Abstract
This single‐arm, open‐label, phase II study in 42 Japanese postmenopausal patients with estrogen receptor‐positive/human epidermal growth factor receptor 2‐negative (ER+/HER2−) advanced breast cancer evaluated the efficacy, safety, and pharmacokinetics of first‐line palbociclib (125 mg once daily, 3 weeks on/1 week off) coadministered with letrozole (2.5 mg once daily). Primary endpoint of investigator‐assessed 1‐year progression‐free survival (PFS) probability was 75.0% (90% CI, 61.3%‐84.4%), far surpassing the 40% lower limit of the 90% CI supporting efficacy. Median duration of treatment was 438 days. Among secondary efficacy measures, median PFS was not reached (95% CI, 16.7: not estimable), 17/42 patients (40.5%) had an objective response, 36/42 (85.7%) maintained disease control, and 27/42 (64.3%) remained in follow‐up. Median overall survival was not reached, and 1‐year survival probability was 92.9% (95% CI, 79.5%‐97.6%). Results of intensive pharmacokinetics in a subset of 6 patients showed palbociclib steady‐state mean area under the plasma concentration‐time curve over the dosing interval [τ] and mean maximum plasma concentration were 1979 ng·h/mL and 124.7 ng/mL, respectively. For day 15 plasma samples from cycles 1 and 2, geometric mean of the within‐patient mean trough concentration was 90.1 ng/mL. The most common treatment‐related adverse events were neutropenia (100%) and stomatitis (73.8%). There was 1 case of treatment‐related febrile neutropenia. Toxicities were generally tolerated and manageable by dose modifications and/or medical care. Efficacy and safety of first‐line palbociclib plus letrozole therapy is supported in Japanese postmenopausal patients with treatment‐naive ER+/HER2− advanced breast cancer.
Keywords: advanced breast cancer, cyclin‐dependent kinase, Japanese, letrozole, palbociclib
Abbreviations
- ABC
advanced breast cancer
- AE
adverse event
- ALT
alanine aminotransferase
- ANC
absolute neutrophil count
- AST
aspartate aminotransferase
- AUCτ
area under the plasma concentration‐time curve over dosing interval [τ]
- CDK4/6
cyclin‐dependent kinases 4 and 6
- CI
confidence interval
- CL/F
apparent clearance
- Cmax
maximum plasma concentration
- CR
complete response
- Ctrough
trough concentration
- CV
coefficient of variation
- DCR
disease control rate
- DOR
duration of response
- ECG
electrocardiogram
- ECOG PS
Eastern Cooperative Oncology Group performance status
- ER+
estrogen receptor‐positive
- FAS
full analysis set
- HER2‐
human epidermal growth factor receptor 2‐negative
- HPLC
high‐performance liquid chromatography
- HR
hazard ratio
- HR+
hormone receptor‐positive
- mBC
metastatic breast cancer
- MedDRA
Medical Dictionary for Regulatory Activities
- NE
not estimable
- ORR
objective response rate
- OS
overall survival
- PD
progressive disease
- PFS
progression‐free survival
- PK
pharmacokinetics
- PR
partial response
- QD
once daily
- QTc
corrected QT
- RECIST
Response Evaluation Criteria in Solid Tumors
- tmax
time to Cmax
- %CV
percentage coefficient of variation
1. INTRODUCTION
The incidence of breast cancer in Japanese women increased 46% from 1995 to 2015,1 and it is now the most common cancer and the fifth leading cause of cancer‐related deaths among Japanese women.2 Globally, between 5% and 10% of breast cancers are metastatic at the time of diagnosis,3 and ABC, including recurrent disease and mBC, is incurable,4 posing a significant public health burden and considerable treatment challenges.5
For postmenopausal women with HR+ ABC, endocrine therapy with agents such as aromatase inhibitors (eg, anastrozole, letrozole, or exemestane) is the standard first‐line treatment6 and is recommended by the Japanese Breast Cancer Society treatment guidelines.7 However, current first‐line endocrine therapy alone may provide only modest benefit in patients with HR+ ABC, and some patients may not respond at all,6 indicating the need for new treatment options. In a randomized phase III trial of exemestane vs anastrozole as first‐line therapy in Japanese women with HR+ ABC, median time to progression was approximately 12 months in each group.8 Thus, there is interest in improving outcomes by combining first‐line endocrine therapy with other agents.
Palbociclib is a potent, orally active, highly selective inhibitor of CDK4/6; it actively arrests cell cycle progression from the G1 phase to the S phase to inhibit DNA synthesis and the pathologic cellular proliferation characteristic of breast malignancies.9, 10, 11 Palbociclib showed antiproliferative activity alone and synergistic activity with tamoxifen in ER+ luminal breast cancer cell lines.10 In clinical trials in non‐Japanese patients with retinoblastoma protein‐positive solid tumors, single‐agent palbociclib showed antitumor efficacy with an acceptable safety profile and was well tolerated.12, 13 In an open‐label, phase II PALOMA‐1 trial, efficacy and safety of first‐line letrozole (2.5 mg QD) alone or in combination with palbociclib (125 mg QD, 3 weeks on, 1 week off [3/1 schedule]) was investigated in non‐Japanese postmenopausal women with ER+ and HER2− ABC.14 Median PFS was significantly longer with combination therapy than with letrozole alone (20.2 vs 10.2 months, respectively; HR, 0.49; 95% CI, 0.32‐0.75; one‐sided P = .0004). AEs with combination therapy were predictable and manageable; neutropenia was the most commonly reported AE.14 These findings were confirmed in the phase III PALOMA‐2 trial in postmenopausal women with ER+/HER2− ABC who received letrozole with or without palbociclib (3/1 schedule) as first‐line therapy.15 Consistent with PALOMA‐1, median PFS was significantly longer with combination therapy than with letrozole monotherapy (24.8 vs 14.5 months; HR, 0.58; 95% CI, 0.46‐0.72; two‐sided P < .001). AEs were similar to those reported in the PALOMA‐1 trial, with uncomplicated neutropenia being the most commonly reported toxicity.15 Based on these findings, palbociclib was approved in the USA and Europe for the treatment of HR+/HER2− mBC or ABC in combination with an aromatase inhibitor as initial endocrine‐based therapy.16, 17
To investigate the efficacy, PK, and safety of palbociclib plus letrozole in Japanese patients, a phase I/II study was conducted in postmenopausal Japanese women with ER+/HER2− ABC.18 In part 1 of the phase I dose‐finding study, single‐agent palbociclib was given to patients with advanced solid tumors that were refractory to standard therapy. Maximum tolerated dose of palbociclib was 125 mg, and safety findings were consistent with previous studies.18 In part 2, palbociclib plus letrozole (same dosage regimen as in PALOMA‐1 and PALOMA‐2 trials) was assessed as first‐line therapy in postmenopausal Japanese patients with ER+/HER2− ABC. There was no evidence of a drug‐drug interaction between palbociclib and letrozole; thus, the recommended palbociclib dosage in Japanese patients is 125 mg QD (3/1 schedule) in combination with letrozole 2.5 mg daily,18 the same dosing regimen used to treat Western patients.
