013.6 LURET, an open‐label, multicenter, phase II trial of vandetanib for RET fusion‐positive lung cancer, also commenced in 2013. The LURET results were published in 2017.3 TKI, tyrosine kinase inhibitor
In the USA, the FDA approved the Oncomine Dx Target Test in June 2017 as a companion diagnostic test that simultaneously diagnoses alterations in three oncogenes, EGFR, BRAF, and ROS1, in lung cancer. Theoretically, this test can detect alterations of 23 cancer‐associated genes (https://www.accessdata.fda.gov/cdrh_docs/pdf16/p160045c.pdf). At present, it is used as a companion diagnostic tool to select lung cancer patients suitable for treatment with the approved targeted therapies for crizotinib (ROS1 fusion), dabrafenib and trametinib (BRAF mutation), and gefitinib (EGFR mutation) (Table 1).
Table 1.
Next‐generation sequencing‐based tumor‐profiling multiplex gene panels
| Test | Number of genes tested | Tumor sample | Non‐tumor sample | FDA approval | Companion diagnostic indications linked to gene alterations |
|---|---|---|---|---|---|
| Oncomine Dx target test | 23 | Tissue DNA/RNA | Not used | Yes | Lung cancer: EGFR, ROS1, and BRAF |
| FoundationOne CDx | 324 | Tissue DNA | Not used | Yes |
Lung cancer: EGFR, ALK, and BRAF
Melanoma: BRAF Breast cancer: HER2 (ERBB2) Colorectal cancer: KRAS and NRAS Ovarian cancer: BRCA1 and BRCA2 |
| MSK‐IMPACT | 468 | Tissue DNA | Peripheral blood | Yes | Unknown |
| Guardant360 | 73 | Cell‐free DNA | Not used | Unknown | Unknown |
| NCC oncopanel | 114 | Tissue DNA | Peripheral blood | Unknown | Unknown |
| Oncoprime | 215 | Tissue DNA | Not used | Unknown | Unknown |
Hundreds of actionable mutations with a potential response to molecular‐targeted drugs in kinase and other cancer‐associated genes have been identified. However, the majority are detected in only a small proportion of cases. This is exemplified by a hotspot AKT1 mutation, with a well replicated association to the efficacy of specific AKT inhibitors.8, 9 Approximately 40% of cancers are likely to have at least one actionable gene mutation associated with approved or experimental targeted drugs.10, 11, 12 Actionable mutations in cancer‐associated genes are therefore of critical importance in terms of therapy selection.
2. CLINICAL SEQUENCING USING NEXT‐GENERATION SEQUENCING (NGS) PANELS IN THE USA
In the clinical setting, sequencing is often undertaken to detect actionable gene mutations in patients with advanced cancer. At this point, oncologists propose sequencing to identify alternative therapies. The introduction of massively parallel NGS has enabled the simultaneous examination of more than 100 genes, in which actionable mutations have been detected. Clinical laboratories in the USA have developed and implemented a variety of NGS‐based tests (tumor‐profiling multiplex gene panels), ranging from targeted “hotspot” panels (Table 1) to comprehensive genome‐scale platforms.13, 14, 15, 16
To ensure that the results can be applied in clinical practice, these NGS‐based tests are carried out in Clinical Laboratory Improvement Amendments (CLIA)‐certified laboratories. Importantly, CLIA‐certified laboratories can undertake laboratory developed tests that have not been submitted for FDA approval (Figure 2). Profiling provides information concerning diagnosis or prognosis, and facilitates the identification of potential treatment regimens involving molecular‐targeted drugs. Such regimens include FDA‐approved medication for defined tumor types, off‐label treatment for non‐approved tumor types, and targeted therapy in clinical trials with investigational agents.
Figure 2.
