Abstract
Background
LZTS2 (leucine zipper tumor suppressor 2), a candidate tumor suppressor gene, suppresses cell growth and plays a vital role in the carcinogenesis and development of tumors. No studies to date have described methylation of the LZTS2 promoter in human cancers, including LSCC (laryngeal squamous cell carcinoma). Therefore, the aim of this study was to explore the relationship between LZTS2 promoter methylation and risk of LSCC.
Methods
In our study, LZTS2 promoter methylation levels in LSCC tumor and adjacent normal tissues from 96 patients were measured using quantitative methylation-specific polymerase chain reaction (qMSP) assays.
Results
The qMSP analyses revealed that LZTS2 promoter methylation levels in the LSCC tumor samples were significantly higher than those in paired adjacent healthy tissue samples. Furthermore, LZTS2 methylation levels were elevated in smokers, advanced T classified, and clinically staged patients, as well as in patients with lymph node metastases. In addition, Kaplan-Meier survival curves results showed that overall survival of LSCC patients with hypomethylated LZTS2 promoters was significantly higher than that in patients with hyper-methylated LZTS2 promoters (log-rank test P = 0.028). Meanwhile, the area under the receiver operating characteristic curve was 0.920. The diagnostic threshold value for LZTS2 methylation was 11.63% (94.7% sensitivity and 80.4% specificity).
Conclusions
LZTS2 promoter hypermethylation is associated with risk, progression, and prognosis of LSCC in a cohort of 96 human subjects; LZTS2 promoter hypermethylation is a candidate diagnostic and prognostic biomarker for LSCC.
Keywords: Laryngeal squamous cell carcinoma, Leucine zipper tumor suppressor 2, DNA methylation, Biomarkers, Tumor suppressor gene
Background
Laryngeal cancer is one of the most common head and neck tumors and has the second highest mortality rates of all respiratory system malignancies [1]. More than 95% of laryngeal carcinomas exhibit laryngeal squamous cell carcinoma (LSCC) pathological features [2]. The pathogenesis of LSCC is complicated and involves genetic, epigenetic, and environmental factors, such as alcohol, tobacco, and asbestos [3]. While recent advancements have been achieved in therapeutic strategies that combine surgery, chemotherapy, and radiotherapy, the 5-year overall survival rate of LSCC remains poor [1, 2].
DNA methylation is a well-studied mechanism driving epigenetic regulation of gene expression [4]. In addition to mutations and deletions, the abnormal methylation of tumor suppressor gene (TSG) promoter is considered to be the third mechanism of inactivation of TSG [4], which mainly happens in the CpG island near the original site of the gene transcription and can lead to the abnormal expression of TSG. Numerous studies have shown that hypermethylation of CpG islands in TSG plays a crucial role in carcinogenesis and progression in various solid and liquid tumors and can be a molecular marker used to identify LSCC [4], such as acute myeloid leukemia [5], bile duct carcinoma [6], and breast cancer [7].
LZTS gene family members share functions related to transcription regulation and controlling the cell cycle [8]. LZTS2 is located at human 10q24.3 (Fig. 1), which is proximate to 10q23.3, the site of the prototypical tumor suppressor gene PTEN [9]. Previous studies have shown that expressions of both regions are frequently downregulated in a variety of tumors, suggesting that other tumor susceptibility genes may exist in these areas in addition to PTEN [10]. Mounting evidence supports that LZTS2 regulates cell growth through protein-protein interactions with β-catenin [11]. β-catenin, one of the subunits of the cadherin protein complex, also performs critical functions in the Wnt signaling pathway. Accumulation of β-catenin in the nucleus plays a vital role in tumorigenesis and progression [12]. LZTS2 interacts with β-catenin to both repress its transcriptional activity and regulate its subcellular localization and signaling; together, these activities suppress cell growth [11]. In addition, Johnson et al. demonstrated, in LZTS2 knock-out mouse embryonic fibroblasts, that a lack of LZTS2 expression promoted cell survival and proliferation [10]. Thus, evidence supports that LZTS2 is a tumor suppressor gene and that aberrant expression contributes to the genesis and development of some tumors [10].
Fig. 1.
