Abstract
Background
Evidence on the changes in the absolute counts of monocyte subpopulations in sepsis is missing.
Methods
Firstly, absolute counts of circulating CD14pos/HLA-DRpos/CD45pos monocytes were measured by flow cytometry in 70 patients with Gram-negative sepsis and in 10 healthy volunteers. In the second phase, immunophenotyping was performed and the absolute count of circulating inflammatory monocytes and of circulating CD14dim/CD16pos/CD45pos patrolling monocytes were measured in another 55 patients and 10 healthy volunteers. Measurements were repeated on days 3, 7, and 10. Results were correlated with survival after 28 days.
Results
Greater numbers of CD14pos/HLA-DRpos/CD45pos monocytes were found on day 1 in survivors compared to nonsurvivors (p = 0.030). Receiver operating characteristic (ROC) analysis showed that a cutoff higher than 337 cells/mm3 on day 1 could discriminate between survivors and nonsurvivors with a positive predictive value (PPV) of 91.1%. Logistic regression including Sequential Organ Failure Assessment (SOFA) score and Acute Physiology and Chronic Health Evaluation (APACHE) II score showed that an absolute count greater than 337 cells/mm3 was independently associated with unfavorable outcome (odds ratio (OR) 0.19, p = 0.050). The absolute counts of inflammatory and of CD14dim/CD16pos/CD45pos monocytes were greater in patients than healthy controls during the entire 10 days of follow-up. The absolute counts on day 3 of CD14dim/CD16pos/CD45pos monocytes were greater in survivors than nonsurvivors (p = 0.027). ROC analysis revealed that the cutoff at 27 cells/mm3 could discriminate between survivors and nonsurvivors with PPV of 94.1%. Logistic regression including age, SOFA score, and APACHE II score showed that an absolute count greater than 27 cells/mm3 was independently associated with unfavorable outcome (OR 0.06, p = 0.033). Logistic regression analysis showed that intra-abdominal infection on day 1 was predictive of low CD14dim/ CD16pos/CD45pos count on day 3.
Conclusion
Circulating counts of inflammatory and patrolling monocytes are greatly increased in Gram-negative sepsis. Absolute counts of CD14pos/HLA-DRpos/CD45pos monocytes on day 1 and CD14dim/CD16pos/CD45pos monocytes on day 3 are independently associated with final outcome.
Trial registration
ClinicalTrials.gov, NCT01223690. Registered retrospectively on 18 October 2010.
Electronic supplementary material
The online version of this article (10.1186/s13054-018-1977-1) contains supplementary material, which is available to authorized users.
Keywords: Sepsis, Survival, Inflammatory monocytes, Patrolling monocytes, Gram-negative
Background
Immune status in sepsis varies during the course of the disease. The early stages are characterized by a phase of excessive inflammation also known as the “cytokine storm”. In this stage, blood monocytes are overfunctioning for the production of cytokines. As the syndrome progresses, the proinflammation stage is followed by a phase of immune-suppression that is characterized by the inability for cytokine secretion [1]. In this stage monocytes are functionally deactivated, and this is shown by the decreased expression of the human leukocyte antigen (HLA) class II on their cell surface [2]. However, it is also possible that these two phases of proinflammation and anti-inflammation may occur simultaneously. Monocytes can be classified into three phenotypical and functionally distinct subpopulations based on the expression of the lipopolysaccharide receptor CD14 and of the FcγIII receptor CD16 on their cell membranes. According to this classification, monocytes are divided into the “classical or inflammatory monocytes” characterized by a bright expression of CD14 and lack of expression of CD16, the “intermediate monocytes” that express both receptors; and the “nonclassical” monocytes with patrolling function that express predominantly CD16 [3, 4]. Data on the contribution of patrolling monocytes to the pathogenesis of sepsis are still missing. Regarding the role of classical monocytes, available data are mainly focused on the change in their percentage whereas data on their absolute counts are missing [5, 6].
The aim of the present study was to investigate the changes in the absolute counts of monocyte subpopulations in sepsis. For reasons of study homogeneity, the study was limited to patients with infections either proven or highly suspected to be caused by Gram-negative bacteria.
Methods
Study design
This is a nested observational clinical study that was approved by the Ethics Committee of the ATTIKON University hospital (266/27-07-09). Written informed consent was provided by patients or by their legal representatives for patients unable to consent. This study was approved as a substudy of a randomized clinical study investigating the role of intravenous clarithromycin for the management of patients with proven or suspected Gram-negative sepsis [7]. The study is registered at https://clinicaltrials.gov/ct2/show/NCT01223690 (NCT01223690).
The study design comprised two phases. In the first phase, we investigated how early counts of CD14pos/HLA-DRpos/CD45pos monocytes are associated with final outcome. If analysis of this phase yielded significant results, the study was allowed to proceed to the second phase. In the second phase, immunophenotyping of monocytes subpopulations was performed and absolute counts of inflammatory and patrolling monocytes were determined. The inclusion criteria for both phases were: 1) age more than or equal to 18 years; 2) severe sepsis or septic shock as defined by the Sepsis-2 definitions that were applying at that time [8]; 3) blood sampling blood within 24 h from the first organ failure; and 4) presence of acute pyelonephritis, intra-abdominal infection, and primary Gram-negative bacteremia. Patients with primary Gram-negative bacteremia were enrolled provided that within the time frame of 24 h since the first organ failure information was given by the microbiology laboratory on the growth of a Gram-negative species in blood culture.