The PALOMA‐1 trial did not include Japanese patients, and the results of the PALOMA‐2 trial were not available when this study started. Thus, the primary objective of the phase II portion of this phase I/II study was to evaluate the efficacy of palbociclib in combination with letrozole using 1‐year PFS probability in postmenopausal Japanese patients with ER+/HER2− ABC.
2. MATERIALS AND METHODS
2.1. Study design
This was the phase II portion of a phase I/II, single‐country, non‐randomized, open‐label, single‐arm, multicenter study in Japanese patients (NCT01684215). The phase I part of this study has been published.18 The primary objective of the phase II part of the study was to evaluate the efficacy of palbociclib in combination with letrozole using 1‐year PFS probability in postmenopausal Japanese patients with ER+/HER2− ABC. There were several secondary objectives. We assessed ORR, DCR, DOR, PFS, 1‐year survival, and OS. Additional assessments included safety and tolerability, evaluation of health‐related quality of life (not reported), determination of plasma Ctrough of palbociclib combined with letrozole in all patients, and evaluation of the full PK profile of palbociclib in a subset of treated patients (n = 6). We also characterized biomarkers of tumor sensitivity and/or resistance in tumor tissue samples, such as the Ki‐67 index, for which a 20% cutoff has been determined to be optimal (a significant factor for overall survival), or the most effective prognostic factor for luminal/HER2− breast cancer based on a study of 4329 Japanese patients with primary breast cancer.19
The study protocol was approved by the Institutional Review Board at each participating center, and written informed consent was obtained from all patients before enrollment. The study was conducted according to applicable local laws and regulatory requirements, the International Conference on Harmonisation Good Clinical Practice guidelines, and the Declaration of Helsinki.
2.2. Patients
Key inclusion criteria included Japanese postmenopausal women (≥20 years of age) with histologically or cytologically confirmed ER+ ABC (locoregionally recurrent or metastatic disease not suitable for resection or radiation therapy with curative intent) who had received no prior systemic anticancer therapy for ER+ ABC, and who had measurable disease (per RECIST, version 1.120) or bone‐only disease, ECOG PS 0‐2, and adequate bone marrow, renal, and liver function.
Key exclusion criteria were a HER2+ tumor identified by protocol‐specified diagnostics; advanced, symptomatic, visceral spread; and risk of life‐threating complications, known active uncontrolled or symptomatic central nervous system metastases, carcinomatous meningitis, or leptomeningeal disease. Patients who had received prior (neo)adjuvant treatment with a non‐steroidal aromatase inhibitor and had disease recurrence during treatment or ≤12 months after completing treatment, or prior treatment with CDK4/6 inhibitors were also excluded. Patients with baseline QTc >480 ms (based on the mean value of triplicate ECGs), family or personal history of QT syndrome were also ineligible.
2.3. Study treatment
Patients received 125 mg palbociclib orally QD with food on days 1 to 21 of every 28‐day cycle followed by 7 days off palbociclib treatment, coadministered with letrozole 2.5 mg orally QD. Study treatments were continued until radiologically documented PD according to RECIST (v1.1), symptomatic deterioration, unacceptable toxicity, or withdrawal of consent.
Palbociclib dose interruptions or delays were permitted if patients experienced the following protocol‐specified treatment‐related AEs: uncomplicated grade 3 neutropenia (ANC <1000/mm3), grade 3 neutropenia associated with a documented infection or fever ≥38.5°C, grade 4 neutropenia (ANC <500/mm3), grade 4 thrombocytopenia (platelet count <25 000/mm3), grade ≥3 non‐hematologic toxicity, or grade 3 QTc prolongation (QTc ≥501 ms on ≥2 separate ECGs). Retreatment after dose interruption for treatment‐related toxicity or at the start of a new cycle was permitted when all of the following criteria were met: platelet count ≥50 000/mm3, ANC ≥1000/mm3 without fever, grade ≥3 treatment‐related non‐hematologic AE that had recovered to grade ≤1 or baseline (or at the investigator's discretion or if grade ≤2 AEs were not considered a safety risk), QTc <501 ms and potential reversible causes (eg, electrolyte imbalance, concomitant medications known to prolong QTc) were corrected. If QTc remained >480 ms, more frequent ECG monitoring occurred per the investigator's best medical judgment until QTc was ≤480 ms. If these criteria were met within 2 weeks of the dose interruption or cycle delay, palbociclib treatment was resumed. If not, resumption or permanent discontinuation of palbociclib treatment was based on the investigator's best medical judgment of whether the patient was deriving clinical benefit from treatment.
Protocol‐specified palbociclib dose reductions were permitted, depending on the type and severity of toxicity reported. The recommended first dose reduction was from 125 to 100 mg/d, and the recommended second dose reduction was from 100 to 75 mg/d. Patients requiring >2 palbociclib dose reductions were discontinued from the study but remained in the follow‐up phase. Subsequent treatment cycles were given at the reduced dose level; dose re‐escalation was not permitted. Patients who continued receiving letrozole remained in the active treatment phase of the study.
Letrozole dose interruptions for treatment‐related toxicities were permitted based on the investigator's judgment. Letrozole dose reductions were not permitted. Patients who discontinued letrozole because of treatment‐related toxicity also discontinued palbociclib and, consequently, were discontinued from the active treatment phase of the study and instead entered the follow‐up phase.
2.4. Assessments
2.4.1. Efficacy
Disease assessments included computed tomography or magnetic resonance imaging of the chest, abdomen, pelvis, and any other sites of disease as clinically indicated; clinical assessment of superficial disease (including lesion measurements); and radionucleotide bone scans. All baseline assessments were carried out before giving study drugs (palbociclib and letrozole). Postbaseline tumor assessments were done every 12 weeks and bone scans (if applicable) every 24 weeks until evidence of disease progression. Radiographic disease progression using RECIST or clinical disease progression (if photographed or palpable lesions) was assessed. Objective tumor response (using RECIST) was reported as ORR (percentage of patients with CR + PR) and DCR (percentage of patients with CR + PR + stable disease lasting ≥24 weeks). All radiographic or clinical (where applicable) efficacy evaluations were based on investigator assessments. OS was determined every 6 months from the last dose of study treatment.
2.4.2. Pharmacokinetics
A subset of 6 patients underwent intensive PK sampling, and blood samples were taken on cycle 1/day 15 at predose and at 1, 2, 4, 6, 8, 10, and 24 hours after dosing, then at predose on cycle 2/day 15. For this subset, plasma concentration‐time data obtained after study drug administration on cycle 1/day 15 were analyzed using non‐compartmental analysis methods to determine the following PK parameters: Cmax, tmax, AUCτ, and CL/F. For assessment of palbociclib plasma Ctrough, blood samples from all patients were collected on day 15 of cycles 1 and 2 before giving study drug. Plasma samples were analyzed for palbociclib concentrations using a validated HPLC method with tandem mass spectrometry at PPD Development (Richmond, VA, USA). Lower limit of quantification for palbociclib was 1.00 ng/mL.
