Abstract
Immune checkpoint inhibitor therapy has been clinically introduced for several malignancies, and its effectiveness has been confirmed by clinical trials. In particular, programmed cell death protein 1 (PD‐1) and programmed death‐ligand 1 (PD‐L1) are widely known as important immune checkpoint molecules associated with the mechanisms of immune escape by malignant tumor cells. In addition, liquid biopsy of blood specimens has the clinical benefit of providing a simple, repeatable sampling tool. Non‐invasive liquid biopsy has recently been spotlighted as a promising approach to predicting tumor progression and prognosis. This study assessed the clinical significance of PD‐L1 mRNA expression in blood specimens obtained from patients with gastric cancer. Peripheral blood specimens were collected before treatment from 124 patients with gastric cancer. The PD‐L1 mRNA expression was evaluated by quantitative RT‐PCR. Programmed death‐ligand 1 mRNA expression was significantly higher in patients with advanced gastric cancer than in patients with early gastric cancer (P = .002). Moreover, PD‐L1 expression correlated significantly with depth of tumor invasion, distant metastasis, and stage (P = .001, P < .001, and P < .001, respectively). Patients with high PD‐L1 expression showed significantly poorer prognosis than those with low PD‐L1 expression (P < .0001). Multivariate analysis indicated PD‐L1 expression as an independent prognostic factor. Expression of PD‐L1 in peripheral blood may offer an immunological predictor of tumor progression and disease outcome in patients with gastric cancer.
Keywords: gastric cancer, liquid biopsy, peripheral blood, prognosis, programmed death‐ligand 1
1. INTRODUCTION
The incidence of patients with gastric cancer has gradually decreased over recent decades.1 Gastric cancer represents the third‐ and fifth‐leading cause of cancer death in male and female individuals, respectively, worldwide.1 This indicates that patients with unresectable advanced and recurrent gastric cancer have poor prognosis despite recent advances in chemotherapeutic treatments, including the introduction of novel molecularly targeted drugs such as trastuzumab and ramucirumab.2 Novel therapeutic strategies based on new antitumor agents are thus important for patients with advanced gastric cancer.
Immune checkpoint blockade has gained attention as a new molecularly targeted therapy in patients with various malignancies, including gastric cancer.3, 4 Surprisingly, immune checkpoint inhibitor therapy appears to offer the clinical potential to control tumor growth and distant metastasis.3, 4 According to a phase III controlled study of nivolumab, an anti‐programmed cell death protein 1 (PD‐1) antibody, in previously untreated patients with advanced melanoma, 1‐year overall survival rates were 72.9% and 42.1% in the nivolumab and dacarbazine groups, respectively.5 In addition, objective response rates were 40.0% and 13.9% in the nivolumab and dacarbazine groups, respectively.5 Accordingly, that CheckMate‐066 clinical trial reported the clinical utility of immune checkpoint blockade as a promising antitumor agent in patients with metastatic melanoma. Programmed cell death protein‐1 and programmed death‐ligand 1 (PD‐L1) are representative immune checkpoint molecules and the PD‐1/PD‐L1 signaling pathway plays a principal role in the inhibition of T cell‐mediated immune response.6, 7, 8
To date, immunohistochemical analyses have indicated that PD‐L1 is overexpressed in tumor cells with melanoma, non‐small‐cell lung cancer, renal cell carcinoma, and gastric cancer.9, 10, 11, 12 Recent studies have shown a close relationship between PD‐L1 expression in primary tumor cells of several malignancies and well‐known prognostic clinicopathological factors, such as depth of tumor invasion, lymph node metastasis, distant metastasis, and stage.9, 10, 11, 12 However, the clinical significance of PD‐L1 expression in blood specimens of patients with gastric cancer has not yet been assessed. Naturally, non‐invasive liquid biopsy using blood specimens has the clinical attractiveness of being a simple and repeatable sampling tool.13
The aim of this study was to investigate PD‐L1 expression in blood specimens and to assess the relationships between PD‐L1 expression and clinicopathological features, including prognosis, in patients with gastric cancer.
2. MATERIALS AND METHODS
2.1. Gastric cancer cell lines
Three gastric cancer cell lines (MKN‐7, MKN‐74, and NCI‐N87) were cultured in RPMI‐1640 (Nissui Pharmaceutical, Tokyo, Japan) supplemented with 10% FCS (Mitsubishi Kasei, Tokyo, Japan), 100 U/mL penicillin, and 100 U/mL streptomycin. All cell lines were incubated at 37°C in a humidified atmosphere containing 5% CO2, then assessed by immunocytochemistry and RT‐PCR.
