Abstract
Use of peptide‐based vaccines as therapeutics aims to elicit immune responses through antigenic epitopes derived from tumor antigens. Peptide‐based vaccines are easily synthesized and lack significant side‐effects when given in vivo. Peptide‐based vaccine therapy against several cancers including urological cancers has made progress for several decades, but there is no worldwide approved peptide vaccine. Peptide vaccines were also shown to induce a high frequency of immune response in patients accompanied by clinical efficacy. These data are discussed in light of the recent progression of immunotherapy caused by the addition of immune checkpoint inhibitors thus providing a general picture of the potential therapeutic efficacy of peptide‐based vaccines and their combination with other biological agents. In this review, we discuss the mechanism of the antitumor effect of peptide‐based vaccine therapy, development of our peptide vaccine, recent clinical trials using peptide vaccines for urological cancers, and perspectives of peptide‐based vaccine therapy.
Keywords: cancer peptide vaccine therapy, oncoantigen, prostate cancer, renal cell carcinoma, urothelial cancer
Abbreviations
- AE
adverse event
- anti‐PD‐1
anti‐programmed cell death‐1
- anti‐PD‐L1
anti‐programmed death‐ligand 1
- APC
antigen presenting cell
- BCG
Bacillus Calmette‐Guérin
- BSC
best supportive care
- CDCA1
cell division associated 1
- CRPC
castration resistant prostate cancer
- DC
dendritic cell
- ELISPOT
Enzyme‐Linked ImmunoSpot
- G3‐4 AE
grade 3‐4 adverse event
- G3
grade 3
- GM‐CSF
granulocyte macrophage colony‐stimulating factor
- HIF
hypoxia‐inducing factor
- HIG2
hypoxia‐inducible protein 2
- HLA
human leukocyte antigen
- ICI
immune checkpoint inhibitor
- IFN
interferon
- IL
interleukin
- LP
long peptide
- mUC
metastatic urothelial cancer
- MVAC
methotrexate, vinblastine, adriamycin and cisplatin
- OS
overall survival
- PFS
progression‐free survival
- PPV
personalized peptide vaccine
- RCC
renal cell carcinoma
- SP
short peptide
- TAA
tumor‐associated antigen
- TCR
T‐cell receptor
- TKI
tyrosine kinase inhibitor
- UC
urothelial cancer
- VEGFR1
vascular endothelial growth factor receptor 1
1. INTRODUCTION
Although immunotherapy for urological cancers is not a new treatment,1 recent clinical advances have confirmed the value of immunotherapy as a urological cancer treatment. Use of therapeutic cancer vaccines for prostate cancer and ICI for RCC and UC is providing evidence that immune‐based treatments may drastically improve survival or the antitumor effect for patients with advanced urological cancers. In the field of cancer immunotherapy, increasing attention has been focused on the use of cancer vaccines that activate T cells to treat growing tumors.2
The development of peptide‐based vaccines has taken more than 20 years. A vaccine specific for tumor antigens may have wide application and utility in the prevention of recurrence in numerous different malignancies. Peptide‐based vaccines are designed to elicit specific T cells against antigens selectively expressed by tumor cells.3 Peptide‐based vaccines might prolong overall survival rate and spare normal tissue because of its low toxic effect. Table 1 shows more recent clinical trials using peptide‐based vaccine therapy in urological cancers. Although peptide‐based cancer vaccines have sometimes shown survival advantages with few adverse side‐effects, this immunotherapy as a monotherapy is considered to be insufficient to elicit durable control of cancers and cures. Until now there is not the peptide vaccine therapy which showed efficacy in Phase 3 trial including urological cancers. Combination immunotherapy with peptide‐based cancer vaccines and immune‐checkpoint blockade therapies are designed concurrently to activate tumor‐specific immune responses.14
Table 1.
Recent clinical trials using peptide‐based vaccine therapy in urological cancer
| Disease status | Setting | Peptide | Phase | HLA genotype | Total no. patients | Immunological response | OS | G3‐4 AE | References |
|---|---|---|---|---|---|---|---|---|---|
| Advanced CRPC | Resistant to docetaxel chemotherapy | CDCA1 | PI | A24 | 12 | 66.7 | 11 | 0 | 4 |
| Advanced CRPC | Resistant to docetaxel chemotherapy | PPV | PII | A2/A24/A3sup/A26 | 42 | 44 | 17.8 | 0 | 5 |
| Advanced CRPC | Pre‐docetaxel chemotherapy | PPV | PII | A2/A24 | 57 | 64 | 22.4 | 0 | 6 |
| Early CRPC | Pre‐docetaxel chemotherapy | PPV | PII | A2/A24/A3sup | 37 | Unknown | 73.9 | 0 | 7 |
| Metastatic RCC | Resistant to cytokine or TKI | HIG2 | PI | A2 | 9 | 88.9 | 25.8 | 0 | 8 |
| Metastatic RCC | Resistant to cytokine or TKI | VEGFR1 | PI | A2/A24 | 18 | 83.3 | 21 | 0 | 9 |
| Metastatic RCC | First line | IMA901 (+ sunitinib) | PIII | A2 | 204 | Unknown | 33.17 | 55% | 10 |
| Advanced UC | Resistant to platinum‐based chemotherapy | PPV | PI | A2/A24 | 10 | 80 | 24 | 0 | 11 |
| Advanced UC | Resistant to platinum‐based chemotherapy | S‐288310 | PI/II | A24 | 38 | 88.9 | 9.4 | G3 9.4% | 12 |
| Advanced UC | Resistant to platinum‐based chemotherapy | PPV | PII | A2/A3/A11/A24/A26/A31/A33 | 39 | 45 | 7.9 | G3 17% | 13 |
CDCA1, cell division associated 1; CRPC, castration resistant prostate cancer; G3, grade 3; G3‐4 AE, grade 3‐4 adverse event; HIG2, hypoxia‐inducible protein 2; HLA, human leukocyte antigen; OS, overall survival; PPV, personalized peptide vaccine; RCC, renal cell carcinoma; TKI, tyrosine kinase inhibitor; UC, urothelial cancer; VEGFR1, vascular endothelial growth factor receptor 1.
In this review, we introduce the mechanism of peptide‐based vaccine therapy, recent clinical trials for urological cancers using peptide vaccines, and perspectives of peptide‐based vaccine therapy.
