Abstract
Objectives
While various monocyte chemokine systems are increased in expression in osteoarthritis (OA), the hierarchy of chemokines and chemokine receptors in mediating monocyte/macrophage recruitment to the OA joint remains poorly defined. Here, we investigated the relative contributions of the CCL2/CCR2 versus CCL5/ CCR5 chemokine axes in OA pathogenesis.
Methods
Ccl2-, Ccr2-, Ccl5- and Ccr5-deficient and control mice were subjected to destabilisation of medial meniscus surgery to induce OA. The pharmacological utility of blocking CCL2/CCR2 signalling in mouse OA was investigated using bindarit, a CCL2 synthesis inhibitor, and RS-504393, a CCR2 antagonist. Levels of monocyte chemoattractants in synovial tissues and fluids from patients with joint injuries without OA and those with established OA were investigated using a combination of microarray analyses, multiplexed cytokine assays and immunostains.
Results
Mice lacking CCL2 or CCR2, but not CCL5 or CCR5, were protected against OA with a concomitant reduction in local monocyte/macrophage numbers in their joints. In synovial fluids from patients with OA, levels of CCR2 ligands (CCL2, CCL7 and CCL8) but not CCR5 ligands (CCL3, CCL4 and CCL5) were elevated. We found that CCR2+ cells are abundant in human OA synovium and that CCR2+ macrophages line, invade and are associated with the erosion of OA cartilage. Further, blockade of CCL2/CCR2 signalling markedly attenuated macrophage accumulation, synovitis and cartilage damage in mouse OA.
Conclusions
Our findings demonstrate that monocytes recruited via CCL2/CCR2, rather than by CCL5/CCR5, propagate inflammation and tissue damage in OA. Selective targeting of the CCL2/CCR2 system represents a promising therapeutic approach for OA.
INTRODUCTION
Osteoarthritis (OA) is the most common form of arthritis for which there are currently no disease-modifying therapies available.1 A growing body of evidence suggests that chronic, low-grade inflammation involving both the innate and adaptive immune systems is critical to the pathogenesis of OA,2–4 yet the precise underlying cellular and molecular mechanisms remain poorly understood. It is well documented that monocytes/macrophages infiltrate OA synovial tissues, and monocyte/ macrophage-derived inflammatory cytokines and chemokines are elevated in OA synovial tissues and fluids.5–7 Moreover, radiographic OA severity and joint symptoms have been shown to correlate with the number of activated macrophages in the synovial tissue of individuals with knee OA.8 However, the principal mechanisms of monocyte recruitment to the joint, namely differential contributions of specific chemokine–chemokine receptor axes, remain unclear.
While monocyte recruitment in the context of acute infectious inflammation has been extensively studied, relatively less is known about monocyte recruitment in chronic inflammatory diseases (eg, atherosclerosis).9,10 The CC chemokine receptors CCR2 and CCR5 as well as their cognate ligands (eg, CCL2, CCL7 and CCL8 for CCR2, and CCL5, CCL3 or CCL4 for CCR5) have been shown to modulate monocyte/macrophage recruitment in multiple inflammatory diseases.9 Indeed, several chemokines that mediate monocyte/macrophage and their receptors are detected in OA synovial tissues and synovial fluids.11,12 Additional studies have reported that synovium from individuals with joint injuries such as meniscal tears, a major risk factor for OA development, have elevated chemokine expression suggesting that these chemokines might instigate inflammatory responses in such individuals.13,14 Furthermore, a recent study reported that synovial fluid levels of CCL2 (or MCP-1) correlated positively with pain and physical disability in patients with OA.15 In line with this, it has been shown that deficiency in CCR2, the main receptor for CCL2, reduces OA-related pain mouse OA.16 Nevertheless, the functional involvement of CCL2 in the OA disease process and the relative contribution of the CCL2/CCR2 versus the CCL5/CCR5 chemokine systems to monocyte recruitment in OA remains unknown. Understanding the processes, driving monocyte recruitment could aid in the development of novel targeted therapies to selectively inhibit pathological responses in OA.
METHODS
Study approval
We studied human samples under protocols approved by the Stanford Institutional Review Board (IRB) and the University of Padova IRB, and with the subjects’ informed consent.
Animals
C57BL/6J, Ccr2−/− (B6.129S4-Ccr2tm1Ifc/J), Ccl2−/− (B6.129S4-Ccl2tm1Rol/J), Ccr5−/− (B6.129P2-Ccr5tm1Kuz/J) and Ccl5−/−(B6.129P2-Ccl5tm1Hso/J) mice were purchased from The Jackson Laboratory. Destabilisation of the medial meniscus (DMM) was performed as described previously.17–19 All animal studies were performed under protocols approved by the Stanford Committee of Animal Research and in accordance with National Institutes of Health guidelines.
Statistics
Data were analysed using two-tailed Student’s t-test or Mann-Whitney U test for parametric and non-parametric data, respectively. p<0.05 was considered statistically significant.
Detailed methods are described in online supplementary materials.
RESULTS
CCL2 deficiency protects against mouse OA and is associated with reduced synovial macrophages and inflammatory mediators
Recently, it was reported that levels of CCL2, a major monocyte chemoattractant in serum and synovial fluid obtained from individuals with OA correlated positively with pain and physical disability.15 Further, microarray analyses of genes expressed in knees of mice with surgery-induced OA showed that Ccl2 is upregulated as early as 6 hours and 7 days after surgery when there is no apparent histological changes) relative to sham-operated controls (see online supplementary figure S1A).18,20 To evaluate whether increased Ccl2 gene expression was sustained through the progression of OA, we analysed synovial Ccl2 expression at 12 weeks after DMM, when significant cartilage degradation is apparent.18,19,21 We found that synovial Ccl2 mRNA was significantly upregulated in DMM-operated compared with sham-operated mice (see online supplementary figure S1B). Notably, mining analyses of microarray data from knees of STR/ort mice, which spontaneously develop progressive OA similar to human OA starting at roughly 12 weeks of age,22 also revealed significant upregulation of Ccl2 relative to similarly aged OA-resistant CBA mice (see online supplementary figure S1C). Together, these data suggest that CCL2 might be involved in mechanical trauma-induced OA and in ageing-related spontaneous OA.