2.2. Patients
The present study enrolled 124 patients (81 men, 43 women; age range, 37‐88 years; average, 66 years) with gastric cancer who had undergone curative gastrectomy with lymph node dissection or chemotherapy at Kagoshima University Hospital (Kagoshima, Japan) between 2010 and 2015. Patients who had undergone endoscopic treatments were excluded from this study. Blood specimens were collected within 2 weeks prior to starting treatment. Patients were classified and staged based on the UICC criteria of TNM classification for gastric carcinoma.14 All patients were followed up every 3‐6 months by regular clinical diagnostic examinations, such as tumor marker studies of serum carcinoembryonic antigen (CEA) and carbohydrate antigen (CA)19‐9, radiography, ultrasonography, and computed tomography at Kagoshima University Hospital. The median follow‐up period was 31 months (range, 3‐81 months).
The Ethics Committee at Kagoshima University approved this study and all patients provided written, informed consent to blood specimen collection.
2.3. Immunocytochemistry
Three gastric cancer cells were cultured on Lab‐Tek II chamber slides (Nalge Nunc International, Naperville, IL, USA), washed with PBS, and fixed with 4% paraformaldehyde in PBS for 10 minutes. Cells were washed in PBS again, then stained using anti‐human PD‐L1 mAb (ab205921; Abcam, Cambridge, MA, USA) and Alexa Fluor‐conjugated goat anti‐rabbit IgG secondary antibody (ab150077; Abcam) at room temperature for 1 hour. Slides were mounted with Vectashield Mounting Medium containing DAPI (Vector Laboratories, Burlingame, CA, USA) for nuclear staining, then cells were assessed under fluorescence microscopy (UIS2; Olympus, Tokyo, Japan).
2.4. Blood processing and RNA extraction for RT‐PCR assay
Blood (5 mL) was withdrawn from each patient into tubes containing sodium citrate before treatment. Blood specimens were processed within 30 minutes after drawing. Blood cells were then collected using lymphocyte separation buffer (Gentra Systems, Minneapolis, MN, USA). All blood and cell lines were mixed with 1 mL Isogen (Nippon Gene, Toyama, Japan) and immediately cryopreserved at −80°C until RNA extraction. Total RNA was isolated and purified using phenol‐chloroform, as previously described.15 The concentration and purity of total RNA were examined using a NanoDrop Lite spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA).
2.5. Primers and probes
Primer and probe sequences of PD‐L1 and GAPDH were designed to determine the mRNA expression of each marker for RT‐PCR assay in blood specimens. The forward primers, fluorescence resonance energy transfer probe sequence, and reverse primers for PD‐L1 and GAPDH were as follows: PD‐L1 (forward), 5′‐GGTCATCCCAGAACTACCTCTG‐3′; (probe), 5′‐FAM‐TGGGAGCCATCTTATTATGCCTTGG‐TAMRA‐1‐3′; (reverse), 5′‐CGGAAGATGAATGTCAGTGC‐3′; GAPDH (forward), 5′‐GGGTGTGAACCATGAGAAGT‐3′; (probe), 5′‐FAM‐CAGCAATGCCTCCTGCACCACCAA‐TAMRA‐1‐3′; and (reverse), 5′‐GACTGTGGTCATGAGTCCT‐3′. The integrity of RNA was verified by RT‐PCR assay using GAPDH.