Exclusion criteria were: 1) infection by the human immunodeficiency virus (HIV)-1; 2) neutropenia defined as less than 1000 neutrophils/mm3; 3) intake of chemotherapy; and 4) chronic corticosteroid intake defined as more than 0.4 mg/kg equivalent prednisone daily for more than 15 consecutive days.
Severe sepsis was defined as infection accompanied by at least two signs of the systemic inflammatory response syndrome (SIRS) and aggravated by at least one organ dysfunction. Septic shock was defined as severe sepsis aggravated with systolic blood pressure below 90 mmHg necessitating the administration of vasopressors despite adequate fluid resuscitation [8]. Acute pyelonephritis was defined as the presence of at least two of the following: 1) dysuria and pain on palpation of the left or right costovertebral angle; 2) more than 10 white blood cells/high-power field (hpf) of centrifuged urine; and 3) compatible findings on renal ultrasound [8]. An intra-abdominal infection was defined as the presence of both clinical signs and radiological findings on abdominal ultrasound or computed tomography compatible with an intra-abdominal infection [8]. Demographics, severity scores, white blood cell count, biochemistry, blood gases, type of infection, microbiology, comorbidities, and type of organ dysfunction were recorded. Survival was also recorded for 28 days.
Twenty healthy volunteers well-matched to sepsis patients for age and gender were also studied; 10 were studied at each phase of the study. At least one volunteer sample was running on the same day as patient samples.
From each patient and healthy volunteer, 2 ml whole blood were collected in one EDTA tube (Becton Dickinson, Cockeysville, MD, USA) under sterile conditions after venipuncture of one forearm vein at four time intervals: during the first 24 h from the first organ failure (day 1), two days later (day 3), 6 days later (day 7), and 9 days later (day 10).
Laboratory investigation
Whole blood was incubated with the following combinations of monoclonal antibodies: a) anti-CD14 at the fluorochrome fluorescein isothiocyanate (FITC, emission 525 nm, clone RMO25, Immunotech, Marseille, France), anti-HLA-DR-DP-DQ at the fluorochrome RD1 (phycoerythrin (PE), emission 575 nm, clone 9-49 I3, CYTO-STAT, Miami, Florida, USA), and anti-CD45 at the fluorochrome phycoerythrin-cyanine 5 (PC5, emission 650 nm, clone J.33, Immunotech); and b) anti-CD14 at the fluorochrome FITC (clone RMO25, Immunotech), anti-CD16 at the fluorochrome PE (clone 3G8, Immunotech), and anti-CD45 at the fluorochrome PC5 (clone J.33, Immunotech). Red blood cells were lysed using VersaLyse Lysing Solution (Beckman Coulter, Immunotech, Marseille, France). White blood cells were analyzed after running through the Cytomics FC-500 flow cytometer with gating for monocytes based on their characteristic SS/CD45 expression. Fluorospheres (Immunotech) were used for the determination of absolute counts. Cells stained with IgG isotypic controls at the fluorochromes FITC and PE (clone 679.1Mc7, Immunotech) were running in parallel so that background staining could be subtracted. The technicians were blind to the clinical data of the patients.
Power of the study
We hypothesized that after receiver operator characteristic (ROC) curve analysis of the absolute counts of CD14pos/HLA-DRpos/CD45pos a cutoff associated with positive predictive value (PPV) more than 90% for survival could be found. To achieve this, with power more than 80% at the 10% level of significance, we calculated that at least 60 patients should be enrolled at the first phase. To adjust for patients dying prior to day 3, 70 patients were studied at the first phase. It was predefined that if analysis was positive at the end of the first phase, the study would proceed to the second phase with a similar number of patients.
Statistical analysis
The absolute cell counts are expressed as medians and 95% confidence intervals (CIs) or interquartile range (IQR) and compared by the Mann-Whitney U test. Comparisons of baseline demographics between patients enrolled in each phase of the study and between survivors and nonsurvivors were performed by the Fisher exact test for qualitative variables and by the Student’s test for quantitative variables. ROC curve analysis was also performed to identify a cutoff for the absolute count of CD14pos/HLA-DRpos/CD45pos on day 1 and of CD14dim/CD16pos/CD45pos patrolling monocytes on day 3 that could discriminate between survivors and nonsurvivors with PPV more than 90%. Odds ratio (ORs) and 95% CIs for unfavorable outcome based on the cutoffs were determined according to Mantel-Haenszel’s statistics. Comparative survival analysis was performed by the log-rank test. Comparisons of baseline characteristics between patients above or below these cutoffs were performed as described above. Variables with a p value lower than 0.050 entered the equations of step-wise forward logistic regression analysis as independent variables; the cutoffs of monocytes or 28-day mortality were the dependent variable. ORs and 95% CIs were calculated. Any value of p below 0.05 was considered statistically significant.