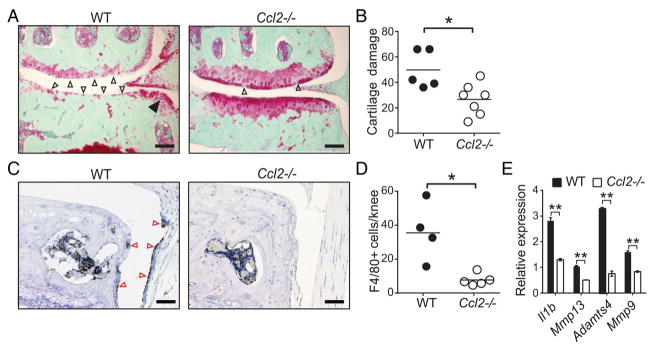
To directly demonstrate a pathogenic role for CCL2 in OA, we surgically induced OA via DMM in CCL2-deficient (Ccl2−/−) and age-matched wild-type (WT) mice. Histological analyses of knee joints 20 weeks after DMM revealed that Ccl2−/− mice exhibit less severe cartilage damage (figure 1A, B), osteophyte formation (figure 1A and online supplementary figure S2B) and synovitis (see online supplementary figures S2A, B) compared with WT controls. Consistent with CCL2’s function as a potent monocyte chemoattractant, we found that macrophage numbers were significantly reduced in knee joints of Ccl2−/− mice compared with WT mice (figure 1C, D). Furthermore, analysis of synovial mRNA revealed significant reductions in inflammatory and degradative enzyme expression in Ccl2−/− compared with WT mice (figure 1E), suggesting that CCL2 deficiency downregulates local inflammatory responses in experimental mouse OA.
Figure 1.
CCL2 deficiency protects against development of osteoarthritis in mice, and is associated with reduced synovial macrophages and inflammatory mediators. (A) Representative safranin-o stained knee joint sections showing extensive cartilage damage (open arrows) and osteophytes (filled arrows) and (B) quantification of cartilage damage in wild-type (WT, n=5) but not Ccl2−/− (n=7) mice 20 weeks after destabilisation of the medial meniscus (DMM) surgery by a blinded investigator. (C, D) Representative immunostains and quantification of F4/80+ (brown) macrophages in the synovium (red arrows) in WT and Ccl2−/− mice 16 weeks after DMM surgery. Symbols represent individual mice and bars denote the mean in (B) and (D). (E) Quantitative PCR (qPCR) analyses of proinflammatory gene expression in synovium of WT (n=3) and Ccl2−/− (n=3) mice 10 weeks after DMM surgery. qPCR data are mean±SEM. Scale bars 200 μm. *p<0.05, **p<0.01 by the Mann-Whitney U test for (B), (D) and by Student’s t-test for (E). The presented data are representative of two independent experiments with similar results.
Reduced mouse OA and local macrophage numbers in CCR2-deficient mice
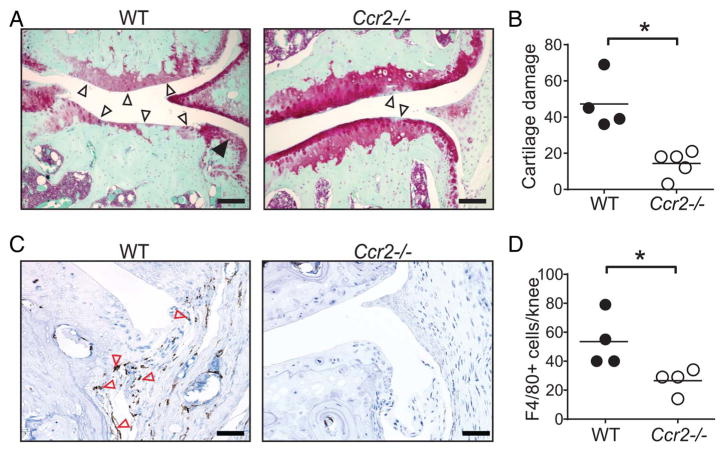
Because CCR2 is the main receptor for CCL2 and CCR2-induced monocyte recruitment has been shown to mediate pain in the DMM model,16 we tested whether CCR2-mediated monocyte recruitment contributes to the development of mouse OA. Whereas WT mice developed severe cartilage damage after DMM surgery, Ccr2−/− mice exhibited significantly less cartilage damage (figure 2A, B), synovitis (see online supplementary figures S3A, B) and osteophyte formation (see online supplementary figure S3B). The role of CCR2 in macrophage recruitment was confirmed by immunohistochemical analysis: whereas the knee joints of WT mice contained substantial numbers of macrophages after DMM, those of Ccr2−/− mice were largely devoid of such cells (figure 2C, D). Together, these findings demonstrate that both CCL2 and CCR2 promote inflammation and tissue damage in OA via mechanism(s) linked to monocyte/macrophage recruitment.
Figure 2.
CCR2 deficiency attenuates osteoarthritis in mice. (A) Representative safranin-o stained knee joint sections showing severe cartilage damage (open arrows) and osteophytes (filled arrows) and (B) quantification of cartilage damage in wild-type (WT, n=4) but not Ccr2−/− (n=5) mice 20 weeks after destabilisation of the medial meniscus surgery by a blinded investigator. (C) Representative immunohistochemistry and (D) quantification of F4/80+ (brown) macrophages lining the synovium (red arrows) in WT and Ccr2−/− mice. Symbols denote individual mice, and bars denote the mean of indicated groups in (B) and (D). Scale bars denote 200 μm. *p<0.05 by the Mann-Whitney U test for (B) and by Students t-test ‘ for (D). The presented data are representative of two independent experiments with similar results.