2.6. Quantitative RT‐PCR assay
All total RNA samples were reverse transcribed using the Advantage RT‐for‐PCR kit (Takara Bio USA, Terra Bella, CA, USA), as previously described.15 Samples were analyzed by quantitative RT‐PCR (qRT‐PCR) using the LightCycler Nano System (Roche Diagnostics, Mannheim, Germany). Reaction mixtures contained cDNA from 250 ng RNA, each primer, probe, MgCl2, and LightCycler FastStart DNA Master hybridization probes (Roche Diagnostics). The amplification profile consisted of a precycling hold at 95°C for 10 minutes, followed by 45 cycles of denaturation at 95°C for 10 seconds, annealing at 63°C for 20 seconds, and extension at 72°C for 10 seconds. Plasmids for each marker were synthesized using pT7Blue‐2 T‐Vector (Novagen, Madison, WI, USA) according to the instructions from the manufacturer. Standard curves for each assay were generated using the threshold cycles of four serial dilutions of plasmid templates (106‐103 copies). The mRNA copy number was measured using LightCycler software (Roche Diagnostics). Each assay was repeated in duplicate with positive (gastric cancer cell line), negative (H2O), and reagent (without DNA) controls to verify qRT‐PCR assays. Absolute copy numbers were calculated based on standard curves with four serial dilutions of plasmid templates. Programmed death‐ligand 1 mRNA copy numbers were normalized by GAPDH mRNA copy numbers (relative PD‐L1 mRNA copies: absolute PD‐L1 mRNA copies / absolute GAPDH mRNA copies).
2.7. Statistical analysis
Differences in PD‐L1 mRNA expression between blood specimens from patients with early gastric cancer and from those with advanced gastric cancer were assessed using the Wilcoxon rank‐sum test. Receiver operating characteristic (ROC) curves were constructed, then the area under the curve was calculated to evaluate the predictive ability of PD‐L1 mRNA expression for distinguishing patients with and without distant metastasis. The relationship between the status of PD‐L1 expression and categorical clinicopathological features was statistically analyzed using the chi‐squared‐test and Fisher's exact tests. Survival curves were generated using the Kaplan–Meier method and differences in survival were examined by the log–rank test. Prognostic factors were determined by univariate and multivariate analyses (Cox proportional hazard regression modeling). All data were significantly analyzed using SAS statistical software (SAS Institute, Cary, NC, USA). A value of P < .05 was considered significant.
3. RESULTS
3.1. Immunocytochemistry for PD‐L1 protein expression in cell lines
We examined morphological PD‐L1 protein expression based on immunocytochemical analysis in gastric cancer cell lines, such as MKN‐7, MKN‐74, and NCI‐N87. Programmed death‐ligand 1 protein expression was found in all gastric cancer cell lines (Figure 1).
Figure 1.
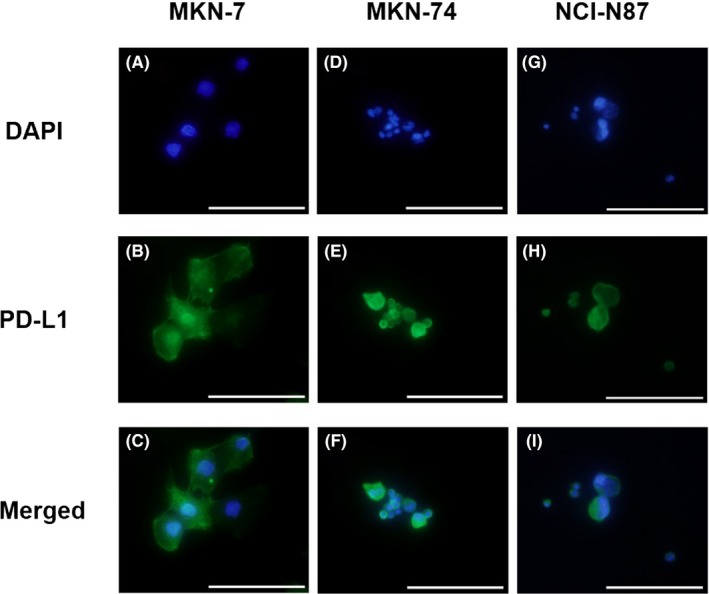
Immunocytochemical staining for programmed death‐ligand 1 (PD‐L1) expression in gastric cancer cell lines using DAPI (A,D,G) and anti‐PD‐L1 mAb–phycoerythrin staining (B,E,H). C,F,I, Merged staining of PD‐L1‐PE and DAPI. PD‐L1 is expressed in tumor cells of MKN‐7, MKN‐74, and NCI‐N87 cell lines. Scale bar = 100 μm (original magnification, ×400)
3.2. Quantitative RT‐PCR assay for PD‐L1 mRNA expression in cell lines and clinical blood specimens
The PD‐L1 mRNA expression in three gastric cancer cell lines and 124 blood specimens from patients with gastric cancer were evaluated by qRT‐PCR assay.