2. MECHANISM OF ANTITUMOR EFFECT BY PEPTIDE‐BASED VACCINES
Tumor‐associated antigens are expressed in tumor cells and can be recognized by T lymphocytes, resulting in activation of the immune system.15 A TAA peptide vaccine, when injected into cancer patients, binds with the restricted MHC molecule expressed in APC.16 The peptide/MHC complex is then transported to the cell surface after intracellular processing and is recognized by the TCR on the surface of T cells, leading to activation of T lymphocytes.17 Therefore, a peptide cancer vaccine may elicit a specific immune response against tumors.
Because CTL have the ability to recognize TAA‐derived CTL epitope peptides consisting of 8‐10 amino acids (SP) in the context of HLA class I molecules expressed on malignant cells and kill them, many cancer immunotherapies have focused primarily on how to activate the CTL to attack malignant cells. To develop effective CTL‐mediated cancer vaccines, many HLA class I‐binding SP derived from various TAA have been identified for clinical application as cancer vaccines.18, 19, 20 Accumulating these clinical data of several SP‐based cancer vaccines has shown that SP given as cancer vaccines can indeed elicit tumor‐targeting immune responses of CTL in cancer patients. However, in spite of the detection of vaccine‐induced T‐cell responses, these immune responses were rarely associated with antitumor effects of cancer vaccines, and the effects of SP‐based cancer vaccines have been limited to a small fraction (<10%) of cancer patients.21, 22 These failures may be a result of many factors, including poor immunogenicity of TAA, immune escape of tumor cells, and tumor heterogeneity.23
Vaccination with HLA class I‐restricted SP alone does not always elicit a sufficient immune response to induce effective antitumor immunity.21 One of the causes for this ineffectiveness of SP‐based cancer vaccines is considered to be the induction of immunological tolerance in CD8+ T cells. SP can induce tolerance or anergy of CD8+ T cells when they are presented by HLA class I molecules expressed on non‐professional APC because of the lack of signaling from costimulatory molecules,21, 24 whereas an extended LP encompassing several epitopes recognized by both CTL and T‐helper cells may overcome this problem because LP cannot bind directly to HLA class I molecules expressed on non‐professional APC because of their long amino acid sequence. After injection of an LP, professional APC, such as DC, take up the LP, process it, then present CTL and T‐helper cell epitopes in the context of HLA class I and HLA class II molecules, respectively.21
3. DEVELOPMENT OF PEPTIDE VACCINE THERAPY USING ONCOANTIGEN
Figure 1 shows the development of peptide vaccine therapy for several urological cancers. We reported a genome‐wide expression profile analysis of urological cancers using cDNA microarray, and identified several TAA.25, 26, 27, 28 These TAA have been shown to be frequently overexpressed in various cancer tissues including urological cancer. We defined these TAA as oncoantigens, which are shown to be essential for tumor growth/survival. Hence, cancer cells are unable to escape from the immune attack from CTL because the loss of their expression leads to the death of cancer cells. As a result of these reasons described above, vaccines we developed are expected to show better clinical efficacy than previously used antigens. In order to identify the epitope peptide corresponding to oncoantigens, we apply the “BIMAS” program for prediction of possible peptide fragments that would bind to the HLA‐A molecule. According to the prediction program, we synthesized many oncoantigen‐derived candidate peptides. Through experiments using human lymphocytes, we identified that specific peptides could induce potent CD8+ T‐cell responses, and established oncoantigen‐derived peptide‐specific CTL clones. To further examine CTL responses to the HLA‐A matched cells that express oncoantigens, we constructed plasmid clones designed to express HLA‐A matched oncoantigens, and transfected into COS7 cells. The oncoantigen‐specific CTL clones produced a substantial amount of IFN‐γ. Moreover, IFN‐γ production was observed when oncoantigen‐specific‐CTL clones were mixed with HLA‐A‐positive cancer cells with endogenous expression of oncoantigens. These results strongly indicated that vaccination with oncoantigen‐derived peptides can induce specific CTL potently cytotoxic to HLA‐A matched cancer cells expressing oncoantigen. Thus, we identified several oncoantigen‐derived epitope peptides by measuring the induction of activation of specific CTL.29, 30, 31 We have also tested these peptides in clinical investigations against several urological cancers.
Figure 1.
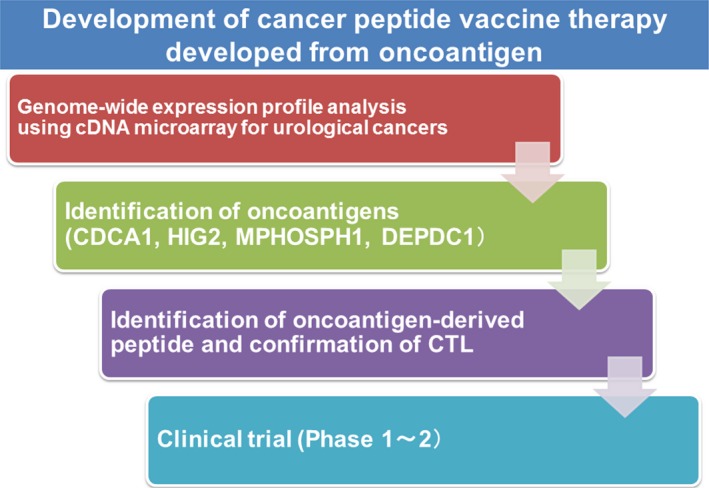
Overview of developed peptide vaccine treatment by our strategy. We constructed a genome‐wide expression profile of bladder cancer using cDNA microarray, and identified several oncoantigens. We subsequently detected that stimulation using human leukocyte antigen (HLA)‐A‐restricted oncoantigen‐derived epitope peptides induces specific CTL. We conducted a clinical study using these novel peptide vaccines for patients with urological cancer. CDCA1, cell division associated 1; DEPDC1, DEP domain‐containing 1; HIG2, hypoxia‐inducible protein 2; MPHOSPH1, M‐phase phosphoprotein 1
4. PROSTATE CANCER
A greater understanding of basic immunological principles and advances in immunological and molecular techniques led to the development of therapeutic cancer vaccines for prostate cancer. Sipuleucel‐T (Provenge) was the first cancer vaccine approved by the FDA for the treatment of CRPC in 2010.32 This agent is designed to work, at least in part, by generating or augmenting an antitumor immune response. Sipuleucel‐T, an autologous cellular therapy, showed an OS benefit of 4.1 months in patients with asymptomatic or minimally symptomatic CRPC (25.8 vs 21.7 months for placebo). Recently, a subsequent analysis suggested that patients in the placebo group who were later given cryopreserved immune cells as part of a planned crossover may have performed better clinically than would have been expected.33 Therefore, the results of the phase III study may have underestimated the actual therapeutic benefit. There has been remarkable progress in cancer immunotherapy using anti‐CTLA4 antibody for prostate cancer. However, a phase III trial using anti‐CTLA4 antibody (ipilimumab) for CRPC patients showed no significant difference between the ipilimumab group and the placebo group in terms of overall survival.34
Fenoglio et al35 confirmed the safety and immunological response against prostate cancers by using a multipeptide, dual‐adjuvant telomerase vaccine called GX301, which is composed of four telomerase peptides (peptide 540‐548, peptide 611‐626, peptide 672‐686, and peptide 766‐780) and two adjuvants, MontanideISA‐51 and Imiquimod. Another approach is a PPV, which uses multiple tumor‐associated antigens based on the pre‐existing host immunity. Two phase II studies have been reported in patients post‐docetaxel,6 with one showing a longer OS time.5 Recently, another phase II study showed a delay in PFS of PSA in patients with chemotherapy naïve CRPC.7 Thirty‐seven patients received peptide vaccinations and 35 received dexamethasone alone. PSA PFS was significantly longer in the vaccination group than in the dexamethasone group (22.0 vs 7.0 months; P = .0076). Median OS was also significantly longer in the vaccination group (73.9 vs 34.9 months; P = .00084). The researchers suggested that early‐stage CRPC with PS of 0 or 1 and PSA <10 ng/mL may receive preferable clinical benefits from peptide vaccine treatment. A randomized phase III study is now progressing for patients with early‐stage CRPC.