CCR5 or CCL5 deficiencies do not attenuate experimental mouse OA development or diminish macrophage burden in the OA knee joints
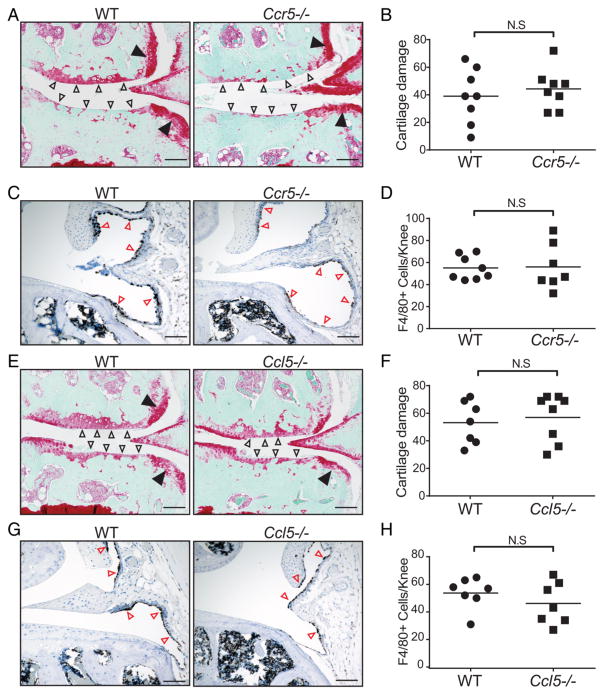
It has been well documented that while CCL2 is a potent monocyte chemoattractant, other chemokine–chemokine receptor systems such as CCL5/CCR5 are also involved in monocyte recruitment during inflammation.9,10 To further investigate the relative contribution of the CCR5/CCL5 chemokine axis in mouse OA, we surgically induced OA in CCR5 or CCL5-deficient mice and found that 20 weeks after DMM surgery, cartilage damage was indistinguishable between CCR5-deficient (Ccr5−/−) and WT mice (figure 3A, B). Remarkably, we found no differences in macrophage numbers between WT and Ccr5−/− mice (figure 3C, D). Similar to mice deficient in CCR5, we found that mice deficient in CCL5, a major CCR5 ligand, also develop severe OA similar to that observed in WT controls (figure 3E, F) and have no apparent reduction in macrophage numbers in their knee joints (figure 3G, H). Together, our data indicate that neither CCL5 nor CCR5 is required for the development of OA in mice.
Figure 3.
CCR5 or CCL5 deficiency does not modulate the severity of experimental osteoarthritis following destabilisation of the medial meniscus (DMM). (A) Representative safranin-o stained knee joint sections showing severe cartilage damage (open arrows) and osteophytes (filled arrows) in both wild-type (WT) and Ccr5−/− mice 20 weeks after DMM surgery. (B) Quantification of cartilage damage in WT (n=8) and Ccr5−/− (n=8) mice by a blinded investigator. (C) Representative immunohistochemistry showing F4/80+ (brown) macrophages lining the synovium (red arrows) in WT and Ccr5−/− mice. (D) Quantification of F4/80+ macrophages in WT and Ccr5−/− mice. (E) Representative safranin-o stained knee joint sections showing marked cartilage damage (open arrows) and osteophytes (filled arrows) in WT and Ccl5−/− mice 20 weeks after DMM surgery. (F) Quantification of cartilage damage in WT (n=7) and Ccl5−/− (n=8) mice by a blinded investigator. (G) Representative immunohistochemistry showing F4/80+ (brown) macrophages lining the synovium (red arrows) in WT and Ccl5−/− mice. (H) Quantification of F4/80+ macrophages in WT and Ccl5−/− mice. Symbols denote individual mice, and bars denote the mean. Scale bars denote 200 μm. *p<0.05 by the Mann-Whitney U test in (B) and (F) and Student’s t-test in (D) and (H).
Increased monocyte chemoattractant proteins in human OA synovial tissue and fluid
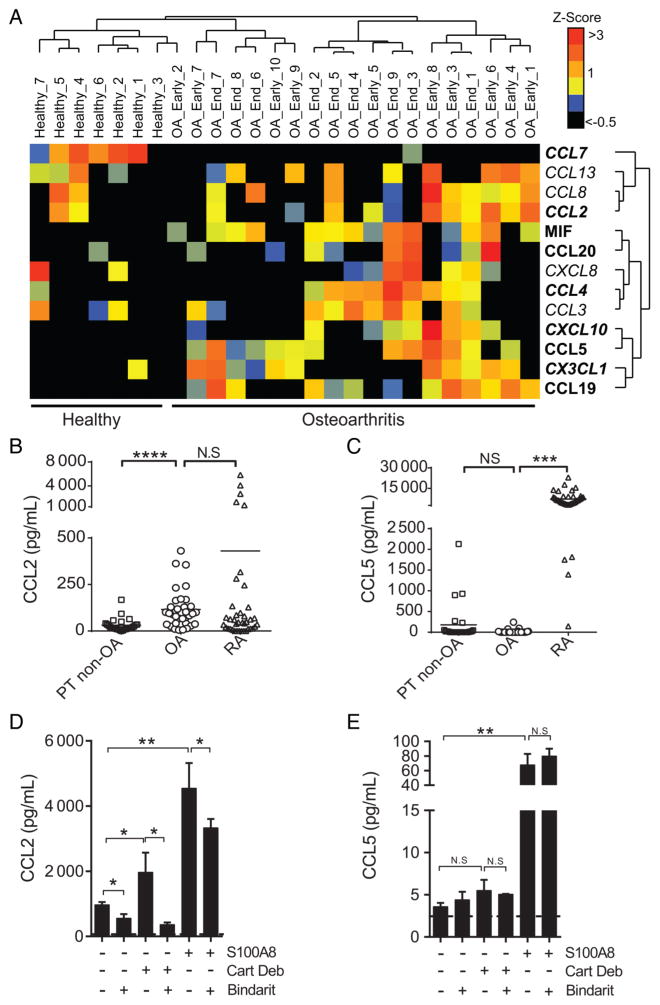
In human OA, levels of various chemokines and chemokine receptors are abnormally high in the synovial fluid and synovial tissue, but little is known about the relative contribution of these molecules to OA pathology.11,23 Unsupervised cluster analyses of publicly available gene expression dataset GSE32317 revealed that multiple chemokines involved in monocyte recruitment or macrophage accumulation are overexpressed in synovial membranes derived from individuals with early or end-stage OA as compared with membranes from ‘healthy’ joints (figure 4A). To validate this, we performed analyses of another independent dataset (GSE46750) and demonstrated that the same putative pathogenic monocyte chemokine encoding genes (eg, CCL2, CCL5, CCL19 and CXCL8) were also statistically upregulated in inflamed OA synovium as compared with non-inflamed OA synovium (see online supplementary table S1).
Figure 4.
