Abstract
Adverse environmental conditions limit various aspects of plant growth, productivity, and ecological distribution. To get more insights into the signaling pathways under low temperature, we identified 10 C-repeat binding factors (CBFs), 9 inducer of CBF expression (ICEs) and 10 cold-responsive (CORs) genes from Aegilops–Triticum composite group under cold stress. Conserved amino acids analysis revealed that all CBF, ICE, COR contained specific and typical functional domains. Phylogenetic analysis of CBF proteins from Triticeae showed that these CBF homologs were divided into 11 groups. CBFs from Triticum were found in every group, which shows that these CBFs generated prior to the divergence of the subfamilies of Triticeae. The evolutionary relationship among the ICE and COR proteins in Poaceae were divided into four groups with high multispecies specificity, respectively. Moreover, expression analysis revealed that mRNA accumulation was altered by cold treatment and the genes of three types involved in the ICE–CBF–COR signaling pathway were induced by cold stress. Together, the results make CBF, ICE, COR genes family in Triticeae more abundant, and provide a starting point for future studies on transcriptional regulatory network for improvement of chilling tolerance in crop.
Electronic supplementary material
The online version of this article (10.1007/s12298-017-0495-y) contains supplementary material, which is available to authorized users.
Keywords: Triticeae, Cold stress, CBF, ICE, COR
Introduction
Low temperature is a vital environmental factor that restricts growth, productivity, and ecological distribution of plants (Zhuang et al. 2015). Upon exposure to low non-freezing temperatures, many plants have evolved sophisticated mechanisms with the acquisition of freezing tolerance, a process known as cold acclimation (Thomashow 1999; Zhao et al. 2016). During this complex process, extensive changes are involved, including gene expression and regulation, biochemical and metabolic changes (Chinnusamy et al. 2007; Shi et al. 2015). Identifying genes involved in freezing tolerance is crucial for understanding how plants regulate growth at low temperature. Transcription factors and effector genes are precisely regulated in plant cells suffering from cold stress (Ma et al. 2014; Zhou et al. 2011). These regulation cascades or signaling pathways play pivotal roles in adaptation to cold stress (Wingler 2014). C-repeat binding factor/dehydration responsive element binding factor (CBF/DREB) are critical transcription factors that specifically interplay with the C-repeat (CRT)/dehydration-responsive element (DRE) and positively regulated the expression of downstream cold-responsive (COR) genes during cold acclimation (An et al. 2017; Liu et al. 1998; Park et al. 2015; Sakuma et al. 2002; Stockinger et al. 1997; Yamaguchi-Shinozaki and Shinozaki 1994). At the same time, CBF/DREB genes are activated by inducers of CBF expression (ICEs) through specific binding to the MYC recognition cis-elements (CANNTG) in the promoter (Chinnusamy et al. 2003). These entities constitute the signal regulatory pathway, ICE–CBF–COR transcriptional cascade, which mediates the cold acclimation process (Chinnusamy et al. 2007; Lissarre et al. 2010; Lu et al. 2017; Ryu et al. 2014; Zhu et al. 2007).
CBF/DREBs which belong to the AP2/ERF (APETALA2/Ethylene-Responsive Factor) superfamily have characteristic signature sequence motifs flanking the AP2 domain, PKK/RPAGRxKFxETRHP and DSAWR (An et al. 2017; Gilmour et al. 1998; Jaglo et al. 2001; Jia et al. 2016; Monroe et al. 2016; Park et al. 2015; Skinner et al. 2005). ICE proteins, member of the MYC family transcription factor (Lee et al. 2015; Lu et al. 2017), have a MYC basic helix–loop–helix (bHLH) domain and localize in the nucleus (Peng et al. 2014). CORs generally refer to the proteins encoded by all cold regulated genes (Lee et al. 1999; Uemura et al. 1996), including late embryogenesis abundant protein (LEA) (Tsuda et al. 2000), stress responsive protein (SRP) (Unpublished, from GenBank), cold induced (KIN), low temperature induced (LTI) (Dong and Pei 2014; Nordin et al. 1993; Welin et al. 1995), and so on. In Arabidopsis, three CBF genes are cold stress-inducible genes (Liu et al. 1998; Ryu et al. 2014; Stockinger et al. 1997), and the three CBFs are not redundant (Zhou et al. 2011). CBF1 and CBF3 are regulated in a different way from CBF2, and CBF2 is a negative regulator of CBF1 and CBF3 expression during cold acclimation (Novillo et al. 2004, 2007; Qin et al. 2011). Furthermore, the recent research using the CRISPR/Cas9 technology reveals the broad mechanism of CBF genes that CBF1 and CBF3 negatively regulate CBF2 expression (Zhao and Zhu 2016). Coordinately, ICE proteins have distinct functions rather than redundant functions (Rahman et al. 2014). In Arabidopsis, ICE1 can activate the expression of CBF3/DREB1A during cold acclimation (Chinnusamy et al. 2003), and ICE2 triggers the expression of CBF1 (Fursova et al. 2009). Finally, many COR genes are induced to resist ambient cold temperature. These elaborate signaling modules constitute a regulatory pathway, which enhances tolerance to cold stress in plants.
Many close wild relatives of common wheat in Triticeae carry abundant and valuable stress resistant genes to facilitate genetic improvement, and particularly in species from Aegilops with strong resistance to cold stress (Jia et al. 2013). Many CBF genes have been characterized from some Triticeae species, including 37 from hexaploid wheat (Triticum aestivum) (Badawi et al. 2007), 13 from Triticum monococcum (Miller et al. 2006), 10 from durum wheat (Triticum durum) (Leonardis et al. 2007), 20 from barley (Hordeum vulgare) (Skinner et al. 2005), 11 from rye (Secale cereale) (Siddiqua and Nassuth 2011), 4 from Brachypodium distachyon (Li et al. 2012; Ryu et al. 2014), 1 from Aegilops tauschii (Badawi et al. 2007) and 9 from Aegilops biuncialis (Unpublished, from GenBank). The previous research indicated that CBF14 gene in A. tauschii associated with resistance to freezing stress (Masoomi-Aladizgeh et al. 2015), however, the lack of essential functional domains of the CBF14 results in many uncertainties. In addition, the functions of CBF genes from A. biuncialis remain largely unknown. Some ICE, COR genes also have been identified from these Triticeae species. In Triticeae, 2 ICE genes and 5 COR genes were obtained from wheat (T. aestivum), including TaICE41, TaICE87, WCS120, Wcor410, Wcor14, WRAB15 and WRAB18 (Badawi et al. 2008; Ganeshan et al. 2008; Soltesz et al. 2013; Talanova et al. 2013), as well as 4 COR genes from barley (H. vulgare) including HVA1, DHN5, DHN8 and COR14b (Dal Bosco et al. 2003; Jeknic et al. 2014; Koag et al. 2009; Kosova et al. 2013). However, the deeper researches on ICE, COR genes in Triticeae are less. To get better understanding of ICE–CBF–COR pathway and its involvement in the chilling tolerance, more genes related to this regulatory network await identification.
In this study, we identified and characterized 10 CBFs, 9 ICEs and 10 CORs in Aegilops–Triticum composite group. The results suggest that all CBF, ICE, COR amino acid sequences contained specific and representative conserved domains. We analyzed phylogenetic relationship of different CBF genes in Triticeae, as well as ICE and COR genes in Poaceae. Moreover, expression analysis revealed that mRNA accumulation was altered by cold treatment and the genes of three types involved in the ICE–CBF–COR signaling pathway were induced by cold stress. This study concluded that CBF genes may be activated by ICE, and may induce the expression of COR genes to enhance plant tolerance to cold. Consequently, the results provide candidate genes and transcriptional regulatory network for improvement of chilling tolerance in Triticeae.
Materials and methods
Plant materials, growth condition and RNA isolation
Four Aegilops species together with one winter-hardy wheat cultivar (T. aestivum) were selected as research materials in this study, which are presented in Table 1. The winter-hardy wheat cultivar Mironovska 808 (abbreviated as M808) was originally screened for its strong cold tolerance in the Ukraine (Tsvetanov et al. 2000). All seeds were germinatied in a petri dish covered with distilled water at 28 °C, then were planted in plastic boxes filled with soil substrates and placed in a controlled growth chamber with the temperature set at 25 °C, a 16 h light/8 h dark photoperiod, and a humidity of 45%. Ten centimeter seedlings were treated with 4 °C cold stress for 6 h before being frozen in liquid nitrogen for total RNA isolation. Total RNA of samples was extracted by TaKaRa MiniBEST Plant RNA Extraction Kit (TaKaRa, Japan) according to the manufacturer’s instruction. First strand cDNA synthesis was performed using PrimeScript™ RT reagent Kit with gDNA Eraser (Takara), and stored at − 20 °C until use.
Table 1.
Research materials in this study
| Species | Cultivar | Ploidy | Source | Origin |
|---|---|---|---|---|
| Triticum aestivum | Mironovska 808 | 6X | Shenyang Agriculture university, China | Ukraine |
| Triticum aestivum | China Spring | 6X | Shenyang Agriculture university, China | China |
| Aegilops umbellulata | – | 2X | National Clonal Germplasm Repository, USA | Turkey |
| Aegilops tauschii | – | 2X | National Clonal Germplasm Repository, USA | Unknown |
| Aegilops speltoides | – | 2X | National Clonal Germplasm Repository, USA | Turkey |
| Aegilops neglecta | – | 4X | National Clonal Germplasm Repository, USA | France |
Gene isolation and bioinformatic analyses
To isolate the Aegilops and T. aestivum CBF, ICE and COR genes, homologous sequences from other species in the GenBank were aligned with the Basic Local Alignment Search Tool (BLAST). A set of primers for gene amplification were designed by Primer 5 software (http://www.Premierbiosoft.com) (Table 2). The cDNA clone of all CBF and COR genes were amplified via Polymerase Chain Reaction (PCR) and then were sequenced to test whether they contain identical sequence or not. To obtain the whole sequence of ICE genes, 5′-RACE and 3′-RACE experiments were performed using a Full RACE Kit (TaKaRa) after the acquisition of the conserved sequence. To validate the whole coding sequence of ICE genes, primers were designed on the basis of the assembled results, and several clones of two independent PCR reactions were sequenced to correct errors introduced during PCR.
Table 2.