We previously identified CDCA1, which was also overexpressed in various cancers including prostate cancer.25 We screened and identified an HLA‐A*2402‐restricted epitope peptide, CDCA1‐A24‐56‐64, that has a high antigenic activity to induce CTL.29 Recently, we reported a phase I clinical trial for patients with CRPC using a CDCA1 peptide vaccination.4 Twelve patients having HLA‐A*2402 with CRPC after failure of docetaxel chemotherapy were enrolled. They received s.c. administration of the CDCA1 peptide as an emulsion with Montanide ISA 51 VG once a week in a dose‐escalation method (doses of 1.0 or 3.0 mg/body, 6 patients received each dose). Primary endpoint was safety, and secondary endpoints were immunological and clinical responses. Vaccination with CDCA1 peptide was well tolerated without any serious adverse events (AE). Peptide‐specific CTL responses using ELISPOT assay and dextramer assay were observed in three patients receiving the 1.0 mg dose and in five patients receiving the 3.0 mg dose (Figure 2). Median overall survival was 11.0 months and specific CTL reacting to CDCA1 peptide were recognized in long‐surviving patients. CDCA1‐derived peptide vaccine treatment was tolerable and might effectively induce peptide‐specific CTL for CRPC patients. This oncoantigen‐derived peptide vaccine therapy might also provide clinical benefit by extending survival and maintaining the quality of life of CRPC patients. In future, a randomized, controlled clinical trial will be essential to show the clinical benefits.
Figure 2.
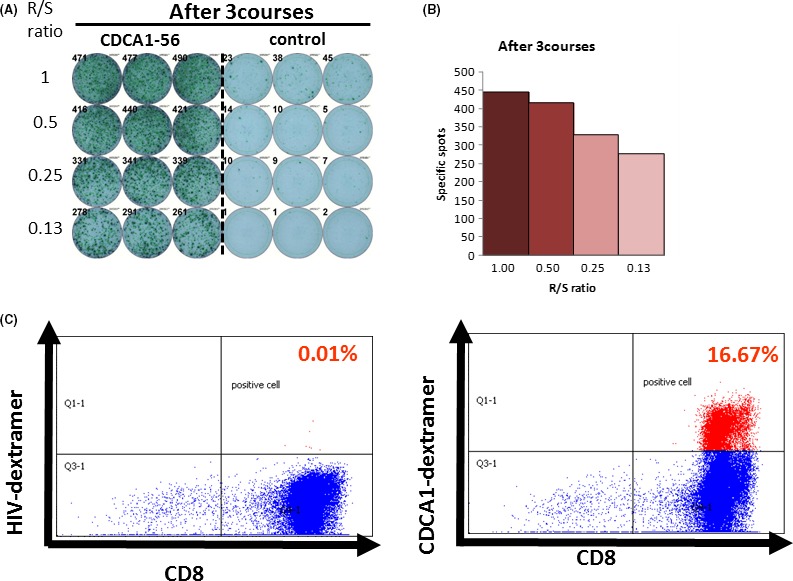
Representative cell division associated 1 (CDCA1) peptide‐specific CTL responses. (A) Cultured lymphocytes were subjected to Enzyme‐Linked ImmunoSpot (ELISPOT) assay after depletion of CD4‐positive cells by magnetic beads. (B) TISI cells were incubated with responder cells in the presence of CDCA1 peptide or HIV peptide as an irrelevant control, and the spot counts were quantified. (C) Cultured lymphocytes were analyzed with human leukocyte antigen (HLA)‐A2402/HIV‐dextramer prevaccination (left) or HLA‐A2402/CDCA1‐dextramer (right) combined with anti‐CD8 and ‐CD3 mAbs by flow cytometry. Value of dextramer (+)/CD8(+) cells among CD3(+) cells is shown. R/S, responder/stimulator
5. RENAL CELL CARCINOMA
Renal cell carcinoma is an immunologically sensitive tumor. Cytokine therapy, as IFN‐α and IL‐2, was the main treatment of metastatic RCC until approval of molecular‐targeted medicines. Recently, nivolumab (anti‐PD‐1) showed an OS benefit in patients previously treated with TKI for metastatic RCC, which led to approval.36
Several RCC‐associated antigens as well as HLA class I‐restricted epitope peptides have previously been reported.37 However, only a limited number of clinical studies using the peptide‐based vaccine for RCC have been reported,9, 38, 39, 40, 41, 42, 43 and the clinical benefit of vaccine therapy for RCC is likely to be limited to a small subset of patients. More recently, Minami et al44 reported that HIF‐1α‐derived peptides can induce RCC‐reactive CTL from HLA‐A24+ RCC patients. Among five peptides derived from HIF‐1α, a HIF‐1α278‐287 peptide induced peptide‐specific CTL from peripheral blood mononuclear cells of HLA‐A24+ RCC patients most effectively.