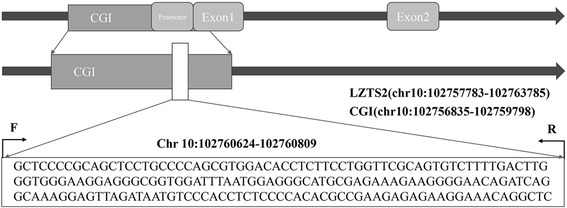
The location of the CpG island and LZTS2 gene promoter. F forward primer, R reverse primer
To date, no reports have investigated methylation of the LZTS2 promoter in human cancers, including LSCC. Therefore, the aim of the present study was to explore whether correlations exist between LZTS2 promoter methylation and risk of LSCC.
Methods
Patient demographics and tissue sample collection
The study recruited 96 patients who were diagnosed with resectable LSCC tumors. Patients were recruited from the Ear, Nose, Throat, Head, and Neck Surgery Departments of Ningbo Medical Center, Lihuili Hospital. The patients’ median age was 60 years (range 40–86 years; Table 1). The majority of subjects were male (96%). All patients were definitively diagnosed according to criteria established by the World Health Organization [13]. None of the patients received neoadjuvant chemotherapy or radiotherapy, nor did any patient have a family history of LSCC. Patients were followed for up to 58 months. The median follow-up time was 39 months (inter-quartile month range 31–50 months). Fourteen patients were lost to follow-up; twenty-three patients died. Tumor specimens were comprised of 45 well-differentiated cases, 38 moderately differentiated cases, and 13 poorly differentiated cases. Using TMN staging criteria, there were 27 Stage I, 18 Stage II, 11 Stage III, and 40 Stage IV cases. Pathological diagnoses of tumor and paired normal specimens were made in strict accordance with the Union for International Cancer Control classification guidelines (TNM 2002). Specimens were obtained from fresh tissue and then preserved at − 80 °C. Participants signed written informed consent documents. Experimental procedures were reviewed and approved by the Ethics Committee of Ningbo Lihuili Hospital.
Table 1.
Associations between LZTS2 promoter methylation and LSCC patient clinicopathological characteristics
| Variable | Number | Mean ± SD | P value |
|---|---|---|---|
| Gender | |||
| Female | 4 | 20.11 ± 6.39 | 0.364 |
| Male | 92 | 27.37 ± 15.80 | |
| Age | |||
| ≥ 60 | 49 | 24.05 ± 14.15 | 0.995 |
| < 60 | 47 | 27.07 ± 17.09 | |
| Smoking behavior | |||
| Yes | 78 | 28.57 ± 15.94 | 0.049 |
| No | 18 | 20.56 ± 12.21 | |
| Histological classification | |||
| Well | 45 | 27.98 ± 15.35 | 0.590 |
| Moderately/poorly | 51 | 26.25 ± 15.88 | |
| T classification | |||
| T 1 + 2 | 57 | 23.26 ± 13.47 | 0.003 |
| T 3 + 4 | 39 | 32.62 ± 12.90 | |
| Clinical stage | |||
| Stage I + II | 45 | 22.44 ± 11.64 | 0.005 |
| Stage III + IV | 51 | 31.14 ± 17.48 | |
| Lymph metastasis | |||
| Yes | 33 | 32.85 ± 18.62 | 0.019 |
| No | 63 | 24.03 ± 12.87 | |
LZTS2 promoter methylation levels were significantly elevated in advanced stage and advanced T classified patients, in patients who were smokers, as well as cases with lymph node metastasis. Italicized entries indicate statistical significance
DNA extraction and bisulfite modification
Genomic DNA samples were extracted from tissue specimens using QIAamp DNA Mini Kits (Qiagen, Hilden, Germany) in strict accordance with the manufacturer’s protocols. DNA concentrations and qualities were estimated using a NanoDrop 1000 spectrophotometer (Thermo Fish Scientific Co. Ltd., Wilmington, USA). Eluted DNA was bisulfite-treated using EZ DNA Methylation-Gold Kits following the manufacturer’s protocols (Zymo Research, Irvine, CA, USA).