Results
The study flowchart is shown in Fig. 1. The study was divided into two phases. In the first phase, absolute counts of CD14pos/HLA-DRpos/CD45pos monocytes were determined in 70 patients and 10 healthy volunteers. Demographic characteristics of patients participating in both study phases are shown in Additional file 1 (Table S1).
Fig. 1.
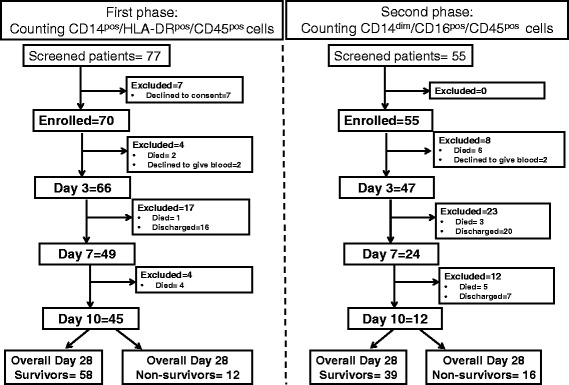
Study flow during the two phases
Absolute cell counts of CD14pos/HLA-DRpos/CD45pos monocytes on days 1 and 3 did not differ between patients allocated to the two groups of treatment (Additional file 2: Figure S1A). Since all-cause mortality was similar in the two groups [7], data from both groups were pooled and analyzed together.
Absolute cell counts of CD14pos/HLA-DRpos/CD45pos monocytes on day 1 were significantly higher in survivors compared to nonsurvivors (Fig. 2a). Based on this observation, a ROC curve was designed and a cutoff value of 337 cells/mm3 could discriminate between survivors and nonsurvivors with PPV of 91.1% (Fig. 2b, c). These results were further confirmed after survival analysis showing prolonged survival among patients with more than 337 cells/mm3 on day 1 (Fig. 2d). The OR for unfavorable outcome among patients with more than 337 cells/mm3 was 0.21 (95% CI 0.06–0.78; p = 0.020). Median (IQR) count of CD14pos/HLA-DRpos/CD45pos monocytes on day 1 was 533.7 (846.1)/mm3 among patients without acute kidney injury (AKI) and 852.7 (1181.9)/mm3 among patients with AKI (p = 0.275). Among patients remaining in phase 1 of the study after day 3, median (IQR) count of CD14pos/HLA-DRpos/CD45pos monocytes on day 7 was 192 (494.8)/mm3 among survivors and 321.9 (365.3)/mm3 among nonsurvivors (p = 0.620); the respective values on day 10 were 900.5 (704.8)/mm3 and 254.4 (820.3)/mm3 (p = 0.312).
Fig. 2.
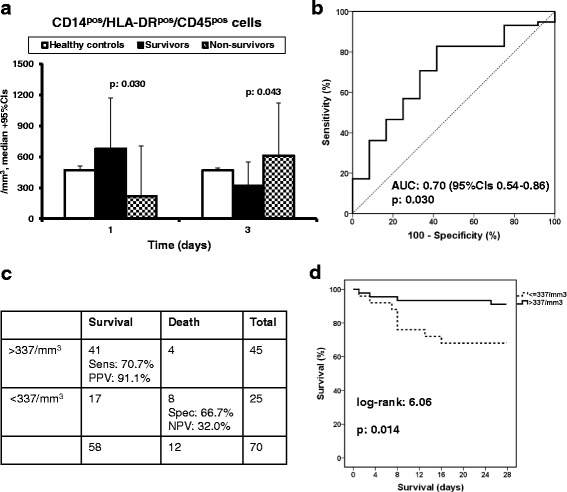
Association between final outcome and absolute counts of circulating CD14pos/HLA-DRpos/CD45pos monocytes. a Absolute counts of CD14pos/HLA-DRpos/CD45pos monocytes of survivors and nonsurvivors on days 1 and 3 and healthy volunteers. P values refer to comparisons between survivors and nonsurvivors. b ROC curve of the absolute count of CD14pos/HLA-DRpos/CD45pos monocytes on day 1 to predict survival after 28 days. c Predictive value of an absolute count of CD14pos/HLA-DRpos/CD45pos monocytes more than 337 cells/mm3 on day 1 for survival after 28 days. d) Comparative survival in relation to the absolute count of CD14pos/HLA-DRpos/CD45pos monocytes on day 1. AUC area under curve, CI confidence interval, NPV negative predictive value, PPV positive predictive value, Sens sensitivity, Spec specificity
These results generated the question whether the defined cutoff for CD14pos/HLA-DRpos/CD45pos monocytes on day 1 is an independent predictor of final outcome. As shown in Additional file 3 (Table S2), the only variables that differed significantly between nonsurvivors and survivors at baseline were Acute Physiology and Chronic Health Evaluation (APACHE) II and Sequential Organ Failure Assessment (SOFA) scores. In the logistic regression analysis, APACHE II score, SOFA score, and an absolute CD14pos/HLA-DRpos/CD45pos monocyte count on day 1 greater than 337 cells/mm3 were entered as independent variables; 28-day outcome was the dependent variable. Analysis showed that an absolute CD14pos/HLA-DRpos/CD45pos monocyte count on day 1 greater than 337 cells/mm3 was independently associated with favorable 28-day outcome (Table 1).
