Abstract
We started a cell suspension culture from magenta coloured calli of cockscomb to study the effect of biotic and abiotic elicitors on the biosynthesis of betalain pigments. The cultures were grown in a flask containing 30 ml MS media fortified with 13.5 μM 2,4-D and 0.44 μM BAP. These cultures were elicited during its log-phase of growth using fungal elicitors (prepared from mycelia of Fusarium oxysporum), yeast extract, copper sulphate and cobalt chloride. The elicitation reduced the cell count, cell viability and percent pigmented cell in the suspension culture. Similarly, it also resulted in reduced betalain content by all the elicitors except 0.125 × 10−3% fungal elicitor. Rather, fungal elicitor at this concentration significantly enhanced the amaranthin, betanin, betalamic acid and betaxanthin content in the culture. Besides this, copper sulphate doubled the pigment contribution (ratio of particular pigment content to total pigment content) of betaxanthin at all the concentrations. Therefore, we conclude that fungal elicitor can further be investigated to enhance the content of betalain pigments in suspension culture at a larger scale.
Keywords: Cockscomb, Elicitor, Suspension culture, Betacyanin, Betaxanthin
Introduction
Cockscomb (Celosia cristata L.) is commonly grown in tropical and subtropical gardens as bedding or potted plant. It is also used as cut flower in flower arrangements. The value of cockscomb is for its vibrant colours varying from yellow to various shades of reds and violets (Schliemann et al. 2001). The colouration of the inflorescence of cockscomb is because of the presence of water soluble pigment—betalain (Bohm 1998; Schliemann et al. 2001). Chemically, these are conjugates of a chromophore betalamic acid and occur in two forms viz. red-violet coloured betacyanin and yellow coloured betaxanthin (Strack et al. 2003).
Use of betalains as natural colourants in dairy food products, confectionary, jams, sausages and cannery is known from many centuries. Presently, betalains are used as safe food colourants under the E-162 code in European Union and as additive 73.40 in the 21 CFR section of the food and Drug Administration (FDA) in the United States (Castellar et al. 2003). Apart from this, betalains have high antioxidant, anti-inflammatory, hepatoprotective and cancer chemo-preventive activities and the capacity to protect low-density lipoproteins (LDLs) from oxidation (Georgiev et al. 2008).
Currently, natural food colourants are more in demand compared to their synthetic counterparts because they are safe. In cockscomb, betalain production mainly depends on the presence of number and size of inflorescence in fully developed plant. Besides, cultivating plants is season- and region-dependent, which is a main hurdle in the supply of raw material for pigment extraction. Reports about production of betalain pigments in in vitro cultures of Chenopodium rubrum (Berlin et al. 1986), Portulaca grandiflora (Bohm et al. 1991), Beta vulgaris L. (Leathers et al. 1992; Savitha et al. 2006), Portulaca sp. (Noda and Adachi 2000) and Pereskia aculeata (de Aguiar et al. 2015) are available. Production of betalains from cockscomb in fermenters is a promising approach. However, prior to this, a study establishing the effectiveness of an elicitor in enhancing the betalain content in the cell suspension is needed.
In our previous communication we reported induction and isolation of callus lines of cockscomb displaying pigment production of variable degree. We have isolated and maintained three callus lines viz. magenta coloured, red coloured and dark-red coloured, initiated from shoot-tip explants over Murashige and Skoog (MS) medium fortified with 13.5 μM 2,4-Dichlorophenoxyacetic acid (2,4-D) and 0.44 μM 6-benzylaminopurine (BAP). As obvious, the dark-red coloured callus line was rich in betalain content followed by red and magenta coloured callus lines (Warhade and Badere 2015). The present study reports initiation of cell suspension culture from these callus lines and subsequent studies on the effect of biotic and abiotic elicitors on betalain synthesis in the suspension culture.
Materials and methods
Initiation of suspension culture
Three callus lines of cockscomb viz. magenta, red and dark-red were isolated and maintained over MS medium containing 13.5 μM 2,4-D and 0.44 μM BAP (Warhade and Badere 2015). The suspension culture was initiated from these three callus lines. For this, the calli were sub-cultured in 30 ml liquid MS medium containing 13.5 μM 2,4-D and 0.44 μM BAP and agitated at 120 rpm and 25 ± 2º C under 16 h photoperiod in an incubating shaker. The suspension thus obtained was used to seed a fresh suspension culture by transferring 2 ml of it to the fresh medium.
Parameters used to study the growth kinetics of suspension culture
Homogeneous suspension was obtained after three subcultures. Later, 50,000 viable cells from suspension were seeded in 30 ml medium to study growth kinetics of the suspension. The growth kinetics was studied by recording cell count, cell viability, packed cell volume (PCV), fresh weight and percent pigmented cell at the interval of 3 days up to 18 days. All experiments were performed in triplicate.
Cell count
The cell count was determined using a hemocytometer (Supratek) according to the manufacturer’s manual and expressed as cells per ml of culture.
Cell viability
The percent viability of cells in the suspension was estimated by Evans blue staining method which preferentially stains non-viable cells (Mustafa et al. 2011).