We previously reported HIG2 as an oncofetal protein that was highly expressed in RCC and fetal kidney as determined by genome‐wide expression profile analysis.26 Because HIG2 expression was specific to RCC and had an expression that was hardly detectable in normal organs, we considered HIG2 to be a good candidate for the development of molecular‐targeted therapies against RCC. We screened and identified a human leukocyte antigen (HLA)‐A*0201/0206‐restricted epitope peptide, named HIG2‐9‐4 peptide, that could have a high antigenic activity to induce CTL.30 Recently, we reported a phase I clinical trial using the HIG2‐9‐4 peptide for patients with advanced RCC.8 Nine patients having HLA‐A*02 with metastatic or unresectable RCC after failure of the cytokine and/or TKI therapies were enrolled in this study. The patients received s.c. administration of the peptide as an emulsion form with Montanide once a week in a dose‐escalation method. HIG‐2 derived peptide vaccine therapy was well tolerated without severe AE. Peptide‐specific CTL responses were detected in eight of the nine patients. Doses of 1.0 or 3.0 mg/bodyweight seemed to induce a CTL response better than did a dose of 0.5 mg/bodyweight. Disease control rate was 77.8%, and median PFS was 10.3 months (Figure 3). HIG2‐9‐4 peptide vaccine treatment was tolerable and effectively induced peptide‐specific CTL in RCC patients. This novel peptide vaccine therapy for RCC seems to be promising.
Figure 3.
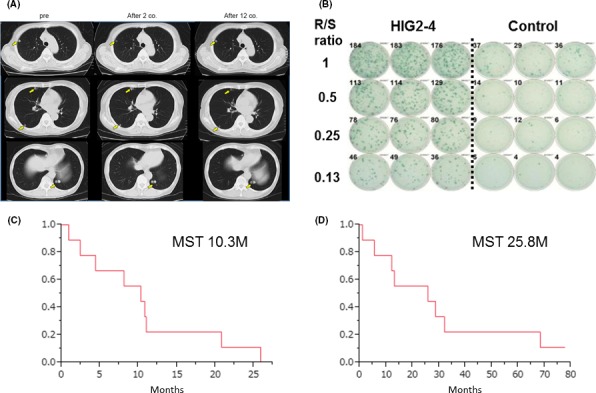
Hypoxia‐inducible protein 2 (HIG2) peptide vaccine therapy for advanced renal cell carcinoma. (A) Chest computed tomography shows multiple lung metastases as indicated by arrows before the vaccine treatment. After 2 and 12 courses of peptide vaccine treatment, sizes of multiple lung metastases were unchanged. (B) HIG2‐9‐4‐specific CTL response after one course of vaccine treatment. MST; median survival time, R/S ratio; responder/stimulator ratio. Kaplan‐Meier estimates of (C) progression‐free survival and overall (D) survival on HIG2 peptide vaccine therapy
6. UROTHELIAL CANCER
Intravesical BCG therapy against bladder cancer, which has been used for a long time, is a non‐specific immunotherapy and is known to activate a cellular immune response. More recently, atezolizumab (anti‐PD‐L1) and nivolumab (anti‐PD‐1) in patients with mUC who had progressed on platinum‐based chemotherapy showed a high response rate and longer survival effect. Based on these trials, FDA approved these immune checkpoint inhibitors for the treatment of mUC progressing after platinum‐based chemotherapy.45, 46
As for peptide vaccine therapy, Matsumoto et al reported a phase I study of PPV for advanced urothelial carcinoma patients who failed treatment with MVAC. Ten patients with MVAC‐refractory advanced or metastatic UC were treated with weekly PPV 12 times using positive peptides chosen from 14 and 16 peptides in patients with HLA A24 and A2, respectively. The peptide vaccination was safe and well tolerated with no major adverse effects. Increased CTL response and antipeptide IgG titer were seen in post‐vaccination sera in eight patients.11 More recently, Noguchi et al13 reported a randomized phase II trial of PPV in patients with bladder cancer that progressed after platinum‐based chemotherapy. Eighty patients were randomly assigned to receive either PPV plus BSC (n = 39) or BSC only (n = 41). No significant improvement in PFS was noted. For the secondary endpoints, PPV plus BSC significantly prolonged OS compared with BSC only, with median OS of 7.9 months in the PPV plus BSC group and 4.1 months in the BSC‐only group. PPV treatment was well tolerated, without serious adverse drug reactions.
We have previously reported two novel oncoantigens, DEP domain‐containing 1 (DEPDC1) and M‐phase phosphoprotein 1 (MPHOSPH1), through expression profile analysis of bladder cancers.27, 28 HLA‐A*24:02‐restricted epitope peptides from these antigens have been shown to induce strong CTL, which were able to kill tumor cells expressing these antigens in a HLA‐restricted way.31 More recently, we reported a phase I/II study using these peptide vaccines in advanced UC patients who were resistant to cisplatin‐based chemotherapy, as a sponsored initiated clinical trial (Figure 4).12 S‐288310, a cancer peptide vaccine composed of two HLA‐A*24:02‐restricted peptides derived from two oncoantigens was investigated. Thirty‐eight HLA‐A*24:02‐positive patients with progressive UC were enrolled in this study. In the phase I part of the study, three patients each were treated with S‐288310 at 1 mg or 2 mg/peptide s.c. once a week to evaluate safety and tolerability. In the phase II part, 32 patients were randomized to receive either 1 mg or 2 mg to evaluate the difference in CTL induction and safety. S‐288310 was safe and well tolerated. There was no difference in CTL induction rate between the 1‐mg (100%) and the 2‐mg (80.0%) groups. Of the 32 patients receiving S‐288310 in the phase II part, the most frequent drug‐related AE was an injection site reaction that was observed in 29 patients (90.6%), but none of the patients discontinued treatment as a result of these reactions and no dose relationship in the frequency and severity was observed. Objective response rate of the 32 patients was 6.3% and disease control rate was 56.3%. Median OS rates for patients vaccinated with S‐288310 after one regimen of chemotherapy, two regimens, or three or more were 14.4, 9.1 and 3.7 months, respectively, and 32.2% of patients post first‐line treatment were alive at 2 years. OS of patients who showed CTL induction to both peptides was longer than that of those with CTL induction to no or one peptide. We concluded that S‐288310 was well tolerated and effectively induced peptide‐specific CTL, which were correlated with longer survival for patients with UC of the bladder. Our findings support the concept of cancer peptide vaccine to prime antitumor responses and warrant further clinical trials.
Figure 4.