Quantification of LZTS2 DNA methylation with quantitative methylation-specific polymerase chain reaction
We measured LZTS2 gene promoter DNA methylation by applying quantitative methylation-specific polymerase chain reaction (qMSP) technology. The primer sequences were GTTTTTCGTAGTTTTTGTTTTAGCG for the forward primer and AAACCTATTTCCTTCTCTCTTCGAC for the reverse primer (see Fig. 1 for genomic mapping details). Bisulfite-treated DNA from each specimen served as template, and qMSP was performed with FastStart Universal SYBR Green Master Mix (Roche Diagnostics, Mannheim, Germany) and LightCycler 480 real-time PCR amplifier (Roche Diagnostics, Mannheim, Germany) following the operating protocols strictly. The internal controls were designed to ACTB (the forward primer sequences of ACTB were CCTAGAAGCA-TTTGCGGTGG, and the reverse primer sequences were GAGCTACGAGCTGCCTGACG). Bisulfite-treated human genomic DNA served as positive control specimens (Zymo Research, Irvine, CA, USA). Two microliters of bisulfite-treated patient’s DNA was added in 18 μl of PCR reaction mixture containing 10 μl of FastStart Universal SYBR Green Master Mix, 6 μl water, and 1 μl of each forward and reverse primers. The reaction protocol was as follows: 10 min at 95 °C, followed by 50 cycles of 95 °C for 20 s, 60 °C for 40 s, and 72 °C for 30 s. All the procedures were performed in triplicate. The specificity of amplification products was determined with melting curve analyses according to fluorescence data acquired during dissociation steps. Results from the positive control samples were used to construct standard curves for quantification. Each specimen’s percentage of methylated reference (PMR) was calculated by entering cycle threshold (Ct) values for each specimen, internal control, and positive control into the following formula: PMR = 2−[(CTsample − CTinternal) − (CTpositive − CTinternal)] × 100%.
Statistical analyses
All statistical tests were performed with SPSS v19.0 (SPSS Inc., Chicago, IL, USA) software. Student’s t tests were performed to determine the likelihood that differences observed in LZTS2 promoter methylation levels between malignant and paired normal tissues occurred due to chance alone. Further paired-samples t test was performed on LZTS2 promoter methylation rates taking into consideration the following clinicopathological characteristics: gender, age, smoking status, histological classification, T classification, presence of lymph node metastases, and clinical stage. Overall survival curves were evaluated by Kaplan-Meier analyses, and statistical differences between curves were evaluated using log-rank tests. Univariate and multivariate Cox proportional hazard models were used to test the prognostic value of LZTS2 methylation for LSCC patients. A two-tailed P < 0.05 was considered to be significant. Figures were illustrated using SPSS v19.0 and GraphPad Prism 7 software (GraphPad, San Diego, CA, USA).
Results
LZTS2 promoter methylation levels were evaluated in 96 LSCC specimens and paired adjacent normal tissues using qMSP technologies. The present data revealed that LZTS2 promoter methylation levels were significantly higher in LSCC cancer tissues when compared to paired adjacent normal tissues (Fig. 2, P = 1.37e−06). Given that smokers are at risk of developing LSCC [14], the cohort was stratified according to smoking status. Subgroup analyses revealed that the observed LZTS2 hypermethylation patterns occurring in LSCC (when compared to paired normal tissues) were more clearly evident in smokers than non-smokers (Fig. 2, smoking P = 4.74e−18; non-smokers P = 0.029). Next, associations between clinicopathological characteristics (e.g., age, gender, smoking behavior, histological classification, T classification, clinical stage, and lymph node metastases) and LZTS2 promoter methylation rates were further explored (Table 1). As expected, the subgroup analyses confirmed that LZTS2 promoter methylation levels of LSCC in smokers were significantly elevated, relative to non-smokers (P = 0.049). Additionally, T classification, clinic stage, and presence of lymph metastases all served as significant explanatory variables. The PMR in advanced clinical stages (stages III + IV) and T classifications (T III + IV) were significantly greater than specimens exhibiting earlier clinical stages (stages I + II; P = 0.005) and lower T classifications (T I + II; P = 0.003). In contrast to patients without lymph node metastases, methylation levels of patients with lymph node metastases were significantly elevated (P = 0.019). The mean of PMR in male subjects, subjects over 60 years old, and subjects with well-differentiated histologies were numerically higher than female subjects, subjects under 60 years old, and subjects with moderately and poorly differentiated histologies, respectively; however, these differences were not statistically significant (Table 1).
Fig. 2.