Table 1.
Logistic regression analysis of variables associated with unfavorable outcome in each phase of the study
| Odds ratio | 95% confidence interval | p value | |
|---|---|---|---|
| Phase 1 | |||
| SOFA score on day 1 | 1.60 | 1.15–2.22 | 0.005 |
| APACHE II score on day 1 | 0.98 | 0.86–1.13 | 0.825 |
| CD14pos/HLA-DRpos/CD45pos > 337/mm3 on day 1 | 0.19 | 0.04–1.00 | 0.050 |
| Phase 2 | |||
| Age | 1.07 | 0.98–1.17 | 0.105 |
| SOFA score on day 1 | 1.73 | 0.99–3.00 | 0.050 |
| APACHE II score on day 1 | 1.05 | 0.91–1.21 | 0.526 |
| CD14dim/CD16bri/CD45pos > 27/mm3 on day 3 | 0.06 | 0.00–0.79 | 0.033 |
APACHE Acute Physiology and Chronic Health Evaluation, SOFA Sequential Organ Failure Assessment
Since the impact of the absolute count of CD14pos/HLA-DRpos/CD45pos monocytes on survival was confirmed after the first phase, we proceeded with the second phase where absolute counts of subpopulations of monocytes were measured in 55 patients and 10 healthy volunteers (Fig. 1). Three monocyte subpopulations were defined: CD14pos/CD16neg/CD45pos and CD14pos/CD16pos/CD45pos monocytes analyzed and reported together as inflammatory monocytes, and CD14dim/CD16pos/CD45pos reported as patrolling monocytes. The absolute counts of both inflammatory and patrolling monocytes were significantly greater among patients than healthy controls (Fig. 3a, b); no differences were found between patients without and with AKI (Fig. 3c, d). Among patients remaining in phase 2 of the study after day 3, median (IQR) counts of inflammatory monocytes was 641 (592.3)/mm3 on day 7 (p = 0.204 vs controls) and 669 (519)/mm3 on day 10 (p = 0.049 vs controls). Those of CD14dim/CD16pos/CD45pos patrolling monocytes were 14 (20)/mm3 on day 7 (p = 0.043 vs controls) and 25.5 (28.5)/mm3 on day 10 (p = 0.011 vs controls).
Fig. 3.
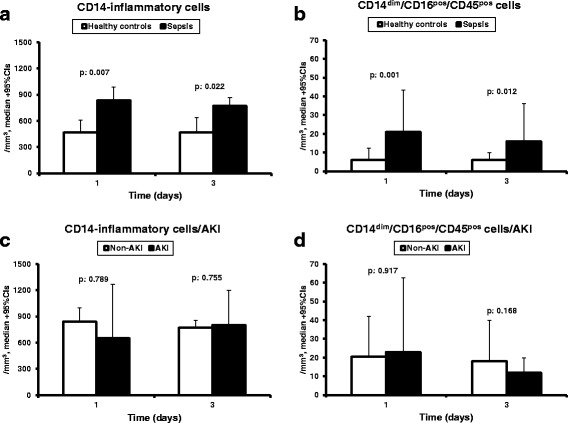
Absolute counts of monocyte subpopulations in sepsis. a Absolute counts of inflammatory monocytes and of b CD14dim/CD16pos/CD45pos patrolling monocytes of healthy controls and of patients with sepsis on days 1 and 3. c Absolute counts of inflammatory monocytes and of d CD14dim/CD16pos/CD45pos patrolling monocytes of patients with sepsis on days 1 and 3 divided into those without and those with acute kidney injury (AKI). P values refer to the indicated comparisons
As a next step, we explored if the described changes in the subpopulations of monocytes are associated with final outcome. The absolute cell counts on days 1 and 3 did not differ between patients allocated to placebo or clarithromycin treatment (Additional file 2: Figure S1B and C). Since all-cause mortality was similar in the two groups [7], data from both groups were pooled and analyzed together.
No differences in cell counts of inflammatory monocytes were observed between survivors and nonsurvivors for any day of sampling (Fig. 4a). Regarding CD14dim/CD16pos/CD45pos patrolling monocytes, significant differences between survivors and nonsurvivors were found on day 3 (Fig. 4b). ROC analysis revealed that a cutoff value of 27 cells/mm3 could discriminate between survivors and nonsurvivors with PPV 94.1% (Fig. 4c, d). This finding was further confirmed after survival analysis (Fig. 4e). The OR for unfavorable outcome among patients with more than 27 cells/mm3 was 0.09 (95% CI 0.01–0.80; p = 0.031).
Fig. 4.
