Abstract
The influence of 2,4-dichlorophenoxyacetic acid (2,4-D), benzyladenine (BA), and thidiazuron (TDZ) on direct rhizome induction and shoot formation from rhizome explants of Cymbidium goeringii was explored. Rhizome segments obtained from in vitro seed cultures of C. goeringii were placed on Murashige and Skoog (MS) medium incorporated with 5, 10, 20, or 40 µM 2,4-D and 1, 2, 4, or 8 µM BA or TDZ alone or in combination with 20 µM 2,4-D. The explants developed only rhizomes on MS medium with or without 2,4-D. The highest percent of rhizome formation (100%) was obtained on MS medium incorporated with 20 μM of 2,4-D. The morphology and number of rhizomes varied with the level of 2,4-D in the medium. Direct adventitious shoot formation was achieved on medium incorporated with BA or TDZ. The adventitious shoots produced per explant significantly increased with the supplementation of 2,4-D to cytokinin-containing medium. The highest mean of 21.8 ± 1.8 shoot buds per rhizome segment was obtained in medium fortified with 20 μM 2,4-D and 2 μM TDZ. The greatest percent of root induction (100%) and the mean of 5.3 ± 1.1 roots per shoot were achieved on ½ MS medium incorporated with 2 μM of α-naphthaleneacetic acid. About 97% of the in vitro-produced plantlets acclimatized in the greenhouse. An efficient in vitro propagation protocol was thus developed for C. goeringii using rhizome explants.
Keywords: 2,4-D; Benzyladenine; Direct shoot regeneration; Orchidaceae; Rhizome explants; Thidiazuron
Introduction
The genus Cymbidium (Orchidaceae) comprises of 52 species of both ornamental and medicinal importance. Cymbidium goeringii Reichenbach fil., commonly called as spring orchid, is distributed across the Himalayas and in China, Taiwan, Japan, and Korea. C. goeringii has been widely cultivated for its beautiful and fragrant flowers. In the recent times, C. goeringii has disappeared in the wild because of habitat deterioration. Moreover, these plants are over collected by plant sellers and enthusiasts (Chung and Chung 1999). C. goeringii was designated as an endangered species (Wu et al. 2010; Tsuji and Kato 2010). Thus, efficient method for mass propagation is required to meet the growing demand and conservation of this species. Although it is propagated by seeds, its germination rate is extremely low under natural conditions, and fungal infection is required for the germination of seeds (Kang and Yang 2003). Furthermore, the multiplication rate of this species by a sexual method of conventional division is also very low under natural conditions (Paek and Kozai 1998). In vitro culture technique has therefore been exploited for the extensive commercial propagation and conservation of rare orchids (Koirala et al. 2013; Devi et al. 2015; Bhattacharyya et al. 2016; Bembemcha et al. 2016; Diengdoh et al. 2017; Jiang et al. 2017; Kim et al. 2017). However, the Asiatic temperate orchids such as Cymbidium has received less attention for commercial propagation using in vitro culture methods than other orchid species because of the rareness and shortage of plant materials (Chugh et al. 2009).
In vitro propagation methods have been established using seeds, flower buds, rhizomes, and shoot tips explants of C. goeringii (Ueda and Torikata 1969; Hasegawa and Goi 1987; Shimasaki and Uemoto 1991; Kang and Yang 2003). In most of these studies, rhizome segments obtained from in vitro cultures of flower buds, shoot tips, and seeds were used for regeneration of temperate Cymbidium. However, species, cultivars, culture media, plant growth regulators (PGRs), and culture environment affected the shoot regeneration ability of rhizome explants (Paek and Kozai 1998). Previous reports have shown that supplementation of cytokinin to the culture media alone or in combination with auxin required for shoot formation in vitro from rhizome explants of C. goeringii (Ueda and Torikata 1969; Shimasaki and Uemoto 1991). The authors studied the effect of N6-benzyladenine (BA), kinetin (Kin) and α-naphthaleneacetic acid (NAA). However, none of these authors used thidiazuron (TDZ) and 2,4-dichlorophenoxyacetic acid (2,4-D) for micropropagation.