PCV
The PCV of the suspension culture was determined by centrifuging 10 ml of cell suspension in a graduated centrifuge tube at 10,000 rpm for 5 min. It was expressed as ml of volume occupied by the cells per ml of culture.
Fresh weight
To determine fresh weight of cells in culture, 10 ml of it was centrifuged at 10,000 rpm for 5 min. The weight of resulting pellet was expressed as grams per ml of culture.
Pigmented cell count
Percentage of pigmented cells in suspension culture was determined by counting number of pigmented cells among all the cells in culture under a microscope.
Preparation of elicitors
The effect of biotic elicitors like fungal cell residue and yeast extract, and abiotic elicitors like cobalt chloride and copper suplhate was studied on the biosynthesis of betalain pigments in the suspension culture.
The culture of Fusarium oxysporum Schleeht (NFCCI–745) was obtained from Agharkar Research Institute, Pune (India). The fungus was cultured over liquid Potato-Dextrose medium for about a month. Later it was autoclaved at 121 °C for 20 min and filtered through Whatman No. 1 filter paper. The fungal residue was dried at room temperature for 48 h and the dried sample ground in mortar and pestle. The elicitor from this powder was prepared in distilled water at concentration of 1 mg/ml. In the same manner, aqueous solutions of copper sulphate and cobalt chloride were also prepared at concentration of 1 mg/ml. The solution of yeast extract was prepared at concentration of 2 mg/ml in distilled water. The elicitors were sterilized by autoclaving before use.
Elicitation of cell suspension culture
The suspension culture was elicited 12 days after its initiation when the culture entered the log phase of its growth by aseptically adding elicitor to it. The concentrations of elicitor used were as follows: fungal cell elicitor [0.125 × 10−3, 0.250 × 10−3 and 0.500 × 10−3% (w/v)], yeast extract (150, 200 and 250 mg/l), copper sulphate (6.4, 12.8 and 25.6 µM) and cobalt chloride (6.7, 13.4 and 26.8 µM). Before elicitation, cell count, cell viability and percent pigmented cells were determined. Unelicited cultures served as control. Each experiment was performed in triplicate.
Harvesting of suspension culture and quantification of betalain pigments
The cell count, cell viability and percent pigmented cells in suspension was determined after 5 days of elicitation and harvested by centrifuging it at 10,000 rpm for 10 min. at 4o C. The betalain pigments (amaranthin, betanin, betalamic acid and betaxanthin) were photometrically quantified as described by Cai et al. (1998) with a slight modification in the residue obtained after centrifugation. The original formula calculated the pigment content per 100 g fresh weight of tissue, whereas we have calculated per g of fresh weight. Similarly, we have extracted the pigments in 10 ml of solvent. The residue obtained after centrifugation was extracted with five volumes of 1% methanolic HCl followed by centrifugation at 10,000 rpm for 12 min. The supernatant was collected and read at 538, 536, 480 and 430 nm against the solvent blank. The complete process was carried out under diffused light. The content of individual pigment was determined by the following formula:
where, A is the absorption at given wavelength, M is molecular weight of the pigment, D is dilution factor, Va is total volume of fresh pulp solution (ml), Wa is fresh weight of pulp solution, ε is molecular extinction coefficient of the pigment and L is path length.
The λMax of amaranthin, betanin, betalamic acid and betaxanthin were 536, 538, 430 and 480 nm, respectively. Similarly, the molecular weight (g/mol) of these pigments were 726.6 (amaranthin), 550 (betanin), 212 (betalamic acid) and 309 (betaxanthin). The molar extinction coefficient (L/mol/cm) of amaranthin, betanin, betalamic acid and betaxanthin were 5.66 × 104, 6.00 × 104, 2.40 × 104 and 48.00 × 106, respectively (Girod and Zryd 1991; Cai et al. 1998; Castellanos-Santiago and Yahia 2008; Biswas et al. 2013).
Similarly, pigment contribution and pigment ratio in the elicited suspension culture were also determined according to the formula mentioned in our earlier communication (Warhade and Badere 2105). The formulae used were:
Statistical analysis
Statistical computations like mean, standard error and Student’s t-value were performed using MS-Excel and XL-STAT.
Results
Growth kinetics of cell suspension culture
In the first attempt, we initiated the cell suspension from dark-red coloured callus line. However, the cells did not proliferate and eventually turned black. The same was the consequence when suspension was initiated using red coloured callus line. Lastly, the cell suspension culture was initiated from magenta coloured callus line. In this, the cells multiplied and remained viable. Hence, the suspension culture was initiated from magenta coloured callus line for further studies. First we studied the growth kinetics of suspension culture using parameters like cell count, percent cell viability, percent pigmented cells, PCV and fresh weight.