Primers in this study
| Primer name | Forward primer sequence (5′–3′) | Rerverse primer sequence (5′–3′) | Purpose | |
|---|---|---|---|---|
| CBF1 | ATGGACGTCGCCGACG | TTAGTCAAACAAATAGCTCCATAGC | Amplification of CBFs | |
| CBF2 | GATGGACGTCGCCGACGC | TTAGTCGAACAAGTAGCTC | ||
| ICE-C | GAAGAA(A/G)GG(T/A/G)ATGCC(T/G)GC(T/C)AAGAA | GG(A/G)CACA(A/G)CTC(T/C)TCCTTGA(C/T)(A/G)CG | Amplification of the conserved sequence of ICEs | |
| ICE-3F | CCAATAGCTTCCTCCCGTCGA | TCGCCTACATCGCGTTCTGGA | 3′ RACE of ICEs | 1st round |
| CTAGACCGCTGGATGGAACCCC | ||||
| ICE-3S | GCCGCATCAAGGAGGAGTTGTG | CTGGAGACCGGCGCAGTGCA | 2nd round | |
| CCTACATCGTGGGATGGAACC | ||||
| ICE-5F | AGGGCGTGATGGAGAACTCGG | TGTGCAGGAGCTCCTTGAGGTA | 5′ RACE of ICEs | 1st round |
| CGATGCTTGATGACGACAGCT | ||||
| ICE-5S | GGGGGCGACTGGGGGTACCT | AAGCCCTGTCCATTTTGCTGATC | 2nd round | |
| CGATGCTTCCCGCTCCTCAACC | ||||
| ICE-IF1 | GCAGAGATAAGTTGCAGTAACTGA | GCGAGCCTCATCCCATCA | Verification of the whole coding sequence of ICEs | 1st round |
| ICE-IS1 | GAACTTGCCAAGAATCCGT | AAAGGAGCGGAACAGCACA | 2nd round | |
| ICE-IF2 | GCTATGCTGTCGCAGTTCAATAG | AAACCATGGGTACTTATTGCAAAT | 1st round | |
| ICE-IS2 | TTGGGTGCAGGAAGGCGA | TTCCTTTGGTATGCTGTCCTCT | 2nd round | |
| COR1 | ATGTCTGGTTGGTTTCAAGAGAAG | TCACCCTCCCATCCCGAG | Amplification of CORs | |
| COR2 | ATGTCGGGTTGGTTCGGGGGTAAC | |||
| 18S | TGAGAAACGGCTACCACATC | TCGGCATCGTTTATGGTTGA | qRT-PCR analysis | |
| CBF4 | AGCCCGAGCAACACTCTTT | GCGCCAAGCTGTCGTAGTA | ||
| CBF9 | GCAGGGTATGCTCGTGTCA | GTCGAACAAGCAGCTCCATA | ||
| ICE41 | AGGGGCAGGCGGTGAACA | GGCGAAGCCATCGAAGCAG | ||
| ICE87 | AAGGCCTTGGTCTTGACGTG | CGCCTTGATTTCCTCAGCCT | ||
| COR1 | TGAGAAAAGCGAGGCTGTCA | TGATCCTTACCGGCTTCCAC | ||
| COR2 | GACCAGACGTTCGGCTTCTT | CGGTCTCCTCGACACACTTT | ||
Multiple sequence alignments were done using DNAMAN 8 (http://www.lynnon.com) and MEGA 5.10 (http://www.megasoftware.net/mega.php). Conserved motifs were identified using MEME Suite 4.10.1 (http://meme-suite.org/) and WebLogo 3.4 (http://weblogo.threeplusone.com/). To compare evolutionary relationship of the isolated Triticeae CBF, ICE, COR family members, the phylogenetic trees were constructed by MEGA 5.10 (http://www.megasoftware.net/mega.php) based on the neighbor-joining (NJ) method and bootstrap analysis (1000 replicates). Highly similar homologous genes were downloaded from NCBI (http://www.ncbi.nlm.nih.gov/) and presented in Tables 3, 4, and 5.
Table 3.
Description of CBF genes used in the study
| Proposed gene name | Gene name | Gene accession | Protein accession | Gene fragment | Protein fragment | Species | References |
|---|---|---|---|---|---|---|---|
| TaCBFIVd-4A | TaCBFIVd-4A | KY931522 | 672 | 223 | Triticum aestivum | This study | |
| AeuCBFIVd-4 | AeuCBFIVd-4 | KY931528 | 672 | 223 | Aegilops umbellulata | This study | |
| AetCBFIVd-4 | AetCBFIVd-4 | KY931534 | 672 | 223 | Aegilops tauschii | This study | |
| AesCBFIVd-4 | AesCBFIVd-4 | KY931540 | 672 | 223 | Aegilops speltoides | This study | |
| AenCBFIVd-4 | AenCBFIVd-4 | KY931546 | 678 | 225 | Aegilops neglecta | This study | |
| TaCBFIVd-9D | TaCBFIVd-9D | KY931521 | 810 | 269 | Triticum aestivum | This study | |
| AeuCBFIVd-9 | AeuCBFIVd-9 | KY931527 | 765 | 254 | Aegilops umbellulata | This study | |
| AetCBFIVd-9 | AetCBFIVd-9 | KY931533 | 765 | 254 | Aegilops tauschii | This study | |
| AesCBFIVd-9 | AesCBFIVd-9 | KY931539 | 765 | 254 | Aegilops speltoides | This study | |
| AenCBFIVd-9 | AenCBFIVd-9 | KY931545 | 765 | 254 | Aegilops neglecta | This study | |
| AebCBFIVd-4 | AebCBF7 | FR719739 | CBX87021 | 555 | 184 | Aegilops biuncialis | Unpublished, 2010 |
| AebCBFIVd-9 | AebCBF9 | FR719741 | CBX87023 | 768 | 255 | Aegilops biuncialis | Unpublished, 2010 |
| AebCBFIIIa-6 | AebCBF1 | FR719733 | CBX87015 | 729 | 242 | Aegilops biuncialis | Unpublished, 2010 |
| AebCBFIIIc-3 | AebCBF2 | FR719734 | CBX87016 | 741 | 246 | Aegilops biuncialis | Unpublished, 2010 |
| AebCBFIIIc-10 | AebCBF3 | FR719735 | CBX87017 | 714 | 237 | Aegilops biuncialis | Unpublished, 2010 |
| AebCBFIIId-12 | AebCBF4 | FR719736 | CBX87018 | 720 | 239 | Aegilops biuncialis | Unpublished, 2010 |
| AebCBFIIId-19 | AebCBF5 | FR719737 | CBX87019 | 711 | 236 | Aegilops biuncialis | Unpublished, 2010 |
| AebCBFIVa-2 | AebCBF6 | FR719738 | CBX87020 | 678 | 225 | Aegilops biuncialis | Unpublished, 2010 |
| AebCBFIVd-22 | AebCBF8 | FR719740 | CBX87022 | 855 | 284 | Aegilops biuncialis | Unpublished, 2010 |
| AtCBFV-1 | AtCBF1 | U77378 | AAC49662 | 642 | 213 | Arabidopsis thaliana (thale cress) | Stockinger et al. (1997) |
| AtCBFV-2 | AtCBF2 | AF074601 | AAD15976 | 651 | 216 | Arabidopsis thaliana (thale cress) | Gilmour et al. (1998) |
| AtCBFV-3 | AtCBF3 | AF074602 | AAD15977 | 651 | 216 | Arabidopsis thaliana (thale cress) | Gilmour et al. (1998) |
| BdCBFIIId-1 | BdCBF1 | JQ180470 | AFD96407 | 735 | 244 | Brachypodium distachyon (stiff brome) | Li et al. (2012) |
| BdCBFIIId-2 | BdCBF2 | JQ180470 | AFD96408 | 777 | 258 | Brachypodium distachyon (stiff brome) | Li et al. (2012) |
| BdCBFIIId-3 | BdCBF3 | JQ180470 | AFD96409 | 753 | 250 | Brachypodium distachyon (stiff brome) | Li et al. (2012) |
| BdCBFIIId-4 | BdCBF4 | JQ180470 | AFD96410 | 747 | 248 | Brachypodium distachyon (stiff brome) | Li et al. (2012) |
| HvCBFIa-1 | HvCBF1 | AF418204 | AAL84170 | 654 | 217 | Hordeum vulgare subsp. vulgare (domesticated barley) | Xue (2002) |
| HvCBFIVa-2 | HvCBF2 | AF442489 | AAM13419 | 666 | 221 | Hordeum vulgare subsp. vulgare (domesticated barley) | Xue (2003) |
| HvCBFIIIc-3 | HvCBF3 | AF239616 | AAG59618 | 750 | 249 | Hordeum vulgare subsp. vulgare (domesticated barley) | Choi et al. (2002) |
| HvCBFIVd-4 | HvCBF4B | AY785848 | AAX28948 | 678 | 225 | Hordeum vulgare subsp. vulgare (domesticated barley) | Skinner et al. (2006) |
| HvCBFII-5 | HvCBF5 | AY785857 | AAX23700 | 645 | 214 | Hordeum vulgare subsp. vulgare (domesticated barley) | Skinner et al. (2006) |
| HvCBFIIIa-6 | HvCBF6 | EU331996 | ACA29480 | 735 | 244 | Hordeum vulgare subsp. vulgare (domesticated barley) | 251 |
| HvCBFV-7 | HvCBF7 | AY785866 | AAX23706 | 660 | 219 | Hordeum vulgare subsp. vulgare (domesticated barley) | Skinner et al. (2006) |
| HvCBFIVd-9 | HvCBF9 | AY785880 | AAX23709 | 876 | 291 | Hordeum vulgare subsp. vulgare (domesticated barley) | Skinner et al. (2006) |
| HvCBFIIIc-10A | HvCBF10A | AY785884 | AAX23713 | 684 | 227 | Hordeum vulgare subsp. vulgare (domesticated barley) | Skinner et al. (2006) |
| HvCBFIIIc-10B | HvCBF10B | AY785887 | AAX23716 | 723 | 240 | Hordeum vulgare subsp. vulgare (domesticated barley) | Skinner et al. (2006) |
| HvCBFIa-11 | HvCBF11 | AY785892 | AAX23720 | 657 | 218 | Hordeum vulgare subsp. vulgare (domesticated barley) | Skinner et al. (2006) |
| HvCBFIIId-12 | HvCBF12 | DQ095157 | ABA01491 | 735 | 244 | Hordeum vulgare subsp. vulgare (domesticated barley) | Skinner et al. (2005) |
| HvCBFIIIc-13 | HvCBF13 | DQ095158 | ABA01492 | 759 | 252 | Hordeum vulgare subsp. vulgare (domesticated barley) | Skinner et al. (2005) |
| HvCBFIVc-14 | HvCBF14 | DQ095159 | ABA01493 | 645 | 214 | Hordeum vulgare subsp. vulgare (domesticated barley) | Skinner et al. (2005) |
| ScCBFIa-11 | ScCBFIa-11 | EU194240 | ABY59777 | 615 | 204 | Secale cereale (rye) | Campoli et al. (2009) |
| ScCBFII-5 | ScCBFII-5 | EU194241 | ABY59778 | 642 | 213 | Secale cereale (rye) | Campoli et al. (2009) |
| ScCBFIIIa-6 | ScCBFIIIa-6 | EU194242 | ABY59779 | 690 | 229 | Secale cereale (rye) | Campoli et al. (2009) |
| ScCBFIIIc-10 | ScCBFIIIc-10 | EU194243 | ABY59780 | 666 | 221 | Secale cereale (rye) | Campoli et al. (2009) |
| ScCBFIIIc-3A | ScCBFIIIc-3A | EU194244 | ABY59781 | 687 | 228 | Secale cereale (rye) | Campoli et al. (2009) |
| ScCBFIIIc-3B | ScCBFIIIc-3B | EU194245 | ABY59782 | 696 | 231 | Secale cereale (rye) | Campoli et al. (2009) |
| ScCBFIIId-12 | ScCBFIIId-12 | EU194246 | ABY59783 | 699 | 232 | Secale cereale (rye) | Campoli et al. (2009) |
| ScCBFIIId-15 | ScCBFIIId-15 | EU194247 | ABY59784 | 663 | 220 | Secale cereale (rye) | Campoli et al. (2009) |
| ScCBFIIId-19 | ScCBFIIId-19 | EU194248 | ABY59785 | 666 | 221 | Secale cereale (rye) | Campoli et al. (2009) |