The positive effect of TDZ in callus induction, shoot regeneration, somatic embryo formation and protocorm-like bodies (PLBs) induction have been reported in orchids, including in Cymbidium (Nayak et al. 1997; Chang and Chang 2000a; Huan et al. 2004; Chugh et al. 2009; Roy et al. 2012). In the in vitro cultures of orchids, 2,4-D has been shown to play a significant role in callus induction, seed germination, rhizome induction, and somatic embryogenesis (Novak et al. 2014). The number of shoots produced per rhizome segment (0.8–3.0) was very few (Ueda and Torikata 1969; Shimasaki and Uemoto 1991). Therefore, an efficient in vitro propagation method is needed for the mass propagation of C. goeringii. The aim of this study was to develop an efficient method for in vitro propagation of C. goeringii using rhizome explants.
Materials and methods
Mature seed (green-capsules) collected at 180–210 days after self-pollination from greenhouse-grown plants of C. goeringii were surface disinfected with 70% (v/v) ethanol solution for 60 s, washing three times with sterile distilled water (sDH2O), followed by 1.0% (v/v) sodium hypochlorite solution for 10 min, and then washing four times with sDH2O. The seeds obtained from the capsules were placed in culture bottle containing Murashige and Skoog (1962, MS) medium with 0.2% (w/v) activated charcoal (AC), 0.2% (v/v) coconut water, 3% (w/v) sucrose, and 0.8% (w/v) plant agar (Duchefa Biochemie, The Netherlands). The pH of the medium was adjusted to 5.6, 120 mL of the MS medium was dispensed in 500-mL culture bottles, and autoclaved. The culture bottles were kept at 22 ± 2 °C under a 16-h photoperiod (10 µmol m−2 s−1). Seeds germinated within 8–12 weeks to develop PLBs (green) were transferred to the same fresh medium (MS) for rhizome development. After 8 weeks of culturing, the rhizomes (0.5 cm) were transferred to MS medium incorporated with 0.2% (w/v) AC, 3% (w/v) sucrose, and 4 µM NAA and cultured for 4–6 months.
Rhizome (2.0–3.0-cm long) explants were placed in culture bottles containing 120 mL of MS medium incorporated with 0.2% (w/v) AC; 3% (w/v) sucrose; 5, 10, 20, or 40 µM 2,4-D; and 1, 2, 4, or 8 µM BA or TDZ with or without 20 µM 2,4-D. The MS medium devoid of PGR served as control. The experiment was conducted in triplicate with 25 rhizomes for each treatment. The cultures were kept at 22 ± 2 °C for a 16-h photoperiod (45 µmol m−2 s−1). The number of rhizomes and shoots were recorded after 12 weeks of culturing.
The in vitro-produced shoot buds were cultured on ½ MS medium incorporated with 0.2% (w/v) AC; 3% (w/v) sucrose; and 0, 1, 2, or 4 µM NAA for root induction. The experiment was conducted in triplicate with 50 shoot buds for each treatment. The frequency of root induction and the number of roots were recorded after 12 weeks of culturing. The rooted shoots were subcultured onto the same medium (fresh). After 12 weeks, well-developed plantlets (5–6-cm tall) were transplanted into 72-cell plug trays containing sphagnum moss, irrigated at 2-day intervals, and maintained in the greenhouse at 22 ± 2 °C for a 16-h photoperiod (45 µmol m−2 s−1) under a relative humidity of 70–80%. After 7 days, plants were fertigated with Hyponex (N–P–K; 20–20–20) solution, and the survival was recorded after 6 weeks. Data were subjected to Duncan’s multiple range test and analysis of variance (ANOVA). The percent values were transformed using arcsine square root (√P) to normalize the error distribution before ANOVA (Compton 1994).