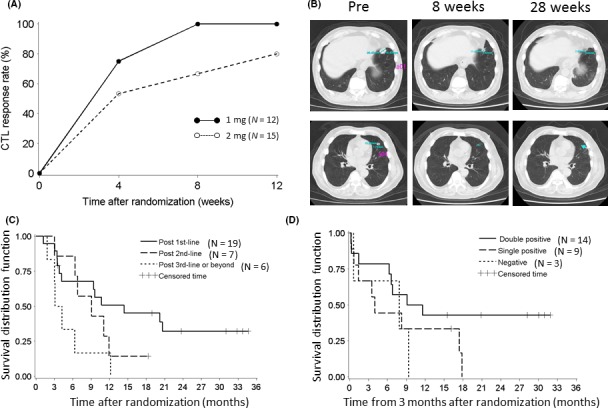
M‐phase phosphoprotein 1 (MPHOSPH1) and DEP domain‐containing 1 (DEPDC1)‐derived peptide vaccine therapy for advanced bladder cancer. (A) Time‐dependent dynamics of CTL induction rate in patients showing positive CTL activity to either one of the two peptides. (B) Chest computed tomography images show durable tumor shrinkage of multiple lung metastases until 28 wk. (C) Subgroup analysis, Kaplan‐Meier curve for survival by number of lines of prior chemotherapy. (D) Landmark analysis, Kaplan‐Meier curve for survival by number of peptides with CTL induction. Double positive means patients in whom CTL induction to both peptides was observed; Single positive, those showing CTL induction to one peptide; Negative, patients showing no CTL induction
7. COMBINATION THERAPY USING PEPTIDE‐BASED VACCINE
The best combination appears to be that involving immunomodulatory agents47 which can amplify T‐cell expansion over time and increase the duration of the effect of vaccination.48 In fact, the only peptide‐based successful phase III trial included a combination of the gp100 peptide and a high dose of IL‐2 in metastatic melanoma patients49 that was crucial for the in vivo maintenance and expansion of T cells induced by the peptide. These immunotherapeutic combinations are being tested in several phase I‐II trials by different groups of researchers worldwide in the hope of increasing the clinical efficacy of cancer vaccination.
A new and promising area of studies is the combination of peptide vaccines with other biotherapeutics such as immunomodulating or antivascular antibodies,48, 50 and even radio/chemotherapy.51 IMA901 is a therapeutic vaccine consisting of nine different HLA class I‐binding tumor‐associated peptides and one HLA class II‐binding tumor‐associated peptide.52 Genes targeted by peptides contained in IMA901 were chosen on the basis of their strong overexpression on renal tumor cells compared with normal cells.53 As IMA901 contains ten different tumor‐associated peptides, it is intended to induce expansion of multiple T cells with different antigen specificities. These T‐cell responses are expected to circumvent the ability of tumors to adapt to and evade a cytotoxic T‐cell response. Furthermore, co‐application of immunological adjuvants with tumor‐associated peptides is crucial to elicit a strong and sustained immune response against tumor cells. GM‐CSF indirectly stimulates T‐cell responses against these tumor‐associated peptides by attracting and stimulating DC in skin loaded with tumor‐associated peptides upon vaccination. Results from several clinical trials54 of multipeptide vaccine for resected stage II melanoma, mostly with known melanoma peptides, showed that the addition of GM‐CSF resulted in better T‐cell responses than the peptide alone or in combination with other immunomodulators. Additionally, evidence from clinical studies55, 56 suggests that low‐dose cyclophosphamide given before vaccination is of benefit to patients with cancer, most likely through inhibition of regulatory T cells and subsequent enhanced immune responses, or clinical response to vaccination, or both. In the past decade, results from in vitro experiments and animal studies showed positive immunomodulatory effects of cyclophosphamide on DC, APC, tumor‐infiltrating cells, and myeloid‐derived suppressor cells.10
Recently, a phase III open‐label trial in metastatic RCC patients that used IMA901 vaccine was reported.10 Before the start of this trial, findings from in vitro and in vivo models57, 58 showed that sunitinib concentrations similar to those measured in the serum of sunitinib‐treated patients do not interfere with the capacity of APC to induce potent T‐cell responses and to stimulate T‐cell proliferation. Moreover, levels of immunosuppressive regulatory T cells in the blood are reduced after treatment with sunitinib in mice and in the peripheral blood of patients with RCC.58 Thus, sunitinib was considered a suitable candidate for immunotherapeutic combinations on the basis of its established antitumor effect in RCC and potentially favorable immune modulatory properties. As a first‐line setting following one cycle of sunitinib, patients were randomized 3:2 for up to 10 intradermal vaccinations of IMA901, a peptide vaccine, plus 75 μg GM‐CSF plus sunitinib versus sunitinib alone. Patients in the vaccination arm were given a single infusion of cyclophosphamide before the first vaccination to reduce regulatory T cells. Three hundred and thirty‐nine patients were randomized to treatment of sunitinib plus IMA901 (n = 204) or sunitinib monotherapy (n = 135). Mean number of vaccinations received was 9.3, and 162 (80%) of 202 patients in the safety population received all 10 scheduled vaccinations with IMA901 and GM‐CSF. Unfortunately, median OS did not differ significantly between the groups (33.17 months in the sunitinib plus IMA901 group vs not reached in the sunitinib monotherapy group; P = .087). Similarly, data for PFS (15.22 months vs 15.12 months), objective tumor response (36% vs 42%), and safety did not show relevant differences between the groups. IMA901 has an acceptable safety profile overall, and frequencies of treatment‐emergent AE were similar in both groups. Transient injection site reactions (eg erythema and pruritus) were the most frequent AE related to IMA901. No significant differences in any AE with a frequency of greater than 5% were seen between the groups. IMA901‐specific CD8‐positive T‐cell responses were reduced threefold in magnitude compared with data from phase I‐II trials of the same vaccine in metastatic RCC and other solid tumors.52, 59 Moreover, by contrast with the previous phase II metastatic RCC trial, we did not find a clear association of T‐cell responses with clinical outcome. Finally, a significant decrease in monocytes after the first treatment cycle with sunitinib was observed. The numerically shorter overall survival in the sunitinib plus IMA901 group than in the sunitinib group might indicate a potentially harmful effect of vaccination; however, the more likely explanation is the exceptionally good outcome in the sunitinib monotherapy group, especially in intermediate‐risk patients, compared with historical controls. Absence of a harmful effect of vaccination is also supported by the similar overall survival results in patients who did and did not develop a T‐cell response to IMA901 vaccination.