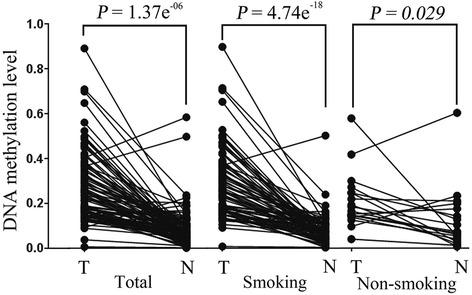
Comparison of LZTS2 methylation levels between LSCC malignant tissues and paired adjacent normal tissues: LZTS2 promoter methylation levels were significantly higher in LSCC tissues compared to normal tissues (n = 96, P = 1.37e−06). Stratification analyses according to smoking status indicated the difference of LZTS2 promoter methylation levels between LSCC tissues and normal tissues was more significant in smokers (n = 78, P = 4.74e−18) versus non-smokers (n = 18, P = 0.029). T tumor specimen, N normal adjacent specimen
To evaluate the potential diagnostic value of LZTS2 methylation, the receiver operating characteristic (ROC) curve was plotted. This technique served to identify a diagnostic threshold value for LZTS2 promoter methylation. The area under the ROC curve (AUC) was 0.920 (Fig. 3). The diagnostic threshold value (cut-off value) for LZTS2 methylation was 11.63% (94.7% sensitivity and 80.4% specificity). The values over the cut-off were defined as positive diagnostic indicators, while those below the cut-off were considered negative indicators. The false positive and false negative rates were 18.8 and 5.2%, respectively. The positive predictive value was 83.5% and negative predictive value was 94.0%. The diagnostic accordance rate was 88.0%.
Fig. 3.
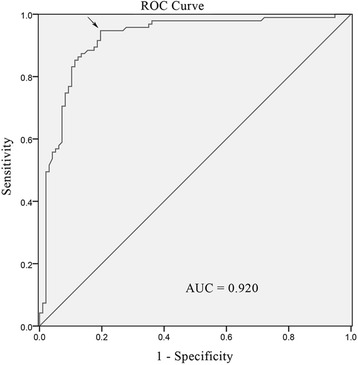
The ROC analysis of the curve. The cut-off point was defined as the maximum Youden index, which was demarcated by the arrow in the figure
To evaluate whether an association existed between patient overall survival and LZTS2 methylation, survival analyses were performed on two subcohorts that were split at the average LSCC PMR value (average value: 0.2706). Fifty-nine patients were classified into the hypomethylation group and 37 were assigned to the hypermethylation group. Using Kaplan-Meier analyses, overall survival of LSCC patients with hypomethylated LZTS2 promoters was significantly higher than that of patients with hypermethylated LZTS2 promoters (Fig. 4, log-rank test P = 0.028). Univariate Cox proportional hazards analysis also revealed an obviously increased risk of death for LSCC patients with hypermethylated LZTS2 (HR = 44.366; 95% CI = 4.586–429.237; P = 0.001). Subsequently, we performed a multivariate Cox proportional hazard analysis by adjusting for smoking behavior, histological differentiation, clinical stage, and lymphatic metastasis. The results confirmed that LZTS2 promoter hypermethylation could be an independent factor to predict a poorer overall survival of LSCC patients (HR = 6.671; 95% CI = 2.087–21.324; P = 0.001, Table 2).
Fig. 4.
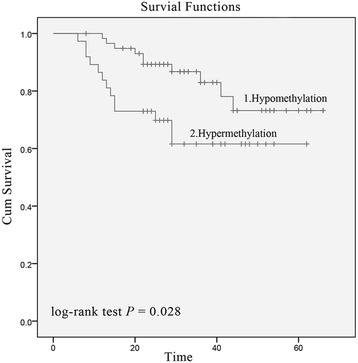
Kaplan-Meier curves of overall survival in LZTS2 promoter hypomethylated and hypermethylated LSCC patients. Log-rank test results indicated that LSCC patients with LZTS2 hypermethylated promoters (n = 37) had significantly worse overall survival rates than those who had LZTS2 hypomethylated promoters (n = 59) (P = 0.028)
Table 2.