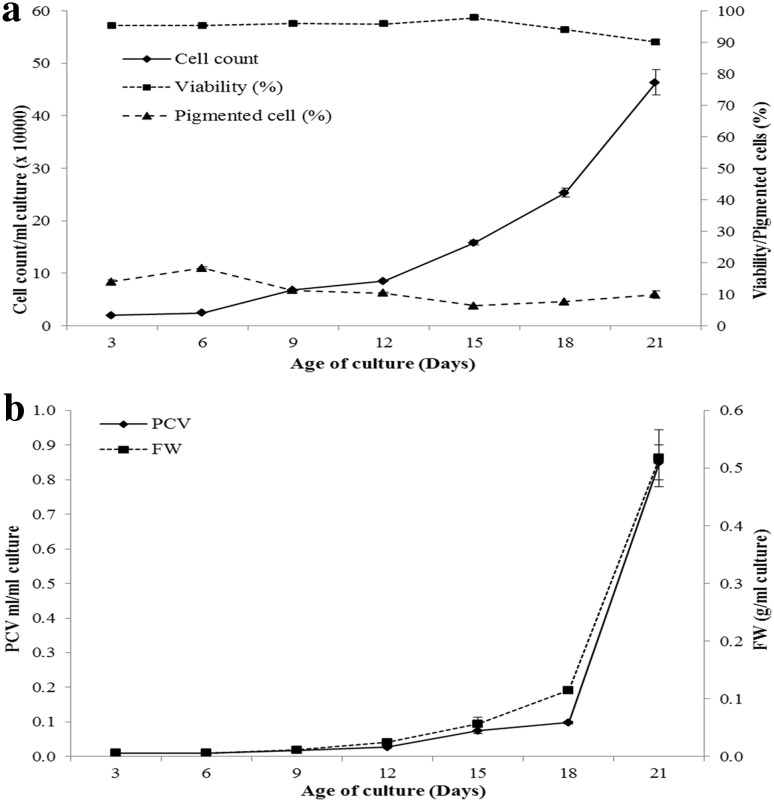
Three days after seeding the culture, cell count reached 19,800 per ml, which increased slightly in next 3 days. However, later the growth picked-up and gradually the cell count increased from 68,000 per ml on 9th day of seeding to reach its maximum of 463,200 per ml on 21st day of seeding the culture (Fig. 1a). The cell viability remained unaffected during 21 days of culture. Markedly, up to 15 days after seeding the culture, cell viability remained around 96%. However, later a slight decrease of about 6% in cell viability was recorded (Fig. 1a). Percent pigmented cells also followed the similar trend. The cell suspension consisted of 13.97% pigmented cells 3 days after seeding the culture. In the next 3 days, 18.28% of cells in the suspension were pigmented. However, later a slight decrease in proportion of pigmented cells was observed (Fig. 1a).
Fig. 1.
Growth kinetics of cell suspension culture. a Growth in terms of cell count, cell viability and percent pigmented cells, b growth in terms of PCV (packed cell volume) and FW (fresh weight)
The growth curve plotted using PCV and fresh weight followed the identical pattern. Both, PCV and fresh weight remained unchanged till 9th day of seeding the suspension. Later, PCV and fresh weight increased gradually till 18th day, where after both these parameters increased remarkably in 21-day old suspension (Fig. 1b).
Effect of elicitors on growth of suspension culture
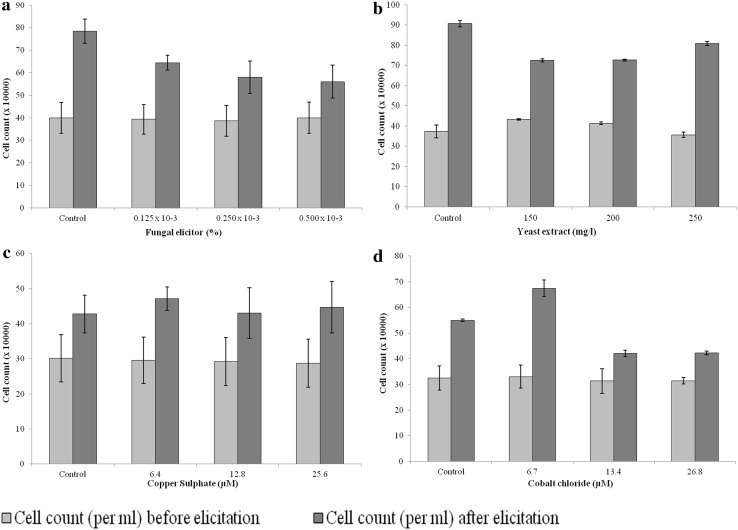
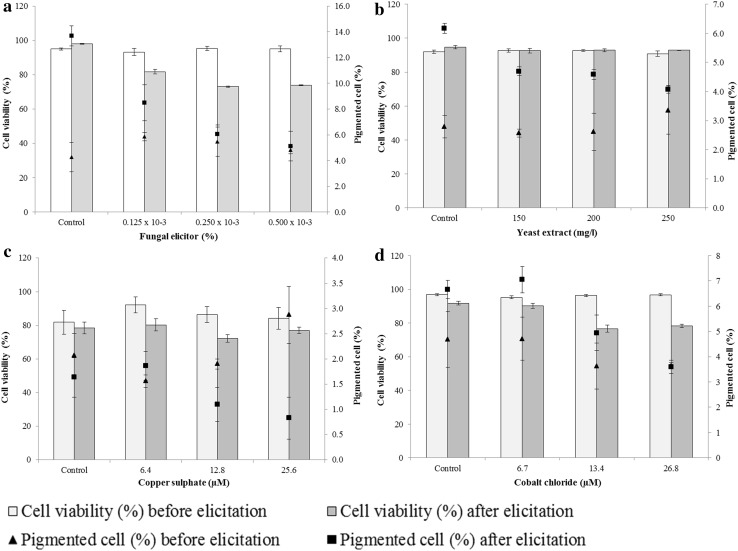
Since the cell count and cell viability seemed reliable parameters to assess the growth of culture, we studied the effect of elicitors only on these two parameters. In addition, we also estimated percent pigmented cells, as these are the cells containing betalain pigment. Eliciting the culture with biotic elicitors (fungal elicitor and yeast extract) led to the decrease in cell count, cell viability and percent pigmented cells in the suspension. Moreover, the decrease was concentration dependent in most of the cases (Figs. 2a, b, 3a, b). The effect of two abiotic elicitors namely copper sulphate and cobalt chloride was contrasting. While, copper sulphate did not affect the cell count and cell viability; it reduced the percent pigmented cells in the suspension at 25.6 μM concentration (Figs. 2c, 3c). In contrast to this, cobalt chloride increased the cell count slightly at 6.7 µM concentration. The other two higher concentrations, however, had an adverse effect on cell count in the culture (Fig. 2d). It was also remarkable that 6.7 µM cobalt chloride did not, at least, affect the cell viability and percent pigmented cells adversely as did the other two higher concentrations of cobalt chloride (Fig. 3d).