| ScCBFIVa-2A | ScCBFIVa-2A | EU194249 | ABY59786 | 582 | 193 | Secale cereale (rye) | Campoli et al. (2009) |
| ScCBFIVa-2B | ScCBFIVa-2B | EU194250 | ABY59787 | 582 | 193 | Secale cereale (rye) | Campoli et al. (2009) |
| TaCBFIa-11 | TaCBFIa-A11 | EF028751 | ABK55354 | 657 | 218 | Triticum aestivum (bread wheat) | Badawi et al. (2007) |
| TaCBFII-5A | TaCBFII-5.1 | EF028752 | ABK55355 | 648 | 215 | Triticum aestivum (bread wheat) | Badawi et al. (2007) |
| TaCBFII-5B | TaCBFII-5.2 | EF028753 | ABK55356 | 660 | 219 | Triticum aestivum (bread wheat) | Badawi et al. (2007) |
| TaCBFII-5C | TaCBFII-5.3 | EF028754 | ABK55357 | 657 | 218 | Triticum aestivum (bread wheat) | Badawi et al. (2007) |
| TaCBFIIIa-6B | TaCBFIIIa-6.1 | EF028755 | ABK55358 | 711 | 236 | Triticum aestivum (bread wheat) | Badawi et al. (2007) |
| TaCBFIIIa-6C | TaCBFIIIa-6.2 | EF028756 | ABK55359 | 729 | 242 | Triticum aestivum (bread wheat) | Badawi et al. (2007) |
| TaCBFIIIa-6A | TaCBFIIIa-D6 | EF028757 | ABK55360 | 717 | 238 | Triticum aestivum (bread wheat) | Badawi et al. (2007) |
| TaCBFIIIc-3B | TaCBFIIIc-3.1 | EF028758 | ABK55361 | 708 | 235 | Triticum aestivum (bread wheat) | Badawi et al. (2007) |
| TaCBFIIIc-3C | TaCBFIIIc-3.2 | EF028759 | ABK55362 | 741 | 246 | Triticum aestivum (bread wheat) | Badawi et al. (2007) |
| TaCBFIIIc-10 | TaCBFIIIc-B10 | EF028761 | ABK55364 | 723 | 240 | Triticum aestivum (bread wheat) | Badawi et al. (2007) |
| TaCBFIIIc-3A | TaCBFIIIc-D3 | EF028760 | ABK55363 | 738 | 245 | Triticum aestivum (bread wheat) | Badawi et al. (2007) |
| TaCBFIIId-12B | TaCBFIIId-12.1 | EF028762 | ABK55365 | 738 | 245 | Triticum aestivum (bread wheat) | Badawi et al. (2007) |
| TaCBFIIId-15B | TaCBFIIId-15.2 | EF028765 | ABK55368 | 726 | 241 | Triticum aestivum (bread wheat) | Badawi et al. (2007) |
| TaCBFIIId-15A | TaCBFIIId-A15 | EF028764 | ABK55367 | 720 | 239 | Triticum aestivum (bread wheat) | Badawi et al. (2007) |
| TaCBFIIId-19B | TaCBFIIId-A19 | EF028766 | ABK55369 | 705 | 234 | Triticum aestivum (bread wheat) | Badawi et al. (2007) |
| TaCBFIIId-12A | TaCBFIIId-B12 | EF028763 | ABK55366 | 738 | 245 | Triticum aestivum (bread wheat) | Badawi et al. (2007) |
| TaCBFIIId-19C | TaCBFIIId-B19 | EF028767 | ABK55370 | 705 | 234 | Triticum aestivum (bread wheat) | Badawi et al. (2007) |
| TaCBFIIId-19A | TaCBFIIId-D19 | EF028768 | ABK55371 | 705 | 234 | Triticum aestivum (bread wheat) | Badawi et al. (2007) |
| TaCBFIVa-2C | TaCBFIVa-2.2 | EF028770 | ABK55373 | 693 | 230 | Triticum aestivum (bread wheat) | Badawi et al. (2007) |
| TaCBFIVa-2A | TaCBFIVa-2.3 | EF028771 | ABK55374 | 579 | 192 | Triticum aestivum (bread wheat) | Badawi et al. (2007) |
| TaCBFIVa-2B | TaCBFIVa-A2 | EF028769 | ABK55372 | 678 | 225 | Triticum aestivum (bread wheat) | Badawi et al. (2007) |
| TaCBFIVb-21A | TaCBFIVb-21.1 | EF028775 | ABK55378 | 609 | 202 | Triticum aestivum (bread wheat) | Badawi et al. (2007) |
| TaCBFIVb-20A | TaCBFIVb-A20 | EF028772 | ABK55375 | 654 | 217 | Triticum aestivum (bread wheat) | Badawi et al. (2007) |
| TaCBFIVb-20B | TaCBFIVb-B20 | EF028773 | ABK55376 | 639 | 212 | Triticum aestivum (bread wheat) | Badawi et al. (2007) |
| TaCBFIVb-20C | TaCBFIVb-D20 | EF028774 | ABK55377 | 639 | 212 | Triticum aestivum (bread wheat) | Badawi et al. (2007) |
| TaCBFIVb-21B | TaCBFIVb-D21 | EF028776 | ABK55379 | 609 | 202 | Triticum aestivum (bread wheat) | Badawi et al. (2007) |
| TaCBFIVc-14C | TaCBFIVc-14.1 | EF028777 | ABK55380 | 639 | 212 | Triticum aestivum (bread wheat) | Badawi et al. (2007) |
| TaCBFIVc-14A | TaCBFIVc-14.3 | EF028779 | ABK55382 | 645 | 214 | Triticum aestivum (bread wheat) | Badawi et al. (2007) |
| TaCBFIVc-14 B | TaCBFIVc-B14 | EF028778 | ABK55381 | 645 | 214 | Triticum aestivum (bread wheat) | Badawi et al. (2007) |
| TaCBFIVd-4C | TaCBFIVd-4.1 | EF028780 | ABK55383 | 669 | 222 | Triticum aestivum (bread wheat) | Badawi et al. (2007) |
| TaCBFIVd-9B | TaCBFIVd-9.1 | EF028782 | ABK55385 | 810 | 269 | Triticum aestivum (bread wheat) | Badawi et al. (2007) |
| TaCBFIVd-22 A | TaCBFIVd-A22 | EF028785 | ABK55388 | 828 | 275 | Triticum aestivum (bread wheat) | Badawi et al. (2007) |
| TaCBFIVd-22 B | TaCBFIVd-B22 | EF028786 | ABK55389 | 873 | 290 | Triticum aestivum (bread wheat) | Badawi et al. (2007) |
| TaCBFIVd-4B | TaCBFIVd-B4 | EF028781 | ABK55384 | 669 | 222 | Triticum aestivum (bread wheat) | Badawi et al. (2007) |
| TaCBFIVd-9A | TaCBFIVd-B9 | EF028783 | ABK55386 | 810 | 269 | Triticum aestivum (bread wheat) | Badawi et al. (2007) |
| TaCBFIVd-22 C | TaCBFIVd-D22 | EF028787 | ABK55390 | 828 | 275 | Triticum aestivum (bread wheat) | Badawi et al. (2007) |
| TaCBFIVd-9C | TaCBFIVd-D9 | EF028784 | ABK55387 | 810 | 269 | Triticum aestivum (bread wheat) | Badawi et al. (2007) |
| TmCBFIIIc-3 | TmCBF3 | AY951949 | AAY32553 | 741 | 246 | Triticum monococcum | Miller et al. (2006) |
| TmCBFII-5 | TmCBF5 | AY951947 | AAY32551 | 633 | 210 | Triticum monococcum | Miller et al. (2006) |
| TmCBFV-7 | TmCBF7 | AY785904 | AAX28965 | 828 | 275 | Triticum monococcum | Skinner et al. (2005) |
| TmCBFIIIc-10 | TmCBF10 | AY951950 | AAY32554 | 720 | 239 | Triticum monococcum | Miller et al. (2006) |
| TmCBFIIId-12 | TmCBF12 | EU076381 | ABW87011 | 744 | 247 | Triticum monococcum | Knox et al. (2008) |
| TmCBFIIIc-13 | TmCBF13 | AY951951 | AAY32555 | 720 | 239 | Triticum monococcum | Miller et al. (2006) |
| TmCBFIVc-14A | TmCBF14 | EU076382 | ABW87012 | 639 | 212 | Triticum monococcum | Knox et al. (2008) |
| TmCBFIVc-14B | TmCBF14 | AY951948 | AAY32552 | 639 | 212 | Triticum monococcum | Miller et al. (2006) |
| TmCBFIIId-15 | TmCBF15 | EU076383 | ABW87013 | 726 | 241 | Triticum monococcum | Knox et al. (2008) |
| TmCBFIIId-16 | TmCBF16 | EU076384 | ABW87014 | 864 | 287 | Triticum monococcum | Knox et al. (2008) |
| TmCBFIIIb-18 | TmCBF18 | AY951946 | AAY32550 | 738 | 245 | Triticum monococcum | Miller et al. (2006) |
Table 4.
Description of ICE genes used in the study
| Proposed gene name | Gene name | Gene accession | Protein accession | Gene fragment | Protein fragment | Species | References |
|---|---|---|---|---|---|---|---|
| AeuICE1 | AeuICE1 | KY931524 | 1149 | 382 | Aegilops umbellulata | This study | |
| AetICE1 | AetICE1 | KY931530 | 1151 | 383 | Aegilops tauschii | This study | |
| AesICE1 | AesICE1 | KY931536 | 1149 | 382 | Aegilops speltoides | This study | |
| AenICE1 | AenICE1 | KY931542 | 1151 | 382 | Aegilops neglecta | This study | |
| TaICE2A | TaICE2A | KY931518 | 1329 | 442 | Triticum aestivum | This study | |
| AeuICE2 | AeuICE2 | KY931523 | 1331 | 443 | Aegilops umbellulata | This study | |
| AetICE2A | AetICE2A | KY931529 | 1331 | 443 | Aegilops tauschii | This study | |
| AesICE2 | AesICE2 | KY931535 | 1331 | 443 | Aegilops speltoides | This study | |
| AenICE2 | AenICE2 | KY931541 | 1331 | 443 | Aegilops neglecta | This study | |
| AetICE2B | AetICE1-like | XM_020312906 | XP_020168495 | 1503 | 500 | Aegilops tauschii | RefSeq, 2017 |
| AtICE1 | AtICE1 | AY195621 | AAP14668 | 1485 | 494 | Arabidopsis thaliana | Chinnusamy et al. (2003) |
| AtICE2 | AtICE2 | KM210288 | AIU34717 | 1335 | 444 | Arabidopsis thaliana | Unpublished, 2014 |
| BdICE1 | BdICE1 | XM_003567379 | XP_003567427 | 1116 | 371 | Brachypodium distachyon (stiff brome) | RefSeq, 2015 |
| HvICE2 | HvICE2 | DQ151537 | ABA25897 | 579 | 192 | Hordeum vulgare subsp. vulgare (domesticated barley) | Skinner et al. (2006) |
| OsICE1 | OsICE1 | XM_015795517 | XP_015651003 | 1239 | 412 | Oryza sativa Japonica Group (Japanese rice) | RefSeq, 2016 |
| SbICE1 | SbICE1-like | XM_002458977 | XP_002459022 | 1131 | 376 | Sorghum bicolor (sorghum) | Paterson et al. (2009) |
| SiICE1 | SiICE1-like | XM_004971094 | XP_004971151 | 1131 | 376 | Setaria italica (foxtail millet) | RefSeq, 2015 |
| TaICE1 | TaICE41 | EU562183 | ACB69501 | 1146 | 381 | Triticum aestivum (bread wheat) | Badawi et al. (2008) |
| TaICE2B | TaICE87 | EU562184 | ACB69502 | 1332 | 443 | Triticum aestivum (bread wheat) | Badawi et al. (2008) |
| ZmICE2 | ZmICE2 | EU974475 | ACG46593 | 1131 | 376 | Zea mays | Alexandrov et al. (2009) |
Table 5.