Ranges of relative PD‐L1 mRNA copies were from 8.43 × 10−3 to 2.51 × 10−2, from 1.00 × 10−4 to 6.10 × 10−3, and from 1.12 × 10−4 to 2.83 × 10−2 in gastric cancer cell lines, blood specimens from patients with early gastric cancer, and blood specimens from patients with advanced gastric cancer, respectively. Mean relative numbers of PD‐L1 mRNA copies (±SD) were 1.43 × 10−2 ± 9.44 × 10−3, 1.02 × 10−3 ± 1.22 × 10−3, and 2.71 × 10−3 ± 4.38 × 10−3 in gastric cancer cell lines, blood specimens from patients with early gastric cancer, and blood specimens from patients with advanced gastric cancer, respectively (Figure 2). Accordingly, the relative number of PD‐L1 mRNA copies was significantly lower in patients with early gastric cancer than in advanced gastric cancer (P = .002).
Figure 2.
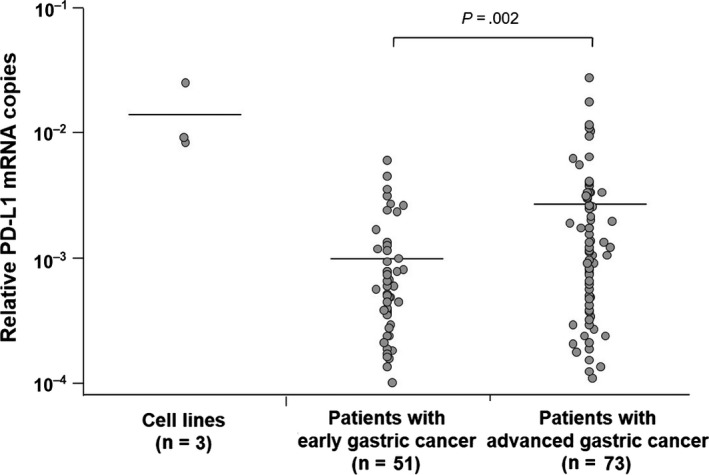
Reverse transcription–PCR assay for programmed death‐ligand 1 (PD‐L1) mRNA expression in gastric cancer cell lines and clinical blood specimens obtained from patients with early and advanced gastric cancer. Horizontal bars indicate mean PD‐L1 mRNA copy number
3.3. Relationship between PD‐L1 expression and clinicopathological features
To assess the relationship between the status of PD‐L1 mRNA expression and clinicopathological factors, we divided all patients into groups with high (n = 62) and low expression (n = 62) based on the median relative number of PD‐L1 mRNA copies. This binary classification for PD‐L1 mRNA expression was used in subsequent analyses.
The status of PD‐L1 expression correlated significantly with depth of tumor invasion, distant metastasis, and UICC stage (P = .001, P < .001, and P < .001, respectively) (Table 1). According to the ROC analysis, the area under the curve for detecting patients with distant metastasis was 0.772 (Figure 3). The sensitivity and specificity of PD‐L1 mRNA expression for detecting patients with distant metastasis were 0.814 and 0.667, respectively.
Table 1.
Relationship between programmed death‐ligand 1 (PD‐L1) expression and clinicopathological factors in 124 patients with gastric cancer
| Factors | PD‐L1 expression (%) | P‐value | |
|---|---|---|---|
| Low (n = 62) | High (n = 62) | ||
| Gender | |||
| Male | 41 (66.1) | 40 (64.5) | 1.000 |
| Female | 21 (33.9) | 22 (35.5) | |
| Age, years | |||
| ≤70 | 39 (62.9) | 36 (58.1) | .714 |
| >70 | 23 (37.1) | 26 (41.9) | |
| Tumor location | |||
| Upper | 17 (24.4) | 19 (30.6) | .657 |
| Middle | 26 (41.9) | 28 (45.2) | |
| Lower | 19 (30.7) | 15 (24.2) | |
| Histological type | |||
| Differentiated | 18 (29.0) | 18 (29.0) | 1.000 |
| Undifferentiated | 44 (71.0) | 44 (71.0) | |
| Depth of tumor invasion | |||
| T1‐T2 | 38 (61.3) | 19 (30.7) | .001 |
| T3‐T4 | 24 (38.7) | 43 (69.3) | |
| Lymph node metastasis | |||
| Negative | 40 (64.5) | 31 (50.0) | .146 |
| Positive | 22 (35.5) | 31 (50.0) | |
| Distant metastasis | |||
| Negative | 54 (87.1) | 31 (50.0) | <.001 |
| Positive | 8 (12.9) | 31 (50.0) | |
| Stage | |||
| I‐II | 45 (72.6) | 23 (37.1) | <.001 |
| III‐IV | 17 (27.4) | 39 (62.9) | |
| Serum CEA levels (<5 ng/mL) | |||
| Negative | 54 (87.2) | 46 (74.2) | .110 |
| Positive | 8 (12.9) | 16 (25.8) | |
| Serum CA19‐9 levels (<37 U/mL) | |||
| Negative | 57 (91.9) | 50 (80.7) | .115 |
| Positive | 5 (8.1) | 12 (19.3) | |
CA19‐9, carbohydrate antigen 19‐9; CEA, carcinoembryonic antigen; T1, invasion of lamina propria or submucosa; T2, invasion of muscularis propria; T3, invasion of subserosa; T4, penetration of serosa or invasion of adjacent structures.