Fig. 2.
Effect of elicitation on cell count in the cell suspension culture. a Fungal elicitor, b yeast extract, c copper sulphate, d cobalt chloride
Fig. 3.
Effect of elicitation on cell viability and percent pigmented cells in the cell suspension culture. a Fungal elicitor, b yeast extract, c copper sulphate, d cobalt chloride
Effect of elicitors on the biosynthesis of betalain pigments in suspension culture
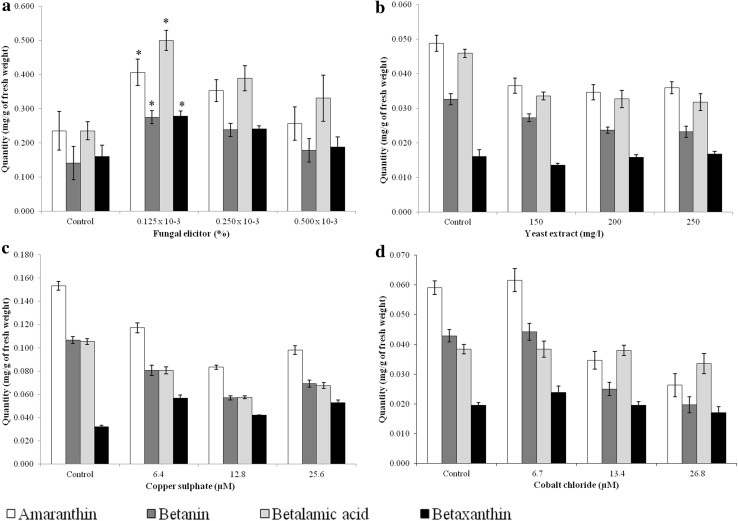
The fungal elicitor, among all, had a positive effect on the biosynthesis of betalain pigments in cell suspension culture as obvious from the increase in the content of amaranthin, betanin, betalamic acid and betaxanthin. Fungal elicitor at the concentration of 0.125 × 10−3% almost doubled the content of all these four pigments. 0.250 × 10−3% fungal elicitor also enhanced betalain content in culture but it was lower than the previous concentration. The highest concentration of fungal elicitor, however, had no significant effect on the content of betalain pigments. In contrast to the fungal elicitor, other three elicitors viz. yeast extract, copper sulphate and cobalt chloride adversely affected the biosynthesis of betalain pigments in the suspension culture (Fig. 4).
Fig. 4.
Effect of elicitation on the betalain pigments in the cell suspension culture. a fungal elicitor, b yeast extract, c copper sulphate, d Cobalt chloride. *Significant at 90% level of confidence according to Student’s t test
After assessing the effect of elicitors on absolute betalain content in cell suspension, we evaluated their effect on relative content of these pigments. For this, two parameters namely pigment contribution and pigment ratio were used. Pigment contribution suggests the proportion of a particular pigment among all in the sample. On the other hand, pigment ratio suggests the relative content of two pigments in the sample. Elicitation kept the pigment contribution unaffected. The only exception to this was copper sulphate, which almost doubled pigment contribution of betaxanthin in the suspension (Table 1). This increase in the content of betaxanthin led to a noticeable decrease in the pigment ratio of amaranthin-betaxanthin, betanin-betaxanthin and betalamic acid-betaxanthin in the suspension elicited with copper sulphate (Table 2). Apart from this the pigment ratio of amaranthin-betalamic acid and betanin-betalamic acid was more or less halved in the suspension elicited with cobalt chloride. The biotic elicitors, on contrary, maintained the pigment ratio in the suspension similar to the control (Table 2).