2.4.3. Safety
Safety was assessed by treatment‐related AEs and clinically significant changes in physical examination findings or abnormal laboratory assessments. AEs were reported throughout the study using MedDRA version 18.1 (International Council for Harmonisation, Geneva, Switzerland), and protocol‐specified clusters of preferred terms, based on incidence, severity (using Common Terminology Criteria for Adverse Events, version 4.0), and investigator‐assessed relationship to study treatment. Physical examinations and 12‐lead ECGs were carried out at baseline, day 1 of each cycle, and at the end of treatment. Laboratory assessments (hematology and blood chemistry) were conducted at baseline, days 1 and 15 of cycle 1, and on day 1 of each subsequent cycle.
2.5. Statistical analyses
Primary endpoint of this study was the 1‐year PFS probability of the lower limit of the 90% CI exceeding 40%, which would correspond to a median PFS of 9 months, assuming that PFS had an exponential distribution. Sample size for this study was 32 patients to meet the above criteria with a >80% probability based on the assumption that median PFS for patients receiving palbociclib plus letrozole was 18 months. Accounting for a 20% potential patient drop‐out, total enrollment target was 40 patients.
The FAS, defined as all patients who received ≥1 dose of palbociclib, was used for all efficacy analyses. Primary endpoint of 1‐year PFS was defined as the proportion of patients without PFS events (ie, PD or death from any cause) at 12 months after the first palbociclib dose. PFS and 90% CI were estimated using the Kaplan‐Meier method.
Subgroup analyses of PFS were carried out to investigate the effects of baseline disease characteristics (ie, visceral vs non‐visceral metastases, length of disease‐free interval since completion of prior treatment [≤12 months vs >12 months vs de novo metastatic disease]). Exploratory analysis of the association between baseline Ki‐67 status (ie, Ki‐67‐positive cells ≤20% vs >20%, central laboratory assessed) and PFS was also conducted. All subgroup analyses of PFS, median PFS, and 95% CI were estimated using the Kaplan‐Meier method.
Objective response rate and DCR were estimated. DOR was based on the subset of patients in the FAS who had an objective response (CR or PR) and was defined as the time from first objective response to first objective PD or death. OS was estimated using the Kaplan‐Meier method, and 1‐year OS was calculated in the same way as for 1‐year PFS.
The PK analysis set was defined as all patients with ≥1 PK parameter of primary interest in ≥1 PK sampling period. PK parameters are presented using descriptive statistics. For the full palbociclib PK profile subset (n = 6), plasma concentration‐time data obtained after giving palbociclib on cycle 1/day 15 were also summarized descriptively and are presented graphically. Steady‐state palbociclib Ctrough values for all patients were calculated as geometric mean and geometric %CV.
The study is still ongoing for the collection of survival data.
3. RESULTS
3.1. Patient disposition
Forty‐three patients were enrolled at 16 centers in Japan, and patient accrual for this study was conducted between June 24, 2014 and February 9, 2015. Of the 43 enrolled patients, 42 were treated with palbociclib plus letrozole and included in the efficacy analyses. The enrolled but untreated patient became ineligible for treatment after an early unplanned examination showed the presence of another malignancy. At the time of data cutoff (March 4, 2016), 27 of 42 patients (64.3%) were continuing their treatment, and 15 of 42 (35.7%) had discontinued the study treatment. The most common reason for discontinuing treatment was objective disease progression or relapse (n = 10 for both palbociclib and letrozole). Four patients discontinued the study (2 of 3 patients died as a result of underlying disease and 1 patient refused further follow up after objective progression or relapse, her last dose of palbociclib was on day 42). The remaining 38 patients in the study were ongoing at the data cutoff.
Baseline demographics and clinical characteristics of patients in the FAS are shown in Table 1. Median age of women was 62.5 years (range, 43‐84 years), and median weight was 50.4 kg (range, 38.6‐74.5 kg; Table 1). Twelve patients (28.6%) had Stage IV disease at study entry. At baseline, most patients (93%) had an ECOG PS of 0, 20 patients (47.6%) had visceral disease, and many (28 patients, 66.7%) had received ≥1 prior (neo)adjuvant systemic therapies, including 20 patients (47.6%) who had prior chemotherapy.
Table 1.
Baseline demographics and disease characteristics of enrolled Japanese postmenopausal patients with ER+/HER2− ABC
| Characteristic | Palbociclib + Letrozole N = 42 |
|---|---|
| Median age (range), y | 62.5 (43‐84) |
| Median weight (range), kg | 50.4 (38.6‐74.5) |
| ECOG performance status, n (%) | |
| 0 | 39 (92.9) |
| 1 | 3 (7.1) |
| Disease site (visceral involvement), n (%)a | |
| Visceral | 20 (47.6) |
| Non‐visceral | 22 (52.4) |
| Disease site (bone involvement), n (%) | |
| Bone only | 6 (14.3) |
| Other with measurable disease | 36 (85.7) |
| Disease site, n (%)b | |
| Bone | 24 (57.1) |
| Breast | 17 (40.5) |
| Pleural effusion | 4 (9.5) |
| Lung | 14 (33.3) |
| Pleura | 4 (9.5) |
| Lymph node | 23 (54.8) |
| Liver | 5 (11.9) |
| Other | 7 (16.7) |
| No. involved disease site(s), n (%) | |
| 1 | 12 (28.6) |
| 2 | 13 (31.0) |
| 3 | 12 (28.6) |
| 4 | 2 (4.8) |
| 5 | 2 (4.8) |
| 6 | 1 (2.4) |
| Disease‐free interval, n (%)c | |
| ≤12 mo | 8 (19.0) |
| >12 mo | 20 (47.6) |
| De novo metastatic disease | 14 (33.3) |
| Stage at initial diagnosis, n (%) | |
| I/IA | 6 (14.3) |
| IIA | 8 (19.0) |
| IIB | 9 (21.4) |
| IIIA | 2 (4.8) |
| IIIB | 1 (2.4) |
| IIIC | 1 (2.4) |
| IV | 12 (28.6) |
| Unknown | 3 (7.1) |
| Biomarkers | |
| Ki‐67‐positive cells, n (%)d | 42 (100) |
| ≤20% | 19 (45.2) |
| >20% | 23 (54.8) |
| Prior therapies for primary diagnosis, yes, n (%) | |
| Prior surgery | 30 (71.4) |
| Prior radiation therapy | 19 (45.2) |
| Prior systemic therapye | 28 (66.7) |
| Chemotherapy | 20 (47.6) |
| Endocrine | 27 (64.3) |
ABC, advanced breast cancer; ECOG, Eastern Cooperative Oncology Group; ER+, estrogen receptor‐positive; HER2−, human epidermal growth factor receptor 2‐negative.
Visceral refers to lung (including pleura) and/or liver involvement.
Involved sites include both target and non‐target sites. Sites with multiple lesions were counted once. Patients may have contributed to >1 category.