Figure 3.
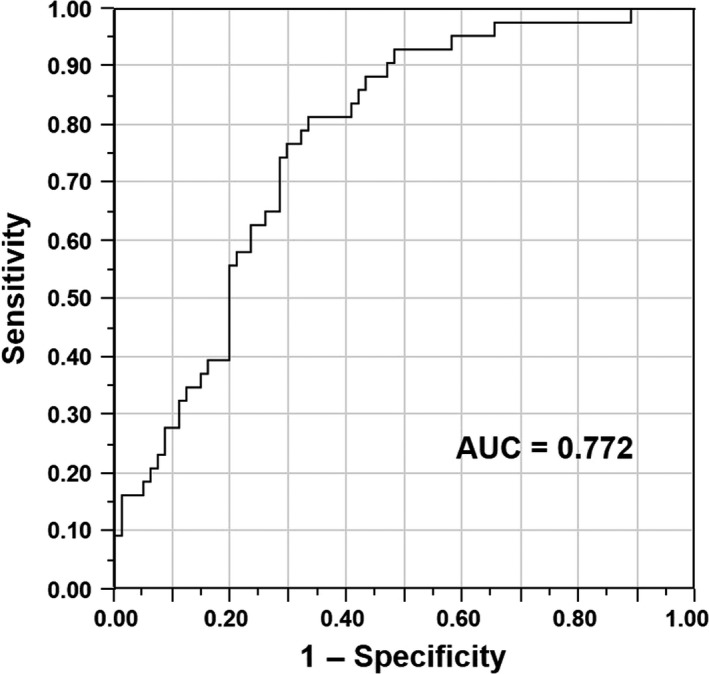
Receiver operating characteristic curve for discriminating gastric cancer patients with and without distant metastasis based on programmed death‐ligand 1 mRNA expression. Area under the curve was 0.772
3.4. Relationship between PD‐L1 expression and prognosis
The 5‐year survival rate was significantly lower in patients with high PD‐L1 expression than in those with low PD‐L1 expression (50.0% vs 84.1%, P < .0001; Figure 4).
Figure 4.
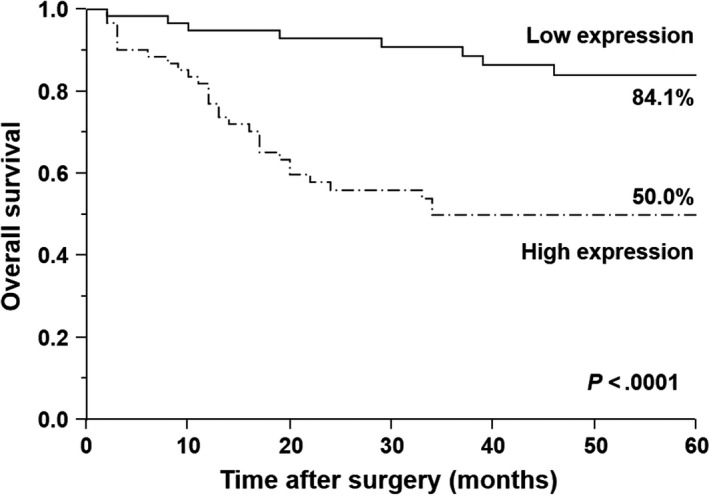
Kaplan–Meier survival curves for patients with gastric cancer based on status of programmed death‐ligand 1 (PD‐L1) mRNA expression. Patients with high PD‐L1 expression had significantly poorer prognosis than those with low PD‐L1 expression (P < .0001)
3.5. Univariate and multivariate analyses of survival
Univariate analysis revealed that the depth of tumor invasion, lymph node metastasis, distant metastasis, serum CEA level, serum CA19‐9 level, and PD‐L1 expression correlated significantly with postoperative survival (P < .0001 each) (Table 2). Moreover, multivariate analysis selected distant metastasis, serum CEA level, and PD‐L1 expression as independent prognostic factors (P < .0001, P = .020, and P = .024, respectively) (Table 2).