Table 1.
Pigment contribution in the suspension cultures after elicitation
| Elicitor and its concentration | Amaranthin | Betanin | Betalamic acid | Betaxanthin |
|---|---|---|---|---|
| Fungal elicitor (%) | ||||
| Control | 0.30 | 0.18 | 0.31 | 0.21 |
| 0.125 × 10−3 | 0.28 | 0.19 | 0.34 | 0.19 |
| 0.250 × 10−3 | 0.29 | 0.19 | 0.32 | 0.20 |
| 0.500 × 10−3 | 0.27 | 0.19 | 0.35 | 0.20 |
| Yeast extract (mg/l) | ||||
| Control | 0.34 | 0.23 | 0.32 | 0.11 |
| 150 | 0.33 | 0.24 | 0.30 | 0.13 |
| 200 | 0.32 | 0.22 | 0.31 | 0.15 |
| 250 | 0.33 | 0.21 | 0.30 | 0.16 |
| Copper sulphate (μM) | ||||
| Control | 0.39 | 0.27 | 0.26 | 0.08 |
| 6.4 | 0.35 | 0.24 | 0.24 | 0.17 |
| 12.8 | 0.35 | 0.24 | 0.24 | 0.18 |
| 25.6 | 0.34 | 0.24 | 0.24 | 0.18 |
| Cobalt chloride (μM) | ||||
| Control | 0.37 | 0.27 | 0.24 | 0.13 |
| 6.7 | 0.37 | 0.26 | 0.23 | 0.14 |
| 13.4 | 0.30 | 0.21 | 0.32 | 0.17 |
| 26.8 | 0.27 | 0.21 | 0.35 | 0.18 |
Table 2.
Pigment ratio in the suspension cultures after elicitation
| Elicitor and its concentration | Am:Be | Am:Ba | Am:Bx | Be:Ba | Be:Bx | Ba:Bx |
|---|---|---|---|---|---|---|
| Fungal elicitor (%) | ||||||
| Control | 1.67 | 1.00 | 1.46 | 0.60 | 0.88 | 1.47 |
| 0.125 × 10−3 | 1.47 | 0.81 | 1.46 | 0.55 | 0.99 | 1.80 |
| 0.250 × 10−3 | 1.48 | 0.91 | 1.46 | 0.61 | 0.99 | 1.62 |
| 0.500 × 10−3 | 1.44 | 0.78 | 1.37 | 0.54 | 0.95 | 1.76 |
| Yeast extract (mg/l) | ||||||
| Control | 1.48 | 1.07 | 3.06 | 0.72 | 2.06 | 2.88 |
| 150 | 1.37 | 1.09 | 2.64 | 0.79 | 1.93 | 2.43 |
| 200 | 1.46 | 1.06 | 2.19 | 0.73 | 1.50 | 2.06 |
| 250 | 1.57 | 1.13 | 2.12 | 0.72 | 1.35 | 1.88 |
| Copper sulphate (μM) | ||||||
| Control | 1.43 | 1.46 | 4.78 | 1.02 | 3.34 | 3.28 |
| 6.4 | 1.44 | 1.44 | 2.05 | 1.00 | 1.42 | 1.42 |
| 12.8 | 1.46 | 1.46 | 1.98 | 1.00 | 1.36 | 1.36 |
| 25.6 | 1.42 | 1.44 | 1.85 | 1.01 | 1.30 | 1.28 |
| Cobalt chloride (μM) | ||||||
| Control | 1.37 | 1.55 | 2.95 | 1.13 | 2.15 | 1.90 |
| 6.7 | 1.41 | 1.63 | 2.58 | 1.16 | 1.83 | 1.58 |
| 13.4 | 1.40 | 0.92 | 1.75 | 0.66 | 1.25 | 1.90 |
| 26.8 | 1.30 | 0.76 | 1.53 | 0.59 | 1.18 | 2.00 |
Am:Be amaranthin to betanin ratio, Am:Ba amaranthin to betalamic acid ratio, Am:Bx amaranthin to betaxanthin ratio, Be:Ba betanin to betalamic acid ratio, Be:Bx betanin to betaxanthin ratio, Ba:Bx betalamic acid to betaxanthin ratio
Discussion
We have tested cell count, cell viability, PCV and fresh weight to study the growth kinetics of cell suspension culture of cockscomb in the present investigation. However, we found use of cell count and cell viability to be more practical; because cell count is a direct method, which considers only the intact cells in culture. Also, reliability of cell count increases on coupling cell viability data with it. In contrast to this, PCV considers live as-well-as dead cells, cell debris and other particulate matter. Similarly, estimation of fresh weight is time-consuming and thus at times becomes impractical. Since, our aim in this study was to study the effect of elicitors on biosynthesis of betalain pigments in suspension culture; we also recorded the percent pigmented cells during the investigation. The elicitation decreased percent pigmented cell in the culture with an increase in elicitor concentration. This decrease in percent pigmented cells coincided with the decrease in betalain content of the cultures after elicitation with all elicitors except the fungal elicitor. In case of fungal elicitor, although, the percent pigmented cells decreased because of elicitation; the content of betalain pigments increased (at least at lower concentration). This suggests that mere pigmented cell percentage cannot be considered as the sole indicator of betalain content. The pigment content in the cultures might have increased after elicitation with fungal elicitor because of increase in accumulation of pigments inside the cell rather than increase in the number of pigment-synthesizing cells.