Disease‐free interval was calculated as the time between end of neoadjuvant or adjuvant treatment and onset of metastatic disease or disease recurrence.
Tumor tissue samples were from metastatic/recurrent tumor lesions whenever possible; when unavailable, a de novo fresh biopsy was recommended if judged by the investigator to be feasible and safe. Original diagnostic tissue was also collected when available.
Neoadjuvant or adjuvant therapy.
3.2. Efficacy
At the March 4, 2016 data cutoff, 1‐year PFS probability was 75.0% (90% CI, 61.3%‐84.4%). Of the 42 patients in the FAS, 12 (28.6%) had PFS events and most events (11/12) were associated with objective disease progression. Median PFS was not reached (95% CI, 16.7 months: NE; Figure 1A). At the October 31, 2016 data cutoff, the updated 1‐year PFS probability was 75.6% (90% CI, 62.4%‐84.7%). Median PFS was still immature (not reached [95% CI, 21.7 months: NE]; Figure 1B).
Figure 1.
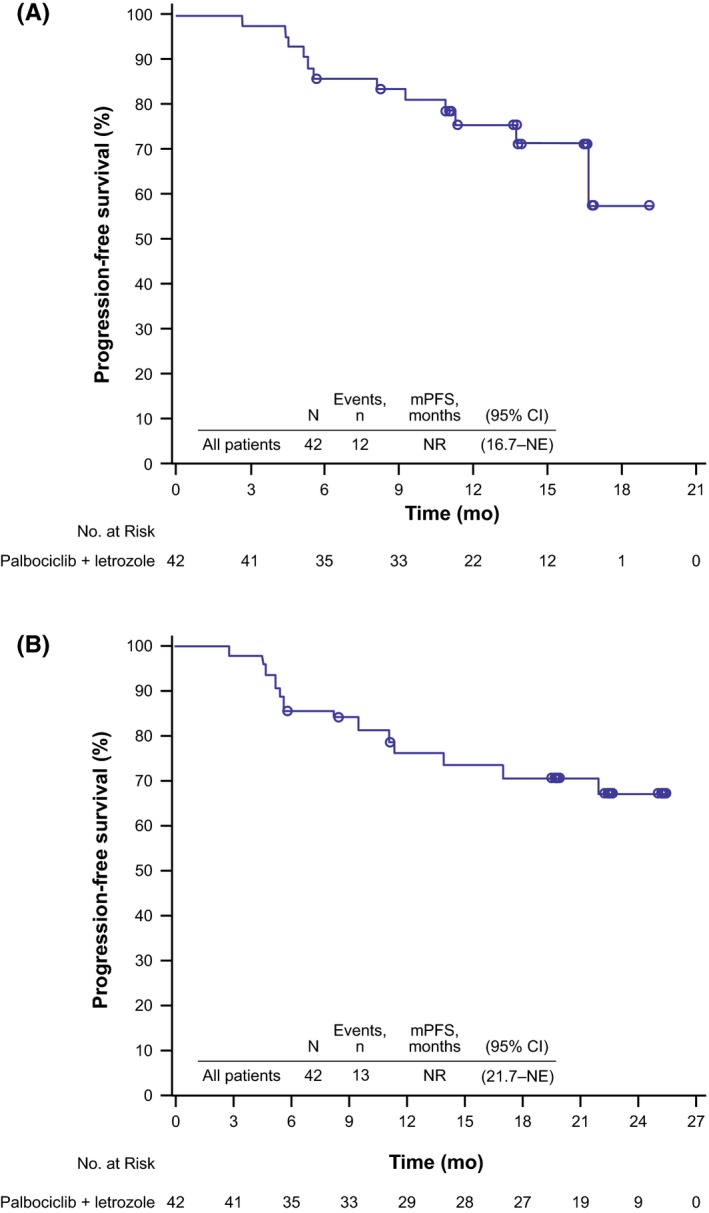
Investigator‐assessed progression‐free survival in Japanese postmenopausal women with estrogen receptor‐positive/human epidermal growth factor receptor 2‐negative advanced breast cancer receiving palbociclib plus letrozole (N = 42) at the (A) March 4, 2016 data cutoff and (B) October 31, 2016 data cutoff. mPFS, median progression‐free survival; NE, not estimable; NR, not reached
Subgroup analyses of PFS by specific baseline characteristics and treatment characteristics are shown in Figure 2. For patients with visceral vs non‐visceral metastases at baseline, 1‐year PFS probability was 51.8% (90% CI, 31.0%‐69.2%) and 95.2% (77.7%‐99.1%), respectively. Median PFS was 16.7 months (95% CI, 5.3–NE) in patients with visceral metastases and was not reached in patients with non‐visceral metastases (Figure 2A). One‐year PFS probability by patient's disease‐free interval at baseline from completion of prior therapy (≤12 months vs >12 months vs de novo metastatic disease) was 60.0% (90% CI, 25.8%‐82.5%), 79.3% (58.9%‐90.4%), and 78.6% (53.5%‐91.1%), respectively. Median PFS was not reached in any of these subgroups (Figure 2B).
Figure 2.
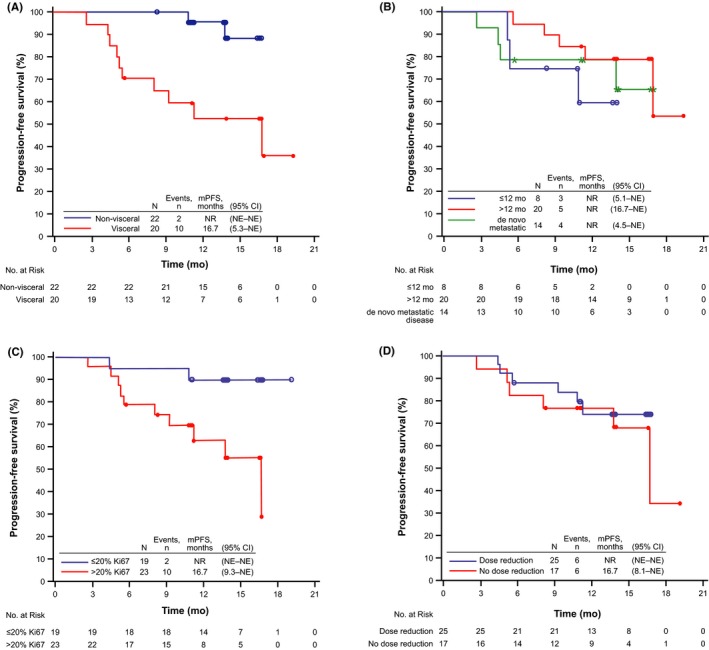
Investigator‐assessed progression‐free survival in Japanese postmenopausal women with estrogen receptor‐positive/human epidermal growth factor receptor 2‐negative advanced breast cancer receiving palbociclib plus letrozole (N = 42). A, Visceral vs non‐visceral metastases at baseline. B, Disease‐free interval (≤12 mo vs >12 mo from end of treatment vs de novo metastatic disease). C, Ki‐67‐positive cells ≤20% vs >20% at baseline. D, Dose reduction vs no dose reduction (at the March 4, 2016 cutoff). mPFS, median progression‐free survival; NE, not estimable; NR, not reached
In patients with tumor biopsies expressing Ki‐67‐positive cells ≤20% vs >20%, median PFS was not reached in the former and was reached at 16.7 months (95% CI, 9.3–NE) in the latter (Figure 2C). Median PFS was not reached in patients who had a dose reduction and was 16.7 months (95% CI, 8.1–NE) in patients who did not have a dose reduction (Figure 2D).