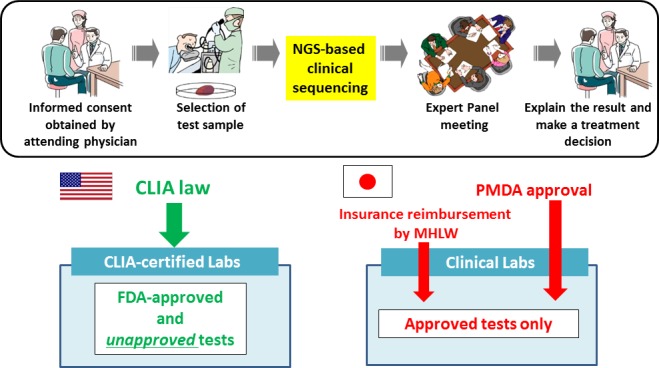
Differences in laboratory test regulations in the USA and Japan. In the USA, Clinical Laboratory Improvement Amendments (CLIA)‐certified laboratories can deploy laboratory developed tests that have not been submitted for FDA approval. In Japan, every test must be approved by the Pharmaceuticals and Medical Devices Agency (PMDA) and the Ministry of Health, Labor, and Welfare (MHLW) prior to application in clinical settings. NGS, next‐generation sequencing
In the USA, scientists at the Memorial Sloan Kettering (MSK) Cancer Center have developed and implemented the MSK‐IMPACT (Integrated Mutation Profiling of Actionable Cancer Targets) test, which detects alterations in cancer‐associated genes using tumor and matched normal peripheral blood, and the Illumina HiSeq 2500 Sequencer. When first developed, the MSK‐IMPACT panel could detect alterations in 341 genes, and this number has increased over time. In a 2017 publication,12 MSK researchers reported that 37% of 10 000 investigated patients harbored at least one actionable mutation, and that 11% of the first 5009 patients who received the MSK‐IMPACT test were subsequently enrolled on genomically matched clinical trials. These results do not include those of several hundred additional MSK patients, who received FDA‐approved targeted therapies outside the clinical trial context. The MSK‐IMPACT test, which currently examines 468 cancer‐associated genes, has now been approved by the FDA (https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm585347.htm). The US molecular diagnostics company Foundation Medicine developed the FoundationOne CDx test, in which 324 cancer‐associated genes are investigated using DNA from tumor tissue. To date, the company has analyzed more than 100 000 cases and indicates that the NGS panel test provides an accurate assessment of high tumor mutational burden,17 which is an emerging biomarker of sensitivity to immune checkpoint blockade therapy.18, 19 The FoundationOne test has now also been approved by the FDA (https://www.fda.gov/medicaldevices/productsandmedicalprocedures/deviceapprovalsandclearances/recently-approveddevices/ucm590331.htm). This is also intended as a companion diagnostic test to identify patients suitable for treatment with targeted therapies (listed in Table 1) in accordance with the approved therapeutic product labeling. The FDA approval of these NGS‐based tests will facilitate cancer genome medicine in the US by promoting insurance reimbursement in clinical oncology.20
3. CLINICAL SEQUENCING IN JAPAN
In Japan, clinical research and regulatory frameworks have reduced the delay in the introduction of new drugs and medical devices (“drug and device lags”).21 However, tumor‐profiling multiplex gene panel tests have not yet been implemented in routine oncological practice. Due to the lack of CLIA‐like regulation, each test must be approved by the Pharmaceuticals and Medical Devices Agency (PMDA) before insurance reimbursement can be approved by the Japanese Ministry of Health, Labor, and Welfare.
Several research teams in Japan, including that of the author, are engaged in clinical research to facilitate the implementation of clinical sequencing.22, 23 The author's group has initiated a prospective cohort study to investigate the feasibility and utility of the NGS‐based analysis of 114 cancer‐associated genes (NCC oncopanel system) in patients with advanced solid tumors (Table 1). This study is termed TOP‐GEAR (Trial of Onco‐Panel for Gene‐profiling to Estimate both Adverse events and Response during cancer treatment, UMIN 000011141). In an analysis of the first 131 cases, actionable mutations were detected in 45% of the cohort.23 Patients were enrolled on genomically matched phase I clinical trials at a rate of 8% (11 of the 131 cases). The median progression‐free survival (PFS) was longer in patients with matched therapy than in patients with non‐matched therapy (5.5 vs 1.9 months). This study, together with another clinical sequencing study,22 indicates the feasibility and utility of NGS‐based testing in clinical oncology in Japan. In collaboration with a Japanese diagnostics company, the NCC oncopanel system is now being improved prior to submission for PMDA approval. This work is being carried out within the context of the SAKIGAKE program of the Japanese Ministry of Health, Labor, and Welfare. In 2018, the research team plans to validate the clinical utility of the NCC oncopanel system within the Advanced Medical Care B system.