Induction of secondary metabolite synthesis in suspension cultures has been reported by many workers. Moreover, enhanced metabolite production in suspension after modification of media and elicitation of culture has also been reported. Akita et al. (2000, 2001, 2002) found that reduction in nitrogen and zinc concentration, modification of ammonium-to-nitrate ratio and removing cobalt and copper from the medium increased betacyanin production in B. vulgaris. Similarly, Noda and Adachi (2000) improved the betacyanin content in P. grandiflora by fortifying the medium with ascorbic acid. In contrast to this Guadarrama-Flores et al. (2015) reported synthesis of dopaxanthin, miraxanthin V, betanidin and decarboxy-betanidin in suspension culture of Celosia argentea var. plumosa over MS medium supplemented with BAP (6.66 µM) and 2,4-D (6.79 µM). Similarly, Kriznik and Pavokovic (2010) showed effectiveness of sucrose in improving the betanin content in suspension culture of B. vulgaris. On the other hand, Savitha et al. (2006) found Ca2+ to be more effective in betalain synthesis in B. vulgaris. Fungal cell wall elicitor is well-known for triggering plant secondary metabolism. Kadoo and Badere (2016) have proved earlier the induction of secondary metabolism in cucumber seedlings using cell wall elicitor of Fusarium oxysporum. Hence, in the present study we have used Fusarium oxysporum cell wall elicitor. Initially we had used higher concentration of fungal cell wall elicitor (Savitha et al. 2006). However, these concentrations inhibited the growth of cell suspension. Therefore, we attempted our studies with lower concentration of fungal cell wall elicitor.
A considerable difference in the pigment content between the various sets of control was apparent in the present investigation. To study the effect of elicitor on pigment synthesis, a fresh suspension was initiated from the mother culture obtained after 4–5 subcultures of the suspension initiated from friable calli. The mother culture was obtained afresh for each elicitor we have investigated. Therefore, possibly the somaclonal variation occurring during callusing might have resulted in the variation of pigment content of control. Hence, to reduce the error we have compared the results of elicitor treatment to its respective control to draw any conclusion out of it.
Among all the elicitors used, fungal elicitor had the positive effect on biosynthesis of betalain pigments in the present investigation. Many times the fungal elicitor triggers the defense mechanism of plant cells, which enhances metabolite production (Goyal and Ramawat 2008; Baldi et al. 2009). Therefore, in the present study the fungal elicitor might have increased the content of precursors of betalains viz. tyrosine, DOPA, dopamine and betalamic acid in the suspension culture. This accumulation of precursors may further enhance betalain content in suspension culture by altering the biosynthetic pathway. Savitha et al. (2006) found that culture filtrate and dry cell powder of Aspergillus niger and Penicillium notatum enhances betalain production in culture of B. vulgaris. However, simultaneously, this reduced the cell mass of the suspension. Besides this, improvement of secondary metabolites like naphtodianthrones in Hypericum perforatum (Simic et al. 2014), forskolin in Coleus forskohlii (Swaroopa et al. 2013), psoralene in Psoralea corylifolia (Ahmed and Baig 2014) by fungal elicitors has also been reported. Although, in the present investigation yeast extract, copper sulphate and cobalt chloride could not trigger the cellular metabolism but reports about the utility of these elicitors have been made. Elicitation with yeast extract enhanced saponin production in suspension culture of Panax ginseng (Kim et al. 2001) and flavonoids in hairy root culture of Fagopyrum tataricum (Zhao et al. 2014). Similarly, Mukundan et al. (1999) found enhancement of betalain accumulation in hairy root culture of B. vulgaris by copper sulphate.
Although, elicitation with copper sulphate reduced total pigment content in suspension, it doubled the pigment contribution of betaxanthin. This indicates preferential enhancement in betaxanthin synthesis in the suspension on elicitation with copper sulphate. In the biosynthetic pathway of betalains, betalamic acid is an intermediate, which condenses with Cyclo-DOPA and dopamine or their derivatives to form various betalain pigments. Betacyanins are formed by the condensation of betalamic acid with Cyclo-DOPA or its derivatives. On the other hand, betaxanthins are formed when betalamic acid condenses with dopamine or its derivatives (Schliemann et al. 2001). Thus, it seems the relative activity of enzymes involved in the pigment synthesis sets the equilibrium, which determines the proportion of various pigments accumulated in the cells. Preferential accumulation of betaxanthin in suspension elicited with copper sulphate suggests that copper sulphate either inhibits the activity of enzymes involved in synthesis of betacyanins or enhances the activity of enzymes involved in synthesis of betaxanthins.