The ORR was 40.5% (95% CI, 25.6%‐56.7%) and the DCR was 85.7% (95% CI: 71.5%‐94.6%); no patient had achieved a CR. In the response evaluable analysis set, which was in patients with measurable disease, ORR was 47.2% (95% CI, 30.4%‐64.5%) and DCR was 83.3% (95% CI, 67.2%‐93.6%). Median DOR was not reached (95% CI, 6.5 months–NE). Only 3 OS events (7.1%) had occurred, and median OS was not reached. Most patients (90.5%) were still in follow up for OS at the data cutoff. Survival probability at 12 months was 92.9% (95% CI, 79.5%‐97.6%).
3.3. Pharmacokinetics
For the PK analyses, data cutoff was September 25, 2015. Palbociclib steady‐state mean AUCτ and Cmax were 1979 ng·h/mL and 124.7 ng/mL, respectively, for the subset of patients who underwent intensive PK sampling (n = 6; Table 2). Interpatient variability (geometric %CV) of palbociclib steady‐state AUCτ (ng·h/mL) and Cmax (ng/mL) were 16% and 26%, respectively. Median palbociclib steady‐state concentration‐time profile on cycle 1/day 15, when coadministered with letrozole, is shown in Figure 3 (n = 6).
Table 2.
Plasma palbociclib steady‐state pharmacokinetics in a subset of 6 Japanese postmenopausal patients with ER+/HER2− ABC receiving palbociclib plus letrozole
| Summary statisticsa | |
|---|---|
| Parameter (units) | Palbociclib + Letrozole Cycle 1/Day 15 n = 6 |
| AUCτ (ng·h/mL) | 1979 (16) |
| Cmax (ng/mL) | 124.7 (26) |
| tmax (h) | 4.90 (2.00‐8.20) |
| CL/F (L/h) | 63.2 (16) |
ABC, advanced breast cancer; AUCτ, area under the plasma concentration‐time curve over dosing interval τ using the linear/log trapezoidal method; CL/F, apparent clearance determined by dose/AUCτ; Cmax, maximum plasma concentration observed directly from the data; ER+, estrogen receptor‐positive; HER2−, human epidermal growth factor receptor 2‐negative; tmax, time to Cmax observed directly from the data as time of first occurrence.
Data are geometric mean (geometric % coefficient of variation) except for median tmax (median [range]).
Figure 3.
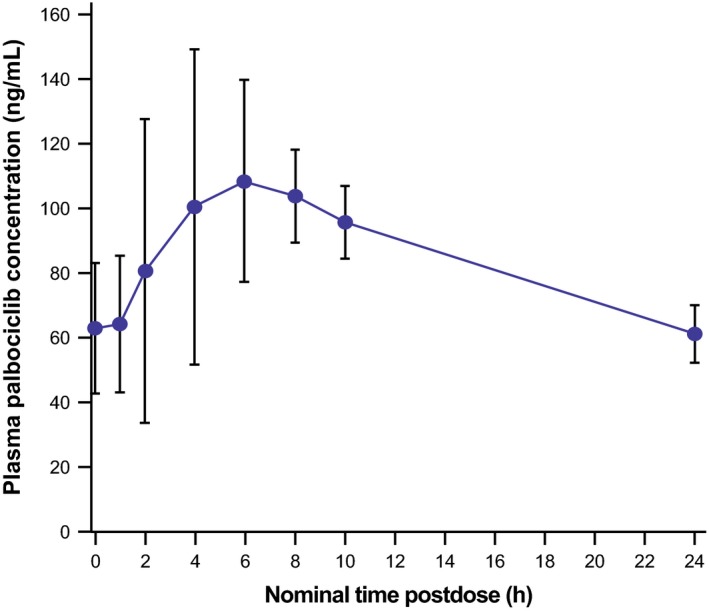
Mean plasma palbociclib steady‐state concentration‐time profile in Japanese postmenopausal women with estrogen receptor‐positive/human epidermal growth factor receptor 2‐negative advanced breast cancer on cycle 1/day 15 following palbociclib 125 mg daily oral doses plus letrozole 2.5 mg daily oral doses (n = 6). Error bars represent ± standard deviation
Mean palbociclib Ctrough in combination with letrozole on cycle 1/day 15 and cycle 2/day 15 were determined for all patients in the PK population (n = 38; Table 3). Mean Ctrough values on each sampling day overlapped (89.4 ng/mL in cycle 1 and 86.8 ng/mL in cycle 2), and the geometric mean Ctrough obtained from day 15 of cycles 1 and 2 at steady state was 90.1 ng/mL.
Table 3.
Mean palbociclib plasma Ctrough in combination with letrozole in cycles 1 and 2 on day 15
| Parameter | Parameter summary statisticsa | ||
|---|---|---|---|
| Predose concentration on cycle 1/day 15 | Predose concentration on cycle 2/day 15 | Predose concentration on day 15, cycles 1 and 2b | |
| N | 32 | 30 | 38 |
| Ctrough (ng/mL) | 89.4 (44) | 86.8 (40) | 90.1 (42) |
Ctrough, predose concentration during multiple dosing; N, number of patients evaluated with the steady‐state Ctrough.
The calculation required 2 conditions to be met for the steady state Ctrough evaluable criteria: (i) Ctrough following at least 7 consecutive days of palbociclib 125 mg daily dose without dosing interruption, and (ii) Ctrough to have the actual sampling time, which was ≤10% time deviation of the nominal time from 24 h after the last dose on day 14.
Geometric mean (geometric % coefficient of variation) for all.
Geometric mean of within‐patient mean Ctrough on cycles 1 and 2 on day 15.
3.4. Safety
3.4.1. Exposure
Median duration of treatment was 438.0 days (range: 56‐585), and patients received a median of 14 (range, 2‐21) treatment cycles as of data cutoff date of March 4, 2016 (Table 4). Median relative dose intensity was 74.2% for palbociclib and 99.7% for letrozole. In total, 25 patients (59.5%) required ≥1 palbociclib dose reduction. Letrozole dose reductions were not permitted, but dosing interruptions for letrozole‐related toxicities were allowed per the investigator's best medical judgment. Dose modifications (dose reductions, dose interruptions, and cycle delays) are also summarized in Table 4.
Table 4.