Enhanced monocyte chemoattractant mRNA expression in human osteoarthritis (OA) synovial tissue and increased CCL2, but not CCL5, levels in human OA synovial fluids and synovial fibroblasts. (A) Unsupervised cluster analyses of gene expression of various chemokines involved in monocyte chemoattraction in microarray datasets from synovial membranes of individuals with prior traumatic joint injury but no radiographic OA (healthy) and from individuals with early-stage or end-stage OA (downloaded from gene expression omnibus (GEO), accession code GSE32317). Scale represents Z scores. Bold font indicates significant difference between ‘healthy’ and ‘ osteoarthritis’ groups by significance analysis of microarrays (SAM) analyses with q-value (false discovery rate) cut-off set at 0.05. Quantification of (B) CCL2 and (C) CCL5 in synovial fluids of individuals with posttraumatic joint injuries (PT non-OA, n=37), OA (n=35) or rheumatoid arthritis (n=26). Analyses of (D) CCL2 and (E) CCL5 levels in supernatants of human OA-derived primary synovial fibroblasts stimulated with 20 mg/mL OA cartilage debris (Cart Deb), 1 μg/mL S100A8 (positive control) or media alone in the presence or absence of 300 μM bindarit for 24 hours. In vitro stimulation assays were performed in triplicate and are representative of data obtained from four independent synovial fibroblast cultures derived from four different individuals with OA undergoing total knee arthroplasty. *p<0.05, **p<0.01, ****p<0.0001 Student’s t-test, NS, not significant.
Chemokines that signal via the CCR2 receptor are elevated in the synovial fluids of individuals with OA
Next, we analysed protein levels of various chemokines in synovial fluids of those with established OA, with rheumatoid arthritis (RA) or obtained from individuals with post-traumatic joint injuries that are at increased risk for developing OA24 (labelled ‘PT non-OA’). Consistent with the concept that OA is associated with low-grade inflammation,25,26 we found that chemokine levels were elevated in OA synovial fluids compared with PT non-OA samples but generally lower than the levels in RA synovial fluids (figure 4B, C and online supplementary figures S7A, B). Importantly, the levels of multiple CCR2 ligands such as CCL2 (figure 4B), CCL7 (see online supplementary figure S4B) and CCL8 (see online supplementary figure S4C) were significantly elevated in OA synovial fluids. By contrast, OA levels of the major CCR5 ligands CCL5 (figure 4C) and CCL3 (see online supplementary figure S4D) were either undetectable or very low in these very same synovial fluids.
CCL2 but not CCL5 is secreted by human OA synovial fibroblasts on stimulation with cartilage debris
CCL2 is predominantly produced by immune cells in humans and mice. In the context of OA, previous studies demonstrated that chondrocytes upregulate CCL2 expression following inflammatory stimuli.17,27,28 Consistent with this, we found that chondrocytes within OA cartilage tissues abundantly express CCL2 (see online supplementary figure S4E). We next tested whether synovial fibroblasts might also produce CCL2 in response to tissue injury by stimulating OA synovial fibroblasts with OA cartilage-derived debris, or the alarmin S100A8. First, we observed that unstimulated OA synovial fibroblasts abundantly secreted CCL2 (figure 4D), but not CCL5 (figure 4E). Next, we found that while synovial fibroblasts stimulated with cartilage debris or S100A8 secreted CCL2 (figure 4D), only stimulation with S100A8 induced robust CCL5 secretion (figure 4E). Finally, we found that CCL2 production by both unperturbed and stimulated fibroblasts could be effectively limited using bindarit, a specific CCL2 synthesis inhibitor (figure 4D). Consistent with previous reports showing that bindarit is highly specific to the monocyte chemoattractant protein (MCP) subfamily which includes CCL2,29 bindarit did not mitigate CCL5 secretion from synovial fibroblasts stimulated with S100A8 (figure 4E).
CCR2+ macrophages are abundant in OA synovium and in close physical association with articular cartilage tissues
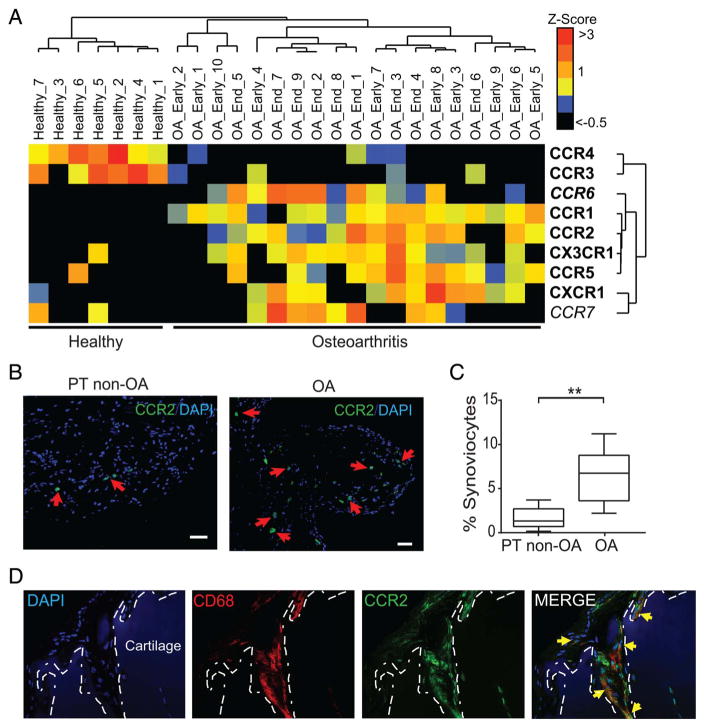
To further analyse whether CCL2 signalling is upregulated in OA synovial tissue, we performed analyses of the GSE32317 dataset and found that gene expression of CCR2 was also upregulated in OA synovium relative to healthy synovium (figure 5A). Supporting this observation, immunofluorescent analysis showed that the number of CCR2-expressing cells (CCR2+) was also significantly higher in OA as compared with PT non-OA samples (figure 5B, C), suggesting an important role for CCR2-expressing cells in human OA pathogenesis. It is well described that CCR2-expressing inflammatory monocytes recruited to tissues differentiate into proinflammatory macrophages and drive local inflammation and tissue damage.30–32 Thus, we hypothesised that CCR2+ macrophages could be directly involved in OA tissue damage. In agreement with this, we found many CCR2-expressing cells in close proximity to the surface of articular cartilage (figure 5D, green). Further, we found that these CCR2+ cells are haematopoietic in origin as they also expressed CD45 (data not shown) and are indeed macrophages as evidenced by CD68 staining (figure 5D, red). Importantly, as seen in online supplementary figure S5, this inflammatory layer of cells was found adjacent to articular cartilage surfaces. However, further analyses needed to define their precise role in cartilage degradation.