Description of COR genes used in the study
| Proposed gene name | Gene name | Gene accession | Protein accession | Gene fragment | Protein fragment | Species | References |
|---|---|---|---|---|---|---|---|
| TaCOR1 | TaCOR1 | KY931520 | 515 | 163 | Triticum aestivum | This study | |
| AeuCOR1 | AeuCOR1 | KY931526 | 518 | 164 | Aegilops umbellulata | This study | |
| AetCOR1 | AetCOR1 | KY931532 | 518 | 164 | Aegilops tauschii | This study | |
| AesCOR1 | AesCOR1 | KY931538 | 518 | 164 | Aegilops speltoides | This study | |
| AenCOR1 | AenCOR1 | KY931544 | 518 | 164 | Aegilops neglecta | This study | |
| TaCOR2 | TaCOR2 | KY931519 | 528 | 166 | Triticum aestivum | This study | |
| AeuCOR2 | AeuCOR2 | KY931525 | 525 | 165 | Aegilops umbellulata | This study | |
| AetCOR2 | AetCOR2 | KY931531 | 528 | 166 | Aegilops tauschii | This study | |
| AesCOR2 | AesCOR2 | KY931537 | 528 | 166 | Aegilops speltoides | This study | |
| AenCOR2 | AenCOR2 | KY931543 | 528 | 166 | Aegilops neglecta | This study | |
| AcLEA | AcLEA | JN007028 | AEJ88291 | 630 | 209 | Agropyron cristatum | Unpublished, 2011 |
| AetPHV A1-like | AetPHV A1-like | XM_020294635 | XP_020150224 | 501 | 166 | Aegilops tauschii | RefSeq, 2017 |
| AtCOR15a | AtCOR15a | U01377 | AAB87705 | 420 | 139 | Arabidopsis thaliana | Baker et al. (1994) |
| AtCOR6.6 | AtCOR6.6 | X55053 | CAA38894 | 201 | 66 | Arabidopsis thaliana | Gilmour et al. (1992) |
| EtLEA3 | EtLEA3 | KJ123698 | AIZ11400 | 600 | 199 | Eremopyrum triticeum | Sun et al. (2014) |
| HvES2A | HvES2A | X79466 | CAA55976 | 534 | 177 | Hordeum vulgare | Speulman and Salamini (1995) |
| OsLEA-like | OsLEA-like | NM_001062730 | NP_001056195 | 603 | 200 | Oryza sativa Japonica Group (Japanese rice) | Rice Annotation et al. (2008) |
| PpLEA3 | PpLEA3 | GU947647 | ADF36679 | 552 | 183 | Pogonatherum paniceum | Wang et al. (2012) |
| TaLEA | TaLEA | HQ718763 | ADW41578 | 570 | 189 | Triticum aestivum (bread wheat) | Min et al. (2012) |
| TaSRP3 | TaSRP3 | JQ923472 | AFN10738 | 570 | 189 | Triticum aestivum (bread wheat) | Unpublished, 2012 |
| WCOR615 | WCOR615 | U73217 | AAB18208 | 528 | 175 | Triticum aestivum (bread wheat) | Unpublished, 1996 |
| Wrab17 | Wrab17 | AF255053 | AAF68628 | 501 | 166 | Triticum aestivum (bread wheat) | Tsuda et al. (2000) |
| Wrab19 | Wrab19 | AF255052 | AAF68627 | 540 | 179 | Triticum aestivum (bread wheat) | Tsuda et al. (2000) |
Expression analysis of ICE–CBF–COR genes
To detect the expression of ICE, CBF, COR genes under cold stress, A. tauschii, Aegilops neglecta, winter-hardy wheat M808 and spring wheat T. aestivum L. cultivar China Spring (CS) were placed in growth chambers under long-day conditions (16 h light, 8 h dark) at 25 °C before cold stress. Then, the plants were removed from the growth chambers to a 4 °C cold room, and the leaves were harvested at intervals of 0, 2, 4, 8, 12, 24 h after cold treatment. Total RNA was extracted by TaKaRa MiniBEST Plant RNA Extraction Kit (TaKaRa). First strand cDNA synthesis was derived using PrimeScript™ RT reagent Kit with gDNA Eraser (Takara). Gene-specific primers (Table 2) were designed according to the 3′-untranslated regions (UTR) for qPCR amplification. 18S (AJ315041) was selected as the reference gene from the GenBank database. According to the instructions of the SYBR R Premix Ex Taq™ (Takara), qRT-PCR was performed using an iQ5 Real-Time PCR System (Bio-Rad, USA). The relative expression level of genes were calculated using iQ5 software and the Microsoft Excel program. Mean values and standard errors of three biological replicates were presented in this analysis.
Results
Identification of CBF genes and nomenclature
To enrich the gene family of Triticeae cereal CBF, a total of 10 distinct CBF genes have been isolated from five materials (Aegilops species and T. aestivum) (Table 3) using PCR amplification with degenerate primers (CBF1 and CBF 2, Table 2). The gene names and GenBank accession numbers are summarized in Table 3. Comparative analysis of the cDNA sequence with genomic sequence confirmed that CBF genes are intronless. The identified CBFs have amino acid residue numbers ranging from 223 to 269. BLAST searches using the reported CBF sequences showed that all CBFs displayed strong similarity to the Triticeae cereal CBFs. Following homology comparison and phylogenetic analysis (CBFIa, CBFII, CBFIIIa, CBFIIIb, CBFIIIc, CBFIIId, CBFIVa, CBFIVb, CBFIVc, and CBFIVd) with the established nomenclature of CBFs from H. vulgare, S. cereale, T. aestivum, and T. monococcum (Badawi et al. 2007; Miller et al. 2006; Skinner et al. 2005), we assigned consistent gene numbers to these identical orthologous genes which belong to the same gene subfamily (Table 3). When the same gene number of several genes arose, a letter designating its order trails CBF gene number (e.g. TaCBFIVd-4A).
Phylogenetic analysis of CBF genes in Triticeae
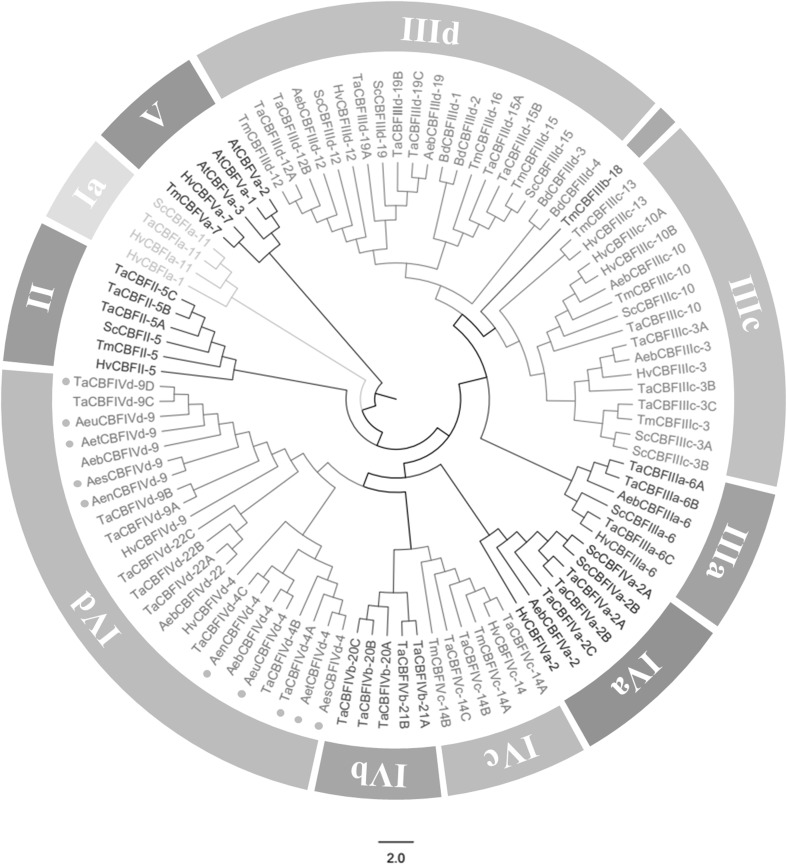
To establish the evolutionary relationship among the different CBFs in Triticeae, phylogenetic analysis was performed using Triticeae CBF homologs (Table 3). The results revealed that these CBF homologs were divided into 11 monophyletic groups (CBFIa, CBFII, CBFIIIa, CBFIIIb, CBFIIIc, CBFIIId, CBFIVa, CBFIVb, CBFIVc, CBFIVd, and CBFV) (Fig. 1). All 10 CBF members isolated in this study belonged to Group IVd, with another 8 CBFs from T. aestivum, 3 CBFs from A. biuncialis and 2 CBFs from H. vulgare. In Group IVd, three subgroups were presented (CBFIVd-9, CBFIVd-22 and CBFIVd-4), and 5 genes isolated in this study belonged to Subgroup CBFIVd-9, while another 5 belonged to CBFIVd-4.
Fig. 1.
Phylogenetic analysis of CBF proteins in Triticeae. The tree was constructed using the neighbor-joining (NJ) method with a Poisson correction model and 1000 bootstrap replicates. The tree was divided into 11 groups, and the solid points before the sequence names indicate the gene isolated in this study
In the phylogenetic analysis, only one group contains a single member (TmCBFIIIb-18), which was designated Group IIIb. Group IVb is the only one that contains CBFs only from T. aestivum. Group IIId is the only one that contains CBFs from the whole five Triticeae subfamilies used in this study. Groups IIIa, IIIc and IVa are the ones that contain CBFs from four Triticeae subfamilies (Aegilops, Hordeum, Triticum, Secale), suggesting that the ancestral CBFIIIa, CBFIIIc and CBFIVa genes were already present before divergence of these subfamilies. Similarly, Groups Ia and II are the ones that contain CBFs from three Triticeae subfamilies (Hordeum, Triticum, Secale), manifesting that the ancestral CBFIa and CBFII genes were already present before divergence of these subfamilies. In addition, 5 CBF homologues belonging to Group V are somewhat distant from those belonging to the rest of the groups, and it may reveal that sequence discrepancies of amino acids play a critical role in the classification reflecting their evolutionary relationships. CBFs from Triticum were found in every group, showing that these CBFs generated prior to the divergence of Triticum and other members of Triticeae. At present, all four genes from B. distachyon were found in the CBFIIId group, indicating that BdCBFs had appeared inferior to the radiation of these subfamilies.
Sequence structure analysis of CBF proteins
To identify some of the structural differences associated with the CBF genes, the genes involved in the subgroup CBFIVd-9 and CBFIVd-4 were selected to perform multiple sequences alignments on the basis of the evolutionary relationship of Triticeae CBFs. Conserved amino acids analysis was performed to highlight conservation of domains by MEME and WebLogo. It was showed that all CBF amino acid sequences contained an AP2 DNA binding domain in the N-terminal and an acidic C-terminal domain (Fig. 2a). The AP2 DNA binding domain directly flanked by two special signature motifs, which were used to distinguish the CBF subfamily from other members of the AP2/ERF superfamily (Fig. 2b). The former is a leader sequence of 16 amino acids (PKRPAGRTKFxExRHP) and the latter is the DSAWR motif, with the exception of AenCBFIVd-4 possessing the first amino acid position of an alanine (A) in place of the more common aspartic acid (D). Analysis of all placed CBFs revealed that three distinct group CBF genes were present with the C-terminal sequence discrepancies (Fig. 2a, c). As shown in the Fig. 2a, c, the Cluster I includes AenCBFIVd-9, AesCBFIVd-9, AetCBFIVd-9 and AeuCBFIVd-9 with the presence of fragment 2, 3, but the absence of fragment 4. The rest of members from the subgroup CBFIVd-9 belonged to the Cluster II with fragment 1, 2, 3 but no fragment 4. All CBF amino acid sequences from the subgroup CBFIVd-4 fall into the Cluster III with the unique fragment 4. Another conserved domain is typically present in the C-terminal, the LWSC/Y motif (Fig. 2c). Members of the subgroup CBFIVd-9 (Cluster I and Cluster II) have a preference for cysteine (C) at the end of this motif, compared to the tyrosine (Y) of the subgroup CBFIVd-4 (Cluster III). The phylogenetic relationship, structural conservation and consistent function of these CBF proteins would reflect the close genetic relationship among Triticeae.