Exposure to study drugs and time and duration of dose modifications in Japanese postmenopausal patients with ER+/HER2− ABC receiving palbociclib plus letrozole
| Overall exposure | Palbociclib + Letrozole N = 42 |
|---|---|
| Duration of treatment in days, na | |
| 1‐28 | 0 |
| 29‐90 | 1 |
| 91‐180 | 6 |
| 181‐364 | 6 |
| ≥365 | 29 |
| Median duration (range), d | 438.0 (56‐585) |
| Median number of cycles (range), n | 14 (2‐21) |
| Drug‐specific dose modifications and exposures |
Palbociclib N = 42 |
Letrozole N = 42 |
|---|---|---|
| Dose reductions | ||
| ≥1 dose reduction, n (%)b , c | 25 (59.5) | — |
| Patients with a dose reduction to 100 mg | 17 (40.5) | — |
| Patients with a dose reduction to 75 mg | 8 (19.0) | — |
| Median time to dose reduction (range), dd | — | |
| First dose reduction | 64 (29‐232) | |
| Second dose reduction | 114 (64‐415) | |
| Dose interruption, n (%)e | 35 (83.3) | 30 (71.4) |
| Median average duration of dose interruption (range), df | 5.5 (1.0‐8.5) | 1.0 (1.0‐24.5) |
| Median time to first dose interruption (range), dd | 15 (5‐124) | 107 (5‐404) |
| Cycle delay, n (%) | 39 (92.9) | 8 (19.0) |
| Median average duration of cycle delay, d (range) | 7 (5‐47) | 7 (5‐47) |
| Median relative dose intensity per week (range), %g | 74.2 (38.0‐99.8) | 99.7 (69.6‐100) |
ABC, advanced breast cancer; d, day; ER+, estrogen receptor‐positive; HER2, human epidermal growth factor receptor 2‐negative.
Total number of dosing days (from the first day until and including the last day) of each study treatment.
Any dose reduction from the initial prescribed dose, regardless of duration (does not include dose interruptions).
Total number of patients categorized at the maximum dose reduction.
Time to first event (dose reduction/dose interruption) = (start date of first occurrence minus first dose date of cycle 1) + 1; for dose interruption, time could not be calculated for some patients as a result of unknown dates for missing doses.
0 mg given on a planned dosing day. Could not be calculated for some patients as a result of unknown dates for missing doses.
During total cycles.
Overall relative dose intensity = (sum of overall cycles of actual total dose per cycle/sum over all cycles of the actual number of weeks in cycle)/(sum over all cycles of intended total dose per cycle/sum over all cycles of intended number of weeks per cycle) × 100. —, not applicable because a dose reduction for letrozole was not allowed per the study protocol.
3.4.2. Treatment‐related adverse events
The most common treatment‐related AEs (by clustered preferred term) were neutropenia (100% of patients), stomatitis (73.8%), and leukopenia (71.4%), and the most common treatment‐related grade 3 or 4 AEs were neutropenia (90.5%), and leukopenia (50.0%; Table 5). There was 1 case of treatment‐related grade 3 febrile neutropenia (onset day 18) that resolved by day 29.
Table 5.
Investigator‐assessed treatment‐related adverse events of all grades and grades 3/4 occurring in ≥10% of patients (all cycles) in Japanese postmenopausal patients with ER+/HER2− ABC receiving palbociclib plus letrozole
| Preferred term/Clustered preferred termsa , b , c | Palbociclib + Letrozole N = 42 | |
|---|---|---|
| All grades | Grade 3/4 | |
| Any AE, n (%) | 42 (100)d | 38 (90.5) |
| Hematologic AE | ||
| Neutropeniaa | 42 (100) | 38 (90.5) |
| Leukopeniaa | 30 (71.4) | 21 (50.0) |
| Thrombocytopeniaa | 11 (26.2) | 1 (2.4) |
| Anemia | 8 (19.0) | 2 (4.8) |
| Non‐hematologic AE | ||
| Stomatitisa | 31 (73.8) | 0 |
| Infectionsa | 10 (23.8) | 0 |
| Constipation | 9 (21.4) | 0 |
| Rasha | 8 (19.0) | 0 |
| ALT increased | 8 (19.0) | 4 (9.5) |
| Alopecia | 7 (16.7) | NA |
| AST increased | 7 (16.7) | 1 (2.4) |
| Malaise | 7 (16.7) | NA |
| Headache | 6 (14.3) | 0 |
ABC, advanced breast cancer; AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ER+, estrogen receptor‐positive; HER2, human epidermal growth factor receptor 2‐negative; MedDRA, Medical Dictionary for Regulatory Activities, version 18.1; NA, grade 3 or 4 is not applicable.
Clustered preferred terms (PTs) were used to represent multiple PTs as follows: Infections included any event with a PT that is part of the MedDRA system organ class “Infections and Infestations,” and in this study, related PTs included angular cheilitis, cellulitis, gingivitis, laryngitis, lip infection, nasopharyngitis, oral herpes, otitis media, pharyngitis, or upper respiratory tract infection; Leukopenia included the PT leukopenia or white blood cell count decreased; Neutropenia included the PT neutropenia or neutrophil count decreased; Rash included the PT rash, rash maculopapular, dermatitis, dermatitis or acneiform; Stomatitis included the PT cheilitis, glossitis, oropharyngeal pain, or stomatitis; Thrombocytopenia included the PT thrombocytopenia or platelet count decreased.
MedDRA.
National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0 Grade ≤1 (except alopecia or other toxicities not considered a safety risk for the patient at investigator's discretion).
Among all reported events, 1 (2.4%) patient had a grade 5 subarachnoid hemorrhage.
3.4.3. Serious adverse events and deaths
Serious AEs were reported in 3 patients (7.1%) and included vomiting, malaise, dizziness, and subarachnoid hemorrhage (in 1 patient), febrile neutropenia (1 patient), and cerebral hemorrhage (1 patient). Two of these events were considered treatment‐related (subarachnoid hemorrhage and febrile neutropenia). The patient who experienced the treatment‐related subarachnoid hemorrhage died; the last doses of palbociclib and letrozole were given 12 and 5 days, respectively, before death.
Treatment‐related AEs leading to permanent discontinuation of treatment were reported in 3 patients (7.1%) who permanently discontinued palbociclib only (neutropenia in each patient) and in 3 patients (7.1%) who discontinued both palbociclib and letrozole (malaise, ALT and AST increased, and subarachnoid hemorrhage in 1 patient each; Table S1). Treatment‐related AEs leading to dose reductions were reported in 25 patients (59.5%); the most common reason was neutropenia (20 patients [47.6%]).
No patients had a QTc interval of ≥500 ms, and there were no clinically relevant changes in vital signs during the study.