Figure 5.
Increased mRNA expression of chemokine receptors and increased CCR2+ macrophages in synovium and cartilage tissues obtained from humans with osteoarthritis (OA). (A) Unsupervised cluster analyses of gene expression of various chemokine receptors involved in monocyte chemoattraction in microarray datasets from synovial membranes of individuals with prior traumatic joint injury but no radiographic OA (healthy) and from individuals with early-stage or end-stage OA (downloaded from GEO, accession code GSE32317). Scale represents Z scores. Bold font indicates significant difference between ‘healthy’ and ‘osteoarthritis’ groups by SAM analyses with q-value (false discovery rate) cut-off set at 0.05. (B) Representative immunofluorescent staining of CCR2 (red arrows) in sections of PT non-OA or OA synovial tissues. (C) Quantification of CCR2+ cells in synovial tissue as a percentage of total DAPI-stained synoviocytes per random low power field (LPF). Ten LPFs were quantitated per synovial tissue section (n=5 individual samples per group). Error bars indicate maximum and minimum values, and line denotes their mean. (D) Representative immunostaining of CD68 (red) and CCR2 (green) adjacent to the lesional articular cartilage samples from patients with OA (n=6) showing CD68+CCR2+ double-positive macrophages (yellow arrows) invading the cartilage tissue. Dotted white lines demarcate the cartilage tissue lined by invading macrophages. Scale bar denotes 200 μm. **p<0.01 Student’s t-test.
Pharmacological intervention at the level of CCL2 synthesis or its binding to CCR2 attenuates mouse OA development and severity
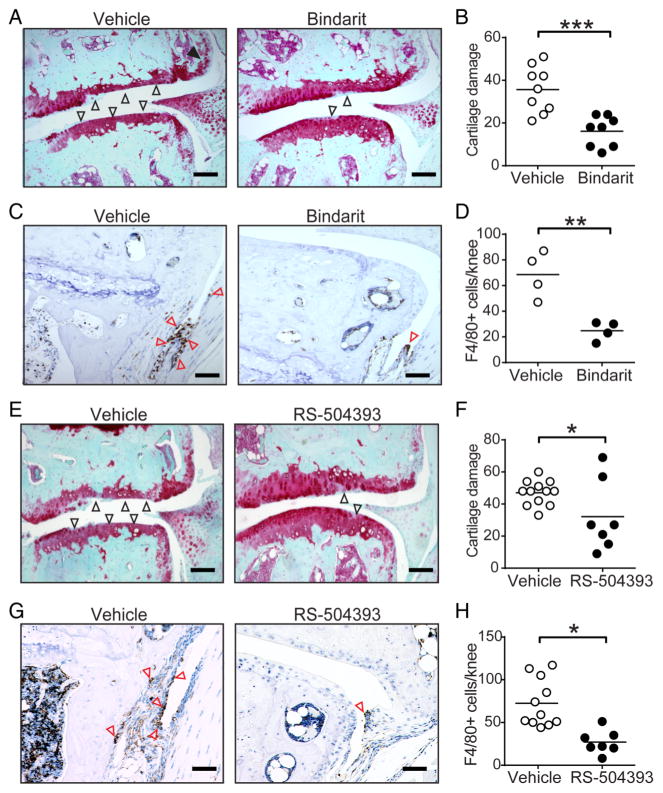
Based on our findings that genetic deficiency of CCL2 attenuates local inflammation and tissue damage in experimental OA and that the CCL2/CCR2 axis is upregulated in human OA, we evaluated the utility of blocking CCL2 synthesis in mouse OA using bindarit, a molecule previously shown to inhibit CCL2 induction in vivo.33,34 While vehicle-treated mice developed severe OA, mice treated with bindarit for 12 weeks exhibit markedly diminished cartilage damage (figure 6A, B), synovitis and osteophyte formation (see online supplementary figure S6A, B). In addition, bindarit treatment significantly reduced macrophage accumulation in mouse knee joints (figure 6C, D).
Figure 6.
Pharmacological blockage of CCL2 synthesis or binding to CCR2 protects against osteoarthritis development in mice. (A) Representative safranin-o stained knee joint sections and (B) quantification of cartilage damage from mice receiving vehicle (n=10) or 50 mg/kg/day bindarit (n=8) by oral gavage for 12 weeks after destabilisation of the medial meniscus (DMM) surgery. Open and filled arrows indicate cartilage damage and osteophytes, respectively. (C) Representative immunostains and (D) quantification of F4/80+ (brown) macrophages in the synovium (red arrows) of vehicle-treated but not bindarit-treated mice. (E) Representative safranin-o stained knee joint sections and (F) quantification of cartilage damage from mice receiving vehicle (n=12) or 4 mg/kg/day RS-504393 (n=7) by oral gavage for 12 weeks after DMM surgery. Open and filled arrows indicate cartilage damage and osteophytes, respectively. (G) Representative immunostains and (H) quantification of F4/80+ (brown) macrophages in the synovium (red arrows) of vehicle-treated but not RS-504393-treated mice. Symbols denote individual mice and bars denote the mean of indicated groups in (B) and (F). Scale bars denote 200 μm. *p<0.05 by the Mann-Whitney U test for (B) and (F) and by Student’s t-test for (D) and (H).
To further confirm a pathogenic role for CCL2/CCR2 signalling in the pathogenesis of mouse OA, we tested whether RS-504393, a CCR2 antagonist that potently inhibits CCL2 binding to CCR2 but not to other CCL2 receptors,35 could attenuate OA disease progression and/or severity. We found that RS-504393 significantly diminished cartilage damage (figure 6E, F), synovitis and osteophyte formation (see online supplementary figures S7A,B). Indeed, the number of F4/80-positive macrophages was significantly lower in RS-504393-treated mice as compared with vehicle-treated mice (figure 6G, H). Thus, pharmacological blockade of the CCL2/CCR2 chemokine system effectively diminishes mouse OA in part by reducing synovial macrophage accumulation.