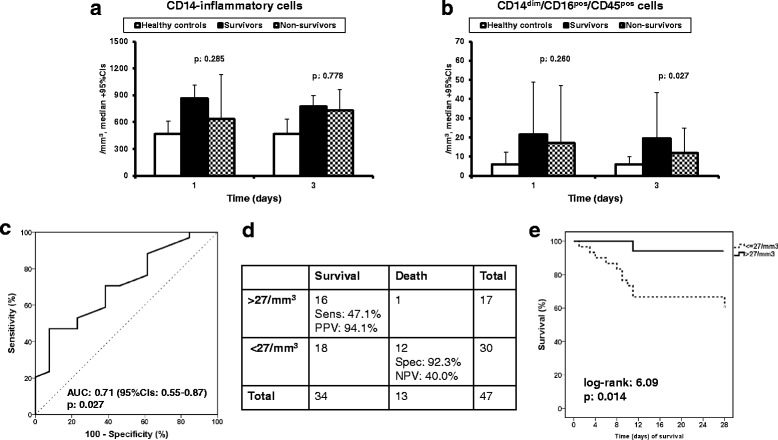
Association between final outcome and absolute counts of circulating CD14dim/CD16pos/CD45pos patrolling monocytes on day 3. a Absolute counts of inflammatory monocytes of survivors and nonsurvivors on days 1 and 3 and healthy volunteers. P values refer to comparisons between survivors and nonsurvivors. b Absolute counts of CD14dim/CD16pos/CD45pos patrolling monocytes of survivors and nonsurvivors on days 1 and 3 and healthy volunteers. P values refer to comparisons between survivors and nonsurvivors. c ROC curve of the absolute count of patrolling monocytes on day 3 to predict survival after 28 days. d Predictive value of an absolute count of patrolling monocytes greater than 27 cells/mm3 on day 3 for survival after 28 days. e Comparative survival for patients according to the number of patrolling monocytes on day 3. AUC area under curve, CI confidence interval, NPV negative predictive value, PPV positive predictive value, Sens sensitivity, Spec specificity
These results generated the question whether the defined cutoff for CD14dim/CD16pos/CD45pos patrolling monocytes on day 3 is an independent predictor of final outcome. As shown in Additional file 4 (Table S3), the only variables that differed significantly between nonsurvivors and survivors at baseline were age, APACHE II score, and SOFA score. In the logistic regression analysis, age, APACHE II score, SOFA score, and an absolute count of CD14dim/CD16pos/CD45pos patrolling monocytes on day 3 greater than 27 cells/mm3 were entered as independent variables; 28-day outcome was the dependent variable. Analysis showed that an absolute count of CD14dim/CD16pos/CD45pos patrolling monocytes on day 3 greater than 27 cells/mm3 was independently associated with favorable 28-day outcome (Table 1).
Next, we asked the question whether clinical signs at admission could predict low counts of patrolling monocytes on day 3. The frequencies of acute pyelonephritis and intra-abdominal infection were significantly different between patients with ≤ 27 cells/mm3 or greater than 27 cells/mm3 CD14dim/CD16pos/CD45pos patrolling monocytes (Table 2). These two clinical characteristics were entered into the logistic regression analysis as independent variables. Analysis revealed that the presence of an intra-abdominal infection was the only variable associated with low CD14dim/CD16pos/CD45pos patrolling monocyte counts on day 3 (Table 3).
Table 2.
Differences in baseline characteristics of patients in relation to the absolute number of CD14dim/CD16bri/CD45pos monocytes on day 3
| > 27 cells/mm3 (n = 17) | ≤ 27 cells/mm3 (n = 30) | p | |
|---|---|---|---|
| Male gender | 6 (35.3%) | 14 (46.7%) | 0.546 |
| Age (years) | 69.5 ± 15.9 | 71.8 ± 17.4 | 0.658 |
| APACHE II score | 11.0 ± 8.2 | 13.3 ± 8.1 | 0.378 |
| SOFA score | 2.4 ± 2.1 | 3.2 ± 2.5 | 0.266 |
| White blood cells (/mm3) | 13,262.9 ± 6016.2 | 12,728.5 ± 4211.1 | 0.750 |
| pO2/FiO2 | 326.7 ± 109.1 | 209.9 ± 129.3 | 0.094 |
| C-reactive protein (mg/l) | 136.4 ± 99.3 | 121.3 ± 118.1 | 0.679 |
| Type of infection | |||
| Acute pyelonephritis | 13 (76.5%) | 9 (30.0%) | 0.003 |
| Primary Gram-negative bacteremia | 3 (17.6%) | 4 (13.3%) | 0.692 |
| Acute intra-abdominal infection | 1 (5.9%) | 17 (57.6%) | 0.001 |
| Presence of at least one chronic disordera | 8 (47.1%) | 15 (50.0%) | 0.758 |
| Presence of multiple organ failure | 5 (35.7%) | 7 (31.7%) | 1.00 |
Values are shown as mean ± SD or n (%)
APACHE Acute Physiology and Chronic Health Evaluation, FiO2 fractional inspired oxygen, pO2 arterial oxygen tension, SOFA Sequential Organ Failure Assessment
a Type 2 diabetes mellitus, chronic obstructive pulmonary disorder, chronic heart failure, chronic renal disease, solid tumor malignancy
Table 3.