4. STANDARDIZING THE INTERPRETATION OF MUTATION RESULTS
Widespread clinical sequencing in the USA has highlighted the importance and potential benefits of standardizing the interpretation and reporting of mutation results across laboratories.24 For this purpose, the Japanese Society of Medical Oncology, the Japanese Society of Clinical Oncology, and the Japanese Cancer Association issued consensus clinical practice guidance for NGS‐based cancer tests (http://www.jsmo.or.jp/about/kanko.html#guideline). This guidance proposes that gene alterations should be classified on the basis of clinical significance. The following levels of evidence were suggested: level 1A, PMDA‐approved biomarker in the tumor type; 1B, FDA‐approved biomarker in the tumor type (not approved by the PMDA) or biomarker shown by a prospective molecularly driven clinical trial; 2A, biomarker shown by subgroup analysis in a prospective clinical trial; 2B, approved biomarker in a different tumor type or biomarker with evidence of clinical utility; 3A, biomarker with evidence of proof of concept in at least one case report; 3A, biomarker with evidence obtained by in vitro/in vivo experiments; and 4, other gene mutations in cancer. This guidance is intended to facilitate and standardize the interpretation of clinical sequencing results.
Prior to reporting, clinical sequencing results must be discussed by a tumor board to provide expert consensus on interpretation. Ideally, the tumor board should comprise experts from a range of specialties, such as clinical oncology, pathology, genome science, bioinformatics, medical genetics, and genetic counseling. The volume of available cancer genomic data is increasing rapidly. Therefore, the significance of gene alterations in terms of diagnosis, therapy, and prognosis requires continuous re‐evaluation. Clinical oncologists must consider an ever‐expanding list of actionable mutations and investigational agents. Furthermore, reports of the molecular basis of pronounced treatment responses in single patients or small cohorts (exceptional responders) may generate new hypotheses concerning actionability.25 However, researchers must exercise caution and avoid over‐interpretation, as a given mutation might not be actionable in a different setting due to tissue‐specific effects. For instance, BRAF inhibitors are effective in BRAF‐mutant melanoma but not in colorectal cancer.26
5. OUTLOOK
In Japan, the implementation of tumor‐profiling multiplex gene panel tests in cancer genome medicine will be realized in the near future. At present, a gap exists between the number of patients with actionable mutations and those receiving genomically matched therapy. This gap is attributable to the lack of availability/accessibility of relevant trials and drugs, and the poor performance status of the respective patients. The approval of novel targeted therapies and subsequent proliferation of molecularly driven clinical trials will increase demand for clinical sequencing. The clinical relevance of sequencing tests will also be enhanced by the development of therapies for gene alterations that are frequent but currently “undruggable”, such as deleterious mutations in the chromatin regulator genes.27, 28, 29 Functional annotation of mutations that are currently classified as variants of unknown significance will also facilitate clinical sequencing by prioritizing further actionable mutations.30, 31
Of course, finding actionable mutations in a patient's tumor does not imply that the patient will respond to a therapeutic agent against that target. It is important to examine whether or not a treatment based on the gene profile of an individual patient can really improve the clinical course of his or her disease. As reported recently,32 comparing the PFS of a treatment regimen based on the gene profiles of the patient's tumor with that of the PFS of the most recent treatment regimen that resulted in a decrease in disease progression (ie, by calculating the PFS ratio using the outcome of the patient's previous treatment history as a control) might be a way to address this issue.
The implementation of clinical sequencing in Japan presents several challenges. First, germline mutations can be detected as secondary findings in a small percentage of patients, as reported in a recent study of East Asians.33 Implications in terms of informed consent, genetic counseling, and total care must therefore be taken into account. Second, many NGS‐based gene panel tests, including the ones to examine cell‐free DNA, such as the Guardant360 test, are expensive.20 Japan operates a universal health‐care system, in which patients are expected to pay 30% of the total cost of treatment.34 The cost of clinical sequencing tests will therefore be a major economic issue for patients and the government. Ongoing discussion between representatives from industry, academia, and relevant regulatory bodies is warranted to facilitate the implementation of cancer genome medicine in Japan.