Fig. 2.
Sequence structure analysis of CBF proteins. a Structure schematic of CBF proteins. All CBF amino acid sequences were divided into two parts including the N-terminal and the C-terminal domain. In the N-terminal, all CBF proteins contained an AP2 DNA binding domain, signature 1 (PKRPAGRTKFxExRHP) and signature 2 (DSAWR). In the acidic C-terminal, three distinct groups were present depending on the C-terminal sequence discrepancies (fragment 1, 2, 3, 4), and all CBF proteins contained another conserved domain (LWSC/Y). The length of schemes does not reflect protein size. b Details of the N-terminal structure of CBF proteins. All characteristics were marked in the figture. c Details of the C-terminal structure of CBF proteins. All characteristics were marked in the figture
Identification of ICE genes and phylogeny of ICE genes in Poaceae
As master regulators of CBF expression, ICEs bind to the promoter of CBF genes, and positively induce the expression of CBF genes. We therefore isolated ICEs from the above five contextual materials. Firstly, a partial conserved cDNA fragment of 230 bp was produced. Then 5′ end (650 bp) and 3′ end (360 bp) of ICE were obtained. The assembled whole coding sequences of ICE genes were verified by several clones and sequencing of two independent PCR reactions. We identified 9 potential ICE homologues, and the gene names and GenBank accession numbers were summarized in Table 4. Nomenclature of ICEs was similar to the previous description, but group information of phylogenetic analysis was not displayed in the name. These ICEs encode proteins ranging from 382 to 443. ICE1s shared higher homology with the published TaICE1 (ACB69501), but ICE2s shared lower homology with the TaICE1. Similarly, ICE2s shared higher homology with the published TaICE2B (ACB69502), but ICE1s shared lower homology with the TaICE87. By further analysis, we found that ICE1s and ICE2s had low homology with the full amino acid sequences for less conservative N-terminal sequences. However, these two groups of proteins are characterized by the presence of the identically C-terminal conserved regions. To get rid of the influence of less conservative N-terminal sequences, we retained the conserved C-terminal domain to perform the phylogenetic analysis.
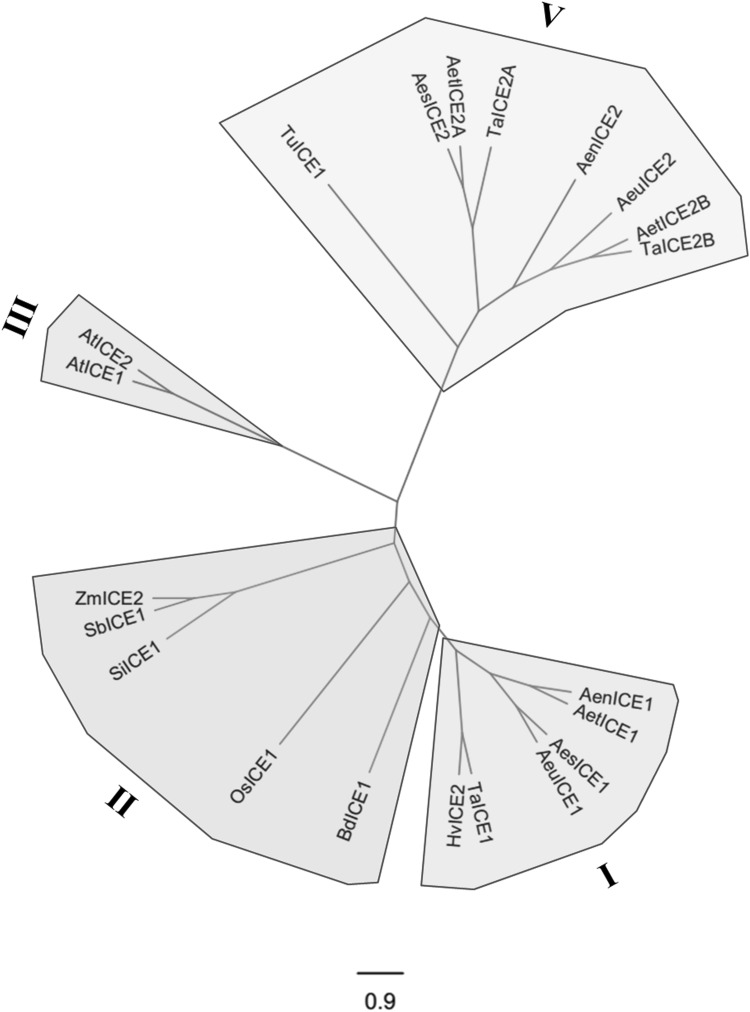
Various ICEs from Poaceae and two ICEs in Arabidopsis were selected to establish the relationship, and these candidate proteins were listed in Table 4. The evolutionary relationship among the ICE proteins showed that these ICEs were divided into 4 groups (Group I, Group II, Group III, Group V) (Fig. 3). Four ICE1s isolated in this study belong to Group I with the published TaICE1 and HvICE2, but five ICE2s isolated in this study belong to Group V with the published TuICE1, AetICE2B and TaICE2B. ICEs from B. distachyon, Oryza sativa, Setaria italica, Sorghum bicolor and Zea mays were classified into the same cluster, Group II. Two ICEs in Arabidopsis fell into Group III. Group I and Group V are the ones that contain ICEs from two Triticeae subfamilies (Aegilops and Triticum), suggesting that the ancestral Group I and Group V genes were already present before divergence of these subfamilies. Interestingly, ICE1s were distantly relative to ICE2s, and the results indicated that ICE1s may have large sequences differences in the C-terminal regions with ICE2s.
Fig. 3.
Phylogenetic analysisof ICE proteins in Poaceae. The tree was constructed using the NJ method with a Poisson correction model and 1000 bootstrap replicates. The tree was divided into 4 groups
Further analysis of the conserved domains was performed. As other ICE-like proteins, these proteins contain a highly conserved basic helix-loop-helix (bHLH) signature domain, which contains the ICE-specific sequence KMDRASILGDAID/EYLKELL (Fig. S1). These structurally different ICE proteins all possess a predicted SUMO binding domain, but members of the Group I have a preference for IKEE, compared to VKEE of the other groups.
Identification of COR genes and phylogeny of COR genes in Poaceae
As CBF target genes or effector molecules, COR genes have a decisive role in enhancing crop cold tolerance. To dissect potential impacts on the downstream cold responsive genes of CBFs, we isolated 10 CORs from the above five contextual materials (Table 5). The details of the gene names were summarized in Table 5. These CORs encode proteins ranging from 163 to 166. COR1s shared higher homology with the published AetPHV A1-like (XM_020294635), Wrab17 (AF255053), TaSRP3 (JQ923472), TaLEA (HQ718763) and HvES2A (X79466). COR2s shared higher homology with the published AetPHV A1-like (XM_020294636) and WCOR615 (U73217).
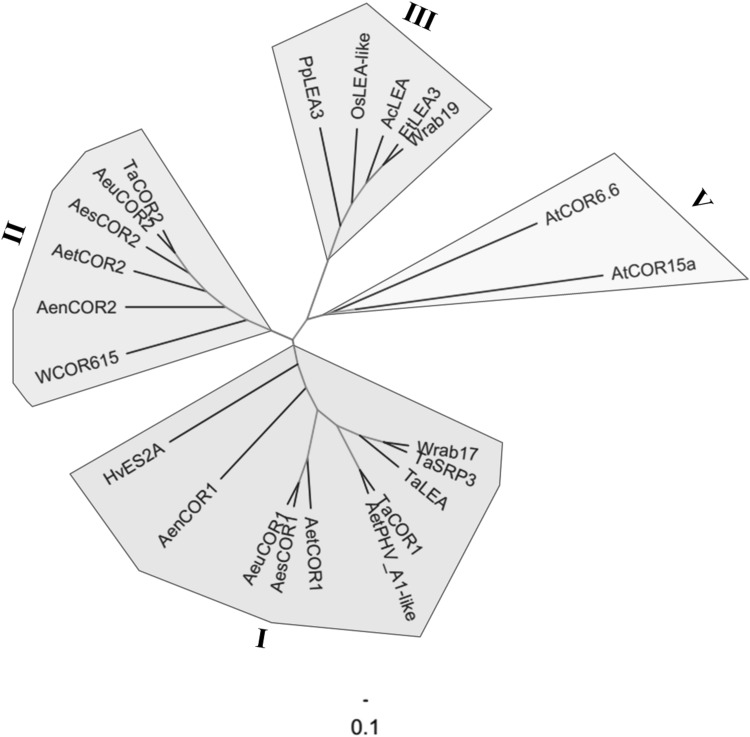
Various kinds of CORs from Poaceae and two CORs in Arabidopsis were chosen to construct the relationship, and 23 total candidate proteins were listed in Table 5. The results indicated that these CORs were assigned into four groups (Group I, Group II, Group III, and Group V) (Fig. 4). Five COR1s deriving from this study belong to Group I with the published Wrab17, TaSRP3, TaLEA, AetPHV A1-like and HvES2A. However, five COR2s deriving from this study and the only WCOR615 fell into the same cluster, Group II. CORs from Pogonatherum paniceum, O. sativa, Agropyron cristatum, Eremopyrum triticeum and Wrab19 from T. aestivum were classified into Group III. The rest of CORs from Arabidopsis belong to Group V. Generally, the evolutionary relationship of CORs showed high multispecies specificity. Exceptionally, unlike other members of COR from T. aestivum which either belong to Group I or Group II, Wrab19 belongs to Group III. The appearance of being distantly related to the core group genes stated that the wheat COR genes continued to evolve following the divergence of species.
Fig. 4.
Phylogenetic analysisof COR proteins in Poaceae. The tree was constructed using the NJ method with a Poisson correction model and 1000 bootstrap replicates. The tree was divided into 4 groups with high multispecies specificity
Sequences variations on proteins from Group I and Group II were performed. Further analysis of amino acid composition showed that these fragments had high hydrophilic amino acid contents, including alanine (A), aspartic acid (D), glutamic acid (E), glycine (G), lysine (K) and threonine (T) (Fig. S2). The maximum of hydrophilic amino acid contents are as high as 70% with the absence of praline (P), cysteine (C) and tryptophan (W).
Expression analysis of ICE, CBF, COR genes
To comprehensively understand the precise mechanism and further details of the ICE–CBF–COR transcriptional cascade in Triticeae, expression analysis of the related genes was assessed. Overall, mRNA accumulation was altered by cold treatment (Fig. 5). The changing pattern of three kinds of genes was similar. Without cold treatment, the basal expression of CBF, ICE, COR genes were flat in all four materials. At the preliminary stage of cold treatment (2 h), these genes were not induced by low temperature. With the continuous cold stress, transcript levels of six genes are slightly higher than that without cold induction. After 4 additional hours of cold treatment, the expression of CBF and ICE genes were significantly elevated in A. tauschii, A. neglecta and winter wheat M808, compared with the spring wheat China Spring (CS). The expression of COR genes after cold treatment for 4 h had no obvious changes in comparison to that for 2 h. Then, the expression of six related genes reached the peak level after 4 °C for 12 h. At this peak point, transcript levels of genes in A. tauschii and A. neglecta are moderately higher than that in winter wheat. Whereas, we noticed that transcript levels of six genes were slightly reduced after longer exposure to cold stress (4 °C for 24 h). In Triticeae, transcript levels of all genes in spring wheat were the lowest, followed by winter wheat and A. neglecta, and transcript levels are the highest in A. tauschii. Together, these results indicate that all genes (ICE, CBF, COR) were cold-induced.