Multivariate Cox proportional hazards analysis of the 96 LSCC patients
| Characteristics | Number | P value | HR | 95% CI |
|---|---|---|---|---|
| Smoking behavior | ||||
| No (Ref) | 18 | – | 1 | – |
| Yes | 78 | 0.888 | 1.084 | 0.356–3.295 |
| Histological classification | ||||
| Well (Ref) | 45 | – | 1 | – |
| Moderately/poorly | 51 | 0.995 | 1.003 | 0.421–2.392 |
| Clinical stage | ||||
| Stage I + II (Ref) | 45 | – | 1 | – |
| Stage III + IV | 51 | 0.822 | 1.181 | 0.278–5.012 |
| Lymph metastasis | ||||
| No (Ref) | 63 | – | 1 | – |
| Yes | 33 | 0.127 | 2.657 | 0.757-9.327 |
| LZTS2 methylation | 96 | 0.011 | 20.184 | 1.979-205.847 |
Ref reference category, HR hazard ratio, CI confidence interval
Discussion
For LSCC patients, early-stage disease often goes undetected because symptoms are mild and non-specific. Consequently, roughly 40% of newly diagnosed patients present with stage III or IV disease [15]. Currently, the prevailing initial therapy for LSCC is total laryngectomy combined with postoperative radiotherapy. Given that the larynx plays an indispensable role in human communication and that the operative treatment damages laryngeal structure and function, current treatment paradigms greatly reduce patients’ quality of life [13]. Patients for whom disease is identified in T1 or T2 stages have an 80–90% cure rate; for advanced LSCC, cure rates drop to about 60% [2]. Therefore, novel methods are needed to allow for earlier identification and possible prevention of LSCC, while cancers remain curable and laryngeal function can be best preserved.
Abnormal TSG promoter methylation is an early and frequent event occurring in LSCC tumorigenesis [4]. Accumulating evidence indicates that hypermethylated gene promoters exist in LSCC tissue, as well as in the serum and saliva of LSCC patients [4]. It stands to reason that hypermethylation of a panel of TSGs could serve LSCC patients as sensitive diagnostic and prognostic biomarkers, or as markers following the natural history of curative therapies [4].
Here, a novel TSG was evaluated. LZTS2 is located at human 10q24.3, is expressed in most normal tissues [8], suppresses cell growth by interacting with β-catenin [11], and plays a vital role in the carcinogenesis and development of tumors [10]. To date, research has focused on the LZTS2 TSG functions; however, the relationship between LZTS2 promoter methylation events and tumorigenesis was largely unstudied. This study investigated associations between LZTS2 promoter methylation and risk of LSCC, as well as LZTS2 clinical diagnostic value. Previous studies have shown that LZTS2 cDNA overexpression suppressed cell growth and reduced colony formation rates of several cancer cell lines, including HEK-293T, AT6.2, LNCaP, PC3, TRSUPr1, Rat-1, and U2OS [8]. Additionally, LZTS2 protein has low expression levels in human prostate cancer cells [10]. The present results revealed that LZTS2 promoter methylation levels in LSCC tissues were significantly higher than in non-cancerous paired control specimens. Thus, the data suggested that LZTS2 promoter hypermethylation decreased LZTS2 protein expression, which may have regulated LSCC carcinogenic mechanisms and increased risk of developing the disease.
Next, clinicopathologic parameters were identified that served as explanatory variables contributing to LZTS2 methylation levels. Previous studies demonstrated that, in addition to lung cancer, cigarette smoking is a risk factor for upper aero-digestive tract cancers including laryngocarcinomas, hypopharyngeal carcinomas, and esophageal cancers [14]. Additionally, smoking gives rise to extensive genome-wide DNA changes especially DNA methylation, which play important roles in tumorigenesis [16]. Therefore, the current study specifically investigated a relationship between LZTS2 promoter methylation and cigarette smoking. LZTS2 promoter methylation levels in LSCC specimens from smokers were significantly elevated, relative to non-smokers (P = 0.049). Thus, smoking behavior may have elevated LZTS2 promoter methylation levels and thus increased risk of LSCC in those patients. Additionally, LZTS2 promoter methylation levels were significantly increased in patients with lymph node metastases, advanced clinical stages, and advanced T classifications. It is well-understood that T classification and lymph node metastases are crucial to the prognosis of LSCC patients [17]. The present results suggested that LZTS2 promoter methylation also contributed to LSCC progression and ultimate prognosis. Furthermore, previous studies have reported that abnormal TSG methylation was associated with poor survival outcomes in several tumor types [5–7]. Survival analyses presented here indicated that patients with LZTS2 promoter hypermethylation have worsened outcomes compared to those with hypomethylation in the same promoter regions (Fig. 3), which was consistent with the results of our univariate Cox proportional hazards analysis. Furthermore, the multivariate Cox proportional hazard analysis we performed confirmed that LZTS2 methylation was an independent adverse factor for LSCC outcomes. These findings suggested that LZTS2 promoter hypermethylation could be a potential biomarker for prognosis of LSCC.