Results
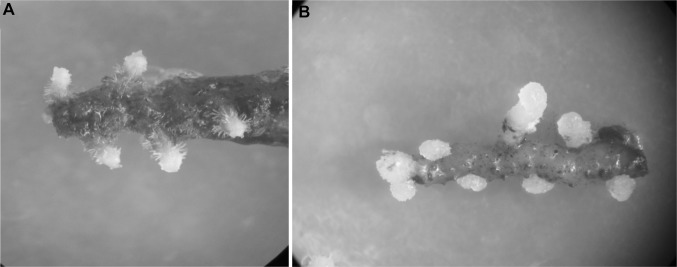
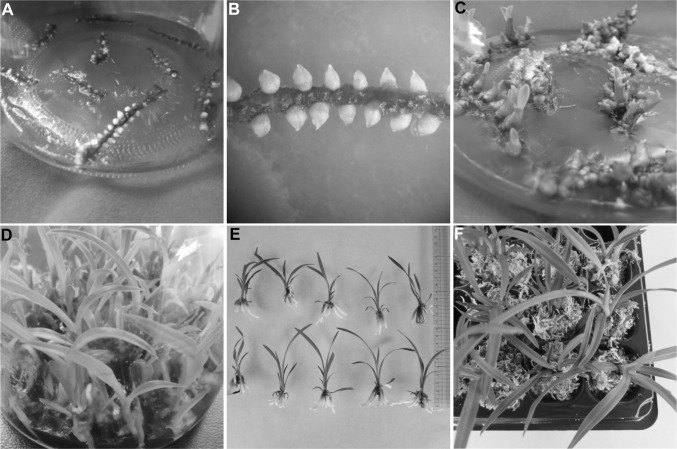
The rhizome segments did not develop shoots on MS medium incorporated with 2,4-D after 12-weeks of culturing. However, rhizomes were formed from the explants within 3-week of incubation. The percent of rhizome induction improved as the level of 2,4-D in the culture medium increased from 0 to 20 μM and then declined with further increase of 2,4-D level (40 μM). The highest percent of rhizome formation (100%) was achieved on MS medium incorporated with 20 μM of 2,4-D. The morphology and number of rhizomes varied with the level of 2,4-D in the MS medium (Table 1). In 5 or 10 μM concentration of 2,4-D, the developed rhizomes demonstrated chlorophyll synthesis and several trichomes on their surfaces (Fig. 1a). The culture medium containing 20-40 μM of 2,4-D produced a creamy white rhizome without trichomes (Fig. 1b). Differences were noted in the number of induced rhizomes on MS medium incorporated with various levels of 2,4-D (Fig. 2a, b). The maximum number of rhizomes (16.6 ± 2.6) was produced on MS medium incorporated with 5 μM of 2,4-D. The induced rhizomes were subcultured on PGR-free MS medium for further growth. The shoots developed from the tip of the rhizomes after 6 months of incubation (Fig. 2c, data not shown).
Table 1.
Effect of 2,4-D on rhizome and shoot induction from rhizome explants of C. goeringii
| 2,4-D (µM) | Rhizome induction (%) | No. of rhizomes/explant | No. of shoot buds/explant |
|---|---|---|---|
| 0 | 68.1 ± 3.6c | 4.4 ± 1.7d | 0.0 ± 0.0 |
| 5 | 85.6 ± 3.5b | 16.6 ± 2.6a | 0.0 ± 0.0 |
| 10 | 98.3 ± 1.6a | 12.5 ± 3.1b | 0.0 ± 0.0 |
| 20 | 100 ± 0.0a | 8.8 ± 1.2c | 0.0 ± 0.0 |
| 40 | 54.6 ± 5.3d | 7.7 ± 1.3c | 0.0 ± 0.0 |
Value (mean ± standard error) within a column followed by the same letters are not significantly different at p ≤ 0.05
Fig. 1.
Rhizome induction from seed-derived rhizome explants of C. goeringii. a Induction of rhizome on MS medium containing 5 µM 2,4-D after 8 weeks; b induction of rhizome on MS medium containing 20 µM 2,4-D after 8 weeks
Fig. 2.
Influence of 2,4-D on rhizome formation C. goeringii. a Induction of rhizome on MS medium after 4 months; b induction of rhizome on MS medium containing 5 µM 2,4-D after 4 months; c shoots developed from the tip of the rhizomes in hormone-free MS medium after 6 months
When the medium was incorporated with BA or TDZ, the rhizome explants developed both direct adventitious shoot buds and rhizomes within 4 weeks of culturing. However, the culture medium containing BA in low concentration (1 or 2 μM) did not develop shoot buds, but the explants produced rhizomes. Whereas, the MS medium containing BA in high concentration produced both adventitious shoot buds and rhizomes (Table 2). Differences were noted in the number of shoot buds per rhizome on the culture medium supplemented with 1-8 μM of TDZ (Table 2). The maximum of 7.2 ± 1.2 shoot buds per rhizome was obtained on MS medium incorporated with 2 μM of TDZ. However, the mean number of shoot buds significantly decreased with increasing level of TDZ (4.0 or 8.0 μM) in MS medium. The supplementation of auxin (2,4-D) to the MS medium containing cytokinin (BA, TDZ) increased the shoot buds number per rhizome segment. The combination of BA and 2,4-D maximized the shoot bud number as compared to BA alone. A large number of shoot buds (8.9 ± 1.7) was produced per rhizome segment on MS medium incorporated with 20 μM of 2,4-D and 8 μM of BA (Table 2). Similarly, the presence of 2,4-D and TDZ increased the yield of shoot buds (Fig. 3a–c). The highest mean of 21.8 ± 1.8 shoots per rhizome was achieved on MS medium fortified with 2 μM of TDZ and 20 μM of 2,4-D (Table 2).