Cobalt chloride halved betanin to betalamic acid pigment ratio in the suspension cultures. Similarly, it also affected the other pigment ratios, although to a lesser extent. A careful analysis of data on pigment contribution reveals that elicitation with cobalt chloride enhanced the pigment ratio of betalamic acid, whereas reduced the pigment ratio of amaranthin and betanin. In contrast to this, the pigment ratio of betaxanthin was also slightly enhanced by cobalt chloride. Please note that betalamic acid is a central molecule in the biosynthetic pathway of betalain pigments. Betacyanins and betaxanthins are synthesized by condensation of Cyclo-DOPA/its derivates and dopamine/its derivatives with betalamic acid, respectively (Schliemann et al. 2001). Thus, it might be possible that cobalt chloride has somehow prevented the condensation of betalamic acid with Cyclo-DOPA/its derivates and reduced the levels of betacyanins and/or has favoured the condensation of betalamic acid with dopamine/its derivatives, thus enhancing the levels of betaxanthins.
Thus, based on the findings of current investigation, it seems likely that fungal elicitor may prove helpful in enhancing the betalain content in the suspension culture of cockscomb at larger scale. Similarly, a thorough investigation on role of copper sulphate and cobalt chloride might provide a better insight on the regulation of betalain biosynthesis in cockscomb.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Ahmed SA, Baig MMV. Biotic elicitor enhanced production of psoralen in suspension culture of Psolralea corylifolia L. Saudi J Biol Sci. 2014;21:499–504. doi: 10.1016/j.sjbs.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akita T, Hika Y, Nishi T. Production of betacyanins by a cell suspension culture of table beet (Beta vulgaris L.) Biosci Biotechnol Biochem. 2000;64:1807–1812. doi: 10.1271/bbb.64.1807. [DOI] [PubMed] [Google Scholar]
- Akita T, Hina Y, Nishi T. Effect of zinc deficiency on betacyanin production in a cell suspension culture of Table Beet (Beta vulgaris L.) Biosci Biotechnol Biochem. 2001;65:962–965. doi: 10.1271/bbb.65.962. [DOI] [PubMed] [Google Scholar]
- Akita T, Hina Y, Nishi T. New medium composition for high betacyanin production by cell suspension culture of Table Beet (Beta vulgaris L.) Biosci Biotechnol Biochem. 2002;66:902–905. doi: 10.1271/bbb.66.902. [DOI] [PubMed] [Google Scholar]
- Baldi A, Shrivastava AK, Bisaria VS. Fungal elicitors for enhanced production of secondary metabolites in plant cell suspension cultures. In: Varma A, Kharkwal AC, editors. Symbiotic fungi: principles and practice. Berlin: Springer; 2009. pp. 373–380. [Google Scholar]
- Berlin J, Sieg S, Strack D, Bokern M, Harms H. Production of betalains by suspension cultures of Chenopodium rubrum L. Plant Cell Tissue Organ Culture. 1986;5:163–174. doi: 10.1007/BF00040126. [DOI] [Google Scholar]
- Biswas M, Dey S, Sen R. Betalains from Amaranthus tricolor L. J Phrmacognosy Phytochem. 2013;1:87–95. [Google Scholar]
- Bohm BA. Introduction to flavonoids. Amsterdam: Harwood Academic Publishers; 1998. [Google Scholar]
- Bohm H, Bohm L, Rink E. Establishment and characterization of a betaxanthin-producing cell culture from Portulaca grandiflora. PCTOC. 1991;26:75–82. doi: 10.1007/BF00036109. [DOI] [Google Scholar]
- Cai Y, Sun M, Wu H, Huang R, Corke H. Characterization and quantification of betacyanin pigments from diverse Amaranthus species. J Agric Food Chem. 1998;46:2063–2070. doi: 10.1021/jf9709966. [DOI] [Google Scholar]
- Castellanos-Santiago E, Yahia M. Identificaion and quantification of betalains from the fruits of 10 Mexican prickly pear cultivars by high-performance liquid chromatography and electrospray ionization mass spectrometry. J Agric Food Chem. 2008;56:5758–5764. doi: 10.1021/jf800362t. [DOI] [PubMed] [Google Scholar]
- Castellar R, Obon JM, Alacid M, Fernandez-Lopez JA. Color properties and stability of betacyanins from Opuntia fruits. J Agric Food Chem. 2003;51:2772–2776. doi: 10.1021/jf021045h. [DOI] [PubMed] [Google Scholar]
- de Aguiar Lage D, da Silva Tirado M, Vanicore SR, Sabino KC, Alberelo N. Production of betalains from callus and cell cultures of Pereskia aculeata Miller, and unconventional leafy vegetable. PCTOC. 2015;122:341–350. doi: 10.1007/s11240-015-0771-x. [DOI] [Google Scholar]