Step-wise forward logistic regression analysis of admission characteristics on day 1 to predict the chance for the presence of low circulating patrolling monocyte cell counts on day 3
| Odds ratio | 95% confidence interval | p value | |
|---|---|---|---|
| Acute pyelonephritis | 0.52 | 0.09–2.90 | 0.453 |
| Acute intra-abdominal infection | 12.75 | 1.03–2.90 | 0.047 |
Discussion
Circulating counts of inflammatory and patrolling monocytes are significantly increased up to at least 10 days in the course of Gram-negative sepsis. Exploratory analysis also identified an association between circulating CD14dim/CD16pos/CD45pos patrolling monocytes on the third day of follow-up and 28-day outcome; the greater number of this subpopulation, the lower is the risk for unfavorable outcome. The presence of acute intra-abdominal infection on admission is a predictive sign for low circulating patrolling monocytes counts on day 3. It was also found that more than 337 CD14pos/HLA-DRpos/CD45pos monocytes/mm3 on admission is associated with favorable outcome.
HLA-DR is expressed on activated CD14 monocytes that are capable of antigen presentation and perpetuation of the inflammatory reaction. In sepsis, the expression of HLA-DR on circulating CD14 monocytes and the number of HLA-DR molecules (mHLA-DR) on the monocyte surface are decreased and this is interpreted as an index of sepsis-induced immunosuppression [9, 10]. To date, decreased mHLA-DR is considered the gold standard for the identification of immunosuppression [11]. However, several problems seem to apply for the universal application of mHLA-DR as a diagnostic tool, which are mainly associated with the inter-laboratory variability of the assay.
Recent studies enrolling between 79 and 93 patients [12–14] (i.e., close to the enrolment of the present study) showed that the decrease in HLA-DR expression is greater in septic shock and among nonsurvivors as early as the first day of sampling. The expression of HLA-DR on CD14 monocytes was further deepening upon worsening on days 3 and 7 of follow-up. However, in these studies the percentage of CD14 cells expressing HLA-DR and mHLA-DR were studied and the absolute count of circulating CD14pos/HLA-DRpos/CD45pos cells was not reported as in our study. This may explain why the absolute count of circulating CD14pos/HLA-DRpos/CD45pos cells was different between survivors and nonsurvivors only on day 1 and not on the next days of follow-up. It may also be the case that the provided association of the absolute count of circulating CD14pos/HLA-DRpos/CD45pos cells on day 1 is a reflection of the pathobiology of the host but that it cannot serve as a biomarker for the follow-up of the patient.
An expansion of CD16-expressing monocytes usually occurs in sepsis with a simultaneous reduction in inflammatory monocytes [5]. In two previous studies, one in sepsis and another in acute pancreatitis, the absolute counts of cells highly expressing both CD14 and CD16 were measured [15, 16]; however, the counts of patrolling monocytes were not reported.
At a functional level, patrolling monocytes are poorly phagocytic, have high antigen-presenting capacity, and express higher levels of Toll-like receptors (TLRs) compared with the other CD14 monocyte subpopulations. In addition, they bear a proinflammatory function by producing high levels of tumor necrosis factor (TNF)α and interleukin (IL)-1β but not IL-10 upon stimulation with lipopolysaccharide (LPS) [5, 17]. In a recent publication from our group we found that failure of adequate production of proinflammatory cytokines by circulating mononuclear cells 48 h after admission for sepsis was interpreted as sustaining the immunosuppression and it was associated with poor outcome. This defective production continued for at least 10 days [18]. Since CD14dim/CD16pos/CD45pos cells are the monocyte subpopulation providing most of the TNFα production, the low cell count of patrolling monocytes on the third day in nonsurvivors could be associated with the immunosuppression of sepsis.
The current study is powered to define the absolute count of CD14pos/HLA-DRpos/CD45pos on day 1 that impact on final outcome. Although the number of patients enrolled during the second phase of the study is rather small to provide definitive associations with final outcome, it clearly reveals a main pathobiological feature of Gram-negative sepsis—i.e., the increase in the studied monocyte subpopulations. However, the independent associations of the proposed cutoffs of circulating CD14pos/HLA-DRpos/CD45pos cells on day 1 and of the circulating CD14dim/CD16pos/CD45pos cells on day 3 with the final outcome proved through logistic regression analysis are remarkable.
Conclusions
This study shows that circulating counts of inflammatory and patrolling monocytes are greatly increased in Gram-negative sepsis. Low absolute counts of CD14dim/CD16pos/CD45pos patrolling monocytes on day 3 are associated with unfavorable outcome. The presence of acute intra-abdominal infection at baseline can predict the low absolute count of patrolling monocytes on day 3. In addition, an association with unfavorable outcome is also provided by low absolute counts of CD14pos/HLA-DRpos/CD45pos on day 1. The present findings cannot support the use of the defined cutoffs of CD14pos/HLA-DRpos/CD45pos and of CD14dim/CD16pos/CD45pos cells as biomarkers for sepsis since validation by another independent cohort is warranted. However, they strongly provide evidence for the independent role of CD14pos/HLA-DRpos/CD45pos cells at baseline and of the CD14dim/CD16pos/CD45pos cells during progression of sepsis as determinants of the outcome of the septic host.