The advent of tumor-specific biomarkers has made major strides in advancing clinic diagnostic practices in a variety of tumors, including CEA and CA19-9 for colorectal cancer [18], AFP for hepatocellular carcinoma [19], and PSA for prostate cancer [20]. However, no biomarker currently exists to assist in clinical prognostication of laryngeal cancer. Here, the AUC of the curve of ROC was 0.920; a large AUC is indicative of a high diagnostic value. The sensitivity, specificity, and diagnostic accordance rates were 94.7, 80.4, and 88.0%, respectively. These data provided strong evidence that LZTS2 promoter hypermethylation could be clinically applicable for use in early identification of LSCC.
Conclusion
LZTS2 promoter hypermethylation was associated with LSCC risk, progression, and prognosis, with the strongest associations observed in the subgroup of patients who smoked. Taken together, LZTS2 promoter hypermethylation has the potential to become an LSCC diagnostic and prognostic biomarker.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the Zhejiang Provincial Natural Science Foundation of China (LY14H160003), the Scientific Innovation Team Project of Ningbo (2012B82019), the Ningbo Social Developmental Key Research Project (2012C5015), the Ningbo Natural Science Foundation (2012A610208 and 2012A610217), and the Medical and Health Research Project of Zhejiang Province (2012ZDA042 and 2014PYA017).
Availability of data and materials
These data and materials are available.
Abbreviations
- AUC
Area under the ROC curve
- CI
Confidence interval
- CT
Cycle threshold
- HR
Hazard ratio
- LSCC
Laryngeal squamous cell carcinoma
- PMR
Percentage of methylated reference
- qMSP
Quantitative methylation-specific polymerase chain reaction
- ROC
Receiver operating characteristic
- TSG
Tumor suppressor gene
Authors’ contributions
ZS designed the study, searched literature, performed the experimental procedure, analyzed and interpreted the data, and wrote the manuscript; LL collected specimens, performed the experimental procedure, searched literature, and wrote the manuscript; BC collected specimens, performed the experimental procedure, and searched literature; CZ collected specimens, performed the experimental procedure, and searched literature; WH performed the experimental procedure; DY collected specimens. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Experimental procedures were reviewed and approved by the Ethics Committee of Ningbo Lihuili Hospital. All participants signed written informed consent documents.
Consent for publication
The authors agree for the publication of this article.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zhisen Shen, Email: szs7216@163.com.
Lexi Lin, Email: 15968737070@163.com.
Bing Cao, Email: cbb7417@163.com.
Chongchang Zhou, Email: zhou900709900709@163.com.
Wenjuan Hao, Email: 664941487@qq.com.
Dong Ye, Email: yedong518@sina.com.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Marioni G, Marchese-Ragona R, Cartei G, Marchese F, Staffieri A. Current opinion in diagnosis and treatment of laryngeal carcinoma. Cancer Treat Rev. 2006;32:504–515. doi: 10.1016/j.ctrv.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Muscat JE, Wynder EL. Tobacco, alcohol, asbestos, and occupational risk factors for laryngeal cancer. Cancer. 1992;69:2244–2251. doi: 10.1002/1097-0142(19920501)69:9<2244::AID-CNCR2820690906>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y, Liu HG. Progress in research on DNA methylation and laryngeal carcinoma. Zhonghua Bing Li Xue Za Zhi. 2011;40:67–70. [PubMed] [Google Scholar]
- 5.Qu X, Othus M, Davison J, Wu Y, Yan L, Meshinchi S, Ostronoff F, Estey EH, Radich JP, Erba HP, et al. Prognostic methylation markers for overall survival in cytogenetically normal patients with acute myeloid leukemia treated on SWOG trials. Cancer. 2017;123;2472-81. [DOI] [PMC free article] [PubMed]