DISCUSSION
The objective of this study was to examine the differential involvement of chemokine–chemokine receptor systems in monocyte recruitment in OA using experimental mouse model systems and human OA tissues. Here, we report that the CCL2/ CCR2 signalling axis preferentially mediates monocyte trafficking and promotes inflammation and tissue damage in OA. Importantly, we show that as in mouse OA, in human OA CCR2 ligands such as CCL2 and CCL8, but not CCR5 ligands such as CCL5, are preferentially elevated. Finally, we demonstrate for the first time that pharmacological inhibition of CCL2 synthesis or its binding to CCR2 protects against development of mouse OA in part by attenuating macrophage accumulation in the synovial joints.
In several mouse models of chronic inflammation (eg, chronic kidney disease, RA, asthma, etc), deficiencies in CCL2 or CCR2 protect against inflammation and tissue damage.36 Previous reports also suggest that specific chemokine–chemokine receptor systems may differentially contribute to monocyte recruitment in a time and context-dependent manner. For instance, in a model of high fat diet-induced atherosclerosis, CCR1 and CCR5 but not CCR2 or CX3CR1 were found to be crucial for monocyte recruitment.31 In a setting of DSS-induced colitis in mice, both CCR2 and CCR5 were found to be important for driving inflammatory responses.37 Here, we report that in OA there exists an apparent hierarchy in chemokine-driven mechanisms in that CCL2/CCR2-driven but not CCL5/CCR5-driven monocyte recruitment promotes mouse and human OA pathogenesis. CCR2 has additional ligands in humans and mice (eg, CCL7, CCL8, CCL13 and CCL14), but studies in mice lacking different combinations of these ligands suggest that CCL2 and CCL7 are critically involved in monocyte mobilisation from the bone marrow.38 Nevertheless, it remains to be experimentally determined whether these additional CCR2 ligands, as well as other CCL2 receptors such as CCR1 and CCR4, modulate the pathogenesis of OA. Further, while monocyte/macrophage accumulation within the inflamed synovial joints is thought to be fundamentally critical for OA pathogenesis, other tightly controlled processes such as survival, activation and polarisation could also be crucial in modulating OA pathogenesis.
The chemokine CCL5 and its receptor CCR5 also possess strong monocyte chemoattractive properties. Here, we show that neither CCR5 nor CCL5 deficiency confers protection against the development or severity of OA in mice. This finding is in direct contrast to prior findings by Takebe et al39 showing reduced cartilage damage in Ccr5−/− mice. These discrepancies can potentially be attributed to differences in experimental design, including (1) age at OA induction (20-week-old mice used in our studies vs 10-week-old mice in studies by Takebe et al,39 as older mice develop more severe OA),40,41 (2) duration of OA development (DMM-associated pathologies were assessed 20 weeks after surgery, whereas Takebe et al39 characterised changes at 8 and 12 weeks after DMM) and (3) possible sex-related differences in disease severity (male mice, used in our study, suffer more OA pathology compared with female mice.41,42 It is unclear which sex was used by Takebe et al).39
Identifying the source of inflammatory chemokines such as CCL2 is fundamentally important to understanding disease biology and developing therapeutic interventions. However, the cell types responsible for CCL2 secretion in OA remain unclear. While it is known that CCL2 is predominantly made by immune cells in humans and mice, other non-immune cell types have also been shown to express CCL2. For instance, previous studies have demonstrated that cultured murine and human chondrocytes upregulate CCL2 expression following inflammatory stimuli.27,28 Furthermore, it is well known that ageing, a major risk factor for OA development, is associated with systemic low-grade inflammation including elevated local and circulating cytokines.43 In this regard, senescent cells such as ageing articular chondrocytes release cytokines including CCL2 that could contribute to inflammatory mechanisms that promote OA as shown in the studies presented here.44 Here, we report that CCL2 is abundantly secreted by human OA synovial fibroblasts on stimulation with cartilage debris. Based on our observations, we propose a model where joint-resident cells such as chondrocytes and/or synovial fibroblasts are the initial source of CCL2 following injury, thereby recruiting CCR2-expressing inflammatory monocytes that propagate OA pathogenesis via production of inflammatory cytokines and tissue degradative enzymes.
We show that CCL2 secretion can be abrogated using bindarit, a synthetic molecule previously shown to potently and selectively inhibit expression of the monocyte chemoattractants CCL2, CCL8 and CCL7 as well as attenuate inflammation in various mouse models of inflammatory disease including experimental autoimmune encephalomyelitis (EAE) and 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced colitis.29,34 We provide proof of concept that pharmacological suppression of CCL2 expression using bindarit is effective in reducing inflammation, cartilage damage and monocyte/macrophage accumulation in mouse OA. We further demonstrate that pharmacological blockage of CCR2 signalling using RS-504393, a CCR2 antagonist that has been shown to specifically inhibit CCL2/CCR2 interactions,35 significantly attenuates OA disease progression and/or severity in mice through a mechanism linked to synovial macrophage accumulation. Notably, RS-504393 has been previously demonstrated to reduce pain in this mouse model in part by inhibiting macrophage recruitment to the dorsal root ganglion. Given the critical role of the CCL2/CCR2 system in recruiting inflammatory monocytes, long-term systemic blockade of CCL2/CCR2 could potentially increase susceptibility to infections, impair wound healing and tissue repair. Thus, local inhibition of CCL2 or CCR2 might be a more effective and safe strategy for the treatment of OA.
Indeed, the prominent role of the CCL2/CCR2 chemokine system in inflammation has rendered it an attractive target for therapeutic intervention in multiple diseases including RA. However, pharmacological inhibition of CCL2 binding to CCR2 has failed in clinical trials to date, most conspicuously in RA.45–47 The reasons for failure are likely multifold, have been reviewed elsewhere and can be in part attributed to off-target effects on CCR5.48,49 It is noteworthy that OA immunopathogenesis is distinct from that of RA, including the use of distinct chemokine–chemokine receptor systems for the recruitment of monocytes/ macrophages to the ‘low-grade’ inflamed OA joint. Based on our results, we propose that selective targeting of the CCL2/CCR2 system, either alone or in combination with other therapies, has the potential to provide therapeutic benefit in OA.
Supplementary Material
Acknowledgments
The authors thank Dr T Lindstrom for critically reviewing and editing the manuscript and Dr D Rajamani for contributing to microarray data analysis.
Funding These studies were supported by VA RR&D Merit Review Awards I01BX002345, I01RX000934 and I01RX000588 to WHR.