Additional files
Table S1. Demographic characteristics of patients in both study phases. (DOCX 16 kb)
Figure S1. Absolute counts of circulating CD14pos/HLA-DRpos/CD45pos monocytes and subpopulations of monocytes in relation to treatment allocation. Absolute counts of (A) CD14pos/HLA-DRpos/CD45pos monocytes, (B) inflammatory monocytes, and (C) CD14dim/CD16pos/CD45pos patrolling monocytes on days 1 and 3 between patients allocated to treatment with placebo and patients allocated to treatment with clarithromycin. P values refer to the indicated comparisons. (DOCX 105 kb)
Table S2. Differences of baseline characteristics of day 1 between survivors and nonsurvivors of the first phase of the study. (DOCX 18 kb)
Table S3. Differences of baseline characteristics of day 1 between survivors and nonsurvivors of the second phase of the study. (DOCX 18 kb)
Acknowledgements
Not applicable.
Funding
The study was funded by the Hellenic Institute for the Study of Sepsis.
Availability of data and materials
Data are available upon request from the corresponding author.
Abbreviations
- AKI
Acute kidney injury
- APACHE
Acute Physiology and Chronic Health Evaluation
- CD
Cluster of differentiation
- CI
Confidence interval
- FcγIII
Fragment crystallizable-γ III
- FITC
Fluorescein isothiocyanate
- HLA-DR
Human leukocyte antigen-D related
- hpf
High-power field
- IL
Interleukin
- IQR
Interquartile range
- LPS
Lipopolysaccharide
- OR
Odds ratio
- PC5
Phycoerythrin-cyanine 5
- PE
Phycoerythrin
- PPV
Positive predictive value
- ROC
Receiver operating characteristic
- SD
Standard deviation
- SOFA
Sequential Organ Failure Assessment
- SS
Side scatter
- TLR
Toll-like receptor
- TNF
Tumor necrosis factor
Authors’ contributions
GG, AP, ML, and IT participated in patient enrollment, critically reviewed the manuscript for intellectual content, and gave final approval for the version to be submitted. EJGB designed the study, participated in data analysis, drafted the manuscript, and gave final approval for the version to be submitted. AP performed the flow cytometric analysis, analyzed the data, wrote the manuscript, and gave final approval for the version to be submitted.
Ethics approval and consent to participate
This study constitutes a substudy of a large prospective randomized clinical trial (registration NCT012236690). The study was approved by the ethics committee of “ATTIKON” University Hospital (266/27-07-9). Patients were enrolled after written informed consent provided by themselves or by their legal representative for patients unable to consent.
Consent for publication
Patients or legal representatives consented to the publication of clinical data.
Competing interests
EJGB has received honoraria for providing scientific advice to AbbVie (Chicago IL, USA), Astellas (Athens, Greece), Biotest AG (Dreieich, Germany), and ThermoFisher Scientific GmbH (Henningdorf, Germany). He has received unrestricted educational funding from Biotest AG, ThermoFisher Scientific GmbH, Sanofi SA, (Athens, Greece), the Seventh Framework European Program HemoSpec, and the Horizon 2020 Marie-Curie grant European Sepsis Academy. The remaining authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s13054-018-1977-1) contains supplementary material, which is available to authorized users.
Contributor Information
Gabriela Gainaru, Email: gabriellagainaru@yahoo.com.
Antonios Papadopoulos, Email: antpapa1@otenet.gr.
Iraklis Tsangaris, Email: itsagkaris@med.uoa.gr.
Malvina Lada, Email: malvinalada@gmail.com.
Evangelos J. Giamarellos-Bourboulis, Phone: 0030 210 58 31 994, Email: egiamarel@med.uoa.gr
Aikaterini Pistiki, Email: aipistiki@gmail.com.
References
- 1.Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, Bricker TL, Jarman SD, 2nd, Kreisel D, Krupnick AS, Srivastava A, Swanson PE, Green JM, Hotchkiss RS. Immunosupression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306:2594–2605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeigler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol. 2007;81:584–592. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
- 3.Ziegler-Heitbrock L, Anucuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJ, Liu YJ, MacPherson G, Randolph GJ, Scherberich J, Schmitz J, Shortman K, Sozzani S, Strobl H, Zembala M, Austyn JM, Lutz MB. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74–e80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- 4.Lund H, Boysen P, Akesson CP, Lewandowska-Sabat AM, Storset AK. Transient migration of large numbers of CD14(++)CD16(+) monocytes to the draining lymph node after onset of inflammation. Front Immunol. 2016;7:322. doi: 10.3389/fimmu.2016.00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mukherjee R, Kanti Barman P, Kumar Thatoi P, Tripathy R, Kumar Das B, Ravindran B. Non-classical monocytes display inflammatory features: validation in sepsis and systemic lupus erythematosus. Sci Rep. 2015;5:13886. doi: 10.1038/srep13886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krychtiuk KA, Lenz M, Koller L, Honeder MC, Wutzlhofer L, Zhang C, Chi L, Maurer G, Niessner A, Huber K, Wojta J, Heinz G, Speidl WS. Monocyte subset is associated with mortality in critically ill patients. Thromb Haemost. 2016;116:949–957. doi: 10.1160/TH16-05-0405. [DOI] [PubMed] [Google Scholar]