Fig. 5.
Expression analysis of ICE, CBF, COR genes under cold stress. The Y-axis indicates the normalized fold change in expression of each ICE, CBF, COR genes. The X-axis indicates the cold (4 °C) treatments for 0, 2, 4, 8, 12, 24 h. Error bars represented the standard error of the mean, and the asterisks represented the significance
Discussion
Wheat is an interesting model species for studying cold tolerance, however as a typical allohexaploid plant, complex genome has limited the further research on its tolerance. Aegilops, a progenitor of the allohexaploid wheat, is a close wild relative of common wheat with cold hardiness. Aegilops tauschii is D genome donor of common wheat with abundant genetic diversity and many important and excellent genes related to the stress resistance, which has been sequenced with a smaller genome (Jia et al. 2013). Consequently, the Aegilops–Triticum composite group is useful for the study and dissection of molecular, transcriptional, and evolutionary components of cold tolerance in cereals. Low temperature induces the expression of many genes, and identifying and comparing the functional domains of key genes involved in the transcriptional cascade are necessary.
To better understand the exact functions and the evolutionary relationships of gene families involved in ICE–CBF–COR pathway during cold stress in the Poaceae, we initiated a study to identify and characterize CBF, ICE, COR genes from the Aegilops–Triticum composite group and obtained 10 CBFs, 9 ICEs and 10 CORs. Conserved amino acids analysis revealed that all amino acid sequences had typical functional domains. Generally, all CBF amino acid sequences contained an AP2 DNA binding domain and an acidic C-terminal domain (Fig. 2a). The above two features were also generally observed in CBFs from other species, which indicated the possible functional significance. The structural conservation and phylogenetic relationship of the CBF proteins would reflect the close genetic relationship among members of Triticeae. However, there existed differences on one of special signature motifs among different protein members, with ASAWR directly flanked of AP2 DNA binding domain of AenCBFIVd-4 (Fig. 2b). Similarly, sequence discrepancies on the CBFs C-terminal with various combinations of four fragments were obvious (Fig. 2a, c). At the end of C-terminal, members of the subgroup CBFIVd-9 (Cluster I and Cluster II) had a preference for LWSC rather than LWSY of subgroup CBFIVd-4 (Cluster III) (Fig. 2c). As transactivation domain, the structural differences described on C-terminal of CBFs in this study may have significant impacts on transcription activities. With less conservative N-terminal sequences of ICEs, we cloned ICE genes through cloning three fragments respectively (a partial conserved fragment, 5′ end and 3′ end), and obtained 9 assembled ICE sequences. These ICE proteins contain a highly conserved bHLH domain, which includes a DNA binding motif and a dimerization motif. The former contributes to binding to the MYC consensus sequence and activates the expression of target genes (Peng et al. 2014), and the latter functions as the formation of homodimers or heterodimers (Mullen et al. 1994; Xu et al. 2014). The ICE-specific sequence of 19 amino acids is highly conserved, but has only an amino acid difference between orthologous genes (D/E). Sumoylation and ubiquitination motifs were essential elements and present in most of ICE proteins (Miura et al. 2007). Structural differentiation of the predicted SUMO binding domain (IKEE/VKEE) may have impacts on the activity and stability of ICE proteins. As downstream effectors, COR genes in this study putatively encoded hydrophilic proteins, which are different from the hydrophobic protein Wrab19 (pI = 10.3) (Tsuda et al. 2000). It has been revealed that the ICE–CBF–COR transcriptional cascade in cold regulation have differences among various organs in Arabidopsis including pollen, leaves and roots (Chinnusamy et al. 2007; Liao et al. 2014), and we also discovered that structure of the members of the transcriptional networks differ among Triticeae subspecies, as well as notable structural differences are displayed by these proteins may have influences on the functions.
On the basis of previous studies on CBFs in Poaceae, we further established the relationship among the different CBFs in Triticeae, and our results revealed that these CBFs were subdivided into 11 groups with the extra CBFV (Fig. 1). A highly conserved AP2 DNA binding domain were observed among CBFs, which demonstated that members of CBF families in Triticeae should also bind the CRT motif, then promote the expression of target genes. Meanwhile, CBFs belonging to Group V are somewhat distant from other groups with larger sequence discrepancies including AtCBFVa-1, AtCBFVa-2, AtCBFVa-3, HvCBFVa-7, and TmCBFVa-7. CBFs in Arabidopsis belong to Brassicaceae, which is evolutionarily distant from Triticeae and serves as members of outgroup. HvCBFVa-7 and TmCBFVa-7 are highly conserved in AP2 DNA binding domain, but the C-terminal and N-terminal of which are not conserved in comparison to other CBFs. Different ICEs from Poaceae and two ICEs in Arabidopsis were divided into 4 groups (Fig. 3). The analysis on multiple conserved domains of ICE proteins indicated that these ICEs can act on initiating the expression of CBFs through binding the MYC motif. In Arabidopsis, different ICE proteins are not redundant but have distinct functions (Rahman et al. 2014). As it turns out, ICE1s in this study were distantly relative to ICE2s, which could imply that the functions of ICE1s and ICE2s are diverse. Similarly, CORs in Poaceae and two CORs in Arabidopsis were assigned into four groups (Fig. 4). Compared with ICE and CBF proteins, as functional proteins rather than transcription factors, CORs displayed greater sequences diversity, which may shed light on important evolutionary trends coupling the functions. Unlike other CORs with high multispecies specificity, Wrab19 presented a long genetic distance away from the core group members, showing that the wheat COR genes continued to evolve following the divergence of species. Recently, functions of every members involved in this pathway in Arabidopsis were explicit, but size and complexity of these genes families in Triticeae are enormous without further research on concrete function of every member so far, as well as functional redundancy of genes families.
The diversity or complexity of different plants depends not on genes but on various regulatory networks and the research on regulation of gene expression under low temperature have recently received more attention. Cold tolerance of plants, as a complex quantitative trait, is regulated by multi-gene, and improvement of single-gene is difficult to completely enhance cold resistance of plants. As a result, this study implies that the ICE–CBF–COR transcriptional networks are likely present in all higher plants and could be a primary and essential component of cereal cold tolerance. After expose to low temperature, all genes in this study involved in the ICE–CBF–COR pathway were cold induced. Under cold conditions, ICE genes were induced and activated downstream CBF genes through binding the MYC recognition motifs in the promoter, subsequently, the target COR genes were accumulated to a higher level of contents. Previous studies had already noted that expression of the upstream regulatory factors occurred before that of the downstream genes in signaling pathways (Lee et al. 2005). In this study, the expression of COR genes after cold treatment for 4 h had no obvious changes in comparison to that for 2 h, but the expression of ICE and CBF genes were slightly elevated during this period, which is consistent with the aforementioned study.
In conclusion, recent studies focus on urgently seeking to improve plant chilling tolerance, and the ICE–CBF–COR transcriptional cascade play a pivotal role. The results showed that the cold stress signaling pathway is conserved in Triticeae. To be specific, ICE genes were activated under cold stress, then CBF genes are induced, and COR genes are ultimately induced to enhance plants chilling tolerance after the two-step cascade of transcriptional activators (Thomashow et al. 2001). In Arabidopsis, cold-activated OST1 kinase can phosphorylate AtICE1 and modulate chilling tolerance by enhancing ICE1 stability (Ding et al. 2015). PHYA (Benedict et al. 2006) and AtSIZ1 protein (Rahman et al. 2014) also can increase the stability of AtICE1, but JAZ1/4 suppresses transcriptional activity of ICE1 (Hu et al. 2013). Moreover, ICE1/SCRM and ICE2/SCRM2 in Arabidopsis are related to stomatal development (Kanaoka et al. 2008). Thus, as a central component in cold signaling pathway or a convergence point integrating cold and other signaling pathways, how ICE1 elaborately modulated covering couple pathways still remains unclear. Cold tolerance is a complex multigenic trait, we should consider it from many aspects of pathways with an aim of extending our understanding on temperature responses in plant, rather than view it as a single entity. In addition, it is notable that how plants response to cold stimulation is unknown. On the whole, this study enriches members of CBF, ICE, COR genes family in Triticeae, and provide a starting point for future studies on transcriptional regulatory network for improvement of chilling tolerance in crop.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1 Sequence structure analysis of ICE proteins. All ICE proteins contained a highly conserved basic helix-loop-helix (bHLH) signature domain and a SUMO binding domain (IKEE/VKEE). All characteristics were marked in the figture (TIFF 11955 kb)
Fig. S2 Sequence structure analysis of COR proteins (TIFF 10375 kb)
Acknowledgements
We express our gratitude to the anonymous reviewers for helpful comments to improve the manuscript. This work was supported by the Youth Science and Technology Innovation Personnel Training Project in Agricultural Field in Liaoning Province from Science and Technology Department of Liaoning Province (Grant No. 2015038) and the Doctoral Fund of Ministy of Education of China (Grant No. 20132103120003).
Footnotes
Ya’nan Jin and Shanshan Zhai have contributed equally to this paper.
Electronic supplementary material
The online version of this article (10.1007/s12298-017-0495-y) contains supplementary material, which is available to authorized users.
Contributor Information
Zhifu Guo, Phone: +86-24-88487164, Email: zfguo@syau.edu.cn.
Liping Bai, Phone: +86-24-88487163, Email: bailp2003@126.com.