Table 2.
Univariate and multivariate analyses of survival in gastric cancer patients (n = 124)
| Independent factor | Univariate analysis | P‐value | Multivariate analysis | P‐value | ||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | Hazard ratio | 95% CI | |||
| Depth of tumor invasion | ||||||
| T1‐T2/T3‐T4 | 42.15 | 9.12‐748.54 | <.0001 | 5.98 | 0.91‐117.89 | .0640 |
| Lymph node metastasis | ||||||
| Negative/positive | 4.20 | 2.14‐8.86 | <.0001 | 1.53 | 0.71‐3.49 | .2810 |
| Distant metastasis | ||||||
| Negative/positive | 51.50 | 19.64‐177.57 | <.0001 | 15.60 | 5.10‐63.56 | <.0001 |
| Serum CEA level | ||||||
| Negative/positive | 6.96 | 3.56‐13.46 | <.0001 | 2.42 | 1.15‐5.10 | .0200 |
| Serum CA19‐9 level | ||||||
| Negative/positive | 6.16 | 2.95‐12.44 | <.0001 | 1.06 | 0.51‐2.26 | .8810 |
| PD‐L1 expression | ||||||
| Low/high | 4.64 | 2.23‐10.88 | <.0001 | 2.60 | 1.13‐6.63 | .0240 |
CA19‐9, carbohydrate antigen 19‐9; CEA, carcinoembryonic antigen; CI, confidence interval; T1, invasion of lamina propria or submucosa; T2, invasion of muscularis propria; T3, invasion of subserosa; T4, penetration of serosa or invasion of adjacent structures.
4. DISCUSSION
The present study examined PD‐L1 mRNA expression by qRT‐PCR assay in peripheral blood specimens from patients with early and advanced gastric cancer. We also investigated the relationship between PD‐L1 mRNA expression and tumor properties to assess the clinical impact of expression in patients with gastric cancer. Although many investigators have reported the clinical significance of PD‐L1 expression in primary tumor sites, this represents the first study to analyze mRNA expression in blood specimens from patients with gastric cancer.
According to a previously published report, PD‐L1 is expressed on antigen‐presenting cells and tumor cells.8 We initially investigated PD‐L1 protein expression using immunocytochemistry in gastric cancer cell lines. This immunocytochemical analysis showed morphological PD‐L1 expression in gastric tumor cells. Moreover, qRT‐PCR assay confirmed PD‐L1 expression in all gastric cancer cell lines. These results indicate that, at least, gastric tumor cells express PD‐L1 as an immune checkpoint inhibitor. Interestingly, patients with advanced gastric cancer showed significantly higher copy numbers of PD‐L1 mRNA than patients with early gastric cancer (P = .002). This finding shows high PD‐L1 mRNA expression within blood vessels in hosts with advanced‐stage tumors. David similarly reported that circulating tumor cells express PD‐L1 in patients with metastatic breast cancer.16 Consequently, PD‐L1 expression in blood specimens may reflect the immune environment including the status of circulating tumor cells and antigen‐presenting cells in patients with gastric cancer.
In this study, patients with high PD‐L1 expression showed significantly deeper tumor invasion and more advanced stage than those with low PD‐L1 expression (P = .001 and P < .001, respectively). In addition, high‐level PD‐L1 expression was shown in 31 (79.5%) of 39 patients with distant metastasis. Surprisingly, the ROC analysis indicated a high sensitivity for predicting distant metastasis based on the status of PD‐L1 expression. These results suggest that PD‐L1 expression in peripheral blood specimens correlated with malignant tumor behavior and that assessment of PD‐L1 expression based on liquid biopsy might have clinical utility for monitoring tumor aggressiveness in the management of patients with gastric cancer. In a study of 465 patients with stage I‐IV disease, Böger et al17 similarly reported that immunohistochemical analysis in primary tumor specimens showed a close relationship between PD‐L1 protein expression and well‐known prognostic clinicopathological factors, such as depth of tumor invasion, distant metastasis, and UICC stage. Accordingly, PD‐L1 represents a promising immune checkpoint inhibitor for predicting tumor progression in patients with gastric cancer.