CONFLICT OF INTEREST
The author is a recipient of a collaborative research grant from the Sysmex Corporation.
ACKNOWLEDGMENTS
This work was supported in part by grants‐in‐aid from the Japanese Agency for Medical Research and Development (AMED) (JP17kk0205004 and JP17lk1403003), and the National Cancer Center Research and Development Fund (27‐A‐1). The author is grateful to the following scholars for fruitful discussion: Atsushi Ochiai, Yasuhiro Fujiwara, Atsushi Ohtsu, Hiroyuki Mano, Noboru Yamamoto, Kenji Tamura, Hitoshi Ichikawa, Kuniko Sunami, Takashi Kubo, Mamoru Kato, Teruhiko Yoshida, Kazuya Tsuchihara, Koichi Goto, Takayuki Yoshino, Kazuto Nishio, Manabu Muto, and Sadakatsu Ikeda. The author is also grateful to many other staff at the National Cancer Center, Japan.
Kohno T. Implementation of “clinical sequencing” in cancer genome medicine in Japan. Cancer Sci. 2018;109:507–512. https://doi.org/10.1111/cas.13486
REFERENCES
- 1. Carr TH, McEwen R, Dougherty B, et al. Defining actionable mutations for oncology therapeutic development. Nat Rev Cancer. 2016;16:319‐329. [DOI] [PubMed] [Google Scholar]
- 2. Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1‐rearranged non‐small‐cell lung cancer. N Engl J Med. 2014;371:1963‐1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yoh K, Seto T, Satouchi M, et al. Vandetanib in patients with previously treated RET‐rearranged advanced non‐small‐cell lung cancer (LURET): an open‐label, multicentre phase 2 trial. Lancet Respir Med. 2017;5:42‐50. [DOI] [PubMed] [Google Scholar]
- 4. Kohno T, Ichikawa H, Totoki Y, et al. KIF5B‐RET fusions in lung adenocarcinoma. Nat Med. 2012;18:375‐377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Planchard D, Smit EF, Groen HJM, et al. Dabrafenib plus trametinib in patients with previously untreated BRAF(V600E)‐mutant metastatic non‐small‐cell lung cancer: an open‐label, phase 2 trial. Lancet Oncol. 2017;18:1307‐1316. [DOI] [PubMed] [Google Scholar]
- 6. Bando H. The current status and problems confronted in delivering precision medicine in Japan and Europe. Curr Probl Cancer. 2017;41:166‐175. [DOI] [PubMed] [Google Scholar]
- 7. Takeuchi S, Murayama T, Yoshimura K, et al. Phase I/II study of alectinib in lung cancer with RET fusion gene: study protocol. J Med Invest. 2017;64:317‐320. [DOI] [PubMed] [Google Scholar]
- 8. Carpten JD, Faber AL, Horn C, et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007;448:439‐444. [DOI] [PubMed] [Google Scholar]
- 9. Davies BR, Guan N, Logie A, et al. Tumors with AKT1E17K mutations are rational targets for single agent or combination therapy with AKT inhibitors. Mol Cancer Ther. 2015;14:2441‐2451. [DOI] [PubMed] [Google Scholar]
- 10. Mendelsohn J. Personalizing oncology: perspectives and prospects. J Clin Oncol. 2013;31:1904‐1911. [DOI] [PubMed] [Google Scholar]
- 11. Hovelson DH, McDaniel AS, Cani AK, et al. Development and validation of a scalable next‐generation sequencing system for assessing relevant somatic variants in solid tumors. Neoplasia 2015;17:385‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zehir A, Benayed R, Shah RH, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23:703‐713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jones S, Anagnostou V, Lytle K, et al. Personalized genomic analyses for cancer mutation discovery and interpretation. Sci Transl Med 2015;7:283ra53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roychowdhury S, Iyer MK, Robinson DR, et al. Personalized oncology through integrative high‐throughput sequencing: a pilot study. Sci Transl Med 2011;3:111ra21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Malek J, Slashinski MJ, Robinson JO, et al. Parental perspectives on whole‐exome sequencing in pediatric cancer: a typology of perceived utility. JCO Precis Oncol. 2017;1:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Millis SZ, Ikeda S, Reddy S, Gatalica Z, Kurzrock R. Landscape of phosphatidylinositol‐3‐kinase pathway alterations across 19784 diverse solid tumors. JAMA Oncol. 2016;2:1565‐1573. [DOI] [PubMed] [Google Scholar]