References
- Alexandrov NN, Brover VV, Freidin S, Troukhan ME, Tatarinova TV, Zhang HY, Swaller TJ, Lu YP, Bouck J, Flavell RB, Feldmann KA. Insights into corn genes derived from large-scale cDNA sequencing. Plant Mol Biol. 2009;69:179–194. doi: 10.1007/s11103-008-9415-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An D, Ma Q, Wang H, Yang J, Zhou W, Zhang P. Cassava C-repeat binding factor 1 gene responds to low temperature and enhances cold tolerance when overexpressed in Arabidopsis and cassava. Plant Mol Biol. 2017;94(1–2):109–124. doi: 10.1007/s11103-017-0596-6. [DOI] [PubMed] [Google Scholar]
- Badawi M, Danyluk J, Boucho B, Houde M, Sarhan F. The CBF gene family in hexaploid wheat and its relationship to the phylogenetic complexity of cereal CBFs. Mol Genet Genomics. 2007;277:533–554. doi: 10.1007/s00438-006-0206-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawi M, Reddy YV, Agharbaoui Z, Tominaga Y, Danyluk J, Sarhan F, Houde M. Structure and functional analysis of wheat ICE (inducer of CBF expression) genes. Plant Cell Physiol. 2008;49:1237–1249. doi: 10.1093/pcp/pcn100. [DOI] [PubMed] [Google Scholar]
- Baker SS, Wilhelm KS, Thomashow MF. The 5′-region of Arabidopsis thalianacor15a has cis-acting elements that confer cold-, drought- and ABA-regulated gene expression. Plant Mol Biol. 1994;24(5):701–713. doi: 10.1007/BF00029852. [DOI] [PubMed] [Google Scholar]
- Benedict C, Geisler M, Trygg J, Huner N, Hurry V. Consensus by democracy. Using meta-analyses of microarray and genomic data to model the cold acclimation signaling pathway in Arabidopsis. Plant Physiol. 2006;141:1219–1232. doi: 10.1104/pp.106.083527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campoli C, Matus-Cadiz MA, Pozniak CJ, Cattivelli L, Fowler DB. Comparative expression of Cbf genes in the Triticeae under different acclimation induction temperatures. Mol Genet Genomics. 2009;282(2):141–152. doi: 10.1007/s00438-009-0451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnusamy V, Ohta M, Kanrar S, Lee B, Hong X, Agarwal M, Zhu JK. ICE1: a regulator of cold-induced transcriptome and freezing tolerance in Arabidopsis. Genes Dev. 2003;17:1043–1054. doi: 10.1101/gad.1077503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnusamy V, Zhu J, Zhu JK. Cold stress regulation of gene expression in plants. Trends Plant Sci. 2007;12:444–451. doi: 10.1016/j.tplants.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Choi DW, Rodriguez EM, Close TJ. Barley Cbf3 gene identification, expression pattern, and map location. Plant Physiol. 2002;129:1781–1787. doi: 10.1104/pp.003046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Bosco C, Busconi M, Govoni C, Baldi P, Stanca AM, Crosatti C, Bassi R, Cattivelli L. cor gene expression in barley mutants affected in chloroplast development and photosynthetic electron transport. Plant Physiol. 2003;131:793–802. doi: 10.1104/pp.014530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Li H, Zhang X, Xie Q, Gong Z, Yang S. OST1 kinase modulates freezing tolerance by enhancing ICE1 stability in Arabidopsis. Dev Cell. 2015;32:278–289. doi: 10.1016/j.devcel.2014.12.023. [DOI] [PubMed] [Google Scholar]
- Dong CH, Pei HX. Over-expression of miR397 improves plant tolerance to cold stress in Arabidopsis thaliana. J Plant Biol. 2014;57:209–217. doi: 10.1007/s12374-013-0490-y. [DOI] [Google Scholar]
- Fursova OV, Pogorelko GV, Tarasov VA. Identification of ICE2, a gene involved in cold acclimation which determines freezing tolerance in Arabidopsis thaliana. Gene. 2009;429:98–103. doi: 10.1016/j.gene.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Ganeshan S, Vitamvas P, Fowler DB, Chibbar RN. Quantitative expression analysis of selected COR genes reveals their differential expression in leaf and crown tissues of wheat (Triticum aestivum L.) during an extended low temperature acclimation regimen. J Exp Bot. 2008;59:2393–2402. doi: 10.1093/jxb/ern112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour SJ, Artus NN, Thomashow MF. cDNA sequence analysis and expression of two cold-regulated genes of Arabidopsis thaliana. Plant Mol Biol. 1992;18:13–21. doi: 10.1007/BF00018452. [DOI] [PubMed] [Google Scholar]
- Gilmour SJ, Zarka DG, Stockinger EJ, Salazar MP, Houghton JM, Thomashow MF. Low temperature regulation of the ArabidopsisCBF family of AP2 transcriptional activators as an early step in cold-induced COR gene expression. Plant J. 1998;16:433–442. doi: 10.1046/j.1365-313x.1998.00310.x. [DOI] [PubMed] [Google Scholar]
- Hu Y, Jiang L, Wang F, Yu D. Jasmonate regulates the inducer of CBF expression-C-repeat binding factor/DRE binding factor1 cascade and freezing tolerance in Arabidopsis. Plant Cell. 2013;25:2907–2924. doi: 10.1105/tpc.113.112631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaglo KR, Kleff S, Amundsen KL, Zhang X, Haake V, Zhang JZ, Deits T, Thomashow MF. Components of the Arabidopsis C-repeat/dehydration-responsive element binding factor cold-response pathway are conserved in Brassica napus and other plant species. Plant Physiol. 2001;127:910–917. doi: 10.1104/pp.010548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeknic Z, Pillman KA, Dhillon T, Skinner JS, Veisz O, Cuesta-Marcos A, Hayes PM, Jacobs AK, Chen TH, Stockinger EJ. Hv-CBF2A overexpression in barley accelerates COR gene transcript accumulation and acquisition of freezing tolerance during cold acclimation. Plant Mol Biol. 2014;84:67–82. doi: 10.1007/s11103-013-0119-z. [DOI] [PubMed] [Google Scholar]
- Jia J, Zhao S, Kong X, Li Y, Zhao G, He W, Appels R, Pfeifer M, Tao Y, Zhang X, Jing R, Zhang C, Ma Y, Gao L, Gao C, Spannagl M, Mayer KF, Li D, Pan S, Zheng F, Hu Q, Xia X, Li J, Liang Q, Chen J, Wicker T, Gou C, Kuang H, He G, Luo Y, Keller B, Xia Q, Lu P, Wang J, Zou H, Zhang R, Xu J, Gao J, Middleton C, Quan Z, Liu G, Wang J, International Wheat Genome Sequencing Consortium. Yang H, Liu X, He Z, Mao L, Wang J. Aegilops tauschii draft genome sequence reveals a gene repertoire for wheat adaptation. Nature. 2013;496(7443):91–95. doi: 10.1038/nature12028. [DOI] [PubMed] [Google Scholar]
- Jia Y, Ding Y, Shi Y, Zhang X, Gong Z, Yang S. The cbfs triple mutants reveal the essential functions of CBFs in cold acclimation and allow the definition of CBF regulons in Arabidopsis. New Phytol. 2016;212:345–353. doi: 10.1111/nph.14088. [DOI] [PubMed] [Google Scholar]
- Kanaoka MM, Pillitteri LJ, Fujii H, Yoshida Y, Bogenschutz NL, Takabayashi J, Zhu JK, Torii KU. SCREAM/ICE1 and SCREAM2 specify three cell-state transitional steps leading to Arabidopsis stomatal differentiation. Plant Cell. 2008;20:1775–1785. doi: 10.1105/tpc.108.060848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox AK, Li C, Vagujfalvi A, Galiba G, Stockinger EJ, Dubcovsky J. Identification of candidate CBF genes for the frost tolerance locus Fr-Am2 in Triticum monococcum. Plant Mol Biol. 2008;67:257–270. doi: 10.1007/s11103-008-9316-6. [DOI] [PubMed] [Google Scholar]
- Koag MC, Wilkens S, Fenton RD, Resnik J, Vo E, Close TJ. The K-segment of maize DHN1 mediates binding to anionic phospholipid vesicles and concomitant structural changes. Plant Physiol. 2009;150:1503–1514. doi: 10.1104/pp.109.136697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosova K, Vitamvas P, Prasilova P, Prasil IT. Accumulation of WCS120 and DHN5 proteins in differently frost-tolerant wheat and barley cultivars grown under a broad temperature scale. Biol Plant. 2013;57:105–112. doi: 10.1007/s10535-012-0237-5. [DOI] [Google Scholar]
- Lee H, Xiong L, Ishitani M, Stevenson B, Zhu JK. Cold-regulated gene expression and freezing tolerance in an Arabidopsis thaliana mutant. Plant J. 1999;17:301–308. doi: 10.1046/j.1365-313X.1999.00375.x. [DOI] [PubMed] [Google Scholar]
- Lee BH, Henderson DA, Zhu JK. The Arabidopsis cold-responsive transcriptome and its regulation by ICE1. Plant Cell. 2005;17:3155–3175. doi: 10.1105/tpc.105.035568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Jung JH, Park CM. Inducer of CBF expression 1 integrates cold signals into Flowering Locus C-mediated flowering pathways in Arabidopsis. Plant J. 2015;84:29–40. doi: 10.1111/tpj.12956. [DOI] [PubMed] [Google Scholar]
- Leonardis AMD, Marone D, Mazzucotelli E, Neffar F, Rizza F, Fonzo ND, Cattivelli L, Mastrangelo AM. Durum wheat genes up-regulated in the early phases of cold stress are modulated by drought in a developmental and genotype dependent manner. Plant Sci. 2007;172:1005–1016. doi: 10.1016/j.plantsci.2007.02.002. [DOI] [Google Scholar]
- Li C, Rudi H, Stockinger EJ, Cheng H, Cao M, Fox SE, Mockler TC, Westereng B, Fjellheim S, Rognli OA, Sandve SR. Comparative analyses reveal potential uses of Brachypodium distachyon as a model for cold stress responses in temperate grasses. BMC Plant Biol. 2012;12:65. doi: 10.1186/1471-2229-12-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao P, Chen QF, Chye ML. Transgenic Arabidopsis flowers overexpressing acyl-CoA-binding protein ACBP6 are freezing tolerant. Plant Cell Physiol. 2014;55:1055–1071. doi: 10.1093/pcp/pcu037. [DOI] [PubMed] [Google Scholar]
- Lissarre M, Ohta M, Sato A, Miura K. Cold-responsive gene regulation during cold acclimation in plants. Plant Signal Behav. 2010;5:948–952. doi: 10.4161/psb.5.8.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K, Shinozaki K. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell. 1998;10:1391–1406. doi: 10.1105/tpc.10.8.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Yang L, Yu M, Lai J, Wang C, McNeil D, Zhou M, Yang C. A novel Zea mays ssp. mexicana L. MYC-type ICE-like transcription factor gene ZmmICE1, enhances freezing tolerance in transgenic Arabidopsis thaliana. Plant Physiol Biochem. 2017;113:78–88. doi: 10.1016/j.plaphy.2017.02.002. [DOI] [PubMed] [Google Scholar]
- Ma LF, Zhang JM, Huang GQ, Li Y, Li XB, Zheng Y. Molecular characterization of cotton C-repeat/dehydration-responsive element binding factor genes that are involved in response to cold stress. Mol Biol Rep. 2014;41:4369–4379. doi: 10.1007/s11033-014-3308-1. [DOI] [PubMed] [Google Scholar]
- Masoomi-Aladizgeh F, Aalami A, Esfahani M, Aghaei MJ, Mozaffari K. Identification of CBF14 and NAC2 genes in Aegilops tauschii associated with resistance to freezing stress. Appl Biochem Biotechnol. 2015;176:1059–1070. doi: 10.1007/s12010-015-1629-8. [DOI] [PubMed] [Google Scholar]
- Miller AK, Galiba G, Dubcovsky J. A cluster of 11 CBF transcription factors is located at the frost tolerance locus Fr-Am2 in Triticum monococcum. Mol Genet Genomics. 2006;275:193–203. doi: 10.1007/s00438-005-0076-6. [DOI] [PubMed] [Google Scholar]
- Min DH, Zhang XH, Xu ZS, Zhao Y, Chen Y, Li LC, Chen M, Ma YZ. Induction kinetics of a novel stress-related LEA Gene in wheat. Plant Mol Biol Rep. 2012;30(6):1313–1321. doi: 10.1007/s11105-012-0446-2. [DOI] [Google Scholar]