To date, several investigators have assessed the prognostic significance of PD‐L1 expression in primary tumor sites of various malignancies, including gastric cancer.9, 10, 11, 12 According to the majority of these reports based on immunohistochemical analyses, patients with high PD‐L1 expression displayed significantly poorer prognosis than those with low PD‐L1 expression.9, 10, 11, 12 In the present study, qRT‐PCR analysis of blood specimens showed significantly worse prognosis in patients with high PD‐L1 mRNA expression than in those with low PD‐L1 mRNA expression (P < .0001). Furthermore, multivariate analysis indicated PD‐L1 expression, as well as distant metastasis and serum CEA level, as an independent prognostic factor. These findings suggest that qRT‐PCR assay for PD‐L1 expression by liquid biopsy might represent a useful tool to select patients for neoadjuvant systemic chemotherapy in the pretherapeutic management of gastric cancer.
Programmed death‐ligand 1 was identified as a third member of the B7 family without interaction with CD28 or cytotoxic T lymphocyte‐associated antigen 4 in 1999.7 It was subsequently identified as the ligand for PD‐1.18 Simultaneously, Freeman et al18 reported that the immune signal between PD‐1 and PD‐L1 leads to the inhibition of T‐cell receptor‐mediated lymphocyte proliferation and cytokine secretion. The PD‐1/PD‐L1 signaling pathway is therefore the focus of attention as a promising immune target in patients with various malignancies. In fact, PD‐1/PD‐L1 checkpoint blockades have dramatically changed the landscape for conventional treatments by chemotherapy and/or radiotherapy in these patients. According to a multicenter phase Ib trial using anti‐PD‐1 antibody pembrolizumab in patients with PD‐L1‐positive recurrent or metastatic adenocarcinoma of the stomach or gastroesophageal junction, the incidence of grade 3 or 4 treatment‐related adverse events and overall response rates were 13% and 22%, respectively.19 The KEYNOTE‐012 study thus suggested that a novel immunotherapy using pembrolizumab had a manageable toxicity profile and positive antitumor effect in patients with advanced gastric cancer.19 However, immune checkpoint inhibitor therapy produces distinctive adverse events, such as pemphigoid and hypothyroidism, that are not commonly encountered in clinical management using conventional chemotherapies. Furthermore, compared with other treatments, immunotherapy is generally expensive in terms of medical economics.20 Accordingly, prediction of appropriate patient cohorts likely to show non‐progressive disease after immunotherapy is clinically important. According to a randomized controlled trial comparing pembrolizumab and docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer, a subgroup analysis of progression‐free survival revealed the clinical utility of PD‐L1 tumor proportion score for predicting responders to pembrolizumab.21 The KEYNOTE‐010 study proposed the status of PD‐L1 expression as a useful biomarker to discriminate responders from non‐responders in PD‐1/PD‐L1 immunotherapy.21 However, heterogeneous expression of PD‐L1 within primary tumor sites is one of the key issues in the clinical management of PD‐1/PD‐L1 checkpoint blockades.22 Supposing the status of PD‐L1 expression in blood specimens correlates with the therapeutic effects of PD‐1/PD‐L1 checkpoint blockades, assessment of PD‐L1 in liquid biopsy may have clinical value in predicting the therapeutic response to immunotherapy in patients with unresectable advanced and recurrent gastric cancer. Moreover, liquid assessments based on repeatable blood sampling may have clinical benefit for monitoring sequential tumor response to immunotherapy.
In conclusion, we showed that PD‐L1 is highly expressed in the peripheral blood of patients with advanced gastric cancer and that expression of PD‐L1 correlates with tumor progression and prognosis. Although larger validation studies are needed to strengthen our results, assessment of PD‐L1 expression in blood biopsy may be useful as a clinical tool to plan therapeutic strategies in patients with gastric cancer. Future studies on the biological behavior of PD‐1/PD‐L1 expression in liquid biopsy might lead to personalized immune checkpoint inhibitor therapy for controlling tumor progression and distant metastasis in patients with unresectable advanced and recurrent gastric cancer.