- Georgiev V, Ilieva M, Bley T, Pavlov A. Betalain production in plant in vitro system. Acta Physiol Plant. 2008;30:581–593. doi: 10.1007/s11738-008-0170-6. [DOI] [Google Scholar]
- Girod PA, Zryd JP. Secondary metabolism in cultured red beet (Beta vulgaris L.) cells: differential regulation of betaxanthin and betacyanin biosynthesis. PCTOC. 1991;25:1–12. doi: 10.1007/BF00033905. [DOI] [Google Scholar]
- Goyal S, Ramawat KG. Increased flavonoids accumulation in cell suspension cultures of Pueraria tuberosa by elicitors. Indian J Biotechnol. 2008;7:378–382. [Google Scholar]
- Guadarrama-Flores B, Rodriguez-Monroy M, Cruz-Sosa F, Garcia-Carmona F, Gandia-Herrero F. Production of dihydroxylated betalains and dopamine in cell suspension cultures of Celosia argentea var. plumosa. J Agric Food Chem. 2015;63(10):2741–2749. doi: 10.1021/acs.jafc.5b00065. [DOI] [PubMed] [Google Scholar]
- Kadoo MR, Badere RS. Induction of phenylalanine ammonia lyase in the seedlings of cucumber and chilli by the elicitors is species- and tissue-specific. J Indian Bot Soc. 2016;95(1&2):203–209. [Google Scholar]
- Kim CY, Im HW, Kim HK, Huh H. Accumulation of 2,5-dimethoxy-1,4-benzoquinone in suspension cultures of Panax ginseng by a fungal elicitor preparation and a yeast elicitor preparation. Appl Microbiol Biotechnol. 2001;56(1–2):239–242. doi: 10.1007/s002530000557. [DOI] [PubMed] [Google Scholar]
- Kriznik B, Pavokovic D. Enhancement of betanin yield in transformed cells of sugar beet (Beta vulgaris L.) Acta Bot Croat. 2010;69:173–182. [Google Scholar]
- Leathers RR, Davin C, Zryd JP. Betalain producing cell cultures of Beta vulgaris L. var. Bikores Monogerm (Red Beet) In vitro Cell Dev Biol. 1992;28:39–45. doi: 10.1007/BF02823016. [DOI] [Google Scholar]
- Mukundan U, Bhide V, Dawda H. Production of betalains by hairy root cultures of Beta vulgaris L. In: Fu T-J, Singh G, Curtis WR, editors. Plant cell and tissue culture for the production of food ingredients. New York: Springer, Kluwer Academic/Plenum Publishers; 1999. pp. 121–127. [Google Scholar]
- Mustafa NR, Winter WD, Iren FV, Verpoorte R. Initiation, growth and cryopreservation plant cell suspension cultures. Nat Protoc. 2011;6:715–742. doi: 10.1038/nprot.2010.144. [DOI] [PubMed] [Google Scholar]
- Noda N, Adachi T. Induction of betacyanin synthesis and pigment accumulation in cell suspension cultures of Portulaca. Plant Biotechnol. 2000;17:27–34. doi: 10.5511/plantbiotechnology.17.27. [DOI] [Google Scholar]
- Savitha BC, Thimmaraju R, Bhagyalakshmi N, Ravishankar GA. Different biotic and abiotic elicitors influence betalain production in hairy root cultures of Beta vulgaris in shake-flask and bioreactor. Process Biochem. 2006;41:50–60. doi: 10.1016/j.procbio.2005.03.071. [DOI] [Google Scholar]
- Schliemann W, Cai Y, Degenkolb T, Schmidt J, Corke H. Betalains of Celosia argentea. Phytochemistry. 2001;58:159–165. doi: 10.1016/S0031-9422(01)00141-8. [DOI] [PubMed] [Google Scholar]
- Simic SG, Tusevski O, Maury S, Delaunay A, Joseph C, Hagege D (2014) Effects of polysachharide elicitors on secondary metabolite production and antioxidant response in Hypericum perforatum L. shoot cultures. Sci World J 1–10. 10.1155/2014/609649 [DOI] [PMC free article] [PubMed]
- Strack D, Vogt T, Schliemann W. Recent advances in betalain research. Phytochemistry. 2003;62:247–269. doi: 10.1016/S0031-9422(02)00564-2. [DOI] [PubMed] [Google Scholar]
- Swaroopa G, Anuradha M, Pullaiah T. Elicitation of forskolin in suspension cultures of Coleus forskohlii (Willd.) Briq. using bacterial elicitors. J Indian Bot Soc. 2013;92(1&2):97–100. [Google Scholar]
- Warhade MI, Badere RS. Isolation of callus lines of Celosia cristata L. with variation in betalain content. J Indian Bot Soc. 2015;94(12):89–96. [Google Scholar]
- Zhao J, Zou L, Zhang C, Li Y, Peng L, Xiang D, Zhao G. Efficient production of flavonoids in Fagopyrum tataricum hairy root cultures with yeast polysaccharide elicitation and medium renewal process. Pharmacogn Mag. 2014;10(39):234–240. doi: 10.4103/0973-1296.137362. [DOI] [PMC free article] [PubMed] [Google Scholar]