Table 2.
Effects of BA, TDZ and 2,4-D on rhizome and shoot induction from rhizome explants of C. goeringii
| PGRs (µM) | No. of rhizomes/explant | No. of shoot buds/explant | ||
|---|---|---|---|---|
| BA | TDZ | 2,4-D | ||
| 0 | 0 | 0 | 4.4 ± 1.7a | 0.0 ± 0.0h |
| 1 | 0 | 0 | 4.1 ± 1.4a | 0.0 ± 0.0h |
| 2 | 0 | 0 | 3.2 ± 1.0a | 0.0 ± 0.0h |
| 4 | 0 | 0 | 3.0 ± 0.7ab | 1.8 ± 0.9g |
| 8 | 0 | 0 | 1.5 ± 0.5c | 2.9 ± 0.7f |
| 1 | 0 | 20 | 1.8 ± 0.8bc | 0.0 ± 0.0h |
| 2 | 0 | 20 | 0.0 ± 0.0d | 4.5 ± 0.9e |
| 4 | 0 | 20 | 0.0 ± 0.0d | 6.5 ± 1.5d |
| 8 | 0 | 20 | 0.0 ± 0.0d | 8.9 ± 1.7c |
| 0 | 1 | 0 | 3.6 ± 1.2a | 3.1 ± 0.9f |
| 0 | 2 | 0 | 2.3 ± 0.9b | 7.2 ± 1.2d |
| 0 | 4 | 0 | 1.9 ± 0.7bc | 4.0 ± 1.6e |
| 0 | 8 | 0 | 0.0 ± 0.0d | 3.6 ± 1.1ef |
| 0 | 1 | 20 | 0.0 ± 0.0d | 10.5 ± 1.8c |
| 0 | 2 | 20 | 0.0 ± 0.0d | 21.8 ± 1.8a |
| 0 | 4 | 20 | 0.0 ± 0.0d | 15.0 ± 2.0b |
| 0 | 8 | 20 | 0.0 ± 0.0d | 9.2 ± 1.3c |
Value (mean ± standard error) within a column followed by the same letters are not significantly different at p ≤ 0.05
Fig. 3.
Micropropagation of C. goeringii. a Induction of direct adventitious shoot buds in MS medium containing 20 µM 2,4-D and 2 µM TDZ after 4 weeks; b a closer view of shoot buds formation from seed-derived rhizome explant; c shoot bud development in MS medium containing 20 µM 2,4-D and 2 µM TDZ after 12 weeks; d in vitro-regenerated shoots developed roots in half-strength MS medium fortified with 2 µM of NAA after 12 weeks; e rooted shoots after 24 weeks of culture in half-strength MS medium containing 2 µM of NAA; f acclimatized plantlets after 6 weeks of cultivation in the greenhouse
The regenerated shoot buds (obtained from MS + 8 μM BA + 20 μM 2,4-D) developed roots in ½ MS medium incorporated with 0–4 µM of NAA within 4 weeks of culturing. However, significant differences were noted in the percent of root induction and the number of roots among the treatments (Table 3). The percent of root induction increased with increasing level of NAA in the MS medium. The greatest percent of root induction (100%) and the mean of 5.3 ± 1.1 roots per shoot were obtained on ½ MS medium incorporated with 2 μM of NAA (Fig. 3d, Table 3). The inclusion of 4 μM NAA into the ½ MS medium did not affect the percent of root induction; however, it significantly decreased the formation of roots (3.4 ± 1.3 per shoot). About 97% of the in vitro-developed plantlets acclimatized in the greenhouse (Fig. 3e, f).
Table 3.