8. PERSPECTIVES OF PEPTIDE‐BASED VACCINE THERAPY
Currently, neoantigens are certainly more cancer‐specific than tumor antigen‐derived peptides. Effective antitumor immunity in humans has been associated with the presence of T cells directed at cancer neoantigens,60 a class of HLA‐bound peptides that arise from tumor‐specific mutations. They are highly immunogenic because they are not present in normal tissues and hence bypass central thymic tolerance. Although neoantigens were long‐envisioned as optimal targets for an antitumor immune response,61 their systematic discovery and evaluation only became feasible with the recent availability of massively parallel sequencing for detection of all coding mutations within tumors, and of machine‐learning approaches to reliably predict those mutated peptides with high‐affinity binding of autologous HLA molecules. Novel technologies provide opportunities for in‐depth knowledge on relevant tumor epitopes, obtaining knowledge about specific tumor antigens forming epitopes recognized by individual T cells.62 The importance of stringent neoantigen prediction and immunogenicity of neoantigen‐based vaccines for advanced melanoma patients has been reported by Carreno et al63 They vaccinated three patients with advanced‐stage melanoma with seven peptides predicted to be immunogenic based on mutation analysis and peptide‐binding experiments, and reported T‐cell responses specific for three peptides post‐vaccination. Two of these responses were not detected before vaccination in these patients. This study shows that mutation‐derived subdominant epitopes can trigger cancer‐specific immune responses through vaccination. More recently, Ott et al64 showed the feasibility, safety, and immunogenicity of a vaccine that targets up to 20 predicted personal tumor neoantigens. Vaccine‐induced polyfunctional CD4+ and CD8+ T cells targeted 58 (60%) and 15 (16%) of the 97 unique neoantigens used across patients, respectively. Of six vaccinated patients, four had no recurrence at 25 months after vaccination, whereas two with recurrent disease were subsequently treated with anti‐PD‐1 therapy and experienced complete tumor regression, with expansion of the repertoire of neoantigen‐specific T cells.
Although neoantigen‐based vaccine therapy is extremely promising, we still do not know which induces a higher level of antitumor immune responses in patients with urological cancer. In addition, it is also certain that HLA‐restricted cancer peptide vaccines derived from oncoantigens can be more widely applicable to a larger subset of cancer patients than individualized neoantigens. We suspect that combination therapies with peptide‐based cancer vaccines including oncoantigen and neoantigen‐targeting cancer vaccines, and immune checkpoint blockade therapies or other immunotherapies are expected to be good candidates for more effective cancer immunotherapy which could enhance clinical benefits. Furthermore, the recent technical progress of genetic analysis enables us to easily evaluate the immunogenicity of tumor cells and the immune status of the tumor microenvironment in individual cancer patients.65 This information is expected to lead to the discovery of predictive biomarkers to select patients for treatment with cancer immunotherapy and the development of personalized peptide‐based cancer vaccines that may improve the efficacy of this immunotherapy.
9. CONCLUSIONS
Peptide‐based vaccines have been used in the past with limited clinical success. However, during the last few years, new knowledge on the biological characteristics of the peptides and their interaction with the immune system to be used clinically has been provided. New protocols have allowed significant immune and clinical responses in patients vaccinated with multiple peptides, particularly by combining the peptides with a variety of other biological therapeutics. This situation is now even more promising than before and we predict that such new peptide‐based trials will provide other clinical successes in urological cancers.
CONFLICTS OF INTEREST
Authors declare no conflicts of interest for this article.
Obara W, Kanehira M, Katagiri T, Kato R, Kato Y, Takata R. Present status and future perspective of peptide‐based vaccine therapy for urological cancer. Cancer Sci. 2018;109:550–559. https://doi.org/10.1111/cas.13506
REFERENCES
- 1. Coley WB. The treatment of inoperable sarcoma by bacterial toxins (the mixed toxins of the streptococcus erysipelas and the Bacillus prodigiosus). Proc R Soc Med. 1910;3:1‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909‐915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Milani A, Sangiolo D, Montemurro F, Aglietta M, Valabrega G. Active immunotherapy in HER2 overexpressing breast cancer: current status and future perspectives. Ann Oncol. 2013;24:1740‐1748. [DOI] [PubMed] [Google Scholar]
- 4. Obara W, Sato F, Takeda K, et al. Phase I clinical trial of cell division associated 1 (CDCA1) peptide vaccination for castration resistant prostate cancer. Cancer Sci. 2017;108:1452‐1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Noguchi M, Moriya F, Suekane S, et al. Phase II study of personalized peptide vaccination for castration‐resistant prostate cancer patients who failed in docetaxel‐based chemotherapy. Prostate. 2012;72:834‐845. [DOI] [PubMed] [Google Scholar]
- 6. Noguchi M, Kakuma T, Uemura H, et al. A randomized phase II trial of personalized peptide vaccine plus low dose estramustine phosphate (EMP) versus standard dose EMP in patients with castration resistant prostate cancer. Cancer Immunol Immunother. 2010;59:1001‐1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yoshimura K, Minami T, Nozawa M, et al. A phase 2 randomized controlled trial of personalized peptide vaccine immunotherapy with low‐dose dexamethasone versus dexamethasone alone in chemotherapy‐naive castration‐resistant prostate cancer. Eur Urol. 2016;70:35‐41. [DOI] [PubMed] [Google Scholar]