Footnotes
Additional material is published online only. To view please visit the journal online (http://dx.doi.org/10.1136/annrheumdis-2016-210426).
Contributors HR and CML performed key studies. HR, QW and HHW conducted the studies of osteoarthritis in mouse models. CML and HR performed immunofluorescent and H&E staining of synovium provided by NJG, SBG and CRC. HR analysed these images. HR and NL conducted ELISA analyses, and NL performed Luminex cytokine profiling using synovial fluids provided by JBS, CRC, LP and FO. HR cultured and performed the in vitro stimulation assays on OA synovial fibroblasts. HR and NL analysed gene expression datasets downloaded from NCBI. CRC and JBS provided key scientific input. HR and WHR wrote and edited the manuscript. All authors reviewed the data and approved the manuscript.
Competing interests None.
Ethics approval Stanford Institutional Review Board, University of Padova IRB.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Felson DT. Clinical practice. Osteoarthritis of the knee. N Engl J Med. 2006;354:841–8. doi: 10.1056/NEJMcp051726. [DOI] [PubMed] [Google Scholar]
- 2.Liu-Bryan R, Terkeltaub R. Emerging regulators of the inflammatory process in osteoarthritis. Nat Rev Rheumatol. 2015;11:35–44. doi: 10.1038/nrrheum.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klareskog L, Johnell O, Hulth A, et al. Reactivity of monoclonal anti-type II collagen antibodies with cartilage and synovial tissue in rheumatoid arthritis and osteoarthritis. Arthritis Rheum. 1986;29:730–8. doi: 10.1002/art.1780290605. [DOI] [PubMed] [Google Scholar]
- 4.Goldring MB, Otero M. Inflammation in osteoarthritis. Curr Opin Rheumatol. 2011;23:471–8. doi: 10.1097/BOR.0b013e328349c2b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kapoor M, Martel-Pelletier J, Lajeunesse D, et al. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 6.Rahmati M, Mobasheri A, Mozafari M. Inflammatory mediators in osteoarthritis: a critical review of the state-of-the-art, current prospects, and future challenges. Bone. 2016;85:81–90. doi: 10.1016/j.bone.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 7.Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!) Osteoarthr Cartil. 2013;21:16–21. doi: 10.1016/j.joca.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Kraus VB, McDaniel G, Huebner JL, et al. Direct in vivo evidence of activated macrophages in human osteoarthritis. Osteoarthr Cartil. 2016;24:1613–21. doi: 10.1016/j.joca.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol. 2014;32:659–702. doi: 10.1146/annurev-immunol-032713-120145. [DOI] [PubMed] [Google Scholar]
- 10.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–74. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Endres M, Andreas K, Kalwitz G, et al. Chemokine profile of synovial fluid from normal, osteoarthritis and rheumatoid arthritis patients: CCL25, CXCL10 and XCL1 recruit human subchondral mesenchymal progenitor cells. Osteoarthr Cartil. 2010;18:1458–66. doi: 10.1016/j.joca.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Haringman JJ, Smeets TJ, Reinders-Blankert P, et al. Chemokine and chemokine receptor expression in paired peripheral blood mononuclear cells and synovial tissue of patients with rheumatoid arthritis, osteoarthritis, and reactive arthritis. Ann Rheum Dis. 2006;65:294–300. doi: 10.1136/ard.2005.037176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scanzello CR, McKeon B, Swaim BH, et al. Synovial inflammation in patients undergoing arthroscopic meniscectomy: molecular characterization and relationship to symptoms. Arthritis Rheum. 2011;63:391–400. doi: 10.1002/art.30137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nair A, Gan J, Bush-Joseph C, et al. Synovial chemokine expression and relationship with knee symptoms in patients with meniscal tears. Osteoarthr Cartil. 2015;23:1158–64. doi: 10.1016/j.joca.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li L, Jiang BE. Serum and synovial fluid chemokine ligand 2/monocyte chemoattractant protein 1 concentrations correlates with symptomatic severity in patients with knee osteoarthritis. Ann Clin Biochem. 2015;52(Pt 2):276–82. doi: 10.1177/0004563214545117. [DOI] [PubMed] [Google Scholar]
- 16.Miller RE, Tran PB, Das R, et al. CCR2 chemokine receptor signaling mediates pain in experimental osteoarthritis. Proc Natl Acad Sci USA. 2012;109:20602–7. doi: 10.1073/pnas.1209294110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Q, Rozelle AL, Lepus CM, et al. Identification of a central role for complement in osteoarthritis. Nat Med. 2011;17:1674–9. doi: 10.1038/nm.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loeser RF, Olex AL, McNulty MA, et al. Disease progression and phasic changes in gene expression in a mouse model of osteoarthritis. PLoS ONE. 2013;8:e54633. doi: 10.1371/journal.pone.0054633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glasson SS, Askew R, Sheppard B, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434:644–8. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- 20.Burleigh A, Chanalaris A, Gardiner MD, et al. Joint immobilization prevents murine osteoarthritis and reveals the highly mechanosensitive nature of protease expression in vivo. Arthritis Rheum. 2012;64:2278–88. doi: 10.1002/art.34420. [DOI] [PubMed] [Google Scholar]
- 21.Xu L, Servais J, Polur I, et al. Attenuation of osteoarthritis progression by reduction of discoidin domain receptor 2 in mice. Arthritis Rheum. 2010;62:2736–44. doi: 10.1002/art.27582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kyostio-Moore S, Nambiar B, Hutto E, et al. STR/ort mice, a model for spontaneous osteoarthritis, exhibit elevated levels of both local and systemic inflammatory markers. Comp Med. 2011;61:346–55. [PMC free article] [PubMed] [Google Scholar]
- 23.Ritter SY, Subbaiah R, Bebek G, et al. Proteomic analysis of synovial fluid from the osteoarthritic knee: comparison with transcriptome analyses of joint tissues. Arthritis Rheum. 2013;65:981–92. doi: 10.1002/art.37823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lotz MK, Kraus VB. New developments in osteoarthritis. Posttraumatic osteoarthritis: pathogenesis and pharmacological treatment options. Arthritis Res Ther. 2010;12:211. doi: 10.1186/ar3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelso EB, Ferrell WR, Lockhart JC, et al. Expression and proinflammatory role of proteinase-activated receptor 2 in rheumatoid synovium: ex vivo studies using a novel proteinase-activated receptor 2 antagonist. Arthritis Rheum. 2007;56:765–71. doi: 10.1002/art.22423. [DOI] [PubMed] [Google Scholar]