Effect NAA on root induction from in vitro-developed shoots of C. goeringii
| NAA (µM) | Root induction (%) | Number of roots per shoot |
|---|---|---|
| 0 | 37.4 ± 4.2c | 2.4 ± 0.7c |
| 1 | 81.3 ± 2.3b | 3.9±1.0b |
| 2 | 100 ± 0.0a | 5.3 ± 1.1a |
| 4 | 100 ± 0.0a | 3.4 ± 1.3b |
Value (mean ± standard error) within a column followed by the same letters are not significantly different at p ≤ 0.05
Discussion
Rhizome formation is an important means of in vitro propagation of terrestrial Cymbidium (Chang and Chang 2000a). In hybrid Cymbidium, the inclusion of 1 or 2 mg L−1 of 2,4-D into the medium resulted in the induction of embryogenic callus from PLBs (Teixeira da Silva 2014). In contrast, rhizome explants of C. goeringii grown on MS medium incorporated with 2,4-D developed only rhizomes (Table 1), which can be attributed to the difference in type and source of explant, genotype, and culture environment. Several studies have demonstrated that exogenous application of auxins is necessary for rhizome induction (Novak et al. 2014). The stimulating effect of auxins in rhizome development has been reported in C. aloifolium (Nayak et al. 1998), C. forrestii (Paek and Yeung 1991), and C. kanran (Shimasaki and Uemoto 1990). These studies have shown that auxin addition inhibits shoot regeneration. In this study, shoots developed from the tip of regenerated rhizomes on the PGR-free medium after 6 months of culturing. The conversion of rhizome meristems into shoots mainly depends on the endogenous levels of PGRs.
In vitro propagation through direct adventitious shoot regeneration is a prerequisite for the extensive commercial propagation and genetic transformation of plants. Exogenous application of cytokinins is necessary for shoot formation from rhizome segments of Cymbidium species. In this work, direct adventitious shoot formation was achieved on MS medium incorporated with BA or TDZ (Table 2). The use of BA has been reported to promote adventitious shoot formation in rhizome explants of C. aloifolium (Nayak et al. 1998), C. faberi (Hasegawa et al. 1985), C. forrestii (Paek and Yeung 1991), and C. kanran (Lee et al. 1986; Shimasaki and Uemoto 1990). In this work, direct adventitious shoot and rhizome induction were observed when the rhizome segments of C. goeringii were grown on MS medium incorporated with greater concentration of BA. Similarly, the presence of 20 μM BA induced upright shoot formation from rhizomes of Cymbidium ensifolium (Lu et al. 2001). In contrast, a maximum number of shoots (8.3) were obtained when the shoot tip of Cymbidium iridioides was grown on MS medium incorporated with a lower concentration (0.5 mg L−1) of BA (Pant and Swar 2011). The number of shoots per rhizome segment was significantly increased when the medium was fortified with TDZ. The explants also developed rhizomes, except in higher concentration of TDZ. Similar results have been observed in C. sinense (Chang and Chang 2000a). Multiple PLBs were induced from pseudostem explants of C. giganteum grown on MS medium containing a low concentration of TDZ (Roy et al. 2012). In Cymbidium hybrid, direct organogenesis was achieved when PLBs were cultured on the medium containing TDZ (Teixeira da Silva 2014). In general, the presence of cytokinin in the medium induced both the shoot and rhizome formation in rhizome explants of Cymbidium. Among the two cytokinins, TDZ was found to be better for direct adventitious shoot formation in C. goeringii. Similarly, TDZ has been used to maximize shoot formation in various orchids such as Cypripedium lentiginosum (Jiang et al. 2017), Dendrobium crepidatum (Bhattacharyya et al. 2016), Dimorphorchis lowii (Jainol and Gansau 2017), Herminium lanceum (Singh and Babbar 2016) and Phalaenopsis ‘Surabaya’ (Balilashaki et al. 2015).