4. DISCUSSION
In this open‐label, phase II study in postmenopausal Japanese women with ER+/HER2− ABC, first‐line treatment with the combination of palbociclib 125 mg daily (3/1 schedule) plus letrozole 2.5 mg daily resulted in a 1‐year PFS probability of 75.0% (90% CI, 61.3%‐84.4%) at the time of the initial March 4, 2016 data cutoff. Thus, the expected efficacy of palbociclib plus letrozole was observed because the lower limit of the 90% CI exceeded 40%. At the later October 31, 2016 data cutoff, 1‐year PFS probability was 75.6% (90% CI, 62.4%‐84.7%). The 1‐year PFS probability in this study was similar to the results in the PALOMA‐1 and PALOMA‐2 studies.21 Median PFS in patients in the PALOMA‐1 study was 20.2 months (95% CI, 13.8‐27.5) and in the PALOMA‐2 trial it was 24.8 months (22.1–NE) after a median follow up of 29.6 and 23 months, respectively.14, 15 The median PFS was not reached by the primary cutoff date (March 4, 2016) or the extended cutoff date (October 31, 2016) in this study, but the lower limit of the 95% CI reached 16.7 and 21.7 months at the respective cutoff dates. Efficacy data for this study after a longer duration of follow up will be further evaluated.
In the current study, subgroup analyses provided valuable insights into the effects of baseline characteristics and study treatment characteristics on PFS. One‐year PFS probability was higher in patients with non‐visceral vs visceral metastases, in patients with de novo metastatic disease or a disease‐free interval >12 months vs ≤12 months. For median PFS, the sample size may have precluded a conclusive result for the subgroup analysis by disease‐free interval. A similar trend, suggesting that de novo metastatic disease or a disease interval >12 months had a favorable effect, was observed in a subgroup analyses of PALOMA‐2 patients.22 In PALOMA‐2, a subgroup analysis suggested palbociclib plus letrozole improved PFS regardless of baseline characteristics, including by disease sites or disease‐free interval.22 In this Japanese study, the Kaplan‐Meier plot was similar in patients who required a dose reduction on treatment vs patients who did not, suggesting palbociclib dose reductions, which were predominantly from 125 to 100 mg, appear not to affect efficacy. Because these data are still immature, the impact of dose reduction will be further investigated when additional follow‐up data are available.
In the subgroup analysis of Ki‐67 positivity (a prognostic biomarker for cell proliferation in breast cancer23), patients with ≤20% Ki‐67‐positive cells at baseline had a prolonged median PFS compared with those with >20% Ki‐67‐positive cells, supporting the prognostic value of this biomarker in patients with breast cancer. In PALOMA‐2, Ki‐67 index values at a 15% or 20% cutoff did not show a patient group with a better or worse PFS with palbociclib plus letrozole.24
At the initial data cutoff, 40.5% of patients had an objective response to treatment and the median DOR had not been reached. These findings are comparable to the objective responses of 43% and 42% in the PALOMA‐1 and PALOMA‐2 trials, respectively. Moreover, the proportion of patients with disease control in the current study (85.7%) was similar to those in PALOMA‐1 (81%) and PALOMA‐2 (84.9%).14, 15
In the current study, median OS also had not been reached by March 4, 2016. The 1‐year survival probability was 92.9%, with only 3 deaths reported, and with most patients remaining in the follow‐up phase at the time of data cutoff. Overall, the efficacy data from the current phase II study in postmenopausal Japanese women with ER+/HER− ABC who received first‐line palbociclib plus letrozole are consistent with data from the 2 large PALOMA‐1 and PALOMA‐2 trials in non‐Japanese patients, or predominantly non‐Japanese patients, respectively.14, 15
When coadministered with letrozole, the full PK analysis conducted in 6 Japanese patients in the current study showed a PK profile that was remarkably similar to that reported in the PALOMA‐1 study in non‐Japanese patients.25 The Ctrough of plasma palbociclib in all patients was 89.4 and 86.8 ng/mL on day 15 of cycles 1 and 2, respectively. These data are highly consistent with the exploratory findings from the phase I portion of this study when the plasma palbociclib Ctrough was 72.8 ng/mL after multiple oral doses on day 8 of cycles 1 and 2 when coadministered with letrozole.18 In the phase II portion of PALOMA‐1, the Ctrough of plasma palbociclib was 63.5 and 62.4 ng/mL on day 14 of cycles 1 and 2, respectively, collectively indicating that the plasma palbociclib mean Ctrough appeared to be slightly higher in Japanese patients; however, the distribution of plasma Ctrough in this study and PALOMA‐1 overlap.
In the current study, palbociclib in combination with letrozole was well tolerated. The most commonly reported AE was uncomplicated neutropenia, and AEs were manageable by implementing palbociclib dose interruptions or reductions and/or standard medical therapy. These findings are consistent with the known safety profile of palbociclib in non‐Japanese patients with advanced ER+/HER2− breast cancer and other solid tumors12, 13, 14, 15 and similar to the findings from the phase I part of this study in Japanese patients.18
Limitations of the current study include the open‐label and single‐arm design and the fact that all efficacy evaluations were based on investigator assessments. However, the efficacy data are consistent with those from other studies of this therapeutic combination.14, 15 In addition, because the median duration of treatment was only 438.0 days (range: 56‐585) as of the data cutoff date of March 4, 2016, it was not possible to observe late AEs with palbociclib.
For decades, endocrine therapy has been the preferred first‐line treatment in patients with advanced ER+/HER2− breast cancer because of its confirmed potential to prolong disease control, low toxicity profile, and tolerability.26 Newer hormonal agents have improved outcomes to some degree, but combination therapies of hormonal agents with agents targeting other pathways may enhance outcomes further in patients with advanced disease. This approach has been successful in the second‐line setting following prior endocrine resistance, where median PFS was shown to have not been reached with a combination of palbociclib plus fulvestrant vs 5.8 months with placebo plus fulvestrant (HR, 0.49; 95% CI, 0.27‐0.87; P < .01) in premenopausal and postmenopausal Asian patients (n = 72) with advanced ER+ breast cancer.27
In conclusion, first‐line therapy with palbociclib plus letrozole was effective and AEs were manageable in postmenopausal Japanese women with advanced ER+/HER2− breast cancer. Palbociclib in combination with letrozole should be considered as a first‐line treatment option in Japanese patients in this population.
CONFLICTS OF INTEREST
Authors Y.Mu., Y.Mo., S.H., T.N., and Y.U. are employees of Pfizer; Y.Mo. and Y.U. also own stock in Pfizer. H.I. receives research funding from Pfizer. K.I.'s institution received research funding from Pfizer, Novartis, Puma Biotechnology, Eli Lilly, Chugai Pharma, and MSD. N.M. received research funding from Chugai and AstraZeneca. S.O. received honoraria from Chugai, AstraZeneca, Eisai, and Novartis. M.To. received research funding from Taiho Pharma, Chugai Pharmaceutical Co. Ltd, and is an unsalaried board member of the Japan Breast Cancer Research Group. This study (Study A5481010 NCT01684215) was sponsored by Pfizer. All authors had full access to all of the data in the study and had final responsibility for the decision to submit for publication. Authors M.T. and R.N. have no conflicts of interest to declare.