- 26.Pessler F, Dai L, Diaz-Torne C, et al. The synovitis of “non-inflammatory” orthopaedic arthropathies: a quantitative histological and immunohistochemical analysis. Ann Rheum Dis. 2008;67:1184–7. doi: 10.1136/ard.2008.087775. [DOI] [PubMed] [Google Scholar]
- 27.Appleton CT, Usmani SE, Pest MA, et al. Reduction in disease progression by inhibition of transforming growth factor alpha-CCL2 signaling in experimental posttraumatic osteoarthritis. Ann Rheum Dis. 2015;67:2691–701. doi: 10.1002/art.39255. [DOI] [PubMed] [Google Scholar]
- 28.Xu YK, Ke Y, Wang B, et al. The role of MCP-1-CCR2 ligand-receptor axis in chondrocyte degradation and disease progress in knee osteoarthritis. Biol Res. 2015;48:64. doi: 10.1186/s40659-015-0057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mirolo M, Fabbri M, Sironi M, et al. Impact of the anti-inflammatory agent bindarit on the chemokinome: selective inhibition of the monocyte chemotactic proteins. Eur Cytokine Netw. 2008;19:119–22. doi: 10.1684/ecn.2008.0133. [DOI] [PubMed] [Google Scholar]
- 30.Swirski FK, Libby P, Aikawa E, et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tacke F, Alvarez D, Kaplan TJ, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–94. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dal-Secco D, Wang J, Zeng Z, et al. A dynamic spectrum of monocytes arising from the in situ reprogramming of CCR2+ monocytes at a site of sterile injury. J Exp Med. 2015;212:447–56. doi: 10.1084/jem.20141539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen JZ, Morgan J, Tesch GH, et al. CCL2-dependent macrophage recruitment is critical for mineralocorticoid receptor-mediated cardiac fibrosis, inflammation, and blood pressure responses in Male mice. Endocrinology. 2014;155:1057–66. doi: 10.1210/en.2013-1772. [DOI] [PubMed] [Google Scholar]
- 34.Ge S, Shrestha B, Paul D, et al. The CCL2 synthesis inhibitor bindarit targets cells of the neurovascular unit, and suppresses experimental autoimmune encephalomyelitis. J Neuroinflammation. 2012;9:171. doi: 10.1186/1742-2094-9-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mirzadegan T, Diehl F, Ebi B, et al. Identification of the binding site for a novel class of CCR2b chemokine receptor antagonists: binding to a common chemokine receptor motif within the helical bundle. J Biol Chem. 2000;275:25562–71. doi: 10.1074/jbc.M000692200. [DOI] [PubMed] [Google Scholar]
- 36.Pérez de Lema G, Maier H, Franz TJ, et al. Chemokine receptor Ccr2 deficiency reduces renal disease and prolongs survival in MRL/lpr lupus-prone mice. J Am Soc Nephrol. 2005;16:3592–601. doi: 10.1681/ASN.2005040426. [DOI] [PubMed] [Google Scholar]
- 37.Andres PG, Beck PL, Mizoguchi E, et al. Mice with a selective deletion of the CC chemokine receptors 5 or 2 are protected from dextran sodium sulfate-mediated colitis: lack of CC chemokine receptor 5 expression results in a NK1.1+ lymphocyte-associated Th2-type immune response in the intestine. J Immunol. 2000;164:6303–12. doi: 10.4049/jimmunol.164.12.6303. [DOI] [PubMed] [Google Scholar]
- 38.Tsou CL, Peters W, Si Y, et al. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117:902–9. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takebe K, Rai MF, Schmidt EJ, et al. The chemokine receptor CCR5 plays a role in post-traumatic cartilage loss in mice, but does not affect synovium and bone. Osteoarthr Cartil. 2015;23:454–61. doi: 10.1016/j.joca.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loeser RF, Olex AL, McNulty MA, et al. Microarray analysis reveals age-related differences in gene expression during the development of osteoarthritis in mice. Arthritis Rheum. 2012;64:705–17. doi: 10.1002/art.33388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fang H, Beier F. Mouse models of osteoarthritis: modelling risk factors and assessing outcomes. Nat Rev Rheumatol. 2014;10:413–21. doi: 10.1038/nrrheum.2014.46. [DOI] [PubMed] [Google Scholar]
- 42.Ma HL, Blanchet TJ, Peluso D, et al. Osteoarthritis severity is sex dependent in a surgical mouse model. Osteoarthr Cartil. 2007;15:695–700. doi: 10.1016/j.joca.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 43.Calçada D, Vianello D, Giampieri E, et al. The role of low-grade inflammation and metabolic flexibility in aging and nutritional modulation thereof: a systems biology approach. Mech Ageing Dev. 2014;136–137:138–47. doi: 10.1016/j.mad.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 44.Loeser RF, Collins JA, Diekman BO. Ageing and the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12:412–20. doi: 10.1038/nrrheum.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haringman JJ, Kraan MC, Smeets TJ, et al. Chemokine blockade and chronic inflammatory disease: proof of concept in patients with rheumatoid arthritis. Ann Rheum Dis. 2003;62:715–21. doi: 10.1136/ard.62.8.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haringman JJ, Gerlag DM, Smeets TJ, et al. A randomized controlled trial with an anti-CCL2 (anti-monocyte chemotactic protein 1) monoclonal antibody in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:2387–92. doi: 10.1002/art.21975. [DOI] [PubMed] [Google Scholar]
- 47.Vergunst CE, Gerlag DM, Lopatinskaya L, et al. Modulation of CCR2 in rheumatoid arthritis: a double-blind, randomized, placebo-controlled clinical trial. Arthritis Rheum. 2008;58:1931–9. doi: 10.1002/art.23591. [DOI] [PubMed] [Google Scholar]
- 48.Szekanecz Z, Koch AE. Successes and failures of chemokine-pathway targeting in rheumatoid arthritis. Nat Rev Rheumatol. 2016;12:5–13. doi: 10.1038/nrrheum.2015.157. [DOI] [PubMed] [Google Scholar]
- 49.Schall TJ, Proudfoot AE. Overcoming hurdles in developing successful drugs targeting chemokine receptors. Nat Rev Immunol. 2011;11:355–63. doi: 10.1038/nri2972. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.