A combination of cytokinin and auxin has often been used to promote shoot regeneration in Cymbidium species (Peak and Kozai 1998). Nayak et al. (1998) reported that a combination of BA and NAA maximized adventitious shoot production in C. aloifolium. In C. giganteum, the maximum number of shoots or PLBs were obtained in medium incorporated with BA and NAA (Hossain et al. 2010). In Cymbidium hybrids, the highest frequency of regeneration (62%) with a mean of 3.3 shoots per explant was obtained in SH medium with 1.0 mg L−1 BA and 0.1 mg L−1 NAA (Pal et al. 2016). Similarly, the presence of BA and NAA in medium induced multiple shoots from rhizome explants of C. goeringii (Shimasaki and Uemoto 1991), C. forrestii (Paek and Yeung 1991), C. kanran (Shimasaki and Uemoto 1990), and C. sinense (Gao et al. 2014). In this study, BA in combination with 2,4-D was more useful for direct adventitious shoot production compared to BA alone. However, more shoot buds per rhizome explant of C. goeringii was achieved on MS medium incorporated with TDZ and 2,4-D. The beneficial effect of TDZ in combination with other PGRs has also been reported for other Cymbidium species. In C. faberi, TDZ combination with NAA was effective for adventitious shoot production from PLB (Tao et al. 2011). The combination of N6-(2-isopentenyl) adenine, TDZ, 6-aminopurine adenine, and NAA was highly effective for shoot bud regeneration from rhizome explants of C. ensifolium var. misericors (Chang and Chang 2000b).
Rooting of in vitro-regenerated shoots and acclimatization of plantlets are crucial steps in micropropagation. Root induction mainly depends on PGRs that are used to induce shoots and the composition of culture medium. In this study, 37.4 ± 4.2% of the shoots developed roots obtained from MS medium incorporated with 20 μM of 2,4-D and 8 μM of BA when grown on ½ MS medium. Whereas, ½ MS medium incorporated with NAA showed a significant improvement in the rooting of in vitro-developed shoots of C. goeringii. The promoting effect of NAA on root induction has also been reported for C. aloifolium (Nayak et al. 1998) and C. sinense (Chang and Chang 2000a; Gao et al. 2014). On the contrary, rooting of C. kanran was inhibited by the inclusion of auxin to the culture medium (Shimasaki and Uemoto 1990). The acclimatized plants (97%) grew well in the greenhouse.
This is the first report on direct adventitious shoot regeneration of C. goeringii using seed-derived rhizome explants. The combination of TDZ and 2,4-D was found to inhibit rhizome induction and promote direct adventitious shoot formation. In vitro-produced shoots rooted the best on ½ MS medium incorporated with NAA. The protocol proposed in this study could be useful for germplasm conservation, in genetic transformation studies, and for large-scale commercial propagation of C. goeringii.
Acknowledgements
This research was supported by Technology Development Program for Agriculture and Forestry, Ministry for Food, Agriculture, Forestry and Fisheries, Republic of Korea. Project No: 315025-3-3-HD040. This article was supported by the KU Research Professor Program of Konkuk University.
Authors contributions
Han Yong Park and Iyyakkannu Sivanesan designed the experiment, analyzed the data and prepared the manuscript. Kyung Won kang and Doo Hwan Kim edited the manuscript.
References
- Balilashaki K, Vahedi M, Karimi R. In vitro direct regeneration from node and leaf explants of Phalaenopsis cv. ‘Surabaya’. Plant Tissue Cult Biotechnol. 2015;25:193–205. doi: 10.3329/ptcb.v25i2.26254. [DOI] [Google Scholar]
- Bembemcha P, Kishor R, Bai N. In vitro immature embryo germination and propagation of Vanda stangeana Rchb. f., an orchid endemic to India. Hortic Environ Biotechnol. 2016;57:615–624. doi: 10.1007/s13580-016-0051-7. [DOI] [Google Scholar]