- 8. Obara W, Karashima T, Takeda K, et al. Effective induction of cytotoxic T cells recognizing an epitope peptide derived from hypoxia‐inducible protein 2 (HIG2) in patients with metastatic renal cell carcinoma. Cancer Immunol Immunother. 2017;66:17‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yoshimura K, Minami T, Nozawa M, Uemura H. Phase I clinical trial of human vascular endothelial growth factor receptor 1 peptide vaccines for patients with metastatic renal cell carcinoma. Br J Cancer. 2013;108:1260‐1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rini BI, Stenzl A, Zdrojowy R, et al. IMA901, a multipeptide cancer vaccine, plus sunitinib versus sunitinib alone, as first‐line therapy for advanced or metastatic renal cell carcinoma (IMPRINT): a multicentre, open‐label, randomised, controlled, phase 3 trial. Lancet Oncol. 2016;17:1599‐1611. [DOI] [PubMed] [Google Scholar]
- 11. Matsumoto K, Noguchi M, Satoh T, et al. A phase I study of personalized peptide vaccination for advanced urothelial carcinoma patients who failed treatment with methotrexate, vinblastine, adriamycin and cisplatin. BJU Int. 2011;108:831‐838. [DOI] [PubMed] [Google Scholar]
- 12. Obara W, Eto M, Mimata H, et al. A phase I/II study of cancer peptide vaccine S‐288310 in patients with advanced urothelial carcinoma of the bladder. Ann Oncol. 2017;28:798‐803. [DOI] [PubMed] [Google Scholar]
- 13. Noguchi M, Matsumoto K, Uemura H, et al. An open‐label, randomized phase ii trial of personalized peptide vaccination in patients with bladder cancer that progressed after platinum‐based chemotherapy. Clin Cancer Res. 2016;22:54‐60. [DOI] [PubMed] [Google Scholar]
- 14. Hirayama M, Nishimura Y. The present status and future prospects of peptide‐based cancer vaccines. Int Immunol. 2016;28:319‐328. [DOI] [PubMed] [Google Scholar]
- 15. Parmiani G, Castelli C, Dalerba P, et al. Cancer immunotherapy with peptide‐based vaccines: what have we achieved? Where are we going? J Natl Cancer Inst. 2002;94:805‐818. [DOI] [PubMed] [Google Scholar]
- 16. Aranda F, Vacchelli E, Eggermont A, et al. Trial watch: peptide vaccines in cancer therapy. Oncoimmunology. 2013;2:e26621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mohme M, Neidert MC, Regli L, Weller M, Martin R. Immunological challenges for peptide‐based immunotherapy in glioblastoma. Cancer Treat Rev. 2014;40:248‐258. [DOI] [PubMed] [Google Scholar]
- 18. Jager E, Chen YT, Drijfhout JW, et al. Simultaneous humoral and cellular immune response against cancer‐testis antigen NY‐ESO‐1: definition of human histocompatibility leukocyte antigen (HLA)‐A2‐binding peptide epitopes. J Exp Med. 1998;187:265‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ohminami H, Yasukawa M, Fujita S. HLA class I‐restricted lysis of leukemia cells by a CD8(+) cytotoxic T‐lymphocyte clone specific for WT1 peptide. Blood. 2000;95:286‐293. [PubMed] [Google Scholar]
- 20. Rongcun Y, Salazar‐Onfray F, Charo J, et al. Identification of new HER2/neu‐derived peptide epitopes that can elicit specific CTL against autologous and allogeneic carcinomas and melanomas. J Immunol. 1999;163:1037‐1044. [PubMed] [Google Scholar]
- 21. Melief CJ, van der Burg SH. Immunotherapy of established (pre)malignant disease by synthetic long peptide vaccines. Nat Rev Cancer. 2008;8:351‐360. [DOI] [PubMed] [Google Scholar]
- 22. Melero I, Gaudernack G, Gerritsen W, et al. Therapeutic vaccines for cancer: an overview of clinical trials. Nat Rev Clin Oncol. 2014;11:509‐524. [DOI] [PubMed] [Google Scholar]
- 23. Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S. Escape of human solid tumors from T‐cell recognition: molecular mechanisms and functional significance. Adv Immunol. 2000;74:181‐273. [DOI] [PubMed] [Google Scholar]
- 24. Bijker MS, van den Eeden SJ, Franken KL, Melief CJ, van der Burg SH, Offringa R. Superior induction of anti‐tumor CTL immunity by extended peptide vaccines involves prolonged. DC‐focused antigen presentation. Eur J Immunol. 2008;38:1033‐1042. [DOI] [PubMed] [Google Scholar]
- 25. Hayama S, Daigo Y, Kato T, et al. Activation of CDCA1‐KNTC2, members of centromere protein complex, involved in pulmonary carcinogenesis. Cancer Res. 2006;66:10339‐10348. [DOI] [PubMed] [Google Scholar]
- 26. Togashi A, Katagiri T, Ashida S, et al. Hypoxia‐inducible protein 2 (HIG2), a novel diagnostic marker for renal cell carcinoma and potential target for molecular therapy. Cancer Res. 2005;65:4817‐4826. [DOI] [PubMed] [Google Scholar]
- 27. Kanehira M, Katagiri T, Shimo A, et al. Oncogenic role of MPHOSPH1, a cancer‐testis antigen specific to human bladder cancer. Cancer Res. 2007;67:3276‐3285. [DOI] [PubMed] [Google Scholar]
- 28. Kanehira M, Harada Y, Takata R, et al. Involvement of upregulation of DEPDC1 (DEP domain containing 1) in bladder carcinogenesis. Oncogene. 2007;26:6448‐6455. [DOI] [PubMed] [Google Scholar]
- 29. Tomita Y, Yuno A, Tsukamoto H, et al. Identification of CDCA1‐derived long peptides bearing both CD4+ and CD8+ T‐cell epitopes: CDCA1‐specific CD4+ T‐cell immunity in cancer patients. Int J Cancer. 2014;134:352‐366. [DOI] [PubMed] [Google Scholar]
- 30. Yoshimura S, Tsunoda T, Osawa R, et al. Identification of an HLA‐A2‐restricted epitope peptide derived from hypoxia‐inducible protein 2 (HIG2). PLoS One. 2014;9:e85267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Obara W, Ohsawa R, Kanehira M, et al. Cancer peptide vaccine therapy developed from oncoantigens identified through genome‐wide expression profile analysis for bladder cancer. Jpn J Clin Oncol. 2012;42:591‐600. [DOI] [PubMed] [Google Scholar]
- 32. Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel‐T immunotherapy for castration‐resistant prostate cancer. N Engl J Med. 2010;363:411‐422. [DOI] [PubMed] [Google Scholar]
- 33. George DJ, Nabhan C, DeVries T, Whitmore JB, Gomella LG. Survival outcomes of sipuleucel‐T phase III studies: impact of control‐arm cross‐over to salvage immunotherapy. Cancer Immunol Res. 2015;3:1063‐1069. [DOI] [PubMed] [Google Scholar]
- 34. Kwon ED, Drake CG, Scher HI, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration‐resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184‐043): a multicentre, randomised, double‐blind, phase 3 trial. Lancet Oncol. 2014;15:700‐712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fenoglio D, Traverso P, Parodi A, et al. A multi‐peptide, dual‐adjuvant telomerase vaccine (GX301) is highly immunogenic in patients with prostate and renal cancer. Cancer Immunol Immunother. 2013;62:1041‐1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal‐cell carcinoma. N Engl J Med. 2015;373:1803‐1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gouttefangeas C, Stenzl A, Stevanovic S, Rammensee HG. Immunotherapy of renal cell carcinoma. Cancer Immunol Immunother. 2007;56:117‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Uemura H, Fujimoto K, Tanaka M, et al. A phase I trial of vaccination of CA9‐derived peptides for HLA‐A24‐positive patients with cytokine‐refractory metastatic renal cell carcinoma. Clin Cancer Res. 2006;12:1768‐1775. [DOI] [PubMed] [Google Scholar]