CONFLICT OF INTEREST
The authors have no conflict of interest.
Amatatsu M, Arigami T, Uenosono Y, et al. Programmed death‐ligand 1 is a promising blood marker for predicting tumor progression and prognosis in patients with gastric cancer. Cancer Sci. 2018;109:814–820. https://doi.org/10.1111/cas.13508
REFERENCES
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87‐108. [DOI] [PubMed] [Google Scholar]
- 2. Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388:2654‐2664. [DOI] [PubMed] [Google Scholar]
- 3. Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56‐61. [DOI] [PubMed] [Google Scholar]
- 4. Martin‐Liberal J, Ochoa de Olza M, Hierro C, Gros A, Rodon J, Tabernero J. The expanding role of immunotherapy. Cancer Treat Rev. 2017;54:74‐86. [DOI] [PubMed] [Google Scholar]
- 5. Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320‐330. [DOI] [PubMed] [Google Scholar]
- 6. Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD‐1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887‐3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dong H, Zhu G, Tamada K, Chen L. B7‐H1, a third member of the B7 family, co‐stimulates T‐cell proliferation and interleukin‐10 secretion. Nat Med. 1999;5:1365‐1369. [DOI] [PubMed] [Google Scholar]
- 8. Zang X, Allison JP. The B7 family and cancer therapy: costimulation and coinhibition. Clin Cancer Res. 2007;13:5271‐5279. [DOI] [PubMed] [Google Scholar]
- 9. Thompson RH, Kuntz SM, Leibovich BC, et al. Tumor B7‐H1 is associated with poor prognosis in renal cell carcinoma patients with long‐term follow‐up. Cancer Res. 2006;66:3381‐3385. [DOI] [PubMed] [Google Scholar]
- 10. Daud AI, Wolchok JD, Robert C, et al. Programmed death‐ligand 1 expression and response to the anti‐programmed death 1 antibody pembrolizumab in melanoma. J Clin Oncol. 2016;34:4102‐4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eto S, Yoshikawa K, Nishi M, et al. Programmed cell death protein 1 expression is an independent prognostic factor in gastric cancer after curative resection. Gastric Cancer. 2016;19:466‐471. [DOI] [PubMed] [Google Scholar]
- 12. Takada K, Toyokawa G, Okamoto T, et al. A comprehensive analysis of programmed cell death ligand‐1 expression with the clone SP142 antibody in non‐small‐cell lung cancer patients. Clin Lung Cancer. 2017;18:572‐582. [DOI] [PubMed] [Google Scholar]
- 13. Arigami T, Uenosono Y, Yanagita S, et al. Clinical significance of circulating tumor cells in blood from patients with gastric cancer. Ann Gastroenterol Surg. 2017;1:60‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, eds. AJCC Cancer Staging Manual, 7th edn New York, NY: Springer; 2010. [Google Scholar]
- 15. Arigami T, Natsugoe S, Uenosono Y, et al. Evaluation of sentinel node concept in gastric cancer based on lymph node micrometastasis determined by reverse transcription‐polymerase chain reaction. Ann Surg. 2006;243:341‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. David R. PD‐L1 expression by circulating breast cancer cells. Lancet Oncol. 2015;16:e321. [DOI] [PubMed] [Google Scholar]
- 17. Böger C, Behrens HM, Mathiak M, Krüger S, Kalthoff H, Röcken C. PD‐L1 is an independent prognostic predictor in gastric cancer of Western patients. Oncotarget. 2016;7:24269‐24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD‐1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Muro K, Chung HC, Shankaran V, et al. Pembrolizumab for patients with PD‐L1‐positive advanced gastric cancer (KEYNOTE‐012): a multicentre, open‐label, phase 1b trial. Lancet Oncol. 2016;17:717‐726. [DOI] [PubMed] [Google Scholar]
- 20. Choudhury N, Nakamura Y. Importance of immunopharmacogenomics in cancer treatment: patient selection and monitoring for immune checkpoint antibodies. Cancer Sci. 2016;107:107‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): a randomised controlled trial. Lancet. 2016;387:1540‐1550. [DOI] [PubMed] [Google Scholar]
- 22. McLaughlin J, Han G, Schalper KA, et al. Quantitative assessment of the heterogeneity of PD‐L1 expression in non‐small‐cell lung cancer. JAMA Oncol. 2016;2:46‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]