- Bhattacharyya P, Kumaria S, Job N, Tandon P. En-masse production of elite clones of Dendrobium crepidatum: a threatened, medical orchid used in Traditional Chinese Medicine (TCM) J Appl Res Med Aromat Plants. 2016;3:168–176. [Google Scholar]
- Chang C, Chang WC. Effect of thidiazuron on bud development of Cymbidium sinense Willd in vitro. Plant Growth Regul. 2000;30:171–175. doi: 10.1023/A:1006341300416. [DOI] [Google Scholar]
- Chang C, Chang WC. Micropropagation of Cymbidium ensifolium var. misericors through callus-derived rhizomes. In Vitro Cell Dev Biol Plant. 2000;36:517–520. doi: 10.1007/s11627-000-0092-5. [DOI] [Google Scholar]
- Chugh S, Guha S, Rao IU. Micropropagation of orchids: a review on the potential of different explants. Sci Hortic. 2009;122:507–520. doi: 10.1016/j.scienta.2009.07.016. [DOI] [Google Scholar]
- Chung MY, Chung MG. Allozyme diversity and population structure in Korean populations of Cymbidium goeringii (Orchidaceae) J Plant Res. 1999;112:139–144. doi: 10.1007/PL00013868. [DOI] [Google Scholar]
- Compton ME. Statistical methods suitable for the analysis of plant tissue culture data. Plant Cell Tissue Organ Cult. 1994;37:217–241. [Google Scholar]
- Devi NP, Lisipriya B, Bai N. Asymbiotic seed germination and mass multiplication of Taprobanea spathulata (L.) Christenson (Asparagales: Orchidaceae): a medicinally important epiphytic orchid. Braz J Biol Sci. 2015;31:271–286. [Google Scholar]
- Diengdoh RV, Kumaria S, Tandon P, Das MC. Asymbiotic germination and seed storage of Paphiopedilum insigne, an endangered lady’s slipper orchid. South Afr J Bot. 2017;112:215–224. doi: 10.1016/j.sajb.2017.05.028. [DOI] [Google Scholar]
- Gao R, Wu SQ, Piao XC, Park SY, Lian ML. Micropropagation of Cymbidium sinense using continuous and temporary airlift bioreactor systems. Acta Physiol Plant. 2014;36:117–124. doi: 10.1007/s11738-013-1392-9. [DOI] [Google Scholar]
- Hasegawa A, Goi M. Rhizome formation in Cymbidium goeringii Reichenbach fil. and Cymbidium kanran Makino in shoot-tip culture. J Jpn Soc Hortic Sci. 1987;56:70–78. doi: 10.2503/jjshs.56.70. [DOI] [Google Scholar]
- Hasegawa A, Ohashi H, Go M. Effect of BA, rhizome length, mechanical treatment and liquid shaking culture on the shoot formation from rhizome in Cymbididum faberi Rolfe. Acta Hortic. 1985;166:25–40. doi: 10.17660/ActaHortic.1985.166.3. [DOI] [Google Scholar]
- Hossain MM, Sharma M, Teixeria da Silva JA, Pathak P. Seed germination and tissue culture of Cymbidium giganteum Wall. Ex Lindl. Sci Hortic. 2010;123:479–487. doi: 10.1016/j.scienta.2009.10.009. [DOI] [Google Scholar]
- Huan LVT, Takamura T, Tanaka M. Callus formation and plant regeneration from callus through somatic embryo structures in Cymbidium orchid. Plant Sci. 2004;166:1443–1449. doi: 10.1016/j.plantsci.2004.01.023. [DOI] [Google Scholar]
- Jainol JE, Gansau JA. Embryogenic callus induction from leaf tip explants and protocorm-like body formation and shoot proliferation of Dimorphorchis lowii: Borneon endemic orchid. AGRIVITA J Agric Sci. 2017;39:1–10. [Google Scholar]
- Jiang H, Chen M-C, Lee Y-I. In vitro germination and low-temperature seed storage of Cypripedium lentiginosum P.J. Cribb & S.C. Chen, a rare and endangered lady’s slipper orchid. Sci Hortic. 2017;225:471–479. doi: 10.1016/j.scienta.2017.07.040. [DOI] [Google Scholar]
- Kang TJ, Yang DC. Days to germination and effect of growth regulator on rhizome growth in Cymbidium goeringii Hybrid. Korean J Plant Res. 2003;6:144–148. [Google Scholar]
- Kim DH, Kang KW, Sivanesan I. In vitro propagation of Cymbidium hybrid. Propag Ornam Plants. 2017;17:48–54. [Google Scholar]
- Koirala D, Pradhan S, Pant B. Asymbiotic seed germination and plantlet development of Coelogyne fuscescens Lindl., a medicinal orchid of Nepal. Sci World. 2013;11:97–100. doi: 10.3126/sw.v11i11.8561. [DOI] [Google Scholar]
- Lee JS, Shim KK, Yoo MS, Lee JS, Kim YJ. Studies on rhizome growth and organogenesis of Cymbidium kanran cultured in vitro. J Korean Soc Hortic Sci. 1986;27:174–180. [Google Scholar]