- 7.Giamarellos-Bourboulis EJ, Mylona V, Antonopoulou A, Tsangaris I, Koutelidakis I, Marioli A, Raftogiannis M, Kopterides P, Lymberopoulou K, Mouktaroudi M, Papageorgiou C, Papaziogas B, Georgopoulou AP, Tsaganos T, Papadomichelakis E, Gogos C, Ladas M, Savva A, Pelekanou A, Baziaka F, Koutoukas P, Kanni T, Spyridaki A, Maniatis N, Pelekanos N, Kotsaki A, Vaki I, Douzinas EE, Koratzanis G, Armaganidis A. Effect of clarithromycin in patients with suspected Gram-negative sepsis: results of a randomized controlled trial. J Antimicrob Chemother. 2014;69:1111–1118. doi: 10.1093/jac/dkt475. [DOI] [PubMed] [Google Scholar]
- 8.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definition Conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 9.Papadopoulos P, Pistiki A, Theodorakopoulou M, Christodoulopoulou T, Damoraki G, Goukos D, Briassouli E, Dimopoulou I, Armaganidis A, Nanas S, Briassoulis G, Tsiodras S. Immunoparalysis: clinical and immunological associations in SIRS and severe sepsis patients. Cytokine. 2017;92:83–92. doi: 10.1016/j.cyto.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Monneret G, Lepape A, Venet F. A dynamic view of mHLA-DR expression in management of severe septic patients. Crit Care. 2011;15:198. doi: 10.1186/cc10452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monneret G, Venet F. Sepsis-induced immune alterations monitoring by flow cytometry as a promising tool for individualized therapy. Cytometry B Clin Cytom. 2016;90:376–386. doi: 10.1002/cyto.b.21270. [DOI] [PubMed] [Google Scholar]
- 12.Drewry AM, Ablordeppey EA, Murray ET, Beiter ER, Walton AH, Hall MW, Hotchkiss RS. Comparison of monocyte human leukocyte antigen-DR expression and stimulated tumor necrosis factor-alpha production as outcome predictors in severe sepsis: a prospective observational study. Crit Care. 2016;20:334. doi: 10.1186/s13054-016-1505-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu JF, Ma J, Chen J, Ou-Yang B, Chen MY, Li LF, Liu YJ, Lin AH, Guan XD. Changes of monocyte human leukocyte antigen-DR expression as a reliable predictor of mortality in severe sepsis. Crit Care. 2011;15:R220. doi: 10.1186/cc10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cazalis MA, Friggeri A, Cavé L, Demaret J, Barbalat V, Cerrato E, Lepape A, Pachot A, Monneret G, Venet F. Decreased HLA-DR antigen-associated invariant chain (CD74) mRNA expression predicts mortality after septic shock. Crit Care. 2013;17:R287. doi: 10.1186/cc13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fingerle G, Pforte A, Passlick B, Blumenstein M, Ströbel M, Ziegler-Heitbrock HW. The novel subset of CD14+/CD16+ blood monocytes is expanded in sepsis patients. Blood. 1993;82:3170–3176. [PubMed] [Google Scholar]
- 16.Rahman H, Salter G, Holmfield H, Larvin M, McMahon MJ. Soluble CD14 receptor expression and monocyte heterogeneity but not the C-260T CD14 genotype are associated with severe acute pancreatitis. Crit Care Med. 2004;32:2457–2463. doi: 10.1097/01.CCM.0000148008.99716.9C. [DOI] [PubMed] [Google Scholar]
- 17.Wong KL, Tai JJ, Wong WC, Han H, Sem X, Yeap WH, Kourilsky P, Wong SC. Gene expression profiling reveals the defining feature of the classical, intermediate, and nonclassical human monocyte subset. Blood. 2011;118:e16–e31. doi: 10.1182/blood-2010-12-326355. [DOI] [PubMed] [Google Scholar]
- 18.Antonakos N, Tsaganos T, Oberle V, Tsangaris I, Lada M, Pistiki A, Machairas N, Souli M, Bauer M, Giamarellos-Bourboulis EJ. Decreased production by mononuclear cells after severe gram-negative infections: early clinical sings and associations with final outcome. Crit Care. 2017;21:48. doi: 10.1186/s13054-017-1625-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Demographic characteristics of patients in both study phases. (DOCX 16 kb)
Figure S1. Absolute counts of circulating CD14pos/HLA-DRpos/CD45pos monocytes and subpopulations of monocytes in relation to treatment allocation. Absolute counts of (A) CD14pos/HLA-DRpos/CD45pos monocytes, (B) inflammatory monocytes, and (C) CD14dim/CD16pos/CD45pos patrolling monocytes on days 1 and 3 between patients allocated to treatment with placebo and patients allocated to treatment with clarithromycin. P values refer to the indicated comparisons. (DOCX 105 kb)
Table S2. Differences of baseline characteristics of day 1 between survivors and nonsurvivors of the first phase of the study. (DOCX 18 kb)
Table S3. Differences of baseline characteristics of day 1 between survivors and nonsurvivors of the second phase of the study. (DOCX 18 kb)
Data Availability Statement
Data are available upon request from the corresponding author.