- 17. Chalmers ZR, Connelly CF, Fabrizio D, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA‐4 blockade in melanoma. N Engl J Med. 2014;371:2189‐2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD‐1 blockade in non‐small cell lung cancer. Science. 2015;348:124‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chakradhar S. Tumor sequencing takes off, but insurance reimbursement lags. Nat Med. 2014;20:1220‐1221. [DOI] [PubMed] [Google Scholar]
- 21. Fujiwara Y. Evolution of frameworks for expediting access to new drugs in Japan. Nat Rev Drug Discovery. 2016;15:293‐294. [DOI] [PubMed] [Google Scholar]
- 22. Kou T, Kanai M, Yamamoto Y, et al. Clinical sequencing using a next‐generation sequencing‐based multiplex gene assay in patients with advanced solid tumors. Cancer Sci. 2017;108:1440‐1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tanabe Y, Ichikawa H, Kohno T, et al. Comprehensive screening of target molecules by next‐generation sequencing in patients with malignant solid tumors: guiding entry into phase I clinical trials. Mol Cancer. 2016;15:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li MM, Datto M, Duncavage EJ, et al. Standards and guidelines for the interpretation and reporting of sequence variants in cancer: a Joint Consensus Recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J Mol Diagn. 2017;19:4‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chau NG, Lorch JH. Exceptional responders inspire change: lessons for drug development from the bedside to the bench and back. Oncologist. 2015;20:699‐701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Prahallad A, Sun C, Huang S, et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature. 2012;483:100‐103. [DOI] [PubMed] [Google Scholar]
- 27. St Pierre R, Kadoch C. Mammalian SWI/SNF complexes in cancer: emerging therapeutic opportunities. Curr Opin Genet Dev. 2017;42:56‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lasko LM, Jakob CG, Edalji RP, et al. Discovery of a selective catalytic p300/CBP inhibitor that targets lineage‐specific tumours. Nature. 2017;550:128‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ogiwara H, Sasaki M, Mitachi T, et al. Targeting p300 addiction in CBP‐deficient cancers causes synthetic lethality by apoptotic cell death due to abrogation of MYC expression. Cancer Discov. 2016;6:430‐445. [DOI] [PubMed] [Google Scholar]
- 30. Kohsaka S, Nagano M, Ueno T, et al. A method of high‐throughput functional evaluation of EGFR gene variants of unknown significance in cancer. Sci Transl Med. 2017;9:eaan6566. [DOI] [PubMed] [Google Scholar]
- 31. Foley SB, Rios JJ, Mgbemena VE, et al. Use of whole genome sequencing for diagnosis and discovery in the cancer genetics clinic. EBioMedicine. 2015;2:74‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Von Hoff DD, Stephenson JJ Jr, Rosen P, et al. Pilot study using molecular profiling of patients’ tumors to find potential targets and select treatments for their refractory cancers. J Clin Oncol. 2010;28:4877‐4883. [DOI] [PubMed] [Google Scholar]
- 33. Tang CS, Dattani S, So MT, et al. Actionable secondary findings from whole‐genome sequencing of 954 East Asians. Hum Genet. 2017. [Epub ahead of print]. https://doi.org/10.1007/s00439-017-1852-1. [DOI] [PubMed] [Google Scholar]
- 34. Fujiwara Y, Yonemori K, Shibata T, Okita N, Ushirozawa N. Japanese universal health care faces a crisis in cancer treatment. Lancet Oncol. 2015;16:251‐252. [DOI] [PubMed] [Google Scholar]