- Lu I, Sutter E, Burger D. Relationships between benzyladenine uptake, endogenous free IAA levels and peroxidase activities during upright shoot induction of Cymbidium ensifoilum cv. Yuh Hwa rhizomes in vitro. Plant Growth Regul. 2001;35:161–170. doi: 10.1023/A:1014430715746. [DOI] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Nayak NR, Rath SP, Patnaik S. In vitro propagation of three epiphytic orchids, Cymbidium aloifolium (L.) Sw., Dendrobium aphyllum (Roxb.) Fisch. and Dendrobium moschatum (Buch.-Ham.) Sw. through thidiazuron-induced high frequency shoot proliferation. Sci Hortic. 1997;71:243–250. doi: 10.1016/S0304-4238(97)00075-7. [DOI] [Google Scholar]
- Nayak NR, Chand PK, Rath SP, Patnaik S. Influence of some plant growth regulators on the growth and organogenesis of Cymbidium aloifolium (L.) Sw. seed derived rhizomes in vitro. In Vitro Cell Dev Biol Plant. 1998;34:185–188. doi: 10.1007/BF02822706. [DOI] [Google Scholar]
- Novak SD, Luna LJ, Gamage RN. Role of auxin in orchid development. Plant Signal Behav. 2014;9(10):e972277. doi: 10.4161/psb.32169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paek KY, Kozai T. Micropropagation of temperate Cymbidium via rhizome culture. HortTechnology. 1998;8:283–288. [Google Scholar]
- Paek KY, Yeung EC. The effect of 1- naphthaleneacetic acid and N6-benzyladenine on the growth of Cymbidium forrestii rhizomes in vitro. Plant Cell Tissue Organ Cult. 1991;24:65–71. doi: 10.1007/BF00039732. [DOI] [Google Scholar]
- Pal R, Ram RB, Dayamma M, Barman D, Sing DR, Sharma P. Response of culture media on multiplication of Cymbidium hybrids cultured in vitro. J Ornam Hortic. 2016;19:119–124. [Google Scholar]
- Pant B, Swar S. Micropropagation of Cymbidium iridioides. Nepal J Sci Technol. 2011;12:91–96. [Google Scholar]
- Roy AR, Sajeev S, Pattanayak A, Deka BC. TDZ induced micropropagation in Cymbidium giganteum Wall. Ex Lindl. and assessment of genetic variation in the regenerated plants. Plant Growth Regul. 2012;68:435–445. doi: 10.1007/s10725-012-9732-0. [DOI] [Google Scholar]
- Shimasaki K, Uemoto S. Micropropagation of terrestrial Cymbidium species using rhizomes, developed from seeds and pseudobulbs. Plant Cell Tissue Organ Cult. 1990;22:237–244. doi: 10.1007/BF00033642. [DOI] [Google Scholar]
- Shimasaki K, Uemoto S. Rhizome induction and plantlet regeneration of Cymbidium goeringii from flower bud cultures in vitro. Plant Cell Tissue Organ Cult. 1991;25:49–52. doi: 10.1007/BF00033912. [DOI] [Google Scholar]
- Singh DK, Babbar SB. In vitro propagation and chemical profiling of Herminium lanceum (Thunb. ex Sw.) Vuijk, a medicinally important orchid, for therapeutically important phenolic acids. Plant Biotechnol. 2016;33:153–160. doi: 10.5511/plantbiotechnology.16.0523a. [DOI] [Google Scholar]
- Tao J, Yu L, Kong F, Zhao D. Effect of plant growth regulators on in vitro propagation of Cymbidium faberi Rolfe. Afr J Biotechnol. 2011;10:15639–15646. [Google Scholar]
- Teixeira da Silva JA. Response of hybrid Cymbidium (Orchidaceae) protocorm-like bodies to 26 plant growth regulators. Bot Lith. 2014;20:3–13. [Google Scholar]
- Tsuji K, Kato M. Odor-guided bee pollinators of two endangered winter/early spring blooming orchids, Cymbidium kanran and Cymbidium goeringii, in Japan. Plant Species Biol. 2010;25:249–253. doi: 10.1111/j.1442-1984.2010.00294.x. [DOI] [Google Scholar]
- Ueda H, Torikata H. Organogenesis in the meristem cultures of cymbidiums. III. Histological studies on the shoot formation at the rhizome-tips of Cymbidium goeringii Reichb. F. cultured in vitro. J Jpn Soc Hortic Sci. 1969;38:262–265. doi: 10.2503/jjshs.38.262. [DOI] [Google Scholar]
- Wu J, Ma H, Lü M, Han S, Zhu Y, Jin H, Liang J, Liu L, Xu J. Rhizoctonia fungi enhance the growth of the endangered orchid Cymbidium goeringii. Botany. 2010;88:20–29. doi: 10.1139/B09-092. [DOI] [Google Scholar]