- Miura K, Jin JB, Lee J, Yoo CY, Stirm V, Miura T, Ashworth EN, Bressan RA, Yun DJ, Hasegawa PM. SIZ1-mediated sumoylation of ICE1 controls CBF3/DREB1A expression and freezing tolerance in Arabidopsis. Plant Cell. 2007;19:1403–1414. doi: 10.1105/tpc.106.048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe JG, McGovern C, Lasky JR, Grogan K, Beck J, McKay JK. Adaptation to warmer climates by parallel functional evolution of CBF genes in Arabidopsis thaliana. Mol Ecol. 2016;25:3632–3644. doi: 10.1111/mec.13711. [DOI] [PubMed] [Google Scholar]
- Mullen MA, Wang H, Wilcox K, Herman T. Characterization of a Max:DNA complex by cross-linking to photoactive oligonucleotides. DNA Cell Biol. 1994;13:521–530. doi: 10.1089/dna.1994.13.521. [DOI] [PubMed] [Google Scholar]
- Nordin K, Vahala T, Palva ET. Differential expression of two related, low-temperature-induced genes in Arabidopsis thaliana (L.) Heynh. Plant Mol Biol. 1993;21:641–653. doi: 10.1007/BF00014547. [DOI] [PubMed] [Google Scholar]
- Novillo F, Alonso JM, Ecker JR, Salinas J. CBF2/DREB1C is a negative regulator of CBF1/DREB1B and CBF3/DREB1A expression and plays a central role in stress tolerance in Arabidopsis. Proc Natl Acad Sci USA. 2004;101:3985–3990. doi: 10.1073/pnas.0303029101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novillo F, Medina J, Salinas J. Arabidopsis CBF1 and CBF3 have a different function than CBF2 in cold acclimation and define different gene classes in the CBF regulon. Proc Natl Acad Sci USA. 2007;104:21002–21007. doi: 10.1073/pnas.0705639105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Lee CM, Doherty CJ, Gilmour SJ, Kim Y, Thomashow MF. Regulation of the Arabidopsis CBF regulon by a complex low-temperature regulatory network. Plant J. 2015;82:193–207. doi: 10.1111/tpj.12796. [DOI] [PubMed] [Google Scholar]
- Paterson AH, Paterson AH, Bowers JE, Bruggmann R, Dubchak I, Grimwood J, Gundlach H, Haberer G, Hellsten U, Mitros T, Poliakov A, Schmutz J, Spannagl M, Tang H, Wang X, Wicker T, Bharti AK, Chapman J, Feltus FA, Gowik U, Grigoriev IV, Lyons E, Maher CA, Martis M, Narechania A, Otillar RP, Penning BW, Salamov AA, Wang Y, Zhang L, Carpita NC, Freeling M, Gingle AR, Hash CT, Keller B, Klein P, Kresovich S, McCann MC, Ming R, Peterson DG, Mehboob-ur-Rahman Ware D, Westhoff P, Mayer KF, Messing J, Rokhsar DS. The Sorghum bicolor genome and the diversification of grasses. Nature. 2009;457:551–556. doi: 10.1038/nature07723. [DOI] [PubMed] [Google Scholar]
- Peng PH, Lin CH, Tsai HW, Lin TY. Cold response in Phalaenopsis aphrodite and characterization of PaCBF1 and PaICE1. Plant Cell Physiol. 2014;55:1623–1635. doi: 10.1093/pcp/pcu093. [DOI] [PubMed] [Google Scholar]
- Qin F, Shinozaki K, Yamaguchi-Shinozaki K. Achievements and challenges in understanding plant abiotic stress responses and tolerance. Plant Cell Physiol. 2011;52:1569–1582. doi: 10.1093/pcp/pcr106. [DOI] [PubMed] [Google Scholar]
- Rahman MA, Moody MA, Nassuth A. Grape contains 4 ICE genes whose expression includes alternative polyadenylation, leading to transcripts encoding at least 7 different ICE proteins. Environ Exp Bot. 2014;106:70–78. doi: 10.1016/j.envexpbot.2014.01.003. [DOI] [Google Scholar]
- Rice Annotation P, et al. The Rice Annotation Project Database (RAP-DB): 2008 update. Nucleic Acids Res. 2008;36:D1028–D1033. doi: 10.1093/nar/gkm978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu JY, Hong SY, Jo SH, Woo JC, Lee S, Park CM. Molecular and functional characterization of cold-responsive C-repeat binding factors from Brachypodium distachyon. BMC Plant Biol. 2014;14:15. doi: 10.1186/1471-2229-14-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma Y, Liu Q, Dubouzet JG, Abe H, Shinozaki K, Yamaguchi-Shinozaki K. DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold-inducible gene expression. Biochem Biophys Res Commun. 2002;290:998–1009. doi: 10.1006/bbrc.2001.6299. [DOI] [PubMed] [Google Scholar]
- Shi Y, Ding Y, Yang S. Cold signal transduction and its interplay with phytohormones during cold acclimation. Plant Cell Physiol. 2015;56:7–15. doi: 10.1093/pcp/pcu115. [DOI] [PubMed] [Google Scholar]
- Siddiqua M, Nassuth A. Vitis CBF1 and Vitis CBF4 differ in their effect on Arabidopsis abiotic stress tolerance, development and gene expression. Plant, Cell Environ. 2011;34:1345–1359. doi: 10.1111/j.1365-3040.2011.02334.x. [DOI] [PubMed] [Google Scholar]
- Skinner JS, von Zitzewitz J, Szucs P, Marquez-Cedillo L, Filichkin T, Amundsen K, Stockinger EJ, Thomashow MF, Chen TH, Hayes PM. Structural, functional, and phylogenetic characterization of a large CBF gene family in barley. Plant Mol Biol. 2005;59:533–551. doi: 10.1007/s11103-005-2498-2. [DOI] [PubMed] [Google Scholar]
- Skinner JS, Szucs P, von Zitzewitz J, Marquez-Cedillo L, Filichkin T, Stockinger EJ, Thomashow MF, Chen TH, Hayes PM. Mapping of barley homologs to genes that regulate low temperature tolerance in Arabidopsis. Theor Appl Genet. 2006;112:832–842. doi: 10.1007/s00122-005-0185-y. [DOI] [PubMed] [Google Scholar]
- Soltesz A, Smedley M, Vashegyi I, Galiba G, Harwood W, Vagujfalvi A. Transgenic barley lines prove the involvement of TaCBF14 and TaCBF15 in the cold acclimation process and in frost tolerance. J Exp Bot. 2013;64:1849–1862. doi: 10.1093/jxb/ert050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speulman E, Salamini F. GA3-regulated cDNAs from Hordeum vulgare leaves. Plant Mol Biol. 1995;28(5):915–926. doi: 10.1007/BF00042075. [DOI] [PubMed] [Google Scholar]
- Stockinger EJ, Gilmour SJ, Thomashow MF. Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc Natl Acad Sci USA. 1997;94:1035–1040. doi: 10.1073/pnas.94.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Huang X, Zhu J, Lv X, Zho H, An X, Li C, Zhou X. Cloning and bioinformatics analysis of LEA3 gene from Eremopyrum triticeum. Shihezi Daxue Xuebao. 2014;3:313–318. [Google Scholar]
- Talanova VV, Titov AF, Repkina NS, Topchieva LV. Cold-responsive COR/LEA genes participate in the response of wheat plants to heavy metals stress. Dokl Biol Sci. 2013;448:28–31. doi: 10.1134/S0012496613010080. [DOI] [PubMed] [Google Scholar]
- Thomashow MF. Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:571–599. doi: 10.1146/annurev.arplant.50.1.571. [DOI] [PubMed] [Google Scholar]
- Thomashow MF, Gilmour SJ, Stockinger EJ, Jaglo-Ottosen KR, Zarka DG. Role of the Arabidopsis CBF transcriptional activators in cold acclimation. Physiol Plant. 2001;112:171–175. doi: 10.1034/j.1399-3054.2001.1120204.x. [DOI] [Google Scholar]
- Tsuda K, Tsvetanov S, Takumi S, Mori N, Atanassov A, Nakamura C. New members of a cold-responsive group-3 Lea/Rab-related Cor gene family from common wheat (Triticum aestivum L.) Genes Genet Syst. 2000;75:179–188. doi: 10.1266/ggs.75.179. [DOI] [PubMed] [Google Scholar]
- Tsvetanov S, Ohno R, Tsuda K, Takumi S, Mori N, Atanassov A, Nakamura C. A cold-responsive wheat (Triticum aestivum L.) gene wcor14 identified in a winter-hardy cultivar ‘Mironovska 808’. Genes Genet Syst. 2000;75:49–57. doi: 10.1266/ggs.75.49. [DOI] [PubMed] [Google Scholar]
- Uemura M, Gilmour SJ, Thomashow MF, Steponkus PL. Effects of COR6.6 and COR15am polypeptides encoded by COR (cold-regulated) genes of Arabidopsis thaliana on the freeze-induced fusion and leakage of liposomes. Plant Physiol. 1996;111:313–327. doi: 10.1104/pp.111.1.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WG, Li R, Liu B, Li L, Wang SH, Chen F. Alternatively spliced transcripts of group 3 late embryogenesis abundant protein from Pogonatherum paniceum confer different abiotic stress tolerance in Escherichia coli. J Plant Physiol. 2012;169(15):1559–1564. doi: 10.1016/j.jplph.2012.06.017. [DOI] [PubMed] [Google Scholar]
- Welin BV, Olson A, Palva ET. Structure and organization of two closely related low-temperature-induced dhn/lea/rab-like genes in Arabidopsis thaliana L. Heynh. Plant Mol Biol. 1995;29:391–395. doi: 10.1007/BF00043662. [DOI] [PubMed] [Google Scholar]
- Wingler A. Comparison of signaling interactions determining annual and perennial plant growth in response to low temperature. Front Plant Sci. 2014;5:794. doi: 10.3389/fpls.2014.00794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Zhang N, Jiao Y, Li R, Xiao D, Wang Z. The grapevine basic helix-loop-helix (bHLH) transcription factor positively modulates CBF-pathway and confers tolerance to cold-stress in Arabidopsis. Mol Biol Rep. 2014;41:5329–5342. doi: 10.1007/s11033-014-3404-2. [DOI] [PubMed] [Google Scholar]
- Xue GP. Characterisation of the DNA-binding profile of barley HvCBF1 using an enzymatic method for rapid, quantitative and high-throughput analysis of the DNA-binding activity. Nucleic Acids Res. 2002;30(15):e77. doi: 10.1093/nar/gnf076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue GP. The DNA-binding activity of an AP2 transcriptional activator HvCBF2 involved in regulation of low-temperature responsive genes in barley is modulated by temperature. Plant J. 2003;33(2):373–383. doi: 10.1046/j.1365-313X.2003.01630.x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K, Shinozaki K. A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. Plant Cell. 1994;6:251–264. doi: 10.1105/tpc.6.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Zhu JK. The broad roles of CBF genes: from development to abiotic stress. Plant Signal Behav. 2016;11:e1215794. doi: 10.1080/15592324.2016.1215794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Zhang Z, Xie S, Si T, Li Y, Zhu JK. Mutational evidence for the critical role of CBF transcription factors in cold acclimation in Arabidopsis. Plant Physiol. 2016;171:2744–2759. doi: 10.1104/pp.16.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou MQ, Shen C, Wu LH, Tang KX, Lin J. CBF-dependent signaling pathway: a key responder to low temperature stress in plants. Crit Rev Biotechnol. 2011;31:186–192. doi: 10.3109/07388551.2010.505910. [DOI] [PubMed] [Google Scholar]
- Zhu H, Gao W, Shi YF, Zhang XJ. The CCAAT-binding factor CBF/NF-Y regulates the human acetylcholinesterase promoter activity during calcium ionophore A23187-induced cell apoptosis. Biochim Biophys Acta. 2007;1770:1475–1482. doi: 10.1016/j.bbagen.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Zhuang L, Yuan X, Chen Y, Xu B, Yang Z, Huang B. PpCBF3 from cold-tolerant kentucky bluegrass involved in freezing tolerance associated with up-regulation of cold-related genes in transgenic Arabidopsis thaliana. PLoS ONE. 2015;10(7):e0132928. doi: 10.1371/journal.pone.0132928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Sequence structure analysis of ICE proteins. All ICE proteins contained a highly conserved basic helix-loop-helix (bHLH) signature domain and a SUMO binding domain (IKEE/VKEE). All characteristics were marked in the figture (TIFF 11955 kb)
Fig. S2 Sequence structure analysis of COR proteins (TIFF 10375 kb)