Supporting information
ACKNOWLEDGMENTS
We thank Junichi Tanuma and Naoko Mizutani of Pfizer Japan for data collection and Hiroko Godai of Pfizer Japan for data analysis. This study was sponsored by Pfizer. Editorial support was provided by Susan Reinwald, PhD, of Complete Healthcare Communications, LLC (West Chester, PA, USA), a CHC Group company, and was funded by Pfizer.
Masuda N, Nishimura R, Takahashi M, et al. Palbociclib in combination with letrozole as first‐line treatment for advanced breast cancer: A Japanese phase II study. Cancer Sci. 2018;109:803–813. https://doi.org/10.1111/cas.13507
Funding information
Sponsored by Pfizer; Study A5481010, ClinicalTrials.gov NCT01684215.
REFERENCES
- 1. Katanoda K, Hori M, Matsuda T, et al. An updated report on the trends in cancer incidence and mortality in Japan, 1958‐2013. Jpn J Clin Oncol. 2015;45:390‐401. [DOI] [PubMed] [Google Scholar]
- 2. Nakamura K, Okada E, Ukawa S, et al. Characteristics and prognosis of Japanese female breast cancer patients: the BioBank Japan project. J Epidemiol. 2017;27:S58‐S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cardoso F, Harbeck N, Fallowfield L, Kyriakides S, Senkus E. Locally recurrent or metastatic breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2012;23:vii11‐vii19. [DOI] [PubMed] [Google Scholar]
- 4. Gombos A, Awada A. Advances in chemical pharmacotherapy to manage advanced breast cancer. Expert Opin Pharmacother. 2017;18:95‐103. [DOI] [PubMed] [Google Scholar]
- 5. Global status of advanced/metastatic breast cancer: 2005‐2015 decade report. 2016. http://www.breastcancervision.com/sites/default/files/Global%20Status%20of%20mBC%20Report%20Summary_041416_Digital.pdf. Accessed July 7, 2017.
- 6. Milani A, Geuna E, Mittica G, Valabrega G. Overcoming endocrine resistance in metastatic breast cancer: current evidence and future directions. World J Clin Oncol. 2014;5:990‐1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aihara T, Toyama T, Takahashi M, et al. The Japanese Breast Cancer Society Clinical Practice Guideline for systemic treatment of breast cancer, 2015 edition. Breast Cancer. 2016;23:329‐342. [DOI] [PubMed] [Google Scholar]
- 8. Iwata H, Masuda N, Ohno S, et al. A randomized, double‐blind, controlled study of exemestane versus anastrozole for the first‐line treatment of postmenopausal Japanese women with hormone‐receptor‐positive advanced breast cancer. Breast Cancer Res Treat. 2013;139:441‐451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fry DW, Harvey PJ, Keller PR, et al. Specific inhibition of cyclin‐dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther. 2004;3:1427‐1438. [PubMed] [Google Scholar]
- 10. Finn R, Dering J, Conklin D, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor‐positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Toogood PL, Harvey PJ, Repine JT, et al. Discovery of a potent and selective inhibitor of cyclin‐dependent kinase 4/6. J Med Chem. 2005;48:2388‐2406. [DOI] [PubMed] [Google Scholar]
- 12. Flaherty KT, Lorusso PM, DeMichele A, et al. Phase I, dose‐escalation trial of the oral cyclin‐dependent kinase 4/6 inhibitor PD 0332991, administered using a 21‐day schedule in patients with advanced cancer. Clin Cancer Res. 2012;18:568‐576. [DOI] [PubMed] [Google Scholar]
- 13. DeMichele A, Clark AS, Tan KS, et al. CDK 4/6 inhibitor palbociclib (PD0332991) in Rb+ advanced breast cancer: phase II activity, safety, and predictive biomarker assessment. Clin Cancer Res. 2015;21:995‐1001. [DOI] [PubMed] [Google Scholar]
- 14. Finn RS, Crown JP, Lang I, et al. The cyclin‐dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first‐line treatment of oestrogen receptor‐positive, HER2‐negative, advanced breast cancer (PALOMA‐1/TRIO‐18): a randomised phase 2 study. Lancet Oncol. 2015;16:25‐35. [DOI] [PubMed] [Google Scholar]
- 15. Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925‐1936. [DOI] [PubMed] [Google Scholar]
- 16. IBRANCE® (palbociclib) . Full Prescribing Information. New York, NY: Pfizer Inc.; 2017. [Google Scholar]
- 17. IBRANCE (palbociclib) Summary of Product Characteristics, 2016. 2016. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003853/WC500217196.pdf. Accessed August 7, 2017.
- 18. Tamura K, Mukai H, Naito Y, et al. Phase I study of palbociclib, a cyclin‐dependent kinase 4/6 inhibitor, in Japanese patients. Cancer Sci. 2016;107:755‐763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tashima R, Nishimura R, Osako T, et al. Evaluation of an optimal cut‐off point for the Ki‐67 index as a prognostic factor in primary breast cancer: a retrospective study. PLoS One. 2015;10:e0119565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228‐247. [DOI] [PubMed] [Google Scholar]
- 21. Data on File. New York, NY: Pfizer Inc; 2017. [Google Scholar]
- 22. Finn R, Diéras V, Rugo H, et al. Palbociclib plus letrozole as first‐line therapy in estrogen receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer: efficacy and safety across patient subgroups American Society of Clinical Oncology Annual Meeting; 2017 June 2‐6; Chicago, IL, USA: 2017. [Google Scholar]
- 23. Inwald EC, Klinkhammer‐Schalke M, Hofstädter F, et al. Ki‐67 is a prognostic parameter in breast cancer patients: results of a large population‐based cohort of a cancer registry. Breast Cancer Res Treat. 2013;139:539‐552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Finn R, Jiang Y, Rugo H, et al. Biomarker Analyses from the Phase 3 PALOMA‐2 Trial of Palbociclib with Letrozole Compared with Placebo Plus LeTrozole in Postmenopausal Women with ER+/HER2– Advanced Breast Cancer European Society For Medical Oncology (ESMO) Congress; 2016 October 7‐11; Denmark, Copenhagen: 2016. [Google Scholar]
- 25. Nishimura R, Masuda N, Takahashi M, et al. Palbociclib in combination with letrozole in postmenopausal patients with estrogen receptor‐positive/human epidermal growth factor receptor 2‐negative, advanced breast cancer: results from a Japanese phase 2 study. 25th Annual Meeting of the Japanese Breast Cancer Society; 2017. July 13‐15; Tokyo, Japan.
- 26. Chlebowski RT. Changing concepts of hormone receptor‐positive advanced breast cancer therapy. Clin Breast Cancer. 2013;13:159‐166. [DOI] [PubMed] [Google Scholar]
- 27. Iwata H, Im SA, Masuda N, et al. PALOMA‐3: phase III trial of fulvestrant with or without palbociclib in premenopausal and postmenopausal women with hormone receptor‐positive, human epidermal growth factor receptor 2‐negative metastatic breast cancer that progressed on prior endocrine therapy‐safety and efficacy in Asian patients. J Glob Oncol. 2017;3:289‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