- 6.Cheng W, Qi Y, Tian L, Wang B, Huang W, Chen Y. Dicer promotes tumorigenesis by translocating to nucleus to promote SFRP1 promoter methylation in cholangiocarcinoma cells. Cell Death Dis. 2017;8:e2628. doi: 10.1038/cddis.2017.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCullough LE, Chen J, Cho YH, Khankari NK, Bradshaw PT, White AJ, Teitelbaum SL, Terry MB, Neugut AI, Hibshoosh H, et al. Modification of the association between recreational physical activity and survival after breast cancer by promoter methylation in breast cancer-related genes. Breast Cancer Res. 2017;19:19. doi: 10.1186/s13058-017-0811-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cabeza-Arvelaiz Y, Thompson TC, Sepulveda JL, Chinault AC. LAPSER1: a novel candidate tumor suppressor gene from 10q24.3. Oncogene. 2001;20:6707–6717. doi: 10.1038/sj.onc.1204866. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 10.Johnson DT, Luong R, Lee SH, Peng Y, Shaltouki A, Lee JT, Lin D, Wang Y, Sun Z. Deletion of leucine zipper tumor suppressor 2 (Lzts2) increases susceptibility to tumor development. J Biol Chem. 2013;288:3727–3738. doi: 10.1074/jbc.M112.417568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thyssen G, Li TH, Lehmann L, Zhuo M, Sharma M, Sun Z. LZTS2 is a novel beta-catenin-interacting protein and regulates the nuclear export of beta-catenin. Mol Cell Biol. 2006;26:8857–8867. doi: 10.1128/MCB.01031-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sudo H, Maru Y. LAPSER1/LZTS2: a pluripotent tumor suppressor linked to the inhibition of katanin-mediated microtubule severing. Hum Mol Genet. 2008;17:2524–2540. doi: 10.1093/hmg/ddn153. [DOI] [PubMed] [Google Scholar]
- 13.Chu EA, Kim YJ. Laryngeal cancer: diagnosis and preoperative work-up. Otolaryngol Clin N Am. 2008;41:673–695. doi: 10.1016/j.otc.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 14.Bosetti C, Gallus S, Peto R, Negri E, Talamini R, Tavani A, Franceschi S, La Vecchia C. Tobacco smoking, smoking cessation, and cumulative risk of upper aerodigestive tract cancers. Am J Epidemiol. 2008;167:468–473. doi: 10.1093/aje/kwm318. [DOI] [PubMed] [Google Scholar]
- 15.American Society of Clinical O. Pfister DG, Laurie SA, Weinstein GS, Mendenhall WM, Adelstein DJ, Ang KK, Clayman GL, Fisher SG, Forastiere AA, et al. American Society of Clinical Oncology clinical practice guideline for the use of larynx-preservation strategies in the treatment of laryngeal cancer. J Clin Oncol. 2006;24:3693–3704. doi: 10.1200/JCO.2006.07.4559. [DOI] [PubMed] [Google Scholar]
- 16.Zeilinger S, Kuhnel B, Klopp N, Baurecht H, Kleinschmidt A, Gieger C, Weidinger S, Lattka E, Adamski J, Peters A, et al. Tobacco smoking leads to extensive genome-wide changes in DNA methylation. PLoS One. 2013;8:e63812. doi: 10.1371/journal.pone.0063812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Licitra L, Bernier J, Grandi C, Locati L, Merlano M, Gatta G, Lefebvre JL. Cancer of the larynx. Crit Rev Oncol Hematol. 2003;47:65–80. doi: 10.1016/S1040-8428(03)00017-9. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Yang Z, Tian H, Li Y, Li M, Zhao W, Zhang C, Wang T, Liu J, Zhang A, et al. Circulating MIC-1/GDF15 is a complementary screening biomarker with CEA and correlates with liver metastasis and poor survival in colorectal cancer. Oncotarget. 2017;8:24892-901. [DOI] [PMC free article] [PubMed]
- 19.Sauzay C, Petit A, Bourgeois AM, Barbare JC, Chauffert B, Galmiche A, Houessinon A. Alpha-foetoprotein (AFP): a multi-purpose marker in hepatocellular carcinoma. Clin Chim Acta. 2016;463:39–44. doi: 10.1016/j.cca.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Uprimny C, Kroiss AS, Decristoforo C, Fritz J, von Guggenberg E, Kendler D, Scarpa L, di Santo G, Roig LG, Maffey-Steffan J, et al. 68Ga-PSMA-11 PET/CT in primary staging of prostate cancer: PSA and Gleason score predict the intensity of tracer accumulation in the primary tumour. Eur J Nucl Med Mol Imaging. 2017;44:941-9. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
These data and materials are available.