- 39. Iiyama T, Udaka K, Takeda S, et al. WT1 (Wilms’ tumor 1) peptide immunotherapy for renal cell carcinoma. Microbiol Immunol. 2007;51:519‐530. [DOI] [PubMed] [Google Scholar]
- 40. Suekane S, Nishitani M, Noguchi M, et al. Phase I trial of personalized peptide vaccination for cytokine‐refractory metastatic renal cell carcinoma patients. Cancer Sci. 2007;98:1965‐1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Patel PM, Sim S, O'Donnell DO, et al. An evaluation of a preparation of Mycobacterium vaccae (SRL172) as an immunotherapeutic agent in renal cancer. Eur J Cancer. 2008;44:216‐223. [DOI] [PubMed] [Google Scholar]
- 42. Minami T, Shimizu N, Yamamoto Y, et al. Identification of erythropoietin receptor‐derived peptides having the potential to induce cancer‐reactive cytotoxic T lymphocytes from HLA‐A24(+) patients with renal cell carcinoma. Int Immunopharmacol. 2014;20:59‐65. [DOI] [PubMed] [Google Scholar]
- 43. Minami T, Shimizu N, Yamamoto Y, et al. Identification of programmed death Ligand 1‐derived peptides capable of inducing cancer‐reactive cytotoxic T lymphocytes from HLA‐A24+ patients with renal cell carcinoma. J Immunother. 2015;38:285‐291. [DOI] [PubMed] [Google Scholar]
- 44. Minami T, Matsumura N, Sugimoto K, et al. Hypoxia‐inducing factor (HIF)‐1alpha‐derived peptide capable of inducing cancer‐reactive cytotoxic T lymphocytes from HLA‐A24+ patients with renal cell carcinoma. Int Immunopharmacol. 2017;44:197‐202. [DOI] [PubMed] [Google Scholar]
- 45. Sharma P, Retz M, Siefker‐Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single‐arm, phase 2 trial. Lancet Oncol. 2017;18:312‐322. [DOI] [PubMed] [Google Scholar]
- 46. Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti‐PD‐L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558‐562. [DOI] [PubMed] [Google Scholar]
- 47. Maio M, Di Giacomo AM, Robert C, Eggermont AM. Update on the role of ipilimumab in melanoma and first data on new combination therapies. Curr Opin Oncol. 2013;25:166‐172. [DOI] [PubMed] [Google Scholar]
- 48. Fourcade J, Sun Z, Pagliano O, et al. PD‐1 and Tim‐3 regulate the expansion of tumor antigen‐specific CD8(+) T cells induced by melanoma vaccines. Cancer Res. 2014;74:1045‐1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schwartzentruber DJ, Lawson DH, Richards JM, et al. Gp100 peptide vaccine and interleukin‐2 in patients with advanced melanoma. N Engl J Med. 2011;364:2119‐2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Okuyama R, Aruga A, Hatori T, Takeda K, Yamamoto M. Immunological responses to a multi‐peptide vaccine targeting cancer‐testis antigens and VEGFRs in advanced pancreatic cancer patients. Oncoimmunology. 2013;2:e27010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vacchelli E, Senovilla L, Eggermont A, et al. Trial watch: chemotherapy with immunogenic cell death inducers. Oncoimmunology. 2013;2:e23510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Walter S, Weinschenk T, Stenzl A, et al. Multipeptide immune response to cancer vaccine IMA901 after single‐dose cyclophosphamide associates with longer patient survival. Nat Med. 2012;18:1254‐1261. [DOI] [PubMed] [Google Scholar]
- 53. Singh‐Jasuja H, Emmerich NP, Rammensee HG. The Tubingen approach: identification, selection, and validation of tumor‐associated HLA peptides for cancer therapy. Cancer Immunol Immunother. 2004;53:187‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schaed SG, Klimek VM, Panageas KS, et al. T‐cell responses against tyrosinase 368‐376(370D) peptide in HLA*A0201+ melanoma patients: randomized trial comparing incomplete Freund's adjuvant, granulocyte macrophage colony‐stimulating factor, and QS‐21 as immunological adjuvants. Clin Cancer Res. 2002;8:967‐972. [PubMed] [Google Scholar]
- 55. Ghiringhelli F, Menard C, Puig PE, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641‐648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Greten TF, Ormandy LA, Fikuart A, et al. Low‐dose cyclophosphamide treatment impairs regulatory T cells and unmasks AFP‐specific CD4+ T‐cell responses in patients with advanced HCC. J Immunother. 2010;33:211‐218. [DOI] [PubMed] [Google Scholar]
- 57. Finke JH, Rini B, Ireland J, et al. Sunitinib reverses type‐1 immune suppression and decreases T‐regulatory cells in renal cell carcinoma patients. Clin Cancer Res. 2008;14:6674‐6682. [DOI] [PubMed] [Google Scholar]
- 58. Hipp MM, Hilf N, Walter S, et al. Sorafenib, but not sunitinib, affects function of dendritic cells and induction of primary immune responses. Blood. 2008;111:5610‐5620. [DOI] [PubMed] [Google Scholar]
- 59. Kirner A, Mayer‐Mokler A, Reinhardt C. IMA901: a multi‐peptide cancer vaccine for treatment of renal cell cancer. Hum Vaccin Immunother. 2014;10:3179‐3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69‐74. [DOI] [PubMed] [Google Scholar]
- 61. Hacohen N, Fritsch EF, Carter TA, Lander ES, Wu CJ. Getting personal with neoantigen‐based therapeutic cancer vaccines. Cancer Immunol Res. 2013;1:11‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Saini SK, Rekers N, Hadrup SR. Novel tools to assist neoepitope targeting in personalized cancer immunotherapy. Ann Oncol 2017;12:xii3‐xii10. [DOI] [PubMed] [Google Scholar]
- 63. Carreno BM, Magrini V, Becker‐Hapak M, et al. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen‐specific T cells. Science. 2015;348:803‐808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ott PA, Hu Z, Keskin DB, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547:217‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Linnemann C, van Buuren MM, Bies L, et al. High‐throughput epitope discovery reveals frequent recognition of neo‐antigens by CD4+ T cells in human melanoma. Nat Med. 2015;21:81‐85. [DOI] [PubMed] [Google Scholar]


